Cicer arietinum Extract Suppresses Lung Sepsis Induced by Cecal Ligation and Puncture in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. In Silico Study
2.2. In Vitro Study
2.2.1. Preparation of CAE
2.2.2. Antibacterial Activity of CAE
2.3. In Vivo Study
2.3.1. Animals
2.3.2. CLP Septic Model
2.3.3. Animal Grouping and Treatment Regimen
2.3.4. Sample Preparations
2.3.5. Preparation of Lung Homogenate
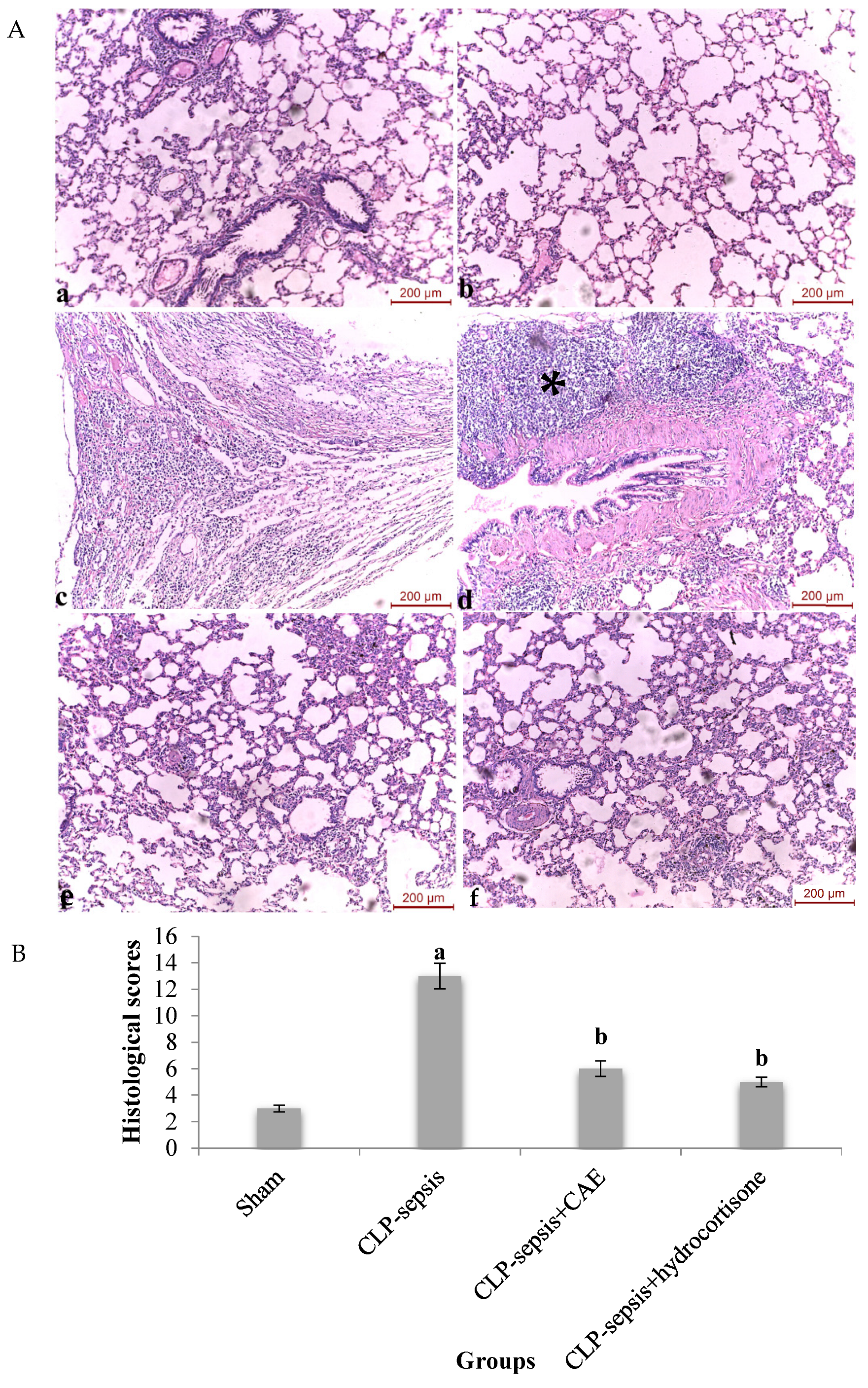
2.3.6. Histological Examination
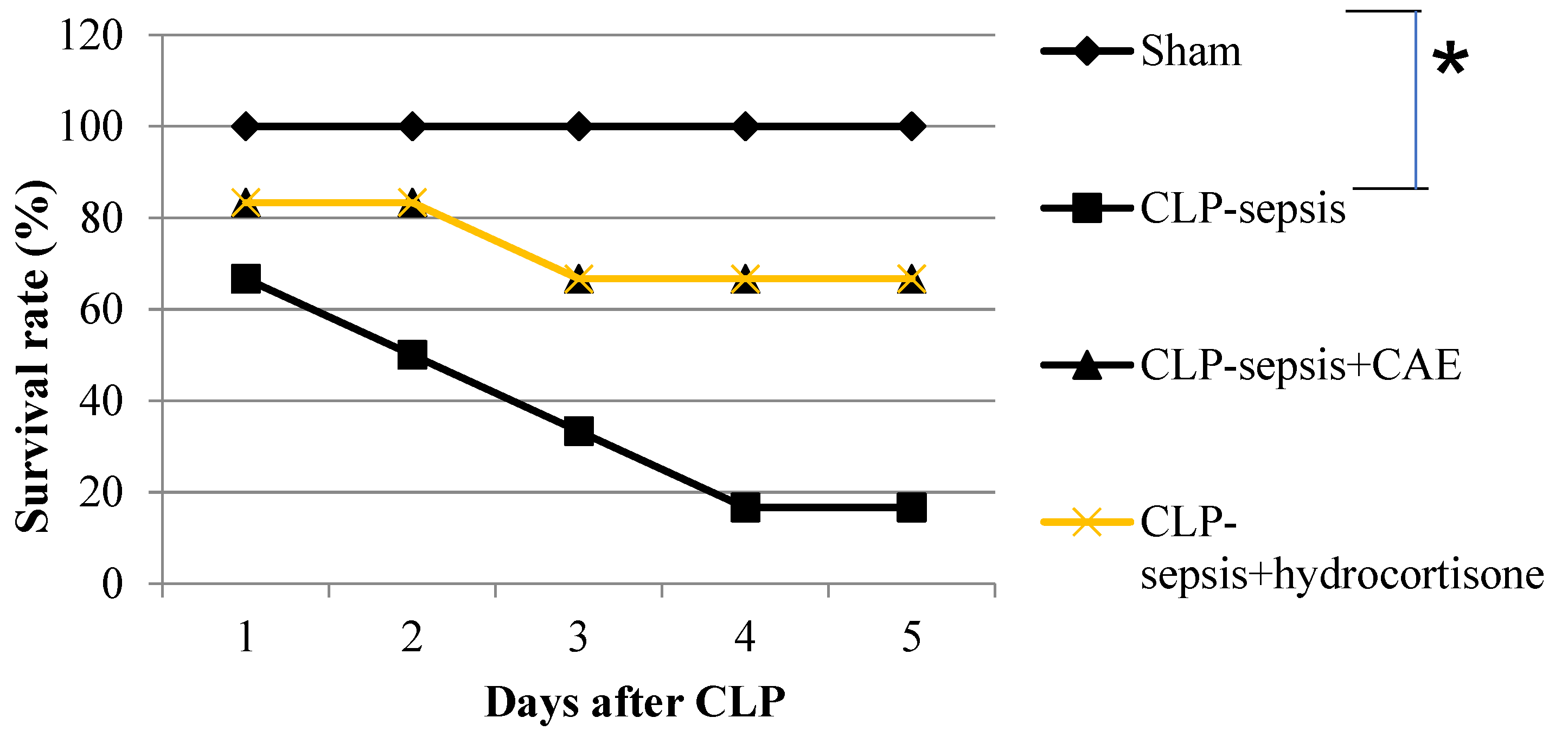
2.3.7. Survival Study
2.4. Statistical Analyses
3. Results
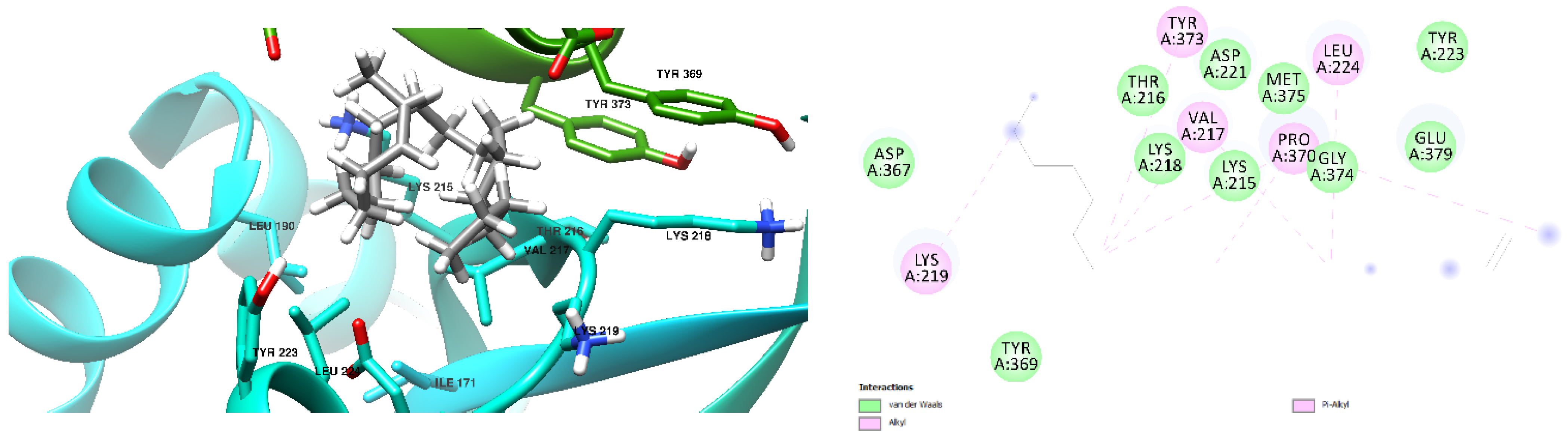
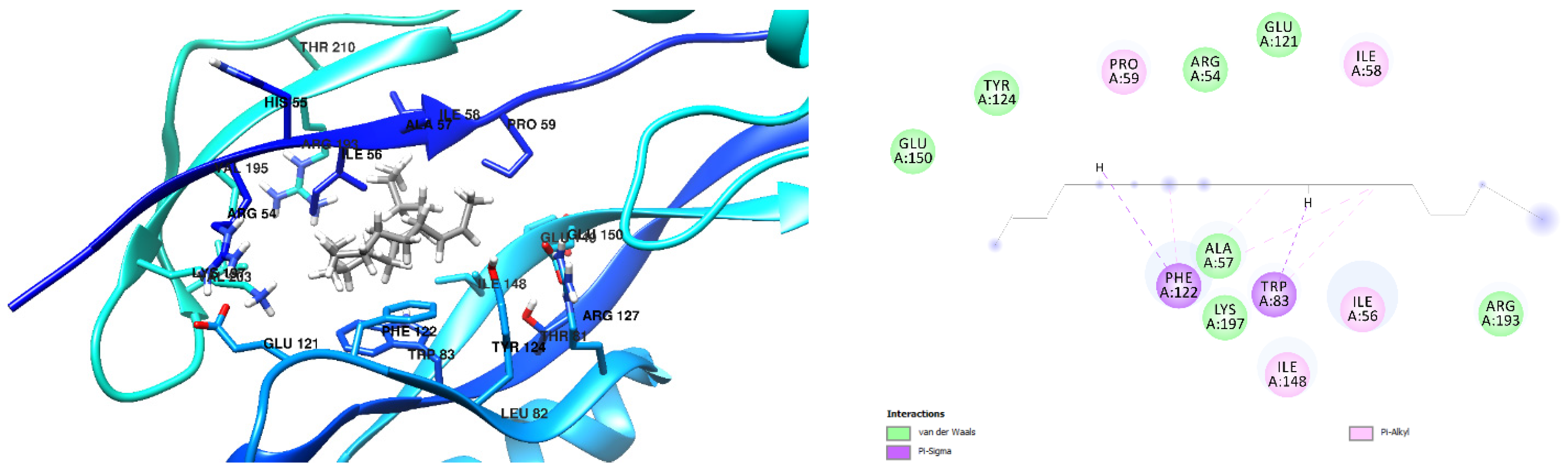
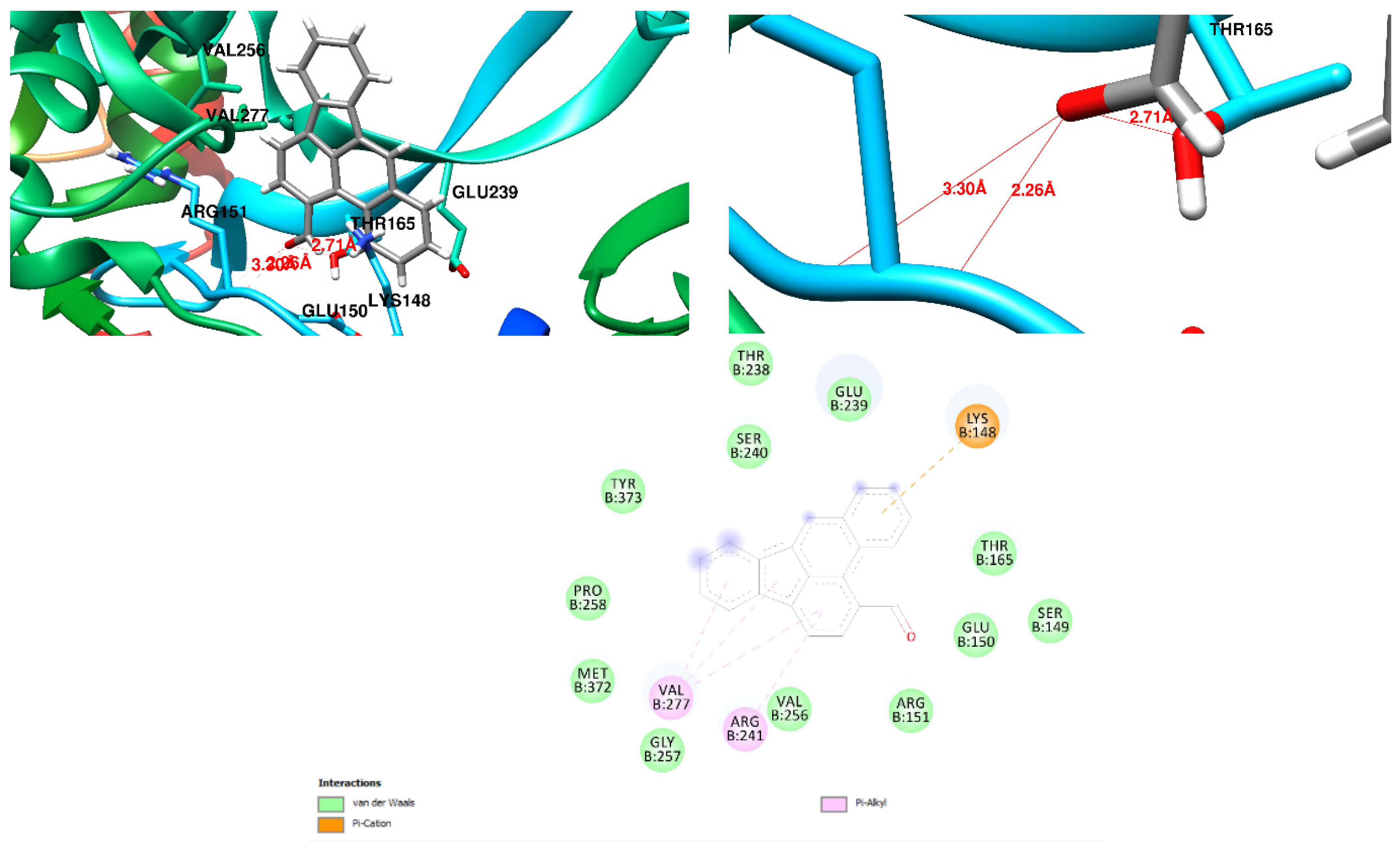
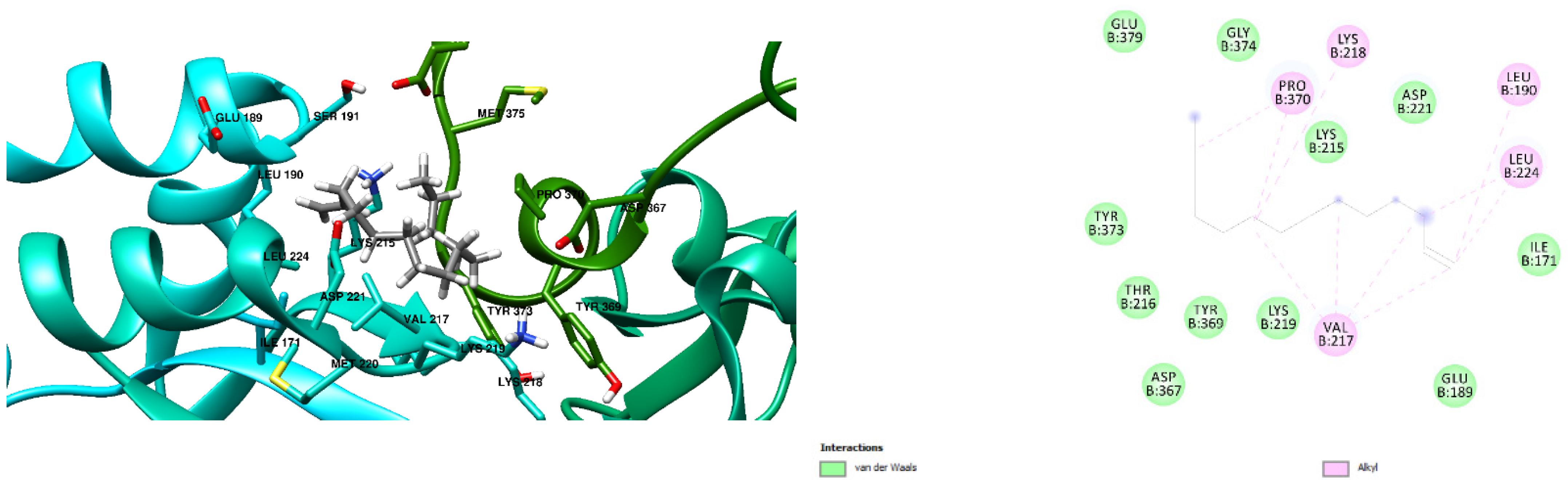
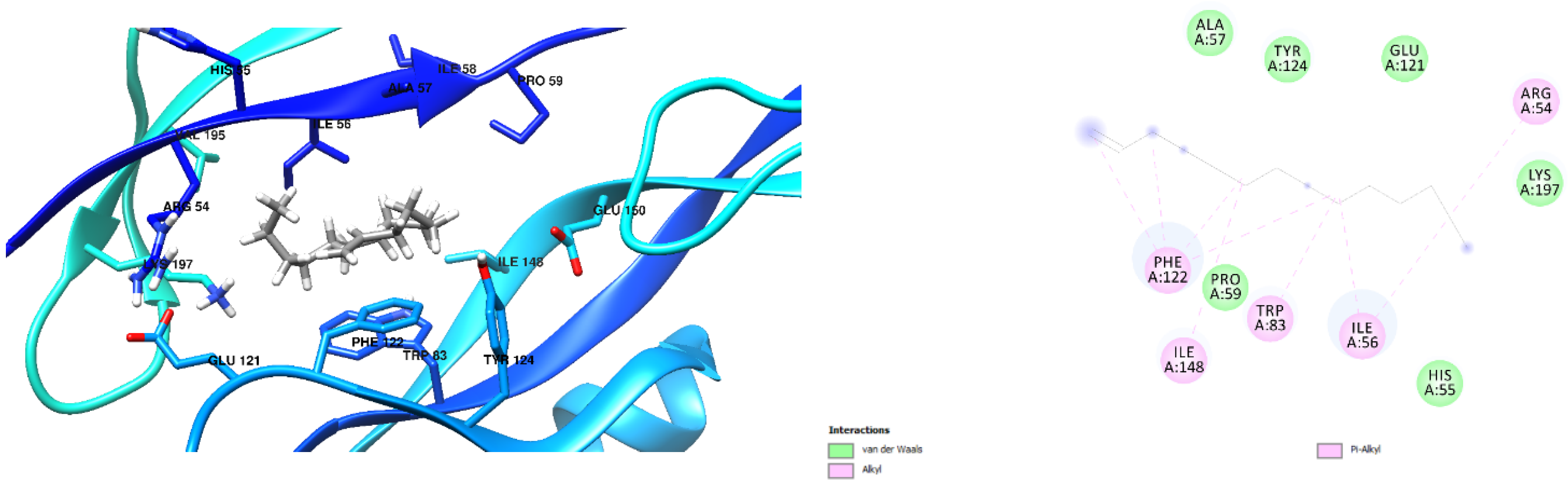
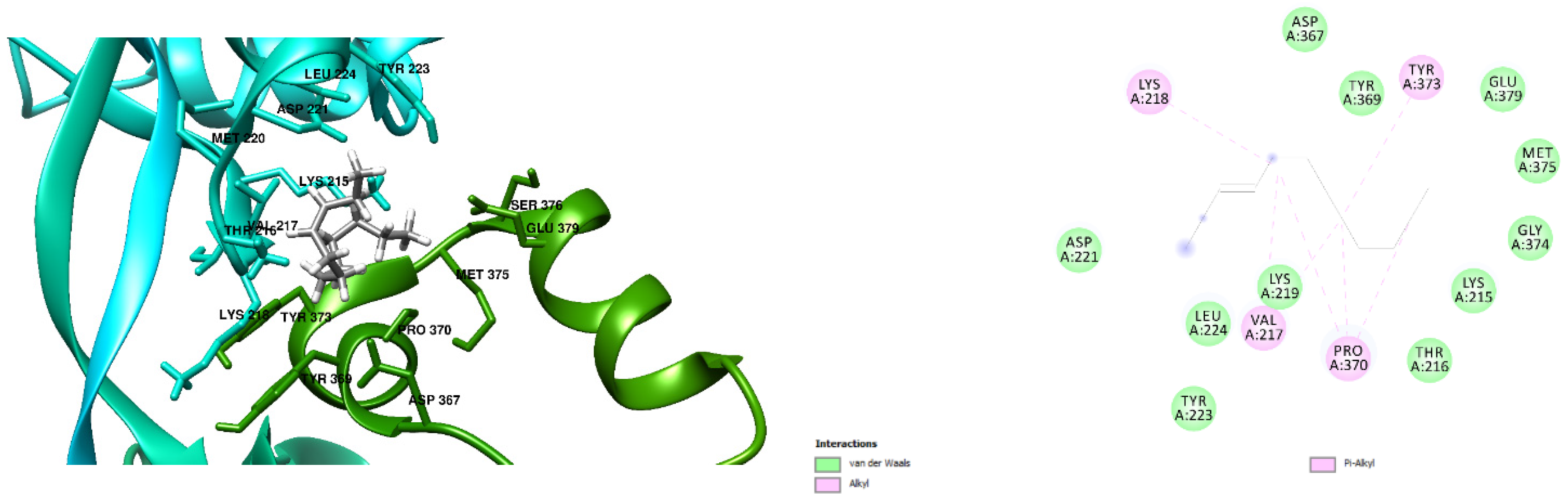
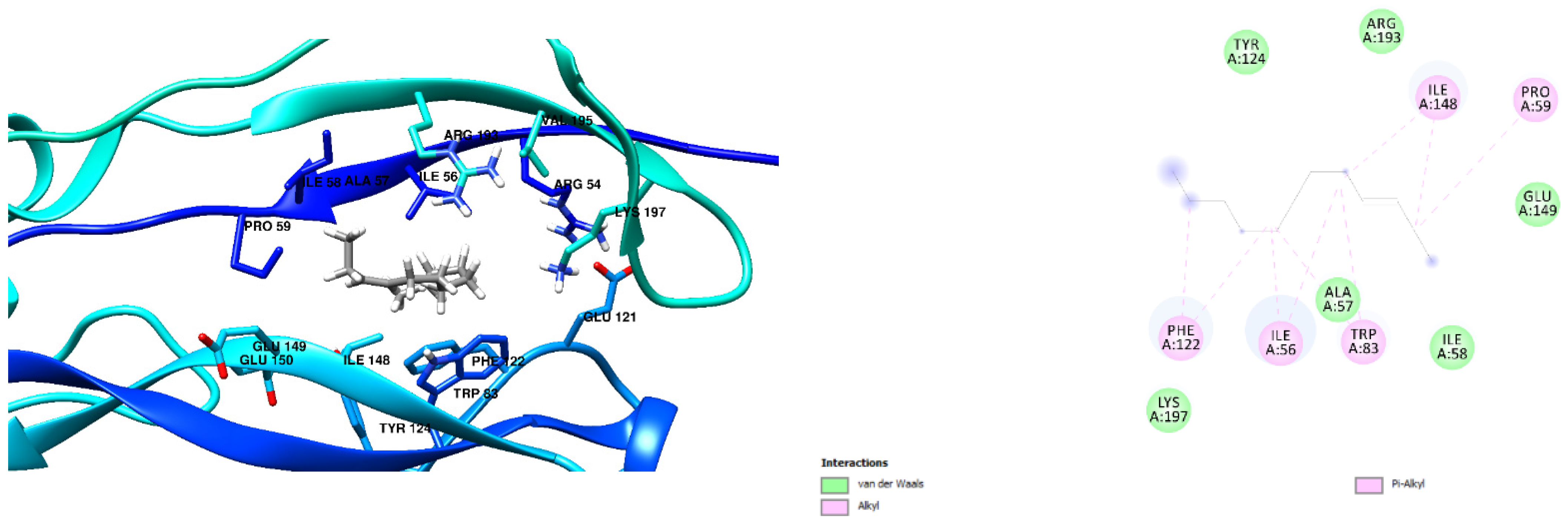
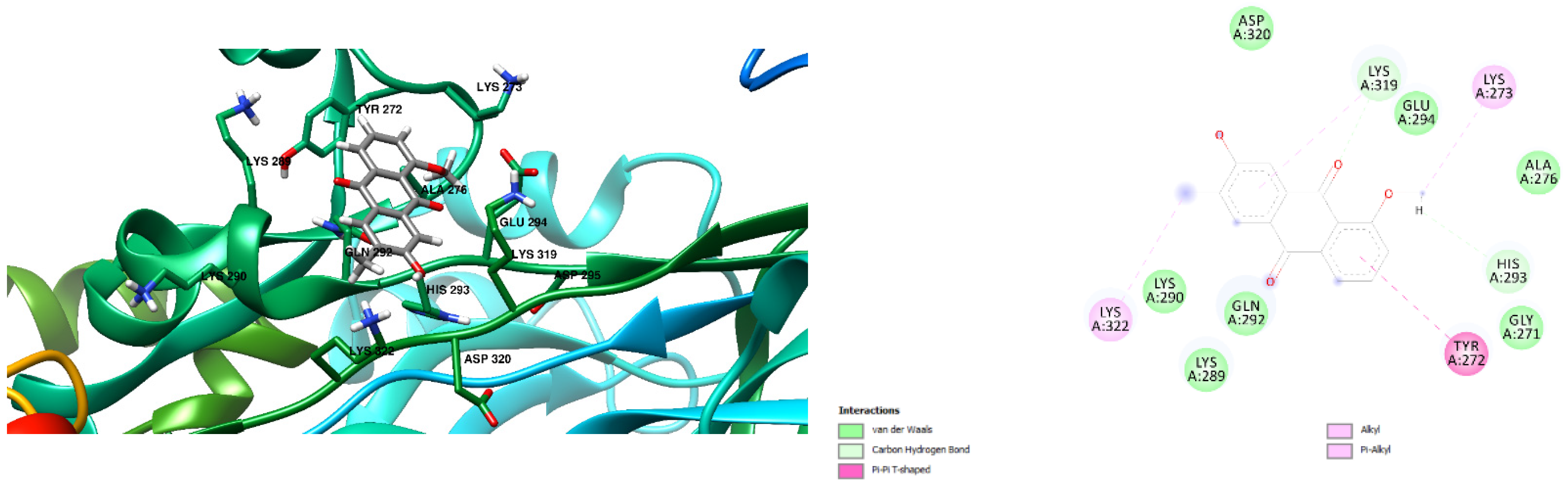
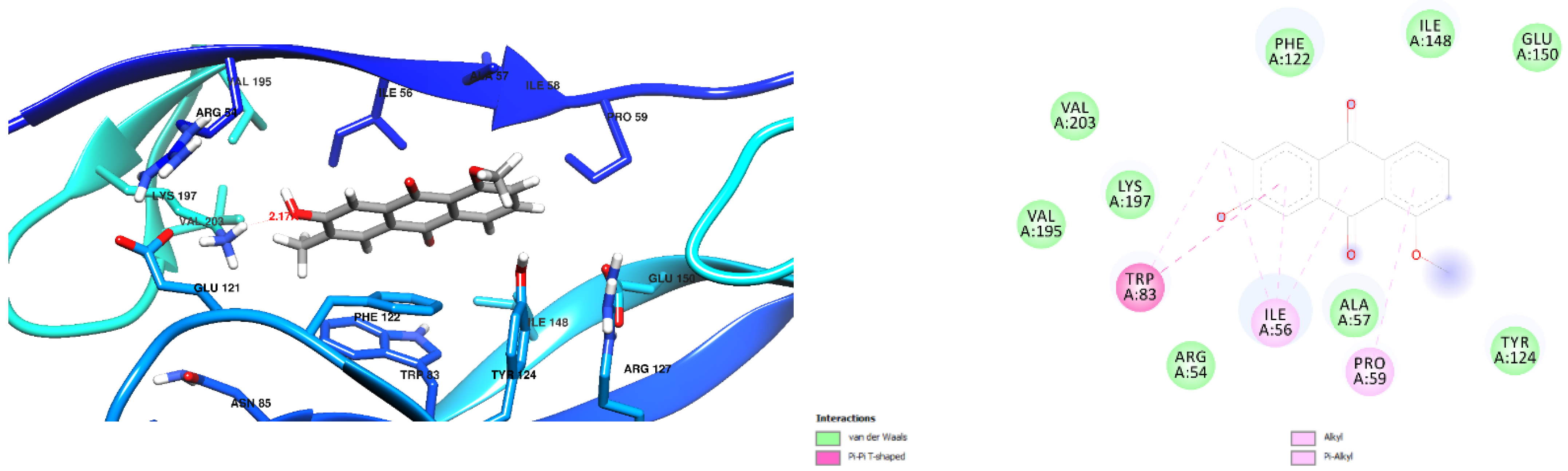
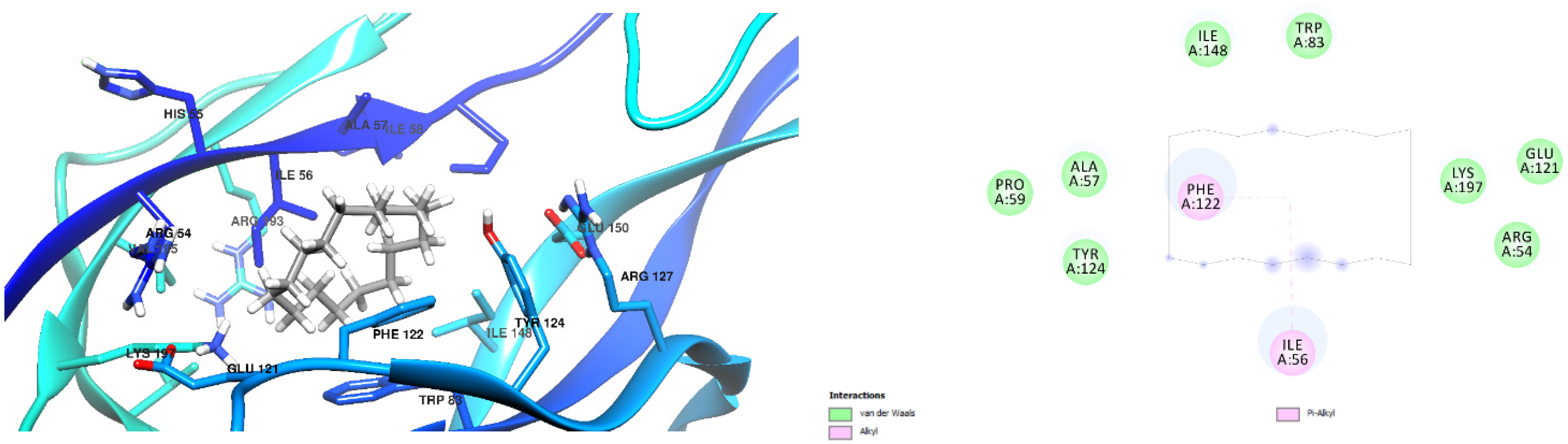
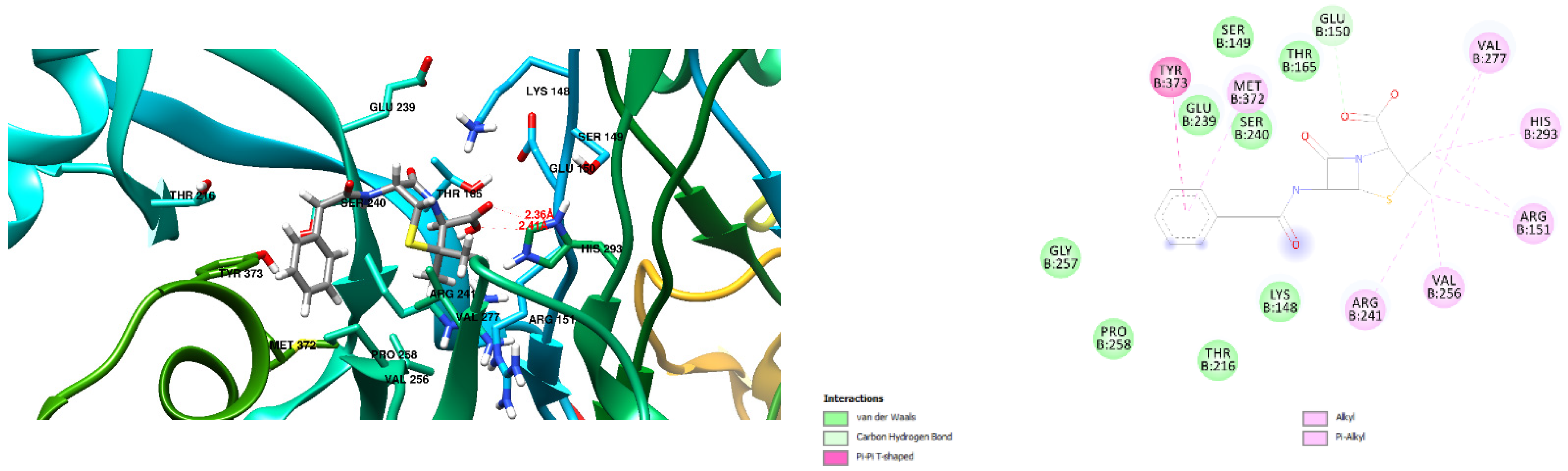
3.1. Docking Results
3.2. Antibacterial Activity of CAE
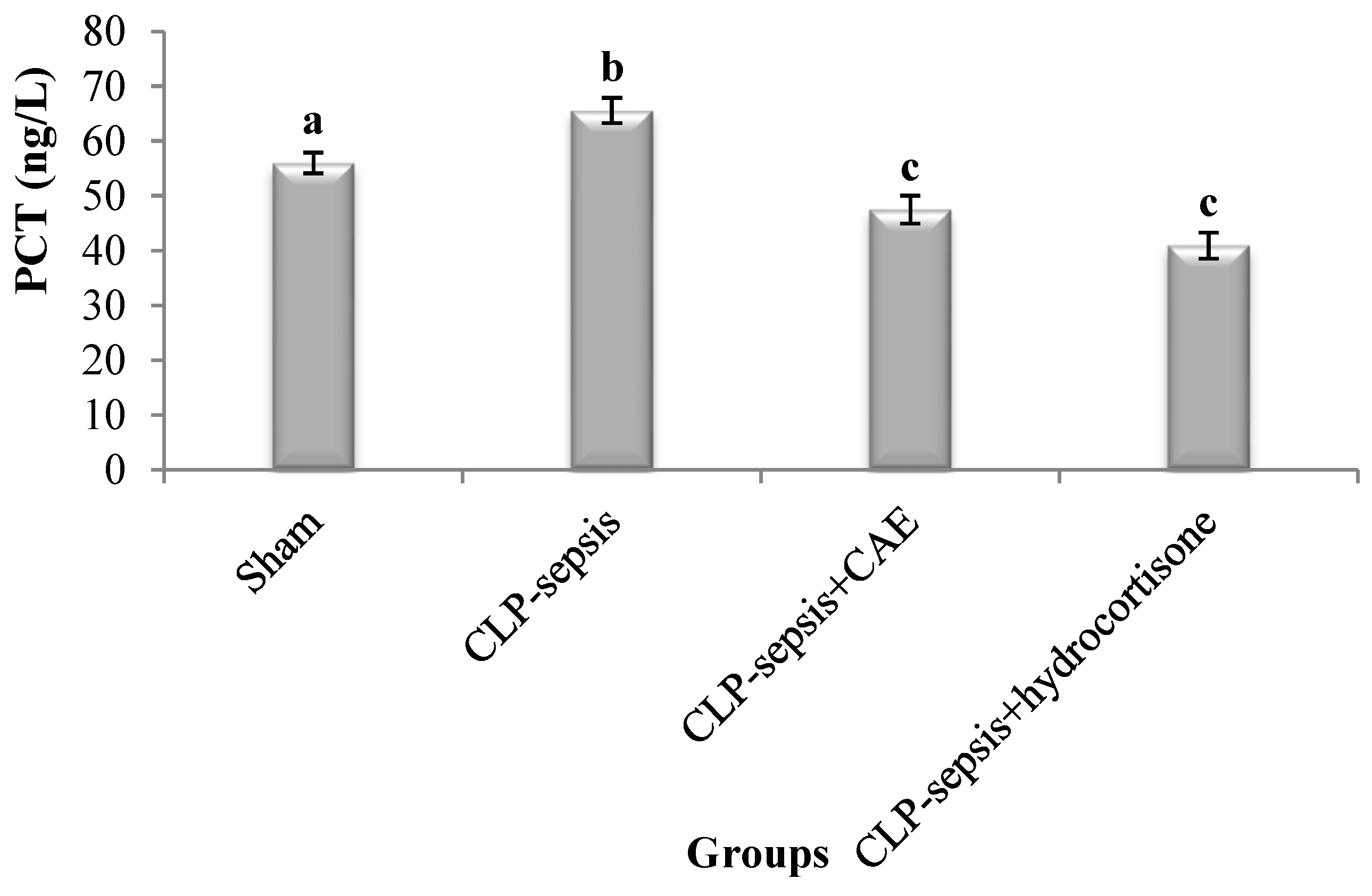
3.3. CAE Inhibits Sepsis by Decreasing Lung PCT Level
3.4. CAE Inhibits Sepsis through Antioxidant System Enhancement
3.5. Histological Evaluation of Lung Tissue
3.6. Treatment with CAE Attenuates CLP-Induced Mortality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dai, W.; Li, Y.; Liu, X.; Wang, N.; Luo, P.; Kong, L. Protective effects of Nigella sativa L. seeds aqueous extract-based silver nanoparticles on sepsis-induced damages in rats. Inorg. Chem. Commun. 2024, 166, 112594. [Google Scholar] [CrossRef]
