Valorization of Residual Babassu Mesocarp Biomass to Obtain Aroma Compounds by Solid-State Fermentation
Abstract
1. Introduction
2. Materials and Methods
2.1. Support Obtaining
2.2. Pre-Treatment of the Supports
2.3. Surface Characterization of the in Natura and Defatted Support—Scanning Electron Microscopy (SEM) and Textural Analysis (BET)
2.4. Microorganisms, Culture Maintenance, and Propagation
2.5. Preparing the Inoculum and Conducting the Fermentation
2.6. Monitoring of the Fermentations on the Supports
2.7. Statistical Analysis
2.8. Surface Analysis of the Fermented Support—Scanning Electron Microscopy (SEM) and Analytical Magnifier
3. Results and Discussion
3.1. Surface Characterization of the Raw and Treated Support—Scanning Electron Microscopy (SEM) and Textural Analysis (BET)
3.2. Inspection of the Fermentations on the Supports
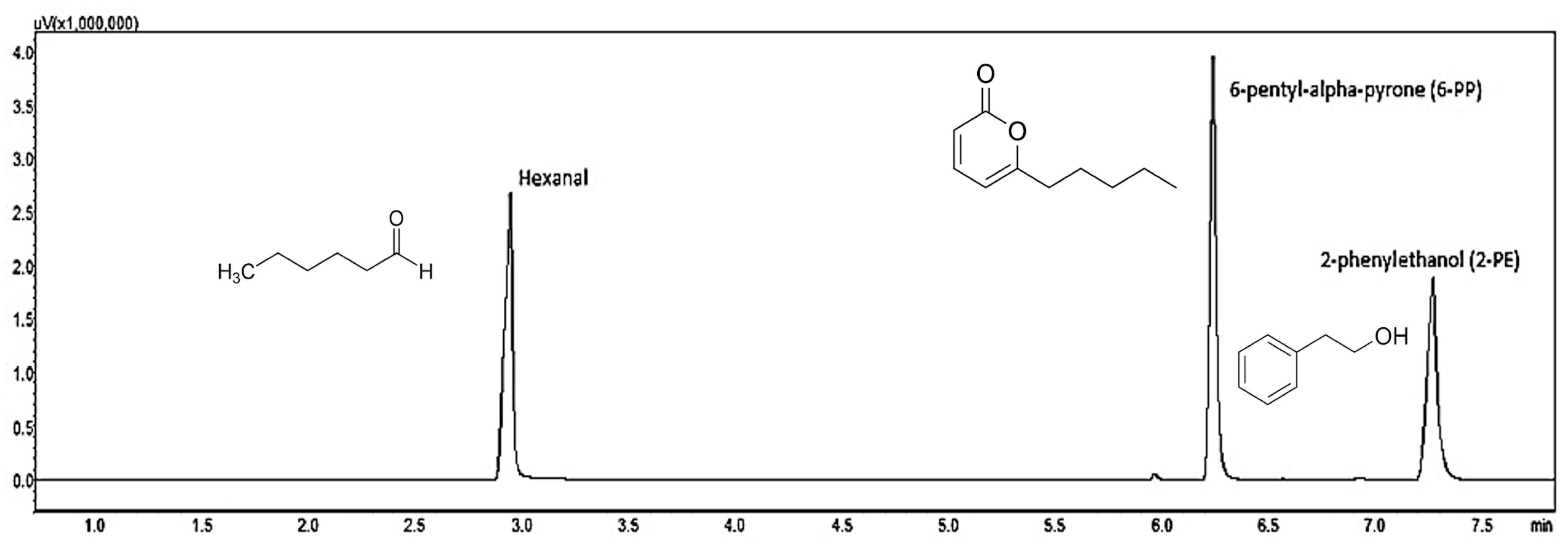
3.3. Extraction, Quantification, and Identification of Aroma Compounds
3.4. Statistical Analysis of Data
3.5. Surface Analysis of the Fermented Supports—Scanning Electron Microscopy (SEM) and Analytical Magnifier
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lobão, M.S.P.; Staduto, J.A.R. Perspectivas sobre o Desenvolvimento Rural Brasileiro: Notas teóricas. Rev. Parana. Desenvolv. 2018, 39, 13–27. [Google Scholar]
- Vanin, A.B.; Marquez, G. Aplicação do sistema de gestão ambiental na minimização dos impactos ambientais gerados por uma agroindústria abatedora de aves. J. Eng. Exact Sci. 2020, 6, 0640–0646. [Google Scholar] [CrossRef]
- Lira, R.K.d.S.; Zardini, R.T.; de Carvalho, M.C.C.; Wojcieszak, R.; Leite, S.G.F.; Itabaiana, I., Jr. Agroindustrial Wastes as a Support for the Immobilization of Lipase from Thermomyces lanuginosus: Synthesis of Hexyl Laurate. Biomolecules 2021, 11, 445. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, P.M.; Baruque, J.R.; Lira, R.K.d.S.; Leite, S.G.F.; Nascimento, R.P.D.; Borges, C.P.; Wojcieszak, R.; Itabaiana, I. Corn Cob as a Green Support for Laccase Immobilization—Application on Decolorization of Remazol Brilliant Blue R. Int. J. Mol. Sci. 2022, 23, 9363. [Google Scholar] [CrossRef] [PubMed]
- CEPEA. Centro de Estudos Avançados em Economia Aplicada (USP) em parceria com a Confederação da Agricultura e Pecuária do Brasil (CNA). 2021. Available online: https://www.cepea.esalq.usp.br/br/pib-do-agronegocio-brasileiro.aspx (accessed on 5 July 2024).
