Abstract
Background: Nowadays, infective endocarditis (IE) is still burdened by a high mortality. In the absence of an adequate prognostic stratification system, it is important to assess new predictors of poor outcomes. The aim of our study is to evaluate which factors were associated with higher mortality in IE patients. Methods: A retrospective cohort study enrolled patients with an IE diagnosis at the Infectious Diseases Clinic of the University ‘G. D’Annunzio’, Chieti, Italy from January 2013 to December 2019. For each patient, demographic, anamnestic and clinical information, embolic phenomena, laboratory and microbiologic data, treatment, and outcomes were collected and analyzed. A correlation analysis was performed. Results: Sixty-eight patients with EI were studied; among them, the mortality was 17.6%, 20.6%, and 23.5%, intra-hospital, at 1 month from discharge and at 6 months from discharge, respectively. Mortality was significantly correlated with age, estimated glomerular filtration rate, and procalcitonin values when considering either basal values (r = 0.266, p = 0.029), or values at 48–72 h from the start of an antibiotic therapy (r = 0.222; p < 0.05), cerebral embolization for 6-month mortality (r = 0.284; p = 0.019), and inadequate antibiotic therapy (r = 0.232, p < 0.05). Conclusions: Procalcitonin values, at EI diagnosis and at 48–72 h after starting antibiotics, are prognostic factors useful for stratifying patient risk, and for setting up a personalized treatment. Of note, cerebral embolization and an inappropriate empirical treatment were associated with a higher mortality in the short- and long-term.
1. Introduction
Infective endocarditis (IE) is an inflammation of the endocardium, usually involving heart valves, due to bacterial or, less frequently, fungal infection [1]. Over the years, considerable progresses have been performed in understanding the main risk factors, as well as in the diagnostic and therapeutic approaches for IE. For example, the distinction between native valve endocarditis (NVE) and prosthetic valve endocarditis (PVE), distinguished in early-onset IE and late-onset IE, allowed different etiological agents to be considered. Moreover, empiric antibiotic schemes are often based on the combination of different bactericidal drugs and, traditionally, require a prolonged parenteral administration [2]; however, in the absence of viable choices, a bacteriostatic agent, such as linezolid, can be used for methicillin-resistant Staphylococcus aureus (MRSA) or vancomycin-resistant enterococci (VRE) [3,4,5,6,7,8]. In spite of progresses on EI diagnosis and treatment, in-hospital IE mortality still remains high (17.7%) [9]. Due to the variability of clinical onset, some IE cases are not promptly recognized or adequately treated on time. IE diagnosis can occur in the presence of an acute complication, i.e., embolization, or otherwise after non-specific signs and symptoms, including fever, malaise, chills, asthenia, gastrointestinal symptoms, and anemia. Staphylococcal etiology is predominant in IE, accounting for 32% of total IE cases, and is often associated with an unfavorable antibiotic susceptibility profile, i.e., MRSA [10]. IE still represents a significant public health problem in terms of morbidity and mortality. Only a few studies examined predictive factors for mortality in IE [11,12,13,14,15,16]. The aim of this study was to assess which risk factors were associated with poorer outcomes in IE patients admitted to an infectious disease unit in Central Italy.
2. Materials and Methods
This is a single-center cohort study, retrospectively enrolling consecutive patients admitted to an infectious diseases clinic, University ‘G. D’Annunzio’, SS Annunziata Hospital of Chieti, Italy, from January 2012 to December 2018. Medical hospital records were examined for each patient, and data regarding anamnesis, clinics, microbiology, diagnosis and therapy were collected and tabulated. The diagnosis of IE was based on Duke’s modified criteria [2] or MRI/PET-Tc positive for IE [17]. Patients with NVEs and PVEs, of any etiology, were included. Patients with suspected diagnosis of IE on admission, not confirmed during hospitalization, were excluded from the study. Furthermore, intra-cardiac device IE cases were excluded from this study. Inappropriate empiric antibiotic therapy was defined when the empirical therapy did not include at least one in vitro active antibiotic against the isolated microorganism. Statistical analysis: Data were analyzed using SPSS Advanced Statistical software version TM 13. Correlation studies were performed for detecting significant associations between mortality in IE patients (at discharge, 30 days and 6 months) and clinical, anamnestic, laboratory and diagnostic features. A Pearson R value was calculated for each parameter, in order to find a linear correlation with mortality. In all statistical tests, the significance threshold was assumed at p ≤ 0.05.