- Ercan, M.; Ozdemir, S. The contribution to studies of the effect of β-glucan on plasma viscosity in a rat sepsis model. Med. Sci. Discov. 2015, 2, 148–153. [Google Scholar] [CrossRef]
- Vincent, J.L.; Nelson, D.R.; Williams, M.D. Is worsening multiple organ failure the cause of death in patients with severe sepsis? Crit. Care Med. 2011, 39, 1050–1055. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Bao, H.-G.; Si, Y.-N.; Han, L.; Zhang, R.; Cai, M.-M.; Shen, Y. Effects of adiponectin on acute lung injury in cecal ligation and puncture-induced sepsis rats. J. Surg. Res. 2013, 183, 752–759. [Google Scholar] [CrossRef]
- Ware, L.B.; Matthay, L.B. The acute respiratory distress syndrome. N. Engl. J. Med. 2000, 342, 1334–1349. [Google Scholar] [CrossRef]
- Fein, A.M.; Calalang-Colucci, M.G. Acute lung injury and acute respiratory distress syndrome in sepsis and septic shock. Crit. Care Clin. 2000, 16, 289–317. [Google Scholar] [CrossRef]
- Bayir, Y.; Albayrak, A.; Can, I.; Karagoz, Y.; Cakir, A.; Suleyman, H.; Uyanik, H.; Yayla, N.; Polat, B.; Karakus, E.; et al. Nigella sativa as a potential therapy for the treatment of lung injury caused by cecal ligation and puncture-induced sepsis model in rats. Cell Mol. Biol. 2012, 58, 1680–1687. [Google Scholar]
- Wheeler, A.P.; Bernard, G.R. Acute lung injury and the acute respiratory distress syndrome: A clinical review. Lancet 2007, 369, 1553–1564. [Google Scholar] [CrossRef]
- Xia, W.; Pan, Z.; Zhang, H.; Zhou, Q.; Liu, Y. ERRα protects against sepsis-induced acute lung injury in rats. Mol. Med. 2023, 29, 76. [Google Scholar] [CrossRef]
- Raoofi, R.; Salmani, Z.; Moradi, F.; Sotoodeh, A.; Sobhanian, S. Procalcitonin as a marker for early diagnosis of sepsis. Am. J. Infect. Dis. 2014, 10, 15–20. [Google Scholar] [CrossRef][Green Version]
