Effects of Aronia melanocarpa-Based Fruit Juices on Metabolic Dysfunction-Associated Fatty Liver Disease in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Preparation of Aronia melanocarpa-Based Fruit Juices (AMBFJs)
2.2.1. Preparation of AM20 and AM60
2.2.2. Preparation of AMRC
2.2.3. Preparation of AMAV
2.3. Polyphenolic Content of Aronia melanocarpa-Based Fruit Juices (AMBFJs)
2.4. Animals
2.5. Experimental Setting
2.6. Histopathological Examination of Liver and Adipose Tissue
2.7. Biochemical Tests
2.8. Statistics
3. Results
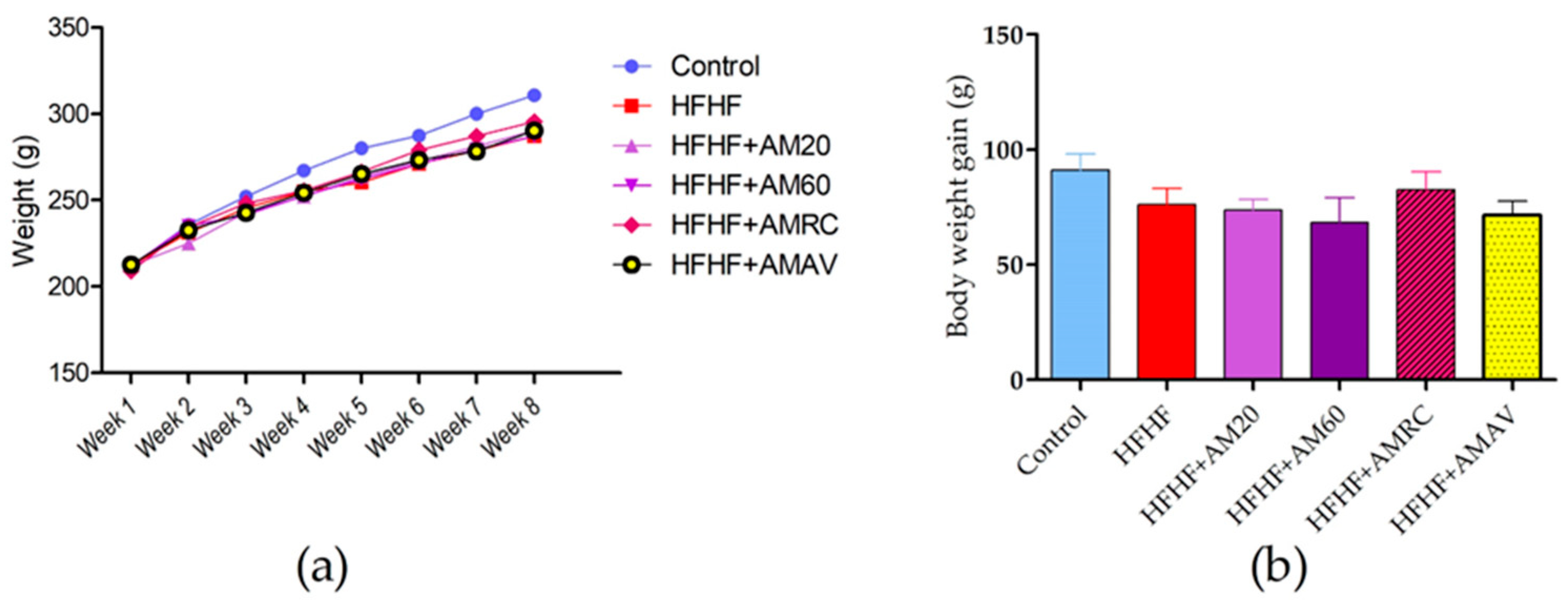
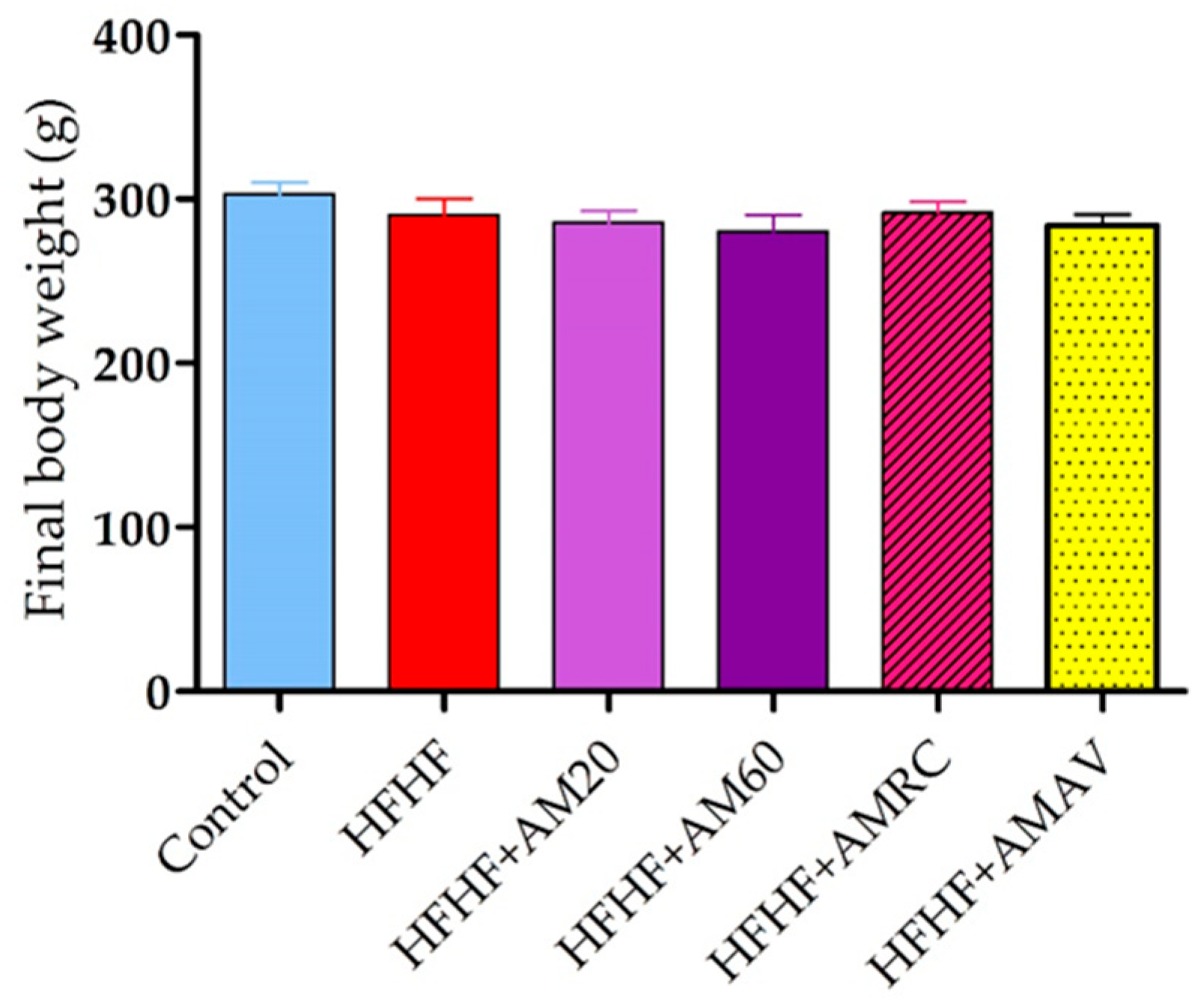
3.1. Body Weight
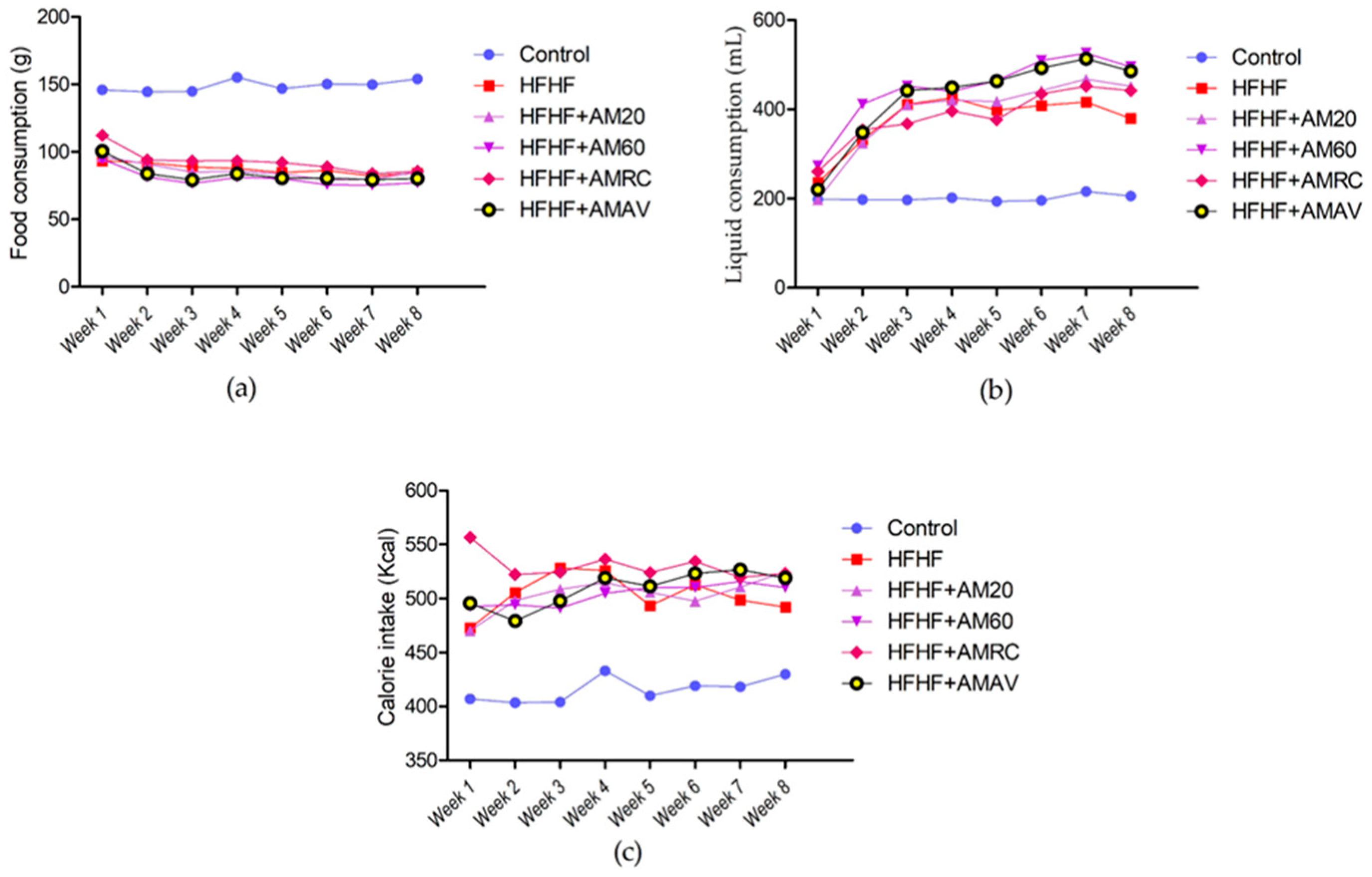
3.2. Food, Liquid, and Calories Consumption
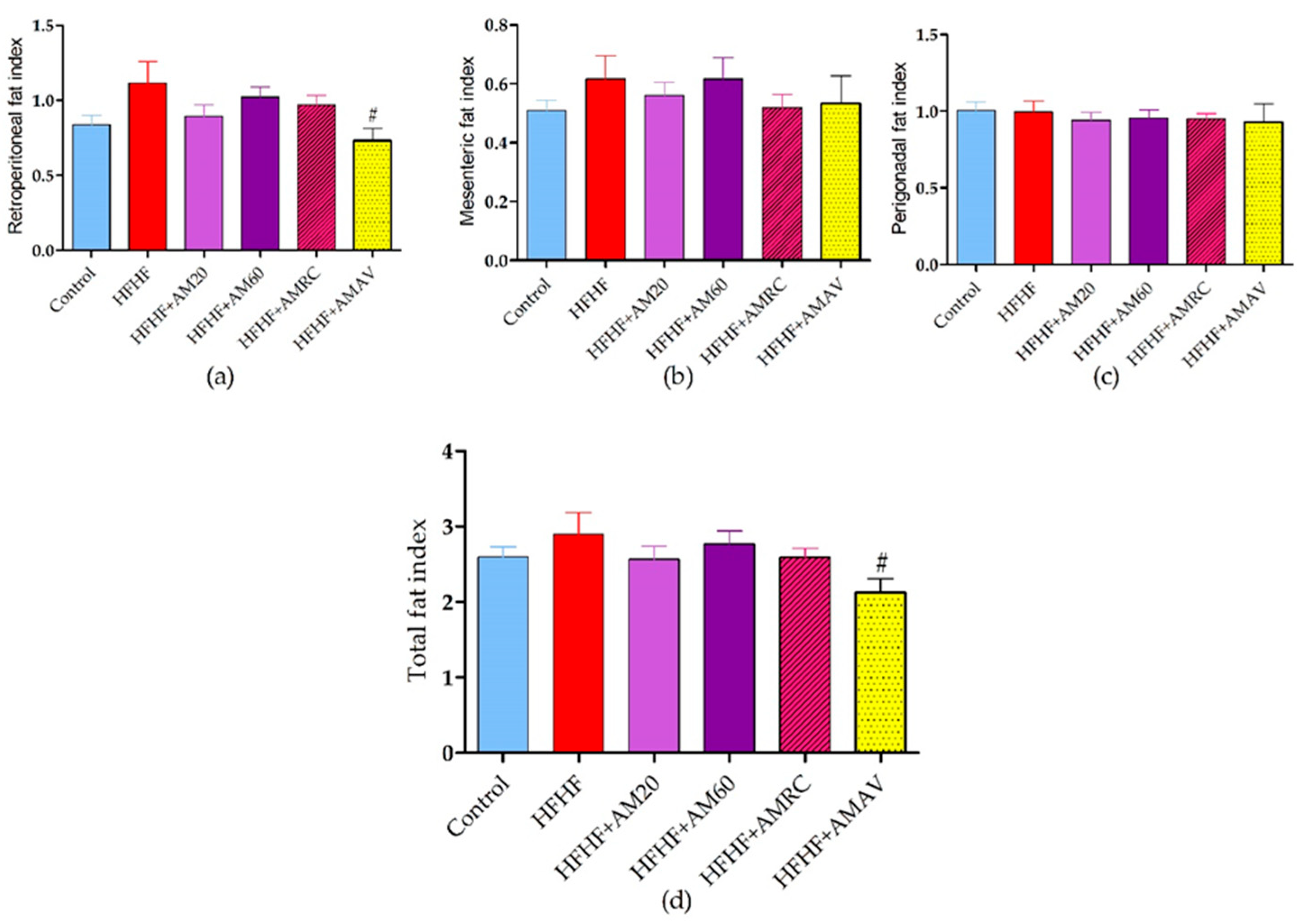
3.3. Fat Indices
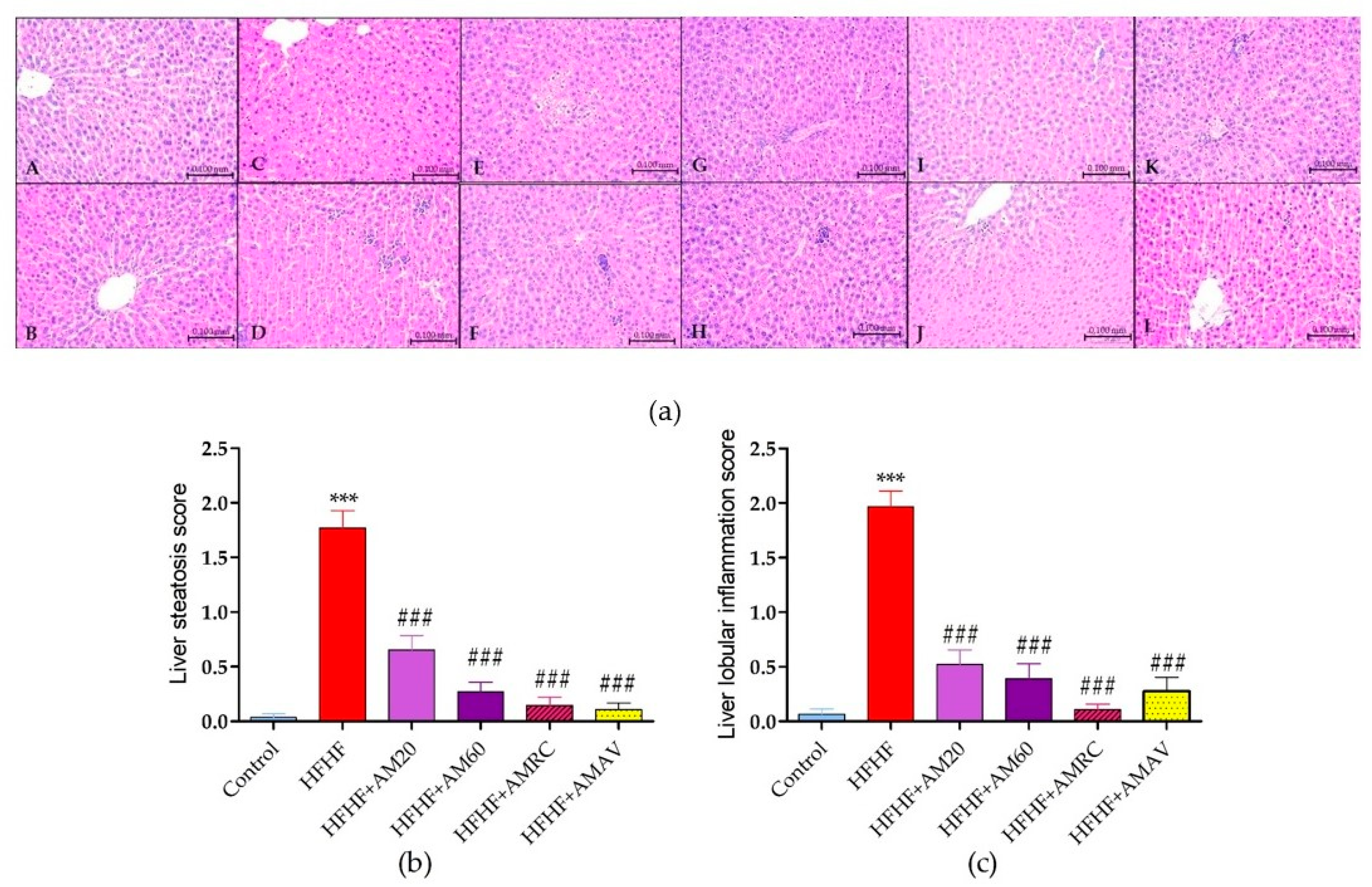
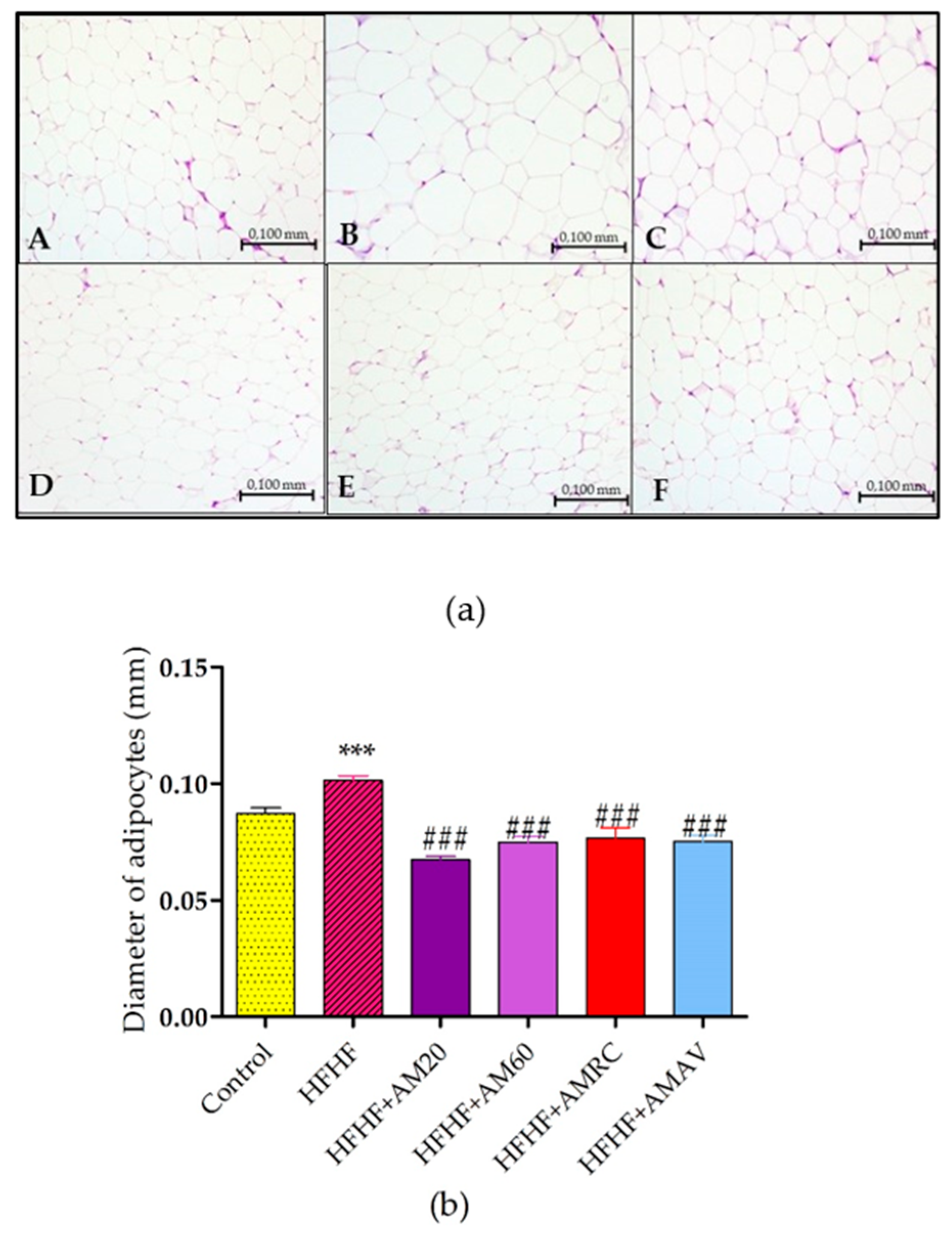
3.4. Histopathological Investigation
3.4.1. Liver
3.4.2. Adipose Tissue
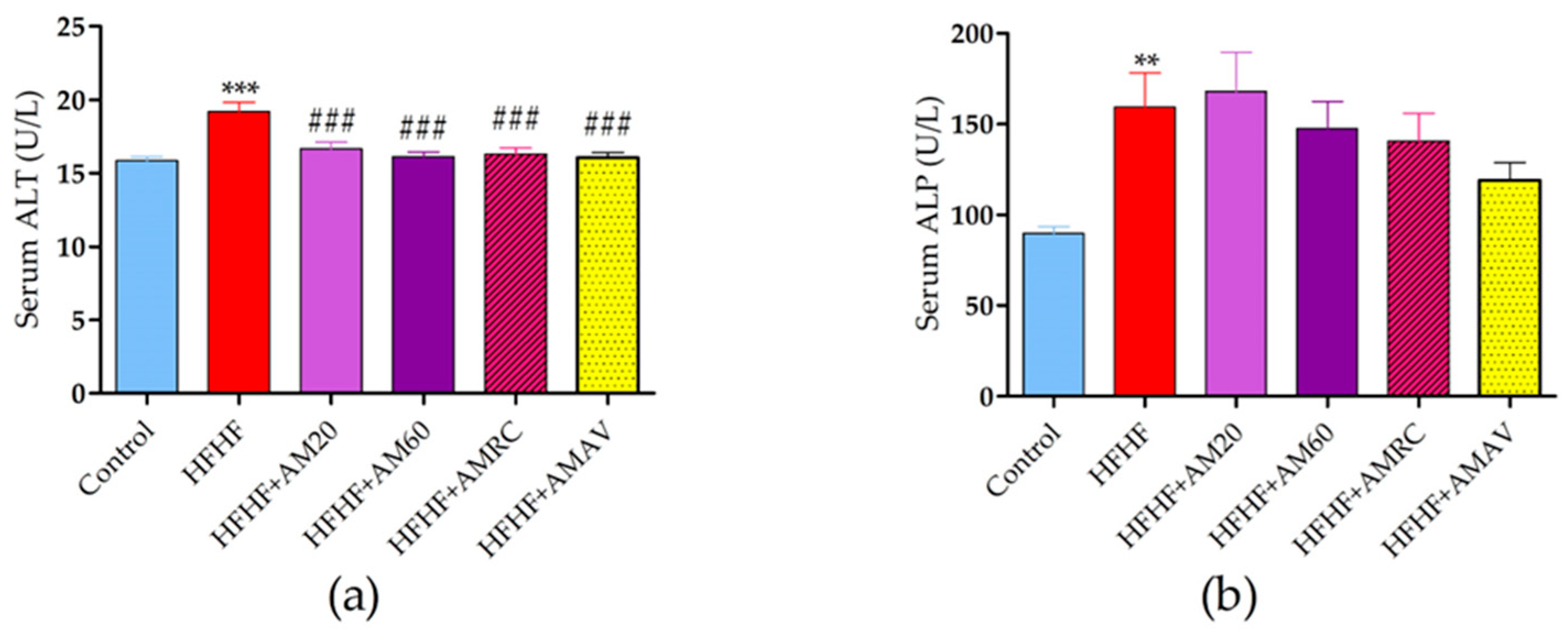
3.5. Biochemical Tests
3.5.1. Serum Lipids
3.5.2. Liver Enzymes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 6 September 2024).
- Murray, C.J.L.; Aravkin, A.Y.; Zheng, P.; Abbafati, C.; Abbas, K.M.; Abbasi-Kangevari, M. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1223–1249. [Google Scholar] [CrossRef] [PubMed]
- Chooi, Y.C.; Ding, C.; Magkos, F. The epidemiology of obesity. Metabolism 2019, 92, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Nutter, S.; Eggerichs, L.A.; Nagpal, T.S.; Salas, X.R.; Chea, C.C.; Saiful, S.; Ralston, J.; Barata-Cavalcanti, O.; Batz, C.; Baur, L.A.; et al. Changing the global obesity narrative to recognize and reduce weight stigma: A position statement from the World Obesity Federation. Obes. Rev. 2024, 25, e13642. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Zhang, X.; Li, G.; Wong, G.L.H.; Wong, V.W.S. Epidemiology and clinical outcomes of metabolic (dysfunction)-associated fatty liver disease. J. Clin. Transl. Hepatol. 2021, 9, 972–982. [Google Scholar] [CrossRef]
- Gancheva, S.; Zhelyazkova-Savova, M.; Galunska, B.; Chervenkov, T. Experimental models of metabolic syndrome in rats. Scr. Sci. Med. 2015, 47, 14–21. [Google Scholar] [CrossRef]
