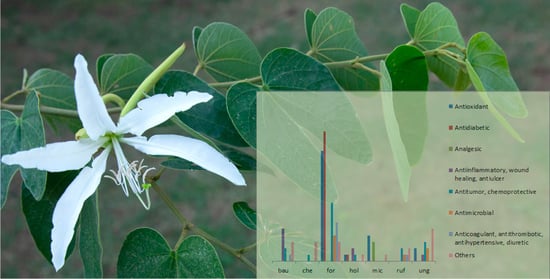
“Cow’s Hoof” (Bauhinia L., Leguminosae): A Review on Pharmacological Properties of Austral South American Species
Abstract
:1. Introduction
2. Methods
3. Biological Activity
3.1. Antioxidant Activity
3.2. Antidiabetic Properties and Related Activities
3.2.1. Bauhinia Forficata
3.2.2. Bauhinia holophylla
3.3. Analgesic Activity
3.4. Anti-inflammatory, Anti-ulcer and Wound Healing Activity
3.5. Antitumor and Chemoprotective Activity
3.6. Antimicrobial Activity
3.7. Anticoagulant, Antithrombotic, Antihypertensive and Diuretic Activity
3.8. Other Biological Activities
3.9. Toxicity and Adverse Effects
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Neto, J.A.S.; Galduróz, J.C.F.; Marques, L.C.; Kato, E.T.; Macrini, T.; Rodrigues, E. Possible adverse reactions to herbal products: A study with individuals who resort to popular medicine in the city of Diadema, SP, Brazil. Phytother. Res. 2014, 28, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Silva, K.L.; Cechinel Filho, V. Plantas do gênero Bauhinia: Composição química e potencial farmacológico. Quim. Nova 2002, 25, 449–454. [Google Scholar] [CrossRef]
- Cechinel Filho, V. Chemical composition and biological potential of plants from the genus Bauhinia. Phytother. Res. 2009, 23, 1347–1354. [Google Scholar] [CrossRef]
- Caffaro, K.M.; Araújo Júnior, J.X.; Santos, J.M.; Santos, R.M.; Campesatto, E.A.; Bastos, M.L.A. Integrative review on medical use and pharmacological activities Bauhinia genus plant. J. Nurs. UFPE 2015, 9, 9399–9405. [Google Scholar]
- Fortunato, R.H. Revisión del género Bauhinia (Cercideae, Caesalpinioideae, Fabaceae). Darwiniana 1986, 27, 527–557. [Google Scholar]
- Fortunato, R.H.; Miotto, S.; Izaguirre, P.; Beyhaut, R.; Bortoluzzi, R.L.C.; Ulibarri, E.; Gómez-Sosa, E. Fabaceae. In Catálogo de las Plantas Vasculares del Cono Sur; Zuloaga, F.O., Morrone, O., Belgrano, M.J., Eds.; Missouri Botanical Garden Press: Buenos Aires, Argentina, 2008; Volume 107, pp. 2078–2319. [Google Scholar]
- Vaz, A.M.S.F.; Bortoluzzi, R.L.C.; Silva, L.A.E. Checklist of Bauhinia sensu stricto (Caesalpiniaceae) in Brazil. Plant Ecol. Evol. 2010, 143, 212–221. [Google Scholar] [CrossRef]
- Rondina, R.V.D.; Bandoni, A.L.; Coussio, J.D. Especies medicinales argentinas con potencial actividad analgésica. Dominguezia 2008, 24, 47–69. [Google Scholar]
- Basualdo, I.; Soria, N. Plantas medicinales comercializadas en el mercado municipal de la ciudad de Pilar, Dpto. Ã ‘eembuc, Paraguay. Dominguezia 2014, 30, 47–53. [Google Scholar]
- Arias Toledo, B.; Trillo, C.; Grilli, M. Uso de plantas medicinales en relación al estado de conservación del bosque en Córdoba, Argentina. Ecología austral. 2010, 20, 235–246. [Google Scholar]
- Morais, S.M.; Dantas, J.D.P.; Silva, A.R.A.; Magalhães, E.F. Ethno-medicinal plants of Tapeba Indians from the State of Ceará-Brazil. Rev. Bras. Farmacogn. 2005, 15, 169–177. [Google Scholar] [CrossRef] [Green Version]
- Ibarrola, D.A.; Degen de Arrúa, R.L. Catálogo Ilustrado de 80 Plantas Medicinales del Paraguay; Facultad de Ciencias Químicas-UNA, Agencia de Cooperación International del Japón (JICA): Asunción, Paraguay, 2011. [Google Scholar]
- Coelho-Ferreira, M. Medicinal knowledge and plant utilization in an Amazonian coastal community of Marudá, Pará State (Brazil). J. Ethnopharmacol. 2009, 126, 159–175. [Google Scholar] [CrossRef] [PubMed]
- Miceli, N.; Buongiorno, L.P.; Celi, M.G.; Cacciola, F.; Dugo, P.; Donato, P.; Mondello, L.; Bonaccorsi, I.; Taviano, M.F. Role of the flavonoid-rich fraction in the antioxidant and cytotoxic activities of Bauhinia forficata Link. (Fabaceae) leaves extract. Nat. Prod. Res. 2016, 30, 1229–1239. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.L.C.; Gaertner, P.; Marson, P.G.; Schwarz, E.D.A.; Santos, C.A.D.M. An ethno-pharmacobotanical survey in Salto Caxias hydroelectric power plant in Paraná State, Brazil, before the flooding. Acta Farm. Bonaerense. 2004, 23, 148–153. [Google Scholar]
- Leitão, F.; Leitão, S.G.; Fonseca-Kruel, V.S.D.; Silva, I.M.; Martins, K. Medicinal plants traded in the open-air markets in the State of Rio de Janeiro, Brazil: An overview on their botanical diversity and toxicological potential. Rev. Bras. Farmacogn. 2014, 24, 225–247. [Google Scholar] [CrossRef]
- Melo, J.D.; Nascimento, V.D.; Amorim, E.D.; Lima, C.S.A.; Albuquerque, U.D. Avaliação da qualidade de amostras comerciais de boldo (Peumus boldus Molina), pata-de-vaca (Bauhinia spp.) e ginco (Ginkgo biloba L.). Rev. Bras. Farmacogn. 2004, 14, 111–120. [Google Scholar] [CrossRef] [Green Version]
- Engel, I.C.; Ferreira, R.A.; Cechinel-Filho, V.; Meyre-Silva, C. Quality control of drugs with Bauhinia forficata Link (Fabaceae). Rev. Bras. Farmacogn. 2008, 18, 258–264. [Google Scholar] [CrossRef]
- Fortunato, R.H.; Varela, B.G.; Castro, M.A.; Nores, M.J. Leaf venation pattern to recognize austral South American medicinal species of “cow’s hoof” (Bauhinia L.; Fabaceae). Rev. Bras. Farmacogn. 2017, 27, 158–161. [Google Scholar] [CrossRef]
- Batista, P.N.; Oliveira, T.C.; Leal, F.R.; Graccedil, M.; Nunes, L.C.C. The influence of using Bauhinia forficata Link in glycemic, lipid and toxicological profile in vivo experimental models: A systematic review. J. Med. Plants Res. 2013, 7, 2343–2348. [Google Scholar]
- Marques, G.S.; Rolim, L.A.; Alves, L.D.S.; Silva, C.C.A.R.; Soares, L.A.L.; Rolim-Neto, P.J. Estado da arte de Bauhinia forficata Link (Fabaceae) como alternativa terapêutica para o tratamento do diabetes mellitus. Rev. Cienc. Farm. Basica Apl. 2013, 34, 313–320. [Google Scholar]
- Cechinel-Zanchett, C.C.; Andrade, S.F.; Cechinel-Filho, V. Ethnopharmacological, phytochemical, pharmacological and toxicological aspects of Bauhinia forficata: A mini-review covering the last five years. Nat. Prod. Commun. 2018, 13, 911–916. [Google Scholar]
- Pontes, M.A.N.; Lima, D.S.; Oliveira, H.M.B.F.; Oliveira Filho, A.A. Bauhinia forficata L. and its hypoglycemic action. Arch. Health Invest. 2017, 6, 509–512. [Google Scholar] [CrossRef] [Green Version]
- Bortoluzzi, R.L.C.; Miotto, S.T.S.; Reis, A. Bauhinia L. In Flora Ilustrada Catarinense 2; Reis, A., Ed.; Herbário Barbosa Rodrigues: Itajaí, Brazil, 2006; pp. 29–67. [Google Scholar]
- RENISUS. Relação Nacional de Plantas Medicinais de Interesse ao SUS; Programa de Fitoterápico e Plantas Medicinais; Ministério da Saúde: Brasília, Brasil; 2009. Available online: https://www.gov.br/saude/pt-br/acesso-a-informacao/acoes-e-programas/programa-de-fitoterapico-e-plantas-medicinais (accessed on 15 August 2019).