- Santos, M.D.F.G.D.; Alves, R.E.; De Brito, E.S.; Silva, S.D.M.; Da Silveira, M.R.S. Quality Characteristics of Fruits and Oils of Palms Native to the Brazilian Amazon. Rev. Bras. Frutic. 2017, 1, 39. [Google Scholar] [CrossRef]
- Magalhães, N.; Cavalcante, A.V.; Andrade, L.S.; Wanderley, C.R.P.; Marinho, G.; Pessoa, K.d.A.R. Produção de ácido cítrico por Aspergillus niger AN 400 a partir de resíduo agroindustrial. Eng. Sanit. Ambient. 2019, 24, 101–107. [Google Scholar] [CrossRef]
- United Nations Environment Programme (UNEP). Food Waste Index Report 2024. Nairob: [s. n.]. 2021. Available online: https://www.unep.org/resources/publication/food-waste-index-report-2024 (accessed on 16 July 2024).
- Diaz, A.B.; Blandino, A.; Caro, I. Value added products from fermentation of sugars derived from agro-food residues. Trends Food Sci. Technol. 2018, 71, 52–64. [Google Scholar] [CrossRef]
- Matei, J.C.; Oliveira, J.A.D.S.; Pamphile, J.A.; Polonio, J.C. Agro-industrial wastes for biotechnological production as potential substrates to obtain fungal enzymes. Ciência E Natura 2021, 43, e72. [Google Scholar] [CrossRef]
- Qian, X.; Yan, W.; Zhang, W.; Dong, W.; Ma, J.; Ochsenreither, K.; Jiang, M.; Xin, F. Current status and perspectives of 2-phenylethanol production through biological processes. Crit. Rev. Biotechnol. 2018, 39, 235–248. [Google Scholar] [CrossRef]
- Damiani, C.; Martins, G.A.D.S.; Becker, F.S. APROVEITAMENTO DE RESÍDUOS VEGETAIS: Potenciais e limitações. Portal De Livros Da Editora 2020, 1, Lv35. [Google Scholar]
- dos Santos, F.P.; de Magalhães, D.C.M.M.; Nascimento, J.d.S.; Ramos, G.L.D.P.A. Use of products of vegetable origin and waste from hortofruticulture for alternative culture media. Food Sci. Technol. 2022, 42, e00621. [Google Scholar] [CrossRef]
- Carboué, Q.; Claeys-Bruno, M.; Bombarda, I.; Sergent, M.; Jolain, J.; Roussos, S. Experimental design and solid state fermentation: A holistic approach to improve cultural medium for the production of fungal secondary metabolites. Chemom. Intell. Lab. Syst. 2018, 176, 101–107. [Google Scholar] [CrossRef]
- Sadh, P.K.; Duhan, S.; Duhan, J.S. Agro-industrial wastes and their utilization using solid state fermentation: A review. Bioresour. Bioprocess. 2018, 5, 1. [Google Scholar] [CrossRef]
- Hamrouni, R.; Molinet, J.; Miché, L.; Carboué, Q.; Dupuy, N.; Masmoudi, A.; Roussos, S. Production of coconut aroma in solid-state cultivation: Screening and identification of Trichoderma strains for 6-pentyl-alpha-pyrone and conidia production. J. Chem. 2019, 2019, 8562384. [Google Scholar] [CrossRef]
