High Transferability of Neutralizing Antibodies against SARS-CoV-2 to Umbilical Cord Blood in Pregnant Women Vaccinated with BNT162b2 XBB.1.5: A Retrospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Enzyme-Linked Immunosorbent Assay (ELISA)
2.2. Clinical Research
2.3. Informed Consent
2.4. Institutional Review Board Approval
2.5. Ethical Compliance with Participants
2.6. Statistical Analysis
2.7. Data Availability
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Callaway, E. Coronavirus variant XBB.1.5 rises in the United States—Is it a global threat? Nature 2023, 613, 222–223. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Twentyman, E.; Koumans, E.; Rosenblum, H.; Griffin-Blake, S.; Jackson, B.; Vagi, S. Information for Persons Who Are Immunocompromised Regarding Prevention and Treatment of SARS-CoV-2 Infection in the Context of Currently Circulating Omicron Sublineages—United States, January 2023. MMWR Morb. Mortal Wkly. Rep. 2023, 72, 128–131. [Google Scholar] [CrossRef]
- Uraki, R.; Ito, M.; Kiso, M.; Yamayoshi, S.; Iwatsuki-Horimoto, K.; Sakai-Tagawa, Y.; Furusawa, Y.; Imai, M.; Koga, M.; Yamamoto, S.; et al. Efficacy of antivirals and bivalent mRNA vaccines against SARS-CoV-2 isolate CH.1.1. Lancet Infect. Dis. 2023, 23, 525–526. [Google Scholar] [CrossRef]
- Perl, S.H.; Uzan-Yulzari, A.; Klainer, H.; Asiskovich, L.; Youngster, M.; Rinott, E.; Youngster, I. SARS-CoV-2-Specific Antibodies in Breast Milk After COVID-19 Vaccination of Breastfeeding Women. JAMA 2021, 325, 2013–2014. [Google Scholar] [CrossRef]
- Munoz, F.M. Can We Protect Pregnant Women and Young Infants from COVID-19 through Maternal Immunization? JAMA Pediatr. 2021, 175, 561–562. [Google Scholar] [CrossRef]
- Gray, K.J.; Bordt, E.A.; Atyeo, C.; Deriso, E.; Akinwunmi, B.; Young, N.; Baez, A.M.; Shook, L.L.; Cvrk, D.; James, K.; et al. Coronavirus disease 2019 vaccine response in pregnant and lactating women: A cohort study. Am. J. Obstet. Gynecol. 2021, 225, 303.e1–303.e17. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, M.; Murphy, E.A.; Sukhu, A.C.; Yee, J.; Singh, S.; Eng, D.; Zhao, Z.; Riley, L.E.; Yang, Y.J. Antibody response to coronavirus disease 2019 (COVID-19) messenger RNA vaccination in pregnant women and transplacental passage into cord blood. Obstet. Gynecol. 2021, 138, 278–280. [Google Scholar] [CrossRef] [PubMed]
- Beharier, O.; Mayo, R.P.; Raz, T.; Sacks, K.N.; Schreiber, L.; Suissa-Cohen, Y.; Chen, R.; Gomez-Tolub, R.; Hadar, E.; Gabbay-Benziv, R.; et al. Efficient maternal to neonatal transfer of antibodies against SARS-CoV-2 and BNT162b2 mRNA COVID-19 vaccine. J. Clin. Investig. 2021, 131, e150319. [Google Scholar] [CrossRef]
- Mithal, L.B.; Otero, S.; Shanes, E.D.; Goldstein, J.A.; Miller, E.S. Cord blood antibodies following maternal coronavirus disease 2019 vaccination during pregnancy. Am. J. Obstet. Gynecol. 2021, 225, 192–194. [Google Scholar] [CrossRef]
- What Is the Effectiveness of the Omicron Strain Vaccine? Comparing Side Effects of Omicron Strain Compatible Vaccine with Conventional Type. Japan Broadcasting Corporation. 2022. December 19. Available online: https://www.nhk.or.jp/shutoken/newsup/20221219a.html (accessed on 19 December 2022).