- Barboza, G.E.; Cantero, J.J.; Núñez, C.; Pacciaroni, A.; Ariza Espinar, L. Medicinal plants: A general review and a phytochemical and ethnopharmacological screening of the native Argentine Flora. Kurtziana 2009, 34, 7–365. [Google Scholar]
- Basualdo, I.; Soria, N.; Ortíz, M.; Degen, R. Plantas medicinales comercializadas en los mercados de Asunción y Gran Asunción. Rojasiana 2004, 6, 95–114. [Google Scholar]
- Almeida, C.; Silva, T.D.L.E.; de Amorim, E.; Maia, M.D.S.; de Albuquerque, U. Life strategy and chemical composition as predictors of the selection of medicinal plants from the caatinga (Northeast Brazil). J. Arid Environ. 2005, 62, 127–142. [Google Scholar] [CrossRef]
- Albuquerque, U.P.; Medeiros, P.M.; Almeida, A.L.S.; Monteiro, J.M.; Neto, E.M.F.L.; Melo, J.G.; Santos, J.P. Medicinal plants of the caatinga (semi-arid) vegetation of NE Brazil: A quantitative approach. J. Ethnopharmacol. 2007, 114, 325–354. [Google Scholar] [CrossRef] [PubMed]
- Pizziolo, V.R.; Brasileiro, B.G.; Oliveira, T.T.; Nagem, T.J. Plantas com possível atividade hipolipidêmica: Uma revisão bibliográfica de libros editados no Brasil entre 1998 e 2008. Rev. Bras. Plant. Med. 2011, 13, 98–109. [Google Scholar] [CrossRef]
- Macedo, J.G.F.; de Menezes, I.R.A.; Ribeiro, D.A.; de Oliveira Santos, M.; de Mâcedo, D.G.; Macêdo, M.J.F.; de Almeida, B.V.; de Oliveira, L.G.S.; Leite, C.P.; Souza, M.M.D.A. Analysis of the variability of therapeutic indications of medicinal species in the Northeast of Brazil: Comparative study. Evid.-Based Complement. Altern. Med. 2018, 2018, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Keller, H.A. Unidades de vegetación y recursos florísticos en una aldea Mbya Guaraní de Misiones, Argentina. Kurtziana 2007, 33, 175–191. [Google Scholar]
- Trojan-Rodrigues, M.; Alves, T.L.S.; Soares, G.L.G.; Ritter, M.R. Plants used as antidiabetics in popular medicine in Rio Grande do Sul, southern Brazil. J. Ethnopharmacol. 2012, 139, 155–163. [Google Scholar] [CrossRef] [Green Version]
- Bolson, M.; Hefler, S.R.; Chaves, E.I.D.; Gasparotto Junior, A.; Cardozo Junior, E.L. Ethno-medicinal study of plants used for treatment of human ailments, with residents of the surrounding region of forest fragments of Paraná, Brazil. J. Ethnopharmacol. 2015, 161, 1–10. [Google Scholar] [CrossRef]
- Soria, N.; Ramos, P. Uso de plantas medicinales en la atención primaria de Salud en Paraguay: Algunas consideraciones para su uso seguro y eficaz. Mem. Inst. Investig. Cienc. Salud. 2015, 13, 8–17. [Google Scholar] [CrossRef]
- Salgueiro, A.C.F.; Folmer, V.; Bassante, F.E.M.; Cardoso, M.H.S.; Rosa, H.S.; Puntel, G.O. Predictive antidiabetic activities of plants used by persons with Diabetes mellitus. Complement. Ther. Med. 2018, 41, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hurrell, J.A.; Ulibarri, E.A.; Puentes, J.P.; Buet Costantino, F.; Arenas, P.M.; Pochettino, M.L. Leguminosas medicinales y alimenticias utilizadas en la conurbación Buenos Aires-La Plata, Argentina. Bol. Latinoam. Caribe Plant. Med. Aromat. 2011, 10, 443–455. [Google Scholar]
- Martínez Crovetto, R.N. Estudios Etnobotánicos, V. Nombres de plantas y su utilidad según los Mbya Guaraní de Misiones, Argentina. Bonplandia 2012, 21, 109–133. [Google Scholar] [CrossRef] [Green Version]
- Tabakian, G. Estudio comparativo de plantas medicinales vinculadas a tradiciones indígenas y europeas en Uruguay. Bonplandia 2019, 28, 135–158. [Google Scholar] [CrossRef]
- Meyre-Silva, C.; Yunes, R.A.; Monache, F.D.; Santos, A.R.S.; Schmeling, L.O.; Gadotti, V.M.; Liz, F.; Cechinel Filho, V. Phytochemical and pharmacological analysis of Bauhinia microstachya (Raddi) Macbr. (Leguminosae). Z. Naturforsch. C 2001, 56, 939–942. [Google Scholar]
- Silva, E.G.; Behr, G.A.; Zanotto-Filho, A.; Lorenzi, R.; Pasquali, M.A.D.B.; Ravazolo, L.G.; Bordignon, C.L., Jr.; Silva, F.A.; Aboy, A.L.; Bassani, V.L.; et al. Antioxidant activities and free radical scavenging potential of Bauhinia microstachya (Raddi) Macbr. (Caesalpinaceae) extracts linked to their polyphenol content. Biol. Pharm. Bull. 2007, 30, 1488–1496. [Google Scholar] [CrossRef] [Green Version]
- Amorozo, M.C.M. Uso e diversidade de plantas medicinais em Santo Antonio do Leverger, M.T. Brasil. Acta Bot. Bras. 2002, 16, 189–203. [Google Scholar] [CrossRef] [Green Version]
- Cunha, S.A.D.; Bortolotto, I.M. Etnobotânica de plantas medicinais no assentamento Monjolinho, município de Anastácio, Mato Grosso do Sul, Brasil. Acta Bot. Brasil. 2011, 25, 685–698. [Google Scholar] [CrossRef] [Green Version]
- Macedo, M.; Ferreira, A.R. Plantas hipoglicemiantes utilizadas por comunidades tradicionais na Bacia do Alto Paraguai e Vale do Guaporé, Mato Grosso-Brasil. Rev. Bras. Farmacogn. 2004, 14, 45–47. [Google Scholar] [CrossRef]
- Silva, M.A.B.; Melo, L.V.L.; Ribeiro, R.V.; Souza, J.P.M.; Lima, J.C.S.; Martins, D.T.O.; Silva, R.M. Levantamento etnobotânico de plantas utilizadas como anti-hiperlipidêmicas e anorexígenas pela população de Nova Xavantina-MT, Brasil. Rev. Bras. Farmacogn. 2010, 20, 549–562. [Google Scholar] [CrossRef] [Green Version]
- Pinto, A.A.D.C.; Maduro, C.B. Produtos e subprodutos da medicina popular comercializados na cidade de boa vista, Roraima. Acta Amazon. 2003, 33, 281–290. [Google Scholar] [CrossRef]
- Souza, B.V.C.; Araújo, R.S.R.M.; Silva, O.A.; Faustino, L.C.; Gonçalves, M.F.B.; Santos, M.L.; Souza, G.R.; Rocha, L.M.; Cardoso, M.L.S.; Nunes, L.C.C. Bauhinia forficata in the treatment of diabetes mellitus: A patent review. Expert Opin. Ther. Pat. 2018, 28, 129–138. [Google Scholar]
- SCImago Journal & Country Rank. Available online: https://www.scimagojr.com/index.php (accessed on 15 January 2021).
- Latindex. Sistema Regional de Información en Línea para Revistas Científicas de América Latina, el Caribe, España y Portugal. Available online: https://www.latindex.org/latindex/Solar/Busqueda (accessed on 29 January 2021).
