Stress Hyperglycemia Ratio as a Prognostic Marker in Diabetic Patients Hospitalized with COVID-19
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Settings
2.2. Inclusion and Exclusion Criteria
2.3. Operational Definitions
2.4. Outcome Measures
2.5. Statistical Analysis
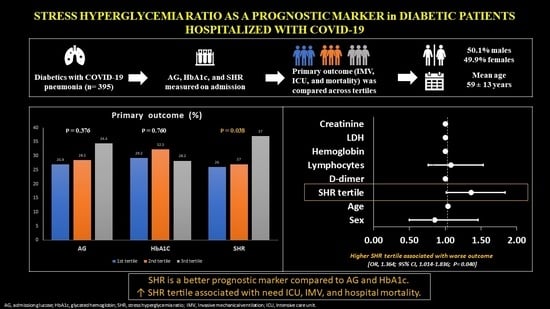
3. Results
3.1. Baseline Characteristics
3.2. Association between SHR and Outcomes
3.3. Association between the Other Glycemia Metrics and Outcomes
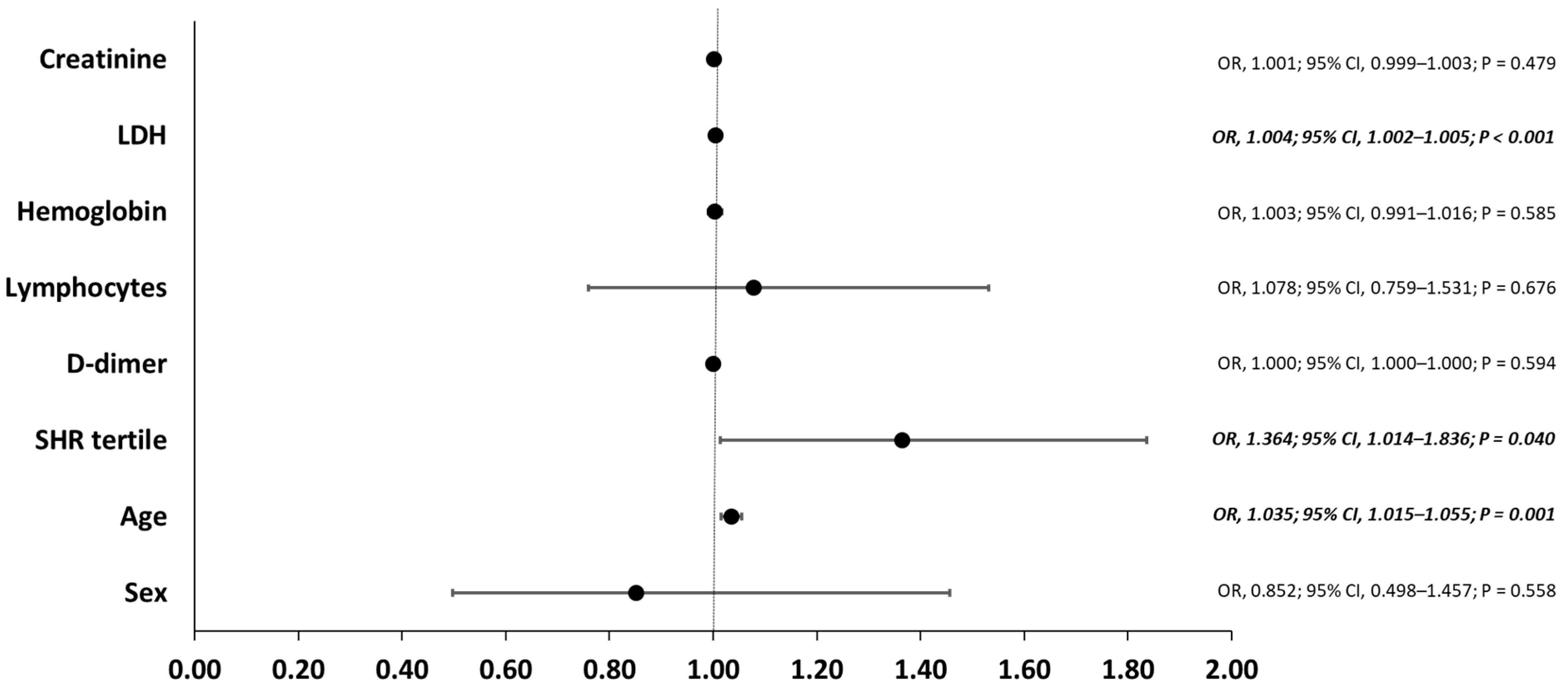
3.4. Risk Factors for the Primary Outcome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Naming the Coronavirus Disease (COVID-19) and the Virus That Causes It. 2020. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it (accessed on 18 February 2022).