- Focused Survey at the Beginning of Administration of the New Coronavirus Vaccine (Cohort Survey). Ministry of Health, Labor and Welfare of Japan. Available online: https://www.mhlw.go.jp/content/10906000/000752514.pdf (accessed on 12 March 2021).
- Shook, L.L.; Atyeo, C.G.; Yonker, L.M.; Fasano, A.; Gray, K.J.; Alter, G.; Edlow, A.G. Durability of Anti-Spike Antibodies in Infants After Maternal COVID-19 Vaccination or Natural Infection. JAMA 2022, 327, 1087–1089. [Google Scholar] [CrossRef]
- Kugelman, N.; Nahshon, C.; Shaked-Mishan, P.; Cohen, N.; Sher, M.L.; Gruber, M.; Marom, I.; Zolotarevsky, A.; Lavie, O.; Damti, A.; et al. Maternal and Neonatal SARS-CoV-2 Immunoglobulin G Antibody Levels at Delivery after Receipt of the BNT162b2 Messenger RNA COVID-19 Vaccine during the Second Trimester of Pregnancy. JAMA Pediatr. 2022, 176, 290–295. [Google Scholar] [CrossRef]
- Jorgensen, S.C.J.; Drover, S.S.M.; Fell, D.B.; Austin, P.C.; D’souza, R.; Guttmann, A.; Buchan, S.A.; Wilson, S.E.; Nasreen, S.; Schwartz, K.L.; et al. Newborn and Early Infant Outcomes Following Maternal COVID-19 Vaccination during Pregnancy. JAMA Pediatr. 2023, 177, 1314–1323. [Google Scholar] [CrossRef] [PubMed]
- Goldshtein, I.; Steinberg, D.M.; Kuint, J.; Chodick, G.; Segal, Y.; Ben David, S.S.; Ben-Tov, A. Association of BNT162b2 COVID-19 Vaccination during Pregnancy with Neonatal and Early Infant Outcomes. JAMA Pediatr. 2022, 176, 470–477. [Google Scholar] [CrossRef]
- Naseh, A.; Ashrafzadeh, S. Possible Vertical Transmission From an Unsuspected SARS-CoV-2-Infected Mother to Her Newborn. Cureus 2021, 13, e15717. [Google Scholar] [CrossRef] [PubMed]
- Vivanti, A.J.; Vauloup-Fellous, C.; Prevot, S.; Zupan, V.; Suffee, C.; Do Cao, J.; Benachi, A.; De Luca, D. Transplacental transmission of SARS-CoV-2 infection. Nat. Commun. 2020, 11, 3572. [Google Scholar] [CrossRef]
- Narayanaswamy, V.; Pentecost, B.T.; Schoen, C.N.; Alfandari, D.; Schneider, S.S.; Baker, R.; Arcaro, K.F. Neutralizing Antibodies and Cytokines in Breast Milk After Coronavirus Disease 2019 (COVID-19) mRNA Vaccination. Obstet Gynecol. 2022 Feb 1;139(2):181-191. [CrossRef]
- Ma, W.; Yang, J.; Fu, H.; Su, C.; Yu, C.; Wang, Q.; de Vasconcelos, A.T.R.; Bazykin, G.A.; Bao, Y.; Li, M. Genomic Perspectives on the Emerging SARS-CoV-2 Omicron Variant. Genom. Proteom. Bioinform. 2022, 20, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Callaway, E. Heavily mutated Omicron variant puts scientists on alert. Nature 2021, 600, 21. [Google Scholar] [CrossRef] [PubMed]
- Seekircher, L.; Bánki, Z.; Kimpel, J.; Rössler, A.; Schäfer, H.; Falkensammer, B.; Bante, D.; Forer, L.; Schönherr, S.; Harthaller, T.; et al. Immune response after two doses of the BNT162b2 COVID-19 vaccine and risk of SARS-CoV-2 breakthrough infection in Tyrol, Austria: An open-label, observational phase 4 trial. Lancet Microbe 2023, 4, e612–e621. [Google Scholar] [CrossRef]
- Modjarrad, k. Monovalent XBB.1.5 BNT162b2 COVID-19 Vaccine. Available online: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2023-09-12/10-covid-modjarrad-508.pdf (accessed on 12 September 2023).