- Tsai, K.; Hsu, T.; Kong, C.; Lin, K.; Lu, F. Is the endogenous peroxyl-radical scavenging capacity of plasma protective in systemic inflammatory disorders in humans? Free. Radic. Biol. Med. 2000, 28, 926–933. [Google Scholar] [CrossRef]
- Macdonald, J.; Galley, H.F.; Webster, N.R. Oxidative stress and gene expression in sepsis. Br. J. Anaesth. 2003, 90, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Pradipta, I.S.; Sodik, D.C.; Lestari, K.; Parwati, I.; Halimah, E.; Diantini, A.; Abdulah, R. Antibiotic resistance in sepsis patients: Evaluation and recommendation of antibiotic use. N. Am. J. Med. Sci. 2013, 5, 344–352. [Google Scholar] [CrossRef]
- Nagar, H.; Piao, S.; Kim, C.S. Role of Mitochondrial Oxidative Stress in Sepsis. Acute Crit. Care 2018, 33, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Sayed, A.A.; Abbas, O.A.; Saad, M.A.; Marie, M.S. Cicer arietinum extract ameliorate γ-irradiation disorders via modulation of oxidative/antioxidative pathway. J. Photochem. Photobiol. B 2018, 183, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Dalal, K.; Ahlawat, S.; Munjal, H.; Patra, A. Antibacterial activity of roots of Cicer arietinum Linn. J. Chem. Pharm. Res. 2010, 2, 43–46. [Google Scholar]
- Zaki, M.; Zaid, A.; Magd, M.; Abou Zid, S. Antibacterial effects of isoflavones isolated from Cicer arietinum. Int. J. Nat. Prod. Res. 2013, 2, 1–5. [Google Scholar]
- Brooks, H.F.; Osabutey, C.K.; Moss, R.F.; Andrews, P.L.; Davies, D.C. Caecal ligation and puncture in the rat mimics the pathophysiological changes in human sepsis and causes multi-organ dysfunction. Metab. Brain Dis. 2007, 22, 353–373. [Google Scholar] [CrossRef]
