Oral Immunization with Yeast-Surface Display of SARS-CoV-2 Antigens in Pichia pastoris Induces Humoral Responses in BALB/C Mice
Abstract
1. Introduction
2. Materials and Methods
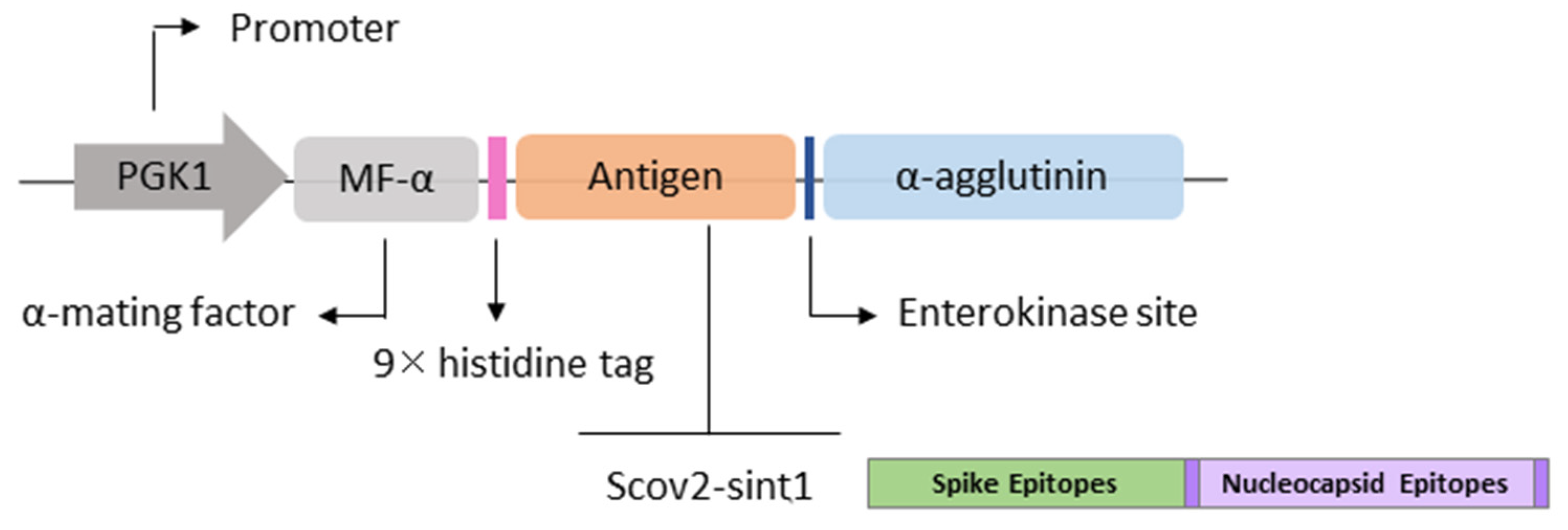
2.1. Gene Design
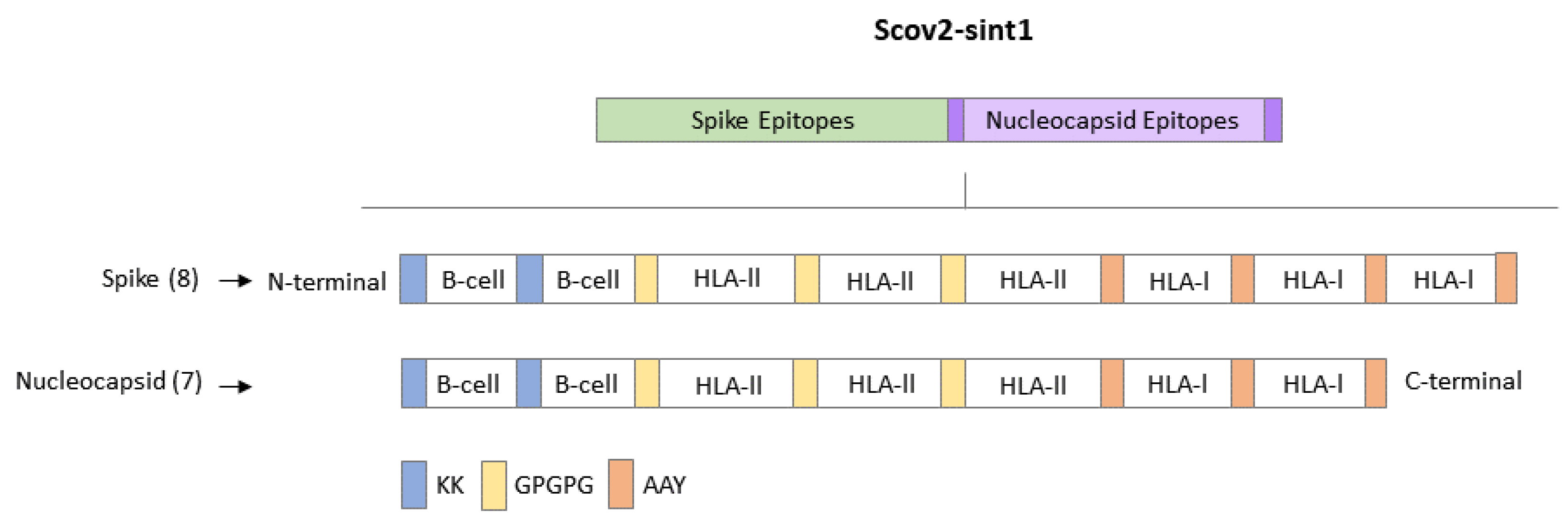
2.2. Obtaining Multi-Epitope Sequence
2.3. Obtaining Expression Vectors
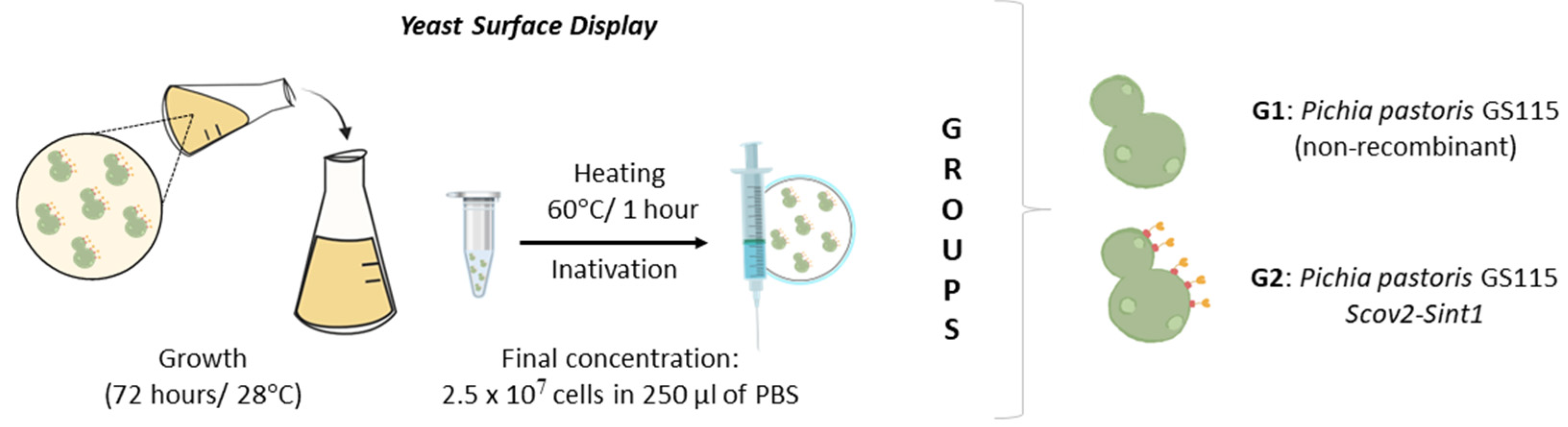
2.4. Microorganism Strains and Culture Conditions
2.5. Obtaining Recombinant Yeast
2.6. Western Blot Analysis
2.7. Immunofluorescence Analysis
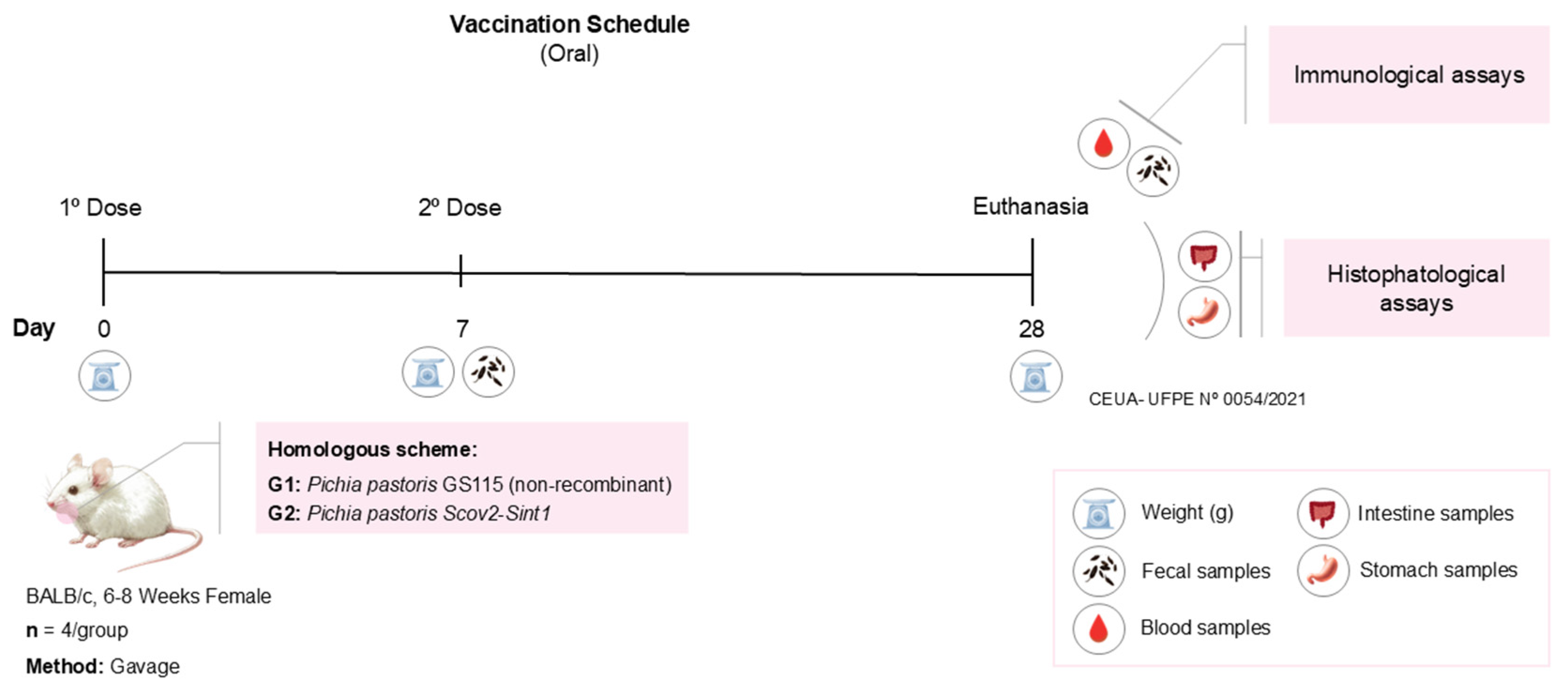
2.8. Preparation of Vaccine Doses, Oral Immunization Regimen, and Sample Collection
2.9. Vaccine Safety Assessment
2.9.1. Myelotoxicity Tests
2.9.2. Histopathological Analyses
2.10. Analysis of Fecal Samples
2.11. Evaluation of the Humoral Response Induced by Vaccines
2.12. Statistical Analysis
3. Results
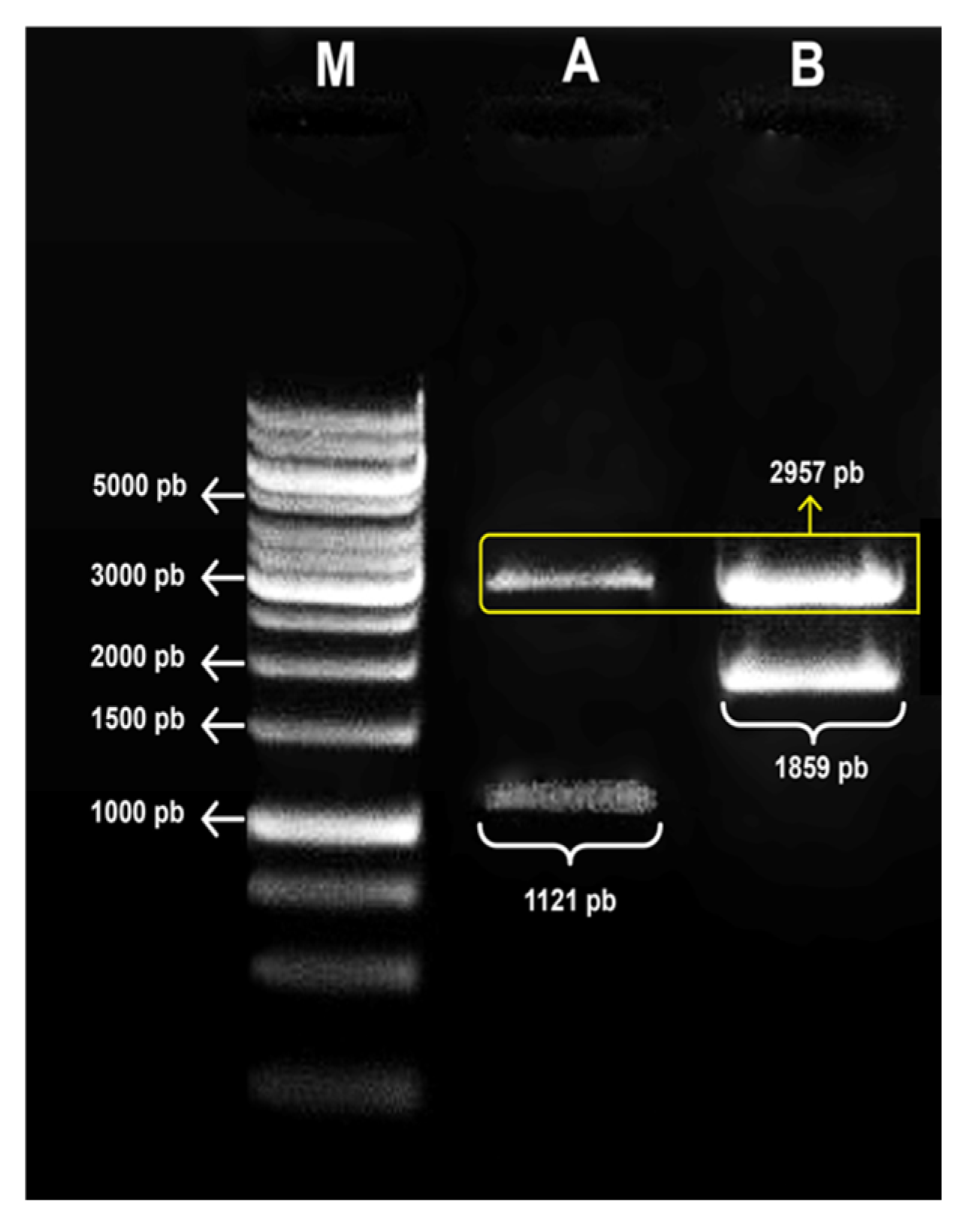
3.1. Confirmation of the Obtaining Expression Vectors
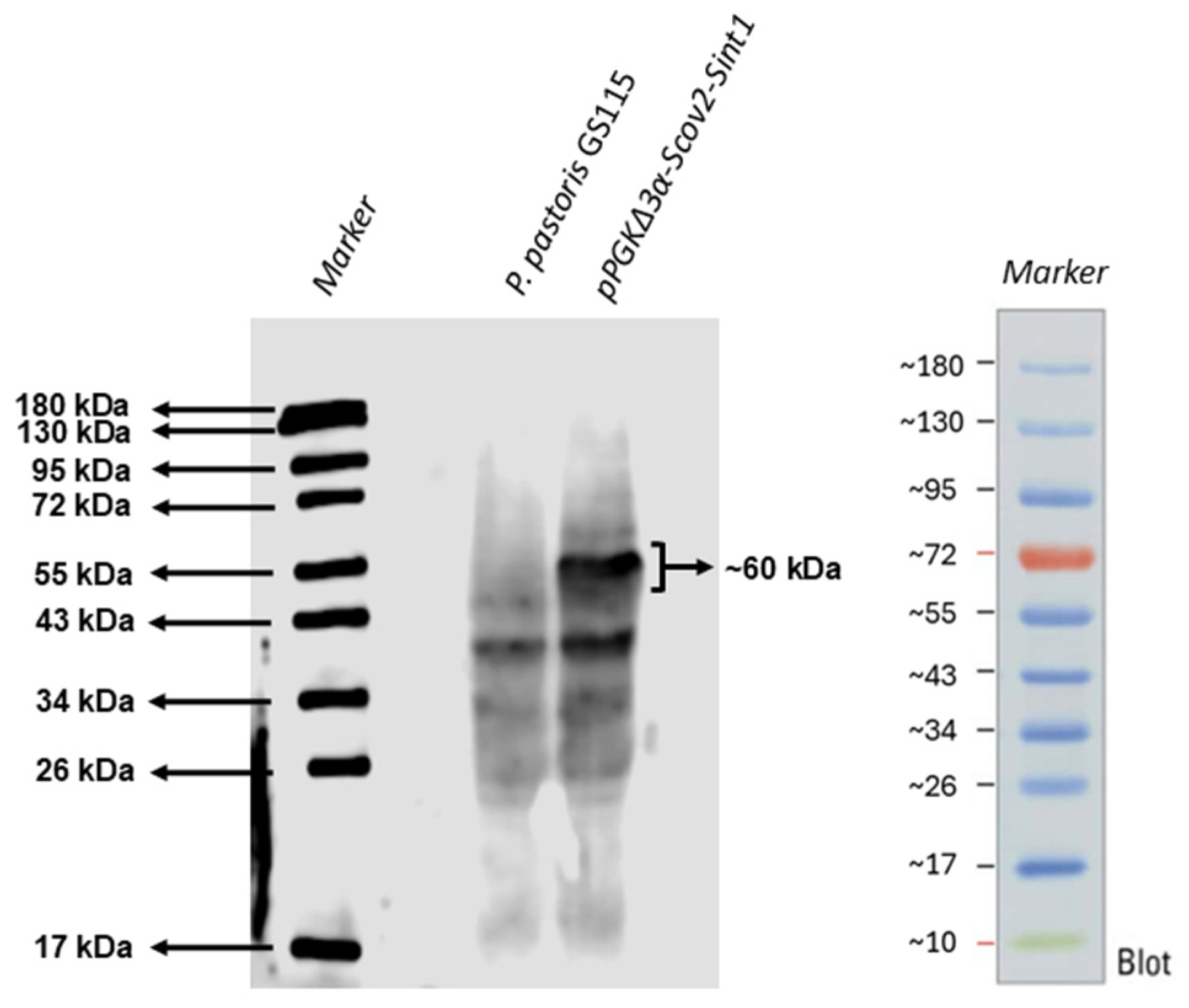
3.2. Recombinant Protein Production
3.3. Immunofluorescence
3.4. Vaccine Safety Profile
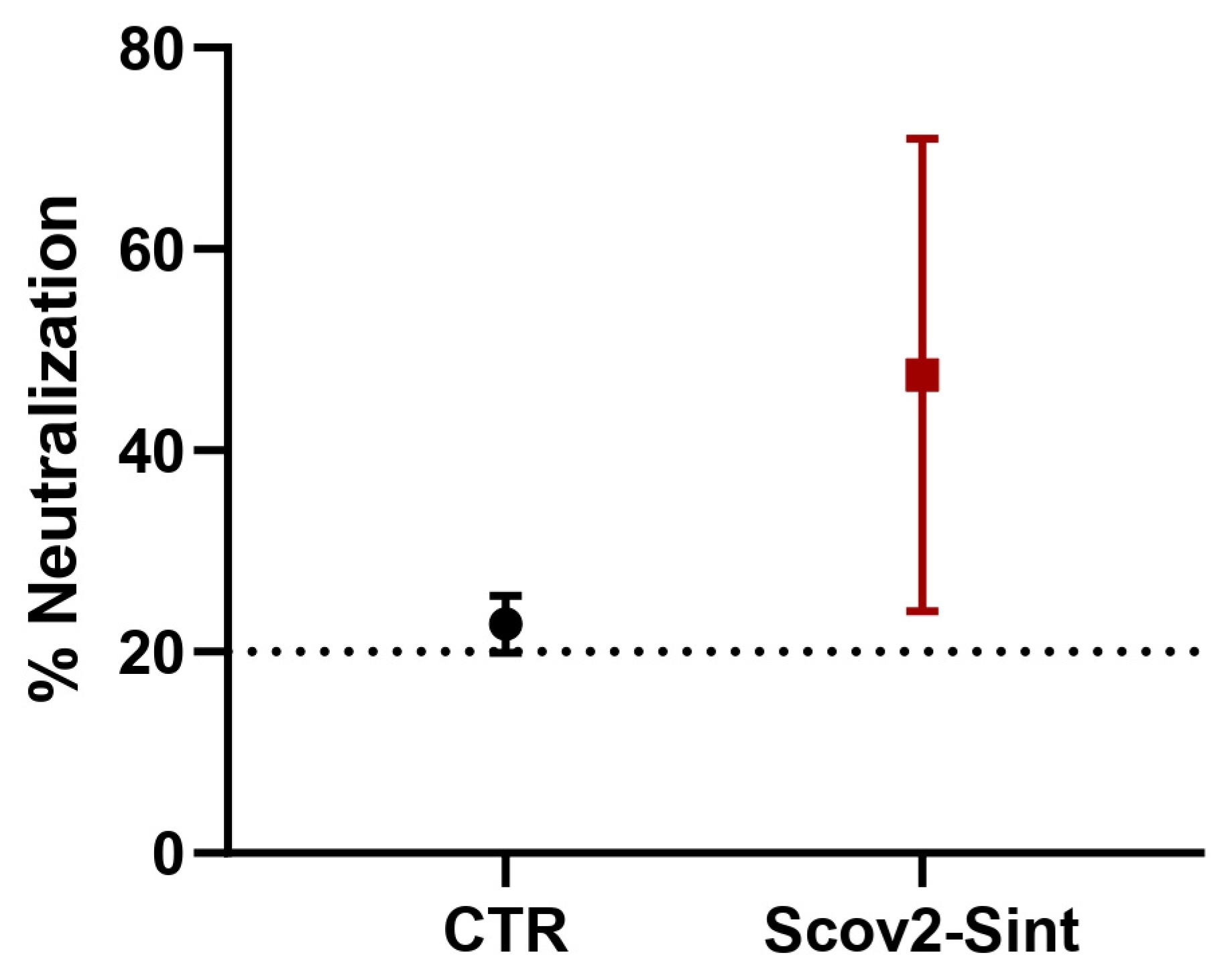
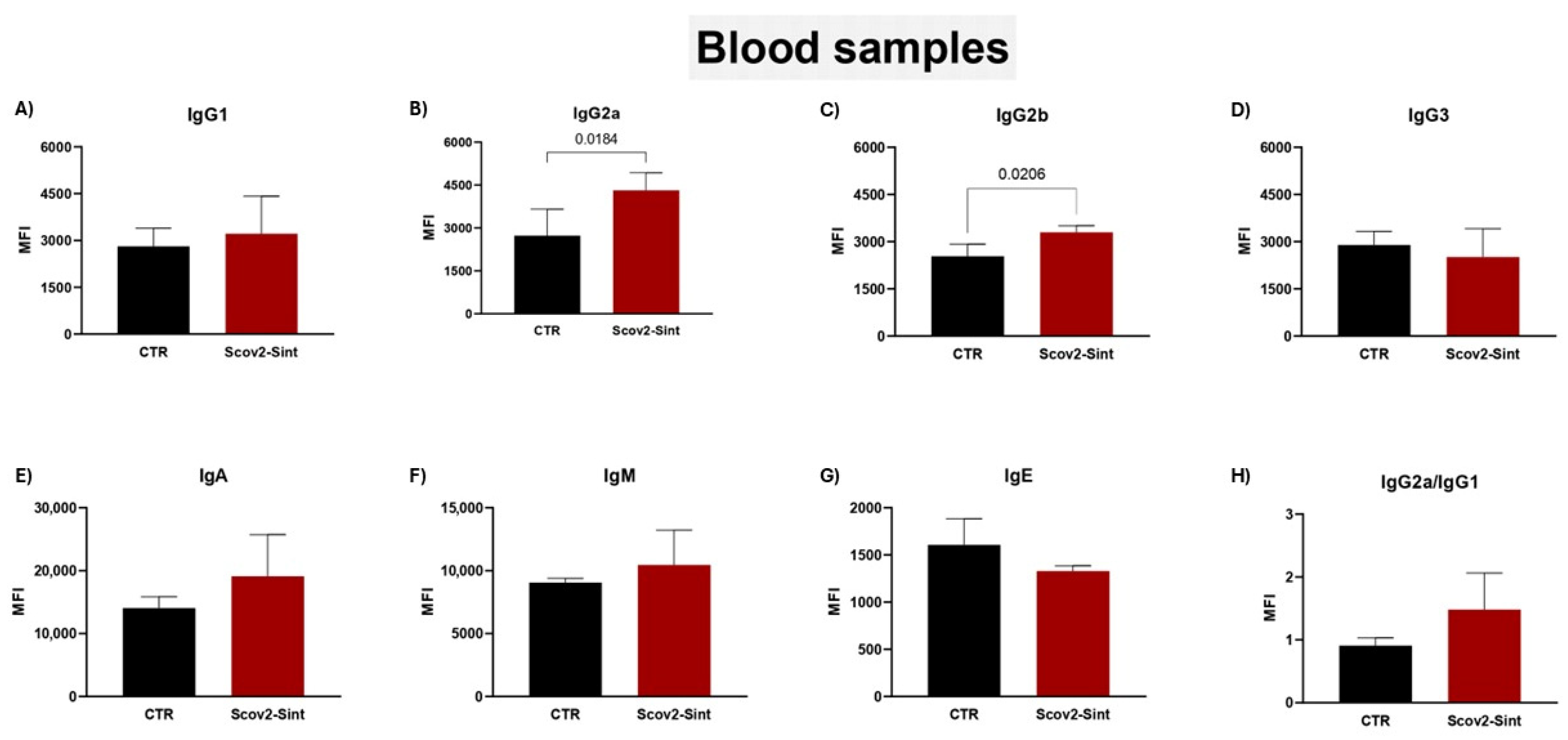
3.5. Assessment of the Humoral Immune Response Induced by Vaccines
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 9xHIS | Histidine Tag |
| Agα | α-Agglutinin |
| APCs | Antigen-Presenting Cells |
| COVID-19 | Coronavirus Disease 2019 |
| DCs | Dendritic Cells |
| EK | Enterokinase |
| FDA | Food and Drug Administration |
| FITC | Fluorescein Isocyanate |
| GALTs | Gut-Associated Immune Tissues |
| GPI | Glycosylphosphatidylinositol |
| GRAS | Generally Recognized As Safe |
| HE | Hematoxylin and Eosin |
| HPV | Human Papillomavirus |
| i.v. | Intravenous |
| L2x | 2× Laemmli Denaturing Buffer |
| LB | Luria–Bertani |
| LBLS | Luria–Bertani Low Salt |
| MHC | Major Histocompatibility Complex |
| MHC-I | Major Histocompatibility Complex Class I |
| MHC-II | Major Histocompatibility Complex Class II |
| PVDF | Polyvinylidene Fluoride |
| RBD | Receptor Binding Domain |
| SARS-CoV-2 | Severe Acute Respiratory Syndrome Coronavirus 2 |
| ShBle | Streptoalloteichus hindustanus-Bleomycin Resistance Protein |
| TAP | Transporter Associated with Antigen Processing |
| α-MF | α-Mating Factor |
References
- Kumar, R.; Kumar, P. Yeast-Based Vaccines: New Perspective in Vaccine Development and Application. FEMS Yeast Res. 2019, 19, foz007. [Google Scholar] [CrossRef]
- Ardiani, A.; Higgins, J.P.; Hodge, J.W. Vaccines Based on Whole Recombinant Saccharomyces cerevisiae Cells: Yeast-Based Vaccines. FEMS Yeast Res. 2010, 10, 1060–1069. [Google Scholar] [CrossRef]
- Walch, B.; Breinig, T.; Schmitt, M.J.; Breinig, F. Delivery of Functional DNA and Messenger RNA to Mammalian Phagocytic Cells by Recombinant Yeast. Gene Ther. 2012, 19, 237–245. [Google Scholar] [CrossRef]