- Neuhof, C.; Oliva, M.L.V.; Maybauer, D.; De Oliveira, C.; Sampaio, M.U.; Sampaio, C.A.M.; Neuhof, H.; Maybauer, M. Effect of plant Kunitz inhibitors from Bauhinia bauhinioides and Bauhinia rufa on pulmonary edema caused by activated neutrophils. Biol. Chem. 2003, 384, 939–944. [Google Scholar] [CrossRef]
- Oliveira, C.; Navarro-Xavier, R.A.; Anjos-Vallota, E.A.; Martins, J.O.; Silveira, V.L.F.; Gonçalves, L.R.C.; Araújo, M.S.; Motta, G.; Sannomiya, P.; Oliva, M.L.V. Effect of plant neutrophil elastase inhibitor on leucocyte migration, adhesion and cytokine release in inflammatory conditions. Br. J. Pharmacol. 2010, 161, 899–910. [Google Scholar] [CrossRef] [PubMed]
- Girão, D.K.F.B.; Cavada, B.S.; Pires, A.D.F.; Martins, T.V.; Franco, X.; Morais, C.M.; Nascimento, K.S.D.; Delatorre, P.; da Silva, H.C.; Nagano, C.S.; et al. The galactose-binding lectin isolated from Bauhinia bauhinioides Mart seeds inhibits neutrophil rolling and adhesion via primary cytokines. J. Mol. Recognit. 2015, 28, 285–292. [Google Scholar] [CrossRef]
- Almeida-Reis, R.; Theodoro-Junior, O.A.; Oliveira, B.T.M.; Oliva, L.V.; Toledo-Arruda, A.C.; Bonturi, C.R.; Brito, M.V.; Lopes, F.D.T.Q.S.; Prado, C.M.; Florencio, A.C.; et al. Plant proteinase inhibitor BbCI modulates lung inflammatory responses and mechanic and remodeling alterations induced by elastase in mice. BioMed. Res. Int. 2017, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Martins-Olivera, B.T.; Almeida-Reis, R.; Theodoro-Júnior, O.A.; Oliva, L.V.; Santos Nunes, N.N.; Olivo, C.R.; Brito, M.V.; Prado, C.M.; Leick, E.A.; Arruda Martins, M.; et al. The plant-derived Bauhinia bauhinioides Kallikrein proteinase inhibitor (rBbKI) attenuates elastase-induced emphysema in mice. Mediat. Inflamm. 2016, 2016, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Brito, M.V.; Oliveira, C.; Salu, B.R.; Andrade, S.A.; Malloy, P.M.; Sato, A.C.; Vicente, C.P.; Sampaio, M.; Maffei, F.H.A.; Oliva, M.L.V. The Kallikrein Inhibitor from Bauhinia bauhinioides (BbKI) shows antithrombotic properties in venous and arterial thrombosis models. Thromb. Res. 2014, 133, 945–951. [Google Scholar] [CrossRef]
- Bilgin, M.; Neuhof, C.; Doerr, O.; Benscheid, U.; Andrade, S.S.; Most, A.; Abdallah, Y.; Parahuleva, M.; Guenduez, D.; Oliva, M.L.; et al. Bauhinia bauhinioides cruzipain inhibitor reduces endothelial proliferation and induces an increase of the intracellular Ca2+ concentration. J. Physiol. Biochem. 2010, 66, 283–290. [Google Scholar] [CrossRef]
- Bilgin, M.; Burgazli, K.M.; Rafiq, A.; Mericliler, M.; Neuhof, C.; Oliva, M.L.; Parahuleva, M.; Soydan, N.; Doerr, O.; Abdallah, Y.; et al. Effect of Bauhinia bauhinioides kallikrein inhibitor on endothelial proliferation and intracellular calcium concentration. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 46–51. [Google Scholar] [PubMed]
- Nakahata, A.M.; Mayer, B.; Neth, P.; Hansen, D.; Sampaio, M.U.; Oliva, M.L.V. Blocking the proliferation of human tumor cell lines by peptidase inhibitors from Bauhinia seeds. Planta Med. 2013, 79, 227–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, D.; Santana, L.A.; Carmona, A.K.; Cezari, M.H.; Sampaio, M.U.; Sampaio, C.A.; Oliva, M.L.V. Structure of cruzipain/cruzain inhibitors isolated from Bauhinia bauhinioides seeds. Biol. Chem. 2001, 382, 847–852. [Google Scholar] [CrossRef] [PubMed]
- Araújo, A.P.U.; Hansen, D.; Vieira, D.F.; Oliveira, C.D.; Santana, L.A.; Beltramini, L.M.; Sampaio, C.A.M.; Sampaio, M.U.; Oliva, M.L.V. Kunitz-type Bauhinia bauhinioides inhibitors devoid of disulfide bridges: Isolation of the cDNAs, heterologous expression and structural studies. Biol. Chem. 2005, 386, 561–568. [Google Scholar] [CrossRef]
- Oliva, M.L.V.; Mendes, C.R.; Santomauro-Vaz, E.M.; Juliano, M.A.; Mentele, R.; Auerswald, E.A.; Sampaio, M.U.; Sampaio, C.A.M. Bauhinia bauhinioides plasma kallikrein inhibitor interaction with synthetic peptides and fluorogenic peptide substrates related to the reactive site sequence. Curr. Med. Chem. 2001, 8, 977–984. [Google Scholar] [CrossRef]
- Silva, A.M.A.; Silva, H.C.; Monteiro, A.O.; Lemos, T.L.G.; Souza, S.M.; Militão, G.C.G.; Santos, H.V.; Alves, P.B.; Romero, N.R.; Santiago, G.M.P. Chemical composition, larvicidal and cytotoxic activities of the leaf essential oil of Bauhinia cheilantha (Bong.) Steud. S. Afr. J. Bot. 2020, 131, 369–373. [Google Scholar] [CrossRef]
- Barbosa, P.B.B.M.; Oliveira, J.M.; Chagas, J.M.; Rabelo, L.M.A.; Medeiros, G.F.; Giodani, R.B.; Silva, E.A.; Uchôa, A.F.; Ximenes, M.F.F. Evaluation of seed extracts from plants found in the Caatinga biome for the control of Aedes aegypti. Parasitol. Res. 2014, 113, 3565–3580. [Google Scholar] [CrossRef]
- Luna, J.D.S.; DOS Santos, A.F.; de Lima, M.; de Omena, M.; de Mendonça, F.; Bieber, L.; Sant’Ana, A. A study of the larvicidal and molluscicidal activities of some medicinal plants from northeast Brazil. J. Ethnopharmacol. 2005, 97, 199–206. [Google Scholar] [CrossRef]
- Silva, M.C.C.; Santana, L.A.; Mentele, R.; Ferreira, R.S.; Miranda, A.; Silva-Lucca, R.A.; Sampaio, M.U.; Correia, M.T.S.; Oliva, M.L. Purification, primary structure and potential functions of a novel lectin from Bauhinia forficata seeds. Process Biochem. 2012, 47, 1049–1059. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, C.Z.; Maiorano, V.A.; Marcussi, S.; Sant’Ana, C.D.; Januário, A.H.; Lourenço, M.V.; Sampaio, S.V.; França, S.C.; Pereira, S.P.; Soares, A.M. Anticoagulant and antifibrinogenolytic properties of the aqueous extract from Bauhinia forficata against snake venoms. J. Ethnopharmacol. 2005, 98, 213–216. [Google Scholar] [CrossRef]
- Anjos, P.J.C.; Pereira, P.R.; Moreira, I.J.A.; Serafini, M.R.; Araújo, A.A.S.; Silva, F.A.; Ramos, C.S.; Antoniolli, A.R.; Santos, M.R.V. Antihypertensive effect of Bauhinia forficata aqueous extract in rats. J. Pharmacol. Toxicol. 2013, 8, 82–89. [Google Scholar] [CrossRef] [Green Version]
- Souza, P.; Silva, L.M.; Boeing, T.; Somensi, L.B.; Cechinel-Zanchett, C.C.; Campos, A.; Krueger, C.M.A.; Bastos, J.K.; Cechinel-Filho, V.; Andrade, S.F. Influence of prostanoids in the diuretic and natriuretic effects of extracts and kaempferitrin from Bauhinia forficata link leaves in rats. Phytother. Res. 2017, 31, 1521–1528. [Google Scholar] [CrossRef] [PubMed]
- Fuentes Mardones, O.; Alarcón Enos, J. Bauhinia candicans improves the endothelium dependent relaxation in aortic rings of alloxan-diabetic rats. Bol. Latinoam. Caribe Plant. Med. Aromat. 2010, 9, 485–490. [Google Scholar]
- Cechinel-Zanchett, C.C.; Silva, R.C.M.V.A.F.; Tenfen, A.; Siebert, D.A.; Micke, G.; Vitali, L.; Cechinel-Filho, V.; Andrade, S.F.; Souza, P. Bauhinia forficata link, a Brazilian medicinal plant traditionally used to treat cardiovascular disorders, exerts endothelium-dependent and independent vasorelaxation in thoracic aorta of normotensive and hypertensive rats. J. Ethnopharmacol. 2019, 243, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Alves, E.P.; de F, L.R.; de Almeida, C.M.; Freires, I.A.; Rosalen, P.L.; Ruiz, A.L.; Granville-Garcia, A.F.; Godoy, G.P.; Pereira, J.V.; de Brito Costa, E.M. Antimicrobial and antiproliferative activity of Bauhinia forficata Link and Cnidoscolus quercifolius extracts commonly used in folk medicine. J. Contemp. Dent. Pract. 2017, 18, 635–640. [Google Scholar]