- Im, Y.R.; Hunter, H.; de Gracia Hahn, D.; Duret, A.; Cheah, Q.; Dong, J.; Fairey, M.; Hjalmarsson, C.; Li, A.; Lim, H.K.; et al. A systematic review of animal models of NAFLD finds high-fat, high-fructose diets most closely resemble human NAFLD. Hepatology 2021, 74, 1884–1901. [Google Scholar] [CrossRef]
- Jakubiak, G.K.; Osadnik, K.; Lejawa, M.; Osadnik, T.; Golawski, M.; Lewandowski, P.; Pawlas, N. Obesity and insulin resistance is the component of the metabolic syndrome most strongly associated with oxidative stress. Antioxidants 2022, 11, 79. [Google Scholar] [CrossRef]
- Martínez-Martínez, E.; Cachofeiro, V. Oxidative stress in obesity. Antioxidants 2022, 11, 639. [Google Scholar] [CrossRef]
- Latorre, J.; Lluch, A.; Ortega, F.J.; Gavaldà-Navarro, A.; Comas, F.; Morón-Ros, S.; Rodríguez, A.; Becerril, S.; Villarroya, F.; Frühbeck, G.; et al. Adipose tissue knockdown of lysozyme reduces local inflammation and improves adipogenesis in high-fat diet-fed mice. Pharmacol. Res. 2021, 166, 105486. [Google Scholar] [CrossRef]
- Tak, Y.J.; Lee, S.Y. Anti-obesity drugs: Long-term efficacy and safety: An updated review. World J. Men’s Health 2021, 3, 208–221. [Google Scholar] [CrossRef]
- Bravo, L. Polyphenols: Chemistry, dietary sources, metabolism, and nutritional significance. Nutr. Rev. 1998, 56, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Boccellino, M.; D’Angelo, S. Anti-obesity effects of polyphenol intake: Current status and future possibilities. Int. J. Mol. Sci. 2020, 21, 5642. [Google Scholar] [CrossRef] [PubMed]
- Benvenuti, S.; Pellati, F.; Melegari, M.; Bertelli, D. Polyphenols, anthocyanins, ascorbic acid, and radical scavenging activity of rubus, ribes, and aronia. J. Food Sci. 2004, 69, 164–169. [Google Scholar] [CrossRef]
- Wangensteen, H.; Bräunlich, M.; Nikolic, V.; Malterud, K.E.; Slimestad, R.; Barsett, H. Anthocyanins, proanthocyanidins and total phenolics in four cultivars of aronia: Antioxidant and enzyme inhibitory effects. J. Funct. Foods 2014, 7, 746–752. [Google Scholar] [CrossRef]
- Sidor, A.; Gramza-Michałowska, A. Black chokeberry Aronia melanocarpa L.—A qualitative composition, phenolic profile and antioxidant potential. Molecules 2019, 24, 3710. [Google Scholar] [CrossRef]
- Zapolska-Downar, D.; Bryk, D.; Małecki, M.; Hajdukiewicz, K.; Sitkiewicz, D. Aronia melanocarpa fruit extract exhibits anti-inflammatory activity in human aortic endothelial cells. Eur. J. Nutr. 2012, 51, 563–572. [Google Scholar] [CrossRef]