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tan, Y.; Ling, Y.; Lu, G.; Liu, F.; Yi, Z.; Jia, X.; Wu, M.; Shi, B.; Xu, S.; et al. Viral and host factors related to the clinical outcome of COVID-19. Nature 2020, 583, 437–440. [Google Scholar] [CrossRef] [PubMed]
- Muller, L.M.A.J.; Gorter, K.J.; Hak, E.; Goudzwaard, W.L.; Schellevis, F.G.; Hoepelman, A.I.M.; Rutten, G.E.H.M. Increased Risk of Common Infections in Patients with Type 1 and Type 2 Diabetes Mellitus. Clin. Infect. Dis. 2005, 41, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, F.Y.; Aleanizy, F.S.; Mohamed, R.A.E.H.; Alanazi, M.S.; Mohamed, N.; Alrasheed, M.M.; Abanmy, N.; Alhawassi, T. Prevalence of comorbidities in cases of Middle East respiratory syndrome coronavirus: A retrospective study. Epidemiol. Infect. 2019, 147, e35. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.K.; Feng, Y.; Yuan, M.Y.; Yuan, S.Y.; Fu, H.J.; Wu, B.Y.; Sun, G.Z.; Yang, G.R.; Zhang, X.L.; Wang, L.; et al. Plasma glucose levels and diabetes are independent predictors for mortality and morbidity in patients with SARS. Diabet. Med. 2006, 23, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Roncon, L.; Zuin, M.; Rigatelli, G.; Zuliani, G. Diabetic patients with COVID-19 infection are at higher risk of ICU admission and poor short-term outcome. J. Clin. Virol. 2020, 127, 104354. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Petrilli, C.M.; Jones, S.A.; Yang, J.; Rajagopalan, H.; O’Donnell, L.; Chernyak, Y.; Tobin, K.A.; Cerfolio, R.J.; Francois, F.; Horwitz, L.I. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: Prospective cohort study. BMJ 2020, 369, m1966. [Google Scholar] [CrossRef] [PubMed]
- Al Saleh, M.; Alotaibi, N.; Schrapp, K.; Alsaber, A.; Pan, J.; Almutairi, F.; Abdullah, M.; Aboelhassan, W.; AlNasrallah, N.; Al-Bader, B.; et al. Risk Factors for Mortality in Patients with COVID-19: The Kuwait Experience. Med. Princ. Pract. 2022, 31, 180–186. [Google Scholar] [CrossRef]
- CDC. Underlying Medical Conditions Associated with Higher Risk for Severe COVID-19: Information for Healthcare Professionals. Centers for Disease Control and Prevention. 11 February 2020. Available online: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/underlyingconditions.html (accessed on 8 March 2022).
- NHS. Who is at High Risk from Coronavirus (COVID-19). nhs.uk. 1 March 2021. Available online: https://www.nhs.uk/conditions/coronavirus-covid-19/people-at-higher-risk/who-is-at-high-risk-from-coronavirus/ (accessed on 7 March 2022).