- Jayaprakash, B.; Das, A. Extraction and Characterization of ChickPEA (Cicer arietinum) Extract with Immunostimulant Activity in BALB/C MICE. Asian Pac. J. Cancer Prev. 2018, 19, 803–810. [Google Scholar]
- Lim, D.C.; Strynadka, N.C.J. Structure of penicillin G acyl-Penicillin binding protein 2a from methicillin resistant Staphylococcus aureus strain 27r at 2.45 A resolution. Nat. Struct. Biol. 2002, 9, 870–876. [Google Scholar]
- Sainsbury, S.; Bird, L.; Stuart, D.I.; Owens, R.J.; Ren, J. Oxford Protein Production Facility (OPPF). Crystal structure of penicillin-binding protein 3 from Pseudomonas aeruginosa. J. Mol. Biol. 2011, 405, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Marzouk, M.; Soliman, A.M.; Fahmy, S.R.; Sayed, A.A. Ameliorative effects of Cicer arietinum extract and Coelatura aegyptiaca shell powder on estrogen sensitive organs in ovariectomized rats. World Appl. Sci. J. 2014, 31, 863–872. [Google Scholar]
- Bauer, A.W.; Kirby, W.M.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Wang, W.; Fang, H.; Yang, Y.; Jiang, X.; Liu, S.; Hu, J.; Hu, Q.; Dahmen, U.; Dirsch, O. Baicalein protects against polymicrobial sepsis-induced liver injury via inhibition of inflammation and apoptosis in mice. Eur. J. Pharmacol. 2015, 748, 45–53. [Google Scholar] [CrossRef]
- Shen, L.; Mo, H.; Cai, L.; Kong, T.; Zheng, W.; Ye, J.; Qi, J.; Xiao, Z. Losartan prevents sepsis induced acute lung injury and decreases activation of nuclear factor kappa B and mitogen-activated protein kinases. Shock. 2009, 31, 500–506. [Google Scholar] [CrossRef]
