Progressive Thoracolumbar Tuberculosis in a Young Male: Diagnostic, Therapeutic, and Surgical Insights
Abstract
1. Introduction
2. Case Presentation
2.1. Brief Case History
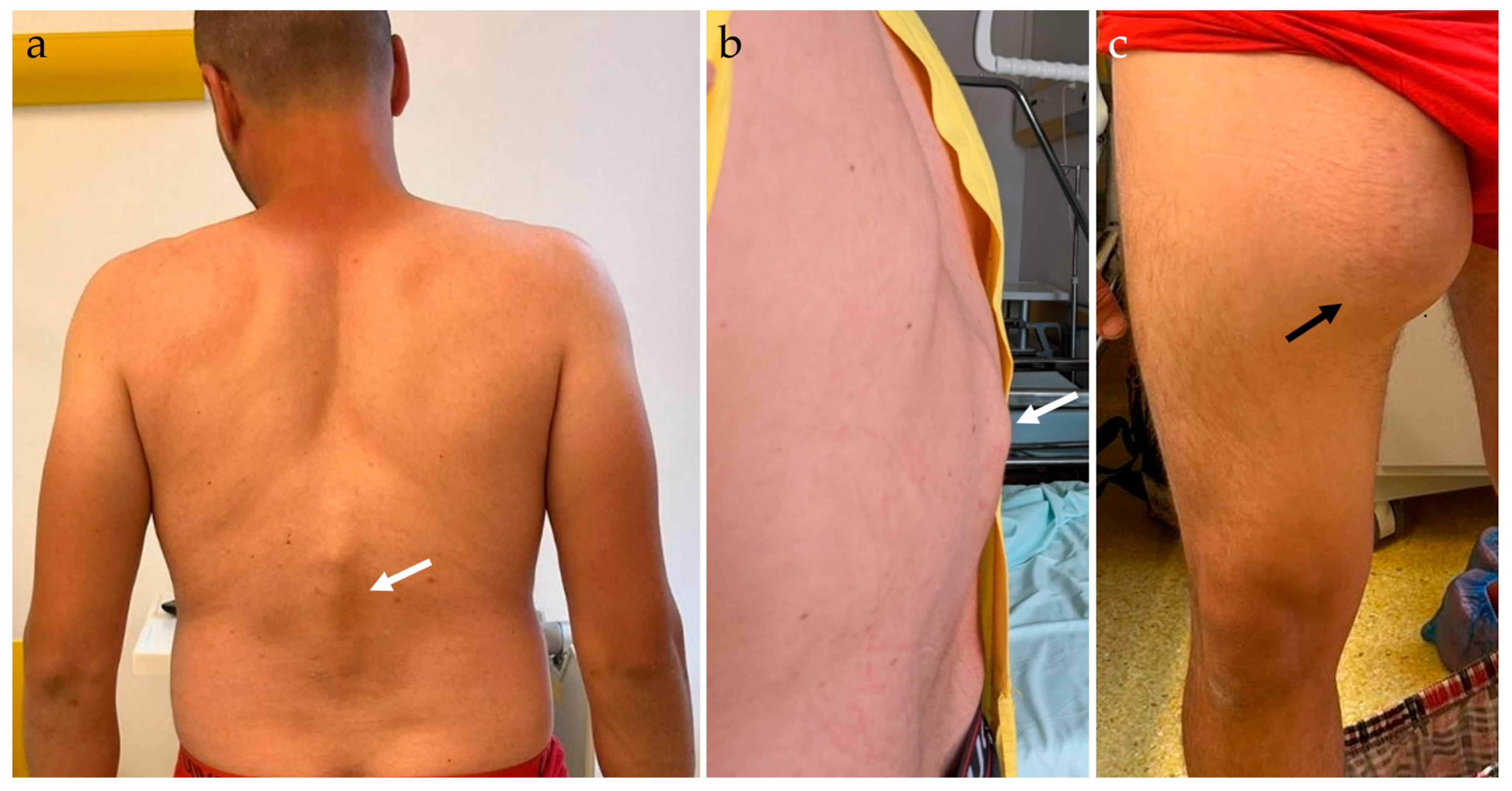
2.2. Physical Examination
2.3. Laboratory Investigations
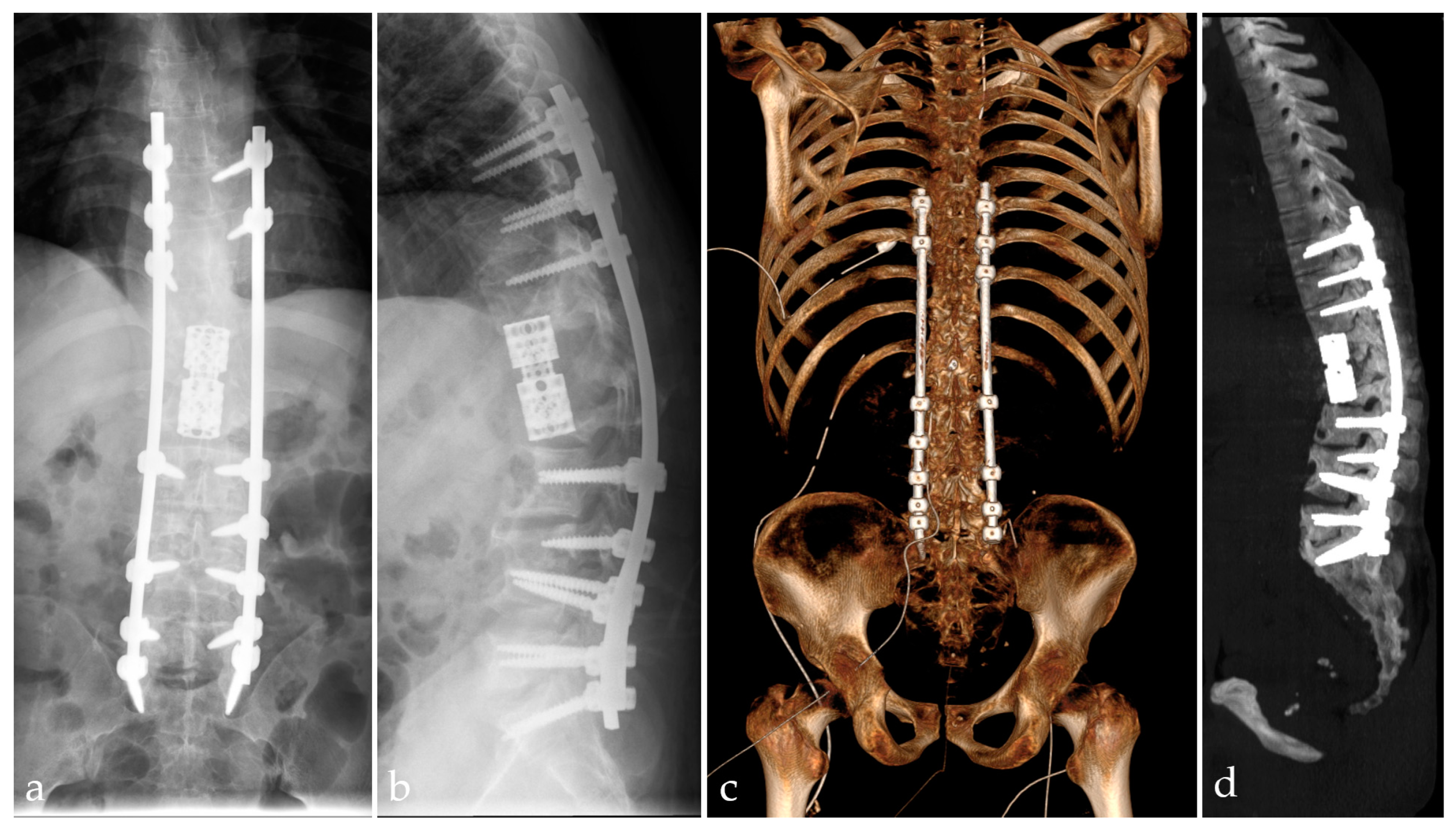
2.4. Imaging Examinations
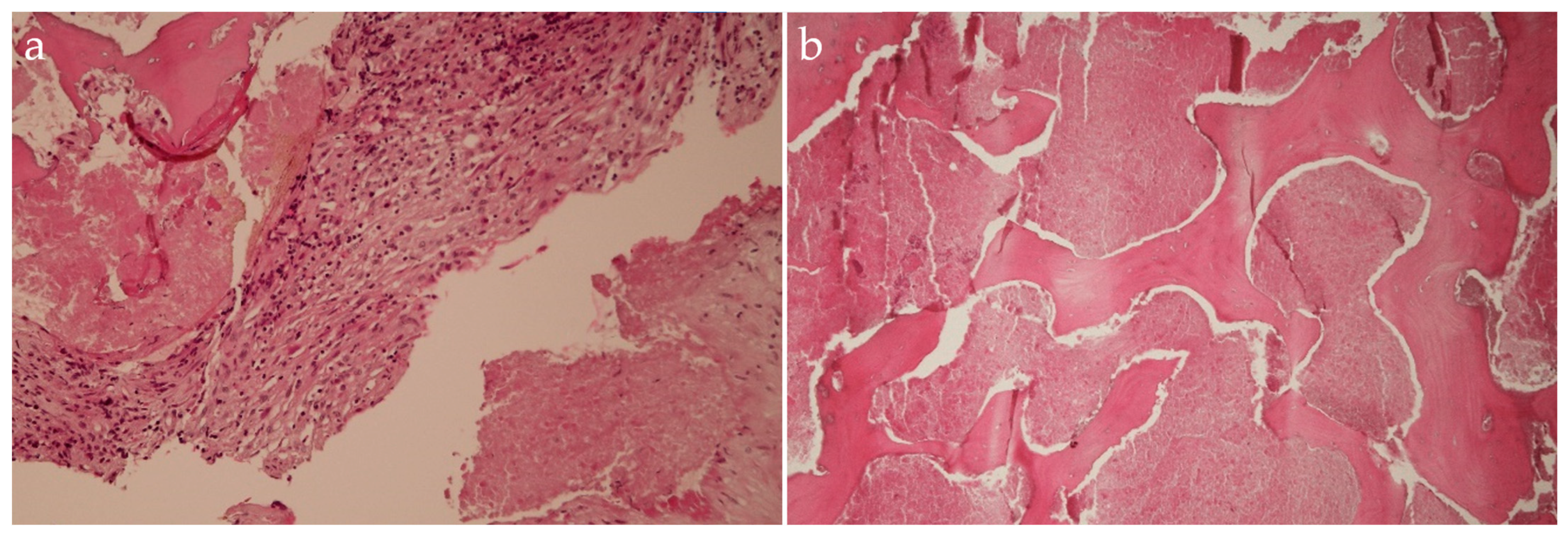
2.5. Management and Surgical Procedures
2.6. Postoperative Management and Recovery
3. Discussions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Global Tuberculosis Report 2023; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- Leowattana, W.; Leowattana, P.; Leowattana, T. Tuberculosis of the spine. World J. Orthop. 2023, 14, 275–293. [Google Scholar] [CrossRef] [PubMed]
- Taha, H.; Durham, J.; Reid, S. Communicable Diseases Prevalence among Refugees and Asylum Seekers: Systematic Review and Meta-Analysis. Infect. Dis. Rep. 2023, 15, 188–203. [Google Scholar] [CrossRef] [PubMed]
- Baykan, A.H.; Sayiner, H.S.; Aydin, E.; Koc, M.; Inan, I.; Erturk, S.M. Extrapulmonary tuberculosıs: An old but resurgent problem. Insights Imaging 2022, 13, 39. [Google Scholar] [CrossRef] [PubMed]
- Shanmuganathan, R.; Ramachandran, K.; Shetty, A.P.; Kanna, R.M. Active tuberculosis of spine: Current updates. N. Am. Spine Soc. J. 2023, 16, 100267. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, J.; Xiu, X.; Shi, Z.; Zhang, Q.; Chen, D. Evaluation of different diagnostic methods for spinal tuberculosis infection. BMC Infect. Dis. 2023, 23, 695. [Google Scholar] [CrossRef]
- Arifin, J.; Biakto, K.T.; Johan, M.P.; Anwar, S.F.Z. Clinical outcomes and surgical strategy for spine tuberculosis: A systematic review and meta-analysis. Spine Deform. 2024, 12, 271–291. [Google Scholar] [CrossRef]
- Pintor, I.A.; Pereira, F.; Cavadas, S.; Lopes, P. Pott’s disease (tuberculous spondylitis). Int. J. Mycobacteriol. 2022, 11, 113–115. [Google Scholar] [CrossRef]
