The Importance of COVID-19/Influenza Vaccines Co-Administration: An Essential Public Health Tool
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Sample Processing
2.3. Immune Response Evaluation
2.4. Personal Opinions Evaluation
2.5. Statistical Analyses
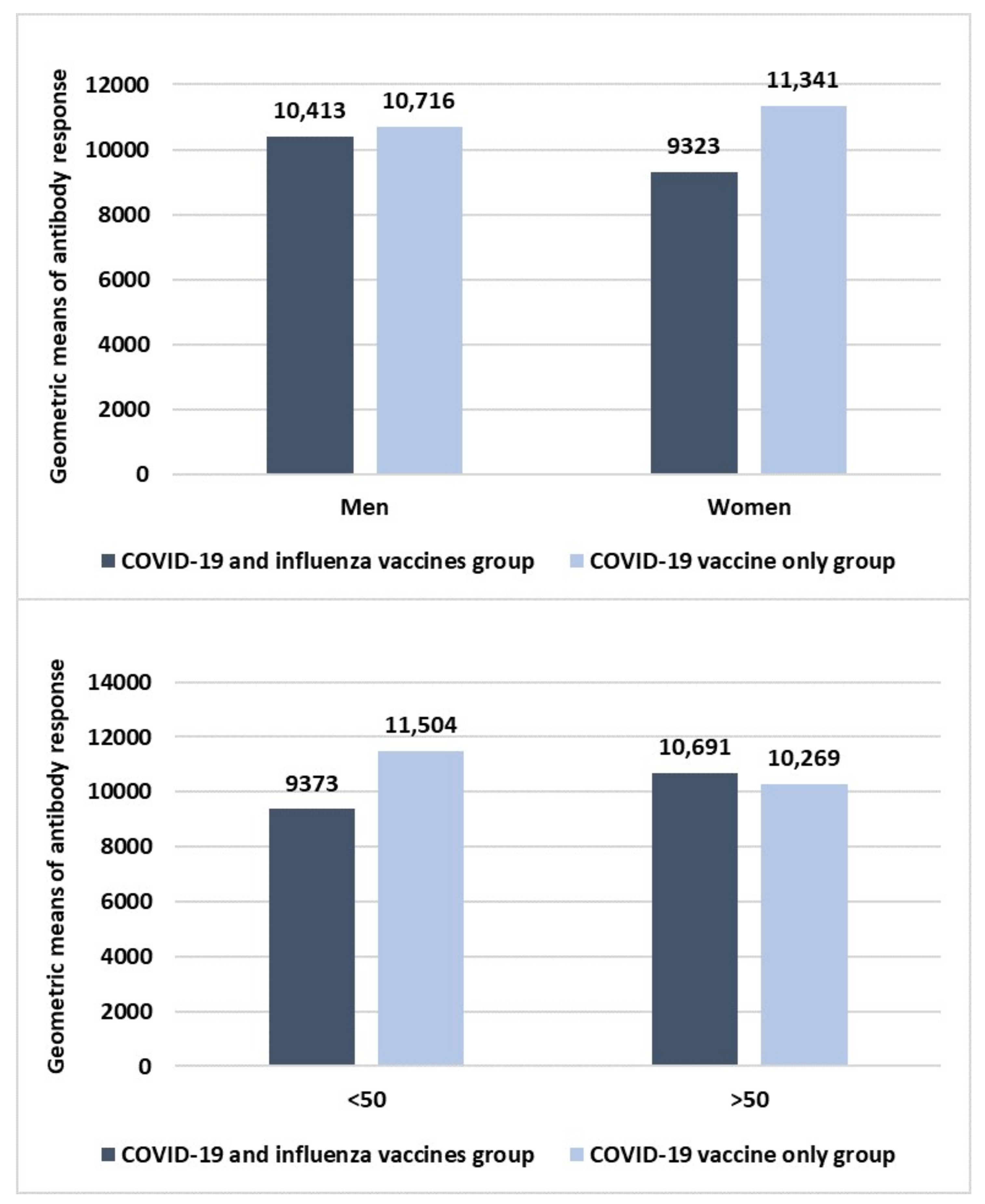
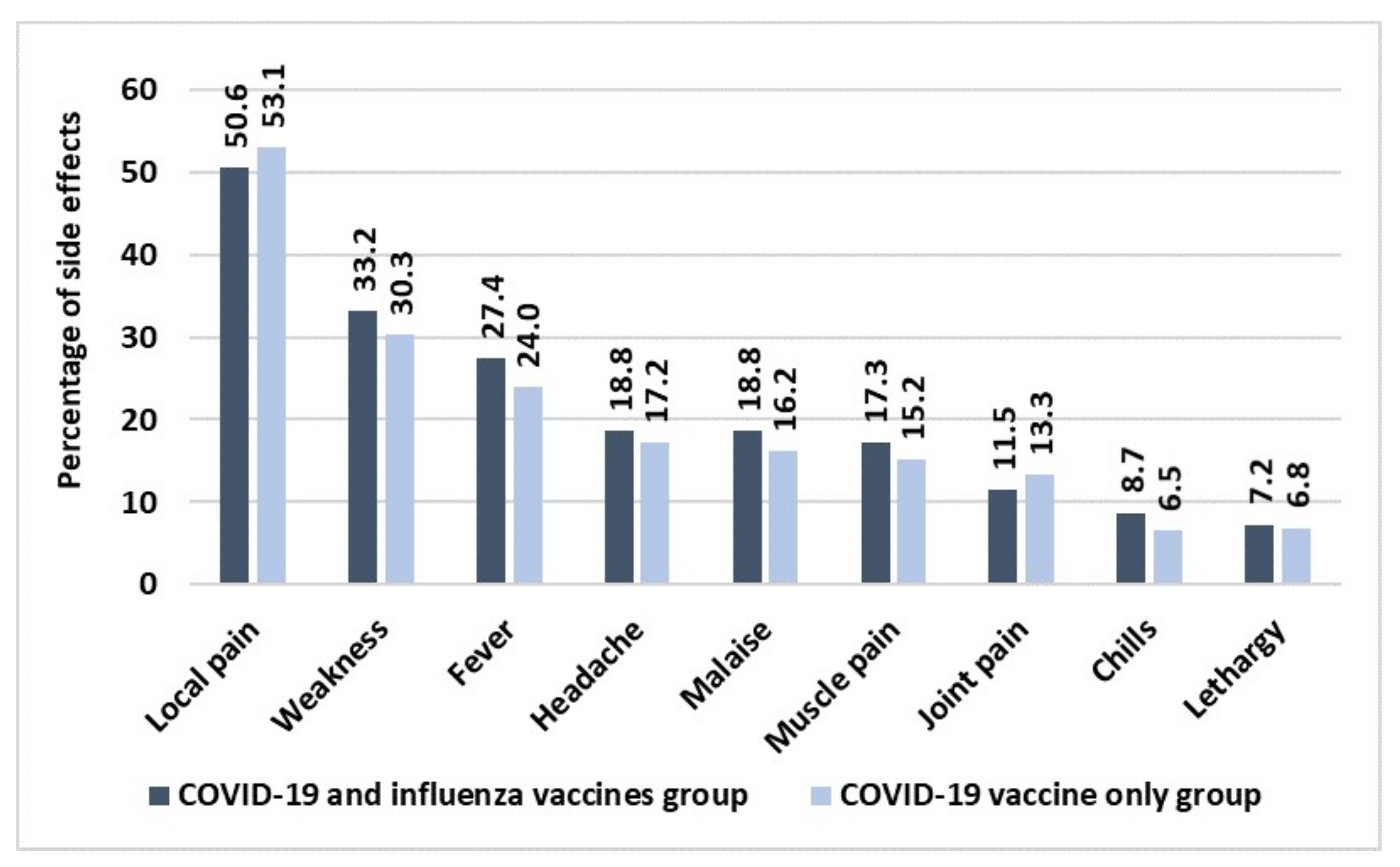
3. Results
4. Discussion and Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rosano, A.; Bella, A.; Gesualdo, F.; Acampora, A.; Pezzotti, P.; Marchetti, S.; Ricciardi, W.; Rizzo, C. Investigating the impact of influenza on excess mortality in all ages in Italy during recent seasons (2013/14-2016/17 seasons). Int. J. Infect. Dis. 2019, 88, 127–134. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). WHO Coronavirus (COVID-19) Dashboard. 2019. Available online: https://covid19.who.int/ (accessed on 20 August 2022).