- Yamanel, L.; Kaldirim, U.; Oztas, Y.; Coskun, O.; Poyrazoglu, Y.; Durusu, M.; Cayci, T.; Ozturk, A.; Demirbas, S.; Yasar, M.; et al. Ozone therapy and hyperbaric oxygen treatment in lung injury in septic rats. Int. J. Med. Sci. 2011, 8, 48–55. [Google Scholar] [CrossRef][Green Version]
- Verdrengh, M.; Collins, L.V.; Bergin, P.; Tarkowski, A. Phytoestrogen genistein as an anti-staphylococcal agent. Microbes Infect. 2004, 6, 86–92. [Google Scholar] [CrossRef]
- Ulanowska, K.; Tkaczyk, A.; Konopa, G.; Wegrzyn, G. Differential antibacterial activity of genistein arising from global inhibition of DNA.; RNA and protein synthesis in some bacterial strains. Arch. Microbiol. 2006, 184, 271–278. [Google Scholar] [CrossRef]
- Hong, H.; Landauer, M.R.; Foriska, M.A.; Ledney, G.D. Antibacterial activity of the soy isoflavone genistein. J. Basic. Microbiol. 2006, 46, 329–335. [Google Scholar] [CrossRef]
- Özkanlar, S.; Koç, F.; Karakuş, E.; Güvenalp, Z.; Oruç, E.; Özbek, H. The protective effects of Peganum harmala extract on lung and kidney in sepsis induced by cecal ligation and perforation in rats. Kafkas Univ. Vet. Fak. Derg. 2015, 21, 367–375. [Google Scholar]
- Crapo, J.D. Oxidative stress as an initiator of cytokine release and cell damage. Eur. Respir. J. Suppl. 2003, 44, 4s–6s. [Google Scholar] [CrossRef] [PubMed]
- Kinnula, V.L.; Crapo, J.D. Superoxide dismutases in the lung and human lung diseases. Am. J. Respir. Crit. Care Med. 2003, 167, 1600–1619. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, S.R.; Soliman, A.M.; Sayed, A.A.; Marzouk, M. Possible antiosteoporotic mechanism of Cicer arietinum extract in ovariectomized rats. Int. J. Clin. Exp. Pathol. 2015, 8, 3477–3490. [Google Scholar] [PubMed]