- Dunn, R.N.; Ben Husien, M. Spinal tuberculosis: Review of current management. Bone Joint J. 2018, 100, 425–431. [Google Scholar] [CrossRef]
- Ahuja, K.; Ifthekar, S.; Mittal, S.; Yadav, G.; Sarkar, B.; Kandwal, P. Defining mechanical instability in tuberculosis of the spine: A systematic review. EFORT Open Rev. 2021, 6, 202–210. [Google Scholar] [CrossRef]
- Roberts, T.T.; Leonard, G.R.; Cepela, D.J. Classifications In Brief: American Spinal Injury Association (ASIA) Impairment Scale. Clin. Orthop. Relat. Res. 2017, 475, 1499–1504. [Google Scholar] [CrossRef]
- Lehuec, J.-C.; Faundez, A.; Dominguez, D.; Hoffmeyer, P.; Aunoble, S. Evidence showing the relationship between sagittal balance and clinical outcomes in surgical treatment of degenerative spinal diseases: A literature review. Int. Orthop. 2014, 39, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Le Huec, J.C.; Aunoble, S.; Philippe, L.; Nicolas, P. Pelvic parameters: Origin and significance. Eur. Spine J. 2011, 20 (Suppl. S5), 564–571. [Google Scholar] [CrossRef] [PubMed]
- Chakaya, J.; Khan, M.; Ntoumi, F.; Aklillu, E.; Fatima, R.; Mwaba, P.; Kapata, N.; Mfinanga, S.; Hasnain, S.E.; Katoto, P.; et al. Global Tuberculosis Report 2020-Reflections on the Global TB burden, treatment and prevention efforts. Int. J. Infect. Dis. 2021, 113 (Suppl. S1), S7–S12. [Google Scholar] [CrossRef]
- Chotmongkol, V.; Wanitpongpun, C.; Phuttharak, W.; Khamsai, S. Intramedullary Conus Medullaris Tuberculoma: A Case Report and Review of the Literature. Infect. Dis. Rep. 2021, 13, 82–88. [Google Scholar] [CrossRef]
- Zhao, C.; Luo, L.; Liu, L.; Li, P.; Liang, L.; Gao, Y.; Luo, F.; Xu, J.; Zhou, Q. Surgical management of consecutive multisegment thoracic and lumbar tuberculosis: Anterior-only approach vs. posterior-only approach. J. Orthop. Surg. Res. 2020, 15, 343. [Google Scholar] [CrossRef] [PubMed]
