State of the Art on Vaccine Development Against Dengue Infection: Scoping Review of the Literature
Abstract
1. Introduction
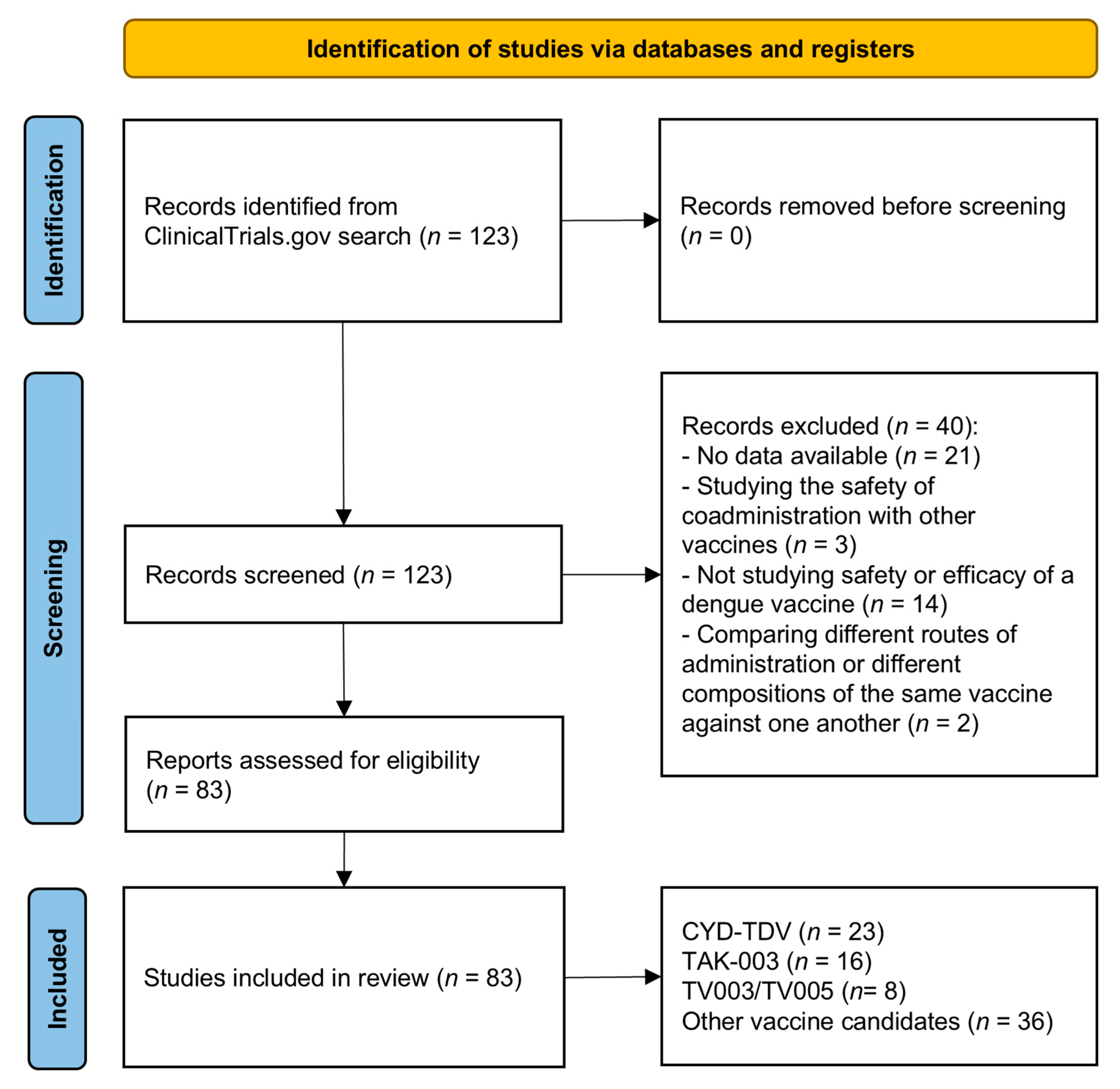
2. Materials and Methods
3. Results
3.1. CYD-TDV
3.2. TAK-003
3.3. TV003/TV005
3.4. TDEN
3.5. DPIV
3.6. V180
3.7. TVDV
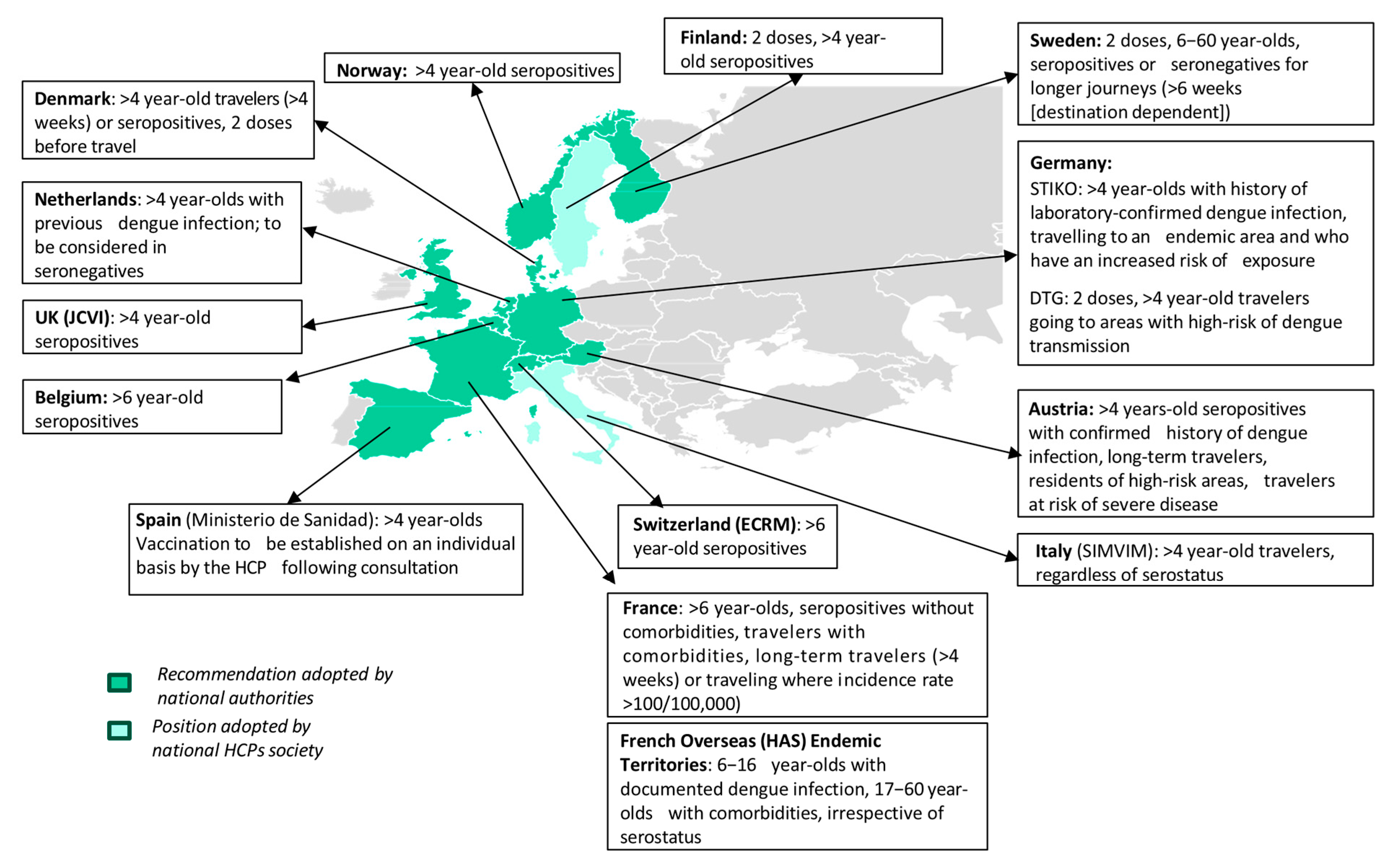
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADE | Antibody-dependent enhancement |
| CYD-TDV | Chimeric yellow fever virus—DENV-tetravalent dengue vaccine |
| DENV | Dengue virus |
| GMTs | Geometric mean titres |
| LATV | Live-attenuated tetravalent vaccine |
| RR | Relative risk |
| SAEs | Serious adverse events |
| VCD | Virologically confirmed dengue |
| VE | Vaccine efficacy |
References
- WHO. Global Dengue Surveillance. Available online: https://worldhealthorg.shinyapps.io/dengue_global/ (accessed on 2 September 2025).
