Bacterial Bioprotectants: Biocontrol Traits and Induced Resistance to Phytopathogens
Abstract
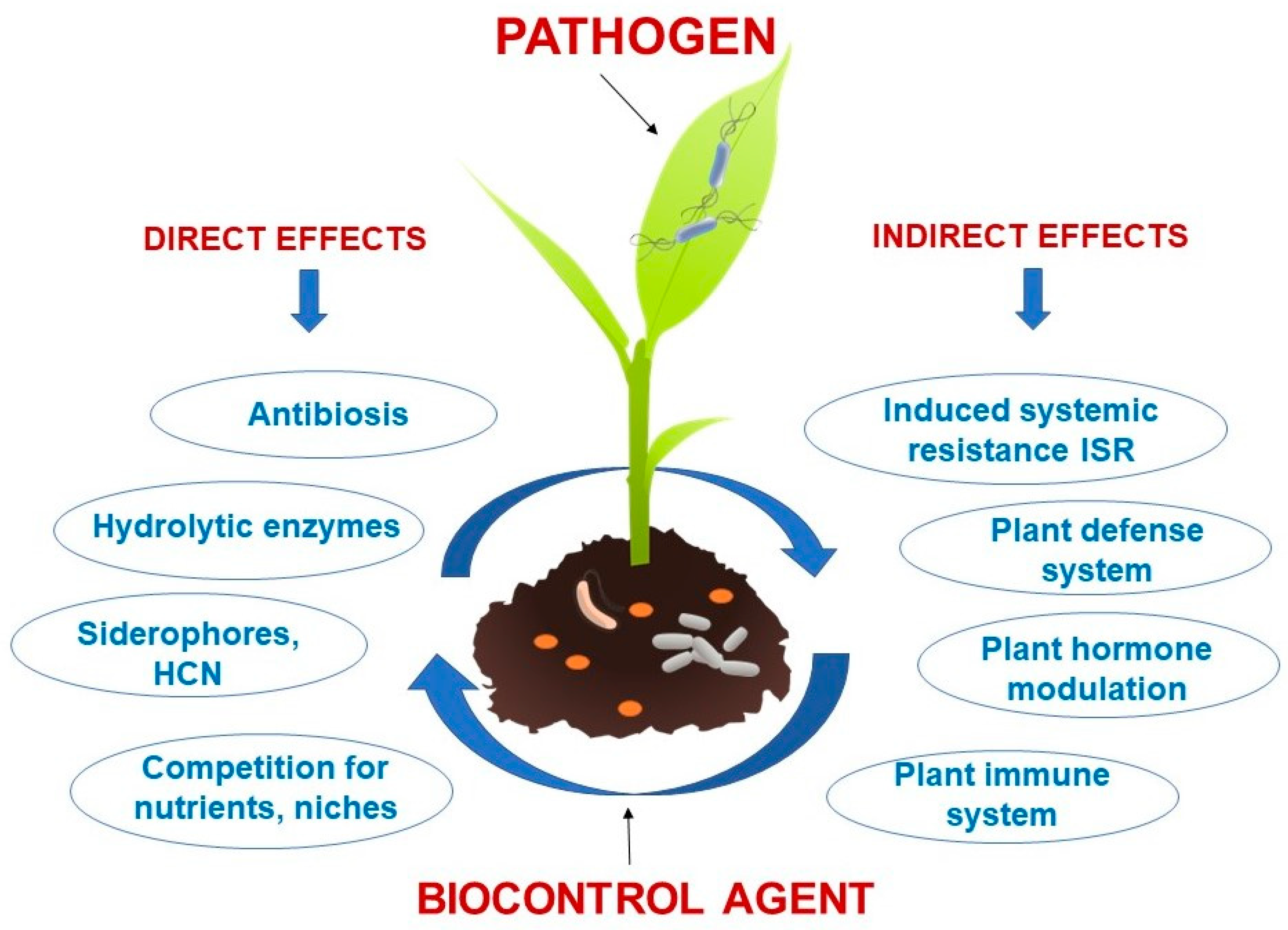
1. Introduction
| Plant Beneficil Bacteria | Host Plant | Pathogen(s) | Reference |
|---|---|---|---|
| Microbispora sp. Streptomyces sp. | Field mustard (Brassica rapa) | Plasmodiophora brassicae | Lee et al. [41] |
| Streptomyces sp. | Tomato (Solanum lycopersicum) | Fusarium proliferatum | Passari et al. [42] |
| Streptomyces sp. | Black kennedia, (Kennedia nigriscans) | Pythium ultimum, Rhizoctonia solani, Phytophthora cinnamomi | Catillo et al. [43] |
| Streptomyces ochraceiscleroticus Leifsonia xyli, Microbacterium sp. | Red sage (Salvia militiorrhiza), Tumeric (Curcuma longa) | Fusarium oxysporum, Curvularia lunata, Botrytis cinerea | Zhao et al. [44] |
| Brevibacterium sp. | Ferula sinkiangensis | Alternaria alternate, Verticillium dahlia | Liu et al. [16] |
| Bacillus sp. | Sugar beet (Beta vulgaris L.) | S. rolfsii | Farhaoui et al. [45] |
| Bacillus licheniformis | Banana (Musa sp.) | Fusarium oxysporum f.sp. cubense | Yadav et al. [46] |
| Lysinibacillus sp., Pseudomonas fluorescens | Potato (Solanum tuberosum) | Ralstonia solanacearum that | Dijaya et al. [47] |
| Bacillus velezensis a | Rice (Oryza sativa) | Burkholderia glumae | Perea-Molina et al. [48] |
| Pseudomonas aeruginosa Bacillus subtilis | Turmeric (Curcuma longa) | Rhizoctonia solani Fusarium solani | Chenniappan et al. [49] |
2. Mechanisms of Action of Microbial Biocontrol Agents
2.1. Production of Phytohormone
2.2. Lytic Enzymes
2.3. Antifungal Compounds
2.4. Siderophore Production
2.5. Induction Systemic Resistance (ISR)
| Endophytes | Properties/Mechanisms | Refernce |
|---|---|---|
| Paecilomyces Variotii SJ1 | Reactive oxygen species accumulation, increased SA and activated SA signaling pathway | Peng et al. [101] |
| Penicillium citrinum LWL4 and Aspergillus terreus LWL5 | Production of SA and JA | Waqas et al. [98] |
| Fusarium Fo47 | Production of SA, JA, and ET | Constantin et al. [107] |
| Burkholderia gladioli | Production of chitinases and β-1,3-glucanase; enhanced endogenous JA levels; overexpression of JA-regulated and other plant defence genes | Ahmad et al. [102] |
| Enterobacter asburiae | Expression of defense-related genes and antioxidant enzymes | Jayaraj et al. [108] |
| Serratia liquefaciens and P. putida | Acyl-homoserine lactones | Schuhegger et al. [104] |
| Azospirillum sp. B510 | The induction of signal transduction pathways triggered by ET | Kusajima et al. [109] |
| P. aeruginosa and P. pseudoalcaligenes | Production of phenolics and flavonoids; induction of PR proteins such as enzymes β-1,3-glucanase and catalase | Jha [103] |
| Bacillus sp. 2P2 | Higher activity of phenylalanine ammonia lyase, peroxidase, polyphenol oxidase, an ascorbate oxidase; upregulated the expression of three pathogenesis-related genes, PR1a, PR2a, and PR3 | Sahu et al. [105] |
| Bacillus velezensis YC7010 | Higher expression of PAD4 with suppression of BIK1 | Rashid et al. [100] |
2.6. Antioxidant Enzymes
2.7. Competition for Nutrient and Niches
3. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Van Bavel, J. The world population explosion: Causes, backgrounds and projections for the future. Facts Views Vis. ObGyn 2013, 5, 281–291. [Google Scholar] [PubMed]
- Kumari, V.V.; Banerjee, P.; Verma, V.C.; Sukumaran, S.; Chandran, M.A.S.; Gopinath, K.A.; Venkatesh, G.; Yadav, S.K.; Singh, V.K.; Awasthi, N.K. Plant Nutrition: An effective way to alleviate abiotic stress in agricultural crops. Int. J. Mol. Sci. 2022, 23, 8519. [Google Scholar] [CrossRef] [PubMed]
- Hashem, A.; Abd-Allah, E.F.; Alqarawi, A.A.; Wirth, S.; Egamberdieva, D. Arbuscular mycorrhizal fungi alleviate salt stress in lupine (Lupinus termis Forsik) through modulation of antioxidant defense systems and physiological traits. Legume Res. 2016, 39, 198–207. [Google Scholar] [CrossRef]
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Ristaino, J.B.; Anderson, P.K.; Bebber, D.P.; Wei, Q. The persistent threat of emerging plant disease pandemics to global food security. Proc. Natl. Acad. Sci. USA 2021, 118, e2022239118. [Google Scholar] [CrossRef]
- Velásquez, A.C.; Castroverde, C.D.M.; He, S.Y. Plant-Pathogen warfare under changing climate conditions. Curr. Biol. 2018, 28, R619–R634. [Google Scholar] [CrossRef]
- Egamberdieva, D. Alleviation of salt stress by plant growth regulators and IAA producing bacteria in wheat. Acta Phys. Plant. 2009, 31, 861–864. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Berg, G.; Lindstrom, K.; Rasanen, L. Co-inoculation of Pseudomonas spp. with Rhizobium improves growth and symbiotic performance of fodder galega (Galega orientalis Lam.). Eur. J. Soil Biol. 2010, 46, 269–272. [Google Scholar] [CrossRef]