- Garg, R.K.; Somvanshi, D.S. Spinal tuberculosis: A review. J. Spinal Cord Med. 2011, 34, 440–454. [Google Scholar] [CrossRef]
- Al-Mahmood, M.R. A case report of a young woman with spinal tuberculosis Pharmacological managements and rehabilitation approach (CARE-compliant). Medicine 2022, 3, e0261. [Google Scholar] [CrossRef]
- Shen, J.; Zheng, Q.; Wang, Y.; Ying, X. One-stage combined anterior-posterior surgery for thoracic and lumbar spinal tuberculosis. J. Spinal Cord Med. 2021, 44, 54–61. [Google Scholar] [CrossRef]
- Swari, R.P.; Wahyudana, I.N.G. Spinal Tuberculosis: A Rare Case Report in a 52-year-old Female without a History of Pulmonary Tuberculosis. Neurol. Spinale Med. Chir. 2023, 6, 23–25. [Google Scholar] [CrossRef]
- Dahlan, R.H.; Ompusunggu, S.E.; Gondowardojo, Y.R.B.; Priambodo, R.; Anugerah, S.W. Spinal tuberculosis: A case series and a literature review. Surg. Neurol. Int. 2022, 13, 196. [Google Scholar] [CrossRef]
- Morris, J.M.; Wentworth, A.; Houdek, M.T.; Karim, S.M.; Clarke, M.J.; Daniels, D.J.; Rose, P.S. The Role of 3D Printing in Treatment Planning of Spine and Sacral Tumors. Neuroimaging Clin. N. Am. 2023, 33, 507–529. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, J.T.; Hsu, W.K. Three-Dimensional Printing in Minimally Invasive Spine Surgery. Curr. Rev. Musculoskelet. Med. 2019, 12, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Eltes, P.E.; Kiss, L.; Bartos, M.; Gyorgy, Z.M.; Csakany, T.; Bereczki, F.; Lesko, V.; Puhl, M.; Varga, P.P.; Lazary, A. Geometrical accuracy evaluation of an affordable 3D printing technology for spine physical models. J. Clin. Neurosci. 2020, 72, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Timofticiuc, I.-A.; Călinescu, O.; Iftime, A.; Dragosloveanu, S.; Caruntu, A.; Scheau, A.-E.; Badarau, I.A.; Didilescu, A.C.; Caruntu, C.; Scheau, C. Biomaterials Adapted to Vat Photopolymerization in 3D Printing: Characteristics and Medical Applications. J. Funct. Biomater. 2024, 15, 7. [Google Scholar] [CrossRef]