3. Results
Study Population
The study enrolled 68 patients; 46 were male (67.6%), and all were of Caucasian ethnicity, with a mean age (±standard deviation, SD) of 65.5 ± 17.4 years. IE population was divided into patients with PVE (n = 24, 35.3%), and patients with an NVE (n = 44, 64.7%). Among the 24 patients with PVE, 3 (12.5%) and 21 (87.5%) had an early-onset and a late-onset endocarditis, respectively. Baseline characteristics of the 68 IE patients are shown in Table 1. In Table 2, clinical manifestations are reported.

Table 1.
Baseline characteristics of 68 patients with infective endocarditis.

Table 2.
Clinical manifestations in the cohort of 68 patients with infective endocarditis.
The organisms most commonly isolated were S. aureus (34% of cases), followed by Enterococci (22%), Streptococcus viridans (13%) and CoNS (9%). We also recorded one fungal IE case due to Candida tropicalis. The number of blood-culture-negative IEs (BCNIEs) was 11 (16.2%) (Figure 1). In terms of valves, the aortic valve was involved in 44%, the mitral valve in 25% and the tricuspid value in 15% of patients; more than one valve was affected in the last 16% of cases. The modified Duke criteria for the IE diagnosis resulted in a 94% diagnosis accuracy, considering definite (n = 47; 69%) and probable (n = 17; 25%) IE cases. Embolic complications occurred in 27 (39.6%) patients, with an extra-cerebral involvement in 20 cases (29.3%). Among these, seven, eight and five patients had an isolated splenic embolization, a pulmonary embolization, and a miscellaneous embolism, respectively (Table 3).
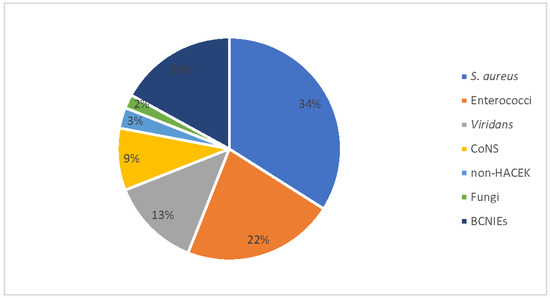
Figure 1.
Population of 68 patients with infective endocarditis divided by aetiologic agent. CoNS: coagulase-negative staphylococci; HACEK: stands for Haemophilus species, Aggregatibacter species, Cardiobacterium hominis, Eikenella corrodens, and Kingella species; BCNIE: blood-culture-negative infective endocarditis.

Table 3.
Embolic complication in 68 patients with infective endocarditis.
Correlation studies showed that the age of patients (p = 0.004) and impaired eGFR values (p = 0.004) were correlated to poorer outcomes, either for intra-hospital or for 1- and 6-month mortality. A linear correlation with mortality in IE patients was found between intra-hospital mortality and Pct levels, either for basal Pct or 48–72 h PCT values. Correlations with mortality were also found with a new-onset heart murmur and a discontinuous antibiotic therapy (i.e., interrupted with modified empirical antibiotic scheme). Furthermore, a significant association with an unfavorable 6-month outcome was discovered with neurological signs and symptoms at diagnosis (p = 0.019) (Table 4). In our IE population, hypertension, gender, affected valve, WBC values, diabetes and previous prosthetic valve implantation were not significantly correlated with mortality.

Table 4.
Significant correlations between discharge, 1-month and 6-month mortality and characteristics of patients.
4. Discussion
Our study confirmed a higher IE mortality rate than that reported in the literature [9]. The analysis of prognostic factors showed a direct proportionality between patient age and poor outcome; eGFR values were inversely correlated with mortality meaning that poor kidney functionality could be used as a predictive factor of a poor outcome, according to the latest European guidelines [2,18]. As already reported in the literature [19], inappropriate empiric antibiotic therapy was associated with a higher in-hospital mortality among our IE patients. Pct values, monitored before (basal Pct, T1) and after (48–72 h from) the beginning of empirical antibiotic therapy, were both associated with an increased intra-hospital mortality. Moreover, as a matter of fact, in our findings, a neurological manifestation was associated with a higher 6-month mortality. On the other hand, our study failed to find a significant correlation between hypertension, gender, affected valve, WBC values, diabetes and previous prosthetic valve implantations with a higher mortality. These findings differ from data reported in the literature [1,2,18,20,21], in which, for example, diabetes has an important prognostic value. As a limitation, the smaller size of our population could have affected the power of our study, which does not allow significant correlations to be found, even in the presence of differences in rates. The findings from our study highlight the critical prognostic role of septic cerebral emboli in IE patients [22,23,24,25]; in particular, the association between cerebral embolic phenomena and 6-month mortality may suggest the important role of the late neurological sequelae in determining a negative outcome. In our study, extra-cerebral embolization was present in 29.4% of IE cases, a percentage higher than previous data from the literature, i.e., 17% according to ICE-PCS [22]. Nevertheless, neither splenic nor pulmonary or renal embolic phenomena were related to a poor intra-hospital outcome; this means that in our experience extra-cerebral embolic involvement could not represent a prognostic factor, as reported in other studies [2,26,27].
