Abstract
Various serovars of Salmonella had been the subject of research for over 150 years; nonetheless, the bacterium has remained an important pathogen of public health concern to date. The tremendous ability of Salmonella to form biofilms on biotic and abiotic surfaces is an important underlying reason for the prevalence of this opportunistic pathogen in healthcare, manufacturing, and the food chain. The current study illustrates that using very common industrial antimicrobial treatments at the highest concentrations suggested by the manufacturers is only efficacious against planktonic and one-day mature biofilms of the pathogen while exhibiting a lack of efficacy for complete removal of bacterial biofilms formed for longer than 2 days. This exhibits the importance of preventive measures against Salmonella biofilm formation in healthcare and manufacturing facilities, schools, nursing homes, and domestic environments. Additionally, our study illustrates the importance of including both planktonic and sessile cells of the pathogen in microbiology validation studies, especially for niche and hard-to-reach surfaces. The current study additionally investigated the suitability of an avirulent strain of the pathogen as a surrogate for pathogenic Salmonella serovars for public health microbiology validation studies when the use of virulent strains is not economically feasible or not possible due to safety concerns.
1. Introduction
As a microorganism with a complex and evolving nomenclature, Salmonella was first isolated in 1855 from the gastrointestinal area of an infected animal [1]. More than one and a half centuries since the discovery of the pathogen, various serovars of Salmonella continue to pose major public health challenges at local, national, and global scales [2]. The nomenclature of Salmonella has been subject to major changes. At the current time, according to the Institute Pasteur and the World Health Organization’s Collaborating Centre for Reference and Research on Salmonella, the bacterium consists of only two species, i.e., Salmonella enterica and Salmonella bongori [3]. While S. bongori has been associated with very limited cases of Salmonellosis in humans, the vast majority of the public health burden of the pathogen is associated with S. enterica, which is further divided into 6 subspecies and more than 2600 serotypes [4].
Various serovars of Salmonella could cause two distinctly different diseases. Based on epidemiological studies, it is well-established that nontyphoidal Salmonella such as Typhimurium, Newport, and Enteritidis serovars are generalist microorganisms with very broad host specificity. Nontyphoidal Salmonella is the causative agent of Salmonellosis worldwide and additionally is epidemiologically dominant in Salmonellae-related diseases in developed nations [4]. In the United States, in a typical year, there are over one million cases of nontyphoidal Salmonellosis with 94% of cases associated with contaminated food [5]. These infections lead to a disability-adjusted life year (DALY) of 32,900, causing the largest public health burden among the major foodborne pathogens in the United States [6].
In contrast to nontyphoidal Salmonella serovars that are very common among various warm-blooded hosts, a few S. enterica serovars including Paratyphi (A, B, or C), Sendai, and Typhi are adapted exclusively to humans as their host. These later serovars are causative agents for enteric fever (known as typhoid and/or paratyphoid fever), potentially life-threatening, invasive, and systematic infections that are more common in the developing world than in developed societies [4]. In a typical year, typhoidal Salmonella could cause 27 million global cases leading to 200,000 annual deaths [7,8]. In the United States, in a typical year, 1821 cases of typhoidal Salmonella occur, with the majority of cases associated with traveling abroad [5]. The hospitalization rate for nontyphoidal and typhoidal Salmonellosis in the United States is 27.2 and 75.7%, respectively [5].
While typhoidal Salmonella is endemic in developing nations and is primarily associated with a lack of clean water, poor hygiene infrastructure, and the fecal–oral route, nontyphoidal Salmonella is the dominant form of Salmonellosis in developed nations and is primarily considered a foodborne disease that could also be contracted via person-to-person contact with infected individuals and contact with infected animals as well [4]. While Salmonellosis can lead to these two broadly different disease phenotypes, there can be a range of pathotypes that could differ in outcome depending on host species and immunity. It is noteworthy that the public health burden of Salmonellosis is expected to be augmented to a great extent under the landscape of climate change [9,10].
In contrast to viral agents that require host cells for reproduction and cause infections, bacterial pathogens including Salmonella serovars have the ability to survive and form complex biofilm structures on abiotic and biotic surfaces [11,12]. Once a biofilm community is formed, bacteria could gain new characteristics through inter- and intraspecies pathways such as vertical and horizontal gene transfer mechanisms and quorum sensing [1,2,9]. Additionally, during the formation of sessile cells, bacterial species are capable of forming complex structures containing exopolysaccharide material, augmenting the resistance of the bacterium to environmental stressors such as antimicrobial treatments [13,14].
Among various serovars of Salmonella, S. Tennessee is an epidemiologically significant serovar that has a very critical role in interstate and intrastate commerce in the United States. Salmonella Tennessee was the causative agent of a multistate outbreak associated with peanut butter from 2006 to 2007 [15]. This outbreak garnered the attention of many popular press media and was one of the leading underlying reasons for the proposal and enactment of the U.S. Food Safety Modernization Act (FSMA) [16]. Various serovars of this bacterium could form biofilm on biotic and abiotic surfaces [1,4].
In addition to FSMA, Salmonella serovars are target microorganisms in other important national and global legislations as well. For example, juice products under the jurisdiction of Hazard Analysis and Critical Control Point (HACCP) would need to provide a validation study for 5-log reduction in nontyphoidal Salmonella serovars [17]. Additionally, it is noteworthy that Salmonella serovars have one of the lowest infective doses among the foodborne microbial pathogens—ingestion of as low as 10 single cells of the pathogen has been associated with human health complications [18]. Thus, the use of non-pathogenic strains of Salmonella, if proven to have comparable characteristics to pathogenic serovars of the pathogen, would be of great importance for microbiological validation studies. An avirulent strain of Salmonella enterica subspecies enterica serovar Typhimurium, with strain designation of LT2 (Salmonella LT2), had been tentatively proposed as a surrogate for virulent serovars of Salmonella [19,20].
Bacterial biofilms are of great importance from a public health perspective. A systematic review and meta-analysis, as an example, shows bacterial biofilms are associated with nearly 80% of chronic wounds [21]. The use of sodium hypochlorite and quaternary ammonium compound-based antimicrobials are among the most common treatments in industrial facilities (e.g., food and pharmaceutical industries), health care facilities (e.g., neonatal and elderly care settings), and domestic environments [11,22,23].
