Antagonistic Interactions in Onychomycosis: Antifungal Activity of Extracts from Pure and Mixed Cultures of Candida parapsilosis and Trichophyton spp.
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganisms
2.2. Extracts
2.3. Susceptibility Profile of Dermatophyte Strains against Extracts and Antifungal Drugs
2.4. Time-Kill Assay
2.5. Toxicity Test with Galleria mellonella
3. Results
3.1. Susceptibility Profile of Dermatophyte Strains against Extracts and Antifungal Drugs
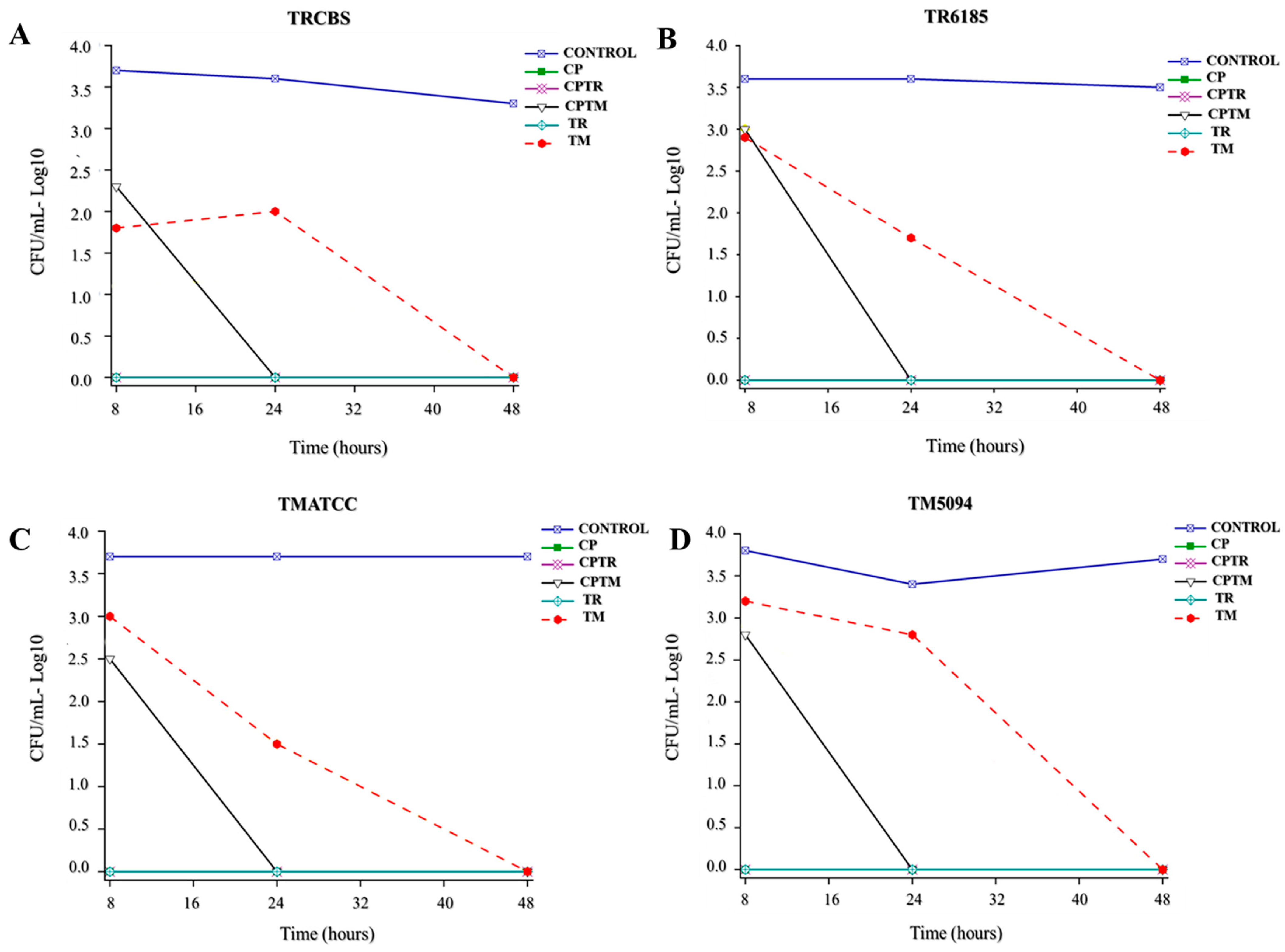
3.2. Time-Kill Assay
3.3. Toxicity Test with Galleria mellonella
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leung, A.K.C.; Lam, J.M.; Leong, K.F.; Hon, K.L.; Barankin, B.; Leung, A.A.M.; Wong, A.H.C. Onychomycosis: An Updated Review. Recent Pat. Inflamm. Allergy Drug Discov. 2020, 14, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Taborda, V.B.A.; Taborda, P.R.O.; Shemer, A.; Summerbell, R.C.; Nakrieko, K.A. High prevalence of mixed infections in global onychomycosis. PLoS ONE 2020, 15, e0239648. [Google Scholar] [CrossRef] [PubMed]
- Andrés, T.S.; Alexandro, B. Candida Onychomycosis: An Old Problem in Modern Times. Curr. Fungal Infect. Rep. 2020, 14, 209–216. [Google Scholar] [CrossRef]
- Rather, S.; Keen, A.; Shah, F.Y.; Yaseen, A.; Farooq, S.; Bakhshi, A. Candidal Onychomycosis: Clinicoepidemiological Profile, Prevailing Strains, and Antifungal Susceptibility Pattern—A Study from a Tertiary Care Hospital. Indian J. Dermatol. 2021, 66, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Stec, N.; Summerbell, R.C.; Shear, N.H.; Piguet, V.; Tosti, A.; Piraccini, B.M. Onychomycosis: A review. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 1972–1990. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Herrera, E.O.; Arroyo-Camarena, S.; Tejada-García, D.L.; Porras-López, C.F.; Arenas, R. Onychomycosis due to opportunistic molds. An. Bras. Dermatol. 2015, 90, 334–337. [Google Scholar] [CrossRef] [PubMed]
- Gaurav, V.; Bhattacharya, S.N.; Sharma, N.; Datt, S.; Kumar, P.; Rai, G.; Singh, P.K.; Taneja, B.; Das, S. Terbinafine resistance in dermatophytes: Time to revisit alternate antifungal therapy. J. Med. Mycol. 2021, 31, 101087. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Stec, N. Recent advances in therapies for onychomycosis and its management. F1000Research 2019, 8, 968. [Google Scholar] [CrossRef] [PubMed]
- Lemes, T.H.; Torrezan, G.S.; Polaquini, C.R.; Octavio, L.; Paziani, H.; von Zeska Kress, M.R.; Siqueira, J.P.Z.; Teresa, M.; Almeida, G. In vitro antifungal activity of Candida culture extracts against Trichophyton rubrum and Trichophyton mentagrophytes. Braz. J. Case Rep. 2021, 1, 135–152. [Google Scholar] [CrossRef]
