New Insights into the Potential Inhibitory Effects of Native Plants from Cyprus on Pathogenic Bacteria and Diabetes-Related Enzymes
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Extraction of Plants
2.3. Assessment of Total Phenolic and Total Flavonoid Contents
2.4. Evaluation of the Antibacterial Potential of Plants
2.5. Evaluation of the Inhibitory Potential of Plants on Diabetes-Related Enzymes
2.6. Statistical Analysis
3. Results and Discussion
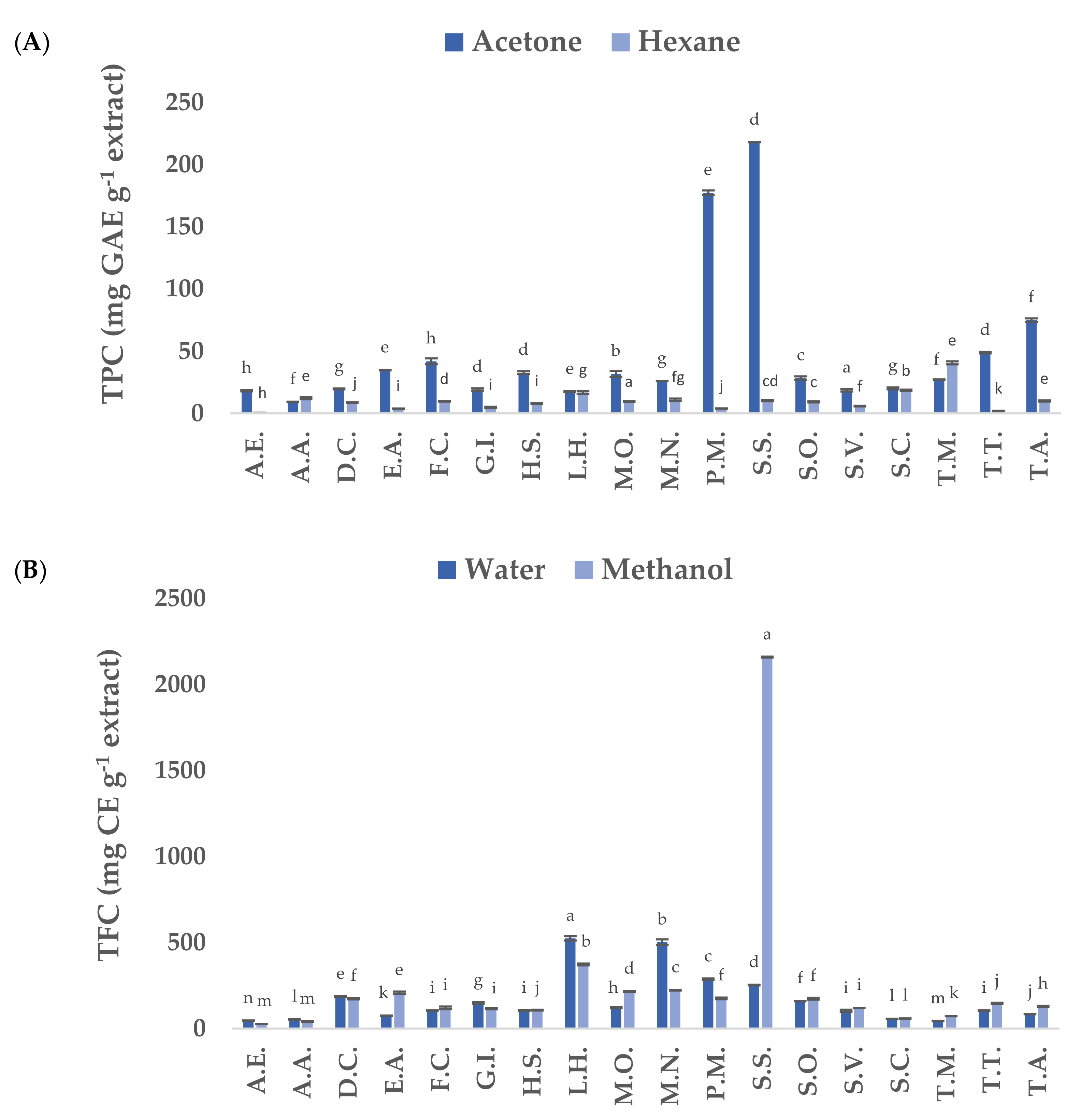
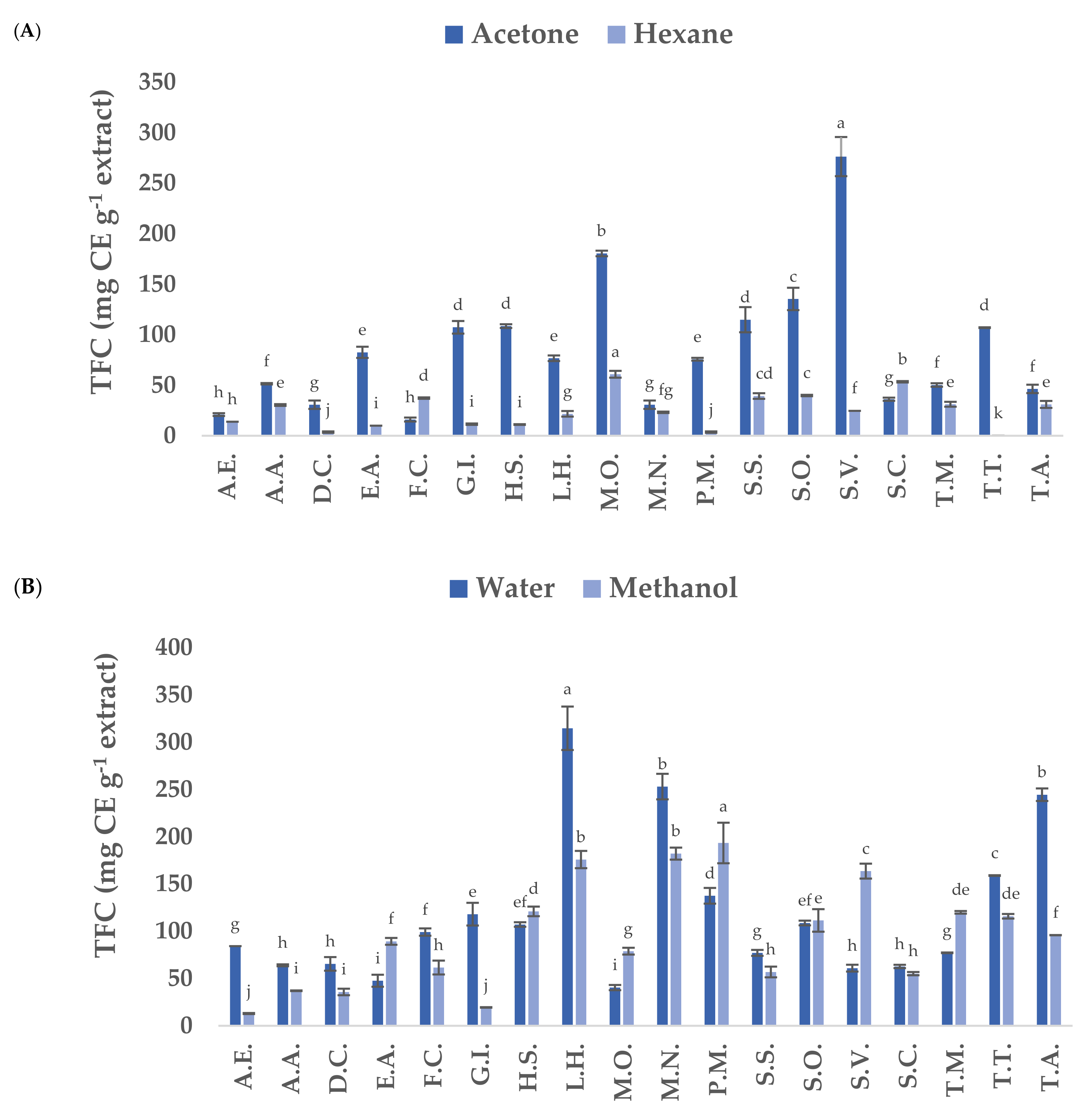
3.1. Total Phenolic Content (TPC) and Total Flavonoid Content (TFC) of Native Plants
3.2. Inhibitory Effects of Native Plants on Gram-Positive and -Negative Bacteria
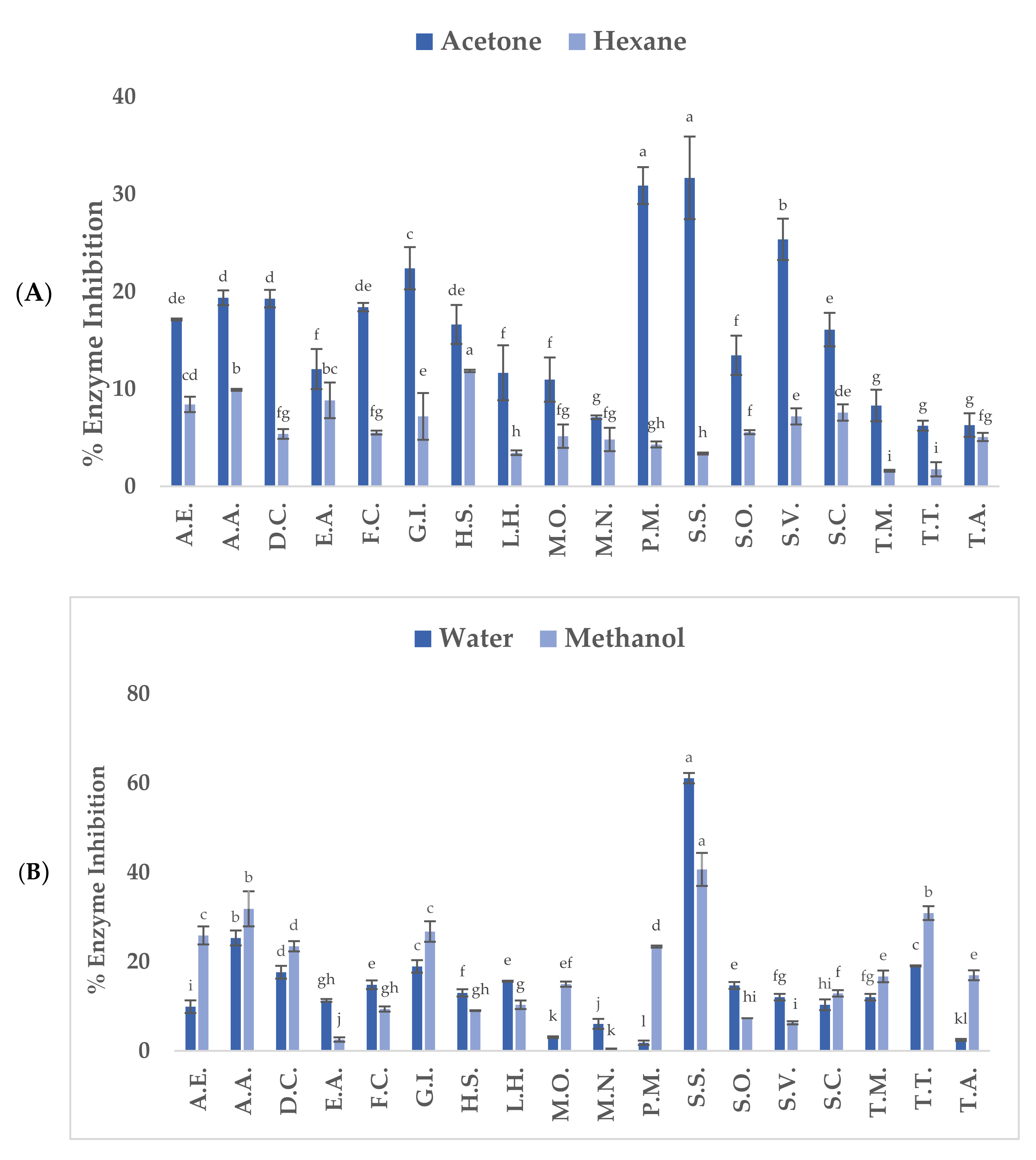
3.3. Inhibitory Effects of Native Plants on Diabetes-Related Enzymes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Orhan, I.E.; Banach, M.; Rollinger, J.M.; Barreca, D.; Weckwerth, W.; Bauer, R.; Bayer, E.A.; et al. Natural Products in Drug Discovery: Advances and Opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Zhu, W.; Huang, W.; Xu, Z.; Cao, M.; Hu, Q.; Pan, C.; Guo, M.; Wei, J.F.; Yuan, H. Analysis of Patents Issued in China for Antihyperglycemic Therapies for Type 2 Diabetes Mellitus. Front. Pharmacol. 2019, 10. [Google Scholar] [CrossRef]
- Bernardini, S.; Tiezzi, A.; Laghezza Masci, V.; Ovidi, E. Natural Products for Human Health: An Historical Overview of the Drug Discovery Approaches. Nat. Prod. Res. 2018, 32, 1926–1950. [Google Scholar] [CrossRef]
- Gunjan, M.; Win Naing, T.; Singh Saini, R.; bin Ahmad, A.; Rameshwar Naidu, J.; Kumar, I. Marketing Trends & Future Prospects of Herbal Medicine in the Treatment of Various Diseases. World J. Pharm. Res. 2015, 4, 132. [Google Scholar]
- Salam, M.A.; Al-Amin, M.Y.; Salam, M.T.; Pawar, J.S.; Akhter, N.; Rabaan, A.A.; Alqumber, M.A.A. Antimicrobial Resistance: A Growing Serious Threat for Global Public Health. Healthcare 2023, 11, 1946. [Google Scholar] [CrossRef]
- Standl, E.; Khunti, K.; Hansen, T.B.; Schnell, O. The Global Epidemics of Diabetes in the 21st Century: Current Situation and Perspectives. Eur. J. Prev. Cardiol. 2019, 26, 7–14. [Google Scholar] [CrossRef]
- Bharti, S.K.; Krishnan, S.; Kumar, A.; Kumar, A. Antidiabetic Phytoconstituents and Their Mode of Action on Metabolic Pathways. Ther. Adv. Endocrinol. Metab. 2018, 9, 81–100. [Google Scholar] [CrossRef]
- Salleh, N.H.; Zulkipli, I.N.; Mohd Yasin, H.; Ja’Afar, F.; Ahmad, N.; Wan Ahmad, W.A.N.; Ahmad, S.R. Systematic Review of Medicinal Plants Used for Treatment of Diabetes in Human Clinical Trials: An ASEAN Perspective. Evid. Based Complement. Altern. Med. 2021, 2021, 5570939. [Google Scholar] [CrossRef] [PubMed]
