1. Introduction
With nearly 180 million cases, the novel coronavirus disease 2019 (COVID-19) has significantly strained healthcare systems worldwide. Approximately 15–30% of patients can develop acute respiratory distress syndrome, requiring prolonged mechanical ventilation and lung recruitment strategies to overcome profound hypoxemia [
1]. Currently, immunomodulators have shown the most promise in reducing the morbidity and mortality of COVID-19 [
2,
3,
4,
5]. However, with the increased use of these medications, there is a heightened concern for immunosuppression and superinfections. In addition, COVID-19 has been shown to cause lymphopenia and impair cytokine circulation during the hyperinflammatory phase of the illness, which may increase the risks of concomitant infections [
6,
7]. In the past, superinfections in the acute setting were linked to increased ICU admissions, hospital length of stay, and mortality. Furthermore, this level of immunosuppression puts patients at an increased risk of bacterial, fungal, viral, and opportunistic infections.
Tuberculosis (TB) remains the number one infectious cause of death worldwide despite global efforts to eradicate the disease. Approximately 25% of the global population has a latent tuberculosis infection (LTBI) with an estimated 5–15% lifetime activation risk [
8]. As countries proceed towards a “new normal”, there may be an increased incidence of TB secondary to active or recovered COVID-19 infections, whether or not immunosuppressant medications are used. Additionally, given that patient access to screening and treatment of TB may be affected by strategies to mitigate the spread of COVID-19, there could be delays in the diagnosis of TB leading to an increased number of cases. Therefore, the World Health Organization (WHO) has recommended simultaneous testing for TB and COVID-19 when indicated as part of their strategy to improve treatment outcomes and reduce the spread of TB [