- Austriaco, N. Yeast Oral Vaccines against Infectious Diseases. Front. Microbiol. 2023, 14, 1150412. [Google Scholar] [CrossRef]
- De Sá Magalhães, S.; Keshavarz-Moore, E. Pichia pastoris (Komagataella phaffii) as a Cost-Effective Tool for Vaccine Production for Low- and Middle-Income Countries (LMICs). Bioengineering 2021, 8, 119. [Google Scholar] [CrossRef] [PubMed]
- Teymennet-Ramírez, K.V.; Martínez-Morales, F.; Trejo-Hernández, M.R. Yeast Surface Display System: Strategies for Improvement and Biotechnological Applications. Front. Bioeng. Biotechnol. 2022, 9, 794742. [Google Scholar] [CrossRef]
- Vogl, T.; Hartner, F.S.; Glieder, A. New Opportunities by Synthetic Biology for Biopharmaceutical Production in Pichia pastoris. Curr. Opin. Biotechnol. 2013, 24, 1094–1101. [Google Scholar] [CrossRef]
- Unver, Y.; Dagci, I. Komagataella phaffii (Pichia pastoris) as a Powerful Yeast Expression System for Biologics Production. Front. Biosci. (Elite Ed) 2024, 16, 19. [Google Scholar] [CrossRef]
- Silva, A.J.D.; de Macêdo, L.S.; Leal, L.R.S.; de Jesus, A.L.S.; Freitas, A.C. Yeasts as a Promising Delivery Platform for DNA and RNA Vaccines. FEMS Yeast Res. 2021, 21, foab018. [Google Scholar] [CrossRef] [PubMed]
- De, S.; Mattanovich, D.; Ferrer, P.; Gasser, B. Established Tools and Emerging Trends for the Production of Recombinant Proteins and Metabolites in Pichia pastoris. Essays Biochem. 2021, 65, 293–307. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.J.D.; Jesus, A.L.S.; Leal, L.R.S.; Silva, G.A.S.; Melo, C.M.L.; Freitas, A.C. Pichia pastoris Displaying ZIKV Protein Epitopes from the Envelope and NS1 Induce in Vitro Immune Activation. Vaccine 2021, 39, 2545–2554. [Google Scholar] [CrossRef]
- Chen, X.; Shi, T.; Chen, F.; Xie, X.; Fang, H.; Wu, Z.; Liu, Y.; Huang, Y.; Wang, Q.; Nie, G.; et al. Orally Antigen-Engineered Yeast Vaccine Elicits Robust Intestinal Mucosal Immunity. ACS Nano 2025, 19, 10841–10853. [Google Scholar] [CrossRef]
- Kumar, R.; Kharbikar, B.N. Lyophilized Yeast Powder for Adjuvant Free Thermostable Vaccine Delivery. Appl. Microbiol. Biotechnol. 2021, 105, 3131–3143. [Google Scholar] [CrossRef]
- Bolhassani, A.; Muller, M.; Roohvand, F.; Motevalli, F.; Agi, E.; Shokri, M.; Rad, M.M.; Hosseinzadeh, S. Whole Recombinant Pichia pastoris Expressing HPV16 L1 Antigen Is Superior in Inducing Protection against Tumor Growth as Compared to Killed Transgenic Leishmania. Hum. Vaccines Immunother. 2014, 10, 3499–3508. [Google Scholar] [CrossRef]
- Bazan, S.B.; Breinig, T.; Schmitt, M.J.; Breinig, F. Heat Treatment Improves Antigen-Specific T Cell Activation after Protein Delivery by Several but Not All Yeast Genera. Vaccine 2014, 32, 2591–2598. [Google Scholar] [CrossRef]