The current study was, thus, designed to compare the biofilm formation of Salmonella Tennessee and Salmonella LT2. Additionally, this study compares the efficacy of common industrial antimicrobials at the highest concentration recommended by the manufacturers against planktonic cells and biofilms of Salmonella Tennessee and Salmonella LT2. The latter comparison is intended to examine if previously validated treatments against planktonic cells are microbiologically efficacious in eliminating sessile cells during various stages of biofilm formation.
2. Materials and Methods
2.1. Strains’ Preparation
Two nontyphoidal Salmonella strains (virulent and avirulent strains) were selected for use in this study. Salmonella enterica subspecies enterica serovar Tennessee (Salmonella Tennessee) was sourced from the American Type Culture Collection with the designation ATCC® 10722™. As discussed earlier, this strain was the causative agent of a very influential outbreak leading to major changes in food policies and regulations [15,16]. In addition to this epidemiologically important pathogen, an avirulent strain of nontyphoidal Salmonella was selected for this study. Salmonella enterica subspecies enterica serovar Typhimurium, strain designation LT2 (Salmonella LT2), was included in the current study as a surrogate for pathogenic Salmonellae. The strain was available in the Public Health Microbiology FoundationSM strains library and had been shown to have comparable sensitivity to heat, antimicrobials, and non-thermal processing when compared to a set of a five-strain diverse mixture of nontyphoidal Salmonella serovars (ATCC® 13076™, 8387™, 6962™, 9270™, 14028™) in the food environment [24]. These two strains were stored, activated, sub-cultured, and purified using the methods detailed in our recent open-access studies [12,25,26]. In short, strains were kept at −80 °C as pathogen/glycerol stock and were transferred aseptically using a microbiological loop into 10 mL of Trypticase Soy Broth (TSB, Difco, Becton Dickinson, Franklin Lakes, NJ, USA) supplemented with 0.6% yeast extract (YE) to minimize acid-stress in the strains [12]. The inoculated TSB + YE tube, for each strain separately, was incubated at 37 °C for 24 h. After this incubation period, the overnight bacterial suspensions were vortexed (3200 RPM) for homogenization, and 100 µL aliquots were sub-cultured into 10 mL of sterilized TSB + YE followed by 24 h aerobic incubation at 37 °C. After homogenization of this incubated bacterial suspension, for each strain separately, the cells were harvested using the centrifugal force of 6000 RPM (3548 g for 88 mm rotor) for 15 min (Model 5424, Eppendorf North America, Hauppauge, NY, USA; Rotor FA-45-24-11). After discarding the supernatant, the microbial pellet for each strain was re-suspended in phosphate-buffered saline (PBS, VWR International, Radnor, PA, USA) and re-centrifuged at the above-mentioned intensity level and time for removal of cell secondary metabolites, remnants of the growth medium, and sloughed cell components and then re-suspended in PBS. These sub-cultured and purified cells were then used for inoculation of stainless-steel coupons for the biofilm formation trials.
2.2. Bacterial Inoculation, Biofilm Formation, and Treatments
The current study was conducted using previously used coupons of stainless steel (type 304, #2b finish) with 1.2 × 5 × 0.3 cm dimensions. These were washed and rinsed with detergent, deionized water, 70% ethanol (Fischer Scientific, Fair Lawn, NJ, USA), and 99% acetone (Fischer Scientific, Fair Lawn, NJ, USA.) for the elimination of any residue and were then autoclaved at 121 °C for 30 min for sterilization [13,27]. The coupons were then spot-inoculated with the above-mentioned two strains (two separate sets) at a target inoculation level of 3 to 4 log CFU/cm2 with 100 μL of inoculum. The spot-inoculated coupons were kept under a previously sterilized biosafety cabinet with a HEPA filter and remained in the sterilized condition at ambient temperature for 60 min (attachment time). The coupons were then aseptically transferred into 15 mL sterilized Eppendorf® conical tubes and 4 mL of sterilized 3% organic milk was added to each tube as a medium for biofilm formation. This medium and arrangement were chosen to ensure that inoculated coupons were half-submerged in a nutrient-dense environment for maximum biofilm formation at the air–liquid interface [28,29]. These coupons remained in the tilted upright position, half-submerged in aerobic condition at 25 °C (ambient temperature formation of sessile cells on abiotic surfaces), and were tested after 2 h (day 0, planktonic cells) and on days 1, 2, 3, 4, and 7 of biofilm formation. Coupons on each of these dates were first rinsed with 10 mL sterilized deionized water (once on each side) to remove loosely attached cells. Subsequently, the coupons, on each treatment day, were assigned into four groups (i) untreated control, (ii) treated controls (DI water treated samples), (iii) treated with sodium hypochlorite (Bleach-Rite, catalog no. BRSPRAY128, Fisher Scientific) at the highest concentration recommended by the manufacturer, and (iv) treated with quaternary ammonium compound (SE66 Disinfectant, Staple Contract & Commercial Inc., Framingham, MA, USA, EPA Establishment No. 8155-OH-1) at the highest concentration recommended by the manufacturer. For treatment samples, after the removal of loosely attached cells [13], 10 mL of chlorine-based (0.525% sodium hypochlorite) or quaternary ammonium compound-based (5% alkyl dimethyl benzyl ammonium chloride, 5% alkyl dimethyl ethyl benzyl ammonium chlorides) solutions were rinsed onto each side of the coupons followed by an exposure time of 60 s at ambient temperature. After the exposure time, the coupons were aseptically placed into 30 mL Dey-Engley (D/E) neutralizing broth (Difco, Becton Dickinson, Franklin Lakes, NJ, USA) containing 10 sterilized 4 mm glass beads. Tubes containing the coupons, neutralizing solutions, and beads were then vortexed for 2 min (3200 revolutions per minute) to detach bacterial biofilm cells from the coupons. Immediately following this separation, a 1 mL aliquot from the tubes was obtained, 10-fold serially diluted in Maximum Recovery Diluent (MRD, Difco, Becton Dickinson, Franklin Lakes, NJ, USA), and spread-plated onto the surface of Trypticase Soy Agar (TSB, Difco, Becton Dickinson, Franklin Lakes, NJ, USA) supplement with 0.6% yeast extract for enumeration of treatment survivors and injured cells. The spread-plated cells were incubated at 37 °C for 24 h, and the colonies were counted based on the U.S. Food and Drug Administration’s Bacteriological Analytical Methods using a Quebec colony counter [30]. A selective medium, Xylose Lysine Deoxycholate (XLD) Agar, was additionally used in this study (HiMedia®, West Chester, PA, USA).
