Abstract
Background/Objectives: Thalassemia, a hereditary blood disorder, leads to reduced hemoglobin levels, impairing oxygen transport and negatively impacting patient health. Recent research suggests a possible association between thalassemia and gastrointestinal (GI) symptoms, such as abdominal pain, diarrhea, and GI bleeding, potentially due to immune compromise and iron overload. This systematic review aims to explore the prevalence and underlying factors of GI pathologies in thalassemia patients, excluding treatment-related effects and iron overload. Methods: A comprehensive search following the PRISMA guidelines was conducted to identify the prevalence and causes of GI disorders in thalassemia patients. Studies assessing non-treatment-related GI symptoms and their links to thalassemia were analyzed. After screening 1902 studies, 13 were included to investigate gastrointestinal manifestations in thalassemia patients. Results: Evidence indicates potential associations between thalassemia and GI disorders, including malabsorption, inflammatory bowel disease, Heliobacter pylori (H. pylori) infection, and celiac disease. Findings highlight immune compromise and iron dysregulation as possible contributing factors. Conclusions: This review highlights the importance of further research into the GI manifestations of thalassemia to enable early detection and improve patient health outcomes and quality of life. Addressing this gap may provide insights into better clinical management strategies for thalassemia patients.
1. Introduction
Thalassemia is a genetic disorder characterized by a dysfunction in hemoglobin production, leading to either abnormal or insufficient hemoglobin production in the blood. It is a common monogenetic disorder most prevalent in the Mediterranean, Middle East, and South Asia [1].
Mutations in the α-globin (HBA1/HBA2) and β-globin (HBB) genes cause α-thalassemia and β-thalassemia, respectively. Both types are usually inherited in an autosomal recessive manner [2]. Hemoglobin is the oxygen-carrying component of red blood cells and consists of two types of proteins: alpha and beta. When the body does not produce enough of either protein, red blood cells cannot form correctly and fail to carry sufficient oxygen, leading to anemia and ineffective red blood cell production that begins in early childhood and persists throughout life [3].
Thalassemia is classified into α-thalassemia and β-thalassemia, according to which globin chain subunit is affected [1]. In alpha-thalassemia, mutations occur in the HBA1 and HBA2 genes on chromosome 16, resulting in decreased alpha-globin production. In contrast, beta-thalassemia is caused by mutations in the HBB gene on chromosome 11, leading to reduced or absent beta-globin synthesis. Therefore, the balance of globin chains is disrupted, leading to ineffective red blood cell production and anemia [4,5].
In alpha-thalassemia, the decreased production of alpha-globin chains results in an excess of beta-globin chains, whereas beta-thalassemia involves reduced beta-globin chain synthesis, leading to an excess of alpha-globin chains. The clinical manifestations of these disorders vary based on the number of affected genes and the severity of the imbalance [6].
β-thalassemia was originally classified as β-thalassemia minor (thalassemia trait/thalassemia carrier), whose patients are usually asymptomatic with mild anemia. β-thalassemia intermedia and major can either be homozygous or compound heterozygous for β-thalassemia mutations. Generally, patients with thalassemia major present earlier in life and have more severe anemia than those with thalassemia intermedia [7].
In a more recent classification of β-thalassemia, patients are classified clinically into transfusion-dependent (TDT) and non-transfusion-dependent (NTDT) groups based on the severity of their condition and the need for frequent transfusions. TDT patients require frequent lifelong transfusions for survival, and NTDT patients are independent of transfusions for survival but may still require occasional transfusions in cases of hemodynamic instability [8]. α-thalassemia is classified into four types based on the number of α-globin gene deletions. Individuals with a single gene mutation are said to have silent α-thalassemia and mild asymptomatic anemia. Those with two genes affected are said to have thalassemia traits and are asymptomatic with mild anemia. Those with three affected genes have hemoglobin H (HbH) disease and develop moderate to severe anemia, classified as NTDT. Those that have four genes affected develop Hb Bart’s hydrops fetalis, with most cases dying before or shortly after delivery [9]. Various studies have investigated the association between thalassemia disease traits, such as beta-thalassemia, with various autoimmune diseases [10]. Others [11] investigated cancer incidence in thalassemia patients and found that thalassemia patients are at a greater risk of developing malignancies, such as hepatocellular carcinoma and lymphoma, than the general population.
Thalassemia is characterized by erythropoiesis dysfunction, a secondary hypercoagulable state, and increased intestinal iron absorption and overload [7]. This could, in turn, affect the prevalence and presentation of gastrointestinal (GI) diseases. Abdominal pain is a common presentation in beta-thalassemia patients with multiple possible etiologies, such as cholelithiasis, hypersplenism, pancreatitis, and kidney stones. Thus, identifying the exact cause of a patient’s symptoms can sometimes be challenging. Furthermore, thalassemia patients with frequent blood transfusions and increased dietary iron absorption can experience iron overload, which is associated with GI symptoms and liver damage [12,13]. Iron chelation therapies, such as deferasirox, have been linked to GI-related side effects, including diarrhea, abdominal pain, nausea and vomiting, and rarely, GI perforation [14,15].
The prevalence of these GI symptoms varies, as iron overload-associated GI injuries are more common in transfusion-dependent patients. GI malabsorption symptoms, such as carbohydrate and histamine intolerance, are less common and seen in patients with β-thalassemia minor, leading to abdominal complaints [16,17].