- Ferreira-Filho, J.C.C.; Marre, A.T.O.; Almeida, J.S.S.; Lobo, L.A.; Farah, A.; Valença, A.M.G.; Fonseca-Gonçalves, A. Treatment of dental biofilm with a tincture of Bauhinia forficata leaves: An ex-vivo study. Nat. Prod. Res. 2019, 33, 3432–3435. [Google Scholar] [CrossRef]
- Ferreira-Filho, J.C.C.; Marre, A.T.O.; Almeida, J.S.S.; Lobo, L.A.; Farah, A.; Romanos, M.T.V.; Maia, L.C.; Valença, A.M.G.; Fonseca-Gonçalves, A. Therapeutic Potential of Bauhinia forficata Link in dental biofilm treatment. J. Med. Food 2020, 23, 998–1005. [Google Scholar] [CrossRef]
- Sousa, J.N.; Oliveira, A.B.M.; Ferreira e Silva, A.K.; Sousa, L.M.S.; Rocha, M.C.F.; Siqueira Júnior, J.P.; Kaatz, G.W.; Almeida, J.R.G.S.; Souza, J.S.N.; Barreto, H.M. Modulation of the resistance to norfloxacin in Staphylococcus aureus by Bauhinia forficata link. Nat. Prod. Res. 2021, 35, 681–685. [Google Scholar] [CrossRef]
- Sousa, E.; Zanatta, L.; Seifriz, I.; Creczynski-Pasa, T.B.; Pizzolatti, M.G.; Szpoganicz, B.; Silva, F.R.M.B. Hypoglycemic effect and antioxidant potential of kaempferol-3, 7-O-(α)-dirhamnoside from Bauhinia forficata leaves. J. Nat. Prod. 2004, 67, 829–832. [Google Scholar] [CrossRef]
- Khalil, N.M.; Pepato, M.T.; Brunetti, I.L. Free radical scavenging profile and myeloperoxidase inhibition of extracts from antidiabetic plants: Bauhinia forficata and Cissus sicyoides. Biol. Res. 2008, 41, 165–171. [Google Scholar] [CrossRef] [Green Version]
- Volpato, G.T.; Damasceno, D.C.; Rudge, M.V.C.; Padovani, C.R.; Calderon, I.D.M.P. Effect of Bauhinia forficata aqueous extract on the maternal-fetal outcome and oxidative stress biomarkers of streptozotocin-induced diabetic rats. J. Ethnopharmacol. 2008, 116, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Souza, C.R.F.; Georgetti, S.R.; Salvador, M.J.; Fonseca, M.J.V.; Oliveira, W.P. Antioxidant activity and physical-chemical properties of spray and spouted bed dried extracts of Bauhinia forficata. Braz. J. Pharm. Sci. 2009, 45, 209–218. [Google Scholar] [CrossRef] [Green Version]
- Ferreres, F.; Gil-Izquierdo, A.; Vinholes, J.; Silva, S.T.; Valentão, P.; Andrade, P.B. Bauhinia forficata Link authenticity using flavonoids profile: Relation with their biological properties. Food Chem. 2012, 134, 894–904. [Google Scholar] [CrossRef]
- Marques, G.S.; Lyra, M.A.M.; Peixoto, M.S.; Monteiro, R.P.M.; Leão, W.F.; Xavier, H.S.; Soares, L.A.L.; Neto, P.J.R. Caracterização fitoquímica e físico-química das folhas de Bauhinia forficata Link coletada em duas regiões brasileiras. Rev. Ciênc. Farm. Básica Apl. 2012, 33, 57–62. [Google Scholar]
- Peroza, L.R.; Busanello, A.; Leal, C.Q.; Röpke, J.; Boligon, A.A.; Meinerz, D.; Libardoni, M.; Athayde, M.L.; Fachinetto, R. Bauhinia forficata prevents vacuous chewing movements induced by haloperidol in rats and has antioxidant potential in vitro. Neurochem. Res. 2013, 38, 789–796. [Google Scholar] [CrossRef]
- Salgueiro, A.C.; Leal, C.Q.; Bianchini, M.C.; Prado, I.O.; Mendez, A.S.; Puntel, R.L.; Folmer, V.; Soares, F.A.; Ávila, D.S.; Puntel, G.O. The influence of Bauhinia forficata Link subsp. pruinosa tea on lipid peroxidation and non-protein SH groups in human erythrocytes exposed to high glucose concentrations. J. Ethnopharmacol. 2013, 148, 81–87. [Google Scholar] [CrossRef]
- Ecker, A.; Vieira, F.A.; Prestes, A.; Dos Santos, M.M.; Ramos, A.; Ferreira, R.D.; De Macedo, G.T.; Klimaczewski, C.V.; Seeger, R.; Da Rocha, J.B.T.; et al. Effect of Syzygium cumini and Bauhinia forficata aqueous-leaf extracts on oxidative and mitochondrial parameters in vitro. EXCLI J. 2015, 14, 1219–1231. [Google Scholar] [PubMed]
- Farag, M.A.; Sakna, S.T.; El-fiky, N.M.; Shabana, M.M.; Wessjohann, L.A. Phytochemical, antioxidant and antidiabetic evaluation of eight Bauhinia L. species from Egypt using UHPLC-PDA-qTOF-MS and chemometrics. Phytochemistry 2015, 119, 41–50. [Google Scholar] [CrossRef]
- Salgueiro, A.C.F.; Folmer, V.; Silva, M.P.; Mendez, A.S.L.; Zemolin, A.P.P.; Posser, T.; Franco, J.L.; Puntel, R.L.; Puntel, G.O. Effects of Bauhinia forficata tea on oxidative stress and liver damage in diabetic mice. Oxid. Med. Cell. Longev. 2016, 2016, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Ecker, A.; Gonzaga, T.K.S.N.; Seeger, R.L.; Santos, M.M.; Loreto, J.S.; Boligon, A.A.; Meinerz, D.F.; Lugokenski, T.H.; Rocha, J.B.T.; Barbosa, N.V. High-sucrose diet induces diabetic-like phenotypes and oxidative stress in Drosophila melanogaster: Protective role of Syzygium cumini and Bauhinia forficata. Biomed. Pharmacother. 2017, 89, 605–616. [Google Scholar] [CrossRef]
- Franco, R.R.; Carvalho, D.S.; Moura, F.B.R.; Justino, A.B.; Silva, H.C.G.; Peixoto, L.G.; Espindola, F.S. Antioxidant and anti-glycation capacities of some medicinal plants and their potential inhibitory against digestive enzymes related to type 2 diabetes mellitus. J. Ethnopharmacol. 2018, 215, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Pinafo, M.S.; Benedetti, P.R.; Gaiotte, L.B.; Costa, F.G.; Schoffen, J.P.F.; Fernandes, G.S.A.; Chuffa, L.G.A.; Seiva, F.R.F. Effects of Bauhinia forficata on glycaemia, lipid profile, hepatic glycogen content and oxidative stress in rats exposed to Bisphenol A. Toxicol. Rep. 2019, 6, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, C.F.; Lucchetta, N.R.; Punhagui, A.P.F.; Banedetti, P.R.; Arakawa, N.S.; Seiva, F.R.F.; Fernandes, G.S.A. Alcohol extract of Bauhinia forficata link reduces lipid peroxidation in the testis and epididymis of adult Wistar rats. Microsc. Res. Tech. 2019, 82, 345–351. [Google Scholar] [CrossRef]
- Franco, R.R.; Alves, V.H.M.; Zabisky, L.F.R.; Justino, A.B.; Martins, M.M.; Saraiva, A.L.; Goulart, L.R.; Espindola, F.S. Antidiabetic potential of Bauhinia forficata Link leaves: A non-cytotoxic source of lipase and glycoside hydrolases inhibitors and molecules with antioxidant and antiglycation properties. Biomed. Pharmacother. 2020, 123, 109798. [Google Scholar] [CrossRef]
- Pinto, L.S.; Cardoso, G.; Kremer, F.S.; Woloski, R.D.S.; Dellagostin, O.A.; Campos, V.F. Heterologous expression and characterization of a new galactose-binding lectin from Bauhinia forficata with antiproliferative activity. Int. J. Biol. Macromol. 2019, 128, 877–884. [Google Scholar] [CrossRef]
- Lim, H.; Kim, M.K.; Lim, Y.; Cho, Y.H.; Lee, C.H. Inhibition of cell-cycle progression in HeLa cells by HY52, a novel cyclin-dependent kinase inhibitor isolated from Bauhinia forficata. Cancer Lett. 2006, 233, 89–97. [Google Scholar] [CrossRef]
- Lim, H.; Lim, Y.; Cho, Y.H.; Lee, C.H. Induction of apoptosis in the HepG2 cells by HY53, a novel natural compound isolated from Bauhinia forficata. J. Microbiol. Biotechnol. 2006, 16, 1262–1268. [Google Scholar]