- Selim, K.A.; El-Beih, A.A.; AbdEl-Rahman, T.M.; El-Diwany, A.I. Biology of endophytic fungi. Curr. Res. Environ. Appl. Mycol. 2012, 2, 31–82. [Google Scholar] [CrossRef]
- Mitter, B.; Pfaffenbichler, N.; Flavell, R.; Compant, S.; Antonielli, L.; Petric, A.; Berninger, T.; Naveed, M.; Sheibani-Tezerji, R.; Von Maltzahn, G. A new approach to modify plant microbiomes and traits by introducing beneficial bacteria at flowering into progeny seeds. Front. Microbiol. 2017, 8, 11. [Google Scholar] [CrossRef]
- Ipek, M.; Pirlak, L.; Esitken, A.; Figen Dönmez, M.; Turan, M.; Sahin, F. Plant growth promoting rhizobacteria (PGPR) increase yield, growth and nutrition of strawberry under high-calcareous soil conditions. J. Plant. Nutr. 2014, 37, 990–1001. [Google Scholar] [CrossRef]
- Cho, S.T.; Chang, H.H.; Egamberdieva, D.; Kamilova, F.; Lugtenberg, B.; Kuo, C.H. Genome analysis of Pseudomonas fluorescens PCL1751: A rhizobacterium that controls root diseases and alleviates salt stress for its plant host. PLoS ONE 2015, 10, e0140231. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Wirth, S.; Alqarawi, A.A.; Abd_Allah, E.F.; Hashem, A. Phytohormones and beneficial microbes: Essential components for plants to balance stress and fitness. Front. Microbiol. 2017, 8, 2104. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Wirth, S.; Behrendt, U.; Parvaiz, A.; Berg, G. Antimicrobial activity of medicinal plants correlates with the proportion of antagonistic endophytes. Front. Microb. 2017, 8, 199. [Google Scholar] [CrossRef]
- Jacoby, R.; Peukert, M.; Succurro, A.; Koprivova, A.; Kopriva, S. The role of soil microorganisms in plant mineral nutrition—Current knowledge and future directions. Front. Plant Sci. 2017, 8, 1617. [Google Scholar] [CrossRef]
- Liu, Y.; Mohamad, O.A.A.; Salam, N.; Zhang, Y.; Guo, J.W.; Li, L.; Egamberdieva, D.; Li, W.J. Diversity, community distribution and growth promotion activities of endophytes associated with halophyte Lycium ruthenicum Murr. 3Biotech 2019, 9, 144. [Google Scholar] [CrossRef]
- De Silva, N.I.; Brooks, S.; Lumyong, S.; Hyde, K.D. Use of endophytes as biocontrol agents. Fungal Biol. Rev. 2019, 33, 133–148. [Google Scholar] [CrossRef]
- Mukherjee, A.; Bhowmick, S.; Yadav, S.; Rashid, M.M.; Chouhan, G.K.; Vaishya, J.K.; Verma, J.K. Re-vitalizing of endophytic microbes for soil health management and plant protection. 3Biotech 2021, 11, 399. [Google Scholar] [CrossRef]
- Castanheira, N.L.; Dourado, A.C.; Pais, I.; Semedo, J.; Scotti-Campos, P.; Borges, N.; Carvalho, G.; Barreto Crespo, M.T.; Fareleira, P. Colonization and beneficial effects on annual ryegrass by mixed inoculation with plant growth promoting bacteria. Microbiol. Res. 2017, 198, 47–55. [Google Scholar] [CrossRef]
- Rangjaroen, C.; Sungthong, R.; Rerkasem, B.; Teaumroong, N.; Noisangiam, R.; Lumyong, S. Untapped endophytic colonization and plant growth-promoting potential of the genus Novosphingobium to optimize rice cultivation. Microbes Environ. 2017, 32, 84–87. [Google Scholar] [CrossRef]
- Herrera, S.D.; Grossi, C.; Zawoznik, M.; Groppa, M.D. Wheat seeds harbour bacterial endophytes with potential as plant growth promoters and biocontrol agents of Fusarium graminearum. Microbiol. Res. 2016, 186, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Naveed, M.; Mitter, B.; Reichenauer, T.G.; Wieczorek, K.; Sessitsch, A. Increased drought stress resilience of maize through endophytic colonization by Burkholderia phytofirmans PsJN and Enterobacter sp. FD 17. Environ. Exp. Bot. 2014, 97, 30–39. [Google Scholar] [CrossRef]
- Parray, A.P.; Jan, S.; Kamili, A.N.; Qadri, R.A.; Egamberdieva, D.; Ahmad, P. Current perspectives on plant growth promoting rhizobacteria. Plant Growth Regul. 2016, 35, 877–902. [Google Scholar] [CrossRef]
- Egamberdiyeva, D.; Höflich, G. The effect of associative bacteria from different climates on plant growth of pea at different soils and temperatures. Arch. Agron. Soil Sci. 2003, 49, 203–213. [Google Scholar] [CrossRef]
- Mantzoukas, S.; Eliopoulos, P.A. Endophytic entomopathogenic fungi: A valuable biological control tool against plant pests. Appl. Sci. 2020, 10, 360. [Google Scholar] [CrossRef]
- Gao, L.; Ma, J.; Liu, Y.; Huang, Y.; Mohamad, O.A.A.; Jiang, H.; Egamberdieva, D.; Li, L. Diversity of cultivable endophytic bacteria associated with halophytes from the west Aral Sea Basin with biocontrol potential. Microorganism 2021, 9, 1448. [Google Scholar] [CrossRef]