- Tian, X.; Ding, H.; Ke, W.; Wang, L. Quorum Sensing in Fungal Species. Annu. Rev. Microbiol. 2021, 75, 449–469. [Google Scholar] [CrossRef]
- M38Ed3 Filamentous Fungi Antifungal Susceptibility Test [Internet]. Clinical & Laboratory Standards Institute. Available online: https://clsi.org/standards/products/microbiology/documents/m38/ (accessed on 21 July 2022).
- Klepser, M.E.; Ernst, E.J.; Lewis, R.E.; Ernst, M.E.; Pfaller, M.A. Influence of test conditions on antifungal time-kill curve results: Proposal for standardized methods. Antimicrob. Agents Chemother. 1998, 42, 1207–1212. [Google Scholar] [CrossRef] [PubMed]
- Ignasiak, K.; Maxwell, A. Galleria mellonella (greater wax moth) larvae as a model for antibiotic susceptibility testing and acute toxicity trials. BMC Res. Notes 2017, 10, 428. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Sáenz, A.; Arenas, R. Mixed Onychomycosis. A Case Report Caused by Trichophyton rubrum, Fusarium sp. and Candida albicans. Dermatol. Cosmet. Medica Quir. 2020, 18, 48–50. [Google Scholar]
- Youssef, A.B.; Kallel, A.; Azaiz, Z.; Jemel, S.; Bada, N.; Chouchen, A.; Belhadj-Salah, N.; Fakhfakh, N.; Belhadj, S.; Kallel, K. Onychomycosis: Which fungal species are involved? Experience of the Laboratory of Parasitology-Mycology of the Rabta Hospital of Tunis. J. Mycol. Med. 2018, 28, 651–654. [Google Scholar] [CrossRef] [PubMed]
- Lemes, T.H.; Maschio-Lima, T.; Brizzotti-Mazuchi, N.S.; Siqueira, J.P.Z.; de Almeida, M.T.G. O estudo da interferência biológica entre dermatófitos e leveduras em onicomicoses. Int. J. Health Sci.-PDVS 2021, 1, 75–86. [Google Scholar]
- Mohammadi, F.; Ghasemi, Z.; Familsatarian, B.; Salehi, E.; Sharifynia, S.; Barikani, A.; Hosseini, M.A. Relationship between antifungal susceptibility profile and virulence factors in Candida albicans isolated from nail specimens. Rev. Soc. Bras. Med. Trop. 2020, 53, e20190214. [Google Scholar] [CrossRef] [PubMed]
- Oliver, J.C.; Laghi, L.; Parolin, C.; Foschi, C.; Marangoni, A.; Liberatore, A.; Dias, A.L.T.; Cricca, M.; Vitali, B. Metabolic profiling of Candida clinical isolates of different species and infection sources. Sci. Rep. 2020, 10, 16716. [Google Scholar] [CrossRef]
- Ciesielska, A.; Kawa, A.; Kanarek, K.; Soboń, A.; Szewczyk, R. Metabolomic analysis of Trichophyton rubrum and Microsporum canis during keratin degradation. Sci. Rep. 2021, 11, 3959. [Google Scholar] [CrossRef]
- Bax, H.I.; Bakker-Woudenberg, I.A.J.M.; de Vogel, C.P.; van der Meijden, A.; Verbon, A.; de Steenwinkel, J.E.M. The role of the time-kill kinetics assay as part of a preclinical modeling framework for assessing the activity of anti-tuberculosis drugs. Tuberculosis 2017, 105, 80–85. [Google Scholar] [CrossRef]