- Gadek, J.E.; DeMichele, S.J.; Karlstad, M.D.; Pacht, E.R.; Donahoe, M.; Albertson, T.E.; Chi, V.H.; Ann, K.W.; Jeffrey, L.N.; Mojtaba, N. Effect of enteral feeding with eicosapentaenoic acid; gamma-linolenic acid; and antioxidants in patients with acute respiratory distress syndrome. Enteral Nutrition in ARDS Study Group. Crit. Care Med. 1999, 27, 1409–1420. [Google Scholar] [CrossRef]
- Rubenfeld, G.D.; Caldwell, E.; Peabody, E.; Weaver, J.; Martin, D.P.; Neff, M.; Stern, E.J.; Hudson, L.D. Incidence and outcomes of acute lung injury. N. Engl. J. Med. 2005, 353, 1685–1693. [Google Scholar] [CrossRef]
- Ritter, C.; Andrades, M.; Frota, M.L.C.; Weaver, J.; Martin, D.P.; Neff, M.; Klamt, F.; Pinheiro, C.T.S.; Menna-Barreto, S.S.; Moreira, J.C.F.; et al. Oxidative parameters and mortality in sepsis induced by cecal ligation and perforation. Intens. Care Med. 2003, 29, 1782–1789. [Google Scholar] [CrossRef]
- Keivanpour, H.; Zamzam, R.; Mojtahedzadeh, M.; Delnavazi, M.R.; Sharifan, A.; Sabzevari, O. 6-Gingerol anti-inflammatory and antioxidant properties protect against heart and liver dysfunction in rats with sepsis. Pharmacol. Res.-Mod. Chin. Med. 2024, 12, 100470. [Google Scholar] [CrossRef]
- Abraham, E. Neutrophils and acute lung injury. Crit. Care Med. 2003, 31, S195. [Google Scholar] [CrossRef]
- Guo, R.; Ward, P.A. Role of oxidants in lung injury during sepsis. Antioxid. Redox Signal. 2007, 9, 1991–2002. [Google Scholar] [CrossRef]





| S.No | RT | Name of Compound | M.Formula | M.Weight |
|---|---|---|---|---|
| 1 | 29.18 | 7-Hydroxy-1-methylanthraquinone | C16H12O4 | 268 |
| 2 | 32.29 | 6-(Aminomethyl)-2-naphthol | C11H11NO | 173 |
| 3 | 13.78 | Cyclohexadecane CAS | C16H32 | 224 |
