Neuropathic Pain in Pancreatic Cancer: An Update of the Last Five Years
Abstract
:1. Introduction
2. Pancreatic Pain Diagnosis and Treatment: What We Know
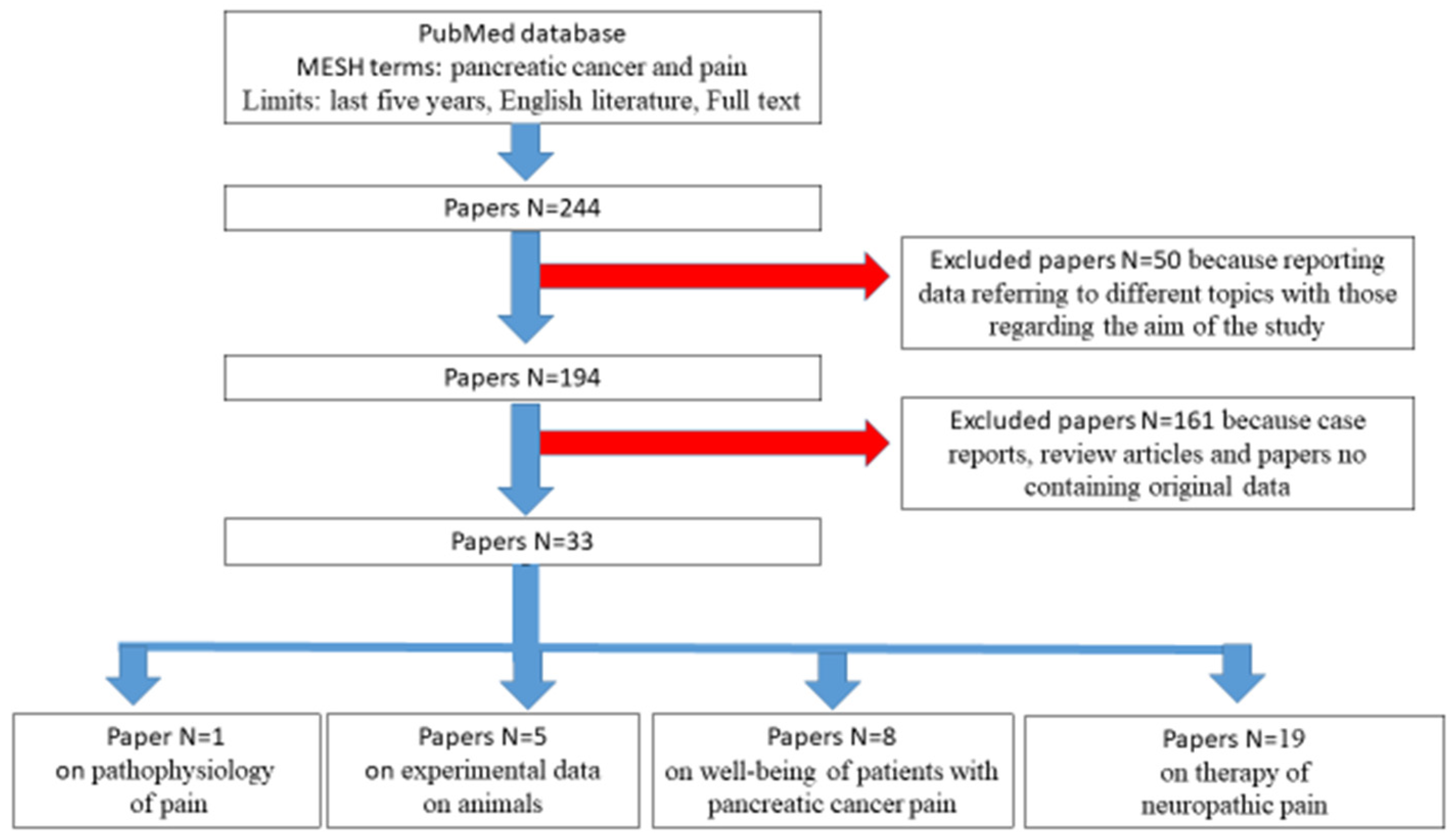
3. Literature Search
4. Experimental Studies of Neuropathic Pain
5. Human Studies
6. How to Assess Well-Being in Patients with Neuropathic Pain
7. Treatment of Neuropathic Pain
7.1. Pharmacological Therapy
7.2. Radiological Therapy
7.3. Celiac Plexus Block
8. Concluding Remarks
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Yan, B.M.; Myers, R.P. Neurolytic celiac plexus block for pain control in unresectable pancreatic cancer. Am. J. Gastroenterol. 2007, 102, 430–438. [Google Scholar] [CrossRef]
- Lebovits, A.H.; Lefkowitz, M. Pain management of pancreatic carcinoma: A review. Pain 1989, 36, 1–11. [Google Scholar] [CrossRef]
- Penman, I.D. Coeliac plexus neurolysis. Best Pract. Res. Clin. Gastroenterol. 2009, 23, 761–766. [Google Scholar] [CrossRef]
- Caraceni, A.; Portenoy, R.K. Pain management in patients with pancreatic carcinoma. Cancer 1996, 78, 639–653. [Google Scholar] [CrossRef]
- Cohen, S.P.; Mao, J. Neuropathic pain: Mechanisms and their clinical implications. BMJ 2014, 348, f7656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baron, R. Neuropathic pain: A clinical perspective. Handb. Exp. Pharmacol. 2009, 194, 3–30. [Google Scholar]
- Adiwinata, R.; Livina, A.; Waleleng, B.J.; Tendean, N.; Gosal, F.; Rotty, L.; Winarta, J.; Waleleng, A. Palliative management of advanced pancreatic cancer: The role of gastroentero-hepatologist. Acta Med. Indones. 2020, 52, 185–191. [Google Scholar]
- Saricaoglu, Ö.C.; Teller, S.; Wang, X.; Wang, S.; Stupakov, P.; Heinrich, T.; Istvanffy, R.; Friess, H.; Ceyhan, G.O.; Demir, I.E. Localisation analysis of nerves in the mouse pancreas reveals the sites of highest nerve density and nociceptive innervation. Neurogastroenterol. Motil. 2020, 32, e13880. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Jiang, J.; Xue, M.; Qin, T.; Xiao, Y.; Wu, E.; Shen, X.; Ma, Q.; Ma, J. Sonic hedgehog signaling pathway promotes pancreatic cancer pain via nerve growth factor. Reg. Anesth. Pain Med. 2020, 45, 137–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Fu, X.; Liu, D.; Yang, M.; Yang, J.; Huo, Y.; Liu, W.; Hua, R.; Sun, Y.; Wang, J. Molecular markers associated with perineural invasion in pancreatic ductal adenocarcinoma. Oncol. Lett. 2020, 20, 5. [Google Scholar] [CrossRef] [PubMed]