- Junqueira, J.C.; Mylonakis, E.; Borghi, E. Galleria mellonella experimental model: Advances and future directions. Pathog. Dis. 2021, 79, ftab021. [Google Scholar] [CrossRef]
- Piatek, M.; Sheehan, G.; Kavanagh, K. Galleria mellonella: The Versatile Host for Drug Discovery, In Vivo Toxicity Testing and Characterising Host-Pathogen Interactions. Antibiotics 2021, 10, 1545. [Google Scholar] [CrossRef] [PubMed]

| MIC (µg/mL) | |||
|---|---|---|---|
| Fluconazole | Itraconazole | Terbinafine | |
| TMATCC | 4 | 0.5 | 0.03 |
| TM5094 | 32 | 2 | 0.03 |
| TM5419 | 16 | 1 | 0.03 |
| TM6007 | 32 | 1 | 0.03 |
| TM6085 | 32 | 1 | 0.03 |
| TM7389 | 16 | 1 | 0.03 |
| TRCBS | 2 | 0.25 | 0.03 |
| TR6185 | 4 | 0.5 | 0.03 |
| TR6195 | 4 | 0.5 | 0.03 |
| TR6284 | 4 | 0.5 | 0.03 |
| TR7259 | 32 | 0.5 | 64.0 |
| TR7604 | 2 | 0.5 | 0.03 |
| TR7984 | 2 | 0.5 | 0.03 |
| MIC (µg/mL) | ||||||
| TMATCC | TM5094 | TM5419 | TM6007 | TM6085 | TM7389 | |
| CP | 1000–2000 | 1000–2000 | 1000–2000 | 1000–2000 | 1000–2000 | 1000–2000 |
| CPTM | 250–8000 | 250–8000 | 500–8000 | 250–8000 | 250–8000 | 250–8000 |
| CPTR | 2000–8000 | 4000–8000 | 4000–8000 | 4000–8000 | 4000–8000 | 4000–8000 |
| TM | 1000–8000 | 2000–8000 | 1000–8000 | 1000–8000 | 1000–8000 | 1000–8000 |
| TR | 2000–8000 | 2000–8000 | 4000–8000 | 4000–8000 | 4000–8000 | 4000–8000 |
| MIC (µg/mL) | ||||||
| TRCBS | TR6185 | TR6195 | TR6284 | TR7604 | TR7984 | |
| CP | 500–2000 | 500–2000 | 500–2000 | 250–500 | 500–2000 | 250–2000 |
| CPTM | 250–8000 | 250–8000 | 250–8000 | 250–1000 | 500–8000 | 250–8000 |
| CPTR | 2000–4000 | 2000–8000 | 2000–4000 | 1000–2000 | 2000–4000 | 2000–4000 |
| TM | 1000–4000 | 1000–8000 | 2000–8000 | 250–2000 | 1000–8000 | 250–4000 |
| TR | 1000–4000 | 1000–8000 | 2000–8000 | 1000 | 2000–8000 | 1000–8000 |
| MFC (µg/mL) | ||||||
| TMATCC | TM5094 | TM5419 | TM6007 | TM6085 | TM7389 | |
| CP | 1000–2000 | 1000–2000 | 1000–2000 | 1000–2000 | 1000–2000 | 1000–2000 |
| CPTM | 500–8000 | 500–8000 | 500–8000 | 500–8000 | 1000–8000 | 500–8000 |
| CPTR | 4000–8000 | 4000–8000 | 4000–8000 | 4000–8000 | 4000–8000 | 4000–8000 |
| TM | 1000–8000 | 2000–8000 | 2000–8000 | 1000–8000 | 1000–8000 | 1000–8000 |
| TR | 2000–8000 | 4000–8000 | 4000–8000 | 4000–8000 | 4000–8000 | 4000–8000 |
| MFC (µg/mL) | ||||||
| TRCBS | TR6185 | TR6195 | TR6284 | TR7604 | TR7984 | |
| CP | 1000–2000 | 1000–2000 | 1000–2000 | 1000–2000 | 500–1000 | 500–2000 |
| CPTM | 250–8000 | 250–8000 | 500–8000 | 500–4000 | 500–8000 | 500–8000 |
| CPTR | 2000–4000 | 2000–8000 | 2000–8000 | 2000 | 4000 | 2000–4000 |
| TM | 1000–4000 | 1000–8000 | 2000–8000 | 1000–4000 | 2000–4000 | 1000–4000 |
| TR | 1000–8000 | 2000–8000 | 2000–8000 | 1000–4000 | 4000–8000 | 2000–4000 |