The potency of treatments iii and iv was verified prior to the application of the antimicrobials at the highest concentration recommended by the supplier’s label. In addition to the certificate of analysis from the supplier, free chlorine and alkyl dimethyl benzyl ammonium compounds concentrations of the antimicrobials were measured and verified using Hanna Instruments Colorimeter for Free Chlorine and Quat™, respectively (Hanna Instruments, Woonsocket, RI, USA). The concentrations of the antimicrobials were additionally semi-quantitatively confirmed to be at least > 5000 and >400 parts per million (mg/L) using free chlorine and alkyl dimethyl benzyl ammonium compounds, respectively, using Free Chlorine and Quat™ test strips.
2.3. Study Design and Descriptive and Inferential Statistical Analyses
The current study was a complete randomized block design, which consisted of two biologically independent repetitions as the blocking factors. Each block in the study further consisted of two replications per day/strain/treatment/bacteriological media with each replication further consisting of two microbiological (instrumental) repetitions. Thus, each value presented in the current study is the mean of 8 independent observations (2 blocks, 2 replicates, and 2 microbiological repetitions). The microbial counts were log-transformed, and the log-normal data were used for the calculation of log reductions (descriptive statistics) and graphical representation (mean ± standard error). For inferential statistics, using the linear model of SAS9.4 software (SAS Inst., Cary, NC, USA), two procedures were utilized. For pair-wise comparisons of all treated samples and treated and untreated controls, an analysis of variance (ANOVA) followed by Tukey-adjusted mean separation was carried out. The largest mean in these analyses received an uppercase letter “A,” (for Salmonella Tennessee) and a lowercase letter “a,” (for Salmonella LT2); thus, the bars in Figure 1 followed by different letters correspond to differences that are statistically significant at a type I error level of 5%. Additionally, and for pathogenic and non-pathogenic strains separately, each treatment and the untreated control (deionized water) were compared to the untreated control using Dunnett’s-adjusted ANOVA, and the result of these analyses, also conduct at a type I error level of 5%, were illustrated using the “*” sign on Figure 1 (i.e., bars marked with the sign are statistically different from the untreated control). In the general linear model (GLM) procedure of SAS9.4, the treatments and medium treatments were placed on class and model statements, respectively. The GLM procedure was sorted by treatment days (i.e., days 0, 1, 2, 3, 4, and 7), and the analysis was repeated for each bacteriological medium (selective and non-selective media) separately. As further detailed above, Tukey and Dunnett were included in the means statement of the model.
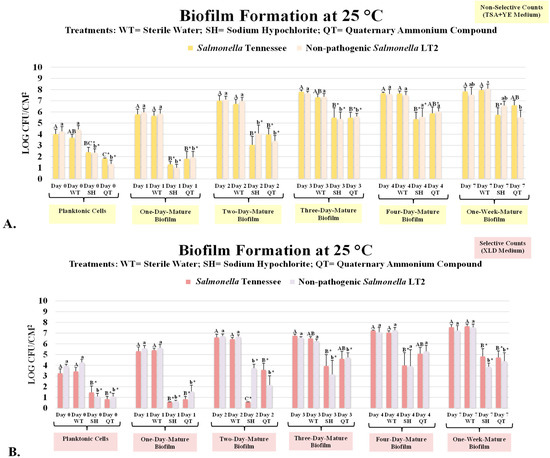
Figure 1.
Biofilm formation and reduction in pathogenic (Salmonella enterica subspecies enterica serovar Tennessee (Salmonella Tennessee)) and non-pathogenic (Salmonella enterica subspecies enterica serovar Typhimurium, strain designation LT2 (Salmonella LT2)) trials. (A) Non-selective (trypticase soy agar supplemented with 0.6% yeast extract) counts (mean ± SE). (B) Selective (xylose lysine deoxycholate [XLD] agar) counts. Columns marked with different uppercase letters are pathogenic counts (Salmonella Tennessee) that are statistically (p < 0.05) different from each other (Tukey-adjusted ANOVA). Columns marked with different lowercase letters are non-pathogenic counts (Salmonella LT2) that are statistically (p < 0.05) different from each other (Tukey-adjusted ANOVA). For pathogenic and non-pathogenic counts separately, columns followed by the “*” sign are statistically different (p < 0.05) from the untreated control (Dunnett’s-adjusted ANOVA).
3. Results and Discussion
Overall, in our experiments, we observed considerably lower counts of the selective medium relative to the supplemented non-selective medium (Figure 1 and Figure 2). This is in agreement with previous studies conducted using Salmonella serovars in the food chain [12,31]. The selective agent of the XLD medium, sodium deoxycholate, that was added to the medium to inhibit the growth of Gram-positive bacteria could additionally inhibit the multiplication of injured but viable cells [12]. Similarly, differential agents in the medium, e.g., xylose and lysine, could have similar effects on inhibiting the multiplication of injured cells. In contrast, the addition of yeast extract to the non-selective medium has been illustrated to have positive effects on the recovery of viable but injured cells [13,14,32]. The use of the selective medium, however, accompanying the non-selective medium, is of great importance since the selective counts provide internal validity that the counts are associated with inoculated pathogens and are not accidental laboratory-induced contamination during the conduct of the trials. These differences between selective and non-selective counts were similar to the information discussed in the literature for Salmonella and other Gram-negative and Gram-positive pathogens of public health concern [33,34].
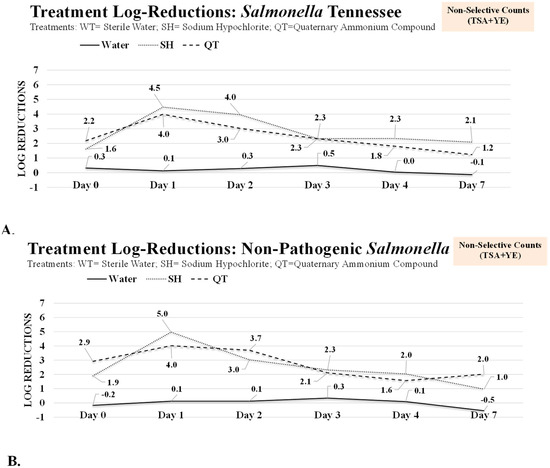
Figure 2.