- Lecomte, C.; Alabouvette, C.; Edel-Hermann, V.; Robert, F.; Steinberg, C. Biological control of ornamental plant diseases caused by Fusarium oxysporum: A review. Biol. Control. 2016, 101, 17–30. [Google Scholar] [CrossRef]
- Dodd, I.C.; Zinovkina, N.Y.; Safronova, V.I.; Belimov, A.A. Rhizobacterial mediation of plant hormone status. Ann. Appl. Biol. 2010, 157, 361–379. [Google Scholar] [CrossRef]
- Erdogan, O.; Benlioglu, K. Biological control of Verticillium wilt on cotton by the use of fluorescent Pseudomonas spp. under field conditions. Biol. Control. 2010, 53, 39–45. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Kucharova, Z.; Davranov, K.; Berg, G.; Makarova, N.; Azarova, T.; Chebotar, V.; Tikhonovich, I.; Kamilova, F.; Validov, S.; et al. Bacteria able to control foot and root rot and to promote growth of cucumber in salinated soils. Biol. Fertil. Soils. 2011, 47, 197–205. [Google Scholar] [CrossRef]
- Khan, N.; Martínez-Hidalgo, P.; Ice, T.A.; Maymon, M.; Humm, E.A.; Nejat, N.; Sanders, E.R.; Kaplan, D.; Hirsch, A.M. Antifungal activity of Bacillus species against fusarium and analysis of the potential mechanisms used in biocontrol. Front. Microbiol. 2018, 9, 2363. [Google Scholar] [CrossRef]
- Romero, D.; De Vicente, A.; Olmos, J.L.; Dávila, J.C.; Pérez-García, A. Effect of lipopeptides of antagonistic strains of Bacillus subtilis on the morphology and ultrastructure of the cucurbit fungal pathogen Podosphaera fusca. J. Appl. Microbiol. 2007, 103, 969–976. [Google Scholar] [CrossRef]
- Baysal, O.; Çalışkan, M.; Yeşilova, O. An inhibitory effect of a new Bacillus subtilis strain (EU07) against Fusarium oxysporum f. sp. radicis-lycopersici. Physiol. Mol. Plant Path. 2008, 73, 25–32. [Google Scholar] [CrossRef]
- Sessitsch, A.; Hardoim, P.; Döring, J.; Weilharter, A.; Krause, A.; Woyke, T. Functional characteristics of an endophyte community colonizing rice roots as revealed by metagenomic analysis. Mol. Plant Microbe Interact. 2012, 25, 28–36. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Wirth, S.; Shurigin, V.; Hashem, A.; Abd_Allah, E.F. Endophytic bacteria improve plant growth, symbiotic performance of chickpea (Cicer arietinum L.) and induce suppression of root rot caused by Fusarium solani under salt stress. Front. Microbiol. 2017, 8, 1887. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Wirth, S.; Bellingrath-Kimura, S.D.; Mishra, J.; Arora, N.K. Salt-tolerant plant growth promoting rhizobacteria for enhancing crop productivity of saline soils. Front. Microbiol. 2019, 10, 2791. [Google Scholar] [CrossRef]
- Spaepen, S.; Vanderleyden, J.; Remans, R. Indole-3-acetic acid in microbial and microorganism-plant signaling. FEMS Microb. Rev. 2007, 31, 425–448. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Berg, G.; Lindström, K.; Räsänen, L.A. Alleviation of salt stress of symbiotic Galega officinalis L. (goat’s rue) by co-inoculation of rhizobium with root colonising Pseudomonas. Plant Soil. 2013, 369, 453–465. [Google Scholar] [CrossRef]
- Berg, G.; Köberl, M.; Rybakova, D.; Müller, H.; Grosch, R.; Smalla, K. Plant microbial diversity is suggested as the key to future biocontrol and health trends. FEMS Microbiol. Ecol. 2017, 93, fix050. [Google Scholar] [CrossRef]
- Kamilova, F.; Validov, S.; Azarova, T.; Mulders, I.; Lugtenberg, B. Enrichment for enhanced competitive plant root tip colonizers selects for a new class of biocontrol bacteria. Environ. Microbiol. 2005, 7, 1809–1817. [Google Scholar] [CrossRef]
- Lee, S.O.; Choi, G.J.; Choi, Y.H.; Jang, K.S.; Park, D.J.; Kim, C.J. Isolation and characterization of endophytic actinomycetes from Chinese cabbage roots as antagonists to Plasmodiophora brassicae. J. Microbiol. Biotechnol. 2008, 18, 1741–1746. [Google Scholar] [PubMed]
- Passari, A.K.; Mishra, V.K.; Leo, V.V.; Gupta, V.K.; Singh, B.P. Phytohormone production endowed with antagonistic potential and plant growth promoting abilities of culturable endophytic bacteria isolated from Clerodendrum colebrookianum Walp. Microbiol. Res. 2016, 193, 57–73. [Google Scholar] [CrossRef] [PubMed]
- Catillo, U.F.; Strobel, G.A.; Ford, E.J.; Hess, W.M.; Porter, H.; Jensen, J.B.; Albert, H. Munumbicins, wide-spectrum antibiotics produced by Streptomyces NRRL 30562, endophytic on Kennedia nigricans. Microbiology 2012, 148, 2675–2685. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Penttinen, P.; Guan, T.; Xiao, J.; Chen, Q.; Xu, J.; Lindström, K. The diversity and anti-microbial activity of endophytic actinomycetes isolated from medicinal plants in Panxi Plateau, China. Curr Microbiol. 2010, 62, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Farhaoui, A.; Adadi, A.; Tahiri, A.; El Alami, N.; Khayi, S.; Mentag, R.; Ezrari, S.; Radouane, N.; Mokrini, F.; Belabess, Z.; et al. Biocontrol potential of plant growth-promoting rhizobacteria (PGPR) against Sclerotiorum rolfsii diseases on sugar beet (Beta vulgaris L.). Physiol. Mol. Plant Path. 2022, 119, 101829. [Google Scholar] [CrossRef]