8]. Screening for LTBI prior to or after a treatment for COVID-19 is not currently recommended in the guidelines for COVID-19 management. This paper describes a case of active TB presenting shortly after a successful treatment of COVID-19.
2. Case Presentation
A 74-year-old Filipino female with a medical history significant for hypertension, hyperlipidemia, and non-insulin-dependent type II diabetes presented to the emergency department (ED) of a South Florida hospital with a one-week history of increased generalized weakness and worsening shortness of breath. Three months prior, the patient was treated in the hospital for COVID-19 with intravenous dexamethasone and remdesivir, and was discharged home with oxygen. She fully recovered to her baseline and no longer required supplemental oxygen two months before the above onset of symptoms. On arrival to the ED, she was lethargic, but not in severe distress. She had an oral temperature of 37.5 °C, a heart rate of 97 beats/min, blood pressure of 133/78 mmHg, a respiratory rate of 20 breaths/min, and oxygen saturation of 91% on 3 L nasal cannula supplemental oxygen. She had decreased breath sounds bilaterally and rales at the bases. Her heart sounds were distant without any murmurs and she had no peripheral edema. The initial laboratory results are available in
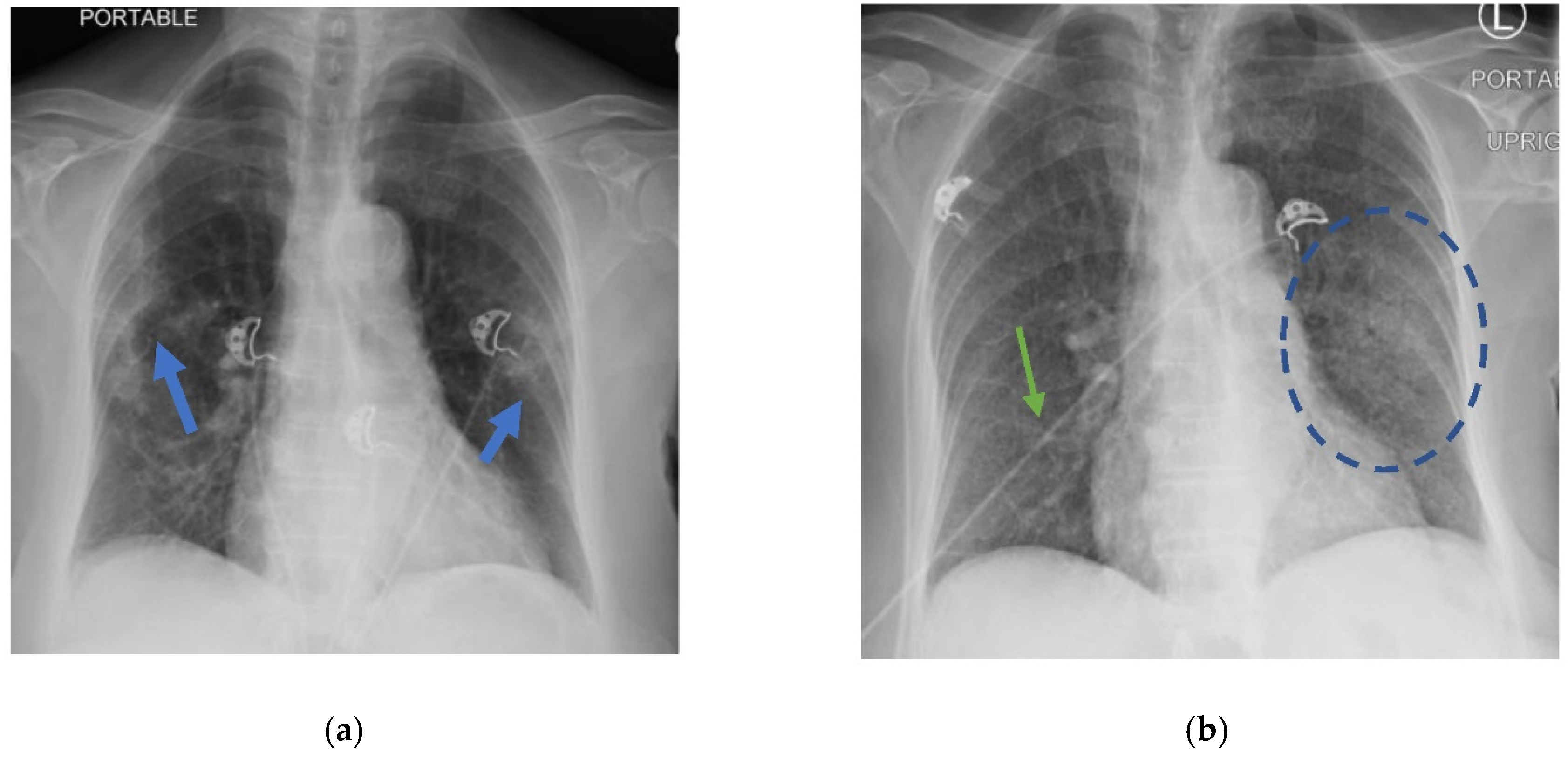
Table 1. Her chest X-ray showed a left mid-lung infiltrate that was new compared with the X-ray three months prior (
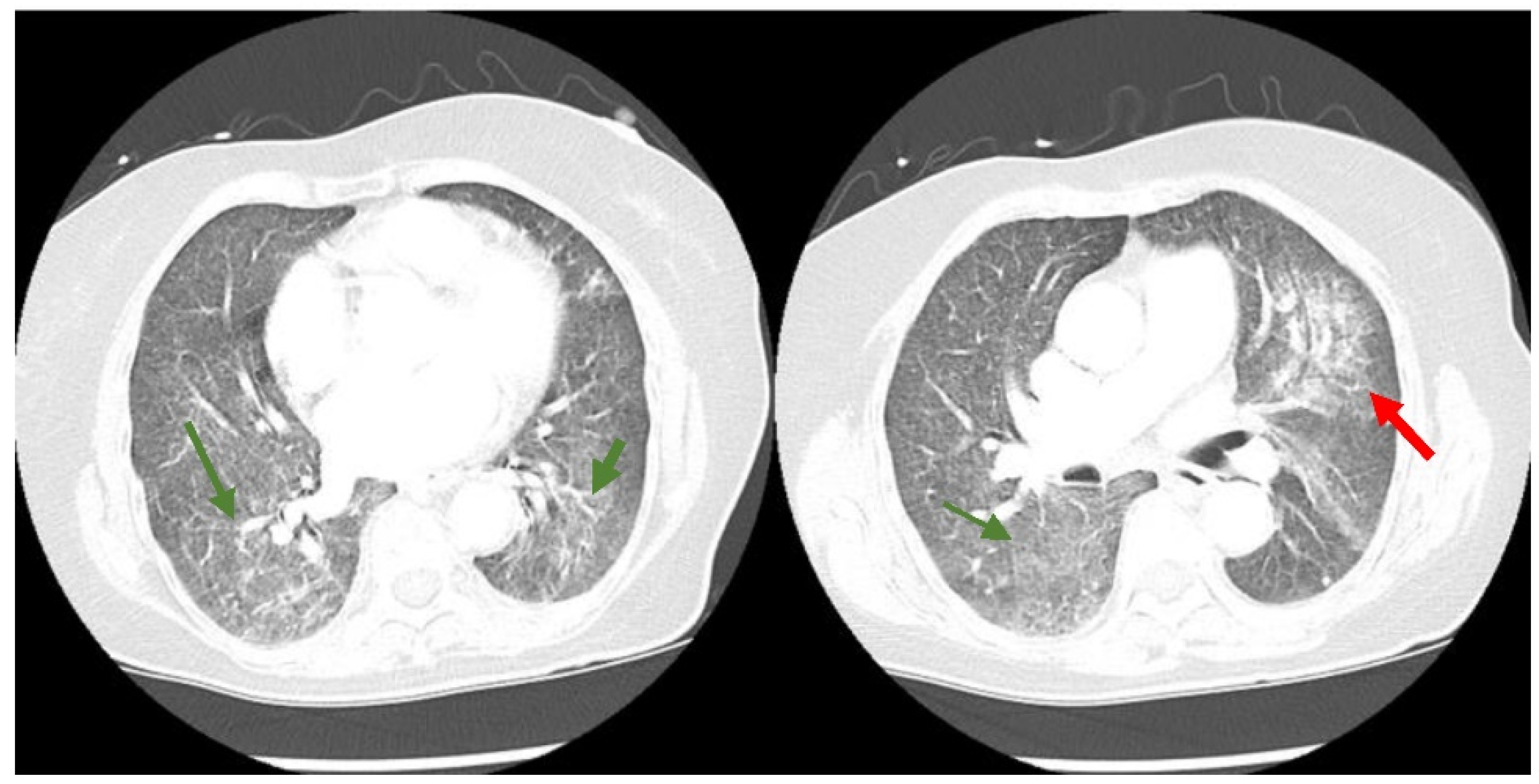
Figure 1). The computed tomography (CT) chest scan with IV contrast showed no evidence of a pulmonary embolism. However, it revealed significant bilateral infiltrates (
Figure 2) that had progressed from her previous CT chest scan three months prior (
Figure 3). Her echocardiogram revealed an ejection fraction of 39% and no other structural abnormalities.