Non-selective (trypticase soy agar supplemented with 0.6% yeast extract) log CFU/cm2 reductions in sessile and planktonic Salmonella serovars for samples treated with deionized water (treated control), sodium hypochlorite, and quaternary ammonium compounds. (A) Pathogenic counts, i.e., Salmonella enterica subspecies enterica serovar Tennessee (Salmonella Tennessee). (B) Non-pathogenic counts, i.e., Salmonella enterica subspecies enterica serovar Typhimurium, strain designation LT2 (Salmonella LT2).
The Tennessee serovar of nontyphoidal Salmonella enterica was chosen in this study due to the historical and regulatory significance of the strain to the stakeholders. Additionally, as discussed earlier, Salmonella enterica serovar Tennessee (Salmonella Tennessee) was the causative agent of one of the most influential foodborne outbreaks associated with food commerce [15,35]. This serovar has exhibited great capability to survive for an extended amount of time even in low-moisture environments, and limited information is available in the literature about the biofilm formation of this epidemiologically important strain. In addition to Salmonella Tennessee, our study utilized Salmonella enterica subspecies enterica serovar Typhimurium, strain designation LT2 (Salmonella LT2). This strain has the ecological and physiological characteristics of pathogenic nontyphoidal Salmonella. In the literature, Salmonella LT2 has been proposed as a potential avirulent surrogate for pathogenic Salmonella [19,20,36,37]. As further detailed in the Methods section, this study evaluated the effects of two common commercial antimicrobial treatments with treated (deionized water) and untreated controls. Sodium hypochlorite and quaternary ammonium compounds are important antimicrobials of choice in healthcare facilities, food and pharmaceutical industries, schools, and nursing homes, as well as domestic environments, and in this study, they were tested at the highest concentration permitted by the regulatory agencies as illustrated on product labels. As detailed in the Methods section, the statistical analyses were conducted for pair-wise comparison of all treatments and controls from various days of the treatments and additionally to compare each treatment and the treated control (DI water treated samples) directly with the untreated control. The former statistical analyses were conducted using a Tukey-based ANOVA and are listed in figures using various uppercase letters, with the letter “A” assigned to the largest value. The later comparisons were achieved using a Dunnett’s-adjusted ANOVA, and in the graphs, these analyses were presented using the “*” sign to indicate a statistical difference (p < 0.05) from the untreated control (Figure 1 and Figure 2). These analyses were conducted on data collected from the selective medium for Salmonella serovars (e.g., XLD agar) and non-selective medium supplemented with yeast extract to ensure maximum recovery of injured cells [33,38]. In addition to these, the pathogen log reductions were presented for selective and non-selective media to afford the opportunity for side-by-side comparisons of the three treatments with the untreated control (Figure 3A,B).
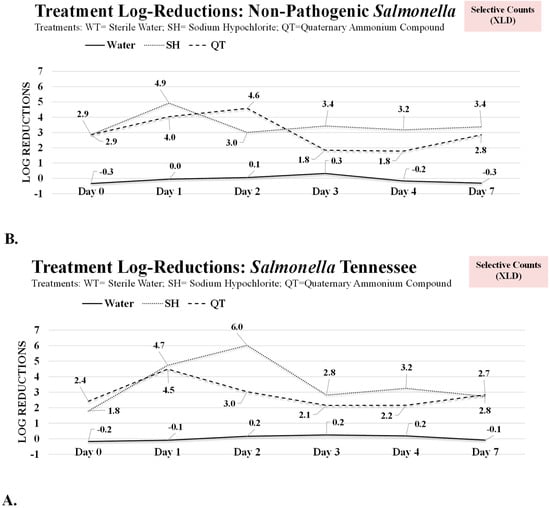
Figure 3.
Selective (xylose lysine deoxycholate (XLD) agar) log CFU/cm2 reductions in sessile and planktonic Salmonella serovars for samples treated with deionized water (treated control), sodium hypochlorite, and quaternary ammonium compounds. (A) Pathogenic counts, i.e., Salmonella enterica subspecies enterica serovar Tennessee (Salmonella Tennessee). (B) Non-pathogenic counts, i.e., Salmonella enterica subspecies enterica serovar Typhimurium, strain designation LT2 (Salmonella LT2).
3.1. Antimicrobial Treatment of Planktonic Cells and One-Day and Two-Day Mature Biofilms of Salmonella Tennessee and Salmonella LT2
The attachment time to biotic and abiotic surfaces could impact the proliferation and susceptibility of bacterial pathogens to environmental stressors such as antimicrobials [39,40]. In our study, the count from the non-selective medium associated with pathogenic Salmonella Tennessee on day 0 (planktonic cells) was 4.01 ± 0.4 log CFU/cm2 (mean ± SE), and the count remained unchanged (p ≥ 0.05) after treatment with deionized water (WT) and was 3.69 ± 0.3 log CFU/cm2 (Figure 1A). The log reductions associated with WT, SH, and QT treatments for planktonic pathogenic Salmonella cells were 0.3, 1.6, and 2.2 log CFU/cm2, respectively, on the non-selective medium (Figure 2A). These results indicate that treatment with SH and QT could eliminate (p < 0.05) up to >99% of the bacterial pathogen (Figure 1A), and the survivors of the treatments could be less than ≤2.5 CFU/cm2, e.g., ≤320 pathogenic cells (e.g., colony forming unit (CFU)) per cm2. This efficacy of the treatment against planktonic cells of the pathogen is in agreement with the existing literature, where cells of foodborne bacterial pathogens were reduced to nearly the detection limit when the antimicrobial tested against planktonic cells [32]. Additionally, our results show that counts of the non-pathogenic Salmonella LT2 strain were similar to those obtained from the pathogenic serovar (Figure 1A,B, Figure 2B and Figure 3B). The non-selective counts of Salmonella LT2 strain on day 0 were 4.26 ± 0.4, 4.43 ± 0.3, 2.35 ± 0.3, and 1.34 ± 0.4 log CFU/cm2 for the untreated control and WT, SH, and QT treatments, respectively (Figure 1A). These similarities between the pathogenic and non-pathogenic counts indicate that Salmonella LT2 could be used as a surrogate for pathogenic Salmonella in public health microbiology validation studies when utilization of pathogenic serovars is not economically feasible or not possible due to safety reasons.