| Geometric Mean (µg/mL) | ||||||
|---|---|---|---|---|---|---|
| TR | TM | TRTM | ||||
| MIC | MFC | MIC | MFC | MIC | MFC | |
| CP | 689 | 1212 | 1137 | 1361 | 885 | 1284 |
| CPTM | 1414 | 1714 | 1759 | 2619 | 1577 | 2119 |
| CPTR | 2245 | 3429 | 4104 | 5040 | 3035 | 4157 |
| TM | 1343 | 2245 | 1805 | 3299 | 1557 | 2722 |
| TR | 2133 | 3429 | 3899 | 5040 | 2883 | 4157 |
| TRCBS | TR6185 | ||||||
| 8 h | 24 h | 48 h | 8 h | 24 h | 48 h | ||
| CONTROL | 5083 | 3812 | 1955 | CONTROL | 3957 | 4258 | 3450 |
| CP | 0 | 0 | 0 | CP | 0 | 0 | 0 |
| CPTR | 0 | 0 | 0 | CPTR | 0 | 0 | 0 |
| CPTM | 183 | 0 | 0 | CPTM | 1117 | 0 | 0 |
| TR | 0 | 0 | 0 | TR | 0 | 0 | 0 |
| TM | 67 | 100 | 1 | TM | 817 | 54 | 1 |
| TMATCC | TM5094 | ||||||
| 8 h | 24 h | 48 h | 8 h | 24 h | 48 h | ||
| CONTROL | 5173 | 5465 | 5217 | CONTROL | 5971 | 2745 | 4753 |
| CP | 0 | 0 | 0 | CP | 0 | 0 | 0 |
| CPTR | 0 | 0 | 0 | CPTR | 0 | 0 | 0 |
| CPTM | 283 | 0 | 0 | CPTM | 667 | 0 | 0 |
| TR | 0 | 0 | 0 | TR | 0 | 0 | 0 |
| TM | 1000 | 33 | 0 | TM | 1533 | 633 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lemes, T.H.; Nascentes, J.A.S.; Regasini, L.O.; Siqueira, J.P.Z.; Rigotto, G.; Tonani, L.; von Zeska Kress, M.R.; de Almeida, M.T.G. Antagonistic Interactions in Onychomycosis: Antifungal Activity of Extracts from Pure and Mixed Cultures of Candida parapsilosis and Trichophyton spp. Microbiol. Res. 2024, 15, 880-888. https://doi.org/10.3390/microbiolres15020057
Lemes TH, Nascentes JAS, Regasini LO, Siqueira JPZ, Rigotto G, Tonani L, von Zeska Kress MR, de Almeida MTG. Antagonistic Interactions in Onychomycosis: Antifungal Activity of Extracts from Pure and Mixed Cultures of Candida parapsilosis and Trichophyton spp. Microbiology Research. 2024; 15(2):880-888. https://doi.org/10.3390/microbiolres15020057
Chicago/Turabian StyleLemes, Thiago Henrique, Julyanna Andrade Silva Nascentes, Luis Octávio Regasini, João Paulo Zen Siqueira, Glaucia Rigotto, Ludmilla Tonani, Marcia Regina von Zeska Kress, and Margarete Teresa Gottardo de Almeida. 2024. "Antagonistic Interactions in Onychomycosis: Antifungal Activity of Extracts from Pure and Mixed Cultures of Candida parapsilosis and Trichophyton spp." Microbiology Research 15, no. 2: 880-888. https://doi.org/10.3390/microbiolres15020057
APA StyleLemes, T. H., Nascentes, J. A. S., Regasini, L. O., Siqueira, J. P. Z., Rigotto, G., Tonani, L., von Zeska Kress, M. R., & de Almeida, M. T. G. (2024). Antagonistic Interactions in Onychomycosis: Antifungal Activity of Extracts from Pure and Mixed Cultures of Candida parapsilosis and Trichophyton spp. Microbiology Research, 15(2), 880-888. https://doi.org/10.3390/microbiolres15020057