- Hirth, M.; Gandla, J.; Höper, C.; Gaida, M.M.; Agarwal, N.; Simonetti, M.; Demir, A.; Xie, Y.; Weiss, C.; Michalski, C.W.; et al. CXCL10 and CCL21 Promote migration of pancreatic cancer cells toward sensory neurons and neural remodeling in tumors in mice, associated with pain in patients. Gastroenterology 2020, 159, 665–681. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, L.; Zhu, J.; Xu, H.; Wei, K.; Chen, Q.; Wu, X.; Miao, X.; Lu, Z. MicroRNA-330 directs downregulation of the gababr2 in the pathogenesis of pancreatic cancer pain. J. Mol. Neurosci. 2020, 70, 1541–1551. [Google Scholar] [CrossRef]
- Fujisawa, T.; Shimamura, T.; Goto, K.; Nakagawa, R.; Muroyama, R.; Ino, Y.; Horiuchi, H.; Endo, I.; Maeda, S.; Harihara, Y.; et al. A novel role of interlekin 13 receptor alpha2 in perineural invasion and its association with poor prognosis of patients with pancreatic ductal adenocarcinoma. Cancers 2020, 12, 1294. [Google Scholar] [CrossRef] [PubMed]
- Mackay, T.M.; Latenstein, A.E.J.; Sprangers, M.A.G.; van der Geest, L.G.; Creemers, G.J.; van Dieren, S.; de Groot, J.B.; Groot Koerkamp, B.; de Hingh, I.H.; Homs, M.Y.V.; et al. Dutch Pancreatic Cancer Group. Relationship between quality of life and survival in patients with pancreatic and periampullary cancer: A multicenter cohort analysis. J. Natl. Compr. Cancer Netw. 2020, 18, 1354–1363. [Google Scholar] [CrossRef] [PubMed]
- Park, S.K.; Park, H.A.; Lee, J. Understanding the public’s emotions about cancer: Analysis of social media data. Int. J. Environ. Res. Public Health 2020, 17, 7160. [Google Scholar] [CrossRef] [PubMed]
- Damm, M.; Weniger, M.; Kölsch, A.K.; Lampert, C.; Ceyhan, G.O.; Beer, S.; Schorn, S.; Moir, J.; Michl, P.; Rosendahl, J. The quality of pain management in pancreatic cancer: A prospective multi-center study. Pancreatology 2020, 20, 1511–1518. [Google Scholar] [CrossRef]
- Ramsey, I.; Eckert, M.; Hutchinson, A.D.; Marker, J.; Corsini, N. Core outcome sets in cancer and their approaches to identifying and selecting patient-reported outcome measures: A systematic review. J. Patient Rep. Outcomes 2020, 4, 77. [Google Scholar] [CrossRef]
- Guan, M.; Gresham, G.; Shinde, A.; Lapite, I.; Gong, J.; Placencio-Hickok, V.R.; Forrest, C.B.; Hendifar, A.E. Priority rankings of patient-reported outcomes for pancreatic ductal adenocarcinoma: A comparison of patient and physician perspectives. J. Natl. Compr. Cancer Netw. 2020, 18, 1075–1083. [Google Scholar] [CrossRef]
- Gustavell, T.; Sundberg, K.; Langius-Eklöf, A. Using an interactive app for symptom reporting and management following pancreatic cancer surgery to facilitate person-centered care: Descriptive study. JMIR Mhealth Uhealth 2020, 8, e17855. [Google Scholar] [CrossRef] [PubMed]