- Cechinel-Zanchett, C.C.; Boeing, T.; Somensi, L.B.; Steimbach, V.M.B.; Campos, A.; Krueger, C.D.M.A.; Schultz, C.; Sant’Ana, D.D.M.G.; Cechinel-Filho, V.; da Silva, L.M.; et al. Flavonoid-rich fraction of Bauhinia forficata Link leaves prevents the intestinal toxic effects of irinotecan chemotherapy in IEC-6 cells and in mice. Phytother. Res. 2019, 33, 90–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lubkowski, J.; Durbin, S.V.; Silva, M.C.; Farnsworth, D.; Gildersleeve, J.C.; Oliva, M.L.; Wlodawer, A. Structural analysis and unique molecular recognition properties of a Bauhinia forficata lectin that inhibits cancer cell growth. FEBS J. 2017, 284, 429–450. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.C.C.; Paula, C.A.; Ferreira, J.G.; Paredes-Gamero, E.J.; Vaz, A.M.; Sampaio, M.U.; Correia, M.T.S.; Oliva, M.L.V. Bauhinia forficata lectin (BfL) induces cell death and inhibits integrin-mediated adhesion on MCF7 human breast cancer cells. Biochim. Biophys. Acta Gen. Subj. 2014, 1840, 2262–2271. [Google Scholar] [CrossRef]
- Düsman, E.; Almeida, I.V.; Coelho, A.C.; Balbi, T.J.; Tonin, L.T.D.; Vicentini, V.E.P. Antimutagenic effect of medicinal plants Achillea millefolium and Bauhinia forficata in vivo. Evid. Based Complement. Alternat. Med. 2013, 2013, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rozza, A.L.; Cesar, D.A.S.; Pieroni, L.G.; Saldanha, L.L.; Dokkedal, A.L.; De-Faria, F.M.; Souza-Brito, A.R.M.; Vilegas, W.; Takahira, R.K.; Pellizzon, C.H. Antiulcerogenic activity and toxicity of Bauhinia holophylla hydroalcoholic extract. J. Evid.-Based. Complement. Altern. Med. 2015, 2015, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribeiro, D.L.; Cilião, H.L.; Specian, A.F.L.; Serpeloni, J.M.; De Oliveira, M.T.; Varanda, E.A.; Vilegas, W.; Saldanha, L.L.; Martínez-López, W.; Dokkedal, A.L.; et al. Phytochemical study and evaluation of cytotoxicity, mutagenicity, cell cycle kinetics and gene expression of Bauhinia holophylla (Bong.) Steud. in HepG2 cells in vitro. Cytotechnology 2018, 70, 713–728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazzeo, G.C.C.; Silva, M.P.O.; Guimarães, L.L.; Brito, A.R.M.S.; Toma, W. Evaluation of anti-ulcerogenic activity in an aqueous extract obtained from Bauhinia forficata leaves. Rev. Cienc. Farm. Basica Apl. 2015, 36, 21–26. [Google Scholar]
- Dos Santos, M.; Teixeira, T.R.; Santos, F.R.D.S.; Lima, W.G.; Ferraz, A.C.; Silva, N.L.; Leite, F.J.; Siqueira, J.M.; Luyten, W.; de Castro, A.H.F.; et al. Bauhinia holophylla (Bong.) Steud. leaves-derived extracts as potent anti-dengue serotype 2. Nat. Prod. Res. 2019, 35, 2804–2809. [Google Scholar] [CrossRef]
- Gadotti, V.M.; Santos, A.R.S.; Meyre-Silva, C.; Schmeling, L.O.; Machado, C.; Liz, F.H.; Filho, V.C. Antinociceptive action of the extract and the flavonoid quercitrin isolated from Bauhinia microstachya leaves. J. Pharm. Pharmacol. 2005, 57, 1345–1351. [Google Scholar] [CrossRef]
- Ramos, S.A.; Remor, C.; Emendörfer, F.; Meyre-Silva, C.; Cechinel Filho, V.; Santos, A.R.S.; Cardozo, A.M. Antispasmodic effects of Bauhinia microstachya on isolated smooth muscle. Pharm. Biol. 2005, 43, 467–470. [Google Scholar] [CrossRef]
- Menezes, P.R.; Schwarz, E.A.; Santos, C.A. In vitro antioxidant activity of species collected in Paraná. Fitoterapia. 2004, 75, 398–400. [Google Scholar] [CrossRef]
- Bianco, E.M.; Santos, C.A.M. Propriedades antioxidantes de folhas e caules de Bauhinia microstachya (Raddi) JF Macbr. R. Bras. Bioci. 2010, 8, 238–241. [Google Scholar]
- Mansur, M.C.P.P.R.; Leitao, S.G.; Lima, L.M.T.R.; Ricci-Junior, E.; Souza, G.R.; Barbi, N.S.; Martins, T.S.; Dellamora-Ortiz, G.M.; Rodrigo, R.T.L.; Vieira, R.C.; et al. Evaluation of the antioxidant and phototoxic potentials of Bauhinia microstachya var. massambabensis Vaz leaf extracts. Lat. Am. J. Pharm. 2012, 31, 200–206. [Google Scholar]
- Mansur, M.C.; Leitão, S.G.; Cerqueira-Coutinho, C.; Vermelho, A.B.; Silva, R.S.; Presgrave, O.A.; Leitão, Á.A.; Leitão, G.G.; Ricci-Júnior, E.; Santos, E.P. In vitro and in vivo evaluation of efficacy and safety of photoprotective formulations containing antioxidant extracts. Rev. Bras. Farmacogn. 2016, 26, 251–258. [Google Scholar] [CrossRef] [Green Version]
- Correia, A.F.; Silveira, D.; Fonseca-Bazzo, Y.M.; Magalhães, P.O.; Fagg, C.W.; Silva, E.C.; Gomes, S.M.; Gandolfi, L.; Pratesi, R.; Nóbrega, Y.K.M. Activity of crude extracts from Brazilian cerrado plants against clinically relevant Candida species. BMC Complement. Altern. Med. 2016, 16, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.G.; Diniz, P.M.M.; Paula, C.A.A.; Lobo, Y.A.; Paredes-Gamero, E.J.; Paschoalin, T.; Nogueira-Pedro, A.; Maza, P.K.; Toledo, M.S.; Suzuki, E.; et al. The impaired viability of prostate cancer cell lines by the recombinant plant kallikrein inhibitor. J. Biol. Chem. 2013, 288, 13641–13654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakahata, A.M.; Bueno, N.R.; Rocha, H.A.; Franco, C.R.; Chammas, R.; Nakaie, C.R.; Jasiulionis, M.; Nader, H.B.; Santana, L.A.; Sampaio, M.U.; et al. Structural and inhibitory properties of a plant proteinase inhibitor containing the RGD motif. Int. J. Biol. Macromol. 2006, 40, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Silveira, D.; Faitanin, R.D.; Gomes, S.M.; Fagg, C.W.; Fonseca-Bazzo, Y.M.; Magalhães, P.O.; Jamal, C.M. Thrombolytic activity evaluation of extracts from Fabaceae species Bauhinia variegata, B. rufa, and Stryphnodendron adstringens. Pharmacol. Online 2016, 3, 56–60. [Google Scholar]
- Neto, J.A.R.; Teixeira, J.L.; Alves, S.N.; Júnior, P.J. Avaliação dos efeitos da cafeína anidra e de diferentes extratos de “pata de vaca” (Bauhinia rufa) e café arábica (Coffea arabica) em larvas de Culex quinquefasciatus. Rev. Eletrônica Biol. 2014, 6, 175–183. [Google Scholar]
- Lacerda, G.M.; Monteiro, A.B.; Tintino, S.R.; Delmondes, G.A.; Fernandes, C.N.; Lemos, I.C.S.; Nascimento, E.P.; Carvalho, T.B.; Coutinho, H.D.M.; Menezes, I.R.A.; et al. Atividade moduladora sobre antibióticos pelo extrato aquoso das folhas de Bauhinia ungulata L. Rev. Cubana Plant. Med. 2016, 21. [Google Scholar]
- Medeiros, S.R.; Filho, A.A.M.; Costa, H.N.R.; Silva, F.S.; Santos, R.C.; Takahashi, J.A.; Ferraz, V.P.; Melo, A.C.G.R.; Ribeiro, P.R.E.; Laranjeira, A.G.A.; et al. Chemical profile, antimicrobial activity, toxicity on Artemia salina and anti-acetylcholinesterase enzyme essential oil from Bauhinia ungulata L.(Fabaceae) leaves. J. Med. Plant Res. 2016, 10, 442–449. [Google Scholar]
- Paula, C.D.S.; Canteli, V.C.D.; Hirota, B.C.K.; Campos, R.; Oliveira, V.B.D.; Kalegari, M.; Silva, C.B.; Silva, G.M.; Miguel, O.G.; Miguel, M.D. Potencial antioxidante in vitro das folhas da Bauhinia ungulata L. Rev. Cienc. Farm. Basica Apl. 2014, 35, 217–222. [Google Scholar]
- Rodrigues, R.O.; Yaochite, J.N.U.; Braga, M.A.; Sousa, A.R.; Sasahara, G.L.; Fonseca, S.G.C.; Araújo, T.D.V.; Santiago, G.M.P.; Sousa, L.M.; Carvalho, J.L.; et al. Antioxidant and antiinflammatory activities of Bauhinia ungulata L. (Fabaceae) on LPS-Stimulated RAW 264.7 cells. Pharmacogn. J. 2019, 11, 37–42. [Google Scholar] [CrossRef] [Green Version]