- Bindu, J.; Narendhirakannan, R.T. Role of Medicinal Plants in the Management of Diabetes Mellitus: A Review. 3 Biotech. 2019, 9, 4. [Google Scholar]
- Riyaphan, J.; Pham, D.-C.; Leong, M.K.; Weng, C.-F. Biomolecules In Silico Approaches to Identify Polyphenol Compounds as α-Glucosidase and α-Amylase Inhibitors against Type-II Diabetes. Biomolecules 2021, 11, 1877. [Google Scholar] [CrossRef]
- World Health Organization. Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report. Available online: https://iris.who.int/bitstream/handle/10665/364996/9789240062702-eng.pdf?sequence=1 (accessed on 23 May 2024).
- Mancuso, G.; Midiri, A.; Gerace, E.; Biondo, C. Bacterial Antibiotic Resistance: The Most Critical Pathogens. Pathogens 2021, 10, 1310. [Google Scholar] [CrossRef]
- Abdallah, E.M.; Alhatlani, B.Y.; de Paula Menezes, R.; Martins, C.H.G. Back to Nature: Medicinal Plants as Promising Sources for Antibacterial Drugs in the Post-Antibiotic Era. Plants 2023, 12, 3077. [Google Scholar] [CrossRef] [PubMed]
- Chassagne, F.; Samarakoon, T.; Porras, G.; Lyles, J.T.; Dettweiler, M.; Marquez, L.; Salam, A.M.; Shabih, S.; Farrokhi, D.R.; Quave, C.L. A Systematic Review of Plants With Antibacterial Activities: A Taxonomic and Phylogenetic Perspective. Front. Pharmacol. 2021, 11, 586548. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, R.; Coppo, E.; Marchese, A.; Daglia, M.; Sobarzo-Sánchez, E.; Nabavi, S.F.; Nabavi, S.M. Phytochemicals for Human Disease: An Update on Plant-Derived Compounds Antibacterial Activity. Microbiol. Res. 2017, 196, 44–68. [Google Scholar] [CrossRef] [PubMed]
- Parham, S.; Kharazi, A.Z.; Bakhsheshi-Rad, H.R.; Nur, H.; Ismail, A.F.; Sharif, S.; Ramakrishna, S.; Berto, F. Antioxidant, Antimicrobial and Antiviral Properties of Herbal Materials. Antioxidants 2020, 9, 1309. [Google Scholar] [CrossRef] [PubMed]
- Walusansa, A.; Asiimwe, S.; Nakavuma, J.L.; Ssenku, J.E.; Katuura, E.; Kafeero, H.M.; Aruhomukama, D.; Nabatanzi, A.; Anywar, G.; Tugume, A.K.; et al. Antibiotic-Resistance in Medically Important Bacteria Isolated from Commercial Herbal Medicines in Africa from 2000 to 2021: A Systematic Review and Meta-Analysis. Antimicrob. Resist. Infect. Control. 2022, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Radulovic, N.S.; Blagojevic, P.D.; Stojanovic-Radic, Z.Z.; Stojanovic, N.M. Antimicrobial Plant Metabolites: Structural Diversity and Mechanism of Action. Curr. Med. Chem. 2013, 20, 932–952. [Google Scholar] [CrossRef] [PubMed]