- Gao, N.; Shu, C.; Wang, Y.; Tian, J.; Lang, Y.; Jin, C.; Cui, X.; Jiang, H.; Liu, S.; Li, Z.; et al. Polyphenol components in black chokeberry (Aronia melanocarpa) as clinically proven diseases control factors—An overview. Food Sci. Hum. Wellness 2024, 13, 1152–1167. [Google Scholar] [CrossRef]
- Reyzov, M.; Eftimov, M.; Gancheva, S.; Todorova, M.; Zhelyazkova-Savova, M.; Tzaneva, M.; Valcheva-Kuzmanova, S. Aronia melanocarpa fruit juice prevents hepatic impairment in rats subjected to metabolic syndrome. Acta Aliment. 2024, 53, 270–280. [Google Scholar] [CrossRef]
- Reyzov, M.; Eftimov, M.; Gancheva, S.; Todorova, M.; Zhelyazkova-Savova, M.; Tzaneva, M.; Valcheva-Kuzmanova, S. Effect of Aronia melanocarpa fruit juice on glucose tolerance, lipid metabolism, and obesity in a rat model of metabolic syndrome. Acta Aliment. 2022, 5, 390–402. [Google Scholar] [CrossRef]
- Kim, N.H.; Jegal, J.; Kim, Y.N.; Chung, D.M.; Heo, J.D.; Rho, J.R.; Yang, M.H.; Jeong, E.J. Antiobesity effect of fermented chokeberry extract in high-fat diet-induced obese mice. J. Med. Food 2018, 21, 1113–1119. [Google Scholar] [CrossRef]
- Lim, S.M.; Lee, H.S.; Jung, J.I.; Kim, S.M.; Kim, N.Y.; Seo, T.S.; Bae, J.S.; Kim, E.J. Cyanidin-3-O-galactoside-enriched Aronia melanocarpa extract attenuates weight gain and adipogenic pathways in high-fat diet-induced obese C57BL/6 mice. Nutrients 2019, 11, E1190. [Google Scholar] [CrossRef] [PubMed]
- Denev, P.; Kratchanova, M.; Petrova, I.; Klisurova, D.; Georgiev, Y.; Ognyanov, M.; Yanakieva, I. Black chokeberry (Aronia melanocarpa (Michx.) Elliot) fruits and functional drinks differ significantly in their chemical composition and antioxidant activity. J. Chem. 2018, 13, 9574587. [Google Scholar] [CrossRef]
- Kerasioti, E.; Apostolou, A.; Kafantaris, I.; Chronis, K.; Kokka, E.; Dimitriadou, C.; Tzanetou, E.N.; Priftis, A.; Koulocheri, S.D.; Haroutounian, S.A.; et al. Polyphenolic composition of Rosa canina, Rosa sempervivens and Pyrocantha coccinea extracts and assessment of their antioxidant activity in human endothelial cells. Antioxidants 2019, 8, 92. [Google Scholar] [CrossRef]