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef]
- Yang, X.; Quam, M.B.M.; Zhang, T.; Sang, S. Global burden for dengue and the evolving pattern in the past 30 years. J. Travel Med. 2021, 28, taab146. [Google Scholar] [CrossRef]
- Brady, O.J.; Gething, P.W.; Bhatt, S.; Messina, J.P.; Brownstein, J.S.; Hoen, A.G.; Moyes, C.L.; Farlow, A.W.; Scott, T.W.; Hay, S.I. Refining the Global Spatial Limits of Dengue Virus Transmission by Evidence-Based Consensus. PLoS Negl. Trop. Dis. 2012, 6, e1760. [Google Scholar] [CrossRef]
- Hussain, S.S.A.; Dhiman, R.C. Distribution Expansion of Dengue Vectors and Climate Change in India. GeoHealth 2022, 6, e2021GH000477. [Google Scholar] [CrossRef]
- Bowman, L.R.; Tejeda, G.S.; Coelho, G.E.; Sulaiman, L.H.; Gill, B.S.; McCall, P.J.; Olliaro, P.L.; Ranzinger, S.R.; Quang, L.C.; Ramm, R.S.; et al. Alarm Variables for Dengue Outbreaks: A Multi-Centre Study in Asia and Latin America. PLoS ONE 2016, 11, e0157971. [Google Scholar] [CrossRef] [PubMed]
- Radici, A.; Hammami, P.; Cannet, A.; L’Ambert, G.; Lacour, G.; Fournet, F.; Garros, C.; Guis, H.; Fontenille, D.; Caminade, C.; et al. Aedes albopictus Is Rapidly Invading Its Climatic Niche in France: Wider Implications for Biting Nuisance and Arbovirus Control in Western Europe. Glob. Change Biol. 2025, 31, e70414. [Google Scholar] [CrossRef]
- de Almeida, M.T.; Merighi, D.G.S.; Visnardi, A.B.; Boneto Gonçalves, C.A.; Amorim, V.M.F.; Ferrari, A.S.A.; de Souza, A.S.; Guzzo, C.R. Latin America’s Dengue Outbreak Poses a Global Health Threat. Viruses 2025, 17, 57. [Google Scholar] [CrossRef]
- Goche, K.S.R.; Castro, M.V.L.; Zanelly, G.A.L.; Camargo, W.M.L.; Velasquez, E.Y.A.; Carrasco, A.G.M.; Diaz-Obregón, D. Epidemiological dynamics of dengue in Peru: Temporal and spatial drivers between 2000 and 2022. PLoS ONE 2025, 20, e0319708. [Google Scholar] [CrossRef]
- Guzman, M.G.; Gubler, D.J.; Izquierdo, A.; Martinez, E.; Halstead, S.B. Dengue infection. Nat. Rev. Dis. Primers 2016, 2, 16055. [Google Scholar] [CrossRef]
- Olkowski, S.; Forshey, B.M.; Morrison, A.C.; Rocha, C.; Vilcarromero, S.; Halsey, E.S.; Kochel, T.J.; Scott, T.W.; Stoddard, S.T. Reduced Risk of Disease During Postsecondary Dengue Virus Infections. J. Infect. Dis. 2013, 208, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- McGuinness, S.L.; Leder, K. Dengue severity in travellers: Challenges and insights. J. Travel Med. 2023, 30, taad146. [Google Scholar] [CrossRef]
- Avrami, S.; Hoffman, T.; Meltzer, E.; Lustig, Y.; Schwartz, E. Comparison of clinical and laboratory parameters of primary vs secondary dengue fever in travellers. J. Travel Med. 2023, 30, taad129. [Google Scholar] [CrossRef]
- Guy, B.; Noriega, F.; Ochiai, R.L.; L’azou, M.; Delore, V.; Skipetrova, A.; Verdier, F.; Coudeville, L.; Savarino, S.; Jackson, N. A recombinant live attenuated tetravalent vaccine for the prevention of dengue. Expert Rev. Vaccines 2017, 16, 671–684. [Google Scholar] [CrossRef]
- Qiu, X.; Bailey, A.L. Two mutations in NS2B are responsible for attenuation of the yellow fever virus (YFV) vaccine strain 17D. PLoS Pathog. 2025, 21, e1013373. [Google Scholar] [CrossRef] [PubMed]
- Bonaldo, M.C.; Sequeira, P.C.; Galler, R. The yellow fever 17D virus as a platform for new live attenuated vaccines. Hum. Vaccines Immunother. 2014, 10, 1256–1265. [Google Scholar] [CrossRef] [PubMed]
- Capeding, M.R.; Tran, N.H.; Hadinegoro, S.R.S.; Ismail, H.I.H.J.M.; Chotpitayasunondh, T.; Chua, M.N.; Luong, C.Q.; Rusmil, K.; Wirawan, D.N.; Nallusamy, R.; et al. Clinical efficacy and safety of a novel tetravalent dengue vaccine in healthy children in Asia: A phase 3, randomised, observer-masked, placebo-controlled trial. Lancet 2014, 384, 1358–1365. [Google Scholar] [CrossRef]
- Villar, L.; Dayan, G.H.; Arredondo-García, J.L.; Rivera, D.M.; Cunha, R.; Deseda, C.; Reynales, H.; Costa, M.S.; Morales-Ramírez, J.O.; Carrasquilla, G.; et al. Efficacy of a Tetravalent Dengue Vaccine in Children in Latin America. N. Engl. J. Med. 2015, 372, 113–123. [Google Scholar] [CrossRef]
- Sabchareon, A.; Wallace, D.; Sirivichayakul, C.; Limkittikul, K.; Chanthavanich, P.; Suvannadabba, S.; Jiwariyavej, V.; Dulyachai, W.; Pengsaa, K.; Wartel, T.A.; et al. Protective efficacy of the recombinant, live-attenuated, CYD tetravalent dengue vaccine in Thai schoolchildren: A randomised, controlled phase 2b trial. Lancet 2012, 380, 1559–1567. [Google Scholar] [CrossRef] [PubMed]