- Bintsis, T. Foodborne Pathogens. AIMS Microbiol. 2017, 3, 529–563. [Google Scholar] [CrossRef]
- Dias, D.A.; Urban, S.; Roessner, U. A Historical Overview of Natural Products in Drug Discovery. Metabolites 2012, 2, 303–336. [Google Scholar] [CrossRef]
- Christou, A.; Stavrou, C.; Michael, C.; Botsaris, G.; Goulas, V. Antibacterial and Carbohydrate Digestive Enzyme Inhibitory Effects of Native Plants Used for Medicinal and Culinary Purposes in Cyprus. Nat. Prod. Commun. 2024, 19, 1934578X231222105. [Google Scholar] [CrossRef]
- Christou, A.; Parisis, N.A.; Venianakis, T.; Barbouti, A.; Tzakos, A.G.; Gerothanassis, I.P.; Goulas, V. Ultrasound-Assisted Extraction of Taro Leaf Antioxidants Using Natural Deep Eutectic Solvents: An Eco-Friendly Strategy for the Valorization of Crop Residues. Antioxidants 2023, 12, 1801. [Google Scholar] [CrossRef] [PubMed]
- Cosme, P.; Rodríguez, A.B.; Espino, J.; Garrido, M. Plant Phenolics: Bioavailability as a Key Determinant of Their Potential Health-Promoting Applications. Antioxidants 2020, 9, 1263. [Google Scholar] [CrossRef] [PubMed]
- Rana, A.; Samtiya, M.; Dhewa, T.; Mishra, V.; Aluko, R.E. Health Benefits of Polyphenols: A Concise Review. J. Food Biochem. 2022, 46, e14264. [Google Scholar] [CrossRef] [PubMed]
- Alibi, S.; Crespo, D.; Navas, J. Plant-Derivatives Small Molecules with Antibacterial Activity. Antibiotics 2021, 10, 231. [Google Scholar] [CrossRef] [PubMed]
- Ćorković, I.; Gašo-Sokač, D.; Pichler, A.; Šimunović, J.; Kopjar, M. Dietary Polyphenols as Natural Inhibitors of α-Amylase and α-Glucosidase. Life 2022, 12, 1692. [Google Scholar] [CrossRef] [PubMed]
- Goulas, V.; Banegas-Luna, A.J.; Constantinou, A.; Pérez-Sánchez, H.; Barbouti, A. Computation Screening of Multi-Target Antidiabetic Properties of Phytochemicals in Common Edible Mediterranean Plants. Plants 2022, 11, 1637. [Google Scholar] [CrossRef]
- Galanakis, C.M.; Goulas, V.; Tsakona, S.; Manganaris, G.A.; Gekas, V. A Knowledge Base for the Recovery of Natural Phenols with Different Solvents. Int. J. Food Prop. 2013, 16, 382–396. [Google Scholar] [CrossRef]
- Yuan, G.; Guan, Y.; Yi, H.; Lai, S.; Sun, Y.; Cao, S. Antibacterial Activity and Mechanism of Plant Flavonoids to Gram-Positive Bacteria Predicted from Their Lipophilicities. Sci. Rep. 2021, 11, 10471. [Google Scholar] [CrossRef]
- Grigonis, D.; Venskutonis, P.R.; Sivik, B.; Sandahl, M.; Eskilsson, C.S. Comparison of Different Extraction Techniques for Isolation of Antioxidants from Sweet Grass (Hierochloë Odorata). J. Supercrit. Fluids 2005, 33, 223–233. [Google Scholar] [CrossRef]