- Penha, M.P. Microencapsulamento de Aroma de Coco (6-Pentil-α-Pirona) e de Aroma de Pêssego (γ-Decalactona) Produzidos Por Trichoderma Harzianum IOC 4042 e Yarrowia Lipolityca ATCC 2060 Utilizando Processos de Fermentação no Estado Sólido e Biotransformação.Tese (Doutorado em Ciência de Alimentos); Universidade Federal do Rio de Janeiro: Rio de Janeiro, Brazil, 2015. [Google Scholar]
- Martínez-Avila, O.; Muñoz-Torrero, P.; Sánchez, A.; Font, X.; Barrena, R. Valorization of agro-industrial wastes by producing 2-phenylethanol via solid-state fermentation: Influence of substrate selection on the process. Waste Manag. 2021, 121, 403–411. [Google Scholar] [CrossRef]
- Oiza, N.; Moral-Vico, J.; Sánchez, A.; Oviedo, E.R.; Gea, T. Solid-State Fermentation from Organic Wastes: A New Generation of Bioproducts. Processes 2022, 10, 2675. [Google Scholar] [CrossRef]
- Harding, M.W.; Marques, L.L.; Howard, R.J.; Olson, M.E. Can filamentous fungi form biofilms? Trends Microbiol. 2009, 17, 475–480. [Google Scholar] [CrossRef]
- Manisha; Yadav, S.K. Technological advances and applications of hydrolytic enzymes for valorization of lignocellulosic biomass. Bioresour. Technol. 2017, 245, 1727–1739. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.M.; Muniz, C.P.L. Universo Cultural da Palmeira de Babaçu, 1st ed.; IPHAN: Brasília, Brazil, 2017; 150p. Available online: http://portal.iphan.gov.br/uploads/publicacao/universo_cultural_da_palmeira_babacu.pdf (accessed on 6 March 2023).
- Cavalcante Neto, A.A. Desenvolvimento de Massa Alimentícia Mista de Farinhas de Trigo e Mesocarpo de Babaçu (Orbignya sp.). Master’s Thesis, Universidade Federal Rural do Rio de Janeiro—UFRRJ, Rio de Janeiro, Brazil, 2012; 82p. [Google Scholar]
- Vinhal, J.O.; Lima, C.F.; Barbosa, L.C. Analytical pyrolysis of the kernel and oil of babassu palm (Orbignya phalerata). J. Anal. Appl. Pyrolysis 2014, 107, 73–81. [Google Scholar] [CrossRef]
- Almeida, E.B., Jr. Coco babaçu: Descrição botânica da palmeira, importância ecológica e abundância regional. In Biocombustíveis de Babaçu: Ensaio Técnico Sobre Oportunidades de Produção de Biocombustíveis a Partir do Coco de Babaçu/Adeilton Pereira Maciel (Org.); EDUFMA: São Luís, Brazil, 2016; p. 45. [Google Scholar]
- Dijkstra, A.J. Lauric Oils. In Encyclopedia of Food and Health; Elsevier: Amsterdam, The Netherlands, 2016; pp. 517–522. [Google Scholar] [CrossRef]
- Santana, A.A.; de Oliveira, R.A.; Pinedo, A.A.; Kurozawa, L.E.; Park, K.J. Microencapsulation of babassu coconut milk. Food Sci. Technol. 2013, 33, 737–744. [Google Scholar] [CrossRef]
- IBGE. Instituto Brasileiro de Geografia e Estatística. Produção de Babaçu—Brasil. Available online: https://www.ibge.gov.br/explica/producao-agropecuaria/babacu/br (accessed on 17 July 2024).
- Markets and Markets. Global Market for Flavor and Fragrance. Available online: https://www.marketsandmarkets.com/Market-Reports/food-flavors-market-93115891.html (accessed on 5 March 2023).