- Silva, H.C.; Pinto, L.D.S.; Teixeira, E.H.; Nascimento, K.S.; Cavada, B.S.; Silva, A.L.C. BUL: A novel lectin from Bauhinia ungulata L. seeds with fungistatic and antiproliferative activities. Process Biochem. 2014, 49, 203–209. [Google Scholar] [CrossRef] [Green Version]
- Sousa, L.M.; Carvalho, J.L.; Gois, R.W.; Silva, H.C.; Santiago, G.M.P.; Lemos, T.L.G.; Arriaga, A.M.C.; Alves, P.B.; Matos, I.L.; Militão, G.C.G.; et al. Chemical composition, larvicidal and cytotoxic activities of the essential oils from two Bauhinia species. Rec. Nat. Prod. 2016, 10, 341–348. [Google Scholar]
- Sousa, L.M.; Carvalho, J.L.; Silva, H.C.; Lemos, T.L.; Arriaga, A.M.; Braz-Filho, R.; Militão, G.C.; Silva, T.D.; Ribeiro, P.R.; Santiago, G.M. New Cytotoxic Bibenzyl and other constituents from Bauhinia ungulata L. (Fabaceae). Chem. Biodivers. 2016, 13, 1630–1635. [Google Scholar] [CrossRef]
- Rodrigues, R.O.; Yaochite, J.N.U.; Sasahara, G.L.; Albuquerque, A.A.; Fonseca, S.G.C.; Araújo, T.D.V.; Santiago, G.M.P.; Sousa, L.M.; Carvalho, J.L.; Alves, A.P.N.N.; et al. Antioxidant, anti-inflammatory and healing potential of ethyl acetate fraction of Bauhinia ungulata L. (Fabaceae) on in vitro and in vivo wound model. Mol. Biol. Rep. 2020, 47, 2845–2859. [Google Scholar] [CrossRef]
- Santos, K.M.; Gonçalves, P.S.; Paiva, M.J.; Lacerda, G.A. Acetylcholinesterase inhibition starting from extracts of Bauhinia variegata L.; Bauhinia variegata var. candida (Aiton) Buch.-Ham., and Bauhinia ungulata L. Rev. Soc. Bras. Med. Trop. 2011, 44, 781–783. [Google Scholar] [CrossRef]
- Santos, K.M.; Nunes, D.A.F.; Gomes, I.N.F.; Silva, S.L.; Ribeiro, R.I.M.A. Inhibition of gelatinase activity of MMP-2 and MMP-9 by extracts of Bauhinia ungulata L. Biosci. J. 2015, 31, 584–590. [Google Scholar] [CrossRef] [Green Version]
- Oliva, M.L.V.; Andrade, S.A.; Juliano, M.A.; Sallai, R.C.; Torquato, R.J.; Sampaio, M.U.; Pott, V.J.; Sampaio, C.A.M. Kinetic characterization of factor Xa binding using a quenched fluorescent substrate based on the reactive site of factor Xa inhibitor from Bauhinia ungulata seeds. Curr. Med. Chem. 2003, 10, 1085–1093. [Google Scholar] [CrossRef]
- Soares, J.C.M.; Costa, S.T.D.; Cecim, M. Níveis glicêmicos e de colesterol em ratos com diabetes mellitus aloxano induzido, tratados com infusão de Bauhinia candicans ou Syzygium jambolanum. Ciência Rural 2000, 30, 113–118. [Google Scholar] [CrossRef]
- Pepato, M.T.; Keller, E.H.; Baviera, A.M.; Kettelhut, I.C.; Vendramini, R.C.; Brunetti, I.L. Anti-diabetic activity of Bauhinia forficata decoction in streptozotocin-diabetic rats. J. Ethnopharmacol. 2002, 81, 191–197. [Google Scholar] [CrossRef]
- Silva, F.R.M.B.; Szpoganicz, B.; Pizzolatti, M.G.; Willrich, M.A.V.; Sousa, E. Acute effect of Bauhinia forficata on serum glucose levels in normal and alloxan-induced diabetic rats. J. Ethnopharmacol. 2002, 83, 33–37. [Google Scholar] [CrossRef]
- Damasceno, D.; Volpato, G.; Calderon, I.D.M.P.; Aguilar, R.; Rudge, M.C. Effect of Bauhinia forficata extract in diabetic pregnant rats: Maternal repercussions. Phytomedicine 2004, 11, 196–201. [Google Scholar] [CrossRef]
- Fuentes, O.; Arancibia-Avila, P.; Alarcón, J. Hypoglycemic activity of Bauhinia candicans in diabetic induced rabbits. Fitoterapia 2004, 75, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Jorge, A.P.; Horst, H.; Sousa, E.; Pizzolatti, M.G.; Silva, F.R.M.B. Insulinomimetic effects of kaempferitrin on glycaemia and on 14 C-glucose uptake in rat soleus muscle. Chem.-Biol. Interact. 2004, 149, 89–96. [Google Scholar] [CrossRef]
- Lino, C.S.; Diógenes, J.P.L.; Pereira, B.A.; Faria, R.A.P.G.; Neto, M.A.; Alves, R.S.; Queiroz, M.G.R.; Sousa, F.C.F.; Viana, G.S.B. Antidiabetic activity of Bauhinia forficata extracts in alloxan-diabetic rats. Biol. Pharm. Bull. 2004, 27, 125–127. [Google Scholar] [CrossRef] [Green Version]
- Vasconcelos, F.; Sampaio, S.V.; Garófalo, M.A.; Guimarães, L.F.; Giglio, J.R.; Arantes, E.C. Insulin-like effects of Bauhinia forficata aqueous extract upon Tityus serrulatus scorpion envenoming. J. Ethnopharmacol. 2004, 95, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Menezes, F.D.S.; Minto, A.B.M.; Ruela, H.S.; Kuster, R.M.; Sheridan, H.; Frankish, N. Hypoglycemic activity of two Brazilian Bauhinia species: Bauhinia forficata L. and Bauhinia monandra Kurz. Rev. Bras. Farmacogn. 2007, 17, 8–13. [Google Scholar] [CrossRef] [Green Version]
- Cunha, A.M.D.; Menon, S.; Menon, R.; Couto, A.G.; Bürger, C.; Biavatti, M.W. Hypoglycemic activity of dried extracts of Bauhinia forficata Link. Phytomedicine 2010, 17, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Pepato, M.T.; Conceição, C.Q.; Gutierres, V.O.; Vendramini, R.C.; Souza, C.R.F.; Oliveira, W.P.; Brunetti, I.L. Evaluation of the spouted bed dried leaf extract of Bauhinia forficata for the treatment of experimental diabetes in rats. Afr. J. Biotechnol. 2010, 9, 7157–7164. [Google Scholar]
- Curcio, S.A.; Stefan, L.F.; Randi, B.A.; Dias, M.A.; da Silva, R.E.; Caldeira, E.J. Hypoglycemic effects of an aqueous extract of Bauhinia forficata on the salivary glands of diabetic mice. Pak. J. Pharm. Sci. 2012, 25, 493–499. [Google Scholar]
- Fuentes, O.; Alarcón, J. Bauhinia candicans stimulation of glucose uptake in isolated gastric glands of normal and diabetic rabbits. Fitoterapia 2006, 77, 271–275. [Google Scholar] [CrossRef]
- Cazarolli, L.H.; Pereira, D.F.; Kappel, V.D.; Folador, P.; Figueiredo, M.S.R.B.; Pizzolatti, M.G.; Silva, F.R.M.B. Insulin signaling: A potential signaling pathway for the stimulatory effect of kaempferitrin on glucose uptake in skeletal muscle. Eur. J. Pharmacol. 2013, 712, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Pozzobon, A.; Rempel, C.; Hoerlle, J.; Strohschoen, A.G.; Bosco, S.M.; Carreno, I. Avaliação do efeito da Bauhinia forficata no perfil glicêmico e verificação dos níveis séricos do cortisol de portadores de diabetes mellitus tipo 2, usuários de unidades básicas de saúde no vale do taquari, RS. Rev. Cad. Pedagógico 2012, 9, 9–23. [Google Scholar]
- Toloza-Zambrano, P.; Avello, M.; Fernández, P. Determinación de rutina y trigonelina en extractos de hojas de Bauhinia forficata subsp. pruinosa y evaluación del efecto hipoglicemiante en humanos. Bol. Latinoam. Caribe Plant. Med. Aromat. 2015, 14, 21–32. [Google Scholar]
- Mariángel, P.C.; Lorca, M.A.; Leon, F.M.; Rocca, P.F.; Zapata, L.V.; Navarrete, E.P. Effects of Bauhinia forficata Link tea on lipid profile in diabetic patients. J. Med. Food 2019, 22, 321–323. [Google Scholar] [CrossRef]
- Pinheiro, M.S.; Rodrigues, L.S.; Neto, L.S.; Moraes-Souza, R.Q.; Soares, T.S.; Americo, M.F.; Campos, K.E.; Damasceno, D.B.; Volpato, G.T. Effect of Bauhinia holophylla treatment in Streptozotocin-induced diabetic rats. An. Acad. Bras. Ciênc. 2017, 89, 263–272. [Google Scholar] [CrossRef]
- Camaforte, N.A.P.; Saldanha, L.L.; Vareda, P.M.P.; Rezende-Neto, J.M.; Senger, M.R.; Delgado, A.Q.; Morgan, H.J.N.; Violato, N.M.; Pieroni, L.G.; Dokkedal, A.L.; et al. Hypoglycaemic activity of Bauhinia holophylla through GSK3-β inhibition and glycogenesis activation. Pharm. Biol. 2019, 57, 269–279. [Google Scholar] [CrossRef] [Green Version]