- Olivera-Botello, G.; Coudeville, L.; Fanouillere, K.; Guy, B.; Chambonneau, L.; Noriega, F.; Jackson, N.; CYD-TDV Vaccine Trial Group. Tetravalent Dengue Vaccine Reduces Symptomatic and Asymptomatic Dengue Virus Infections in Healthy Children and Adolescents Aged 2–16 Years in Asia and Latin America. J. Infect. Dis. 2016, 214, 994–1000. [Google Scholar] [CrossRef]
- Hadinegoro, S.R.; Arredondo-García, J.L.; Capeding, M.R.; Chotpitayasunondh, T.; Dietze, R.; Muhammad Ismail, H.I.; Reynales, H.; Limkittikul, K.; Rivera-Medina, D.M.; Tran, H.N.; et al. Efficacy and Long-Term Safety of a Dengue Vaccine in Regions of Endemic Disease. N. Engl. J. Med. 2015, 373, 1195–1206. [Google Scholar] [CrossRef]
- Sridhar, S.; Luedtke, A.; Langevin, E.; Zhu, M.; Bonaparte, M.; Machabert, T.; Savarino, S.; Zambrano, B.; Moureau, A.; Khromava, A.; et al. Effect of Dengue Serostatus on Dengue Vaccine Safety and Efficacy. N. Engl. J. Med. 2018, 379, 327–340. [Google Scholar] [CrossRef]
- Arredondo-García, J.L.; Hadinegoro, S.R.; Reynales, H.; Chua, M.N.; Rivera Medina, D.M.; Chotpitayasunondh, T.; Tran, N.H.; Deseda, C.C.; Wirawan, D.N.; Cortés Supelano, M.; et al. Four-year safety follow-up of the tetravalent dengue vaccine efficacy randomized controlled trials in Asia and Latin America. Clin. Microbiol. Infect. 2018, 24, 755–763. [Google Scholar] [CrossRef]
- Laydon, D.J.; Dorigatti, I.; Hinsley, W.R.; Nedjati-Gilani, G.; Coudeville, L.; Ferguson, N.M. Efficacy profile of the CYD-TDV dengue vaccine revealed by Bayesian survival analysis of individual-level phase III data. eLife 2021, 10, e65131. [Google Scholar] [CrossRef] [PubMed]
- Vigne, C.; Dupuy, M.; Richetin, A.; Guy, B.; Jackson, N.; Bonaparte, M.; Hu, B.; Saville, M.; Chansinghakul, D.; Noriega, F.; et al. Integrated immunogenicity analysis of a tetravalent dengue vaccine up to 4 y after vaccination. Hum. Vaccines Immunother. 2017, 13, 2004–2016. [Google Scholar] [CrossRef]
- Harenberg, A.; Begue, S.; Mamessier, A.; Gimenez-Fourage, S.; Ching Seah, C.; Wei Liang, A.; Li Ng, J.; Yun Toh, X.; Archuleta, S.; Wilder-Smith, A.; et al. Persistence of Th1/Tc1 responses one year after tetravalent dengue vaccination in adults and adolescents in Singapore. Hum. Vaccines Immunother. 2013, 9, 2317–2325. [Google Scholar] [CrossRef]
- Hss, A.S.; Koh, M.T.; Tan, K.K.; Chan, L.G.; Zhou, L.; Bouckenooghe, A.; Crevat, D.; Hutagalung, Y. Safety and immunogenicity of a tetravalent dengue vaccine in healthy children aged 2–11 years in Malaysia: A randomized, placebo-controlled, Phase III study. Vaccine 2013, 31, 5814–5821. [Google Scholar] [CrossRef]
- Qiao, M.; Shaw, D.; Forrat, R.; Wartel-Tram, A.; Lang, J. Priming Effect of Dengue and Yellow Fever Vaccination on the Immunogenicity, Infectivity, and Safety of a Tetravalent Dengue Vaccine in Humans. Am. J. Trop. Med. Hyg. 2011, 85, 724. [Google Scholar] [CrossRef]
- Gailhardou, S.; Skipetrova, A.; Dayan, G.H.; Jezorwski, J.; Saville, M.; Van der Vliet, D.; Wartel, T.A. Safety Overview of a Recombinant Live-Attenuated Tetravalent Dengue Vaccine: Pooled Analysis of Data from 18 Clinical Trials. PLoS Negl. Trop. Dis. 2016, 10, e0004821. [Google Scholar] [CrossRef]
- Kirstein, J.; Douglas, W.; Thakur, M.; Boaz, M.; Papa, T.; Skipetrova, A.; Plennevaux, E. Immunogenicity of the CYD tetravalent dengue vaccine using an accelerated schedule: Randomised phase II study in US adults. BMC Infect. Dis. 2018, 18, 475. [Google Scholar] [CrossRef]
- Santos, J.; Montellano, M.E.; Solante, R.; Perreras, N.; Meyer, S.; Toh, M.L.; Zocchetti, C.; Vigne, C.; Mascareñas, C. Immunogenicity and Safety of a Tetravalent Dengue Vaccine Administered Concomitantly or Sequentially with Tdap Vaccine: Randomized Phase IIIb Trial in Healthy Participants 9–60 Years of Age in the Philippines. Pediatr. Infect. Dis. J. 2021, 40, 856. [Google Scholar] [CrossRef] [PubMed]
- Study Details. Study of Yellow Fever Vaccine Administered with Tetravalent Dengue Vaccine in Healthy Toddlers. ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT01436396 (accessed on 22 September 2024).