- Coppelli, A.; Giannarelli, R.; Aragona, M.; Penno, G.; Falcone, M.; Tiseo, G.; Ghiadoni, L.; Barbieri, G.; Monzani, F.; Virdis, A.; et al. Hyperglycemia at Hospital Admission Is Associated With Severity of the Prognosis in Patients Hospitalized for COVID-19: The Pisa COVID-19 Study. Diabetes Care 2020, 43, 2345–2348. [Google Scholar] [CrossRef] [PubMed]
- Balik, M.; Svobodova, E.; Porizka, M.; Maly, M.; Brestovansky, P.; Volny, L.; Brozek, T.; Bartosova, T.; Jurisinova, I.; Mevaldova, Z.; et al. The impact of obesity on the outcome of severe SARS-CoV-2 ARDS in a high volume ECMO centre: ECMO and corticosteroids support the obesity paradox. J. Crit. Care 2022, 72, 154162. [Google Scholar] [CrossRef] [PubMed]
- Facciolà, A.; Micali, C.; Visalli, G.; Venanzi Rullo, E.; Russotto, Y.; Laganà, P.; Laganà, A.; Nunnari, G.; Di Pietro, A. COVID-19 and pregnancy: Clinical outcomes and scientific evidence about vaccination. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 2610–2626. [Google Scholar] [PubMed]
- Facciolà, A.; D’Amato, S.; Calimeri, S.; Giudice, D.L.; Micali, C.; Russotto, Y.; Venanzi Rullo, E.; Nunnari, G.; Squeri, R.; Pellicanò, G.F. Efficacy of COVID-19 Vaccination in People Living with HIV: A Public Health Fundamental Tool for the Protection of Patients and the Correct Management of Infection. Infect. Dis. Rep. 2022, 14, 784–793. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.; Ouhtit, A.; Al Khatib, H.A.; Eid, A.H.; Mathew, S.; Nasrallah, G.K.; Emara, M.M.; Al Maslamani, M.A.; Yassine, H.M. Burden and disease pathogenesis of influenza and other respiratory viruses in diabetic patients. J. Infect. Public Health 2022, 15, 412–424. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Vaccines against Influenza WHO Position Paper-November 2012. Wkly. Epidemiol. Rec. 2012, 87, 461–476. [Google Scholar]
- Schaffer DeRoo, S.; Pudalov, N.J.; Fu, L.Y. Planning for a COVID-19 Vaccination Program. JAMA 2020, 323, 2458–2459. [Google Scholar] [CrossRef] [PubMed]
- Ministero Della Salute. Nota n. 45886, 8 Ottobre 2021. Aggiornamento delle Indicazioni Sulla Somministrazione di Dosi Addizionali e di Dosi “Booster” Nell’ambito Della Campagna di Vaccinazione Anti SARS-CoV-2/COVID19. Available online: https://www.trovanorme.salute.gov.it/norme/renderNormsanPdf?anno=2021&codLeg=83176&parte=1%20&serie=null (accessed on 1 September 2022).
- Squeri, R.; Levita, A.; Intelisano, R.; Costa, G.B.; Mancuso, G.; Grasso, L.; D’Amato, S.; Mazzitelli, F.; Squeri, A.; Midiri, A.; et al. Correct management and low rate of contagiousness of healthcare workers in a University Hospital in Southern Italy: From contact tracing to serological investigation. Acta Biomed. 2020, 91, 79–86. [Google Scholar] [PubMed]
- D’Amato, S.; Squeri, R.; La Fauci, V.; Pantò, G.; Esposito, E.M.; Denaro, F.; Visalli, G.; Giunta, I.; Venuto, R.; Privitera, A.; et al. COVID-19 serological evaluation in a cohort of Vaccinated and Seropositive healthcare workers. Acta Biomed. 2021, 92, e2021415. [Google Scholar] [PubMed]
- Ministero della Salute. Nota n. 14614, 8 Aprile 2021. Prevenzione e Controllo Dell’influenza: Raccomandazioni per la Stagione 2021–2022. Available online: https://www.trovanorme.salute.gov.it/norme/renderNormsanPdf?anno=2021&codLeg=79647&parte=1%20&serie=null (accessed on 1 September 2022).