After a 24 h incubation at 25 °C (e.g., day 1), the non-selective counts of the pathogenic and non-pathogenic Salmonella were increased to 5.78 ± 0.5 and 5.97 ± 0.4 log CFU/cm2 (Figure 1A), respectively. Treatment with water did not reduce (p ≥ 0.05) the pathogenic and non-pathogen counts (Figure 1A). In contrast, on day 1, the SH and QT treatments reduced (p < 0.05) the pathogenic Salmonella counts by 4.5 and 4.0 log CFU/cm2, respectively (Figure 2A), and similarly reduced (p < 0.05) the non-pathogenic Salmonella counts by 5.0 and 4.0 log CFU/cm2, respectively (Figure 2B). This indicates that counts of the Salmonella serovars were reduced nearly to the detection limit when the treatments were tested against planktonic cells and one-day mature biofilms.
The efficacy of these industrially prevalent treatments, however, was reduced when they were tested against the 2-day mature biofilms (e.g., day 2). The log-reduction associated with the non-selective counts of pathogenic Salmonella on this day were 0.3, 4.0, and 3.0 log CFU/cm2 for WT, SH, and QT treated samples, respectively (Figure 2A). These treatments left behind 6.72 ± 0.4, 3.05 ± 0.8, and 3.98 ± 0.5 log CFU/cm2 of the pathogen on the surface of stainless-steel coupons after WT, SH, and QT treatments on day 2, respectively (Figure 2A). Very similar reductions and the number of survivors were also observed for the non-pathogenic Salmonella LT2 strain (Figure 2A). It is noteworthy that as low as <10 single cells of Salmonella, if ingested orally, could lead to Salmonellosis in the general population and susceptible individuals [41]. Thus, Salmonella cells surviving a treatment are of great importance from a public health perspective.
3.2. Inactivation of Three-, Four-, and Seven-Day Biofilms of Salmonella Tennessee and Salmonella LT2
Biofilms of the pathogenic and non-pathogenic Salmonella serovars multiplied extensively during the trial (Figure 1A, B). As previously discussed, the overall counts from the non-selective medium were higher than those obtained from the selective medium since the latter has selective and differential additives and former has 0.6% supplemented yeast extract that inhibits and enhances the multiplication of the injured but viable bacteria, respectively. It is noteworthy that presenting data from both media is important to ensure the internal validity of this study, thus verifying that counts are obtained from the inoculated pathogen rather than accidental contamination in the laboratory procedures. The non-selective counts, that more accurately illustrate the existing microbial populations, for the pathogenic Salmonella on days 0, 1, 2, 3, 4, and 7 were 4.01 ± 0.4, 5.78 ± 0.5, 7.01 ± 0.5, 7.81 ± 0.2, 7.69 ± 0.1, and 7.83 ± 0.4 log CFU/cm2, respectively (Figure 1A). These counts for the non-pathogenic Salmonella were similar and were 4.26 ± 0.4, 5.97 ± 0.4, 7.10 ± 0.3, 7.70 ± 0.1, 7.60 ± 0.4, and 7.54 ± 0.7 log CFU/cm2, respectively, for the above order of testing days (Figure 1A). This shows that the quantity of the pathogenic and non-pathogenic biofilms was increased (p < 0.05) by >3 log CFU/ cm2 during the 7-day aerobic trial.
On days 3, 4, and 7, treatments with deionized water were not effective (p ≥ 0.05) to eliminate the biofilm of the pathogen exhibiting log reductions (p ≥ 0.05) with values ≤0.5 logs CFU/cm2 for the three days, for both the pathogenic and non-pathogenic serovars and in both the selective and non-selective media (Figure 2 and Figure 3). Treatment with SH, however, exhibited ≥99% reduction (p < 0.05) in pathogenic and non-pathogenic serovars (Figure 2 and Figure 3). On days 3, 4, and 7, the non-selective pathogenic counts were reduced by 2.3, 2.3, and 2.1 log CFU/cm2, respectively (Figure 2A). The corresponding reductions (p < 0.05) for the non-pathogenic strain were similar and were 2.3, 2.0, and 2.0 log CFU/cm2 (Figure 2B), respectively. Although this treatment was effective for at least 2 log CFU/cm2 reductions (p < 0.05) on days 3, 4, and 7, it is noteworthy that the treatment left behind a significant, both statistical and from a public health perspective, number of survivors after the treatment. In other words, the treatment that could eliminate nearly the entire pathogen population at the planktonic state, exhibits low efficacy for the complete removal of the pathogen biofilms from the surface when tested at the highest concentration recommended by the manufacturers. The pathogenic Salmonella survivors (non-selective counts) on the surface of the coupons after SH treatment on days 3, 4, and 7 were 5.48 ± 0.9, 5.35 ± 0.6, and 5.74 ± 0.6 log CFU/cm2, respectively (Figure 1A). These reductions were very similar for the non-pathogenic Salmonella LT2 (Figure 1A), for both the selective and non-selective counts (Figure 1A, B), and the coupons treated with QT (Figure 1, Figure 2 and Figure 3).
Our data show the importance of protective measures during manufacturing to ensure the prevention of biofilm formation. Our data also show that once the Salmonella biofilms are formed on a surface, common antimicrobial treatments might not be sufficient to remove the biofilm, and additional physical and/or chemical procedures are required to ensure the complete elimination of the pathogen. Additionally, our data highlight the importance of considering pathogens both at planktonic and sessile states during validation studies. This ensures that a validated cleaning procedure is not providing a false sense of security to health practitioners or manufacturing entrepreneurs and is indeed efficacious for the elimination of planktonic and biofilms of pathogens. Manufacturers and suppliers of these antimicrobials could also communicate the efficacy of their products to stakeholders in a manner that clearly indicates these products are tested and are recognized as effective treatments against only planktonic cells and additional validation studies are required to ensure their efficacy against sessile microbial cells. These data are in agreement with the existing literature associated with other pathogens. As an example, O157 and non-O157 Shiga toxin-producing Escherichia coli, as well as pathogenic Cronobacter, exhibited elevated resistance to common sanitizers at a sessile state relative to their corresponding wild-type planktonic cells [12,13].