Throughout the hospitalization, her hypoxemia worsened for which she required BiPAP and subsequently developed paroxysmal atrial fibrillation with a rapid ventricular response. The acute hypoxemic respiratory failure of the patient was thought to be multifactorial and was empirically treated with diuretics, antibiotics, and steroids for a suspected pulmonary edema vs. pneumonia vs. nitrofurantoin-induced pneumonitis as the patient received nitrofurantoin for a UTI one week before arrival to the ED. She was also treated with therapeutic enoxaparin for a non-ST elevation MI although it was likely due to demand ischemia in the setting of severe pulmonary disease. She remained febrile despite optimal treatment, prompting a further workup including a lumbar puncture 15 days after admission; however, the CSF findings were unrevealing for an infectious etiology. The patient had a worsening clinical course requiring intubation and mechanical ventilation. An induced sputum culture sample obtained after intubation showed a positive AFB smear and positive PCR for a Mycobacterium tuberculosis (MTB) complex. Subsequent induced sputum cultures were also positive for MTB, confirming the diagnosis of TB.
Initially, the treatment regimen was rifamycin, isoniazid, pyrazinamide, and ethambutol (RIPE therapy) for MTB. However, the patient developed transaminitis within a few days so she started second-line therapy (linezolid, isoniazid with vitamin B6, and levofloxacin). The patient was also placed on pulse-dose steroids for presumed immune reconstitution inflammatory syndrome (IRIS) and Pneumocystis jirovecii prophylaxis with atovaquone. In order to improve efficacy, rifabutin was added, but ultimately the regimen was changed to ethambutol, rifabutin, and levofloxacin due to ongoing hepatotoxicity. Eventually, the respiratory status of the patient improved so she underwent a tracheostomy placement and subsequently improved enough to have the tracheostomy closed. Finally, the patient was discharged to a specialized TB inpatient center to complete six months of MTB treatment with rifabutin, levofloxacin, and ethambutol.