Thalassemia patients are reported to have alterations in their immune system, with defective phagocyte function, reduced lymphocyte differentiation, and an altered CD4/CD8 ratio [18]. Most published studies focus primarily on GI symptoms directly related to complications from iron overload. Therefore, this paper aimed to investigate other GI pathologies that may be indirectly related to treatment and iron overload, although the exact mechanisms are not fully understood. Conditions such as H. pylori gastritis, malabsorption (including celiac disease), and an increased risk of inflammatory bowel disease (IBD) may be overlooked in these patients. Our systematic review identified few studies that have examined these issues, emphasizing the need for further research in this area.
2. Methodology
This systematic review aimed to investigate gastrointestinal (GI) pathologies associated with thalassemia and to identify the contributing factors for their development. Following the PRISMA guidelines, a comprehensive search was conducted across PubMed, the Cochrane Library, and Google Scholar using the following search terms: (“Thalassemia” OR “Thalassemia major”) AND (“Gastritis” OR “Esophagitis” OR “Colitis” OR “Ischemic colitis” OR “Perforation” OR “Infection” OR “Ileitis” OR “Inflammatory bowel disease” OR “Celiac disease” OR “Gastrointestinal diseases” OR “Hemorrhagic colitis”). As shown in Figure 1, the initial search yielded 2079 studies after the removal of 177 duplicates. 1902 studies were screened. Of these, 1843 studies were excluded for reasons such as a lack of relevance to thalassemia patients with GI manifestations or because they consisted of letters, conference abstracts, review articles, or studies primarily focused on pregnancy- or lactation-related complications, as well as blood transfusion-related pathologies. 59 studies were sought for full-text review, but 9 could not be retrieved. Of the 50 studies assessed for eligibility, 37 were excluded for reasons such as focusing on therapies/drug treatments, no relation of symptoms to thalassemia, or being in languages other than English.
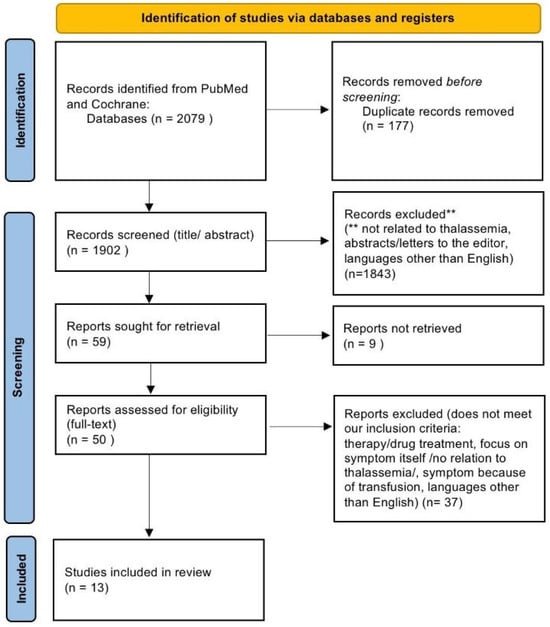
Figure 1.
Study selection process following PRISMA guidelines.
Finally, n = 13 studies were included in this review. Among the 13 studies included, 6 were case reports, 5 were case–control studies, and 2 were cohort studies. Six reviewers independently screened titles and abstracts to assess eligibility, and discrepancies were resolved through discussion. The findings were systematically summarized to address the study’s objectives.
3. Results
3.1. H. pylori and Thalassemia
Helicobacter (H. pylori), a gram-negative bacterium, colonizes the human gastric mucosa. This pathogen is the main cause of chronic gastritis, peptic ulcers, gastric cancer, and dyspepsia [19,20]. Considering the high rate of gastric diseases among thalassemia patients, a correlation between H. pylori and β-thalassemia was postulated. Some articles have shown a probable relationship between H. pylori and recurrent abdominal pain (RAP) in people with hemoglobinopathies, such as sickle cell anemia and β-thalassemia [21,22].
RAP is a clinical term used to describe chronic or intermittent abdominal pain lasting for at least three months and severe enough to interfere with daily activities. The Rome criteria, a standardized diagnostic framework for functional gastrointestinal disorders (FGIDs), classify RAP into specific subtypes, including functional dyspepsia, irritable bowel syndrome (IBS), abdominal migraine, and functional abdominal pain—not otherwise specified (FAP-NOS). However, distinguishing between RAP caused by H. pylori infection and that resulting from β-thalassemia remains complex [23].
In a survey by Karimi et al., H. pylori infection was more common in β-thalassemia major patients with RAP than in non-thalassemia controls with RAP. Although the difference was insignificant, the authors suggested that H. pylori may increase the risk of RAP in thalassemia patients. Overall, the prevalence of H. pylori was 43% of the study population. This rate was greater in the β-thalassemia minor group (53%) than in the control group (33%), and the difference was significant (odds ratio [OR] = 2.29, 95% CI: 1.3–4.06; p = 0.005) [24]. The rate of H. pylori positivity in β-thalassemia major patients with RAP was slightly higher than in healthy controls with the same symptoms (68% compared with 60%, p > 0.05) [24].
Furthermore, another study investigated the prevalence of H. pylori infection in β-thalassemia major patients with RAP among 50 β-thalassemia patients and 50 controls. H. pylori infection was observed more frequently in β-thalassemia patients (68%) than in controls (60%), although this difference was not statistically significant. The study revealed a significant association between H. pylori prevalence and age, duration of blood transfusions, and splenectomy, indicating that older patients, those with longer transfusion histories, and splenectomized individuals were more likely to be infected. Additionally, gastritis was the most common gastrointestinal pathology and was present in 72% of thalassemia patients. However, the presence of H. pylori did not strongly correlate with specific GI symptoms, such as pain intensity or location [21].