To facilitate the assimilation of this, perhaps the biofilm formation of foodborne bacteria could be compared with Streptococcus mutans, the leading cause of dental cavities and the leading cause of worldwide infection, the microorganism that has a tremendous ability to form biofilm on the surfaces of human teeth. Although Streptococcus mutans could be eliminated with common antimicrobials at planktonic states, when the microorganism are forming biofilm in the form of dental plaques, the recommendation is for healthy adults to request a dental assistant to physically remove the biofilm of the bacterium from the surfaces of the teeth [42]. Of course, there is no intention of directly comparing the biofilm formation of a Gram-positive bacterium of oral health concern (Streptococcus mutans) with a Gram-negative pathogen associated with foodborne and waterborne diseases (Salmonella serovars). However, the persisting nature of biofilms in the form of dental plaques highlights that once the bacterial biofilm has formed, antimicrobial treatments, validated against planktonic cells, might not be efficacious for the complete removal of a bacterial pathogen. Unlike viral agents, this biofilm formation capability is uniquely associated with bacterial infections, and recent studies indicate that challenges associated with bacterial diseases and their biofilm formation are expected to be augmented under the landscape of the changing climate [9,10,43]. It is also important to highlight that the current study only evaluated biofilm formation and decontamination of non-typhoidal Salmonella serovars, and future studies could additionally study biofilm formation of typhoidal serovars of this opportunist and prevalent pathogen of public health concern.
It is noteworthy that this study investigated Salmonella Tennessee and Salmonella LT2, which are of importance from public health and industrial perspectives. The pathogenic strain utilized in the current study was recently studied during prolonged storage of food products [44] and for the identification of specific genetic material associated with its biofilm formation [45]. The interaction between the biofilm formation and pathogenicity of Salmonella serovars has additionally been studied extensively in recent years [46]. Future studies using more diverse industrial, plant-based, non-acid, nitric-acid, and phosphoric-acid disinfectants, to name a few, could be of great importance and complement to the current study. Finally, it is important to mention that not all strains of Salmonella with LT2 designation are considered avirulent, and some could exhibit mild pathogenicity for humans and/or mammalian cells. Thus, utilization of LT2 strains as a non-pathogenic surrogate for pathogenic Salmonella could be considered only after a careful and thorough investigation of each specific strain.
4. Conclusions
Under the condition of our trials, we observed that two very common sanitizers, validated in the past against planktonic cells and tested at the highest recommended concentrations, were effective for the elimination of planktonic cells and one-day mature biofilms. However, this efficacy was diminished to a great extent when the antimicrobials were tested against 2-, 3-, 4-, and 7-day mature biofilms. These data show the importance of preventive measures in the healthcare setting and during manufacturing to ensure controlling the proliferation and biofilm formation of bacterial pathogens of public health concern. Additionally, our study shows the importance of the sessile form of bacteria and highlights the need for the incorporation of bacterial biofilms in hurdle and sanitation public health microbiology validation studies. This could be an important part of health and safety plans for healthcare facilities and for manufacturing products under the jurisdiction of the U.S. Food Safety Modernization Act, or those mandated under the regulatory requirements of Hazard Analysis and Critical Control Point, to ensure that these food safety management systems can eliminate planktonic and sessile pathogens of public health concern from the food chain. Comparing the trials conducted using a pathogenic strain of epidemiological significance (Salmonella Tennessee) with a potentially avirulent strain of Salmonella (Salmonella LT2), we observed that the two strains have comparable biofilm formation capability and susceptibility to common antimicrobials. This illustrates that when the use of virulent Salmonella is not economically feasible or not possible due to safety concerns, an avirulent Salmonella LT2 strain could be used interchangeably for public health microbiology validation trials in studies with a similar scope to the current study.
It is important to re-emphasize that ingestion of as low as 10 single cells of Salmonella serovars could potentially lead to human health complications. While these commercially prevalent antimicrobials, used at the highest manufacturer’s recommended concentrations, were able to substantially reduce the pathogen counts of an important and prevalent bacterial pathogen such as Salmonella serovars, the presence and absence of the bacterium is of greater public health importance rather than the reduction in counts. These results further highlight the importance of preventive measures against bacterial biofilm.
Author Contributions
S.A. (Simen Asefaw), S.A. (Sadiye Aras), M.N.K., S.W. and S.C. contributed to conducting the trials and collection of data and assisted in the preparation of the manuscript. S.A. (Simen Asefaw) and S.A. (Sadiye Aras) led the data collection efforts, and S.A. (Simen Asefaw) contributed to co-writing the manuscript. A.C.F. secured extramural funding, designed the trials, provided training for laboratory assays, conducted statistical analyses, and co-wrote and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported in part by the Public Health Microbiology FoundationSM in Nashville, TN, and by the National Institute of Food and Agriculture of the United States Department of Agriculture.
Data Availability Statement
The datasets generated in the current study can be obtained by contacting this study’s corresponding author with reasonable requests. A request could be submitted by obtaining the contact information from the Public Health Microbiology FoundationSM at: https://publichealthmicrobiology.education/ (accessed on 27 May 2023). The SAS codes used for statistical analyses in the current study were derived from no-cost and publicly available sources with needed modifications and can be obtained by contacting the study’s corresponding author with reasonable requests.
Acknowledgments
Contributions of the students and staff of the Public Health Microbiology Laboratory are sincerely appreciated by the corresponding author of this study.
Conflicts of Interest
The authors declare no competing interests.