- Tabaszewska, M.; Najgebauer-Lejko, D. The content of selected phytochemicals and in vitro antioxidant properties of rose hip (Rosa canina L.) tinctures. NFS J. 2020, 21, 50–56. [Google Scholar] [CrossRef]
- Lattanzio, F.; Greco, E.; Carretta, D.; Cervellati, R.; Govoni, P.; Speroni, E. In vivo anti-inflammatory effect of Rosa canina L. extract. J. Ethnopharmacol. 2011, 137, 880–885. [Google Scholar] [CrossRef] [PubMed]
- Kratchanova, M.; Denev, P.; Kratchanov, C. Rosehip extract synergistically increase antioxidant activity of fruit and herb extracts. Bulg. Chem. Commun. 2014, 46, 59–64. [Google Scholar]
- Kanak, S.; Krzemińska, B.; Celiński, R.; Bakalczuk, M.; Dos Santos Szewczyk, K. Phenolic composition and antioxidant activity of Alchemilla species. Plants 2022, 11, 2709. [Google Scholar] [CrossRef]
- Valcheva-Kuzmanova, S.; Denev, P.; Eftimov, M.; Georgieva, A.; Kuzmanova, V.; Kuzmanov, A.; Kuzmanov, K.; Tzaneva, M. Protective effects of Aronia melanocarpa juices either alone or combined with extracts from Rosa canina or Alchemilla vulgaris in a rat model of indomethacin-induced gastric ulcers. Food Chem. Toxicol. 2019, 132, 110739. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdicphosphotungstic acid reagents. Am. J. Emol. Viticult. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescence probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef]
- Hruby, A.; Manson, J.E.; Qi, L.; Malik, V.S.; Rimm, E.B.; Sun, Q.; Willett, W.C.; Hu, F.B. Determinants and consequences of obesity. Am. J. Public Health 2016, 106, 1656–1662. [Google Scholar] [CrossRef]
- Goodman, Z.D.; Pan, X.J.; Ge, C.L.; Guo, Y.T.; Zhang, P.F.; Yan, T.T.; Zhou, J.Y.; He, Q.X.; Goodman, Z.D. The impact of obesity on liver histology. Clin. Liver Dis. 2014, 18, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Matias, A.M.; Estevam, W.M.; Coelho, P.M.; Haese, D.; Kobi, J.B.B.S.; Lima-Leopoldo, A.P.; Leopoldo, A.S. Differential effects of high sugar, high lard or a combination of both on nutritional, hormonal and cardiovascular metabolic profiles of rodents. Nutrients 2018, 10, 1071. [Google Scholar] [CrossRef] [PubMed]
- Miranda, C.A.; Schönholzer, T.E.; Klöppel, E.; Sinzato, Y.K.; Volpato, G.T.; Damasceno, D.C.; Campos, K.E. Repercussions of low fructose-drinking water in male rats. An. Acad. Bras. Ciências 2019, 91, e20170705. [Google Scholar] [CrossRef]