While some studies suggest a possible association between H. pylori and RAP in thalassemia patients, the lack of a correlation between infection and specific gastrointestinal symptoms implies that other factors, such as gastritis or iron-related oxidative stress, may play a more significant role. Further research with well-defined control groups and objective symptom assessment based on the Rome criteria is necessary to clarify this relationship.
3.2. Inflammatory Bowel Disease (IBD) and Thalassemia
Inflammatory bowel disease (IBD) is an idiopathic chronic inflammatory condition of the GI tract, with two main subtypes: ulcerative colitis and Crohn’s disease. Both conditions are characterized by periods of remission and flare-ups, resulting in symptoms such as abdominal pain, diarrhea, and weight loss. The precise pathogenesis of IBD remains unclear, but it is believed to result from inappropriate immune responses in genetically predisposed individuals. Crohn’s disease can affect any part of the GI tract, from the mouth to the anus, and is characterized by transmural inflammation, which can lead to complications such as fissures, strictures, and fistulas. In contrast, ulcerative colitis primarily involves continuous inflammation and ulceration of the colon, typically involving the rectum.
Anemia is one of the most common extra-intestinal manifestations of IBD, with a reported prevalence of 27% in patients with Crohn’s disease and 21% in those with ulcerative colitis [25]. Most commonly, it presents as iron deficiency anemia, and anemia of chronic disease [26,27]. There is limited evidence on the association between IBD and thalassemia major or thalassemia traits; this includes a single cross-sectional study and a few case reports, all of which have shown evidence of thalassemia’s association with Crohn’s disease, except for one showing a possible thalassemia association with ulcerative colitis.
A cross-sectional study explored the prevalence and causes of anemia in patients with inflammatory bowel disease (IBD), including the association between thalassemia and IBD [28]. The study included 965 patients with confirmed IBD diagnoses, comprising 383 with ulcerative colitis and 582 with Crohn’s disease. Disease severity was assessed using the Crohn’s Disease Activity Index (CDAI), allowing for a comparison of the clinical activity of UC and Crohn’s patients. Among the 134 anemic individuals identified, only 9 (6.7%) had thalassemia traits, of whom 6 were diagnosed with Crohn’s disease.
In another case report [29], a 15-year-old female patient presented with fatigue, abdominal discomfort, diarrhea, and rectal blood loss. Laboratory tests showed microcytic anemia consistent with iron deficiency and evidence of thalassemia. A colonoscopy showed pancolitis and lesions in the cecal region. A histological examination confirmed the Crohn’s disease diagnosis. Further investigations, including a 99mTc thyroid scan, revealed diffuse decreased uptake, leading to a diagnosis of Crohn’s disease, autoimmune thyroiditis, and thalassemia. The patient received a treatment regimen of propylthiouracil, levothyroxine, mesalazine, and prednisolone, resulting in the resolution of her symptoms.
A 65-year-old man presented with long-standing anemia that was resistant to iron supplementation. The patient was a known case of thalassemia minor. His CBC tests revealed that his ferritin hemoglobin levels, hematocrit, and red blood cell counts were all within the normal ranges, and his MCH and MCHC indicated hypochromic microcytic anemia. Histological examination of a resected part of the small bowel revealed chronic active enteritis with increased submucosal fibrosis, which is characteristic of Crohn’s disease. The patient was treated for Crohn’s disease, and his gastrointestinal bleeding improved [30].
Moreover, a 28-year-old male was admitted with iron deficiency anemia (IDA) and bloody diarrhea. Electrophoresis revealed thalassemia traits. An abdominal ultrasound of the liver revealed coarse parenchymal echogenicity and splenomegaly. A biopsy from the duodenum revealed inflammation and the reduced fold characteristics of celiac disease. His liver biopsy revealed chronic inflammation. He also underwent a colonoscopy and demonstrated diffuse erythema, pseudo polyps and pathological ulcerative colitis with mucosal dysplasia and adenocarcinoma of the splenic flexure [31].
On the other hand, the prevalence of thalassemia in anemic patients with IBD was 6.7% [28,31]. This is not a significant finding, as the prevalence of thalassemia in Italy, where this study was conducted, is 6–8%, indicating no likely association between thalassemia and IBD.
Case–control studies have shown that the most common presentation for thalassemia patients with IBD is iron deficiency anemia, as these patients are more susceptible to developing anemia than non-thalassemia patients. The thalassemia traits can also make diagnosing anemia more difficult, as it masks iron deficiency as seen in [30].
It has been suggested that patients with thalassemia have lower levels of IL-10, which is an important factor for immune homeostasis in the colon [10]. Consequently, thalassemia patients may be at a higher risk of developing severe colitis, leading to more severe symptoms and greater iron deficiency anemia. While this relationship has not yet been proven and requires further investigation, as no studies specifically explore the severity of anemia in thalassemia patients, the co-existence of the two diseases would likely present with more severe anemia, especially iron deficiency anemia as is reported in cases [29,30,31].
3.3. Malabsorption in Thalassemia Including Celiac Disease
3.3.1. Celiac Disease and Thalassemia
Celiac disease is a gluten-mediated type IV hypersensitivity autoimmune disease. CD4+ T helper cells are triggered by gliadin, leading to an inflammatory process that damages the intestinal lining every time gluten is consumed, which leads to chronic diarrhea, steatorrhea, abdominal pain, bloating, malabsorption, dermatitis herpetiformis, malabsorption symptoms, and other symptoms [32]. Celiac disease has been associated with other autoimmune and non-autoimmune diseases [33]. The association of celiac disease with thalassemia traits was examined in the following three case–control studies.