References
- Eng, S.K.; Pusparajah, P.; Ab Mutalib, N.S.; Ser, H.L.; Chan, K.G.; Lee, L.H. Salmonella: A review on pathogenesis, epidemiology and antibiotic resistance. Front. Life Sci. 2015, 8, 284–293. [Google Scholar] [CrossRef]
- Kumar, A.; Allison, A.; Henry, M.; Scales, A.; Fouladkhah, A.C. Development of salmonellosis as affected by bioactive food compounds. Microorganisms 2019, 7, 364. [Google Scholar] [CrossRef]
- Grimont, P.A.; Weill, F.X. Antigenic Formulae of the Salmonella Serovars; WHO Collaborating Centre for Reference and Research on Salmonella: Paris, France, 2007. [Google Scholar]
- Gal-Mor, O.; Boyle, E.C.; Grassl, G.A. Same species, different diseases: How and why typhoidal and non-typhoidal Salmonella enterica serovars differ. Front. Microbiol. 2014, 5, 391. [Google Scholar] [CrossRef] [PubMed]
- Scallan, E.; Hoekstra, R.M.; Angulo, F.J.; Tauxe, R.V.; Widdowson, M.A.; Roy, S.L.; Jones, J.L.; Griffin, P.M. Foodborne illness acquired in the United States—Major pathogens. J. Emerg. Infect. Dis. 2011, 17, 7. [Google Scholar] [CrossRef]
- Scallan, E.; Hoekstra, R.M.; Mahon, B.E.; Jones, T.F.; Griffin, P.M. An assessment of the human health impact of seven leading foodborne pathogens in the United States using disability adjusted life years. Epidemiol. Infect. 2015, 143, 2795–2804. [Google Scholar] [CrossRef] [PubMed]
- Crump, J.A.; Luby, S.P.; Mintz, E.D. The global burden of typhoid fever. Bull. World Health Organ. 2004, 82, 346–353. [Google Scholar]
- Buckle, G.C.; Walker, C.L.F.; Black, R.E. Typhoid fever and paratyphoid fever: Systematic review to estimate global morbidity and mortality for 2010. J. Glob. Health 2012, 2, 010401. [Google Scholar] [CrossRef]
- Fouladkhah, A.C.; Thompson, B.; Camp, J.S. Safety of food and water supplies in the landscape of changing climate. Microorganisms 2019, 7, 469. [Google Scholar] [CrossRef]
- Fouladkhah, A.C.; Thompson, B.; Camp, J.S. The threat of antibiotic resistance in changing climate. Microorganisms 2020, 8, 748. [Google Scholar] [CrossRef] [PubMed]
- Henry, M.; Fouladkhah, A.C. Outbreak history, biofilm formation, and preventive measures for control of Cronobacter sakazakii in infant formula and infant care settings. Microorganisms 2019, 7, 77. [Google Scholar] [CrossRef]
- Allison, A.; Fouladkhah, A.C. Sensitivity of planktonic cells and biofilm of wild-type and pressure-stressed Cronobacter sakazakii and Salmonella enterica serovars to sodium hypochlorite. Food Prot. Trends 2021, 41, 195–203. [Google Scholar] [CrossRef]
- Fouladkhah, A.; Geornaras, I.; Sofos, J.N. Biofilm formation of O157 and Non-O157 Shiga toxin-producing Escherichia coli and multidrug-resistant and susceptible Salmonella Typhimurium and Newport and their inactivation by sanitizers. J. Food Sci. 2013, 78, M880–M886. [Google Scholar] [CrossRef]
- Kabir, M.N.; Aras, S.; Wadood, S.; Chowdhury, S.; Fouladkhah, A.C. Fate and biofilm formation of wild-type and pressure-stressed pathogens of public health concern in surface water and on abiotic surfaces. Microorganisms 2020, 8, 408. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Multistate outbreak of Salmonella serotype Tennessee infections associated with peanut butter—United States, 2006–2007. MMWR 2007, 56, 521–524. [Google Scholar]
- Fouladkhah, A. The Need for evidence-based outreach in the current food safety regulatory landscape. J. Ext. 2017, 55, 20. [Google Scholar]
- Nummer, B.; Gump, D.; Wells, S.; Zimmerman, S.; Montalbano, A. Hazard Analysis and Critical Control Points (HACCP). In Regulatory Foundations for the Food Protection Professional; Springer: New York, NY, USA, 2015. [Google Scholar]
- Public Health Microbiology FoundationSM. Bacterial Multiplication. Available online: https://publichealthmicrobiology.education/fact-sheets (accessed on 29 December 2022).
- de Moraes, M.H.; Chapin, T.K.; Ginn, A.; Wright, A.C.; Parker, K.; Hoffman, C.; Pascual, D.W.; Danyluk, M.D.; Teplitski, M. Development of an avirulent Salmonella surrogate for modeling pathogen behavior in pre-and postharvest environments. Appl. Environ. Microbiol. 2016, 82, 4100–4111. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ryser, E.T. Quantitative transfer of Salmonella Typhimurium LT2 during mechanical slicing of tomatoes as impacted by multiple processing variables. Int. J. Food Microbiol. 2016, 234, 76–82. [Google Scholar] [CrossRef]
- Malone, M.; Bjarnsholt, T.; McBain, A.J.; James, G.A.; Stoodley, P.; Leaper, D.; Tachi, M.; Schultz, G.; Swanson, T.; Wolcott, R.D. The prevalence of biofilms in chronic wounds: A systematic review and meta-analysis of published data. J. Wound Care 2017, 26, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Buffet-Bataillon, S.; Tattevin, P.; Bonnaure-Mallet, M.; Jolivet-Gougeon. Emergence of resistance to antibacterial agents: The role of quaternary ammonium compounds—A critical review. Int. J. Antimicrob. Agents 2012, 39, 381–389. [Google Scholar] [CrossRef]
- Fukuzaki, S. Mechanisms of actions of sodium hypochlorite in cleaning and disinfection processes. Biocontrol Sci. 2006, 11, 147–157. [Google Scholar] [CrossRef]
- Chowdhury, A.; Aras, S.; Kabir, N.; Wadood, S.; Allison, A.; Chowdhury, S.; Fouladkhah, A.C. Susceptibility of pathogenic nontyphoidal Salmonella serovars and avirulent Salmonella LT2 to elevated hydrostatic pressure and citricidalTM. J. Tenn. Acad. Sci. 2021, 96, 49–54. [Google Scholar] [CrossRef]
- Kabir, M.N.; Aras, S.; Allison, A.; Adhikari, J.; Chowdhury, S.; Fouladkhah, A.C. Interactions of carvacrol, caprylic acid, habituation, and mild heat for pressure-based inactivation of O157 and non-O157 serogroups of Shiga toxin-producing Escherichia coli in acidic environment. Microorganisms 2019, 7, 145. [Google Scholar] [CrossRef]
- Aras, S.; Kabir, M.N.; Chowdhury, S.; Fouladkhah, A.C. Augmenting the pressure-based pasteurization of Listeria monocytogenes by synergism with nisin and mild heat. Int. J. Environ. Res. Public Health 2020, 17, 563. [Google Scholar] [CrossRef]
- Simpson Beauchamp, C.; Dourou, D.; Geornaras, I.; Yoon, Y.; Scanga, J.A.; Belk, K.E.; Smith, G.C.; Nychas, G.J.E.; Sofos, J.N. Transfer, attachment, and formation of biofilms by Escherichia coli O157: H7 on meat-contact surface materials. J. Food Sci. 2012, 77, M343–M347. [Google Scholar] [CrossRef] [PubMed]
- Wijman, J.G.; de Leeuw, P.P.; Moezelaar, R.; Zwietering, M.H.; Abee, T. Air-liquid interface biofilms of Bacillus cereus: Formation, sporulation, and dispersion. Appl. Environ. Microbiol. 2007, 73, 1481–1488. [Google Scholar] [CrossRef]
- Giaouris, E.D.; Nychas, G.J.E. The adherence of Salmonella Enteritidis PT4 to stainless steel: The importance of the air–liquid interface and nutrient availability. Food Microbiol. 2006, 23, 747–752. [Google Scholar] [CrossRef]
- United States Food and Drug Administration. Bacteriological Analytical Methods (FDA BAM). Aerobic Plate Count. 2001. Available online: https://www.fda.gov/food/laboratory-methods-food/bam-chapter-3-aerobic-plate-count (accessed on 29 December 2022).