- Acquaviva, R.; Genovese, C.; Amodeo, A.; Tomasello, B.; Malfa, G.; Sorrenti, V.; Tempera, G.; Addamo, A.P.; Ragusa, S.; Rosa, T.; et al. Biological Activities of Teucrium flavum L., Teucrium fruticans L., and Teucrium siculum Rafin Crude Extracts. Plant Biosyst. 2018, 152, 720–727. [Google Scholar] [CrossRef]
- De, M.C.; Torre, L.A.; Rodriguez, B.; Bruno, M.; Savona, G.; Piozzi, F.; Servettazt, O. Neo-claradone Diterpenes from Teucrium micropodioides. Phytochemistry 1988, 27, 213–216. [Google Scholar] [CrossRef]
- Hanoğlu, D.Y.; Hanoğlu, A.; Demirci, B.; Başer, K.H.C. The Essential Oil Compositions of Teucrium Spp. Belonging to the Section Polium Schreb. (Lamiaceae) Growing in Cyprus. Rec. Nat. Prod. 2023, 17, 113–124. [Google Scholar] [CrossRef]
- Soković, M.; Stojković, D.; Glamočlija, J.; Ćirić, A.; Ristić, M.; Grubišić, D. Susceptibility of Pathogenic Bacteria and Fungi to Essential Oils of Wild Daucus Carota. Pharm. Biol. 2009, 47, 38–43. [Google Scholar] [CrossRef]
- Kavaz, D.; Faraj, R. El Investigation of Composition, Antioxidant, Antimicrobial and Cytotoxic Characteristics from Juniperus Sabina and Ferula Communis Extracts. Sci. Rep. 2023, 13, 7193. [Google Scholar] [CrossRef] [PubMed]
- Matejić, J.S.; Džamić, A.M.; Mihajilov-Krstev, T.M.; Randelović, V.N.; Krivošej, Z.D.; Marin, P.D. Total Phenolic and Flavonoid Content, Antioxidant and Antimicrobial Activity of Extracts from Tordylium Maximum. J. Appl. Pharm. Sci. 2013, 3, 55–59. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Fallah, F.; Setzer, W.N.; Entezari Heravi, R.; Sharifi-Rad, M. Tordylium Persicum Boiss. & Hausskn Extract: A Possible Alternative for Treatment of Pediatric Infectious Diseases. Cell Mol. Biol. 2016, 62, 20–26. [Google Scholar] [CrossRef]
- Koifinas, C.; Chinvu, A.; Loukis, A.; Harvala, C.; Maillari, M.; Hostettmanu, K. Flavanoids and bioactive coumarins of Tordylium apulum. Phytochemistry 1998, 48, 637–641. [Google Scholar] [CrossRef]
- Artasensi, A.; Pedretti, A.; B Vistoli, G.; Fumagalli, K. Type 2 Diabetes Mellitus: A Review of Multi-Target Drugs. Molecules 2020, 25, 1987. [Google Scholar] [CrossRef]
- Elyasiyan, U.; Nudel, A.; Skalka, N.; Rozenberg, K.; Drori, E.; Oppenheimer, R.; Kerem, Z.; Rosenzweig, T. Anti-Diabetic Activity of Aerial Parts of Sarcopoterium Spinosum. BMC Complement. Altern. Med. 2017, 17, 356. [Google Scholar] [CrossRef]
- Kasabri, V.; Afifi, F.U.; Hamdan, I. In Vitro and in Vivo Acute Antihyperglycemic Effects of Five Selected Indigenous Plants from Jordan Used in Traditional Medicine. J. Ethnopharmacol. 2011, 133, 888–896. [Google Scholar] [CrossRef] [PubMed]