In the first case–control study [34], 200 children diagnosed with beta-thalassemia major were compared with 420 healthy control children for celiac disease by measuring tissue transglutaminase (tTG-IGA). The cases had significantly higher levels of tTG than the controls; 11.5% of the cases presented a titer equal to or greater than 20 U/mL (which was the cutoff point for the diagnosis), whereas 3.5% of the controls presented a diagnostic titer.
In the second case–control study [35], 215 high school-aged thalassemia major patients were compared to 1500 controls in the same age group for celiac disease diagnosis. This was performed by measuring tTG levels, followed by a biopsy to confirm the diagnosis. The results revealed that none of the patients had elevated tTG levels, whereas 2% of the controls had elevated tTG levels, with biopsy confirming the diagnosis in 0.6% of total controls.
In the third case–control study [36], 100 B-thalassemia minor children were compared with 200 healthy controls for celiac disease diagnosis; tTG titer levels with a cutoff point of 20 U/mL were used. Compared with 5% of the controls, 26% of the patients had a higher risk for celiac disease, as 26% had a diagnostic titer. There was no statistically significant difference in the BMI between the two groups.
The conflicting evidence in those studies, which are the only case–control studies on the association between thalassemia and celiac disease, indicates the need for further investigation of the association between these two diseases. Importantly, the diagnosis of celiac disease in these studies was reliant mainly on the tTG titer, and all participants, cases and controls, were asymptomatic for celiac disease. This, in turn, can affect the accuracy of the diagnosis.
A 10-year-old child with B-thalassemia major presented to the thalassemia day care center with growth retardation. Malabsorption syndrome is suspected even without GI symptoms [37]. Further investigation revealed elevated levels of tTG, classic jejunal mucosal lesions with an absence of normal intestinal villi on biopsy, flattened epithelial surface loss of the villus structure, and lymphocyte infiltration on histology, confirming the diagnosis.
Additionally, a 17-year-old male patient with B-thalassemia major presented for routine evaluation. As his height was always slightly below the third percentile, he presented delayed bone age and delayed pubertal development. Following a gonadotropin-releasing hormone (GnRH) stimulation test, the patient developed hypogonadotropic hypogonadism and was started on testosterone therapy. Following routine thyroid function tests every 6 months, he developed primary hypothyroidism and was started on L-thyroxin (100 Ug/d). The patient was lost to follow-up until he was 26-years-old when he presented with unrelated events. He presented with primary hypothyroidism, GH deficiency, growth retardation (height: 159 cm, weight: 45 kg), and a pubertal stage of P2 G2 according to Tanner standards. He was started on L-thyroxin again but required doses that were too high for his body weight, which led to a suspicion of malabsorption even in the absence of GI symptoms [38]. Anti-gliadin antibodies (AGA IgA–IgG) and anti-endomysial antibodies (EMAs) were positive, characteristic jejunal lesions were found on biopsy, and grossly, the mucosal surface was flat with the complete absence of normal intestinal villi. The histological examination confirmed the diagnosis of a flattened epithelium, lymphocyte infiltration, loss of villus structure, and intestinal crypt hypertrophy.
In the case report of a 21-year-old girl with thalassemia major, the patient developed recurrent abdominal pain and diarrhea followed by an unexplained drop in ferritin a few months later, which required a lower dose of deferoxamine. A screening for celiac disease revealed anti-transglutaminase IgA > 100 UA/mL and positive anti-endomysium antibodies [39]. A reduction in the number and height of folds was detected in the bulb and duodenum via upper GI endoscopy, and histological examination confirmed the celiac disease diagnosis. After being fed a gluten-free diet, ferritin levels rose again, necessitating a higher dose of deferoxamine.
The following are case reports for two children who presented with thalassemia and celiac disease [40]. A 1-year-old child was positive for anti-endomysium antibodies, and endoscopy confirmed the diagnosis after developing enamel hypoplasia. A 14-year-old girl experienced height and weight decline throughout childhood and developed episodes of abdominal cramps and diarrhea. The serological screening was positive, as was the duodenal endoscopy. The cramps and diarrhea disappeared after the start of a gluten-free diet.
The most common presentation of celiac disease in these studies was failure to thrive, abdominal pain, diarrhea, and other malabsorption symptoms. However, there is no evidence that the presentation of celiac disease differs from that of a non-thalassemic patient. Importantly, celiac disease patients can present with GI symptoms as well as non-GI symptoms. Many of these non-GI symptoms overlap with thalassemia symptoms, such as failure to thrive, delayed puberty, elevated liver enzymes, joint pain, and fatigue, so it is important not to overlook these symptoms in thalassemia patients and consider them for celiac disease screening.
The mechanism for the possible association between these two diseases is poorly understood. In [10], they argued that since both thalassemia and celiac disease are linked to the HLADQA1 and DQB1 alleles it is possible to have an association in the prevalence of the two. They also argued that the decrease in ferritin in celiac disease patients is due to the upregulation of hepcidin by IL-6, which leads to a defect in iron absorption in the GI tract. Another factor for consideration is that both thalassemia and celiac disease are associated with autoimmune endocrine disorders, such as diabetes mellitus, thyroid dysfunction, and glucose intolerance [41,42], which may be evidence of a link between these two diseases.