- Wasilenko, J.L.; Sarmento, L.; Spatz, S.; Pantin-Jackwood, M. Cell Surface Display of Highly Pathogenic Avian Influenza Virus Hemagglutinin on the Surface of Pichia pastoris Cells Using A-agglutinin for Production of Oral Vaccines. Biotechnol. Prog. 2010, 26, 542–547. [Google Scholar] [CrossRef]
- Luo, B.; Jin, M.M.; Li, X.; Makunga, N.P.; Hu, X. Yeast Surface Display for In Vitro Biosynthetic Pathway Reconstruction. ACS Synth. Biol. 2021, 10, 2938–2946. [Google Scholar] [CrossRef] [PubMed]
- Naveed, M.; Sheraz, M.; Amin, A.; Waseem, M.; Aziz, T.; Khan, A.A.; Ghani, M.; Shahzad, M.; Alruways, M.W.; Dablool, A.S.; et al. Designing a Novel Peptide-Based Multi-Epitope Vaccine to Evoke a Robust Immune Response against Pathogenic Multidrug-Resistant Providencia heimbachae. Vaccines 2022, 10, 1300. [Google Scholar] [CrossRef]
- Sharma, A.D.; Magdaleno, J.S.L.; Singh, H.; Orduz, A.F.C.; Cavallo, L.; Chawla, M. Immunoinformatics-Driven Design of a Multi-Epitope Vaccine Targeting Neonatal Rotavirus with Focus on Outer Capsid Proteins VP4 and VP7 and Non Structural Proteins NSP2 and NSP5. Sci. Rep. 2025, 15, 11879. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Xiao, G.; Luo, D.; Kong, L.; Chen, X.; Sun, D.; Yan, J. Chimeric Epitope Vaccine against Leptospira Interrogans Infection and Induced Specific Immunity in Guinea Pigs. BMC Microbiol. 2016, 16, 241. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Zuo, W.; Kang, C.; Zou, Z.; Zhang, K.; Qiu, J.; Shang, X.; Li, J.; Zhang, Y.; Zuo, Q.; et al. Saccharomyces cerevisiae Oral Immunization in Mice Using Multi-Antigen of the African Swine Fever Virus Elicits a Robust Immune Response. Front. Immunol. 2024, 15, 1373656. [Google Scholar] [CrossRef]
- Invenção, M.D.C.V.; Macêdo, L.S.D.; Moura, I.A.D.; Santos, L.A.B.D.O.; Espinoza, B.C.F.; Pinho, S.S.D.; Leal, L.R.S.; Santos, D.L.D.; São Marcos, B.D.F.; Elsztein, C.; et al. Design and Immune Profile of Multi-Epitope Synthetic Antigen Vaccine Against SARS-CoV-2: An In Silico and In Vivo Approach. Vaccines 2025, 13, 149. [Google Scholar] [CrossRef]
- Ayyagari, V.S.; T. C., V.; K., A.P.; Srirama, K. Design of a Multi-Epitope-Based Vaccine Targeting M-Protein of SARS-CoV2: An Immunoinformatics Approach. J. Biomol. Struct. Dyn. 2022, 40, 2963–2977. [Google Scholar] [CrossRef]
- Gu, Y.; Sun, X.; Li, B.; Huang, J.; Zhan, B.; Zhu, X. Vaccination with a Paramyosin-Based Multi-Epitope Vaccine Elicits Significant Protective Immunity against Trichinella Spiralis Infection in Mice. Front. Microbiol. 2017, 8, 1475. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Chu, Z.; Yu, F.; Zha, Y. Contriving Multi-Epitope Subunit of Vaccine for COVID-19: Immunoinformatics Approaches. Front. Immunol. 2020, 11, 1784. [Google Scholar] [CrossRef] [PubMed]