- Coronel-Martínez, D.L.; Park, J.; López-Medina, E.; Capeding, M.R.; Cadena Bonfanti, A.A.; Montalbán, M.C.; Ramírez, I.; Gonzales, M.L.A.; DiazGranados, C.A.; Zambrano, B.; et al. Immunogenicity and safety of simplified vaccination schedules for the CYD-TDV dengue vaccine in healthy individuals aged 9–50 years (CYD65): A randomised, controlled, phase 2, non-inferiority study. Lancet Infect. Dis. 2021, 21, 517–528. [Google Scholar] [CrossRef]
- Coronel, D.; García-Rivera, E.J.; Rivera, M.; Arredondo-García, J.L.; Dietze, R.; Perroud, A.P.; Cortés, M.; Bonaparte, M.; Zhao, J.; Tila, M.; et al. Dengue Vaccine Booster in Healthy Adolescents and Adults in Latin America: Evaluation 4–5 Years After a Primary 3-Dose Schedule. Pediatr. Infect. Dis. J. 2019, 38, e90. [Google Scholar] [CrossRef]
- Park, J.; Archuleta, S.; Oh, M.L.H.; Shek, L.P.; Jin, J.; Bonaparte, M.; Fargo, C.; Bouckenooghe, A. Immunogenicity and safety of a dengue vaccine given as a booster in Singapore: A randomized Phase II, placebo-controlled trial evaluating its effects 5–6 years after completion of the primary series. Hum. Vaccines Immunother. 2020, 16, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Archuleta, S.; Oh, M.L.H.; Shek, L.P.; Wang, H.; Bonaparte, M.; Frago, C.; Bouckenooghe, A.; Jantet-Blaudez, F.; Begue, S.; et al. Humoral and cellular immunogenicity and safety following a booster dose of a tetravalent dengue vaccine 5+ years after completion of the primary series in Singapore: 2-year follow-up of a randomized phase II, placebo-controlled trial. Hum. Vaccines Immunother. 2021, 17, 2107–2116. [Google Scholar] [CrossRef] [PubMed]
- CDC. About a Dengue Vaccine. Dengue. 30 January 2025. Available online: https://www.cdc.gov/dengue/vaccine/index.html (accessed on 1 May 2025).
- Comunicado Sobre a Descontinuação Definitiva da Fabricação/Importação do Medicamento DENGVAXIA® (Vacina Dengue 1, 2, 3 e 4, Recombinante e Atenuada). Available online: https://www.sanofi.com.br/pt/noticias/informacoes-de-produtos/2025-2-28-comunicado-sobre-a-descontinuacao-definitiva-da-fabricacao-importacao-do-medicamento-dengvaxia-vacina-dengue-1-2-3-e-4-recombinante-e-atenuada (accessed on 1 May 2025).
- Tricou, V.; Sáez-Llorens, X.; Yu, D.; Rivera, L.; Jimeno, J.; Villarreal, A.C.; Dato, E.; Saldaña de Suman, O.; Montenegro, N.; DeAntonio, R.; et al. Safety and immunogenicity of a tetravalent dengue vaccine in children aged 2-17 years: A randomised, placebo-controlled, phase 2 trial. Lancet 2020, 395, 1434–1443. [Google Scholar] [CrossRef]
- Sáez-Llorens, X.; Tricou, V.; Yu, D.; Rivera, L.; Jimeno, J.; Villarreal, A.C.; Dato, E.; Mazara, S.; Vargas, M.; Brose, M.; et al. Immunogenicity and safety of one versus two doses of tetravalent dengue vaccine in healthy children aged 2–17 years in Asia and Latin America: 18-month interim data from a phase 2, randomised, placebo-controlled study. Lancet Infect. Dis. 2018, 18, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Biswal, S.; Reynales, H.; Saez-Llorens, X.; Lopez, P.; Borja-Tabora, C.; Kosalaraksa, P.; Sirivichayakul, C.; Watanaveeradej, V.; Rivera, L.; Espinoza, F.; et al. Efficacy of a Tetravalent Dengue Vaccine in Healthy Children and Adolescents. N. Engl. J. Med. 2019, 381, 2009–2019. [Google Scholar] [CrossRef]
- Biswal, S.; Borja-Tabora, C.; Vargas, L.M.; Velásquez, H.; Theresa Alera, M.; Sierra, V.; Johana Rodriguez-Arenales, E.; Yu, D.; Wickramasinghe, V.P.; Duarte Moreira, E., Jr.; et al. Efficacy of a tetravalent dengue vaccine in healthy children aged 4–16 years: A randomised, placebo-controlled, phase 3 trial. Lancet 2020, 395, 1423–1433. [Google Scholar] [CrossRef]
- Rivera, L.; Biswal, S.; Sáez-Llorens, X.; Reynales, H.; López-Medina, E.; Borja-Tabora, C.; Bravo, L.; Sirivichayakul, C.; Kosalaraksa, P.; Martinez Vargas, L.; et al. Three-year Efficacy and Safety of Takeda’s Dengue Vaccine Candidate (TAK-003). Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2022, 75, 107–117. [Google Scholar] [CrossRef]
- Tricou, V.; Yu, D.; Reynales, H.; Biswal, S.; Saez-Llorens, X.; Sirivichayakul, C.; Lopez, P.; Borja-Tabora, C.; Bravo, L.; Kosalaraksa, P.; et al. Long-term efficacy and safety of a tetravalent dengue vaccine (TAK-003): 4·5-year results from a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Glob. Health 2024, 12, e257–e270. [Google Scholar] [CrossRef]
- Biswal, S.; Mendez Galvan, J.F.; Macias Parra, M.; Galan-Herrera, J.F.; Carrascal Rodriguez, M.B.; Rodriguez Bueno, E.P.; Brose, M.; Rauscher, M.; LeFevre, I.; Wallace, D.; et al. Immunogenicity and safety of a tetravalent dengue vaccine in dengue-naïve adolescents in Mexico City. Rev. Panam. Salud Publica Pan. Am. J. Public Health 2021, 45, e67. [Google Scholar] [CrossRef]
- Sirivichayakul, C.; Barranco-Santana, E.A.; Rivera, I.E.; Kilbury, J.; Raanan, M.; Borkowski, A.; Papadimitriou, A.; Wallace, D. Long-term Safety and Immunogenicity of a Tetravalent Dengue Vaccine Candidate in Children and Adults: A Randomized, Placebo-Controlled, Phase 2 Study. J. Infect. Dis. 2020, 225, 1513–1520. [Google Scholar] [CrossRef]
- Patel, S.S.; Winkle, P.; Faccin, A.; Nordio, F.; LeFevre, I.; Tsoukas, C.G. An open-label, Phase 3 trial of TAK-003, a live attenuated dengue tetravalent vaccine, in healthy US adults: Immunogenicity and safety when administered during the second half of a 24-month shelf-life. Hum. Vaccines Immunother. 2023, 19, 2254964. [Google Scholar] [CrossRef]
- Whitehead, S.S.; Blaney, J.E.; Durbin, A.P.; Murphy, B.R. Prospects for a dengue virus vaccine. Nat. Rev. Microbiol. 2007, 5, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, S.S. Development of TV003/TV005, a single dose, highly immunogenic live attenuated dengue vaccine; what makes this vaccine different from the Sanofi-Pasteur CYDTM vaccine? Expert Rev. Vaccines 2016, 15, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Durbin, A.P. Historical discourse on the development of the live attenuated tetravalent dengue vaccine candidate TV003/TV005. Curr. Opin. Virol. 2020, 43, 79–87. [Google Scholar] [CrossRef]