In agreement with other studies [16], in our study, the onset of a new heart murmur was associated with increased mortality; indeed, the finding of a new murmur is commonly associated with valve damage and a more-aggressive endocarditis. This evidence reveals the importance of the clinical presentation of the patient at admission and of his/her frequent cardiac auscultation during hospital stay. Procalcitonin still demonstrates its prognostic importance in patients with IE. In the past, other studies evaluated only the poor PCT diagnostic value, which could not be used to confirm a suspected IE [28]. Our study underlined the prognostic role of monitoring PCT values during the hospital stay and follow-up of IE patients. The higher the admission PCT levels (basal Pct, T0), the higher the mortality rate (recorded for in-hospital stay and at follow-up). Higher PCT values at basal time could mean the presence of bacteremia and sepsis at onset, both conditions with a more-severe prognosis. In our findings, the measurement of PCT levels after 48–72 h from the start of the treatment remains a marker of ineffective therapy resulting in an uncontrolled infection, as in the case of inappropriate empirical antibiotic treatment. Diagnostic accuracy, concordant with modified the Duke criteria of our case series, was higher than that reported in ESC guidelines, i.e., 94% vs. 81% [2]; this confirms the validity of our findings, even though, as a major limitation, our data come from a single center, and the size population is relatively limited. Regarding etiology, in our study, S. aureus was the most common organism isolated, as reported by other European studies [29]; however, the prevalence of enterococcal infections was two-fold higher than that reported in the literature, i.e., 22% vs. 11% [1,10], and BCNIE (n = 11, 16.2%). was higher than expected [2,30]. This may reflect, for enterococcal infections, the peculiar ecology of a single center, and for BCNIEs, the previous at-home and in-hospital stay exposure to antibiotics, due to the the paucisymptomatic presentation of the disease. Indeed, 73% of our IE cases presented only a mild fever lasting weeks from admission.
5. Conclusions
Our findings, even though from a single center, confirmed the importance of an appropriate risk stratification for IE patients; moreover, they underline the critical role of monitoring procalcitonin levels during hospital stay and follow-up as a prognostic marker of disease progression. High Pct levels are likely the expression of an endocardial and systemic infection not adequately controlled by empirical therapy, and its values must guide clinicians towards an earlier and more aggressive treatment of IE patients. Finally, our findings highlight the importance of following a prompt and appropriate empirical antibiotic treatment, in case of microbiological isolations, by a targeted therapy. This could limit local/systemic IE complications and reduce the rate of adverse outcomes.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author Contributions
Conceptualization, C.U. and K.F.; methodology, J.V.; formal analysis, K.F.; data curation, C.C. and A.D.G. writing—original draft preparation, C.U. and A.A.; writing—review and editing, C.U. and K.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Internal Institutional Review Board.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data available upon request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bennett, J.; Dolin, R.; Blaser, M. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases, 9th ed.; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- The 2015 ESC Guidelines for the management of infective endocarditis. Eur. Heart J. 2015, 36, 3036–3037. [CrossRef]