- Macchiaverni, P.; Lloyd, M.; Masters, L.; Divakara, N.; Panta, K.; Imrie, A.; Sánchez-García, L.; Pellicer, A.; Rodriguez, J.M.; Verhasselt, V. Specific IgA, but Not IgG, in Human Milk from COVID-19-infected Mothers Neutralizes SARS-CoV-2. Pediatr. Infect. Dis. J. 2024, 43, 532–535. [Google Scholar] [CrossRef]
- Juncker, H.G.; van Doesburg, M.; de Groot, C.J.; Pajkrt, D.; Korosi, A.; van Gils, M.J.; van Goudoever, J.B.; van Keulen, B.J. Physical activity in lactating women influences SARS-CoV-2-specific antibodies in human milk. Heliyon 2023, 9, e19218. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Gazit, S.; Saciuk, Y.; Perez, G.; Peretz, A.; Ben-Tov, A.; Stuart, E.A.; Patalon, T. Hybrid immunity against reinfection with SARS-CoV-2 following a previous SARS-CoV-2 infection and single dose of the BNT162b2 vaccine in children and adolescents: A target trial emulation. Lancet Microbe 2023, 4, e495–e505. [Google Scholar] [CrossRef] [PubMed]
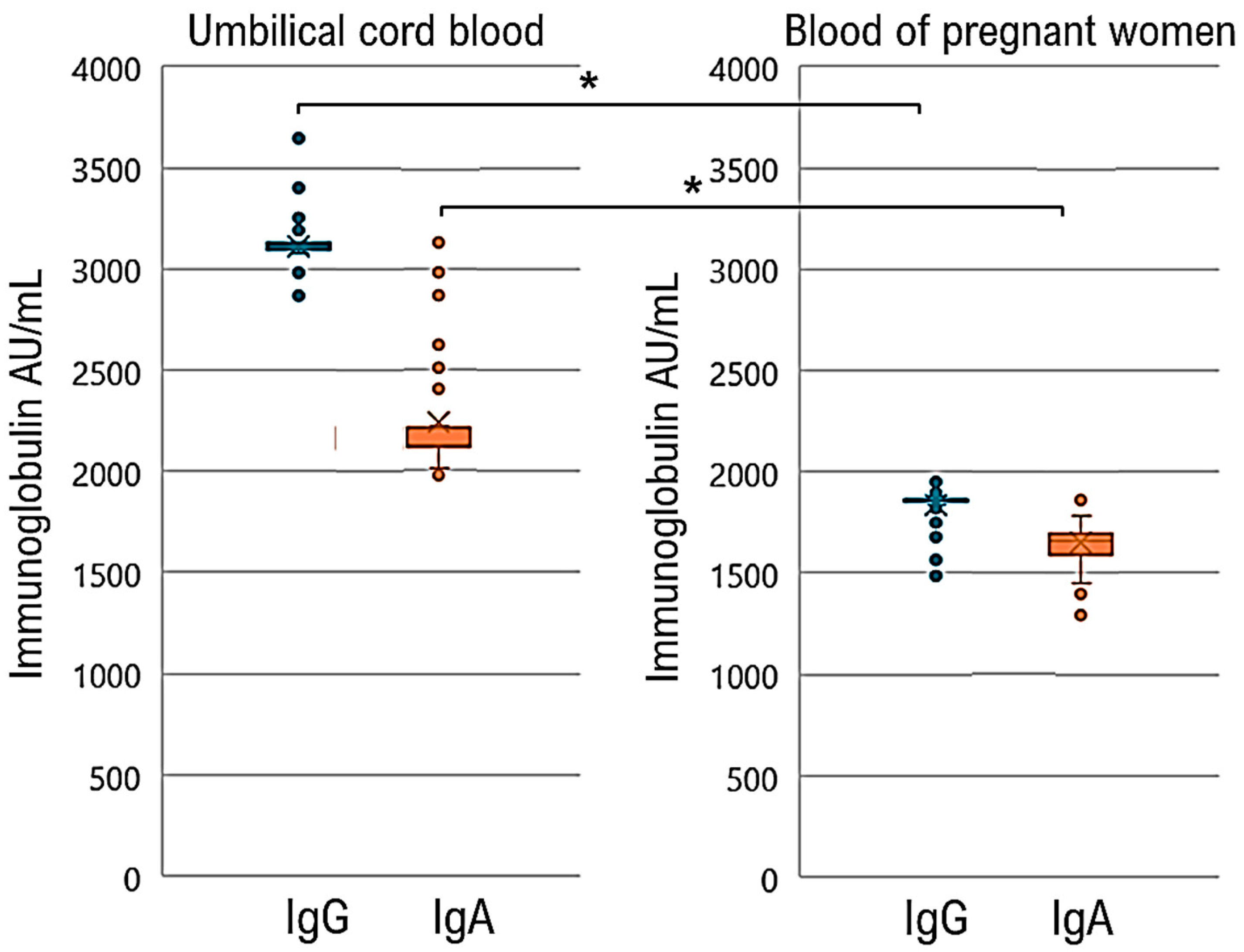
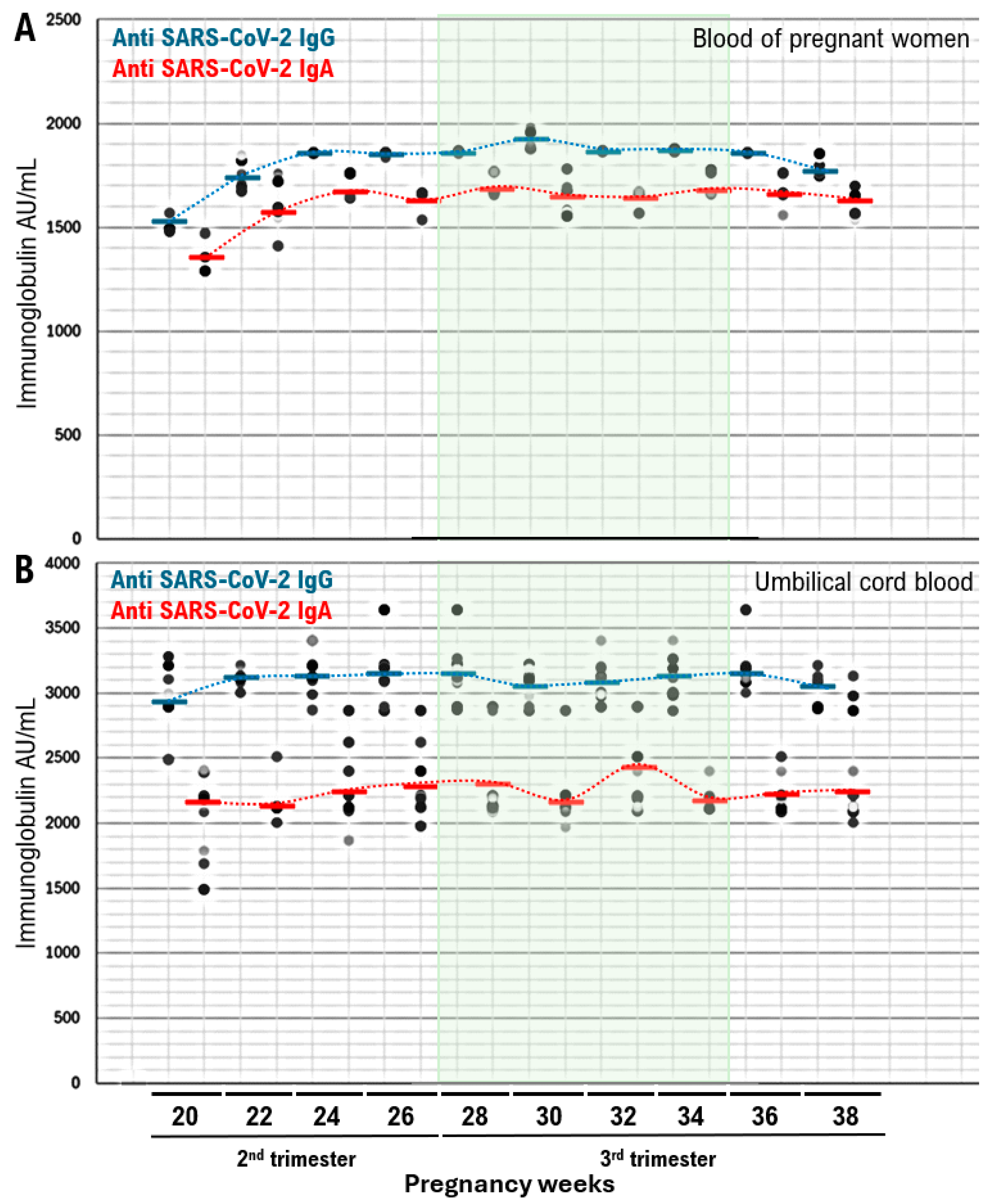
| Characteristics | Women (N = 148) a |
|---|---|
| Maternal age, mean (SD), years | 36.9 (4.9) |