- Kirkpatrick, B.D.; Durbin, A.P.; Pierce, K.K.; Carmolli, M.P.; Tibery, C.M.; Grier, P.L.; Hynes, N.; Diehl, S.A.; Elwood, D.; Jarvis, A.P.; et al. Robust and Balanced Immune Responses to All 4 Dengue Virus Serotypes Following Administration of a Single Dose of a Live Attenuated Tetravalent Dengue Vaccine to Healthy, Flavivirus-Naive Adults. J. Infect. Dis. 2015, 212, 702–710. [Google Scholar] [CrossRef]
- Angelo, M.A.; Grifoni, A.; O’Rourke, P.H.; Sidney, J.; Paul, S.; Peters, B.; de Silva, A.D.; Phillips, E.; Mallal, S.; Diehl, S.A.; et al. Human CD4+ T Cell Responses to an Attenuated Tetravalent Dengue Vaccine Parallel Those Induced by Natural Infection in Magnitude, HLA Restriction, and Antigen Specificity. J. Virol. 2017, 91, e02147-16. [Google Scholar] [CrossRef]
- Weiskopf, D.; Angelo, M.A.; Bangs, D.J.; Sidney, J.; Paul, S.; Peters, B.; de Silva, A.D.; Lindow, J.C.; Diehl, S.A.; Whitehead, S.; et al. The human CD8+ T cell responses induced by a live attenuated tetravalent dengue vaccine are directed against highly conserved epitopes. J. Virol. 2015, 89, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Nivarthi, U.K.; Swanstrom, J.; Delacruz, M.J.; Patel, B.; Durbin, A.P.; Whitehead, S.S.; Kirkpatrick, B.D.; Pierce, K.K.; Diehl, S.A.; Katzelnick, L.; et al. A tetravalent live attenuated dengue virus vaccine stimulates balanced immunity to multiple serotypes in humans. Nat. Commun. 2021, 12, 1102. [Google Scholar] [CrossRef]
- Kirkpatrick, B.D.; Whitehead, S.S.; Pierce, K.K.; Tibery, C.M.; Grier, P.L.; Hynes, N.A.; Larsson, C.J.; Sabundayo, B.P.; Talaat, K.R.; Janiak, A.; et al. The live attenuated dengue vaccine TV003 elicits complete protection against dengue in a human challenge model. Sci. Transl. Med. 2016, 8, 330ra36. [Google Scholar] [CrossRef]
- Kallas, E.G.; Precioso, A.R.; Palacios, R.; Thomé, B.; Braga, P.E.; Vanni, T.; Campos, L.M.A.; Ferrari, L.; Mondini, G.; da Graça Salomão, M.; et al. Safety and immunogenicity of the tetravalent, live-attenuated dengue vaccine Butantan-DV in adults in Brazil: A two-step, double-blind, randomised placebo-controlled phase 2 trial. Lancet Infect. Dis. 2020, 20, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, S.S.; Durbin, A.P.; Pierce, K.K.; Elwood, D.; McElvany, B.D.; Fraser, E.A.; Carmolli, M.P.; Tibery, C.M.; Hynes, N.A.; Jo, M.; et al. In a randomized trial, the live attenuated tetravalent dengue vaccine TV003 is well-tolerated and highly immunogenic in subjects with flavivirus exposure prior to vaccination. PLoS Negl. Trop. Dis. 2017, 11, e0005584. [Google Scholar] [CrossRef] [PubMed]
- Durbin, A.P.; Kirkpatrick, B.D.; Pierce, K.K.; Carmolli, M.P.; Tibery, C.M.; Grier, P.L.; Hynes, N.; Opert, K.; Jarvis, A.P.; Sabundayo, B.P.; et al. A 12-Month-Interval Dosing Study in Adults Indicates That a Single Dose of the National Institute of Allergy and Infectious Diseases Tetravalent Dengue Vaccine Induces a Robust Neutralizing Antibody Response. J. Infect. Dis. 2016, 214, 832–835. [Google Scholar] [CrossRef]
- Kallás, E.G.; Cintra, M.A.T.; Moreira, J.A.; Patiño, E.G.; Braga, P.E.; Tenório, J.C.V.; Infante, V.; Palacios, R.; de Lacerda, M.V.G.; Batista Pereira, D.; et al. Live, Attenuated, Tetravalent Butantan–Dengue Vaccine in Children and Adults. N. Engl. J. Med. 2024, 390, 397–408. [Google Scholar] [CrossRef]
- Durbin, A.P.; Kirkpatrick, B.D.; Pierce, K.K.; Elwood, D.; Larsson, C.J.; Lindow, J.C.; Tibery, C.; Sabundayo, B.P.; Shaffer, D.; Talaat, K.R.; et al. A Single Dose of Any of Four Different Live Attenuated Tetravalent Dengue Vaccines Is Safe and Immunogenic in Flavivirus-naive Adults: A Randomized, Double-blind Clinical Trial. J. Infect. Dis. 2013, 207, 957–965. [Google Scholar] [CrossRef]
- Walsh, M.C.R.; Alam, M.S.; Pierce, K.K.; Carmolli, M.; Alam, M.; Dickson, D.M.; Bak, D.M.; Afreen, S.; Nazib, F.; Golam, K.; et al. Safety and durable immunogenicity of the TV005 tetravalent dengue vaccine, across serotypes and age groups, in dengue-endemic Bangladesh: A randomised, controlled trial. Lancet Infect. Dis. 2024, 24, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Gubler, D.J.; Reed, D.; Rosen, L.; Hitchcock, J.R. Epidemiologic, clinical, and virologic observations on dengue in the Kingdom of Tonga. Am. J. Trop. Med. Hyg. 1978, 27, 581–589. [Google Scholar] [CrossRef]
- Gubler, D.J.; Suharyono, W.; Lubis, I.; Eram, S.; Gunarso, S. Epidemic dengue 3 in central Java, associated with low viremia in man. Am. J. Trop. Med. Hyg. 1981, 30, 1094–1099. [Google Scholar] [CrossRef]
- Pierce, K.K.; Durbin, A.P.; Walsh, M.C.R.; Carmolli, M.; Sabundayo, B.P.; Dickson, D.M.; Diehl, S.A.; Whitehead, S.S.; Kirkpatrick, B.D. TV005 dengue vaccine protects against dengue serotypes 2 and 3 in two controlled human infection studies. J. Clin. Investig. 2024, 134, e173328. [Google Scholar] [CrossRef]
- Eckels, K.H.; Dubois, D.R.; Putnak, R.; Vaughn, D.W.; Innis, B.L.; Henchal, E.A.; Hoke, C.H., Jr. Modification of dengue virus strains by passage in primary dog kidney cells: Preparation of candidate vaccines and immunization of monkeys. Am. J. Trop. Med. Hyg. 2003, 69 (Suppl. 6), 12–16. [Google Scholar] [CrossRef] [PubMed]
- Innis, B.L.; Eckels, K.H. Progress in development of a live-attenuated, tetravalent dengue virus vaccine by the United States Army Medical Research and Materiel Command. Am. J. Trop. Med. Hyg. 2003, 69 (Suppl. 6), 1–4. [Google Scholar] [CrossRef]
- Simasathien, S.; Thomas, S.J.; Watanaveeradej, V.; Nisalak, A.; Barberousse, C.; Innis, B.L.; Sun, W.; Putnak, J.R.; Eckels, K.H.; Hutagalung, Y.; et al. Safety and immunogenicity of a tetravalent live-attenuated dengue vaccine in flavivirus naive children. Am. J. Trop. Med. Hyg. 2008, 78, 426–433. [Google Scholar] [CrossRef]