| 4 | 17.22 | (cis)-2-nonadecene | C19H38 | 266 |
| 5 | 20.99 | 1-formylbenzo[b]fluoranthene | C21H12O | 280 |
| 6 | 10.60 | 1-Tetradecene (CAS) | C14H28 | 196 |
| 7 | 24.54 | (cis)-2-nonadecene | C19H38 | 266 |
| 8 | 7.18 | 3-Dodecene,(Z)- | C12H24 | 168 |
| Free Binding Energy of Temperature (T) = 298.15 K | Staphylococcus aureus-PBP2a (1 MWT) Docking Score | Pseudomonas aeruginosa-PBP3 (3OC2) Docking Score |
|---|---|---|
| Penicillin G | −6.9 kcal/mol (2 Hydrogen Bonds) | −7.1 kcal/mol (1 Hydrogen Bond) ASN 351 Hydrogen to Ligand oxygen, 2.39 Å |
| ||
| (cis)-2-nonadecene | −4.3 kcal/mol (0 Hydrogen Bonds) | −5.1 kcal/mol (0 Hydrogen Bond) |
| 1-formylbenzo[b]fluoranthene | −8.6 kcal/mol (3 Hydrogen Bonds) | −9.0 kcal/mol (0 Hydrogen Bond) |
| ||
| 1-Tetradecene (CAS) | −4.3 kcal/mol (0 Hydrogen Bonds) | −4.7 kcal/mol (0 Hydrogen Bond) |
| 3-Dodecene, (Z)- | −4.3 kcal/mol (0 Hydrogen Bonds) | −4.7 kcal/mol (0 Hydrogen Bond) |
| 6-(Aminomethyl)-2-naphthol | −6.8 kcal/mol (1 Hydrogen Bonds) Ligand Oxygen to Ser 403 Oxygen, 2.24 Å | −6.2 kcal/mol (1 Hydrogen Bond) Ligand hydrogen to Ala 57 Oxygen, 2.29Å |
| 7-Hydroxy-1-methoxy-6-methylanthraquinone | −6.4 kcal/mol (0 Hydrogen Bonds) | −7.2 kcal/mol (1 Hydrogen Bond) Lys 197 Hydrogen to Ligand Oxygen, 2.17Å |
| Cyclohexadecane (CAS) | −6.6 kcal/mol (0 Hydrogen Bonds) | −7.2 kcal/mol (0 Hydrogen Bond) |
| Groups | Distilled Water | CAE (500 mg/kg b.wt) | Hydrocortisone | Administration Time (Days) | Euthanization Time (Days) |
|---|---|---|---|---|---|
| + | − | − | 3 | 4th |
| + | − | − | 3 | 4th |
| − | + | − | 3 | 4th |
| − | − | + | 3 | 4th |
| Test Organism | Zone of Inhibition (mm) | ||
|---|---|---|---|
| CAE | Positive Control | Negative Control | |