| Body mass index, median (IQR) b | 26.5 (23.9–30.1) |
| Underlying systemic disease c | 29 (19.86) |
| Japanese | 145 (99.31) |
| White people | 1 (0.68) |
| Primipara | 59 (40.41) |
| Multipara (2 births) | 108 (73.97) |
| Grand multipara (5 births) | 4 (2.74) |
| Systemic adverse effects after first vaccination d | 35 (23.97) |
| Systemic adverse effects after second vaccination d | 36 (24.32) |
| Gestational age at second vaccine dose, mean (5D), week | 25.6 (3.5) |
| Systemic adverse effects d | |
| After second vaccination | 63 (43.15) |
| After first or second vaccination | 68 (46.58) |
| Gestational age at birth, mean (SD), week | 39.1 (1.4) |
| Duration from second vaccination to birth, mean (SD), week | 14.4 (3.1) |
| SARS-CoV-2 lgG, IgA antibody level, median (range), AU/mL | |
| lntrapartum maternal IgG, IgA | IgG 3115.8 (2867–3403) IgA 2237.5 (1970–3128) |
| Umbilical cord blood IgG, IgA | IgG 1837.34 (1490–1954) IgA 1651.04 (1290–1780) |
| Newborn sex | |
| Male | 77 (52.73) |
| Female | 69 (47.27) |