- Aleixandre, A.; Gil, J.V.; Sineiro, J.; Rosell, C.M. Understanding Phenolic Acids Inhibition of α-Amylase and α-Glucosidase and Influence of Reaction Conditions. Food Chem. 2022, 372, 131231. [Google Scholar] [CrossRef]
- Zbeeb, H.; Khalifeh, H.; Lupidi, G.; Baldini, F.; Zeaiter, L.; Khalil, M.; Salis, A.; Damonte, G.; Vergani, L. Polyphenol-Enriched Extracts of Sarcopoterium Spinosum Fruits for Counteracting Lipid Accumulation and Oxidative Stress in an in Vitro Model of Hepatic Steatosis. Fitoterapia 2024, 172, 105743. [Google Scholar] [CrossRef] [PubMed]
- Toda, M.; Kawabata, J.; Kasai, T. α-Glucosidase Inhibitors from Clove (Syzgium Aromaticum). Biosci. Biotechnol. Biochem. 2000, 64, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Ghosh, R.; Pal, B.C. α-Glucosidase Inhibitory Terpenoids from Potentilla Fulgens and Their Quantitative Estimation by Validated HPLC Method. J. Funct. Foods 2013, 5, 1135–1141. [Google Scholar] [CrossRef]
- Lee, D.; Park, J.Y.; Lee, S.; Kang, K.S. In Vitro Studies to Assess the α-Glucosidase Inhibitory Activity and Insulin Secretion Effect of Isorhamnetin 3-o-Glucoside and Quercetin 3-o-Glucoside Isolated from Salicornia Herbacea. Processes 2021, 9, 483. [Google Scholar] [CrossRef]
- Gunawan-Puteri, M.D.P.T.; Kawabata, J. Novel α-Glucosidase Inhibitors from Macaranga Tanarius Leaves. Food Chem. 2010, 123, 384–389. [Google Scholar] [CrossRef]

| Scientific Name | Common Name | Family | Parts Used | Collection Site | Voucher Specimen |
|---|---|---|---|---|---|
| Allium neapolitanum Cirillo | False garlic | Amaryllidaceae | Flowers, Leaves | 34°41′50.2″ N 32°57′40.5″ E | A-0001 |
| Asphodelus aestivus Brot. | Summer asphodel | Asphodelaceae | Flowers, Leaves | 34°41′59.1″ N 32°57′51.2″ E | A-0002 |
| Daucus carota L. | Wild carrot | Apiaceae | Flowers, Leaves | 34°41′50.2″ N 32°57′36.2″ E | D-0001 |
| Echium angustifolium subsp. Angustifolium | Narrow-leaved bugloss | Boraginaceae | Flowers, Leaves | 34°41′54.2″ N 32°57′44.2″ E | E-0001 |
| Ferula communis subsp. Communis | Giant fennel | Apiaceae | Flowers, Leaves | 34°41′44.3″ N 32°56′58.7″ E | F-0001 |
| Gladiolus italicus Miller | Field gladiolus | Iridaceae | Flowers, Leaves | 34°39′16.7″ N 32°45′04.0″ E | G-0002 |
| Helichrysum stoechas subsp. barrelieri (Ten.) Nyman | Mediterranean strawflower | Asteraceae | Flowers, Leaves | 34°41′45.1″ N 32°56′57.0″ E | H-0001 |
| Lithodora hispidula subsp. versicolor | - | Boraginaceae | Flowers, Leaves | 34°46′05.5″ N 32°56′06.8″ E | L-0002 |
| Mandragora officinarum L. | Mandrake | Solanaceae | Flowers, Fruits, Leaves | 34°41′59.5″ N 32°57′58.4″ E | M-0002 |
| Micromeria nervosa (Desf.) Benth | - | Lamiaceae | Flowers, Leaves | 34°39′16.7″ N 32°45′04.0″ E | M-0003 |