- Yadav, K.; Damodaran, T.; Dutt, K.; Singh, A.; Muthukumar, M.; Rajan, S.; Gopal, R.; Sharma, P.C. Effective biocontrol of banana fusarium wilt tropical race 4 by a bacillus rhizobacteria strain with antagonistic secondary metabolites. Rhizosphere 2021, 18, 100341. [Google Scholar] [CrossRef]
- Djaya, L.; Hersanti; Istifadah, N.; Hartati, S.; Joni, I.M. In vitro study of plant growth promoting rhizobacteria (PGPR) and endophytic bacteria antagonistic to Ralstonia solanacearum formulated with graphite and silica nano particles as a biocontrol delivery system (BDS). Biocat. Agric. Biotech. 2019, 19, 101153. [Google Scholar] [CrossRef]
- Perea-Molina, P.A.; Pedraza-Herrera, L.A.; Beauregard, P.B.; Uribe-Vélez, D. A biocontrol Bacillus velezensis strain decreases pathogen Burkholderia glumae population and occupies a similar niche in rice plants. Biol. Control 2022, 176, 105067. [Google Scholar] [CrossRef]
- Chenniappan, C.; Narayanasamy, M.; Daniel, G.M.; Ramaraj, C.B.; Ponnusamy, P.; Sekar, J.; Ramalingam, P.V. Biocontrol efficiency of native plant growth promoting rhizobacteria against rhizome rot disease of turmeric. Biol. Control 2019, 129, 55–64. [Google Scholar] [CrossRef]
- Saranya, K.; Sundaramanickam, A.; Manupoori, S.; Kanth, S.V. Screening of multi-faceted phosphate-solubilising bacterium from seagrass meadow and their plant growth promotion under saline stress condition. Microbiol. Res. 2022, 261, 127080. [Google Scholar] [CrossRef]
- Raju, S.C.; Aslam, A.; Thangadurai, D.; Sangeetha, J.; Kathiravan, K.; Shajahan, A. Indole acetic acid (IAA) producing endophytic bacteria on direct somatic embryogenesis and plant regeneration of Exacum travancoricum Bedd. Vegetos 2020, 33, 690–702. [Google Scholar] [CrossRef]
- Santoyo, G.; Sánchez-Yáñez, J.M.; Santos-Villalobos, S.D.L. Methods for detecting biocontrol and plant growth-promoting traits in rhizobacteria. In Microbes and Signaling Biomolecules against Plant Stress; Springer: Singapore, 2019; pp. 133–149. [Google Scholar]
- Orozco-Mosqueda, M.d.C.; Flores, A.; Rojas-Sánchez, B.; Urtis-Flores, C.A.; Morales-Cedeño, L.R.; Valencia-Marin, M.F.; Chávez-Avila, S.; Rojas-Solis, D.; Santoyo, G. Plant growth-promoting bacteria as bioinoculants: Attributes and challenges for sustainable crop improvement. Agronomy 2021, 11, 1167. [Google Scholar] [CrossRef]
- Chhaya; Yadav, B.; Jogawat, A.; Gnanasekaran, P.; Kumari, P.; Lakra, N.; Krishan Lal, S.; Pawar, J.; Narayan, O.P. An overview of recent advancement in phytohormones-mediated stress management and drought tolerance in crop plants. Plant Gene 2021, 25, 100264. [Google Scholar] [CrossRef]
- Aci, M.M.; Sidari, R.; Araniti, F.; Lupini, A. Emerging trends in allelopathy: A genetic perspective for sustainable agriculture. Agronomy 2022, 12, 2043. [Google Scholar] [CrossRef]
- Santos, M.; Cesanelli, I.; Diánez, F.; Sánchez-Montesinos, B.; Moreno-Gavíra, A. Advances in the role of dark septate endophytes in the plant resistance to abiotic and biotic stresses. J. Fungi 2021, 7, 939. [Google Scholar] [CrossRef]
- Lubna, S.; Hamayun, M.; Gul, H.; Lee, I.J.; Hussain, A. Aspergillus niger CSR3 regulates plant endogenous hormones and secondary metabolites by producing gibberellins and indoleacetic acid. J. Plant Interact. 2018, 13, 100–111. [Google Scholar] [CrossRef]
- Kapoor, N.; Ntemafack, A.; Chouhan, R.; Gandhi, R.G. Anti-phytopathogenic and plant growth promoting potential of endophytic fungi isolated from Dysoxylum gotadhora. Arch. Phytopath. Plant Prot. 2022, 55, 454–473. [Google Scholar] [CrossRef]
- Srivastava, S.; Bist, V.; Srivastava, S.; Singh, P.C.; Trivedi, P.K.; Asif, M.H.; Chauhan, P.S.; Nautiyal, C.S. Unraveling aspects of Bacillus amyloliquefaciens mediated enhanced production of rice under biotic stress of Rhizoctonia solani. Front. Plant Sci. 2016, 7, 587. [Google Scholar] [CrossRef]
- Zebelo, S.; Song, Y.; Kloepper, J.W.; Fadamiro, H. Rhizobacteria activates (+)-δ-cadinene synthase genes and induces systemic resistance in cotton against beet armyworm (Spodoptera exigua). Plant Cell Environ. 2016, 39, 935–943. [Google Scholar] [CrossRef]