- Varshavsky, A. The N-end Rule Pathway and Regulation by Proteolysis. Protein Sci. 2011, 20, 1298–1345. [Google Scholar] [CrossRef] [PubMed]
- Doytchinova, I.A.; Flower, D.R. VaxiJen: A Server for Prediction of Protective Antigens, Tumour Antigens and Subunit Vaccines. BMC Bioinform. 2007, 8, 4. [Google Scholar] [CrossRef]
- Velders, M.P.; Weijzen, S.; Eiben, G.L.; Elmishad, A.G.; Kloetzel, P.-M.; Higgins, T.; Ciccarelli, R.B.; Evans, M.; Man, S.; Smith, L.; et al. Defined Flanking Spacers and Enhanced Proteolysis Is Essential for Eradication of Established Tumors by an Epitope String DNA Vaccine. J. Immunol. 2001, 166, 5366–5373. [Google Scholar] [CrossRef]
- De Almeida, J.R.M.; De Moraes, L.M.P.; Torres, F.A.G. Molecular Characterization of the 3-phosphoglycerate Kinase Gene (PGK1) from the Methylotrophic Yeast Pichia pastoris. Yeast 2005, 22, 725–737. [Google Scholar] [CrossRef]
- Arruda, A.; Reis, V.C.B.; Batista, V.D.F.; Daher, B.S.; Piva, L.C.; De Marco, J.L.; De Moraes, L.M.P.; Torres, F.A.G. A Constitutive Expression System for Pichia pastoris Based on the PGK1 Promoter. Biotechnol. Lett. 2016, 38, 509–517. [Google Scholar] [CrossRef]
- Barrero, J.J.; Casler, J.C.; Valero, F.; Ferrer, P.; Glick, B.S. An Improved Secretion Signal Enhances the Secretion of Model Proteins from Pichia Pastoris. Microb. Cell Fact. 2018, 17, 161. [Google Scholar] [CrossRef]
- Zhao, H.; Shen, Z.-M.; Kahn, P.C.; Lipke, P.N. Interaction of α-Agglutinin and a-Agglutinin, Saccharomyces cerevisiae Sexual Cell Adhesion Molecules. J. Bacteriol. 2001, 183, 2874–2880. [Google Scholar] [CrossRef]
- Zhang, M.; Gu, L.; Zheng, P.; Chen, Z.; Dou, X.; Qin, Q.; Cai, X. Improvement of Cell Counting Method for Neubauer Counting Chamber. Clin. Lab. Anal. 2020, 34, e23024. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Z.; Xu, H.; Xiang, B.; Dang, R.; Yang, Z. Orally Administrated Whole Yeast Vaccine Against Porcine Epidemic Diarrhea Virus Induced High Levels of IgA Response in Mice and Piglets. Viral Immunol. 2016, 29, 526–531. [Google Scholar] [CrossRef]
- Barbosa, B.D.S.; Praxedes, É.A.; Lima, M.A.; Pimentel, M.M.L.; Santos, F.A.; Brito, P.D.; Lelis, I.C.N.G.; Macedo, M.F.D.; Bezerra, M.B. Haematological and Biochemical Profile of Balb-c Mice. Acta Scientiae. Vet. 2017, 45, 5. [Google Scholar] [CrossRef]
- Silva-Santana, G.; Bax, J.C.; Fernandes, D.C.S.; Bacellar, D.T.L.; Hooper, C.; Dias, A.A.S.O.; Silva, C.B.; De Souza, A.M.; Ramos, S.; Santos, R.A.; et al. Clinical Hematological and Biochemical Parameters in Swiss, BALB/c, C57BL/6 and B6D2F1 Mus musculus. Anim. Models Exp. Med. 2020, 3, 304–315. [Google Scholar] [CrossRef]
- Zhang, L.; Yao, L.; Guo, Y.; Li, X.; Ma, L.; Sun, R.; Han, X.; Liu, J.; Huang, J. Oral SARS-CoV-2 Spike Protein Recombinant Yeast Candidate Prompts Specific Antibody and Gut Microbiota Reconstruction in Mice. Front. Microbiol. 2022, 13, 792532. [Google Scholar] [CrossRef]