- World Health Organization (WHO). Coadministration of Seasonal Inactivated Influenza and COVID-19 Vaccines. Interim Guidance. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccines-SAGE_recommendationcoadministration-influenza-vaccines (accessed on 13 September 2022).
- Ministero della Salute. Nota n. 44591, 2 Ottobre2021. Intervallo Temporale tra la Somministrazione dei Vaccini Anti-SARS-CoV2/COVID-19 e Altri Vaccini. Available online: https://www.trovanorme.salute.gov.it/norme/renderNormsanPdf?anno=2021&codLeg=83013&parte=1%20&serie=null (accessed on 19 September 2022).
- Gilchrist, S.A.; Nanni, A.; Levine, O. Benefits and effectiveness of administering pneumococcal polysaccharide vaccine with seasonal influenza vaccine: An approach for policymakers. Am. J. Public Health 2012, 102, 596–605. [Google Scholar] [CrossRef] [PubMed]
- Bonanni, P.; Boccalini, S.; Bechini, A.; Varone, O.; Matteo, G.; Sandri, F.; Gabutti, G. Co-administration of vaccines: A focus on tetravalent Measles-Mumps-Rubella-Varicella (MMRV) and meningococcal C conjugate vaccines. Hum. Vaccin. Immunother. 2020, 16, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Ministero della Salute. Coperture Vaccinazione Antinfluenzale. Available online: https://www.salute.gov.it/imgs/C_17_tavole_19_3_1_file.pdf (accessed on 20 September 2022).
- Facciolà, A.; Visalli, G.; Orlando, A.; Bertuccio, M.P.; Spataro, P.; Squeri, R.; Picerno, I.; Di Pietro, A. Vaccine hesitancy: An overview on parents’ opinions about vaccination and possible reasons of vaccine refusal. J. Public Health Res. 2019, 8, 1436. [Google Scholar] [CrossRef] [PubMed]
- Domnich, A.; Orsi, A.; Trombetta, C.S.; Guarona, G.; Panatto, D.; Icardi, G. COVID-19 and Seasonal Influenza Vaccination: Cross-Protection, Co-Administration, Combination Vaccines, and Hesitancy. Pharmaceuticals 2022, 15, 322. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, R.; Baos, S.; Cappel-Porter, H.; Carson-Stevens, A.; Clout, M.; Culliford, L.; Emmett, S.R.; Garstang, J.; Gbadamoshi, L.; Hallis, B.; et al. Safety and immunogenicity of concomitant administration of COVID-19 vaccines (ChAdOx1 or BNT162b2) with seasonal influenza vaccines in adults in the UK (ComFluCOV): A multicentre, randomised, controlled, phase 4 trial. Lancet 2021, 398, 2277–2287. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Interim Clinical Considerations for Use of COVID-19 Vaccines Currently Authorized in the United States. Available online: https://www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html#Coadministration (accessed on 25 September 2022).
- Ministero della Salute. FAQ—Influenza e Vaccinazione Antinfluenzale. Available online: https://www.salute.gov.it/portale/p5_1_1.jsp?lingua=italiano&faqArea=influenza&id=103 (accessed on 25 September 2022).