- Ramos, A.d.S.; Martins, P.S.d.O.; Fiaux, S.B.; Leite, S.G.F. Microextração em fase sólida de 6-pentil-α-pirona produzida por fermentação em estado sólido. Food Sci. Technol. 2009, 29, 523–528. [Google Scholar] [CrossRef]
- Ladeira, N.C.; Peixoto, V.J.; Penha, M.P.; Barros, E.B.P.; Leite, S.G.F. Optimization of 6-pentyl-alpha-pyrone production by solid state fermentation using sugarcane bagasse as residue. BioResources 2010, 5, 2297–2306. [Google Scholar] [CrossRef]
- Ramos, A.S. Otimização da Produção de Aroma de Coco Por Fermentação em Estado Sólido. Master’s Thesis, Universidade Federal do Rio de Janeiro (UFRJ), Rio de Janeiro, Brazil, 2006. [Google Scholar]
- Martins, P.S.O. Avaliação da Extração e da Produção de Aroma de Coco Por Trichoderma Harzianum. Master’s Thesis, Universidade Federal do Rio de Janeiro (UFRJ), Rio de Janeiro, Brazil, 2003. [Google Scholar]
- Alcântara, S.R.; Almeida, F.d.A.C.; da Silva, F.L.H.; Gomes, J.P. Isotermas de adsorção do pedúnculo seco do caju. Rev. Bras. Eng. Agric. Ambient. 2009, 13, 81–87. [Google Scholar] [CrossRef]
- Liu, D.-M.; Chen, J.; Shi, Y.-P. Advances on methods and easy separated support materials for enzymes immobilization. TrAC Trends Anal. Chem. 2018, 102, 332–342. [Google Scholar] [CrossRef]
- Zhou, S.; Yang, Q.; Runge, T.M. Ambient-temperature sulfuric acid pretreatment to alter structure and improve enzymatic digestibility of alfalfa stems. Ind. Crop. Prod. 2015, 70, 410–416. [Google Scholar] [CrossRef]
- Lonappan, L.; Rouissi, T.; Das, R.K.; Brar, S.K.; Ramirez, A.A.; Verma, M.; Surampalli, R.Y.; Valero, J.R. Adsorption of methylene blue on biochar microparticles derived from different waste materials. Waste Manag. 2016, 49, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Selvakumar, P.; Soccol, C.; Nigam, P. Solid State Fermentation for the Production of Industrial Enzymes. Curr. Sci. 1999, 77, 149–162. [Google Scholar]
- Raghavarao, K.; Ranganathan, T.; Karanth, N. Some engineering aspects of solid-state fermentation. Biochem. Eng. J. 2003, 13, 127–135. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Oda, S.; Isshiki, K.; Ohashi, S. Production of 6-pentyl-α-pyrone with Trichoderma atroviride and its mutant in a novel extractive liquid-surface immobilization (Ext-LSI) system. Process. Biochem. 2009, 44, 625–630. [Google Scholar] [CrossRef]
- Calasans, P.N. Produção de Aroma de Coco Por Trichoderma Harzianum Utilizando Bagaço de Cana. Master’s Thesis, Universidade Federal de Sergipe, São Cristóvão, Brazil, 2012. [Google Scholar]
- Franco, M.R.B.; Janzantti, N.S. Avanços na metodologia instrumental da pesquisa do sabor. In Aroma e Sabor de Alimentos: Temas Atuais; Franco, M.R.B., Ed.; Livraria Varela: São Paulo, Brazil, 2004; pp. 17–28. [Google Scholar]
- Feron, G.; Bonnarme, P.; Durand, A. Prospects for the microbial production of food flavours. Trends Food Sci. Technol. 1996, 7, 285–293. [Google Scholar] [CrossRef]
- Etschmann, M.; Bluemke, W.; Sell, D.; Schrader, J. Biotechnological production of 2-phenylethanol. Appl. Microbiol. Biotechnol. 2002, 59, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, S.M.M.; Gomes, S.D.; Sene, L.; Christ, D.; Piechontcoski, J. Production of natural aroma by yeast in wastewater of cassava starch industry. Eng. Agric. 2015, 35, 721–732. [Google Scholar] [CrossRef]
- Dimick, P.S.; Hoskin, J.C.; Acree, T.E. Review of apple flavor—State of the art∗. CRC Crit. Rev. Food Sci. Nutr. 1983, 18, 387–409. [Google Scholar] [CrossRef] [PubMed]