- Iversen, K.; Ihlemann, N.; Gill, S.U.; Madsen, T.; Elming, H.; Jensen, K.T.; Bruun, N.E.; Høfsten, D.E.; Fursted, K.; Christensen, J.J.; et al. Partial Oral versus Intravenous Antibiotic Treatment of Endocarditis. N. Engl. J. Med. 2019, 380, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Mancino, P.; Ucciferri, C.; Falasca, K.; Pizzigallo, E.; Vecchiet, J. Methicillin-resistant Staphylococcus epidermidis (MRSE) endocarditis treated with linezolid. Scand. J. Infect. Dis. 2008, 40, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Yeager, S.D.; Oliver, J.E.; Shorman, M.A.; Wright, L.R.; Veve, M.P. Comparison of linezolid step-down therapy to standard parenteral therapy in methicillin-resistant Staphylococcus aureus bloodstream infections. Int. J. Antimicrob. Agents 2021, 57, 106329. [Google Scholar] [CrossRef] [PubMed]
- Rezar, R.; Jirak, P.; Lichtenauer, M.; Jung, C.; Lauten, A.; Hoppe, U.C.; Wernly, B. Partial oral antibiotic therapy is non-inferior to intravenous therapy in non-critically ill patients with infective endocarditis. Wien. Klin. Wochenschr. 2020, 132, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Martí-Carvajal, A.J.; Dayer, M.; O Conterno, L.; Garay, A.G.G.; Martí-Amarista, C.E. A comparison of different antibiotic regimens for the treatment of infective endocarditis. Cochrane Database Syst. Rev. 2020, 2020, CD009880. [Google Scholar] [CrossRef]
- Paz, D.L.; Lakbar, I.; Tattevin, P. A review of current treatment strategies for infective endocarditis. Expert Rev. Anti-Infective Ther. 2020, 19, 297–307. [Google Scholar] [CrossRef]
- Murdoch, D.R.; Corey, G.R.; Hoen, B.; Miro, J.M.; Fowler, V.G., Jr.; Bayer, A.S.; Karchmer, A.W.; Olaison, L.; Pappas, P.A.; Moreillon, P.; et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: The International Collaboration on Endocarditis-Prospective Cohort Study. Arch. Intern. Med. 2009, 169, 463–473. [Google Scholar] [CrossRef]
- Fowler, V.G.; Miro, J.M.; Hoen, B.; Cabell, C.H.; Abrutyn, E.; Rubinstein, E.; Corey, G.R.; Spelman, D.; Bradley, S.F.; Barsic, B.; et al. Staphylococcus aureus Endocarditis. JAMA 2005, 293, 3012–3021. [Google Scholar] [CrossRef]
- Diemberger, I.; Biffi, M.; Lorenzetti, S.; Martignani, C.; Raffaelli, E.; Ziacchi, M.; Rapezzi, C.; Pacini, D.; Boriani, G. Predictors of long-term survival free from relapses after extraction of infected CIED. Europace 2017, 20, 1018–1027. [Google Scholar] [CrossRef]
- Luciani, N.; Mossuto, E.; Ricci, D.; Luciani, M.; Russo, M.; Salsano, A.; Pozzoli, A.; Pierri, M.D.; D’Onofrio, A.; Chiariello, G.A.; et al. Prosthetic valve endocarditis: Predictors of early outcome of surgical therapy. A multicentric study. Eur. J. Cardio-Thorac. Surg. 2017, 52, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Dong, S.; Yuan, J.; Yu, D.; Bei, W.; Chen, R.; Qin, H. Accuracy and Prognosis Value of the Sequential Organ Failure Assessment Score Combined With C-Reactive Protein in Patients With Complicated Infective Endocarditis. Front. Med. 2021, 8. [Google Scholar] [CrossRef] [PubMed]
- Alves, S.G.; Júnior, F.P.; Filippini, F.B.; Dannenhauer, G.P.; Miglioranza, M.H. SHARPEN score accurately predicts in-hospital mortality in infective endocarditis. Eur. J. Intern. Med. 2021, 92, 124–127. [Google Scholar] [CrossRef] [PubMed]
- Marques, A.; Cruz, I.; Caldeira, D.; Alegria, S.; Gomes, A.C.; Broa, A.L.; João, I.; Pereira, H. Fatores de Risco para Mortalidade Hospitalar na Endocardite Infecciosa. Arq. Bras. Cardiol. 2019, 114, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Motoc, A.; Kessels, J.; Roosens, B.; Lacor, P.; Van de Veire, N.; De Sutter, J.; Magne, J.; Droogmans, S.; Cosyns, B. Impact of the initial clinical presentation on the outcome of patients with infective endocarditis. Cardiol. J. 2021. [Google Scholar] [CrossRef]