- Watanaveeradej, V.; Simasathien, S.; Nisalak, A.; Endy, T.P.; Jarman, R.G.; Innis, B.L.; Thomas, S.J.; Gibbons, R.V.; Hengprasert, S.; Samakoses, R.; et al. Safety and immunogenicity of a tetravalent live-attenuated dengue vaccine in flavivirus-naive infants. Am. J. Trop. Med. Hyg. 2011, 85, 341–351. [Google Scholar] [CrossRef]
- Thomas, S.J.; Eckels, K.H.; Carletti, I.; De La Barrera, R.; Dessy, F.; Fernandez, S.; Putnak, R.; Toussaint, J.F.; Sun, W.; Bauer, K.; et al. A phase II, randomized, safety and immunogenicity study of a re-derived, live-attenuated dengue virus vaccine in healthy adults. Am. J. Trop. Med. Hyg. 2013, 88, 73–88. [Google Scholar] [CrossRef] [PubMed]
- Watanaveeradej, V.; Gibbons, R.V.; Simasathien, S.; Nisalak, A.; Jarman, R.G.; Kerdpanich, A.; Tournay, E.; De La Barrerra, R.; Dessy, F.; Toussaint, J.F.; et al. Safety and immunogenicity of a rederived, live-attenuated dengue virus vaccine in healthy adults living in Thailand: A randomized trial. Am. J. Trop. Med. Hyg. 2014, 91, 119–128. [Google Scholar] [CrossRef]
- Moris, P.; Bauer, K.M.; Currier, J.R.; Friberg, H.; Eckels, K.H.; Esquilin, I.O.; Gibbons, R.V.; Innis, B.L.; Jarman, R.G.; Simasathien, S.; et al. Cell-mediated immune responses to different formulations of a live-attenuated tetravalent dengue vaccine candidate in subjects living in dengue endemic and non-endemic regions. Hum. Vaccines Immunother. 2019, 15, 2090–2105. [Google Scholar] [CrossRef]
- Schmidt, A.C.; Lin, L.; Martinez, L.J.; Ruck, R.C.; Eckels, K.H.; Collard, A.; De La Barrera, R.; Paolino, K.M.; Toussaint, J.F.; Lepine, E.; et al. Phase 1 Randomized Study of a Tetravalent Dengue Purified Inactivated Vaccine in Healthy Adults in the United States. Am. J. Trop. Med. Hyg. 2017, 96, 1325–1337. [Google Scholar] [CrossRef]
- Diaz, C.; Lin, L.; Martinez, L.J.; Eckels, K.H.; Campos, M.; Jarman, R.G.; De La Barrera, R.; Lepine, E.; Toussaint, J.F.; Febo, I.; et al. Phase I Randomized Study of a Tetravalent Dengue Purified Inactivated Vaccine in Healthy Adults from Puerto Rico. Am. J. Trop. Med. Hyg. 2018, 98, 1435–1443. [Google Scholar] [CrossRef] [PubMed]
- Diaz, C.; Koren, M.; Lin, L.; Martinez, L.J.; Eckels, K.H.; Campos, M.; Jarman, R.G.; De La Barrera, R.; Lepine, E.; Febo, I.; et al. Safety and Immunogenicity of Different Formulations of a Tetravalent Dengue Purified Inactivated Vaccine in Healthy Adults from Puerto Rico: Final Results after 3 Years of Follow-Up from a Randomized, Placebo-Controlled Phase I Study. Am. J. Trop. Med. Hyg. 2020, 102, 951–954. [Google Scholar] [CrossRef]
- Friberg, H.; Gargulak, M.; Kong, A.; Lin, L.; Martinez, L.J.; Schmidt, A.C.; Paris, R.M.; Jarman, R.G.; Diaz, C.; Thomas, S.J.; et al. Characterization of B-cell and T-cell responses to a tetravalent dengue purified inactivated vaccine in healthy adults. NPJ Vaccines 2022, 7, 132. [Google Scholar] [CrossRef]
- Manoff, S.B.; Sausser, M.; Falk Russell, A.; Martin, J.; Radley, D.; Hyatt, D.; Roberts, C.C.; Lickliter, J.; Krishnarajah, J.; Bett, A.; et al. Immunogenicity and safety of an investigational tetravalent recombinant subunit vaccine for dengue: Results of a Phase I randomized clinical trial in flavivirus-naïve adults. Hum. Vaccines Immunother. 2019, 15, 2195–2204. [Google Scholar] [CrossRef]
- Durbin, A.P.; Pierce, K.K.; Kirkpatrick, B.D.; Grier, P.; Sabundayo, B.P.; He, H.; Sausser, M.; Russell, A.F.; Martin, J.; Hyatt, D.; et al. Immunogenicity and Safety of a Tetravalent Recombinant Subunit Dengue Vaccine in Adults Previously Vaccinated with a Live Attenuated Tetravalent Dengue Vaccine: Results of a Phase-I Randomized Clinical Trial. Am. J. Trop. Med. Hyg. 2020, 103, 855–863. [Google Scholar] [CrossRef]
- Beckett, C.G.; Tjaden, J.; Burgess, T.; Danko, J.R.; Tamminga, C.; Simmons, M.; Wu, S.J.; Sun, P.; Kochel, T.; Raviprakash, K.; et al. Evaluation of a prototype dengue-1 DNA vaccine in a Phase 1 clinical trial. Vaccine 2011, 29, 960–968. [Google Scholar] [CrossRef]
- Danko, J.R.; Kochel, T.; Teneza-Mora, N.; Luke, T.C.; Raviprakash, K.; Sun, P.; Simmons, M.; Moon, J.E.; De La Barrera, R.; Martinez, L.J.; et al. Safety and Immunogenicity of a Tetravalent Dengue DNA Vaccine Administered with a Cationic Lipid-Based Adjuvant in a Phase 1 Clinical Trial. Am. J. Trop. Med. Hyg. 2018, 98, 849–856. [Google Scholar] [CrossRef]
- Wu, R.S.L.; Chan, K.R.; Tan, H.C.; Chow, A.; Allen, J.C.; Ooi, E.E. Neutralization of dengue virus in the presence of Fc receptor-mediated phagocytosis distinguishes serotype-specific from cross-neutralizing antibodies. Antivir. Res. 2012, 96, 340–343. [Google Scholar] [CrossRef]
- Montoya, M.; Gresh, L.; Mercado, J.C.; Williams, K.L.; Vargas, M.J.; Gutierrez, G.; Kuan, G.; Gordon, A.; Balmaseda, A.; Harris, E. Symptomatic versus inapparent outcome in repeat dengue virus infections is influenced by the time interval between infections and study year. PLoS Negl. Trop. Dis. 2013, 7, e2357. [Google Scholar] [CrossRef] [PubMed]
- Endy, T.P.; Yoon, I.K.; Mammen, M.P. Prospective cohort studies of dengue viral transmission and severity of disease. Curr. Top. Microbiol. Immunol. 2010, 338, 1–13. [Google Scholar] [CrossRef]
- Monath, T.P. Dengue and yellow fever—Challenges for the development and use of vaccines. N. Engl. J. Med. 2007, 357, 2222–2225. [Google Scholar] [CrossRef] [PubMed]
- Akter, R.; Tasneem, F.; Das, S.; Soma, M.A.; Georgakopoulos-Soares, I.; Juthi, R.T.; Sazed, S.A. Approaches of dengue control: Vaccine strategies and future aspects. Front. Immunol. 2024, 15, 1362780. [Google Scholar] [CrossRef] [PubMed]
- AR5 Climate Change 2013: The Physical Science Basis—IPCC. Available online: https://www.ipcc.ch/report/ar5/wg1/ (accessed on 2 September 2025).