- Paillard, N.M.M. The flavor of apples, pears, and quinces. In Food Flavours, Part C: The Flavour of Fruits; Morton, L.D., MacLeod, A.J., Eds.; Elsevier Science Publishing Company Inc.: Amsterdam, The Netherlands, 1990; pp. 1–41. [Google Scholar]
- da Penha, M.P.; da Rocha-Leão, M.H.M.; Leite, S.G.F. Sugarcane bagasse as support for production of coconut aroma by solid state fermentation. Bioresources. BioResources 2012, 7, 2366–2375. [Google Scholar] [CrossRef]
- Sarhy-Bagnon, V.; Lozano, P.; Saucedo-Castañeda, G.; Roussos, S. Production of 6-pentyl-α-pyrone by Trichoderma harzianum in liquid and solid state cultures. Process. Biochem. 2000, 36, 103–109. [Google Scholar] [CrossRef]
- Häusler, A.; Münch, T. Microbial production of natural flavours. ASM News 1997, 63, 551–559. [Google Scholar]
- Etschmann, M.; Sell, D.; Schrader, J. Medium optimization for the production of the aroma compound 2-phenylethanol using a genetic algorithm. J. Mol. Catal. B Enzym. 2004, 29, 187–193. [Google Scholar] [CrossRef]
- Fabre, C.E.; Blanc, P.J.; Goma, G. Production of 2-Phenylethyl Alcohol by Kluyveromyces marxianus. Biotechnol. Prog. 1998, 14, 270–274. [Google Scholar] [CrossRef]
- Chung, H.; Lee, S.L.; Chou, C.C. Production and molar yield of 2-phenylethanol by Pichia fermentans L-5 as affected by some medium components. J. Biosci. Bioeng. 2000, 90, 142–147. [Google Scholar] [CrossRef]
- Wittmann, C.; Hans, M.; Bluemke, W. Metabolic physiology of aroma-producing Kluyveromyces marxianus. Yeast 2002, 19, 1351–1363. [Google Scholar] [CrossRef]
- Garavaglia, J.; Flôres, S.H.; Pizzolato, T.M.; Peralba, M.D.C.; Ayub, M.A.Z. Bioconversion of l-phenylalanine into 2-phenylethanol by Kluyveromyces marxianus in grape must cultures. World J. Microbiol. Biotechnol. 2007, 23, 1273–1279. [Google Scholar] [CrossRef]
- Pinto, L.L.L. Produção Biotecnológica de Álcool Feniletílico Por Fungos Filamentosos em Meio de Cultura Desenvolvido Com Utilização de Resíduos de Maçã (Malus Domestica); Faculdade de Engenharia de Alimentos universidade Estadual de Campinas: Campinas, Brazil, 2017. [Google Scholar]
- Martínez-Avila, O.; Sánchez, A.; Font, X.; Barrena, R. Bioprocesses for 2-phenylethanol and 2-phenylethyl acetate production: Current state and perspectives. Appl. Microbiol. Biotechnol. 2018, 102, 9991–10004. [Google Scholar] [CrossRef] [PubMed]
- Chreptowicz, K.; Mierzejewska, J. Enhanced bioproduction of 2-phenylethanol in a biphasic system with rapeseed oil. New Biotechnol. 2018, 42, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Eshkol, N.; Sendovski, M.; Bahalul, M.; Katz-Ezov, T.; Kashi, Y.; Fishman, A. Production of 2-phenylethanol from L-phenylalanine by a stress tolerant Saccharomyces cerevisiae strain. J. Appl. Microbiol. 2009, 106, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Dong, Q.; Meng, C.; Shi, X.A.; Guo, Y. A continuous and adsorptive bioprocess for efficient production of the natural aroma chemical 2-phenylethanol with yeast. Enzym. Microb. Technol. 2011, 48, 404–407. [Google Scholar] [CrossRef] [PubMed]
- Červeňanský, I.; Mihaľ, M.; Markoš, J. Potential application of perfusion and pertraction for in situ product removal in biocatalytic 2-phenylethanol production. Sep. Purif. Technol. 2017, 183, 11–20. [Google Scholar] [CrossRef]
- Rossi, S.; Vandenberghe, L.; Pereira, B.; Gago, F.; Rizzolo, J.; Pandey, A.; Soccol, C.; Medeiros, A. Improving fruity aroma production by fungi in SSF using citric pulp. Food Res. Int. 2009, 42, 484–486. [Google Scholar] [CrossRef]