- Iung, B.; Erba, P.A.; Petrosillo, N.; Lazzeri, E. Common diagnostic flowcharts in infective endocarditis. Q. J. Nucl. Med. Mol. Imaging 2014, 58, 55–65. [Google Scholar]
- Durante-Mangoni, E.; Giuffrè, G.; Ursi, M.P.; Iossa, D.; Bertolino, L.; Senese, A.; Pafundi, P.C.; D’Amico, F.; Albisinni, R.; Zampino, R. Predictors of long-term mortality in left-sided infective endocarditis: An historical cohort study in 414 patients. Eur. J. Intern. Med. 2021, 94, 27–33. [Google Scholar] [CrossRef]
- Fernández-Hidalgo, N.; Almirante, B.; Tornos, P.; González-Alujas, M.; Planes, A.; Larrosa, M.N.; Sambola, A.; Igual, A.; Pahissa, A. Prognosis of left-sided infective endocarditis in patients transferred to a tertiary-care hospital—prospective analysis of referral bias and influence of inadequate antimicrobial treatment. Clin. Microbiol. Infect. 2011, 17, 769–775. [Google Scholar] [CrossRef][Green Version]
- Buburuz, A.-M.; Petris, A.; Costache, I.; Jelihovschi, I.; Arsenescu-Georgescu, C.; Iancu, L. Evaluation of Laboratory Predictors for In-Hospital Mortality in Infective Endocarditis and Negative Blood Culture Pattern Characteristics. Pathogens 2021, 10, 551. [Google Scholar] [CrossRef]
- Li, Z.; Gao, Q.; Ren, Z.; Zhou, H.; Qian, Z.; Peng, J. Nomogram based on neutrophil-to-platelet ratio to predict in-hospital mortality in infective endocarditis. Biomarkers Med. 2021, 15, 1233–1243. [Google Scholar] [CrossRef]
- Arregle, F.; Martel, H.; Philip, M.; Gouriet, F.; Casalta, J.P.; Riberi, A.; Torras, O.; Casalta, A.-C.; Camoin-Jau, L.; Lavagna, F.; et al. Infective endocarditis with neurological complications: Delaying cardiac surgery is associated with worse outcome. Arch. Cardiovasc. Dis. 2021, 114, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Neurological Sequelae of Endocarditis–PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/31194361/ (accessed on 22 September 2021).
- Alegria, S.; Marques, A.; Cruz, I.; Broa, A.L.; Pereira, A.R.F.; João, I.; Simões, O.; Pereira, H. Complicações Neurológicas em Pacientes com Endocardite Infecciosa: Perspectivas de um Centro Terciário. Arq. Bras. De Cardiol. 2021, 116, 682–691. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, T.; Rabinstein, A.; Wijdicks, E. Neurologic complications of infective endocarditis. Neurol. Clin. 2021, 177, 125–134. [Google Scholar] [CrossRef]
- Netzer, R.O.M.; Zollinger, E.; Seiler, C.; Cerny, A. Infective endocarditis: Clinical spectrum, presentation and outcome. An analysis of 212 cases 1980–1995. Heart 2000, 84, 25–30. [Google Scholar] [CrossRef]
- Bui, J.T.; Schranz, A.J.; Strassle, P.D.; Agala, C.B.; Mody, G.N.; Ikonomidis, J.S.; Long, J.M. Pulmonary complications observed in patients with infective endocarditis with and without injection drug use: An analysis of the National Inpatient Sample. PLoS ONE 2021, 16, e0256757. [Google Scholar] [CrossRef]
- Yu, C.-W.; Juan, L.-I.; Hsu, S.-C.; Chen, C.-K.; Wu, C.-W.; Lee, C.-C.; Wu, J.-Y. Role of procalcitonin in the diagnosis of infective endocarditis: A meta-analysis. Am. J. Emerg. Med. 2013, 31, 935–941. [Google Scholar] [CrossRef]
- Primus, C.P.; A Clay, T.; McCue, M.S.; Wong, K.; Uppal, R.; Ambekar, S.; Das, S.; Bhattacharyya, S.; Davies, L.C.; Woldman, S.; et al. 18F-FDG PET/CT improves diagnostic certainty in native and prosthetic valve Infective Endocarditis over the modified Duke Criteria. J. Nucl. Cardiol. 2021, 1–10. [Google Scholar] [CrossRef]
- Hoen, B.; Selton-Suty, C.; Lacassin, F.; Etienne, J.; Briançon, S.; Leport, C.; Canton, P. Infective Endocarditis in Patients with Negative Blood Cultures: Analysis of 88 Cases from a One-Year Nationwide Survey in France. Clin. Infect. Dis. 1995, 20, 501–506. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).