3.3.2. Malabsorption and Nutrient Deficiency in Thalassemia
Intestinal insufficiency or deficiency refers to a decrease in gut absorptive function that does not require intravenous supplementation for health and growth maintenance. Malabsorption is the impaired absorption of nutrients caused by any disruption in the normal absorption process [43,44]. This can lead to deficiencies in essential nutrients, such as vitamin C, which are crucial in various bodily functions. For example, vitamin C deficiency, known as scurvy, can lead to symptoms such as muscle weakness, bleeding gums, anemia, and impaired wound healing [43,44,45]. A study by Liakakos et al. [46] investigated ascorbic acid (vitamin C) malabsorption in children with thalassemia. The findings revealed that thalassemic children had significantly lower plasma levels of ascorbic acid than healthy controls, indicating malabsorption, particularly after oral administration. Thus, this suggests an association between thalassemia and ascorbic acid malabsorption. Understanding malabsorption and its implications is crucial for effectively managing conditions such as thalassemia and preventing deficiencies in essential nutrients.
In patients with thalassemia, a deficiency in vitamin C can worsen anemia by affecting iron absorption, increasing oxidative stress, reducing the effectiveness of iron chelation therapy, and impairing hemoglobin synthesis. As a result, anemia can be severe in such cases. Thus, vitamin C supplementation is crucial to mitigate anemia severity [47,48].
3.4. Immunological Changes in Thalassemia MajorW
Herpes simplex virus (HSV), which belongs to the Herpesvirida family, is a common infection that can cause painful blisters or ulcers and spreads primarily through skin-to-skin contact, causing long-standing infection, which is characterized by primary infection that remains dormant in the nervous system and then reactivates when immunity decreases. The primary infection is typically mild or asymptomatic, despite its ability to cause many symptoms in different organs, such as the mouth (cold sores), gingivostomatitis (sores inside the mouth), esophagus (herpes cogitates), and liver hepatitis, which mostly occurs in immunocompromised patients. A case–control study examined the seroprevalence of herpes simplex 1,2 IgG antibodies in patients with thalassemia major. This study included 45 patients who were previously diagnosed with beta-thalassemia major according to clinical and laboratory findings and 45 healthy controls.
The patients and controls were then divided according to their age into three groups: the first group (2–10 years), the second group (11–20 years), and the third group (21–30 years). After that, they undertook a serological study of HSV-1 and HSV-2 IgG antibodies among cases and controls via ELISA. They reported that the prevalence of HSV1,2 IgG antibodies was estimated to be 88.8% among patients with thalassemia and 77.7% in the control group. The p value was 0.64, indicating no significant difference between the two groups [49,50].
3.5. Alpha-Thalassemia X-Linked Intellectual Disability (ATR-X) Syndrome and GI Complications
Alpha-thalassemia X-linked intellectual disability (ATR-X) syndrome is a rare genetic disorder affecting multiple organ systems of the body [51]. The rare association between alpha-thalassemia and mental retardation (ATR) was recognized in the 1990s [52] and is characterized by severe to profound mental retardation, characteristic facial appearance, genital anomalies, and alpha-thalassemia [53]. Thalassemia arises when there is a defect in the synthesis of the globin chains of adult hemoglobin (HbA, a2b2). When they described three children with mental retardation with thalassemia and a variety of developmental abnormalities, their interest was stimulated by the unusual nature of thalassemia [54]. Patients with ATR-X syndrome often present with a range of GI issues, particularly feeding difficulties, regurgitation and vomiting, abdominal pain, distension, and chronic constipation [55]. In addition to these common complications, some patients experience more serious conditions, such as upper GI bleeding, aspiration of vomiting, intestinal malrotation, ileus, or pseudo-obstruction, which can be fatal in certain cases [56]. The GI management of ATR-X syndrome remains challenging. In some cases, some surgical interventions, such as fundoplication and gastrostomy, have been used to manage severe gastroesophageal reflux (GER). These early interventions resulted in reasonable nutritional management and significantly improved quality of life [56].
It is important to note that most but not all of the listed GI symptoms can be attributed to mental retardation.
3.6. Anomalies in Homozygous Alpha-Thalassemia Patients
Homozygous alpha-thalassemia results from the inactivation or deletion of both alpha-globin genes on chromosome 16, leading to the formation of hemoglobin Bart’s (Hb Bart’s), which predominates in fetal life and has a poor oxygen-carrying capacity [57]. Only one case report was found [58], and they reported a female infant born at 35 weeks, to a mother from China, who presented with severe complications associated with Hb Bart’s disease. The infant presented with aplastic/hypoplastic digits and hepatosplenomegaly upon examination. Persistent pulmonary hypertension was diagnosed, requiring respiratory support and a double-volume exchange transfusion. A double-bubble sign observed on the abdominal X-ray led to the diagnosis of jejunal atresia, a GI complication that required surgical intervention. After laparotomy and jejunal atresia repair, the infant’s pulmonary hypertension temporarily worsened, requiring high-frequency ventilation. However, her lung function gradually improved over the following days. This case highlights a possible association between homozygous alpha-thalassemia and GI abnormalities such as jejunal atresia.
Table 1 below provides a summary of the key findings from our results.

Table 1.
Summary of study results.
4. Conclusions
In conclusion, this systematic review reveals a complex relationship between thalassemia and various GI disorders. The findings indicate that patients with thalassemia, especially those with beta-thalassemia major, may have a higher prevalence of GI conditions such as H. pylori infections, malabsorption syndromes, and autoimmune disorders like celiac disease and inflammatory bowel disease (IBD). Additionally, this study emphasizes the importance of exploring other causes of iron deficiency anemia in these patients. The evidence remains inconsistent due to varying data quality, small sample sizes, and methodological limitations across different studies. Thus, this highlights the need for further research focusing on cohort studies and trials with standardized diagnostic criteria to strengthen the current understanding of these associations, understand the underlying mechanisms, and enhance our understanding.
Moreover, the early management of these GI symptoms can significantly improve the quality of life and overall long-term outcomes for patients with thalassemia.