- Zhu, L.; She, Z.G.; Cheng, X.; Qin, J.J.; Zhang, X.J.; Cai, J.; Lei, F.; Wang, H.; Xie, J.; Wang, W.; et al. Association of Blood Glucose Control and Outcomes in Patients with COVID-19 and Pre-existing Type 2 Diabetes. Cell Metab. 2020, 31, 1068–1077.e3. [Google Scholar] [CrossRef]
- Cariou, B.; Hadjadj, S.; Wargny, M.; Pichelin, M.; Al-Salameh, A.; Allix, I.; Amadou, C.; Arnault, G.; Baudoux, F.; Bauduceau, B.; et al. Phenotypic characteristics and prognosis of inpatients with COVID-19 and diabetes: The CORONADO study. Diabetologia 2020, 63, 1500–1515. [Google Scholar] [CrossRef] [PubMed]
- Smati, S.; Tramunt, B.; Wargny, M.; Gourdy, P.; Hadjadj, S.; Cariou, B. COVID-19 and Diabetes Outcomes: Rationale for and Updates from the CORONADO Study. Curr. Diabetes Rep. 2022, 22, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Holman, N.; Knighton, P.; Kar, P.; O’Keefe, J.; Curley, M.; Weaver, A.; Barron, E.; Bakhai, C.; Khunti, K.; Wareham, N.J.; et al. Risk factors for COVID-19-related mortality in people with type 1 and type 2 diabetes in England: A population-based cohort study. Lancet Diabetes Endocrinol. 2020, 8, 823–833. [Google Scholar] [CrossRef]
- Shi, Q.; Zhang, X.; Jiang, F.; Zhang, X.; Hu, N.; Bimu, C.; Feng, J.; Yan, S.; Guan, Y.; Xu, D.; et al. Clinical Characteristics and Risk Factors for Mortality of COVID-19 Patients With Diabetes in Wuhan, China: A Two-Center, Retrospective Study. Diabetes Care 2020, 43, 1382–1391. [Google Scholar] [CrossRef]
- Wang, S.; Ma, P.; Zhang, S.; Song, S.; Wang, Z.; Ma, Y.; Xu, J.; Wu, F.; Duan, L.; Yin, Z.; et al. Fasting blood glucose at admission is an independent predictor for 28-day mortality in patients with COVID-19 without previous diagnosis of diabetes: A multi-centre retrospective study. Diabetologia 2020, 63, 2102–2111. [Google Scholar] [CrossRef]
- Wu, J.; Huang, J.; Zhu, G.; Wang, Q.; Lv, Q.; Huang, Y.; Yu, Y.; Si, X.; Yi, H.; Wang, C.; et al. Elevation of blood glucose level predicts worse outcomes in hospitalized patients with COVID-19: A retrospective cohort study. BMJ Open Diabetes Res Care 2020, 8, e001476. [Google Scholar] [CrossRef]
- Pescatore, J.M.; Sarmiento, J.; Hernandez-Acosta, R.A.; Skaathun, B.; Quesada-Rodriguez, N.; Rezai, K. Glycemic control is associated with lower odds of mortality and successful extubation in severe COVID-19. J. Osteopath Med. 2022, 122, 111–115. [Google Scholar] [CrossRef]
- Roberts, G.W.; Quinn, S.J.; Valentine, N.; Alhawassi, T.; O’Dea, H.; Stranks, S.N.; Burt, M.G.; Doogue, M.P. Relative Hyperglycemia, a Marker of Critical Illness: Introducing the Stress Hyperglycemia Ratio. J. Clin. Endocrinol. Metab. 2015, 100, 4490–4497. [Google Scholar] [CrossRef]
- American Diabetes Association. 15. Diabetes Care in the Hospital: Standards of Medical Care in Diabetes—2021. Diabetes Care 2021, 44 (Suppl. S1), S211–S220. [Google Scholar] [CrossRef]
- Korytkowski, M.; Antinori-Lent, K.; Drincic, A.; Hirsch, I.B.; McDonnell, M.E.; Rushakoff, R.; Muniyappa, R. A Pragmatic Approach to Inpatient Diabetes Management during the COVID-19 Pandemic. J. Clin. Endocrinol. Metab. 2020, 105, 3076–3087. [Google Scholar] [CrossRef]
- Nathan, D.M.; Kuenen, J.; Borg, R.; Zheng, H.; Schoenfeld, D.; Heine, R.J.; A1c-Derived Average Glucose (ADAG) Study Group. Translating the A1C Assay Into Estimated Average Glucose Values. Diabetes Care 2008, 31, 1473–1478. [Google Scholar] [CrossRef] [PubMed]
- WHO. Novel Coronavirus COVID-19 Therapeutic Trial Synopsis. 2020. Available online: https://www.who.int/publications/i/item/covid-19-therapeutic-trial-synopsis (accessed on 2 January 2022).