- Fritzell, C.; Rousset, D.; Adde, A.; Kazanji, M.; Kerkhove, M.D.V.; Flamand, C. Current challenges and implications for dengue, chikungunya and Zika seroprevalence studies worldwide: A scoping review. PLoS Negl. Trop. Dis. 2018, 12, e0006533. [Google Scholar] [CrossRef] [PubMed]
- Dengvaxia (Dengue Tetravalent Vaccine, Live) FDA Approval History. Drugs.com. Available online: https://www.drugs.com/history/dengvaxia.html (accessed on 30 September 2024).
- Dengvaxia. European Medicines Agency (EMA). 18 December 2018. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/dengvaxia (accessed on 30 September 2024).
- WHO Position Paper on Dengue Vaccines. May 2024. Available online: https://www.who.int/publications/i/item/who-wer-9918-203-224 (accessed on 30 September 2024).
- Vaccines and Immunization: Dengue. Available online: https://www.who.int/news-room/questions-and-answers/item/dengue-vaccines (accessed on 30 September 2024).
- Strategic Advisory Group of Experts on Immunization (SAGE). Dengue. Available online: https://www.who.int/groups/strategic-advisory-group-of-experts-on-immunization/working-groups/dengue_2022 (accessed on 30 September 2024).
- Fukunishi, A.; Machida, M.; Fukushima, S.; Inoue, S. Travel medicine providers’ opinions on the dengue vaccine TAK-003 as a travel vaccine and the need for decision-support information and materials before its launch in Japan. Hum. Vaccines Immunother. 2025, 21, 2483560. [Google Scholar] [CrossRef] [PubMed]
- Eperon, G.; Veit, O.; Antonini, P.; Fehr, J.; Haller, S.; Hatz, C.; Landry, P.; Neumayr, A.; Niederer-Lohrer, A.; Schlagenhauf, P.; et al. Vaccination against dengue fever for travellers: Statement of the Swiss Expert Committee for Travel Medicine, an organ of the Swiss Society for Tropical and Travel Medicine, August 2024. Swiss. Med. Wkly. 2024, 154, 3858. [Google Scholar] [CrossRef]
- Angelin, M.; Sjölin, J.; Kahn, F.; Ljunghill Hedberg, A.; Rosdahl, A.; Skorup, P.; Werner, S.; Woxenius, S.; Askling, H.H. Qdenga®—A promising dengue fever vaccine; can it be recommended to non-immune travelers? Travel Med. Infect. Dis. 2023, 54, 102598. [Google Scholar] [CrossRef]
- Support, S. Indicazioni per L’utilizzo del Vaccino Contro la Dengue. SimVim. 9 April 2024. Available online: https://www.simvim.org/indicazioni-per-lutilizzo-del-vaccino-contro-la-dengue/ (accessed on 2 May 2025).
- The STIKO Recommendation on Vaccination Against Dengue with the Qdenga Vaccine. Available online: https://www.rki.de/EN/Topics/Infectious-diseases/Immunisation/STIKO/STIKO-recommendations/Downloads/STIKO-rec-on-vax-vs-dengue_with-Qdenga_BGR_P.html (accessed on 4 May 2025).
- NaTHNaC—The Green Book Travel Chapters. Available online: https://travelhealthpro.org.uk/factsheet/109/the-green-book-travel-chapters (accessed on 6 May 2025).
- Vaccination Against Dengue. Superior Health Council. Available online: https://www.hgr-css.be/en/report/9739/vaccination-against-dengue (accessed on 6 May 2025).
- Denguefebervaksine. Folkehelseinstituttet. 5 March 2025. Available online: https://www.fhi.no/va/vaksinasjonshandboka/vaksiner-mot-de-enkelte-sykdommene/denguefeber/ (accessed on 20 June 2025).
- Stetens Serum Institut. Denguefeber Vaccine (Qdenga). Available online: https://www.ssi.dk/vaccinationer/vaccineleksikon/d/denguefeber-vaccine (accessed on 20 June 2025).
- Vaccination Med Qdenga Till Svenska Resenärer—En Vägledning. Infektion. net. Available online: https://infektion.net/kunskap/vagledning-for-vaccination-med-qdenga-till-svenska-resenarer/ (accessed on 20 June 2025).
- Impfplan Österreich. Bundesministerium für Arbeit, Soziales, Gesundheit, Pflege und Konsumentenschutz. Available online: https://www.sozialministerium.gv.at/Themen/Gesundheit/Impfen/Impfplan-Oesterreich.html (accessed on 20 June 2025).
- Zur Impfung Gegen Denguefieber mit Qdenga® FAQs Erarbeitet Durch den Ständigen Ausschuss Reisemedizin (StAR) der DTG. Available online: https://www.dtg.org/images/Startseite-Download-Box/StAR_QDENGA_FINAL_160224.pdf (accessed on 20 June 2025).
- Haute Autorité de Santé—Stratégie de Vaccination Contre la Dengue—Place du Vaccin Qdenga. Available online: https://www.has-sante.fr/jcms/p_3461308/fr/strategie-de-vaccination-contre-la-dengue-place-du-vaccin-qdenga (accessed on 20 June 2025).
- Ministerio de Sanidad. Nota Informativa para los Viajeros Internacionales en Relación a la Reemergencia del Dengue en el Mundo, y la Nueva Vacuna Frente al Dengue. Available online: https://www.sanidad.gob.es/areas/sanidadExterior/laSaludTambienViaja/notasInformativas/docs/NI_ReemergenciaDENGUE-NuevaVacunaFrenteAlDengue-11Septiembre24.pdf (accessed on 20 June 2025).
- Superior Health Council. Advisory Report of the Superior Health Council no. 9739. Available online: https://www.health.belgium.be/sites/default/files/uploads/fields/fpshealth_theme_file/20230405_shc_9739_dengue_vaccination_vweb.pdf (accessed on 20 June 2025).
- Een Nieuw Denguevaccin Voor Reizigers/IB 09 2024. RIVM. Available online: https://www.rivm.nl/weblog/ib-nieuw-denguevaccin-voor-reizigers (accessed on 20 June 2025).
- Nohynek, H.; Holmberg, V.; Dengue. Duodecim Terveyskirjasto. Available online: https://www.terveyskirjasto.fi/mat00204 (accessed on 20 June 2025).