- Zhao, L.F.; Xu, Y.; Lai, X.H. Antagonistic endophytic bacteria associated with nodules of soybean (Glycine max L.) and plant growth-promoting properties. Braz. J. Microb. 2018, 49, 269–278. [Google Scholar] [CrossRef]
- Akhtar, S.S.; Mekureyaw, M.F.; Pandey, C.; Roitsch, T. Role of cytokinins for interactions of plants with microbial pathogens and pest insects. Front. Plant Sci. 2020, 10, 1777. [Google Scholar] [CrossRef] [PubMed]
- Karimi, K.; Amini, J.; Harighi, B.; Bahramnejad, B. Evaluation of biocontrol potential of Pseudomonas and Bacillus spp. against Fusarium wilt of chickpea. Aust. J. Crop Sci. 2012, 6, 695–703. [Google Scholar]
- Van Loon, L.C. Plant responses to plant growth-promoting rhizobacteria. Eur. J. Plant Pathol. 2007, 119, 243–254. [Google Scholar] [CrossRef]
- Dixit, R.; Agrawal, L.; Singh, S.P.; Singh, P.C.; Prasad, V.; Chauhan, P.C. Paenibacillus lentimorbus induces autophagy for protecting tomato from Sclerotium rolfsii infection. Microbiol. Res. 2018, 215, 164–174. [Google Scholar] [CrossRef]
- Fadiji, A.E.; Babalola, O.O. Elucidating mechanisms of endophytes used in plant protection and other bioactivities with multifunctional prospects. Front. Bioeng. Biotechnol. 2020, 8, 467. [Google Scholar] [CrossRef]
- Jacob, J.; Krishnan, G.V.; Thankappan, D.; Bhaskaran Nair Saraswathy Amma, D.K. Microbial Endophytes; Elsevier: Amsterdam, The Netherlands, 2020; pp. 75–105. [Google Scholar] [CrossRef]
- Pandey, P.K.; Samanta, R.; Yadav, R.N.S. Inside the plant: Addressing bacterial endophytes in biotic stress alleviation. Arch. Microbiol. 2019, 201, 415–429. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Shurigin, V.; Alaylar, B.; Wirth, S.; Kimura, S.K.B. Bacterial endophytes from horseradish (Armoracia rusticana) with antimicrobial efficacy against pathogens. Plant Soil Environ. 2020, 66, 309–316. [Google Scholar] [CrossRef]
- Muniroh, M.S.; Nusaibah, S.A.; Vadamalai, G.; Siddique, Y. Proficiency of biocontrol agents as plant growth promoters and hydrolytic enzyme producers in Ganoderma boninense infected oil palm seedlings. Curr. Plant Biol. 2019, 20, 100116. [Google Scholar] [CrossRef]
- Woo, S.L.; Ruocco, M.; Vinale, F.; Nigro, M.; Marra, R.; Lombardi, N.; Manganiello, G. Trichoderma-based products and their widespread use in agriculture. Open Mycol. J. 2014, 8, 71–126. [Google Scholar] [CrossRef]
- Xu, W.F.; Ren, H.S.; Ou, T.; Lei, T.; Wei, J.H.; Huang, C.S.; Li, T.; Strobel, G.; Zhou, Z.Y.; Xie, J. Genomic and functional characterization of the endophytic Bacillus subtilis 7PJ-16 Strain, a potential biocontrol agent of mulberry fruit Sclerotiniose. Micro. Ecol. 2019, 77, 651–663. [Google Scholar] [CrossRef]
- Haidar, R.; Fermaud, M.; Calvo-Garrido, C.; Roudet, J.; Deschamps, A. Modes of action for biological control of Botrytis cinerea by antagonistic bacteria. Phytopathol. Mediterr. 2016, 55, 13–34. [Google Scholar]
- Bolívar-Anillo, H.J.; Garrido, C.; Collado, I.G. Endophytic microorganisms for biocontrol of the phytopathogenic fugus Botrytis cinerea. Phytochem. Rev. 2020, 19, 721–740. [Google Scholar] [CrossRef]
- Gouda, S.; Das, G.; Sen, S.K.; Shin, H.S.; Patra, J.K. Endophytes: A treasure house of bioactive compounds of medicinal importance. Front. Microbiol. 2016, 7, 1538. [Google Scholar] [CrossRef]
- Le, K.D.; Yu, N.H.; Park, A.R.; Park, D.-J.; Kim, C.-J.; Kim, J.-C. Streptomyces sp. AN090126 as a biocontrol agent against bacterial and fungal plant diseases. Microorganisms 2022, 10, 791. [Google Scholar] [CrossRef]
- Cho, S.J.; Lim, W.J.; Hong, S.Y.; Park, S.R.; Yun, H.D. Endophytic colonization of balloon flower by antifungal strain Bacillus sp. CY22. Biosci. Biotechnol. Biochem. 2003, 67, 2132–2138. [Google Scholar] [CrossRef]
- Musa, Z.; Ma, J.; Egamberdieva, D.; Mohamad, O.; Liu, Y.H.; Li, W.J.; Li, L. Diversity and antimicrobial potential of cultivable endophytic actinobacteria associated with medicinal plant Thymus roseus. Front. Microbiol. 2020, 11, 191. [Google Scholar] [CrossRef]
- Hilário, S.; Gonçalves, M.F.M. Endophytic Diaporthe as promising leads for the development of biopesticides and biofertilizers for a sustainable agriculture. Microorganisms 2022, 10, 2453. [Google Scholar] [CrossRef]
- Xu, K.; Li, X.Q.; Zhao, D.L.; Zhang, P. Antifungal secondary metabolites produced by the fungal endophytes: Chemical diversity and potential use in the development of biopesticides. Front. Microbiol. 2021, 12, 689527. [Google Scholar] [CrossRef]
- Sumarah, M.W.; Kesting, J.R.; Sørensen, D.; Miller, J.D. Antifungal metabolites from fungal endophytes of Pinus strobus. Phytochemistry 2011, 72, 1833–1837. [Google Scholar] [CrossRef]