3. Discussion
The above case illustrates the likely risk of a LTBI reactivation in a patient who had recently recovered from COVID-19 treated with steroids and remdesivir. Possible causes were the immunosuppressive sequelae of COVID-19, the effects of the treatment, or a combination of both. In any case, it is essential to consider the risk of a TB infection when treating COVID-19 patients, especially those who have emigrated from TB-endemic areas. For example, the patient presented in this case was an immigrant from the Philippines without other risk factors or known recent exposures. Therefore, the most likely conclusion was that it was a reactivation of LTBI triggered by COVID-19 rather than acute TB.
Although TB is less common in the United States (US) than in the rest of the world, it is not rare, especially in areas with large immigrant populations. In 2018, the Centers for Disease Control and Prevention (CDC) reported 9029 new cases of TB in the US with two-thirds of cases in non-US-born people (a rate 14 times greater than US-born people), and 591 cases in the state of Florida [
9]. Unfortunately, although it is required to report cases of active TB to the CDC, it is not required for cases of LTBI. Therefore, there are only incidence/prevalence estimates provided by the CDC via the National Health and Nutrition Examination Survey (NHANES), which includes a medical examination and TB screening tests; these data are extrapolated to the civilian population. In 2015, the estimated national prevalence of LTBI in the US was 13.2 million [
10].
Recently, it has been shown that COVID-19 infections affect the innate immune system and T cells, which are essential to prevent LTBI reactivation. For example, studies have shown that SARS-CoV-2 can cause T cell dysfunction and cytokine storms, leading to immunosuppression. Patients with COVID-19 have decreased numbers of lymphocytes, specifically CD4 and CD8 T cells; there is evidence that these cells undergo “exhaustion”, becoming dysfunctional/ineffective, and express an increased level of immune-inhibitory factors on their surface. These patients also tend to have increased levels of cytokines such as TNF-α, IL-6, and IL-10 [
6,
11]. One case series showed that 46.4% of patients with COVID-19 developed a secondary infection with respiratory and bloodstream infections being the most common source; this risk was higher in patients receiving corticosteroids, but was also present in patients who did not receive corticosteroids. However, this case series had a high number of severe and critically ill patients, so it may not be representative of all COVID-19 infections [
6]. Previous studies in mice have shown that a depletion of CD4 T cells is associated with the reactivation of LTBI. CD4 T cells are critical to the cytotoxic function of CD8 T cells via cytokines, and a depletion of CD8 T cells has also been associated with the reactivation of LTBI [
12]. Multiple viruses—including other coronaviruses such as SARS-CoV and MERS-CoV as well as viruses such as HIV, influenza, and measles—have been shown to increase the risk of a reactivation of LTBI [
13,
14,
15]. Other case series in TB-endemic areas such as China and Italy have increasingly documented COVID-19 and active TB co-infections. The majority of the current studies focus on co-infections or an increased risk of COVID-19 in patients with TB and vice versa, but little is documented on LTBI reactivation after recovery. In a study looking at patients with SARS-CoV, their cell counts took up to three months to normalize for CD8 T cell counts and up to one year for CD4 T cells [
16]. Therefore, it is possible that an infection with SARS-CoV-2 alone could put patients with LTBI at an increased risk of reactivation. There are two other separate case reports of patients that developed an active TB infection several weeks after recovery from COVID-19 and these patients likely had LTBI (both were from endemic areas and one had a known exposure via a household contact two years prior; neither patient had previously been tested or treated for LTBI) [
17,
18].