- Takeda Withdraws US Application for Dengue Vaccine Candidate. Reuters. 11 July 2023. Available online: https://www.reuters.com/business/healthcare-pharmaceuticals/takeda-withdraws-us-application-dengue-vaccine-candidate-2023-07-11/ (accessed on 28 October 2024).
- Liu, X. Opportunities and challenges of mRNA technologies in development of dengue virus vaccine. Front. Immunol. 2025, 16, 1520968. [Google Scholar] [CrossRef]
- Rahman, N.A.A.; Fuaad, A.A.H.A.; Azami, N.A.M.; Amin, M.C.I.M.; Azmi, F. Next-generation Dengue Vaccines: Leveraging Peptide-Based Immunogens and Advanced Nanoparticles as Delivery Platforms. J. Pharm. Sci. 2024, 113, 2044–2054. [Google Scholar] [CrossRef] [PubMed]
| Timepoint | VE vs. VCD (Overall) | VE vs. VCD (Seronegative) | VE vs. VCD (Seropositive) | VE vs. Hospitalized Dengue (Overall) | VE vs. Hospitalized Dengue (Seronegative) | VE vs. Hospitalized Dengue (Seropositive) |
|---|---|---|---|---|---|---|
| 12 months | Overall: 80.9% DENV-1: 73.7% DENV-2: 97.7% DENV-3: 62.2% DENV-4: insufficient data | 74.9% * | 82.2% | 95.4% | 97.2% | 94.4% |
| 18 months | Overall: 73.3% DENV-1: 69.8%, DENV-2: 95.1% DENV-3: 48.9% DENV-4: insufficient data | 66.2% | 76.1% | 90.4% | 89.5% | 89.7% |
| 3 years | Overall: 62.0% DENV-1: 56.2%, DENV-2: 83.4% DENV-3: 52.3% DENV-4: 60.7% | Overall: 54.3% DENV-1: 43.5%, DENV-2: 91.9% DENV-3: insufficient data DENV-4: insufficient data | Overall: 65.0% DENV-1: 56.2%, DENV-2: 83.4% DENV-3: 52.3% DENV-4: 60.7% | 83.6% | Overall: 77.1% DENV-1: 77.2%, DENV-2: non estimable DENV-3: insufficient data DENV-4: non estimable | Overall: 86.0% DENV-1: 69.2%, DENV-2: 95.3% DENV-3: 72.1% DENV-4: non estimable |
| 4.5 years | 61.2% | Overall: 53.5% DENV-1: 45.4%, DENV-2: 88.1% DENV-3: insufficient data DENV-4: insufficient data | Overall: 64.2% DENV-1: 56.1% DENV-2: 80.4% DENV-3: 52.3% DENV-4: 70.6% | 84.1% | Overall: 79.3% DENV-1: 78.4% DENV-2: non estimable DENV-3: insufficient data DENV-4: non estimable | Overall: 85.9% DENV-1: 66.8% DENV-2: 95.8% DENV-3: 74.0% DENV-4: non estimable |
| Vaccine | Type of Vaccine | Producer | Development | Reference |
|---|---|---|---|---|
| CYD-TDV | Recombinant live-attenuated tetravalent vaccine, based on substitution of the yellow fever vaccine (YF17D) prM/E genes, with the genes of each dengue serotype | Sanofi Pasteur | Licensed (manufacturing suspended) | [23] |
| TAK-003 | Live-attenuated tetravalent vaccine, backbone based on DENV-2 primary dog kidney (PDK)–53, and substitution of the pre-membrane and envelope genes of TDV-2 with those from wild-type DENV-1, DENV-1, DENV-3, and DENV-4 strains | Takeda | Licensed | [38] |
| TV003-TV005 | Live-attenuated vaccine, obtained by the deletion of nucleotides from the 3′-untraslated regions of the DENV viral genome | National Institute of Allergy and Infectious Diseases (NIAID) | Phase IIIb | [53,58] |
| TDEN | Tetravalent live-attenuated DENV vaccine, made of four lyophilized monovalent live-attenuated strains representing each DENV viral strain | Walter Reed Army Institute of Research (WRAIR) and GlaxoSmithKline (GSK) | Phase II | [65] |
| DPIV | Tetravalent inactivated DENV vaccine, with three different adjuvants (aluminum hydroxide or AS01E or AS03B) | WRAIR, GSK and Fiocruz (Rio de Janeiro, Brazil) | Phase I | [69] |
| V180 | Recombinant subunit vaccine based on all four DENV strains envelope glycoprotein | Merck & Co. (Rahway, NJ, USA) | Phase I | [70,71] |
| TVDV | Tetravalent DNA vaccine based on prM and E protein coding sequences cloned in VR1012 plasmid (co-administered with Vaxfectin as an adjuvant) | U.S. Army Medical Research and Development Command (Fort Detrick, MD, USA), WRAIR, NMRC and Vical (San Diego, CA, USA) | Phase I | [73] |
| Vaccine | EMA | FDA | WHO |
|---|---|---|---|
| CYD-TDV | Individuals 6–45 years with laboratory-confirmed previous dengue infection | People aged 9–16 years living in endemic areas, who have laboratory-confirmed previous dengue infection | Individuals aged 9–45 years or 9–60 years (depending on the country-specific regulatory approvals) living in dengue-endemic areas, who have laboratory-confirmed previous dengue infection |
| TAK-003 | Adults, adolescents and children from 4 years of age. | Not licensed by FDA | Children aged 6–16 years in high-transmission settings. Persons with comorbidities in dengue-endemic countries could be offered vaccination, even if they fall outside the recommended age range, with the upper limit of 60 years. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marangoni, D.; Barbiero, A.; Spinicci, M.; Bartoloni, A.; Rossanese, A.; Bonanni, P.; Zammarchi, L. State of the Art on Vaccine Development Against Dengue Infection: Scoping Review of the Literature. Infect. Dis. Rep. 2025, 17, 117. https://doi.org/10.3390/idr17050117
Marangoni D, Barbiero A, Spinicci M, Bartoloni A, Rossanese A, Bonanni P, Zammarchi L. State of the Art on Vaccine Development Against Dengue Infection: Scoping Review of the Literature. Infectious Disease Reports. 2025; 17(5):117. https://doi.org/10.3390/idr17050117
Chicago/Turabian StyleMarangoni, Davide, Anna Barbiero, Michele Spinicci, Alessandro Bartoloni, Andrea Rossanese, Paolo Bonanni, and Lorenzo Zammarchi. 2025. "State of the Art on Vaccine Development Against Dengue Infection: Scoping Review of the Literature" Infectious Disease Reports 17, no. 5: 117. https://doi.org/10.3390/idr17050117
APA StyleMarangoni, D., Barbiero, A., Spinicci, M., Bartoloni, A., Rossanese, A., Bonanni, P., & Zammarchi, L. (2025). State of the Art on Vaccine Development Against Dengue Infection: Scoping Review of the Literature. Infectious Disease Reports, 17(5), 117. https://doi.org/10.3390/idr17050117


_Rachiotis.png)