Author Contributions
Supervision: D.A.; Project Leadership: S.F. and A.M.; Team Coordination: R.A. and B.S.; Methodology: S.F.; Investigation: S.F., L.A.-R., A.M., R.A., B.S. and O.A.; Writing: S.F., L.A.-R., A.M., R.A., B.S. and O.A.; Review and editing: D.A., S.F. and L.A.-R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Rao, E.; Kumar Chandraker, S.; Misha Singh, M.; Kumar, R. Global distribution of β-thalassemia mutations: An update. Gene 2024, 896, 148022. [Google Scholar] [CrossRef] [PubMed]
- Angastiniotis, M.; Lobitz, S. Thalassemias: An Overview. Int. J. Neonatal Screen. 2019, 5, 16. [Google Scholar] [CrossRef] [PubMed]
- Bajwa, H.; Basit, H. Thalassemia; StatPearls: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK545151/ (accessed on 24 February 2025).
- MedlinePlus Genetics. Alpha Thalassemia; National Library of Medicine: Bethesda, MD, USA, 2020. Available online: https://medlineplus.gov/genetics/condition/alpha-thalassemia/ (accessed on 19 February 2025).
- MedlinePlus Genetics. Beta Thalassemia; National Library of Medicine: Bethesda, MD, USA, 2020.
- Baird, D.C.; Batten, S.H.; Sparks, S.K. Alpha- and Beta-thalassemia: Rapid Evidence Review. Am. Fam. Physician 2022, 105, 272–280. [Google Scholar] [PubMed]
- Origa, R. Beta-Thalassemia. Genet. Med. 2017, 19, 609–619. [Google Scholar] [CrossRef]
- Musallam, K.M.; Rivella, S.; Vichinsky, E.; Rachmilewitz, E.A. Non-transfusiondependent thalassemias. Haematologica 2013, 98, 833–844. [Google Scholar] [CrossRef]
- Farashi, S.; Harteveld, C.L. Molecular basis of alpha-thalassemia. Blood Cells Mol. Dis. 2018, 70, 43–53. [Google Scholar] [CrossRef]
- El Hasbani, G.; Musallam, K.M.; Uthman, I.; Cappellini, M.D.; Taher, A.T. Thalassemia and autoimmune diseases: Absence of evidence or evidence of absence? Blood Rev. 2022, 52, 100874. [Google Scholar] [CrossRef]
- Hodroj, M.H.; Bou-Fakhredin, R.; Nour-Eldine, W.; Noureldine, H.A.; Noureldine, M.H.A.; Taher, A.T. Thalassemia and malignancy: An emerging concern? Blood Rev. 2019, 37, 100585. [Google Scholar] [CrossRef]
- Cappellini, M.D.; Cohen, A.; Eleftheriou, A.; Piga, A.; Porter, J.; Taher, A. Chapter 8, The Liver in Thalassaemia. In Guidelines for the Clinical Management of Thalassaemia, 2nd Revised ed.; Thalassaemia International Federation: Nicosia, Cyprus, 2008. Available online: https://www.ncbi.nlm.nih.gov/books/NBK173968/ (accessed on 24 February 2025).
- Cappellini, M.D.; Cohen, A.; Eleftheriou, A.; Piga, A.; Porter, J.; Taher, A. Chapter 18, Outline of Diagnostic Dilemmas in Thalassaemia. In Guidelines for the Clinical Management of Thalassaemia, 2nd Revised ed.; Thalassaemia International Federation: Nicosia, Cyprus, 2008. Available online: https://www.ncbi.nlm.nih.gov/books/NBK173977/ (accessed on 24 February 2025).
- Chauhan, D.; Kilic, Y.; Segal, J.; Patel, N.; Koizia, L. An Unusual Cause of Gastrointestinal Perforation in an Adolescent Patient With Beta-Thalassemia on Deferasirox and SARS-CoV-2 Infection. J. Hematol. 2021, 10, 76–79. [Google Scholar] [CrossRef]
- Drugs.com. Deferasirox Side Effects; c2000–2025. Available online: https://www.drugs.com/sfx/deferasirox-side-effects.html (accessed on 19 February 2025).