| S. aureus | 12 | 19 | - |
| P. aeruginosa | 12 | 15 | - |
| E. coli | - | 17 | - |
| Parameter | MDA (nmol/g Tissue) | GSH (mg/g Tissue) | SOD (U/g Tissue) | CAT (U/g Tissue) | |
|---|---|---|---|---|---|
| Groups | |||||
| Sham | 2.127 ± 0.304 a | 0.399 ± 0.075 a | 404.965 ± 42.518 a | 0.925 ± 0.158 a | |
| CLP-sepsis | 5.470 ± 1.390 b | 0.163 ± 0.026 b | 160.813 ± 12.335 b | 0.599 ± 0.090 a | |
| CLP-sepsis + CAE | 1.329 ± 0.265 a | 1.038 ± 0.082 c | 402.040 ± 43.825 a | 0.748 ± 0.086 a | |
| CLP-sepsis + hydrocortisone | 0.998 ± 0.008 a | 1.001 ± 0.067 c | 385.972 ± 34.863 a | 0.698 ± 0.064 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Ali, A.; Abu-Alghayth, M.H.; Ghaleb, K.I.; Ibrahim, S. Cicer arietinum Extract Suppresses Lung Sepsis Induced by Cecal Ligation and Puncture in Rats. Microbiol. Res. 2024, 15, 1939-1956. https://doi.org/10.3390/microbiolres15030130
Al Ali A, Abu-Alghayth MH, Ghaleb KI, Ibrahim S. Cicer arietinum Extract Suppresses Lung Sepsis Induced by Cecal Ligation and Puncture in Rats. Microbiology Research. 2024; 15(3):1939-1956. https://doi.org/10.3390/microbiolres15030130
Chicago/Turabian StyleAl Ali, Amer, Mohammed H. Abu-Alghayth, Khaled I. Ghaleb, and Sara Ibrahim. 2024. "Cicer arietinum Extract Suppresses Lung Sepsis Induced by Cecal Ligation and Puncture in Rats" Microbiology Research 15, no. 3: 1939-1956. https://doi.org/10.3390/microbiolres15030130
APA StyleAl Ali, A., Abu-Alghayth, M. H., Ghaleb, K. I., & Ibrahim, S. (2024). Cicer arietinum Extract Suppresses Lung Sepsis Induced by Cecal Ligation and Puncture in Rats. Microbiology Research, 15(3), 1939-1956. https://doi.org/10.3390/microbiolres15030130