- Periferakis, A.; Periferakis, A.T.; Troumpata, L.; Dragosloveanu, S.; Timofticiuc, I.A.; Georgatos-Garcia, S.; Scheau, A.E.; Periferakis, K.; Caruntu, A.; Badarau, I.A.; et al. Use of Biomaterials in 3D Printing as a Solution to Microbial Infections in Arthroplasty and Osseous Reconstruction. Biomimetics 2024, 9, 154. [Google Scholar] [CrossRef]
- Kabra, A.; Mehta, N.; Garg, B. 3D printing in spine care: A review of current applications. J. Clin. Orthop. Trauma 2022, 35, 102044. [Google Scholar] [CrossRef]
- Dragosloveanu, S.; Petre, M.A.; Gherghe, M.E.; Nedelea, D.G.; Scheau, C.; Cergan, R. Overall Accuracy of Radiological Digital Planning for Total Hip Arthroplasty in a Specialized Orthopaedics Hospital. J. Clin. Med. 2023, 12, 4503. [Google Scholar] [CrossRef]
- Lan, Q.; Li, S.; Zhang, J.; Guo, H.; Yan, L.; Tang, F. Reliable prediction of implant size and axial alignment in AI-based 3D preoperative planning for total knee arthroplasty. Sci. Rep. 2024, 14, 16971. [Google Scholar] [CrossRef]
- Ding, H.; Hai, Y.; Zhou, L.; Liu, Y.; Zhang, Y.; Han, C.; Zhang, Y. Clinical Application of Personalized Digital Surgical Planning and Precise Execution for Severe and Complex Adult Spinal Deformity Correction Utilizing 3D Printing Techniques. J. Pers. Med. 2023, 13, 602. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, Z.; Lan, J.; Tian, J.; Chen, F.; Miao, J. The treatment of tuberculosis in the upper thoracic spine using the small incision technique through the third rib. Front. Surg. 2023, 10, 1236611. [Google Scholar] [CrossRef]
- Vanino, E.; Tadolini, M.; Evangelisti, G.; Zamparini, E.; Attard, L.; Scolz, K.; Terzi, S.; Barbanti Brodano, G.; Girolami, M.; Pipola, V.; et al. Spinal tuberculosis: Proposed spinal infection multidisciplinary management project (SIMP) flow chart revision. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 1428–1434. [Google Scholar] [CrossRef] [PubMed]