- Marinelli, V.; Danzi, O.P.; Mazzi, M.A.; Secchettin, E.; Tuveri, M.; Bonamini, D.; Rimondini, M.; Salvia, R.; Bassi, C.; Del Piccolo, L. Prepare: PreoPerative Anxiety REduction. One-year feasibility rct on a brief psychological intervention for pancreatic cancer patients prior to major surgery. Front. Psychol. 2020, 11, 362. [Google Scholar] [CrossRef] [Green Version]
- Maharaj, A.D.; Samoborec, S.; Evans, S.M.; Zalcberg, J.; Neale, R.E.; Goldstein, D.; Merrett, N.; White, K.; Croagh, D.; Pilgrim, C.H.C.; et al. Patient-reported outcome measures (PROMs) in pancreatic cancer: A systematic review. HPB 2020, 22, 187–203. [Google Scholar] [CrossRef]
- Kajiwara, I.; Sano, M.; Ichimaru, Y.; Oshima, Y.; Kitajima, O.; Hao, H.; Masamune, A.; Kim, J.; Ishii, Y.; Ijichi, H.; et al. Duloxetine improves cancer-associated pain in a mouse model of pancreatic cancer through stimulation of noradrenaline pathway and its antitumor effects. Pain 2020, 161, 2909–2919. [Google Scholar] [CrossRef]
- Kamal, M.; Wang, X.S.; Shi, Q.; Mendoza, T.; Garcia-Gonzalez, A.; Bokhari, R.H.; Cleeland, C.S.; Fogelman, D.R. A randomized, placebo-controlled, double-blind study of minocycline for reducing the symptom burden experienced by patients with advanced pancreatic cancer. J. Pain Symptom Manag. 2020, 59, 1052–1058. [Google Scholar] [CrossRef]
- Fürst, P.; Lundström, S.; Strang, P. Methadone in Swedish specialized palliative care-Is it the magic bullet in complex cancer-related pain? PLoS ONE 2020, 5, e0230845. [Google Scholar] [CrossRef]
- Bandieri, E.; Romero, M.; Ripamonti, C.I.; Artioli, F.; Sichetti, D.; Fanizza, C.; Santini, D.; Cavanna, L.; Melotti, B.; Conte, P.F.; et al. Early strong opioid treatment study (ESOT) investigators. Randomized trial of low-dose morphine versus weak opioids in moderate cancer pain. J. Clin. Oncol. 2016, 34, 436–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steele, G.L.; Dudek, A.Z.; Gilmore, G.E.; Richter, S.A.; Olson, D.A.; Eklund, J.P.; Zylla, D.M. Impact of pain, opioids, and the mu-opioid receptor on progression and survival in patients with newly diagnosed stage IV pancreatic cancer. Am. J. Clin. Oncol. 2020, 43, 591–597. [Google Scholar] [CrossRef]
- Stearns, L.M.; Abd-Elsayed, A.; Perruchoud, C.; Spencer, R.; Hammond, K.; Stromberg, K.; Weaver, T. Intrathecal drug delivery systems for cancer pain: An analysis of a prospective, multicenter product surveillance registry. Anesth. Analg. 2020, 130, 289–297. [Google Scholar] [CrossRef]
- Kone, L.B.; Kunda, N.M.; Tran, T.B.; Maker, A.V. Surgeon-placed continuous wound infusion pain catheters markedly decrease narcotic use and improve outcomes after pancreatic tumor resection. Ann. Surg. Oncol. 2021, 28, 2287–2295. [Google Scholar] [CrossRef] [PubMed]