- Rorabaugh, J.M.; Stratford, J.M.; Zahniser, N.R. Differences in bingeing behavior and cocaine reward following intermittent access to sucrose, glucose or fructose solutions. Neuroscience 2015, 301, 213–220. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, J.Y.; Wei, Y.L.; Hao, J.Y.; Lei, Y.Q.; Zhao, W.B.; Xiao, Y.H.; Sun, A.D. The polyphenol-rich extract from chokeberry (Aronia melanocarpa L.) modulates gut microbiota and improves lipid metabolism in diet-induced obese rats. Nutr. Metab. 2020, 17, 54. [Google Scholar] [CrossRef]
- Kim, H.K.; Jung, J.; Kang, E.Y.; Gang, G.; Kim, W.; Go, G. Aronia melanocarpa reduced adiposity via enhanced lipolysis in high-fat diet-induced obese mice. Korean J. Food Sci. Technol. 2020, 52, 255–262. [Google Scholar] [CrossRef]
- Mikami, N.; Hosotani, Y.; Saso, T.; Ohta, T.; Miyashita, K.; Hosokawa, M. Black chokeberry (Aronia melanocarpa) juice residue and its ethanol extract decrease serum lipid levels in high-fat diet-fed C57BL/6J mice. Int. J. Funct. Nutr. 2020, 2, 10. [Google Scholar] [CrossRef]
- Lee, H.Y.; Suh, K.S.; Kim, Y.I.; Jang, B.K.; Kim, B.H.; Yim, S.V. Bioactive fraction of Aronia melanocarpa fruit inhibits adipogenic differentiation of cultured 3T3-L1 cells. Appl. Sci. 2021, 1, 9224. [Google Scholar] [CrossRef]
- Park, C.H.; Kim, J.H.; Lee, E.B.; Hur, W.; Kwon, O.J.; Park, H.J.; Yoon, S.K. Aronia melanocarpa extract ameliorates hepatic lipid metabolism through PPARγ2 downregulation. PLoS ONE 2017, 12, e0169685. [Google Scholar] [CrossRef]
- Broncel, M.; Koziróg, M.; Duchnowicz, P.; Koter-Michalak, M.; Sikora, J.; Chojnowska-Jezierska, J. Aronia melanocarpa extract reduces blood pressure, serum endothelin, lipid, and oxidative stress marker levels in patients with metabolic syndrome. Med. Sci. Monit. 2010, 16, 34. [Google Scholar]
- Mu, J.; Xin, G.; Zhang, B.; Wang, Y.; Ning, C.; Meng, X. Beneficial effects of Aronia melanocarpa berry extract on hepatic insulin resistance in type 2 diabetes mellitus rats. J. Food Sci. 2020, 85, 1307–1318. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Lamora, H.; Nicola-Llorente, M.; Torres-Oteros, D.; Pérez-Martí, A.; Aghziel, I.; Lozano-Castellón, J.; Vallverdú-Queralt, A.; Canudas, S.; Marrero, P.F.; Haro, D.; et al. The antiobesity effects of rosehip (Rosa canina) flesh by antagonizing the PPAR gamma activity in high-fat diet-fed mice. Mol. Nutr. Food Res. 2024, 68, 2300539. [Google Scholar] [CrossRef]