- Carvalho, D.S. Produção de Aroma Frutal por Linhagens de Neurospora sp em Meios Sintéticos e Resíduos Agroindustriais. Ph.D. Thesis, Faculdade de Engenharia de Alimentos, Universidade Estadual de Campinas, Campinas, Brazil, 2011; 174p. [Google Scholar]
- Araújo, K.B. Avaliação do Potencial Biotecnológico da Farinha de Casca de Mandioca na Obtenção de Acetato de Etila com Microrganismo Ceratocystis Fimbriata. Ph.D. Thesis, Universidade Federal de Sergipe, São Cristóvão, Brazil, 2016; 121p. [Google Scholar]
- McLafferty, F.W.; Stauffer, D.B. The Wiley/NBS Registry of Mass Spectral Data; J. Wiley and Sons: New York, NY, USA, 1989; Volume 3. [Google Scholar]
- Ravindran, R.; Hassan, S.S.; Williams, G.A.; Jaiswal, A.K. A review on bioconversion of agro-industrial wastes to industrially important enzymes. Bioengineering 2018, 5, 93. [Google Scholar] [CrossRef]








| Waste | Surface Area (m² g)−1 | Pore Volume (cm3 g)−1 | Pore Diameter (Å) | Average Particle Size (mm) |
|---|---|---|---|---|
| IN-BM | 0.3042 | 3.19 × 10−4 | 34.273 | 0.48 |
| DEF-BM | 0.3395 | 3.22 × 10−4 | 38.876 | 0.45 |
| Statistical Variable | 6-PP | |||||||
|---|---|---|---|---|---|---|---|---|
| IN-BM | DEF-BM | |||||||
| NS3SG | NS3SS | NS7SG | NS7SS | NS3SG | NS3SS | NS7SG | NS7SS | |
| Mean | 154.24 | 125.18 | 175.76 | 150.22 | 300.34 | 286.43 | 308.17 | 295.89 |
| Median | 154.76 | 125.23 | 175.32 | 150.40 | 301.58 | 287.21 | 307.86 | 295.05 |
| Variance | 5.42 | 1.21 | 2.22 | 0.74 | 7.40 | 5.93 | 7.86 | 4.41 |
| Standard deviation | 2.64 | 1.25 | 1.69 | 0.97 | 3.08 | 2.75 | 3.18 | 2,38 |
| 2-PE | ||||||||
| IN-BM | DEF-BM | |||||||
| NS3SG | NS3SS | NS7SG | NS7SS | NS3SG | NS3SS | NS7SG | NS7SS | |
| Mean | 232.33 | 218.89 | 259.76 | 227.66 | 357.70 | 329.18 | 366.02 | 344.23 |
| Median | 231.08 | 219.72 | 256.17 | 229.72 | 356.46 | 329.59 | 366.56 | 344.63 |
| Variance | 54.91 | 16.68 | 77.98 | 74.34 | 4.74 | 3.74 | 12.29 | 5.34 |
| Standard deviation | 1.38 | 2.08 | 2.13 | 2.01 | 2.47 | 2.19 | 1.96 | 1.54 |
| HEXANAL | ||||||||
| IN-BM | DEF-BM | |||||||
| NS3SG | NS3SS | NS7SG | NS7SS | NS3SG | NS3SS | NS7SG | NS7SS | |
| Mean | 192.93 | 180.70 | 196.50 | 188.19 | 188.88 | 176.41 | 210.75 | 198.02 |
| Median | 192.69 | 180.00 | 196.13 | 188.06 | 189.07 | 176.43 | 210.75 | 197.04 |
| Variance | 3.94 | 4.67 | 3.62 | 1.26 | 3.49 | 1.11 | 3.60 | 3.32 |
| Standard deviation | 2.11 | 2.04 | 1.04 | 0.93 | 1.24 | 2.04 | 1.15 | 1.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
dos Anjos, T.N.; Wojcieszak, R.; Leite, S.G.F.; Itabaiana Jr, I. Valorization of Residual Babassu Mesocarp Biomass to Obtain Aroma Compounds by Solid-State Fermentation. Microbiol. Res. 2024, 15, 1386-1405. https://doi.org/10.3390/microbiolres15030093
dos Anjos TN, Wojcieszak R, Leite SGF, Itabaiana Jr I. Valorization of Residual Babassu Mesocarp Biomass to Obtain Aroma Compounds by Solid-State Fermentation. Microbiology Research. 2024; 15(3):1386-1405. https://doi.org/10.3390/microbiolres15030093
Chicago/Turabian Styledos Anjos, Tamires N., Robert Wojcieszak, Selma G. F. Leite, and Ivaldo Itabaiana Jr. 2024. "Valorization of Residual Babassu Mesocarp Biomass to Obtain Aroma Compounds by Solid-State Fermentation" Microbiology Research 15, no. 3: 1386-1405. https://doi.org/10.3390/microbiolres15030093
APA Styledos Anjos, T. N., Wojcieszak, R., Leite, S. G. F., & Itabaiana Jr, I. (2024). Valorization of Residual Babassu Mesocarp Biomass to Obtain Aroma Compounds by Solid-State Fermentation. Microbiology Research, 15(3), 1386-1405. https://doi.org/10.3390/microbiolres15030093