| Prasium majus L. | Spanish hedge-nettle | Lamiaceae | Flowers, Leaves | 34°39′16.7″ N 32°45′04.0″ E | P-0002 |
| Sarcopoterium spinosum (L.) Spach | Prickly burnet | Rosaceae | Flowers, Fruits, Leaves | 34°42′08.0″ N 32°57′44.2″ E | S-0007 |
| Smyrnium olusatrum L. | Alexanders | Apiaceae | Flowers, Leaves | 34°46′05.5″ N 32°56′06.8″ E | S-0005 |
| Solanum villosum Mill. | Hairy nightshade | Solanaceae | Flowers, Fruits, Leaves | 34°42′02.0″ N 32°57′57.0″ E | S-0002 |
| Stachys cretica L. | Mediterranean woundwort | Lamiaceae | Flowers, Leaves | 34°41′50.2″ N 32°57′40.5″ E | S-0006 |
| Teucrium micropodioides Rouy | Micropodioides germander | Lamiaceae | Flowers, Leaves | 34°41′50.2″ N 32°57′40.5″ E | T-0003 |
| Thymelaea tartonraira subsp. argentea | - | Thymelaeaceae | Flowers, Leaves | 34°39′16.7″ N 32°45′04.0″ E | T-0001 |
| Tordylium aegyptiacum L. | Mediterranean hartwort | Apiaceae | Flowers, Leaves | 34°41′50.2″ N 32°57′40.5″ E | T-0002 |
| Native Plant | Solvent | Bacillus cereus | Listeria monocytogenes | Staphylococcus aureus | Escherichia coli | ||||
|---|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | ||
| Allium neapolitanum Cirillo | Hexane | >2000 | >2000 | >2000 | >2000 | 1000 | >2000 | 1000 | >2000 |
| Acetone | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | |
| Methanol | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | |
| Water | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | |
| Asphodelus aestivus Brot. | Hexane | 1000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 |
| Acetone | >2000 | >2000 | >2000 | >2000 | 1000 | >2000 | >2000 | >2000 | |
| Methanol | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | |
| Water | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | |
| Daucus carota L. | Hexane | 500 | 1000 | >2000 | >2000 | 500 | 1000 | 1000 | >2000 |
| Acetone | 250 | 500 | >2000 | >2000 | 250 | 500 | >2000 | >2000 | |
| Methanol | 250 | 500 | >2000 | >2000 | 250 | 500 | >2000 | >2000 | |
| Water | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | |
| Echium angustifolium subsp. angustifolium | Hexane | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 |
| Acetone | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | |
| Methanol | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | |
| Water | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | |
| Ferula communis subsp. communis | Hexane | 250 | 500 | 250 | 500 | 250 | 500 | 1000 | 2000 |
| Acetone | 250 | 500 | 250 | 500 | 250 | 500 | >2000 | >2000 | |
| Methanol | 250 | 500 | >2000 | >2000 | 500 | 1000 | >2000 | >2000 | |