Additionally, the treatment for patients with COVID-19 who require supplemental oxygen is steroids, which also have immunosuppressive effects. The Infectious Disease Society of America (IDSA) guidelines for COVID-19 treatment recommend corticosteroids for a critical illness or severe non-critical illness as well as tocilizumab, which directly blocks the IL-6 receptor, for severe disease with signs of systemic inflammation [
19]. Corticosteroids mainly cause immunosuppression via effects on the function of phagocytes and temporary reductions in lymphocytes, which are necessary for preventing LTBI from reactivating via granuloma formation and other activities [
20]. There are limited data regarding whether tocilizumab increases the risk of an active TB infection, but corticosteroids have been shown to increase the risk of new TB infections and the reactivation of LTBI, usually with prolonged and high-dose courses. If a patient is going to be on a prolonged course of high-dose corticosteroids and has an increased risk of LTBI, it is recommended that they be screened with an interferon-gamma release assay or tuberculin skin testing [
21]. The same principle may need to be considered for patients treated for COVID-19.
4. Conclusions
In short, we present a case of TB in the US of a patient from the Philippines who recovered from a COVID-19 infection treated with corticosteroids and remdesivir and subsequently developed TB three months after recovery. Given the history and patient risk factors, this was likely a reactivation of LTBI rather than a new acute TB infection. There are currently no guidelines regarding whether screening for TB should be considered in patients with COVID-19, whether they receive treatment with steroids or tocilizumab. However, it seems that, given the increasing number of case reports regarding a co-infection with TB and the possible reactivation of LTBI, it may be prudent to begin screening high-risk patients with COVID-19, especially severe cases that require treatment. There are increasing data in the literature regarding co-infections of COVID-19 and TB, but a paucity of data regarding the possible reactivation of LTBI long after treatment and recovery from COVID-19; this will need further investigation.
Author Contributions
Conceptualization, A.-A.L., K.B., R.P., S.R., A.K. and R.B.; writing—original draft preparation, A.-A.L., K.B., R.P. and S.R.; writing—review and editing, A.-A.L., K.B., A.K. and R.B. All authors have read and agreed to the published version of the manuscript.
Funding
Memorial Physician Group Infectious Disease Department funded the article processing fee. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Institutional Review Board Statement
IRB not required for case reports as per hospital policy.
Informed Consent Statement
Written informed consent was obtained from the patient to publish this paper.
Data Availability Statement
Not applicable.
Acknowledgments
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Attaway, A.H.; Scheraga, R.G.; Bhimraj, A.; Biehl, M.; Hatipoğlu, U. Severe covid-19 pneumonia: Pathogenesis and clinical management. BMJ. 2021, 372, n436. [Google Scholar] [CrossRef] [PubMed]
- Kalil, A.C.; Patterson, T.F.; Mehta, A.K.; Tomashek, K.M.; Wolfe, C.R.; Ghazaryan, V.; Marconi, V.C.; Ruiz-Palacios, G.M.; Hsieh, L.; Kline, S.; et al. Baricitinib plus remdesivir for hospitalized adults with Covid-19. N. Engl. J. Med. 2021, 384, 795–807. [Google Scholar] [CrossRef] [PubMed]
- REMAP-CAP Investigators; Gordon, A.C.; Mouncey, P.R.; Al-Beidh, F.; Rowan, K.M.; Nichol, A.D.; Arabi, Y.M.; Mafham, M.; Bell, J.L.; Linsell, L.; et al. Interleukin-6 Receptor Antagonists in Critically Ill Patients with Covid-19. N. Engl. J. Med. 2021, 384, 1491–1502. [Google Scholar] [CrossRef] [PubMed]
- RECOVERY Collaborative Group; Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, V.; et al. Dexamethasone in Hospitalized Patients with Covid-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [CrossRef] [PubMed]
- WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group; Sterne, J.A.; Murthy, S.; Diaz, J.V.; Slutsky, A.S.; Villar, J.; Angus, D.C.; Annane, D.; Azevedo, L.C.P.; Berwanger, O.; et al. Association Between Administration of Systemic Corticosteroids and Mortality Among Critically Ill Patients With COVID-19: A Meta-analysis. JAMA 2020, 324, 1330–1341. [Google Scholar] [CrossRef] [PubMed]