- Zhao, M.; Guo, D.L.; Liu, G.H.; Fu, X.; Gu, Y.C.; Ding, L.S.; Zhou, Y. Antifungal halogenated cyclopentenones from the endophytic fungus Saccharicola bicolor of Bergenia purpurascens by the one strain-many compounds strategy. J. Agric. Food Chem. 2020, 68, 185–192. [Google Scholar] [CrossRef]
- Chen, Y.M.; Yang, Y.H.; Li, X.N.; Zou, C.; Zhao, P.J. Diterpenoids from the endophytic fungus Botryosphaeria sp. P483 of the Chinese herbal medicine Huperzia serrata. Molecules 2015, 209, 16924–16932. [Google Scholar] [CrossRef]
- Talontsi, F.M.; Dittrich, B.; Schüffler, A.; Sun, H.; Laatsch, H. Epicoccolides: Antimicrobial and antifungal polyketides from an endophytic fungus Epicoccum sp. associated with Theobroma cacao. Eur. J. Org. Chem. 2013, 2013, 3174–3180. [Google Scholar] [CrossRef]
- Xia, Y.; Liu, J.; Chen, C.; Mo, X.; Tan, Q.; He, Y.; Wang, Z.; Yin, J.; Zhou, G. The Multifunctions and future prospects of endophytes and their metabolites in plant disease management. Microorganisms 2022, 10, 1072. [Google Scholar] [CrossRef]
- Gu, S.; Yang, T.; Shao, Z.; Wang, T.; Cao, K.; Jousset, A.; Friman, V.P.; Mallon, C.; Mei, X.; Wei, Z.; et al. Siderophore-mediated interactions determine the disease suppressiveness of microbial consortia. mSystems 2020, 5, e00811-19. [Google Scholar] [CrossRef] [PubMed]
- Ryu, C.M.; Farag, M.A.; Hu, C.H.; Reddy, M.S.; Wei, H.X.; Pare, P.W.; Kloepper, J.W. Bacterial volatiles promote growth in Arabidopsis. Proc. Nat. Acad. Sci. USA 2003, 100, 4927–4932. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Ai, C.; Xin, L.; Zhou, G. The siderophore-producing bacterium, Bacillus subtilis CAS15, has a biocontrol effect on Fusarium wilt and promotes the growth of pepper. Eur. J. Soil Biol. 2011, 47, 138–145. [Google Scholar] [CrossRef]
- Chowdappa, S.; Jagannath, S.; Konappa, N.; Udayashankar, A.C.; Jogaiah, S. Detection and characterization of anti-434 bacterial siderophores secreted by endophytic fungi from Cymbidium aloifolium. Biomolecules 2020, 10, 1412. [Google Scholar] [CrossRef]
- Lugtenberg, B.; Kamilova, F. Plant-growth-promoting rhizobacteria. Ann. Rev. Microbiol. 2009, 63, 541–556. [Google Scholar] [CrossRef]
- Ullah, A.; Nisar, M.; Ali, H.; Hazrat, A.; Hayat, K.; Keerio, A.A.; Ihsan, M.; Laiq, M.; Ullah, S.; Fahad, S.; et al. Drought tolerance improvement in plants: An endophytic bacterial approach. Appl. Micro-Biol. Biotechnol. 2019, 103, 7385–7397. [Google Scholar] [CrossRef]
- Gao, Y.; Ning, Q.; Yang, Y.; Liu, Y.; Niu, S.; Hu, X.; Pan, H.; Bu, Z.; Chen, N.; Guo, J.; et al. Endophytic Streptomyces hygroscopicus OsiSh-2-Mediated balancing between growth and disease resistance 445 in host Rice. mBio 2021, 12, e01566-21. [Google Scholar] [CrossRef]
- Grabka, R.; d’Entremont, T.W.; Adams, S.J.; Walker, A.K.; Tanney, J.B.; Abbasi, P.A.; Ali, S. Fungal endophytes and their role in agricultural plant protection against pests and pathogens. Plants 2022, 11, 384. [Google Scholar] [CrossRef]
- Kloepper, J.W.; Ryu, C.M. Bacterial endophytes as elicitors of induced systemic resistance. In Microbial Root Endophytes; Schulz, B.J.E., Boyle, C.J.C., Sieber, T.N., Eds.; Soil Biology; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Romera, F.J.; García, M.J.; Lucena, C.; Martínez-Medina, A.; Aparicio, M.A.; Ramos, J.; Alcántara, E.; Angulo, M.; Pérez-Vicente, R. Induced systemic resistance (ISR) and Fe deficiency responses in dicot plants. Front. Plant Sci. 2019, 10, 287. [Google Scholar] [CrossRef]
- Gao, F.K.; Dai, C.C.; Liu, X.Z. Mechanisms of fungal endophytes in plant protection against pathogens. Afr. J. Micro-Biol. Res. 2010, 4, 1346–1351. [Google Scholar]
- Quyet-Tien, P.; Park, Y.M.; Seul, K.J.; Ryu, C.M.; Park, S.H.; Kim, J.G. Assessment of root-associated Paenibacillus polymyxa groups on growth promotion and induced systemic resistance in pepper. J. Microbiol. Biotechnol. 2010, 20, 1605–1613. [Google Scholar]
- Waqas, M.; Khan, A.L.; Hamayun, M.; Shahzad, R.; Kang, S.-M.; Kim, J.-G.; Lee, I.-J. Endophytic fungi promote plant growth and mitigate the adverse effects of stem rot: An example of Penicillium citrinum and Aspergillus terreus. J. Plant Interact. 2015, 10, 280–287. [Google Scholar] [CrossRef]
- Kavroulakis, N.; Ntougias, S.; Zervakis, G.I.; Ehaliotis, C.; Haralampidis, K.; Papadopoulou, K.K. Role of ethylene in the protection of tomato plants against soil-borne fungal pathogens conferred by an endophytic Fusarium solani strain. J. Exp. Bot. 2007, 58, 3853–3864. [Google Scholar] [CrossRef]