| Water | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | |
| Gladiolus italicus Miller | Hexane | >2000 | >2000 | >2000 | >2000 | 1000 | >2000 | 1000 | >2000 |
| Acetone | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | |
| Methanol | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | |
| Water | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | |
| Helichrysum stoechas subsp. barrelieri (Ten.) Nyman | Hexane | 1000 | >2000 | >2000 | >2000 | 500 | 1000 | >2000 | >2000 |
| Acetone | 500 | 1000 | >2000 | >2000 | 250 | 500 | >2000 | >2000 | |
| Methanol | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | |
| Water | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | |
| Lithodora hispidula subsp. versicolor | Hexane | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 |
| Acetone | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | |
| Methanol | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | |
| Water | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | |
| Mandragora officinarum L. | Hexane | 1000 | >2000 | >2000 | >2000 | >2000 | >2000 | 1000 | >2000 |
| Acetone | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | |
| Methanol | >2000 | >2000 | >2000 | >2000 | 500 | 1000 | >2000 | >2000 | |
| Water | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | |
| Micromeria nervosa (Desf.) Benth | Hexane | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 |
| Acetone | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | |
| Methanol | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | |
| Water | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | |
| Prasium majus L. | Hexane | 1000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 |
| Acetone | 1000 | >2000 | >2000 | >2000 | 1000 | >2000 | >2000 | >2000 | |
| Methanol | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | |
| Water | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | |
| Sarcopoterium spinosum (L.) Spach | Hexane | 1000 | 2000 | >2000 | >2000 | 500 | 1000 | >2000 | >2000 |
| Acetone | 500 | 1000 | >2000 | >2000 | 250 | 500 | >2000 | >2000 | |
| Methanol | 1000 | 2000 | >2000 | >2000 | 500 | 1000 | >2000 | >2000 | |
| Water | >2000 | >2000 | >2000 | >2000 | 500 | 1000 | >2000 | >2000 | |
| Smyrnium olusatrum L. | Hexane | 1000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 |
| Acetone | 500 | 1000 | >2000 | >2000 | 500 | 1000 | >2000 | >2000 | |
| Methanol | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | |
| Water | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | |
| Solanum villosum Mill. | Hexane | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 |