- Acar, M.; Unver, Y. Constitutive and Extracellular Expression of Pectin Methylesterase from Pectobacterium chrysanthemi in Pichia pastoris. 3 Biotech 2022, 12, 219. [Google Scholar] [CrossRef]
- Peng, X.-B.; Chen, G.-J.; Han, Z.-G.; Yang, J.-K. High-Level Secretive Expression of a Novel Achieved Talaromyces cellulolyticus Endo-Polygalacturonase in Pichia pastoris by Improving Gene Dosage for Hydrolysis of Natural Pectin. World J. Microbiol. Biotechnol. 2019, 35, 84. [Google Scholar] [CrossRef]
- Kao, M.-R.; Yu, S.-M.; Ho, T.-H.U.D. Improvements of the Productivity and Saccharification Efficiency of the Cellulolytic β-Glucosidase D2-BGL in Pichia pastoris via Directed Evolution. Biotechnol. Biofuels 2021, 14, 126. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, C.; Xu, X.; Xu, W.; Liu, S. Structural and Functional Properties of SARS-CoV-2 Spike Protein: Potential Antivirus Drug Development for COVID-19. Acta Pharmacol. Sin. 2020, 41, 1141–1149. [Google Scholar] [CrossRef]
- Li, T.; Zheng, Q.; Yu, H.; Wu, D.; Xue, W.; Xiong, H.; Huang, X.; Nie, M.; Yue, M.; Rong, R.; et al. SARS-CoV-2 Spike Produced in Insect Cells Elicits High Neutralization Titres in Non-Human Primates. Emerg. Microbes Infect. 2020, 9, 2076–2090. [Google Scholar] [CrossRef]
- Esposito, D.; Mehalko, J.; Drew, M.; Snead, K.; Wall, V.; Taylor, T.; Frank, P.; Denson, J.-P.; Hong, M.; Gulten, G.; et al. Optimizing High-Yield Production of SARS-CoV-2 Soluble Spike Trimers for Serology Assays. Protein Expr. Purif. 2020, 174, 105686. [Google Scholar] [CrossRef] [PubMed]
- Bazan, S.B.; Geginat, G.; Breinig, T.; Schmitt, M.J.; Breinig, F. Uptake of Various Yeast Genera by Antigen-Presenting Cells and Influence of Subcellular Antigen Localization on the Activation of Ovalbumin-Specific CD8 T Lymphocytes. Vaccine 2011, 29, 8165–8173. [Google Scholar] [CrossRef]
- Howland, S.W.; Wittrup, K.D. Antigen Release Kinetics in the Phagosome Are Critical to Cross-Presentation Efficiency. J. Immunol. 2008, 180, 1576–1583. [Google Scholar] [CrossRef]
- Lei, H.; Jin, S.; Karlsson, E.; Schultz-Cherry, S.; Ye, K. Yeast Surface-Displayed H5N1 Avian Influenza Vaccines. J. Immunol. Res. 2016, 2016, 4131324. [Google Scholar] [CrossRef]
- Lei, H.; Xie, B.; Gao, T.; Cen, Q.; Ren, Y. Yeast Display Platform Technology to Prepare Oral Vaccine against Lethal H7N9 Virus Challenge in Mice. Microb. Cell Fact. 2020, 19, 53. [Google Scholar] [CrossRef] [PubMed]
- Xing, H.; Zhu, L.; Wang, P.; Zhao, G.; Zhou, Z.; Yang, Y.; Zou, H.; Yan, X. Display of Receptor-Binding Domain of SARS-CoV-2 Spike Protein Variants on the Saccharomyces cerevisiae Cell Surface. Front. Immunol. 2022, 13, 935573. [Google Scholar] [CrossRef] [PubMed]