- Ramon, J.; Llauradó, G.; Güerri, R.; Climent, E.; Ballesta, S.; Benaiges, D.; López-Montesinos, I.; Navarro, H.; Fernández, N.; Carrera, M.J.; et al. Acute-to-Chronic Glycemic Ratio as a Predictor of COVID-19 Severity and Mortality. Diabetes Care 2022, 45, 255–258. [Google Scholar] [CrossRef]
- Apicella, M.; Campopiano, M.C.; Mantuano, M.; Mazoni, L.; Coppelli, A.; Del Prato, S. COVID-19 in people with diabetes: Understanding the reasons for worse outcomes. Lancet Diabetes Endocrinol. 2020, 8, 782–792. [Google Scholar] [CrossRef]
- Lim, S.; Bae, J.H.; Kwon, H.S.; Nauck, M.A. COVID-19 and diabetes mellitus: From pathophysiology to clinical management. Nat. Rev. Endocrinol. 2021, 17, 11–30. [Google Scholar] [CrossRef]
- Langouche, L.; Van den Berghe, G.; Gunst, J. Hyperglycemia and insulin resistance in COVID-19 versus non-COVID critical illness: Are they really different? Crit. Care 2021, 25, 437. [Google Scholar] [CrossRef] [PubMed]
- Drucker, D.J. Coronavirus Infections and Type 2 Diabetes-Shared Pathways with Therapeutic Implications. Endocr. Rev. 2020, 41, bnaa011. [Google Scholar] [CrossRef] [PubMed]
- Williamson, E.J.; Walker, A.J.; Bhaskaran, K.; Bacon, S.; Bates, C.; Morton, C.E.; Curtis, H.J.; Mehrkar, A.; Evans, D.; Inglesby, P.; et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020, 584, 430–436. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, D.; Cheng, B.; Chen, J.; Peng, A.; Yang, C.; Liu, C.; Xiong, M.; Deng, A.; Zhang, Y.; et al. Clinical Characteristics and Outcomes of Patients With Diabetes and COVID-19 in Association With Glucose-Lowering Medication. Diabetes Care 2020, 43, 1399–1407. [Google Scholar] [CrossRef]
- Al Argan, R.; Alkhafaji, D.; Al Elq, A.; Albaker, W.; Alqatari, S.; Alzaki, A.; Alwaheed, A.; Al Said, A.; Bukhari, H.; Al Warthan, S.; et al. The Impact of Diabetes Mellitus and Hyperglycemia on the Severity and Outcome of Patients with COVID-19 Disease: A Single-Center Experience. Int. J. Gen. Med. 2021, 14, 9445–9457. [Google Scholar] [CrossRef]
- Liao, W.I.; Wang, J.C.; Chang, W.C.; Hsu, C.W.; Chu, C.M.; Tsai, S.H. Usefulness of Glycemic Gap to Predict ICU Mortality in Critically Ill Patients With Diabetes. Medicine 2015, 94, e1525. [Google Scholar] [CrossRef]
- Fabbri, A.; Marchesini, G.; Benazzi, B.; Morelli, A.; Montesi, D.; Bini, C.; Rizzo, S.G. Stress Hyperglycemia and Mortality in Subjects With Diabetes and Sepsis. Crit. Care Explor. 2020, 2, e0152. [Google Scholar] [CrossRef] [PubMed]
- Dungan, K.M.; Braithwaite, S.S.; Preiser, J.C. Stress hyperglycaemia. Lancet 2009, 373, 1798–1807. [Google Scholar] [CrossRef]
- Mamtani, M.; Athavale, A.M.; Abraham, M.; Vernik, J.; Amarah, A.R.; Ruiz, J.P.; Joshi, A.J.; Itteera, M.; Zhukovski, S.D.; Madaiah, R.P.; et al. Association of hyperglycaemia with hospital mortality in nondiabetic COVID-19 patients: A cohort study. Diabetes Metab. 2021, 47, 101254. [Google Scholar] [CrossRef] [PubMed]