- Buckarma, E.; Thiels, C.A.; Habermann, E.B.; Glasgow, A.; Grotz, T.E.; Cleary, S.P.; Smoot, R.L.; Kendrick, M.L.; Nagorney, D.M.; Truty, M.J. Preoperative opioid use is associated with increased length of stay after pancreaticoduodenectomy. HPB 2020, 22, 1074–1081. [Google Scholar] [CrossRef] [PubMed]
- Erosa, S.C.; Haffey, P.R.; Mehta, N.; Gulati, A. Tapentadol, Buprenorphine, and Levorphanol for the Treatment of Neuropathic Pain: A Systematic Review. Curr. Pain Headache Rep. 2021, 25, 18. [Google Scholar] [CrossRef]
- Fan, T.; Zhou, J.Y. Computed Tomography-guided 125I Radioactive seed implantation therapy for pancreatic cancer pain. J. Coll. Physicians Surg. Pak. 2020, 30, 364–368. [Google Scholar]
- Kanno, Y.; Koshita, S.; Masu, K.; Ogawa, T.; Kusunose, H.; Murabayashi, T.; Sakai, T.; Kozakai, F.; Ito, K. Efficacy of EUS-guided celiac plexus neurolysis compared with medication alone for unresectable pancreatic cancer in the oxycodone/fentanyl era: A prospective randomized control study. Gastrointest. Endosc. 2020, 92, 120–130. [Google Scholar] [CrossRef]
- Vahedian, J.; Saraee, A.; Baghai Wadji, M.; Safari, S.; Chavoshi Khamneh, A. Pain relieving effect of intraoperative chemical splanchnicectomy of celiac ganglions in patients with resectable pancreatic or gastric masses: A randomized clinical trial. Pain Res. Manag. 2020, 2020, 2675940. [Google Scholar] [CrossRef] [Green Version]
- Yoon, W.J.; Oh, Y.; Yoo, C.; Jang, S.; Cho, S.S.; Suh, J.H.; Choi, S.S.; Park, D.H. EUS-guided versus percutaneous celiac neurolysis for the management of intractable pain due to unresectable pancreatic cancer: A randomized clinical trial. J. Clin. Med. 2020, 9, 1666. [Google Scholar] [CrossRef] [PubMed]
- Ji, K.; Shao, Y.J.; Hao, J.L.; Cheng, X.J.; Guan, B.Q.; Liu, W.S.; Chen, L.; Wang, X.; Song, Y.C.; Wang, K.; et al. Celiac plexus block after stereotactic body radiotherapy improves pain relief in locally advanced pancreatic cancer. J. Pain Res. 2020, 13, 919–925. [Google Scholar] [CrossRef]
- Dumitrescu, A.; Aggarwal, A.; Chye, R. A retrospective case series of patients who have undergone coeliac plexus blocks for the purpose of alleviating pain due to intra-abdominal malignancy. Cancer Rep. 2020, 3, e1265. [Google Scholar]
- Saleh, A.A.G.; Sultan, A.; Hammouda, M.A.; Shawki, A.; El Ghaffar, M.A. Value of adding dexmedetomidine in endoscopic ultrasound-guided celiac plexus neurolysis for treatment of pancreatic cancer-associated pain. J. Gastrointest. Cancer 2021, 52, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Filippiadis, D.; Ptohis, N.; Efthymiou, E.; Kelekis, A. A technical report on the performance of percutaneous cryoneurolysis of splanchnic nerves for the treatment of refractory abdominal pain in patients with pancreatic cancer: Initial experience. Cardiovasc. Interv. Radiol. 2021, 44, 789–794. [Google Scholar] [CrossRef] [PubMed]