- Sobrinho, T.J.S.P.; Silva, C.H.T.P.; Nascimento, J.E.; Monteiro, J.M.; Albuquerque, U.P.; Amorim, E.L.C. Validação de metodologia espectrofotométrica para quantificação dos flavonóides de Bauhinia cheilantha (Bongard) Steudel. Rev. Bras. Cienc. Farm. 2008, 44, 683–689. [Google Scholar] [CrossRef] [Green Version]
- Silva, K.L.D.; Biavatti, M.W.; Leite, S.N.; Yunes, R.A.; Monache, F.D.; Filho, V.C. Phytochemical and pharmacognostic investigation of Bauhinia forficata Link (Leguminosae). Z. Naturforsch. 2000, 55, 478–480. [Google Scholar] [CrossRef]
- Pizzolatti, M.G.; Cunha, A.J.; Szpoganicz, B. Flavonoid glycosides from leaves and flowers of Bauhinia forficata (Leguminosae). Quim. Nova 2003, 26, 466–469. [Google Scholar] [CrossRef] [Green Version]
- Duarte-Almeida, J.M.; Negri, G.; Salatino, A. Volatile oils in leaves of Bauhinia (Fabaceae Caesalpinioideae). Biochem. Syst. Ecol. 2004, 32, 747–753. [Google Scholar] [CrossRef]
- Pinheiro, T.S.; Johansson, L.A.; Pizzolatti, M.G.; Biavatti, M.W. Comparative assessment of kaempferitrin from medicinal extracts of Bauhinia forficata Link. J. Pharm. Biomed. Anal. 2006, 41, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Sartorilli, P.; Correa, D.S. Constituents of essential oil from Bauhinia forficata Link. J. Essent. Oil Res. 2007, 19, 468–469. [Google Scholar] [CrossRef]
- Sayago, C.T.M.; Camargo, V.B.; Barbosa, F.; Gularte, C.; Pereira, G.; Miotto, S.; Cechinel Filho, V.; Puntel, R.L.; Folmer, V.; Mendez, A. Chemical composition and in vitro antioxidant activity of hydro-ethanolic extracts from Bauhinia forficata subsp. pruinosa and B. variegata. Acta Biol. Hung. 2013, 64, 21–33. [Google Scholar] [CrossRef]
- Farias, L.D.S.; Mendez, A.S. LC/ESI-MS method applied to characterization of flavonoids glycosides in Bauhinia forficata subsp. pruinosa. Quim. Nova. 2014, 37, 483–486. [Google Scholar] [CrossRef]
- Santos, M.; Fortunato, R.H.; Spotorno, V.G. Analysis of flavonoid glycosides with potential medicinal properties on Bauhinia uruguayensis and Bauhinia forficata subspecies pruinosa. Nat. Prod. Res. 2019, 33, 2574–2578. [Google Scholar] [CrossRef] [PubMed]
- Bianco, E.M.; Santos, C.A.M. Substances isolated from Bauhinia microstachya (Raddi) Macbr. (Caesalpiniaceae) leaves. Rev. Bras. Farmacogn. 2003, 13, 93–99. [Google Scholar] [CrossRef]
- Neto, M.M.; Neto, M.A.; Filho, R.B.; Lima, M.A.S.; Silveira, E.R. Flavonoids and alkaloids from leaves of Bauhinia ungulata L. Biochem. Syst. Ecol. 2008, 36, 227–229. [Google Scholar] [CrossRef]
- Gramosa, N.V.; Freitas, J.V.B.; Neto, M.N.L.; Silveira, E.R.; Nunes, E.P. Volatile components of the essential oil from Bauhinia ungulata L. J. Essent. Oil Res. 2009, 21, 495–496. [Google Scholar] [CrossRef]
- Maritim, A.C.; Sanders, R.A.; Watkins, J.B., III. Diabetes, oxidative stress, and antioxidants: A review. J. Biochem. Mol. Toxicol. 2003, 17, 24–38. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef]
- Pedrete, T.A.; Hauser-Davis, R.A.; Moreira, J.C. Proteomic characterization of medicinal plants used in the treatment of diabetes. Int. J. Biol. Macromol. 2019, 140, 294–302. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2014, 37 (Suppl. S1), S81–S90. [Google Scholar] [CrossRef] [PubMed]
- Prasad, C.V.; Mohan, S.S.; Banerji, A.; Gopalakrishnapillai, A. Kaempferitrin inhibits GLUT4 translocation and glucose uptake in 3T3-L1 adipocytes. Biochem. Biophys. Res. Commun. 2009, 380, 39–43. [Google Scholar] [CrossRef]
- Silva, D.; Casanova, L.M.; Marcondes, M.C.; Espindola-Netto, J.M.; Paixão, L.P.; Melo, G.O.; Zancan, P.; Sola-Penna, M.; Costa, S.S. Antidiabetic activity of Sedum dendroideum: Metabolic enzymes as putative targets for the bioactive flavonoid kaempferitrin. IUBMB life. 2014, 66, 361–370. [Google Scholar] [CrossRef]
- Tostes, J.B.; Siani, A.C.; Monteiro, S.S.; Melo, V.F.; Costa, J.O.; Valente, L.M.M. Seasonal flavonoid profile and kaempferitrin content in the leaf extracts of Bauhinia forficata subspecies forficata from two locations in southeastern Brazil. Am. J. Plant Sci. 2019, 10, 208–220. [Google Scholar] [CrossRef] [Green Version]
- Sobrinho, T.J.S.P.; Cardoso, K.D.; Gomes, T.D.L.; Albuquerque, U.P.; Amorim, E.L. Análise da pluviosidade e do efeito de borda sobre os teores de flavonóides em Bauhinia cheilantha (Bong.) Steud. Fabaceae. Rev. Bras. Farmacogn. 2009, 19, 740–745. [Google Scholar] [CrossRef] [Green Version]
- Martí, A.; Tapiz, C. Modelos murinos para el estudio de la diabetes tipo 2: Una revisión sistemática. Rev. ALAD 2019, 9, 165–178. [Google Scholar] [CrossRef]
- Sah, S.P.; Singh, B.; Choudhary, S.; Kumar, A. Animal models of insulin resistance: A review. Pharmacol. Rep. 2016, 68, 1165–1177. [Google Scholar] [CrossRef]
- Oliva, M.L.V.; Sampaio, M.U. Bauhinia Kunitz-type proteinase inhibitors: Structural characteristics and biological properties. Biol. Chem. 2008, 389, 1007–1013. [Google Scholar] [CrossRef]
- Oliva, M.L.V.; Sampaio, M.U. Action of plant proteinase inhibitors on enzymes of physiopathological importance. An. Acad. Bras. Ciênc. 2009, 81, 615–621. [Google Scholar] [CrossRef] [Green Version]
- Oliva, M.L.V.; Ferreira, R.S.; Ferreira, J.G.; Paula, C.A.A.; Salas, C.E.; Sampaio, M.U. Structural and functional properties of kunitz proteinase inhibitors from leguminosae: A mini review. Curr. Protein Pept. Sci. 2011, 12, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Srikanth, S.; Chen, Z. Plant protease inhibitors in therapeutics-focus on cancer therapy. Front. Pharmacol. 2016, 7, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Cagliari, R.; Kremer, F.S.; Pinto, L.S. Bauhinia lectins: Biochemical properties and biotechnological applications. Int. J. Biol. Macromol. 2018, 119, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Simões, R.C.; Almeida, S. Estudo fitoquímico de Bauhinia forficata (Fabaceae). Biota Amaz. 2015, 5, 27–31. [Google Scholar] [CrossRef]
- Debenedetti, S.L.; Miño, J.; Rojo, A.; Acevedo, M.C.D. Ensayo del efecto diurético de los extractos acuosos de Amaranthus muricatus (Moquin) Gill. Ex Hicken, Bauhinia candicans Benth. y Smilax campestris Griseb. Acta Farm. Bonaerense. 2000, 19, 17–20. [Google Scholar]
- Cechinel-Zanchett, C.C.; Mariano, L.N.B.; Boeing, T.; Costa, J.C.; Silva, L.M.; Bastos, J.K.; Cechinel-Filho, V.; Souza, P. Diuretic and renal protective effect of kaempferol 3-O-Alpha-l-rhamnoside (Afzelin) in normotensive and hypertensive rats. J. Nat. Prod. 2020, 83, 1980–1989. [Google Scholar] [CrossRef]
- Castro, A.H.F.; Tavares, H.D.S.; Pereira, S.R.F.; Granjeiro, P.A.; da Silva, J.A.; Galdino, A.S. Production and characterization of lectin from Bauhinia holophylla (Fabaceae: Cercideae) calli. Plant Cell Tissue Organ Cult. 2018, 134, 423–432. [Google Scholar] [CrossRef]
- Pepato, M.T.; Baviera, A.M.; Vendramini, R.C.; Brunetti, I.L. Evaluation of toxicity after one-months treatment with Bauhinia forficata decoction in streptozotocin-induced diabetic rats. BMC Complement. Altern. Med. 2004, 4, 7. [Google Scholar] [CrossRef]