- Marenzi, G.; Cosentino, N.; Milazzo, V.; De Metrio, M.; Cecere, M.; Mosca, S.; Rubino, M.; Campodonico, J.; Moltrasio, M.; Marana, I.; et al. Prognostic Value of the Acute-to-Chronic Glycemic Ratio at Admission in Acute Myocardial Infarction: A Prospective Study. Diabetes Care 2018, 41, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Di Luzio, R.; Dusi, R.; Mazzotti, A.; Petroni, M.L.; Marchesini, G.; Bianchi, G. Stress Hyperglycemia and Complications Following Traumatic Injuries in Individuals with/without Diabetes: The Case of Orthopedic Surgery. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 9–17. [Google Scholar] [CrossRef]
- Yang, Y.; Kim, T.H.; Yoon, K.H.; Chung, W.S.; Ahn, Y.; Jeong, M.H.; Seung, K.B.; Lee, S.H.; Chang, K. The stress hyperglycemia ratio, an index of relative hyperglycemia, as a predictor of clinical outcomes after percutaneous coronary intervention. Int. J. Cardiol. 2017, 241, 57–63. [Google Scholar] [CrossRef]
| Total (n = 395) | SHR ≤ 0.89 (1st Tertile, n = 131) | SHR 0.90–1.22 (2nd Tertile, n = 137) | SHR ≥ 1.23 (3rd Tertile, n = 127) | p-Value | Normal Range | |
|---|---|---|---|---|---|---|
| Age (y), mean ± SD | 59.37 ± 13.33 | 60.17 ± 13.14 | 57.72 ± 11.6 | 60.32 ± 15.08 | 0.200 | |
| Sex, n (%) | ||||||
| Male Female | 198 (50.1) 197 (49.9) | 63 (48.1) 68 (51.9) | 72 (52.6) 65 (47.4) | 63 (49.6) 64 (50.4) | 0.758 | |
| Comorbidity, n (%) | ||||||
| Hypertension Renal disease ASCVD a Lung disease Cancer | 218 (55.2) 57 (14.4) 165 (41.8) 40 (10.1) 4 (1) | 84 (64.1) 16 (12.2) 49 (37.4) 13 (9.9) 0 (0) | 66 (48.2) 14 (10.2) 55 (40.1) 12 (8.8) 2 (1.5) | 68 (53.5) 27 (21.3) 61 (48) 15 (11.8) 2 (1.6) | 0.029 0.026 0.200 0.711 0.365 | |
| WBCs | 7 (5–9.5) | 6.5 (5–8.3) | 6.9 (4.8–8.7) | 7.6 (5.1–10.67) | 0.196 | 4–10 × 109/L |
| Neutrophils | 5.1 (3.6–7.5) | 4.4 (3.6–6.7) | 5.2 (3.5–7.2) | 5.75 (3.52–8.37) | 0.167 | 2–7 × 109/L |
| Lymphocytes | 1.1 (0.7–1.5) | 1.2 (0.7–1.5) | 1 (0.7–1.6) | 0.9 (0.6–1.4) | 0.039 | 1–3 × 109/L |
| Hemoglobin | 126 (113–139) | 129 (115–140) | 130 (114–140) | 124 (106.2–136.8) | 0.018 | 130–170 g/L |
| Platelets | 221 (172–275) | 215 (175–265) | 221 (177–287) | 215.5 (167–270) | 0.649 | 150–410 × 109/L |
| Creatinine | 88 (68–116.79) | 82 (65–106.5) | 87 (63–107) | 89 (70–139) | 0.220 | 57–113 mol/L |
| Albumin | 28.1 (25.6–30.8) | 28.6 (25.6–31.4) | 27.9 (26.1–30.7) | 27.4 (24.7–30.8) | 0.250 | 35–55 g/L |
| ALT | 29 (20–46) | 29.5 (20.25–45) | 28 (20–46) | 27 (19–47) | 0.804 | 8–41 IU/L |
| AST | 37 (28–55) | 38.5 (28–53) | 35 (28–63) | 32 (25–55) | 0.203 | 10–40 IU/L |
| Ferritin | 488.5 (240.6–852) | 428 (221.6–893) | 483 (211.1–863.2) | 483 (257.9–885) | 0.415 | 34–310 ng/ml |
| LDH | 307 (237–383) | 295 (228–371) | 309.5 (246.3–399.5) | 291.5 (220–397) | 0.603 | 95–200 IU/L |
| D-dimer | 342 (221–616.6) | 332.5 (187–651.5) | 286.7 (193.5–589.5) | 345 (243.5–665) | 0.185 | <232 ng/ml |
| Admission glucose | 12.2 (8.7–16.5) | 7.7 (6.4–10.2) | 11.5 (9.35–16) | 17.5 (14.3–21) | <0.001 | 4–7 mmol/L |
| HbA1c | 8.6 (7.1–10.6) | 8.4 (7–10.5) | 8.4 (7.1–10.75) | 8.8 (7–10.4) | 0.957 | <6.5% |
| eAG | 11.1 (8.7–14.3) | 10.8 (8.6–14.1) | 10.9 (8.7–14.6) | 11.4 (8.6–14) | 0.885 |
| Total (n = 395) | SHR 1st Tertile | SHR 2nd Tertile | SHR 3rd Tertile | p Value a | p Value b | |