- Zhou, S.; Sheen, S.; Zhao, G.; Chuang, S.; Liu, L. Prediction of Salmonella inactivation in sliced tomato subject to high pressure processing and trans-cinnamaldehyde treatment using selective and non-selective growth media for survival evaluations. Food Control 2020, 118, 107441. [Google Scholar] [CrossRef]
- Allison, A.; Chowdhury, S.; Fouladkhah, A.C. Synergism of mild heat and high-pressure pasteurization against Listeria monocytogenes and natural microflora in phosphate-buffered saline and raw milk. Microorganisms 2018, 6, 102. [Google Scholar] [CrossRef]
- Kabir, M.N.; Aras, S.; George, J.; Wadood, S.; Chowdhury, S.; Fouladkhah, A.C. High-pressure and thermal-assisted pasteurization of habituated, wild-type, and pressure-stressed Listeria monocytogenes, Listeria innocua, and Staphylococcus aureus. LWT 2021, 137, 110445. [Google Scholar] [CrossRef]
- Allison, A.; Daniels, E.; Chowdhury, S.; Fouladkhah, A.C. Effects of elevated hydrostatic pressure against mesophilic background microflora and habituated Salmonella serovars in orange juice. Microorganisms 2018, 6, 23. [Google Scholar] [CrossRef]
- Aviles, B.; Klotz, C.; Smith, T.; Williams, R.; Ponder, M. Survival of Salmonella enterica serotype Tennessee during simulated gastric passage is improved by low water activity and high fat content. J. Food Prot. 2013, 76, 333–337. [Google Scholar] [CrossRef]
- Wilmes-Riesenberg, M.R.; Foster, J.W.; Curtiss, R. An altered rpoS allele contributes to the avirulence of Salmonella typhimurium LT2. Infect. Immun. 1997, 65, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Smolinski, H.S.; Wang, S.; Ren, L.; Chen, Y.; Kowalcyk, B.; Thomas, E.; Doren, J.V.; Ryser, E.T. Transfer and redistribution of Salmonella Typhimurium LT2 and Escherichia coli O157: H7 during pilot-scale processing of baby spinach, cilantro, and romaine lettuce. J. Food Prot. 2018, 81, 953–962. [Google Scholar] [CrossRef]
- Allison, A.; Fouladkhah, A.C. Sensitivity of wild-type and rifampicin-resistant O157 and non-O157 Shiga toxin-producing Escherichia coli to elevated hydrostatic pressure and lactic acid in ground meat and meat homogenate. PLoS ONE 2021, 16, e0246735. [Google Scholar] [CrossRef] [PubMed]
- Kwok, T.Y.; Ma, Y.; Chua, S.L. Biofilm dispersal induced by mechanical cutting leads to heightened foodborne pathogen dissemination. Food Microbiol. 2022, 102, 103914. [Google Scholar] [CrossRef] [PubMed]
- Dantas, S.T.; Rossi, B.F.; Bonsaglia, E.C.; Castilho, I.G.; Hernandes, R.T.; Fernandes, A.; Rall, V.L. Cross-contamination and biofilm formation by Salmonella enterica serovar Enteritidis on various cutting boards. Foodborne Pathog. Dis. 2018, 15, 81–85. [Google Scholar] [CrossRef] [PubMed]
- The U.S. Food and Drug Administration. Bad Bug Book: Handbook of Foodborne Pathogenic Microorganisms and Natural Toxins. 2017. Available online: https://www.fda.gov/files/food/published/Bad-Bug-Book-2nd-Edition-%28PDF%29.pdf (accessed on 29 December 2022).
- The U.S. Centers for Disease Control and Prevention. Oral Health. 2021. Available online: https://www.cdc.gov/oralhealth/index.html (accessed on 29 December 2022).
- Fouladkhah, A. Epidemiology–Laboratory Interactions for Developing Resilience Against Future Infectious Diseases. Research Outreach 2022, p. 129. Available online: https://researchoutreach.org/articles/epidemiology-laboratory-interactions-developing-resilience-future-infectious-diseases/ (accessed on 29 December 2022).
- Aviles, B.; Klotz, C.; Eifert, J.; Williams, R.; Ponder, M. Biofilms promote survival and virulence of Salmonella enterica sv. Tennessee during prolonged dry storage and after passage through an in vitro digestion system. Int. J. Food Microbiol. 2013, 162, 252–259. [Google Scholar] [CrossRef]
- Lee, S.; Chen, J. Identification of the genetic elements involved in biofilm formation by Salmonella enterica serovar Tennessee using mini-Tn10 mutagenesis and DNA sequencing. Food Microbiol. 2022, 106, 104043. [Google Scholar] [CrossRef]
- Jahan, F.; Chinni, S.V.; Samuggam, S.; Reddy, L.V.; Solayappan, M.; Yin, L.S. The Complex Mechanism of the Salmonella typhi Biofilm Formation That Facilitates Pathogenicity: A Review. Int. J. Mol. Sci. 2022, 2, 6462. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).