| Newborn weight, mean (SD), g | 3145.3 (412.6) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayashi, T.; Sano, K.; Konishi, I. High Transferability of Neutralizing Antibodies against SARS-CoV-2 to Umbilical Cord Blood in Pregnant Women Vaccinated with BNT162b2 XBB.1.5: A Retrospective Cohort Study. Infect. Dis. Rep. 2024, 16, 481-490. https://doi.org/10.3390/idr16030036
Hayashi T, Sano K, Konishi I. High Transferability of Neutralizing Antibodies against SARS-CoV-2 to Umbilical Cord Blood in Pregnant Women Vaccinated with BNT162b2 XBB.1.5: A Retrospective Cohort Study. Infectious Disease Reports. 2024; 16(3):481-490. https://doi.org/10.3390/idr16030036
Chicago/Turabian StyleHayashi, Takuma, Kenji Sano, and Ikuo Konishi. 2024. "High Transferability of Neutralizing Antibodies against SARS-CoV-2 to Umbilical Cord Blood in Pregnant Women Vaccinated with BNT162b2 XBB.1.5: A Retrospective Cohort Study" Infectious Disease Reports 16, no. 3: 481-490. https://doi.org/10.3390/idr16030036
APA StyleHayashi, T., Sano, K., & Konishi, I. (2024). High Transferability of Neutralizing Antibodies against SARS-CoV-2 to Umbilical Cord Blood in Pregnant Women Vaccinated with BNT162b2 XBB.1.5: A Retrospective Cohort Study. Infectious Disease Reports, 16(3), 481-490. https://doi.org/10.3390/idr16030036