- Selahvarzian, A.; Alizadeh, A.; Baharvand, P.A.; Eldahshan, O.A.; Rasoulian, B. Medicinal properties of Rosa canina L. Herb. Med. J. 2018, 3, 77–84. [Google Scholar] [CrossRef]
- Mansour, B.; Shaheen, N.; Kmail, A.; Haggag, N.; Saad, S.; Sadiq, O.; Zaid, R.; Saad, B. Anti-inflammatory and anti-adipogenesis effects of Alchemilla vulgaris L., Salvia officinalis L., and Vitis vinifera L. in THP-1-derived macrophages and 3T3-L1 cell line. Immuno 2023, 3, 148–159. [Google Scholar] [CrossRef]
- Changizi-Ashtiyani, S.; Berenji, S.; Zarei, A.; Ramezani, M.; Hosseini, N. The effects of the extract of Rosa canina L. on lipid profile, liver and thyroid functions in hypercholesterolemic rats. J. Kerman Univ. Med. Sci. 2018, 25, 318–327. [Google Scholar]
- Taghizadeh, M.; Rashidi, A.A.; Taherian, A.A.; Vakili, Z.; Mehran, M. The protective effect of hydroalcoholic extract of Rosa canina (dog rose) fruit on liver function and structure in streptozotocin-induced diabetes in rats. J. Diet. Suppl. 2017, 15, 624–635. [Google Scholar] [CrossRef]
- Jurić, T.; Stanković, J.S.K.; Rosić, G.; Selaković, D.; Joksimović, J.; Mišić, D.; Stanković, V.; Mihailović, V. Protective effects of Alchemilla vulgaris L. extracts against cisplatin-induced toxicological alterations in rats. S. Afr. J. Bot. 2020, 128, 141–151. [Google Scholar] [CrossRef]
- Kılıçgün, H.; Altıner, D. Correlation between antioxidant effect mechanisms and polyphenol content of Rosa canina. Pharmacogn. Mag. 2010, 6, 238–241. [Google Scholar] [CrossRef]
- Taneva, I.; Petkova, N.; Dimov, I.; Ivanov, I.; Denev, P. Characterization of Rose hip (Rosa canina L.) fruits extracts and evaluation of their in vitro antioxidant activity. J. Pharmacogn. Phytochem. 2016, 5, 35–38. [Google Scholar]
- Yuan, X.; Wei, G.; You, Y.; Huang, Y.; Lee, H.J.; Dong, M.; Lin, J.; Hu, T.; Zhang, H.; Zhang, C.; et al. Rutin ameliorates obesity through brown fat activation. FASEB J. 2017, 31, 333–345. [Google Scholar] [CrossRef]
- Seo, S.; Lee, M.S.; Chang, E.; Shin, Y.; Oh, S.; Kim, I.H.; Kim, Y. Rutin increases muscle mitochondrial biogenesis with AMPK activation in high-fat diet-induced obese rats. Nutrients 2015, 7, 8152–8169. [Google Scholar] [CrossRef]