|---|---|---|---|---|---|---|
| Admission scale c 3 4 5 | 61 (15.4) 329 (83.3) 5 (1.3) | 21 (16) 107 (81.7) 3 (2.3) | 20 (14.6) 115 (83.9) 2 (1.5) | 20 (15.7) 107 (84.3) 0 (0) | ||
| Primary outcome d | 118 (29.9) | 34 (26) | 37 (27) | 47 (37) | 0.038 | 0.054 |
| Clinical deterioration e | 104 (26.3) | 30 (22.9) | 34 (24.8) | 40 (31.5) | 0.121 | 0.227 |
| IMV | 102 (25.8) | 30 (22.9) | 32 (23.4) | 40 (31.5) | 0.121 | 0.138 |
| ICU | 118 (29.9) | 34 (26) | 37 (27) | 47 (37) | 0.038 | 0.054 |
| In-hospital mortality | 69 (17.5) | 20 (15.3) | 22 (16.1) | 27 (21.3) | 0.212 | 0.227 |
| Outcomes | Admission Glucose ≤ 9.60 mmol/L (1st Tertile, n = 134) | Admission Glucose 9.61–14.90 mmol/L (2nd Tertile, n = 130) | Admission Glucose ≥ 14.91 mmol/L (3rd Tertile, n = 131) | p-Value |
|---|---|---|---|---|
| Primary outcome a | 36 (26.9) | 37 (28.5) | 45 (34.4) | 0.376 |
| Clinical deterioration b | 31 (23.1) | 36 (27.7) | 37 (28.2) | 0.584 |
| IMV | 31 (23.1) | 34 (26.2) | 37 (28.2) | 0.633 |
| ICU | 36 (26.9) | 37 (28.5) | 45 (34.4) | 0.376 |
| In-hospital mortality | 19 (14.2) | 25 (19.2) | 25 (19.1) | 0.467 |
| HbA1c ≤ 7.5% (1st tertile, n = 137) | HbA1c 7.6–9.9% (2nd tertile, 127) | HbA1c ≥ 10% (3rd tertile, 131) | ||
| Primary outcome a | 40 (29.2) | 41 (32.3) | 37 (28.2) | 0.760 |
| Clinical deterioration b | 36 (26.3) | 37 (29.1) | 31 (23.7) | 0.608 |
| IMV | 35 (25.5) | 36 (28.3) | 31 (23.7) | 0.689 |
| ICU | 40 (29.2) | 41 (32.3) | 37 (28.2) | 0.760 |
| In-hospital mortality | 26 (19) | 21 (16.5) | 22 (16.8) | 0.846 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aon, M.; Alsaeedi, A.; Alzafiri, A.; Al-Shammari, A.; Taha, S.; Al-Shammari, O.; Tawakul, M.; Alshammari, J.; Alherz, N.; Alenezi, M.; et al. Stress Hyperglycemia Ratio as a Prognostic Marker in Diabetic Patients Hospitalized with COVID-19. Infect. Dis. Rep. 2022, 14, 675-685. https://doi.org/10.3390/idr14050073
Aon M, Alsaeedi A, Alzafiri A, Al-Shammari A, Taha S, Al-Shammari O, Tawakul M, Alshammari J, Alherz N, Alenezi M, et al. Stress Hyperglycemia Ratio as a Prognostic Marker in Diabetic Patients Hospitalized with COVID-19. Infectious Disease Reports. 2022; 14(5):675-685. https://doi.org/10.3390/idr14050073
Chicago/Turabian StyleAon, Mohamed, Abdullah Alsaeedi, Azeez Alzafiri, Abdelrahman Al-Shammari, Sherif Taha, Omar Al-Shammari, Mahmoud Tawakul, Jarrah Alshammari, Naser Alherz, Monerah Alenezi, and et al. 2022. "Stress Hyperglycemia Ratio as a Prognostic Marker in Diabetic Patients Hospitalized with COVID-19" Infectious Disease Reports 14, no. 5: 675-685. https://doi.org/10.3390/idr14050073
APA StyleAon, M., Alsaeedi, A., Alzafiri, A., Al-Shammari, A., Taha, S., Al-Shammari, O., Tawakul, M., Alshammari, J., Alherz, N., Alenezi, M., Eyadah, M., Aldhafeeri, M., Alharbi, T., Alshammari, D., Alenezi, Z., Aldouseri, S., Albazee, E., Ibrahim, M. M., & Aoun, A. H. (2022). Stress Hyperglycemia Ratio as a Prognostic Marker in Diabetic Patients Hospitalized with COVID-19. Infectious Disease Reports, 14(5), 675-685. https://doi.org/10.3390/idr14050073