- Silva De Macêdo, L.; Sousa De Pinho, S.; Duarte Silva, A.J.; De Moura, I.A.; Flores Espinoza, B.C.; Viana Invenção, M.D.C.; Silva Novis, P.V.; Turiah Machado Da Gama, M.A.; Do Nascimento Carvalho, M.; Rosa Sales Leal, L.; et al. Understanding Yeast Shells: Structure, Properties and Applications. ADMET DMPK 2024, 12, 299–317. [Google Scholar] [CrossRef]
- Alkalbani, N.S.; Osaili, T.M.; Al-Nabulsi, A.A.; Olaimat, A.N.; Liu, S.-Q.; Shah, N.P.; Apostolopoulos, V.; Ayyash, M.M. Assessment of Yeasts as Potential Probiotics: A Review of Gastrointestinal Tract Conditions and Investigation Methods. J. Fungi 2022, 8, 365. [Google Scholar] [CrossRef]
- Silva, A.J.D.; De Sousa, M.M.G.; De Macêdo, L.S.; De França Neto, P.L.; De Moura, I.A.; Espinoza, B.C.F.; Invenção, M.D.C.V.; De Pinho, S.S.; Da Gama, M.A.T.M.; De Freitas, A.C. RNA Vaccines: Yeast as a Novel Antigen Vehicle. Vaccines 2023, 11, 1334. [Google Scholar] [CrossRef] [PubMed]
- Cerutti, A.; Rescigno, M. The Biology of Intestinal Immunoglobulin A Responses. Immunity 2008, 28, 740–750. [Google Scholar] [CrossRef]
- Kobayashi, N.; Takahashi, D.; Takano, S.; Kimura, S.; Hase, K. The Roles of Peyer’s Patches and Microfold Cells in the Gut Immune System: Relevance to Autoimmune Diseases. Front. Immunol. 2019, 10, 2345. [Google Scholar] [CrossRef]
- Kenngott, E.E.; Kiefer, R.; Schneider-Daum, N.; Hamann, A.; Schneider, M.; Schmitt, M.J.; Breinig, F. Surface-Modified Yeast Cells: A Novel Eukaryotic Carrier for Oral Application. J. Control. Release 2016, 224, 1–7. [Google Scholar] [CrossRef]
- Bal, J.; Luong, N.N.; Park, J.; Song, K.-D.; Jang, Y.-S.; Kim, D.-H. Comparative Immunogenicity of Preparations of Yeast-Derived Dengue Oral Vaccine Candidate. Microb. Cell Fact. 2018, 17, 24. [Google Scholar] [CrossRef]
- Chan, R.W.Y.; Liu, S.; Cheung, J.Y.; Tsun, J.G.S.; Chan, K.C.; Chan, K.Y.Y.; Fung, G.P.G.; Li, A.M.; Lam, H.S. The Mucosal and Serological Immune Responses to the Novel Coronavirus (SARS-CoV-2) Vaccines. Front. Immunol. 2021, 12, 744887. [Google Scholar] [CrossRef]
- Bakema, J.E.; Van Egmond, M. The Human Immunoglobulin A Fc Receptor FcαRI: A Multifaceted Regulator of Mucosal Immunity. Mucosal Immunol. 2011, 4, 612–624. [Google Scholar] [CrossRef]
- Cerutti, A.; Cols, M.; Gentile, M.; Cassis, L.; Barra, C.M.; He, B.; Puga, I.; Chen, K. Regulation of Mucosal IgA Responses: Lessons from Primary Immunodeficiencies. Ann. N. Y. Acad. Sci. 2011, 1238, 132–144. [Google Scholar] [CrossRef]
- Jin, Y.; Wang, M.; Zuo, Z.; Fan, C.; Ye, F.; Cai, Z.; Wang, Y.; Cui, H.; Pan, K.; Xu, A. Diagnostic Value and Dynamic Variance of Serum Antibody in Coronavirus Disease 2019. Int. J. Infect. Dis. 2020, 94, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Takamatsu, Y.; Omata, K.; Shimizu, Y.; Kinoshita-Iwamoto, N.; Terada, M.; Suzuki, T.; Morioka, S.; Uemura, Y.; Ohmagari, N.; Maeda, K.; et al. SARS-CoV-2-Neutralizing Humoral IgA Response Occurs Earlier but Is Modest and Diminishes Faster than IgG Response. Microbiol. Spectr. 2022, 10, e02716–e02722. [Google Scholar] [CrossRef] [PubMed]