- Liu, Q.; Pan, R.; Ding, L.; Zhang, F.; Hu, L.; Ding, B.; Zhu, L.; Xia, Y.; Dou, X. Rutin exhibits hepatoprotective effects in a mouse model of non-alcoholic fatty liver disease by reducing hepatic lipid levels and mitigating lipid-induced oxidative injuries. Int. Immunopharmacol. 2017, 49, 132–141. [Google Scholar] [CrossRef]
- Lee, Y.M.; Yoon, Y.; Yoon, H.; Park, H.M.; Song, S.; Yeum, K.J. Dietary anthocyanins against obesity and inflammation. Nutrients 2017, 9, 1089. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Su, H.; Sun, C.; Zheng, X.; Chen, W. Recent advances in understanding the anti-obesity activity of anthocyanins and their biosynthesis in microorganisms. Trends Food Sci. Technol. 2018, 72, 13–24. [Google Scholar] [CrossRef]
- Dias, K.A.; Oliveira, L.A.; Pereira, S.M.S.; Abrantes, L.C.S.; de Souza Vicente, L.C.O.; Gonçalves, R.V.; Della Lucia, C.M. Anti-inflammatory and antioxidant effects of anthocyanins in Nonalcoholic fatty liver disease (NAFLD): A systematic review of in vivo studies. Crit. Rev. Food Sci. Nutr. 2025, 2025, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Santana-Gálvez, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Chlorogenic acid: Recent advances on its dual role as a food additive and a nutraceutical against metabolic syndrome. Molecules 2017, 22, 358. [Google Scholar] [CrossRef]
- Xue, H.; Wei, M.; Ji, L. Chlorogenic acids: A pharmacological systematic review on their hepatoprotective effects. Phytomedicine 2023, 118, 154961. [Google Scholar] [CrossRef]
- Tsai, M.C.; Wang, C.C.; Tsai, I.N.; Yu, M.H.; Yang, M.Y.; Lee, Y.J.; Chan, K.C.; Wang, C.J. Improving the effects of mulberry leaves and neochlorogenic acid on glucotoxicity-induced hepatic steatosis in high fat diet treated db/db mice. J. Agric. Food Chem. 2024, 72, 6339–6346. [Google Scholar] [CrossRef]

| Ingredient | Juice AM20 | Juice AM60 | Juice AMRC | Juice AMAV |
|---|---|---|---|---|
| Total polyphenol content (mg/L) | 7772.7 ± 321.1 | 11,237.4 ± 456.2 | 10,802.1 ± 218.3 | 15,929.1 ± 356.7 |
| Flavonoids | ||||
| Quercetin | 90.4 ± 8.7 | 49.6 ± 3.2 | 42.2 ± 2.5 | 76.7 ± 5.2 |
| Rutin | 593.1 ± 21.3 | 446.5 ± 12.5 | 382.2 ± 15.6 | 2614.0 ± 189.5 |
| Catechin | -- | -- | 1230.4 ± 78.9 | 1731.5 ± 95.2 |