- Schnedl, W.J.; Mangge, H.; Schenk, M.; Enko, D. β-thalassemia minor, carbohydrate malabsorption and histamine intolerance. Hematol. Rep. 2017, 9, 7043. [Google Scholar] [CrossRef]
- Taher, A.T.; Musallam, K.M.; Karimi, M.; El-Beshlawy, A.; Belhoul, K.; Daar, S.; Saned, M.S.; El-Chafic, A.H.; Fasulo, M.R.; Cappellini, M.D. Prevalence and distribution of iron overload in patients with β-thalassemia major: A multicenter study. Eur. J. Haematol. 2014, 93, 520–527. [Google Scholar] [CrossRef]
- Ezer, U.; Gulderen, F.; Culha, V.K.; Akgul, N.; Gurbuz, O. Immunological status of thalassemia syndrome. Pediatr. Hematol. Oncol. 2002, 19, 51–58. [Google Scholar] [CrossRef] [PubMed]
- McColl, K.E. Clininal practice Helicobacter pylori infection. N. Engl. J. Med. 2010, 362, 1597–1604. [Google Scholar] [CrossRef] [PubMed]
- Mentis, A.; Lehours, P.; Mégraud, F. Epidemiology and diagnosis of Helicobacter pylori infection. Helicobacter 2015, 20, 1–7. [Google Scholar] [CrossRef]
- Balci, Y.I.; Aral, Y.Z.; Covut, I.E.; Polat, Y.; Turk, M.; Acimis, N. The frequency of Helicobacter pylori infection in Beta Thalassemia major Patients with recurrent abdominal pain. Pak. J. Med. Sci. 2011, 27, 316–319. [Google Scholar]
- Woods, K.F.; Onuoha, A.; Schade, R.R.; Kutlar, A. Helicobacter pylori infection in sickle cell disease. J. Natl. Med. Assoc. 2000, 92, 361–365. [Google Scholar]
- Drossman, D.A. Functional Gastrointestinal Disorders: History, Pathophysiology, Clinical Features, and Rome IV. Gastroenterology 2016, 150, 1262–1279.e2. [Google Scholar] [CrossRef]
- Karimi, M.; Imanieh, M.H.; Ghiam, A.F.; Hashemi, Z. Investigation of Helicobacter pylori infection in β- thalassaemia major patients with recurrent abdominal pain. Eur. J. Gastroenterol. Hepatol. 2005, 17, 1363–1367. [Google Scholar] [CrossRef]
- Filmann, N.; Rey, J.; Schneeweiss, S.; Ardizzone, S.; Bager, P.; Bergamaschi, G.; Koutroubakis, I.; Lindgren, S.; de la Morena, F.; Moum, B.; et al. Prevalence of anemia in inflammatory bowel diseases in European countries: A systematic review and individual patient data meta-analysis. Inflamm. Bowel Dis. 2014, 20, 936–945. [Google Scholar] [CrossRef]
- Gomollón, F.; Gisbert, J.P. Anemia and inflammatory bowel diseases. World J. Gastroenterol. 2009, 15, 4659–4665. [Google Scholar] [CrossRef]
- Reinisch, W.; Staun, M.; Bhandari, S.; Muñoz, M. State of the iron: How to diagnose and efficiently treat iron deficiency anemia in inflammatory bowel disease. J. Crohns. 2013, 7, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Testa, A.; Rispo, A.; Romano, M.; Riegler, G.; Selvaggi, F.; Bottiglieri, E.; Martorano, M.; Rea, M.; Gravina, A.; Nardone, O.M.; et al. The burden of anaemia in patients with inflammatory bowel diseases. Dig. Liver Dis. 2016, 48, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Bank, I.; Busari, J.O. Crohn’s disease, autoimmune thyroiditis, and beta-thalassemia trait in an adolescent: An unusual combination of diseases. Eur. J. Pediatr. 2008, 167, 1343–1346. [Google Scholar] [CrossRef]
- Dubcenco, E.; Jeejeebhoy, K.N.; Streutker, C.J.; Zalev, A.H.; Garvey, M.B.; Kim, Y.-I.; Baker, J.P. A patient with anemia of obscure origin: Crohn’s disease in disguise. Nat. Clin. Pract. Gastroenterol. Hepatol. 2006, 3, 229–233. [Google Scholar] [CrossRef]
- Valizadeh, N.; Shateri, K. Association of celiac disease with inflammatory bowel disease and colonic cancer and liver involvement in a case of β-thalassemia minor. Shiraz E-Med. J. 2009, 10, 90–92. [Google Scholar]
- Oxentenko, A.S.; Rubio-Tapia, A. Celiac Disease. Mayo Clin. Proc. 2019, 94, 2556–2571. [Google Scholar] [CrossRef]
- Nijhawan, S.; Katiyar, P.; Nagaich, N.; Saradava, V.; Nijhawan, M.; Gupta, G.; Mathur, A.; Sharma, R.; Nepalia, S. Prevalence of associated disorders in Indian patients with celiac disease. Indian J. Gastroenterol. 2013, 32, 330–334. [Google Scholar] [CrossRef]
- Shahramian, I.; Dehghani, S.M.; Haghighat, M.; Noori, N.M.; Teimouri, A.R.; Sharafi, E.; Kalili, M. Serologic evaluation of celiac disease in patients with beta thalassemia major and control. Gastroenterol. Hepatol. Bed Bench 2015, 8, 153–159. [Google Scholar]
- Honar, N.; Kamali, S.; Karimi, M. Frequency of Celiac Disease in Children with Beta Thalassemia major. Iran. J. Ped. Hematol. Oncol. 2014, 4, 48–52. [Google Scholar]
- Noori, N.M.; Shahramian, I.; Teimouri, A.; Haghighat, M.; Dehghani, S.M.; Sharafi, E. Evaluation of Tissue Transglutaminase IgA in Thalassemia Minor Patients. Asian J. Med. Pharm. Res. 2017, 7, 19–24. [Google Scholar]
- Parakh, A.; Sudha, S.; Dubey, A.P.; Gupta, A. Celiac disease in a child with beta-thalassemia major: A need for improved screening and awareness. J. Pediatr. Hematol. Oncol. 2008, 30, 913–914. [Google Scholar] [CrossRef] [PubMed]