- Healy, K.; Pin, E.; Chen, P.; Söderdahl, G.; Nowak, P.; Mielke, S.; Hansson, L.; Bergman, P.; Smith, C.I.E.; Ljungman, P.; et al. Salivary IgG to SARS-CoV-2 Indicates Seroconversion and Correlates to Serum Neutralization in mRNA-Vaccinated Immunocompromised Individuals. Media 2022, 3, 137–153.e3. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Ren, Y.; Li, S.; Lu, X.; Lei, H. Immune Response Induced by Oral Administration with a Saccharomyces cerevisiae-Based SARS-CoV-2 Vaccine in Mice. Microb. Cell Fact. 2021, 20, 95. [Google Scholar] [CrossRef]
- Diem, G.; Jäger, M.; Dichtl, S.; Bauer, A.; Lass-Flörl, C.; Reindl, M.; Wilflingseder, D.; Posch, W. Vaccination and Omicron BA.1/BA.2 Convalescence Enhance Systemic but Not Mucosal Immunity against BA.4/5. Microbiol. Spectr. 2023, 11, e05163-22. [Google Scholar] [CrossRef]
- Bournazos, S.; Gupta, A.; Ravetch, J.V. The Role of IgG Fc Receptors in Antibody-Dependent Enhancement. Nat. Rev. Immunol. 2020, 20, 633–643. [Google Scholar] [CrossRef]
- Moss, P. The T Cell Immune Response against SARS-CoV-2. Nat. Immunol. 2022, 23, 186–193. [Google Scholar] [CrossRef]
- Peng, Y.; Mentzer, A.J.; Liu, G.; Yao, X.; Yin, Z.; Dong, D.; Dejnirattisai, W.; Rostron, T.; Supasa, P.; Liu, C.; et al. Broad and Strong Memory CD4+ and CD8+ T Cells Induced by SARS-CoV-2 in UK Convalescent Individuals Following COVID-19. Nat. Immunol. 2020, 21, 1336–1345. [Google Scholar] [CrossRef]
- Gosselin, E.J.; Bitsaktsis, C.; Li, Y.; Iglesias, B.V. Fc Receptor-Targeted Mucosal Vaccination as a Novel Strategy for the Generation of Enhanced Immunity against Mucosal and Non-Mucosal Pathogens. Arch. Immunol. Ther. Exp. 2009, 57, 311–323. [Google Scholar] [CrossRef]
- Lima, G.G.; Portilho, A.I.; De Gaspari, E. Adjuvants to Increase Immunogenicity of SARS-CoV-2 RBD and Support Maternal–Fetal Transference of Antibodies in Mice. Pathog. Dis. 2022, 80, ftac038. [Google Scholar] [CrossRef] [PubMed]
- Rothen, D.A.; Krenger, P.S.; Nonic, A.; Balke, I.; Vogt, A.S.; Chang, X.; Manenti, A.; Vedovi, F.; Resevica, G.; Walton, S.M.; et al. Intranasal Administration of a Virus like Particles-based Vaccine Induces Neutralizing Antibodies against SARS-CoV-2 and Variants of Concern. Allergy 2022, 77, 2446–2458. [Google Scholar] [CrossRef]
- Cerutti, A.; Chen, K.; Chorny, A. Immunoglobulin Responses at the Mucosal Interface. Annu. Rev. Immunol. 2011, 29, 273–293. [Google Scholar] [CrossRef] [PubMed]