- Rashid, M.H.; Khan, A.; Hossain, M.T.; Chung, Y.R. Induction of systemic resistance against aphids by endophytic Bacillus velezensis YC7010 via Expressing PHYTOALEXIN DEFICIENT4 in Arabidopsis. Front. Plant Sci. 2017, 15, 211. [Google Scholar] [CrossRef]
- Peng, C.; Zhang, A.; Wang, Q.; Song, Y.; Zhang, M.; Ding, X.; Li, Y.; Geng, Q.; Zhu, C. Ultrahigh-activity immune inducer from Endophytic Fungi induces tobacco resistance to virus by SA pathway and RNA silencing. BMC Plant Biol. 2020, 20, 169. [Google Scholar] [CrossRef]
- Ahmad, T.; Bashir, A.; Farooq, S.; Riyaz-Ul-Hassan, S. Burkholderia gladioli E39CS3, an endophyte of Crocus sativus Linn., induces host resistance against corm-rot caused by Fusarium oxysporum. J. Appl. Microbiol. 2022, 132, 495–508. [Google Scholar] [CrossRef]
- Jha, Y. Endophytic bacteria mediated anti-autophagy and induced catalase, β-1,3-glucanases gene in paddy after infection with pathogen Pyricularia grisea. Indian Phytopathol. 2019, 72, 99–106. [Google Scholar] [CrossRef]
- Schuhegger, R.; Ihring, A.; Gantner, S.; Bahnweg, G.; Knappe, C.; Vogg, G.; Hutzler, P.; Schmid, M.; Van Breusegem, F.; Eberl, L.; et al. Induction of systemic resistance in tomato by N-acyl-L-homoserine lactone-producing rhizosphere bacteria. Plant Cell Environ. 2006, 29, 909–918. [Google Scholar] [CrossRef] [PubMed]
- Sahu, K.P.; Singh, S.; Gupta, A.; Singh, U.B.; Brahmaprakash, G.P.; Saxena, A.K. Antagonistic potential of bacterial endophytes and induction of systemic resistance against collar rot pathogen Sclerotium rolfsii in tomato. Biol. Control 2019, 137, 104014. [Google Scholar] [CrossRef]
- Gupta, S.; Pandey, S.; Sharma, S. Decoding the plant growth promotion and antagonistic potential of bacterial endophytes from Ocimum sanctum Linn. against root rot pathogen Fusarium oxysporum in Pisum sativum. Front. Plant Sci. 2022, 14, 813686. [Google Scholar] [CrossRef] [PubMed]
- Constantin, M.E.; de Lamo, F.J.; Vlieger, B.V.; Rep, M.; Takken, F.L.W. Endophyte-mediated resistance in tomato to Fusarium oxysporum Is independent of ET, JA, and SA. Front. Plant Sci. 2019, 10, 979. [Google Scholar] [CrossRef] [PubMed]
- Jayaraj, R.; Anand, T.; Lakshmana Rao, P.V. Activity and gene expression profile of certain antioxidant enzymes to microcystin-LR induced oxidative stress in mice. Toxicology 2006, 220, 136–146. [Google Scholar] [CrossRef]
- Kusajima, M.; Shima, S.; Fujita, M.; Minamisawa, K.; Che, F.-S.; Yamakawa, H.; Nakashita, H. Involvement of ethylene signaling in Azospirillum sp. B510-induced disease resistance in rice. Biosci. Biotechnol. Biochem. 2018, 82, 1522–1526. [Google Scholar] [CrossRef]
- Kasim, W.A.; Osman, M.E.; Omar, M.N.; Abd El-Daim, I.A.; Bejai, S.; Meijer, J. Control of drought stress in wheat using plant-growth-promoting bacteria. J. Plant Growth Regul. 2013, 32, 122–130. [Google Scholar] [CrossRef]
- Imahori, Y.; Takemura, M.; Bai, J. Chilling-induced oxidative stress and antioxidant responses in mume (Prunus mume) fruit during low temperature storage. Postharvest Biol. Technol. 2008, 49, 54–60. [Google Scholar] [CrossRef]
- Wang, X.; Xie, S.; Mu, X.; Guan, B.; Hu, Y.; Ni, Y. Investigating the resistance responses to Alternaria brassicicola in ‘Korla’ fragrant pear fruit induced by a biocontrol strain Bacillus subtilis Y2. Posth. Biol. Technol. 2023, 199, 112293. [Google Scholar] [CrossRef]
- Cavalcanti, V.P.; Aazza, S.; Bertolucci, S.K.V.; Pereira, S.M.A.; Cavalcanti, P.P.; Buttrós, V.H.T.; Oliveira-Silva, A.M.; Pasqual, M.; Dória, J. Plant, pathogen and biocontrol agent interaction effects on bioactive compounds and antioxidant activity in garlic. Physiol. Mol. Plant Pathol. 2020, 112, 101550. [Google Scholar] [CrossRef]
- Wu, Y.; Gao, Y.; Zheng, X.; Yu, T.; Yan, F. Enhancement of biocontrol efficacy of Kluyveromyces marxianus induced by N-acetylglucosamine against Penicillium expansum. Food Chem. 2023, 404, 134658. [Google Scholar] [CrossRef]
- Passardi, F.; Cosio, C.; Penel, C.; Dunand, C. Peroxidases have more functions than a Swiss army knife. Plant Cell Rep. 2005, 24, 255–265. [Google Scholar] [CrossRef]
- Farooq, M.A.; Niazi, A.K.; Akhtar, J.; Saifullah; Farooq, M.; Souri, Z.; Karimi, N.; Rengel, Z. Acquiring control: The evolution of ROS-Induced oxidative stress and redox signaling pathways in plant stress responses. Plant Physiol. Biochem. 2019, 141, 353–369. [Google Scholar] [CrossRef]
- Yan, Y.; Zheng, X.; Apaliya, M.T.; Yang, H.; Zhang, H. Transcriptome characterization and expression profile of de-499 fense-related genes in pear induced by Meyerozyma guilliermondii. Postharvest Biol. Technol. 2018, 141, 63–70. [Google Scholar] [CrossRef]