| Acetone | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | |
| Methanol | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | |
| Water | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | |
| Stachys cretica L. | Hexane | 1000 | >2000 | >2000 | >2000 | 500 | 1000 | >2000 | >2000 |
| Acetone | 500 | 1000 | >2000 | >2000 | 1000 | >2000 | >2000 | >2000 | |
| Methanol | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | |
| Water | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | |
| Teucrium micropodioides Rouy | Hexane | 1000 | >2000 | 500 | 1000 | 250 | 500 | 1000 | >2000 |
| Acetone | 500 | 1000 | >2000 | >2000 | 500 | 1000 | >2000 | >2000 | |
| Methanol | >2000 | >2000 | >2000 | >2000 | 500 | 1000 | >2000 | >2000 | |
| Water | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | |
| Thymelaea tartonraira subsp. argentea | Hexane | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 |
| Acetone | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | |
| Methanol | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | |
| Water | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | |
| Tordylium aegyptiacum L. | Hexane | 250 | 500 | >2000 | >2000 | 500 | 1000 | 1000 | >2000 |
| Acetone | 250 | 500 | >2000 | >2000 | 250 | 500 | >2000 | >2000 | |
| Methanol | >2000 | >2000 | >2000 | >2000 | 1000 | >2000 | >2000 | >2000 | |
| Water | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Christou, A.; Stavrou, C.; Michael, C.; Botsaris, G.; Goulas, V. New Insights into the Potential Inhibitory Effects of Native Plants from Cyprus on Pathogenic Bacteria and Diabetes-Related Enzymes. Microbiol. Res. 2024, 15, 926-942. https://doi.org/10.3390/microbiolres15020061
Christou A, Stavrou C, Michael C, Botsaris G, Goulas V. New Insights into the Potential Inhibitory Effects of Native Plants from Cyprus on Pathogenic Bacteria and Diabetes-Related Enzymes. Microbiology Research. 2024; 15(2):926-942. https://doi.org/10.3390/microbiolres15020061
Chicago/Turabian StyleChristou, Atalanti, Constantina Stavrou, Christodoulos Michael, George Botsaris, and Vlasios Goulas. 2024. "New Insights into the Potential Inhibitory Effects of Native Plants from Cyprus on Pathogenic Bacteria and Diabetes-Related Enzymes" Microbiology Research 15, no. 2: 926-942. https://doi.org/10.3390/microbiolres15020061
APA StyleChristou, A., Stavrou, C., Michael, C., Botsaris, G., & Goulas, V. (2024). New Insights into the Potential Inhibitory Effects of Native Plants from Cyprus on Pathogenic Bacteria and Diabetes-Related Enzymes. Microbiology Research, 15(2), 926-942. https://doi.org/10.3390/microbiolres15020061