- Brandtzaeg, P. Secretory IgA: Designed for Anti-Microbial Defense. Front. Immunol. 2013, 4, 222. [Google Scholar] [CrossRef] [PubMed]
- Becerril-García, M.Á.; Flores-Maldonado, O.E.; González, G.M.; García-González, G.; Hernández-Bello, R.; Palma-Nicolás, J.P. Safety Profile of Intravenous Administration of Live Pichia pastoris Cells in Mice. FEMS Yeast Res. 2022, 22, foac023. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.J.D.; De Jesus, A.L.S.; Leal, L.R.S.; De Macêdo, L.S.; Da Silva Barros, B.R.; De Sousa, G.F.; Da Paz Leôncio Alves, S.; Pena, L.J.; De Melo, C.M.L.; De Freitas, A.C. Whole Yeast Vaccine Displaying ZIKV B and T Cell Epitopes Induces Cellular Immune Responses in the Murine Model. Pharmaceutics 2023, 15, 1898. [Google Scholar] [CrossRef] [PubMed]
- Embregts, C.W.E.; Reyes-Lopez, F.; Pall, A.C.; Stratmann, A.; Tort, L.; Lorenzen, N.; Engell-Sorensen, K.; Wiegertjes, G.F.; Forlenza, M.; Sunyer, J.O.; et al. Pichia pastoris Yeast as a Vehicle for Oral Vaccination of Larval and Adult Teleosts. Fish Shellfish Immunol. 2019, 85, 52–60. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Macêdo, L.S.; Espinoza, B.C.F.; Invenção, M.d.C.V.; Pinho, S.S.d.; Leal, L.R.S.; Silva, M.E.d.S.; Bandeira, B.M.A.; Novis, P.V.S.; Souza, T.H.d.S.; Maux, J.M.d.L.; et al. Oral Immunization with Yeast-Surface Display of SARS-CoV-2 Antigens in Pichia pastoris Induces Humoral Responses in BALB/C Mice. Infect. Dis. Rep. 2025, 17, 104. https://doi.org/10.3390/idr17050104
de Macêdo LS, Espinoza BCF, Invenção MdCV, Pinho SSd, Leal LRS, Silva MEdS, Bandeira BMA, Novis PVS, Souza THdS, Maux JMdL, et al. Oral Immunization with Yeast-Surface Display of SARS-CoV-2 Antigens in Pichia pastoris Induces Humoral Responses in BALB/C Mice. Infectious Disease Reports. 2025; 17(5):104. https://doi.org/10.3390/idr17050104
Chicago/Turabian Stylede Macêdo, Larissa Silva, Benigno Cristofer Flores Espinoza, Maria da Conceição Viana Invenção, Samara Sousa de Pinho, Lígia Rosa Sales Leal, Micaela Evellin dos Santos Silva, Beatriz Mendonça Alves Bandeira, Pedro Vinícius Silva Novis, Tiago Henrique dos Santos Souza, Julliano Matheus de Lima Maux, and et al. 2025. "Oral Immunization with Yeast-Surface Display of SARS-CoV-2 Antigens in Pichia pastoris Induces Humoral Responses in BALB/C Mice" Infectious Disease Reports 17, no. 5: 104. https://doi.org/10.3390/idr17050104
APA Stylede Macêdo, L. S., Espinoza, B. C. F., Invenção, M. d. C. V., Pinho, S. S. d., Leal, L. R. S., Silva, M. E. d. S., Bandeira, B. M. A., Novis, P. V. S., Souza, T. H. d. S., Maux, J. M. d. L., Silva Neto, J. d. C., de Freitas, A. C., & Silva, A. J. D. (2025). Oral Immunization with Yeast-Surface Display of SARS-CoV-2 Antigens in Pichia pastoris Induces Humoral Responses in BALB/C Mice. Infectious Disease Reports, 17(5), 104. https://doi.org/10.3390/idr17050104