- Compagnone, D.; Cecchinato, R.; Pezzi, A.; Langella, F.; Damilano, M.; Redaelli, A.; Vanni, D.; Lamartina, C.; Berjano, P.; Boriani, S. Diagnostic Approach and Differences between Spinal Infections and Tumors. Diagnostics 2023, 13, 2737. [Google Scholar] [CrossRef] [PubMed]
- Bruckner, J.; Edelstein, J. Orthotics: A Comprehensive Clinical Approach; Routledge: London, UK, 2024. [Google Scholar]
- Drago, G.; Pastorello, G.; Gallinaro, P.; Zanata, R.; Del Verme, J.; Stafa, A.; Giordan, E. Novel Polyethylene Terephthalate Screw Sleeve Implant: Salvage Treatment in a Case of Spine Instability after Vertebroplasty Failure. Medicines 2022, 10, 6. [Google Scholar] [CrossRef] [PubMed]
- Dragosloveanu, Ş.; Dragosloveanu, C.D.M.; Stanca, H.T.; Cotor, D.C.; Dragosloveanu, C.I.; Stoica, C.I. A new perspective towards failure of gamma nail systems. Exp. Ther. Med. 2020, 20, 216. [Google Scholar] [CrossRef] [PubMed]
- Schauvliege, H.; Du Bois, M.; Verlooy, J. Implant failure following pedicle based dynamic stabilization of the lumbar spine. Acta Orthop. Belg. 2021, 87, 191–196. [Google Scholar] [CrossRef]
- Zileli, M. Complication Avoidance in Spine Surgery. Acta Neurochir. Suppl. 2023, 130, 141–156. [Google Scholar] [CrossRef]
- Ansorge, A.; Betz, M.; Wetzel, O.; Burkhard, M.D.; Dichovski, I.; Farshad, M.; Uçkay, I. Perioperative Urinary Catheter Use and Association to (Gram-Negative) Surgical Site Infection after Spine Surgery. Infect. Dis. Rep. 2023, 15, 717–725. [Google Scholar] [CrossRef]
- Yamaura, I.; Yone, K.; Nakahara, S.; Nagamine, T.; Baba, H.; Uchida, K.; Komiya, S. Mechanism of destructive pathologic changes in the spinal cord under chronic mechanical compression. Spine 2002, 27, 21–26. [Google Scholar] [CrossRef]