- Acquaviva, A.; Municchi, G.; Marconcini, S.; D’Ambrosio, A.; Morgese, G. Celiac disease in a patient with beta-thalassemia major. J. Pediatr. Gastroenterol. Nutr. 2003, 36, 489–491. [Google Scholar] [CrossRef] [PubMed]
- Montuori, M.; Smacchia, M.P.; Iorfida, D.; Leoni, S.; Trovato, C.M.; Gatti, S.; Celletti, I.; Valitutti, F.; Capogna, M.; Anania, C.; et al. An uncommon diagnosis of celiac disease in a thalassemic girl. Dig. Liver Dis. 2014, 46, e117. [Google Scholar] [CrossRef]
- Ciccocioppo, R.; Bernardo, M.E.; Russo, M.L.; Vanoli, A.; Franco, C.; Martinetti, M.; Catenacci, L.; Giorgiani, G.; Zecca, M.; Piralla, A.; et al. Allogeneic hematopoietic stem cell transplantation may restore gluten tolerance in patients with celiac disease. J. Pediatr. Gastroenterol. Nutr. 2013, 56, 422–427. [Google Scholar] [CrossRef]
- Oerter, K.E.; Kamp, G.A.; Munson, P.J.; Nienhuis, A.W.; Cassorla, F.G.; Manasco, P.K. Multiple hormone deficiencies in children with hemochromatosis. J. Clin. Endocrinol. Metab. 1993, 76, 357–361. [Google Scholar] [CrossRef]
- Hashemi, A.; Ghilian, R.; Golestan, M.; Akhavan, G.M.; Zare, Z.; Dehghani, M.A. The study of growth in thalassemic patients and its correlation with serum ferritin level. Iran. J. Pediatr. Hematol. Oncol. 2011, 1, 147–151. [Google Scholar]
- Pironi, L.; Arends, J.; Bozzetti, F.; Cuerda, C.; Gillanders, L.; Jeppesen, P.B.; Joly, F.; Kelly, D.; Lal, S.; Staun, M.; et al. ESPEN guidelines on chronic intestinal failure in adults. Clin. Nutr. 2016, 35, 247–307. [Google Scholar] [CrossRef]
- Pironi, L. Definitions of intestinal failure and the short bowel syndrome. Best Pract. Res. Clin. Gastroenterol. 2016, 30, 173–185. [Google Scholar] [CrossRef]
- Doseděl, M.; Jirkovský, E.; Macáková, K.; Krčmová, L.K.; Javorská, L.; Pourová, J.; Mercolini, L.; Remião, F.; Nováková, L.; Mladěnka, P.; et al. Vitamin C-Sources, Physiological Role, Kinetics, Deficiency, Use, Toxicity, and Determination. Nutrients 2021, 13, 615. [Google Scholar] [CrossRef]
- Liakakos, D.; Matsaniotis, N.; Karpouzas, J.; Morphis, L.; Agathopoulos, A. Clinica Chimica Acta 197 Ascorbic Acid Malabsorption In Thalassaemia. Clin. Chim. Acta 1969, 26, 197–202. [Google Scholar] [CrossRef]
- Yusof, J.; d’Arqom, A.; Andriani, A.P.; Nasution, M.Z.; Fatimah, N.; Mustika, A.; Handayani, S. Dietary Supplement Consumption and Mental Health in Indonesian Adults During Second Wave of COVID-19 Pandemic. Patient Prefer. Adherence 2023, 17, 1799–1811. [Google Scholar] [CrossRef] [PubMed]
- Chatterjea, B.; Maitra, A.; Banerjee, D.K.; Basu, A.K. Status of Ascorbic Acid in Iron Deficiency Anaemia and Thalassaemia. Acta Haematol. 1980, 64, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Cleveland Clinic. Herpes Simplex. Available online: https://my.clevelandclinic.org/health/diseases/22855-herpes-simplex (accessed on 28 October 2024).
- Atefi, A.; Binesh, F.; Hashemi, A.; Atefi, A.; Aminorroaya, M.M. Seroprevalence of herpes simplex 1, 2 IgG antibodies in patients with beta-thalassemia in a major tertiary care hospital located in Yazd, Iran. Iran. J. Ped. Hematol. Oncol. 2014, 4, 64–67. [Google Scholar] [PubMed]
- National Organization for Rare Disorders (NORD). Alpha-Thalassemia X-Linked Intellectual Disability Syndrome. Available online: https://rarediseases.org/rare-diseases/alpha-thalassemia-x-linked-intellectual-disability-syndrome/ (accessed on 28 October 2024).
- Gibbons, R.J.; Suthers, G.K.; Wilkie, A.O.; Buckle, V.J.; Higgs, D.R. X-linked a-Thalassemia/Mental Retardation (ATR-X) Syndrome: Localization to XqI2-q21.31 by X Inactivation and Linkage Analysis. Am. J. Hum. Genet. 1992, 51, 1136–1149. [Google Scholar] [PubMed]
- Thienpont, B.; de Ravel, T.; Van Esch, H.; Van Schoubroeck, D.; Moerman, P.; Vermeesch, J.R.; Fryns, J.-P.; Froyen, G.; Lacoste, C.; Badens, C.; et al. Partial duplications of the ATRX gene cause the ATR-X syndrome. Eur. J. Hum. Genet. 2007, 15, 1094–1097. [Google Scholar] [CrossRef]
- Gibbons, R.J. Molecular-clinical spectrum of the ATR-X syndrome. Am. J. Med. Genet. 2000, 97, 204–212. [Google Scholar] [CrossRef]
- Martucciello, G. Gastrointestinal phenotype of ATR-X syndrome. Am. J. Med. Genet. A 2006, 140, 1172–1176. [Google Scholar] [CrossRef]
- Watanabe, T. Esophago-gastric motility and nutritional management in a child with ATR-X syndrome. Pediatr. Int. 2014, 56, e48–e51. [Google Scholar] [CrossRef]
- Lee, S.Y.; Chow, C.B.; Li, C.K.; Chiu, M.C. Outcome of intensive care of homozygous alpha-thalassaemia without prior intra- uterine therapy. J. Paediatr. Child. Health 2007, 43, 546–550. [Google Scholar] [CrossRef]
- Lee, S.Y.R. Survival of homozygous alpha-thalassemia with aplasia/hypoplasia of phalanges and jejunal atresia. J. Matern. Fetal Neonatal Med. 2009, 22, 711–713. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).