- Facciorusso, A.; Antonino, M.; Muscatiello, N. Sarcopenia represents a negative prognostic factor in pancreatic cancer patients undergoing EUS celiac plexus neurolysis. Endosc. Ultrasound 2020, 9, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Comlek, S. Pain control with splanchnic neurolysis in pancreatic cancer patients unresponsive to celiac plexus neurolysis. J. Pain Res. 2020, 13, 2023–2031. [Google Scholar] [CrossRef]
- Koulouris, A.I.; Alexandre, L.; Hart, A.R.; Clark, A. Endoscopic ultrasound-guided celiac plexus neurolysis (EUS-CPN) technique and analgesic efficacy in patients with pancreatic cancer: A systematic review and meta-analysis. Pancreatology 2021, 21, 434–442. [Google Scholar] [CrossRef] [PubMed]

| Study Reference | Type of Study | Type of Intervention | Interventional Group | Control Group | Outcomes Measures | Time Evaluation | Differences |
|---|---|---|---|---|---|---|---|
| [32] | Randomized controlled trial | EUS celiac plexus neurolysis vs. nothing | 24 | 22 | Pain, QoL, opiod consumption | 4 weeks | No |
| [33] | Randomized controlled trial | Intraoperative splanchnicectomy using alcohol neurolysis vs. nothing | 23 | 19 | Pain | 4 months | No |
| [34] | Randomized controlled trial | EUS celiac neurolysis vs. percutaneous celiac neurolysis | 30 | 30 | Pain and narcotic consumption | 3 months | No |
| [35] | Retrospective | Stereotactic body radiotherapy plus celiac plexus block vs. stereotactic body radiotherapy only | 23 | 12 | Daily narcotic consumption | 4 months | Significant decrease in celiac plexus block |
| [36] | Retrospective | EUS celiac neurolysis vs. percutaneous celiac neurolysis | 24 | 14 | Pain | 4 weeks | No |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pezzilli, R. Neuropathic Pain in Pancreatic Cancer: An Update of the Last Five Years. Gastroenterol. Insights 2021, 12, 302-309. https://doi.org/10.3390/gastroent12030027
Pezzilli R. Neuropathic Pain in Pancreatic Cancer: An Update of the Last Five Years. Gastroenterology Insights. 2021; 12(3):302-309. https://doi.org/10.3390/gastroent12030027
Chicago/Turabian StylePezzilli, Raffaele. 2021. "Neuropathic Pain in Pancreatic Cancer: An Update of the Last Five Years" Gastroenterology Insights 12, no. 3: 302-309. https://doi.org/10.3390/gastroent12030027
APA StylePezzilli, R. (2021). Neuropathic Pain in Pancreatic Cancer: An Update of the Last Five Years. Gastroenterology Insights, 12(3), 302-309. https://doi.org/10.3390/gastroent12030027