- Izikson, R.; Brune, D.; Bolduc, J.S.; Bourron, P.; Fournier, M.; Moore, T.M.; Pandey, A.; Perez, L.; Sater, N.; Shrestha, A.; et al. Safety and immunogenicity of a high-dose quadrivalent influenza vaccine administered concomitantly with a third dose of the mRNA-1273 SARS-CoV-2 vaccine in adults aged ≥ 65 years: A phase 2, randomised, open-label study. Lancet Respir. Med. 2022, 10, 392–402. [Google Scholar] [CrossRef] [PubMed]
- Vaccaro, M.; Bertino, L.; Squeri, R.; Genovese, C.; Isola, S.; Spatari, G.; Spina, E.; Cutroneo, P. Early atypical injection-site reactions to COVID-19 vaccine: A case series. J. Eur. Acad. Dermatol. Venereol. 2022, 36, e24–e26. [Google Scholar] [CrossRef] [PubMed]
- Stefanizzi, P.; Martinelli, A.; Bianchi, F.P.; Migliore, G.; Tafuri, S. Acceptability of the third dose of an-ti-SARS-CoV-2 vaccine co-administered with influenza vaccine: Preliminary data in a sample of Italian HCWs. Hum. Vaccines Immunother. 2021, 18, 1–2. [Google Scholar]
- Van Buynder, P.G.; Newbound, A.; MacIntyre, C.R.; Kennedy, A.T.; Clarke, C.; Anderson, J. Australian experience of the SH21 flu vaccination program during the COVID-19 vaccine program. Hum. Vaccines Immunother. 2021, 17, 4611–4616. [Google Scholar] [CrossRef] [PubMed]
- Phillips, N. The coronavirus is here to stay—Here’s what that means. Nature 2021, 590, 382–384. [Google Scholar] [CrossRef] [PubMed]

| Differences in Side Effects According to Sex | |||
|---|---|---|---|
| Men (%) | Women (%) | p value | |
| Local pain | 26.7 | 43.2 | 0.017692 |
| Weakness | 16.7 | 29.5 | 0.030157 |
| Headache | 15.0 | 9.1 | // |
| Fever | 16.7 | 20.5 | // |
| Malaise | 13.3 | 13.4 | // |
| Muscle pain | 10.0 | 13.6 | // |
| Joint pain | 5.0 | 11.4 | // |
| Chills | 5.0 | 6.8 | // |
| Lethargy | 3.3 | 6.8 | // |
| Differences in side effects according to type of flu vaccine used | |||
| Egg-based flu vaccine (%) | Culture cell-based flu vaccine (%) | p value | |
| Local pain | 39.0 | 26.7 | // |
| Weakness | 22.3 | 24.4 | // |
| Headache | 15.3 | 8.9 | // |
| Fever | 18.6 | 17.8 | // |
| Malaise | 11.9 | 13.3 | // |
| Muscle pain | 11.9 | 11.1 | // |
| Joint pain | 10.1 | 11.1 | // |
| Chills | 5.1 | 6.7 | // |
| Lethargy | 5.1 | 4.4 | // |
| Questions | Yes (%) | No (%) |
|---|---|---|
| Did you regret having co-administered COVID-19 and influenza vaccines? | 6.7 | 93.3 |
| Would you do it again? | 89.4 | 10.6 |
| Would you recommend it? | 85.6 | 14.4 |
| Would you be favourable to a single combined influenza-COVID-19 vaccine? | 80.8 | 19.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venuto, R.; Giunta, I.; Cortese, R.; Denaro, F.; Pantò, G.; Privitera, A.; D’Amato, S.; Genovese, C.; La Fauci, V.; Fedele, F.; et al. The Importance of COVID-19/Influenza Vaccines Co-Administration: An Essential Public Health Tool. Infect. Dis. Rep. 2022, 14, 987-995. https://doi.org/10.3390/idr14060098
Venuto R, Giunta I, Cortese R, Denaro F, Pantò G, Privitera A, D’Amato S, Genovese C, La Fauci V, Fedele F, et al. The Importance of COVID-19/Influenza Vaccines Co-Administration: An Essential Public Health Tool. Infectious Disease Reports. 2022; 14(6):987-995. https://doi.org/10.3390/idr14060098
Chicago/Turabian StyleVenuto, Roberto, Ioselita Giunta, Rosaria Cortese, Federica Denaro, Giuseppe Pantò, Antonino Privitera, Smeralda D’Amato, Cristina Genovese, Vincenza La Fauci, Francesco Fedele, and et al. 2022. "The Importance of COVID-19/Influenza Vaccines Co-Administration: An Essential Public Health Tool" Infectious Disease Reports 14, no. 6: 987-995. https://doi.org/10.3390/idr14060098
APA StyleVenuto, R., Giunta, I., Cortese, R., Denaro, F., Pantò, G., Privitera, A., D’Amato, S., Genovese, C., La Fauci, V., Fedele, F., Ceccio, C., Squeri, R., & Facciolà, A. (2022). The Importance of COVID-19/Influenza Vaccines Co-Administration: An Essential Public Health Tool. Infectious Disease Reports, 14(6), 987-995. https://doi.org/10.3390/idr14060098