- Behal, M.; Barlow, B.; Mefford, B.; Thompson, B.; Melissa, L.; Donaldson, J.; PharmD, J.C.; Melanie, L.; Brittany, B. Pharmacotherapy in Coronavirus Disease 2019 and Risk of Secondary Infections: A Single-Center Case Series and Narrative Review. Crit. Care Explor. 2021, 3, e0492. [Google Scholar] [CrossRef] [PubMed]
- Kimmig, L.M.; Wu, D.; Gold, M.; Pettit, N.; Pitrak, D.; Mueller, J.; Husain, A.N.; Mutlu, E.A.; Mutlu, G.M. IL-6 Inhibition in Critically Ill COVID-19 Patients Is Associated with Increased Secondary Infections. Front. Med. 2020, 7, 583897. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Tuberculosis Report 2020; Licence: CC BY-NC-SA 3.0 IGO; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Talwar, A.; Tsang, C.A.; Price, S.F.; Pratt, R.H.; Walker, W.L.; Schmit, K.M.; Langer, A.J. Tuberculosis—United States. MMWR Morb Mortal Wkly. Rep. 2018, 68, 257–262. [Google Scholar] [CrossRef]
- Miramontes, R.; Hill, A.N.; Yelk Woodruff, R.S.; Lambert, L.A.; Navin, T.R.; Castro, K.G.; LoBue, P.A. Tuberculosis Infection in the United States: Prevalence Estimates from the National Health and Nutrition Examination Survey, 2011–2012. PLoS ONE 2015, 10, e0140881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diao, B.; Wang, C.; Tan, Y.; Chen, X.; Liu, Y.; Ning, L.; Chen, L.; Li, M.; Liu, Y.; Wang, G.; et al. Reduction and Functional Exhaustion of T Cells in Patients with Coronavirus Disease 2019 (COVID-19). Front. Immunol. 2020, 11, 827. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S. Pathogenesis, immunology, and diagnosis of latent Mycobacterium tuberculosis infection. Clin. Dev. Immunol. 2011, 2011, 814943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Low, J.G.; Lee, C.C.; Leo, Y.S.; Low, J.G.; Lee, C.C.; Leo, Y.S. Severe acute respiratory syndrome and pulmonary tuberculosis. Clin. Infect. Dis. 2004, 38, e123-5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alfaraj, S.H.; Al-Tawfiq, J.A.; Altuwaijri, T.A.; Memish, Z.A. Middle East Respiratory Syndrome Coronavirus and Pulmonary Tuberculosis Coinfection: Implications for Infection Control. Intervirology 2017, 60, 53–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walaza, S.; Cohen, C.; Tempia, S.; Moyes, J.; Nguweneza, A.; Madhi, S.A.; McMorrow, M.; Cohen, A.L. Influenza and tuberculosis co-infection: A systematic review. Influenza Other Respir Viruses 2020, 14, 77–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, L.; Lu, L.; Cao, W.; Li, T. Hypothesis for potential pathogenesis of SARS-CoV-2 infection-a review of immune changes in patients with viral pneumonia. Emerg. Microbes Infect. 2020, 9, 727–732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khayat, M.; Fan, H.; Vali, Y. COVID-19 promoting the development of active tuberculosis in a patient with latent tuberculosis infection: A case report. Respir. Med. Case Rep. 2021, 32, 101344. [Google Scholar] [CrossRef] [PubMed]
- Friedman, A.; DeGeorge, K.C. Reactivation of latent tuberculosis in a COVID-19 patient on corticosteroid treatment. BMJ Case Rep. 2022, 15, e247562. [Google Scholar] [CrossRef] [PubMed]
- Bhimraj, A.; Morgan, R.L.; Shumaker, A.H.; Lavergne, V.; Baden, L.; Cheng, V.C.-C.; Edwards, K.M.; Gandhi, R.; Gallagher, J.; Muller, W.J.; et al. Infectious Diseases Society of America Guidelines on the Treatment and Management of Patients with COVID-19. Infectious Diseases Society of America 2021; Version 4.4.1. Available online: https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/ (accessed on 15 August 2021).
- Barshes, N.R.; Goodpastor, S.E.; Goss, J.A. Pharmacologic immunosuppression. Front Biosci. 2004, 9, 411–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Targeted tuberculin testing and treatment of latent tuberculosis infection. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. This is a Joint Statement of the ATS and the Centers for Disease Control and Prevention (CDC). This statement was endorsed by the Council of the Infectious Diseases Society of America. (IDSA), September 1999, and the sections of this statement. Am. J. Respir. Crit. Care Med. 2000, 161 Pt 2, S221-47. [CrossRef]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).