- Amicosante, M.; Ciccozzi, M.; Markova, R. Rational use of immunodiagnostic tools for tuberculosis infection: Guidelines and cost effectiveness studies. New Microbiol. 2010, 33, 93. [Google Scholar]
- Faye, L.M.; Hosu, M.C.; Oostvogels, S.; Dippenaar, A.; Warren, R.M.; Sineke, N.; Vasaikar, S.; Apalata, T. The Detection of Mutations and Genotyping of Drug-Resistant Mycobacterium tuberculosis Strains Isolated from Patients in the Rural Eastern Cape Province. Infect. Dis. Rep. 2023, 15, 403–416. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nedelea, D.-G.; Vulpe, D.E.; Viscopoleanu, G.; Radulescu, A.C.; Mihailescu, A.A.; Gradinaru, S.; Orghidan, M.; Scheau, C.; Cergan, R.; Dragosloveanu, S. Progressive Thoracolumbar Tuberculosis in a Young Male: Diagnostic, Therapeutic, and Surgical Insights. Infect. Dis. Rep. 2024, 16, 1005-1016. https://doi.org/10.3390/idr16050080
Nedelea D-G, Vulpe DE, Viscopoleanu G, Radulescu AC, Mihailescu AA, Gradinaru S, Orghidan M, Scheau C, Cergan R, Dragosloveanu S. Progressive Thoracolumbar Tuberculosis in a Young Male: Diagnostic, Therapeutic, and Surgical Insights. Infectious Disease Reports. 2024; 16(5):1005-1016. https://doi.org/10.3390/idr16050080
Chicago/Turabian StyleNedelea, Dana-Georgiana, Diana Elena Vulpe, George Viscopoleanu, Alexandru Constantin Radulescu, Alexandra Ana Mihailescu, Sebastian Gradinaru, Mihnea Orghidan, Cristian Scheau, Romica Cergan, and Serban Dragosloveanu. 2024. "Progressive Thoracolumbar Tuberculosis in a Young Male: Diagnostic, Therapeutic, and Surgical Insights" Infectious Disease Reports 16, no. 5: 1005-1016. https://doi.org/10.3390/idr16050080
APA StyleNedelea, D.-G., Vulpe, D. E., Viscopoleanu, G., Radulescu, A. C., Mihailescu, A. A., Gradinaru, S., Orghidan, M., Scheau, C., Cergan, R., & Dragosloveanu, S. (2024). Progressive Thoracolumbar Tuberculosis in a Young Male: Diagnostic, Therapeutic, and Surgical Insights. Infectious Disease Reports, 16(5), 1005-1016. https://doi.org/10.3390/idr16050080