| Epicatechin | 251.4 ± 14.2 | 408.2 ± 25.6 | 383.3 ± 29.1 | 858.6 ± 50.2 |
| Total anthocyanins | 863.8 | 2125.0 | 1359.1 | 2148.4 |
| Cyanidin-3-galactoside | 638.5 ± 21.0 | 1498.4 ± 102.3 | 991.5 ± 45.6 | 1507.0 ± 58.2 |
| Cyanidin-3-glucoside | 44.4 ± 4.1 | 120.1 ± 8.7 | 78.9 ± 3.6 | 133.5 ± 9.8 |
| Cyanidin-3-arabinoside | 177.5 ± 13.2 | 501.9 ± 31.8 | 285.4 ± 14.5 | 502.3 ± 45.6 |
| Cyanidin-3-xyloside | 2.73 ± 0.2 | 4.59 ± 0.2 | 3.25 ± 0.3 | 5.51 ± 0.5 |
| Phenolic acids | ||||
| Chlorogenic acid | 1142.9 ± 81.2 | 1375.6 ± 80.3 | 1262.9 ± 56.2 | 1809.7 ± 103.8 |
| Neochlorogenic acid | 1305.2 ± 102.8 2027.0 ± 131.8 | 1543.1 ± 111.2 | 1499.2 ± 96.5 | 2027.0 ± 131.8 |
| ORAC, μmol TE/L | 81,256 ± 6545 | 122,545 ± 9849 | 138,569 ± 10,253 | 168,456 ± 10,458 |
| Diet | Proteins (Per 100 g) | Fat (Per 100 g) | Carbohydrates | |
|---|---|---|---|---|
| Starch (Per 100 g) | Sugars (Per 100 g) | |||
| Standard diet | 20.48 g | 3 g | 38.3 g | 3 g |
| HFHF diet | 13.65 g | 18.67 g | 25.5 g | 19.55 g |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Georgieva, A.; Eftimov, M.; Stefanova, N.; Tzaneva, M.; Denev, P.; Valcheva-Kuzmanova, S. Effects of Aronia melanocarpa-Based Fruit Juices on Metabolic Dysfunction-Associated Fatty Liver Disease in Rats. Gastroenterol. Insights 2025, 16, 23. https://doi.org/10.3390/gastroent16030023
Georgieva A, Eftimov M, Stefanova N, Tzaneva M, Denev P, Valcheva-Kuzmanova S. Effects of Aronia melanocarpa-Based Fruit Juices on Metabolic Dysfunction-Associated Fatty Liver Disease in Rats. Gastroenterology Insights. 2025; 16(3):23. https://doi.org/10.3390/gastroent16030023
Chicago/Turabian StyleGeorgieva, Antoaneta, Miroslav Eftimov, Nadezhda Stefanova, Maria Tzaneva, Petko Denev, and Stefka Valcheva-Kuzmanova. 2025. "Effects of Aronia melanocarpa-Based Fruit Juices on Metabolic Dysfunction-Associated Fatty Liver Disease in Rats" Gastroenterology Insights 16, no. 3: 23. https://doi.org/10.3390/gastroent16030023
APA StyleGeorgieva, A., Eftimov, M., Stefanova, N., Tzaneva, M., Denev, P., & Valcheva-Kuzmanova, S. (2025). Effects of Aronia melanocarpa-Based Fruit Juices on Metabolic Dysfunction-Associated Fatty Liver Disease in Rats. Gastroenterology Insights, 16(3), 23. https://doi.org/10.3390/gastroent16030023