- Mohammadi, M.; Kazemi, H. Changes in peroxidase and polyphenol oxidase activities in susceptible and resistant wheat heads inoculated with Fusarium graminearum and induced resistance. Plant Sci. 2002, 162, 491–498. [Google Scholar] [CrossRef]
- Sebestyen, D.; Perez-Gonzalez, G.; Goodell, B. Antioxidants and iron chelators inhibit oxygen radical generation in fungal cultures of plant pathogenic fungi. Fungal Biol. 2022, 126, 480–487. [Google Scholar] [CrossRef]
- Compant, S.; Duffy, B.; Nowak, J.; Clement, C.; Barka, E.A. Use of plant growth-promoting bacteria for biocontrol of plant diseases: Principles, mechanisms of action, and future prospects. Appl. Environ. Microbiol. 2005, 1, 4951–4959. [Google Scholar] [CrossRef]
- Lugtenberg, B.J.; Dekkers, L.; Bloemberg, G.V. Molecular determinants of rhizosphere colonization by Pseudomonas. Ann. Rev. Phytopathol. 2001, 39, 461–490. [Google Scholar] [CrossRef]
- Köhl, J.; Kolnaar, R.; Ravensberg, W.J. Mode of action of microbial biological control agents against plant diseases: Relevance beyond efficacy. Front. Plant Sci. 2019, 10, 845. [Google Scholar] [CrossRef]
- Srebot, M.S.; Tano, J.; Carrau, A.; Ferretti, M.D.; Martínez, M.L.; Orellano, E.G.; Rodriguez, M.V. Bacterial wilt biocontrol by the endophytic bacteria Gluconacetobacter diazotrophicus in Río Grande tomato cultivar. Biol. Control 2021, 162, 104728. [Google Scholar] [CrossRef]
- Fokkema, N.J.; Riphagen, I.; Poot, R.J.; De Jong, C. Aphid honeydew, a potential stimulant of Cochliobolus sativus and Septoria nodorum and the competitive role of saprophytic mycoflora. Brit. Mycol. Soc. 1983, 81, 355–363. [Google Scholar] [CrossRef]
- van Dijk, K.; Nelson, E.B. Fatty acid competition as a mechanism by which Enterobacter cloacae suppresses Pythium ultimum sporangium germination and damping-off. Appl. Environ. Microbiol. 2000, 66, 5340–5347. [Google Scholar] [CrossRef] [PubMed]
- Eisendle, M.; Oberegger, H.; Buttinger, R.; Illmer, P.; Haas, H. Biosynthesis and uptake of siderophores is controlled by the PacCmediated ambient-pH regulatory system in Aspergillus nidulans. Euk Cell. 2004, 3, 561–563. [Google Scholar] [CrossRef] [PubMed]
- Chin-A.-Woeng, T.F.C.; Bloemberg, G.V.; van der Bij, A.J.; van der Drift, K.M.-G.-M.-; Schripsema, J.; Kroon, B.; Scheffer, R.J.; Keel, C.; Bakker, P.A.H.M.; Tichy, H.T. Biocontrol by phenazine-1- carboxamide producing Pseudomonas chlororaphis PCL1391 of tomato root rot caused by Fusarium oxysporum f.sp. radicislycopersici. Mol. Plant Microbe Interact. 1998, 11, 1069–1077. [Google Scholar] [CrossRef]
- de Weert, S.; Bloomberg, G.V. Rhizosphere competence and role of root colonization in biocontrol. In Plant-Associated Bacteria; Gnanamanickam, S.S., Ed.; Springer: Dordrecht, The Netherlands, 2006; p. 317. [Google Scholar]
- Innerebner, G.; Knief, C.; Vorholt, J.A. Protection of Arabidopsis thaliana against leaf-pathogenic Pseudomonas syringae by Sphingomonas strains in a controlled model system. Appl. Environ. Microbiol. 2011, 77, 3202–3210. [Google Scholar] [CrossRef]
- Ji, P.; Wilson, M. Assessment of the importance of similarity in carbon source utilization profiles between the biological control agent and the pathogen in biological control of bacterial speck of tomato. Appl. Environ. Microbiol. 2002, 68, 4383–4389. [Google Scholar] [CrossRef]
- Situ, J.; Zheng, L.; Xu, D.; Gu, G.; Xi, P.; Deng, Y.; Hsiang, T.; Jiang, Z. Screening of effective biocontrol agents against postharvest litchi downy blight caused by Peronophythora litchii. Posth. Biol. Technol. 2023, 198, 112249. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Egamberdieva, D.; Eshboev, F.; Shukurov, O.; Alaylar, B.; Arora, N.K. Bacterial Bioprotectants: Biocontrol Traits and Induced Resistance to Phytopathogens. Microbiol. Res. 2023, 14, 689-703. https://doi.org/10.3390/microbiolres14020049
Egamberdieva D, Eshboev F, Shukurov O, Alaylar B, Arora NK. Bacterial Bioprotectants: Biocontrol Traits and Induced Resistance to Phytopathogens. Microbiology Research. 2023; 14(2):689-703. https://doi.org/10.3390/microbiolres14020049
Chicago/Turabian StyleEgamberdieva, Dilfuza, Farkhod Eshboev, Oybek Shukurov, Burak Alaylar, and Naveen Kumar Arora. 2023. "Bacterial Bioprotectants: Biocontrol Traits and Induced Resistance to Phytopathogens" Microbiology Research 14, no. 2: 689-703. https://doi.org/10.3390/microbiolres14020049
APA StyleEgamberdieva, D., Eshboev, F., Shukurov, O., Alaylar, B., & Arora, N. K. (2023). Bacterial Bioprotectants: Biocontrol Traits and Induced Resistance to Phytopathogens. Microbiology Research, 14(2), 689-703. https://doi.org/10.3390/microbiolres14020049