- Cavalcanti, E.M.; Bonafé, C.; Silva, M.G.; Gerenutti, M. Properties of Bauhinia forficata Link in rats: Behavioral evaluations. Pharmacologyonline 2011, 2, 205–211. [Google Scholar]

| Species | Traditional Uses | References | |
|---|---|---|---|
| affinis | Diabetes. | [7,24] | |
| argentinensis * | Analgesic (kidney). Hepatic disorders. Kidney disorders. | [8,25] | |
| bauhinioides | Diuretic. Kidney disorders. Refrigerant. | [8,26] | |
| cheilantha | Analgesic (back, pain in general, headache). Anemia. Anti-inflammatory. Antilipidemic. Asthma. Blood thinner.Cancer. Depurative. Diabetes. Digestive disorders. Dysphonia and throat inflammation. Flu and cough, expectorant. Hemostatic. Helminthiasis. Hypertension. Hypocholesterolemic agent. Inappetence. Kidney disorders. Rheumatism. Sedative. Sexual impotence. Tonic. Triglyceride reducer. Urinary infection, burning in the urethra, uterus. | [3,27,28,29,30] | |
| forficata ** | Antiseptic. Cardiovascular disorders. Diabetes. Hypoglycemic agent. Diuretic. Endocrine disorders. Gastrointestinal disorders. Gynecologic and obstetrics disorders. Hepatic disorders. Hypocholesterolemic agent. Indigestion flatulence. Kidney disorders. Urinary disorders. Weakness. | [9,13,14,24,26,29,31,32,33,34,35] | |
| p | Abluent. Analgesic (headache). Antidandruff. Antihemorrhoidal. Antinephritic. Antitussive expectorant. Astringent. Blood depurative. Cardiotonic. Diabetes. Hypoglycemic agent. Digestive. Diuretic. Genito-urinary and hemolymphatic system. Hypotensive agent. Rheumatism. Vulnerary. | [8,10,25,36,37,38] | |
| c | Cistitis. Diabetes. Hypoglycemic agent. Kidney disorders, kidney stones. | [1,2,3] | |
| holophylla | Anti-obesity. Astringent. Diabetes. Hypoglycemic agent. Diuretic. | [3] | |
| microstachya | Analgesic. Anti-inflammatory. Blood depurative. Diabetes. Hypoglycemic agent. Liver pain, spleen ache. Respiratory disorders. Urinary and gallbladder disorders. | [8,13,15,31,37,39,40] | |
| mollis | nd. | [41,42] | |
| rufa | Anoretic. Antihyperlipidemic agent. Astringent. Diabetes. Hypoglycemic agent. Diuretic. | [3,41,43,44] | |
| ungulata | Analgesic (stomach). Diabetes. Hypoglycemic agent. Hypocholesterolemic agent. Hypolipidemic agent. Laxative. | [3,29,41,45,46] | |
| Species | Biological Activity | Study Type | Extract/ Compound | Part Used | Study Model/Target Species/Cells/Enzymes/Method Investigated | References | |
|---|---|---|---|---|---|---|---|
| Category Detail | |||||||
| bau | Anti-inflammatory | VIV | BbCI | seed | Rabbit-activated neutrophil-induced pulmonary edema. | [49] | |
| VIV | BbCI | seed | Rat carrageenan-induced paw edema and pleurisy. Scrotal microvasculature. | [50] | |||
| VIV | BBL | seed | Rat carrageenan-induced paw edema and carrageenan or TNF-α-induced peritonitis. | [51] | |||
| VIV | rBbCI | Mice elastase-induced pulmonary emphysema. | [52] | ||||
| VIV | rBbKI | Mice elastase-induced pulmonary inflammation. | [53] | ||||
| Antithrombotic | VIV | BbKI | seed | Vein and arterial thrombosis models in rats and mice. | [54] | ||
| Antitumor | Antiproliferative activity. | VIT | BbCI, BbKI | seed | HUVEC human umbilical vein endothelial cells. | [55,56] | |
| VIT | rBbCI, rBbKI | MKN-28 Hs746T (gastric), HCT116 HT29 (colorectal), SkBr-3 MCF-7 (breast), THP-1 and K562 (leukemia) human cancer cells. | [57] | ||||
| Other biological activities | Kunitz-tipe proteinase inhibitors activity. | VIT | BbCI | seed | Elastase cathepsin L and cathepsin G. | [58,59] | |
| VIT | BbKI | seed | Trypsin chymotrypsin plasmin and pancreatic and plasma kallikrein. | [59,60] | |||
| Trypanosoma cruzi cruzipain inhibitor activity. | VIT | BbCI | seed | Trypanosoma cruzi cruzipain. | [58,59] | ||
| che | Antitumor | Cytotoxic activity. | VIT | Essential oils | leaf | HL-60 (leukemia), MCF-7 (breast), NCI-H292 (lung) and HEP-2 (endocervical) human cancer cells. | [61] |
| Other biological activities | Insecticide. | VIT | Crude ext | seed | Aedes aegypti. | [62] | |
| Larvicide. | VIT | Ethanolic ext | wood | Aedes aegypti. | [63] | ||
| VIT | Essential oils | leaf | Aedes aegypti. | [61] | |||
| Anticoagulant, antihypertensive and diuretic | Anticoagulant and antiplatelet aggregating properties. | VIT | BfL | seed | Biological models of homeostasis. Human blood samples. | [64] | |
| Anticoagulant, antifrinogenolytic activities (snake venoms). | VIT | Aqueous ext | leaf | Clotting disturbances in human blood samples induced by snake venoms. | [65] | ||
| Antihypertensive effects. | VIV | Aqueous ext | leaf | Normotensive and hypertensive rats. | [66] | ||
| Diuretic and natriuretic activity. | VIV | Aqueous infusion, methanolic ext, trichloromethane, ethyl acetate-butanolic fra, kaempferitrin | leaf | Normotensive and spontaneously hypertensive rats. | [67] | ||
| Vasorelaxant properties. | VIV c | Hydroethanolic ext | leaf | Aortic rings of alloxan-induced diabetic rats. | [68] | ||
| VIV | Ext and fra, kaempferitrin, kaempferol | leaf | Aortic rings of normotensive and hypertensive rats. | [69] | |||
| Antimicrobial | VIT | Hydroethanolic ext | leaf | Candida albicans. | [70] | ||
| VIT | Hydroethanolic solution | leaf | Oral microorganism strains and mature dental biofilms. | [71,72] | |||
| VIT | Ethanolic ext | leaf | Staphylococcus aureus SA1199-B. | [73] | |||
| Antioxidant | VIT | Kaempferitrin | leaf | Peroxidation induced by ascorbyl radical in microsomes or in asolectin and phosphatidylcholine liposomes. DPPH assays and MPO activity. | [74] | ||
| VIT | Aqueous ext | leaf | Superoxide anion radical scavenging MPO activity and ABTS radical cation assay. | [75] | |||
| VIV | Aqueous ext | leaf | Pregnant streptozotocin-induced diabetic rats blood. DNTB assay. | [76] | |||
| VIT | Spray and spouted bed dried ext | leaf | DPPH and lipid peroxidation assay. | [77] | |||
| VIT f,p | Hydromethanolic ext | leaf | DPPH assay NO and superoxide radicals scavenging activity. | [78] | |||
| VIT | Methanolic ext and fra | leaf | DPPH assay. | [79] | |||
| VIT VIV | Decoction | leaf | Lipid peroxidation (TBARS). Orofacial dyskinesia induced by long-term treatment with haloperidol in rats. | [80] | |||
| VIT p | Aqueous infusion | leaf | Human erythrocytes exposed to high glucose concentrations and egg yolk samples. Non-protein SH levels and lipid peroxidation (TBARS assay). Iron chelating DPPH and deoxyribose degradation assays. | [81] | |||
| VIT | Aqueous ext | leaf | DPPH assay. Fe2+/citrate-mediated mitochondrial lipid peroxidation in isolated rat liver mitochondria. | [82] | |||
| VIT | Hydromethanolic ext | leaf | DPPH assay. | [83] | |||
| VIT | Hydroalcoholic ext, flavonoid-rich fra | leaf | DPPH assay measurement of reducing power and ferrous ions chelating activity. | [35] | |||
| VIV p | Aqueous infusion | leaf | Streptozotocin-induced diabetic mice pancreas. Lipid peroxidation assay (TBARS) DCFH oxidation assay and NADPH quinone oxidoreductase 1 expression levels. | [84] | |||
| VIT | Aqueous infusion | leaf | Drosophila melanogaster fed on high-sucrose diet. | [85] | |||
| VIT | Ethanolic and hexane ext | leaf | DPP and ORAC assays. | [86] | |||
| VIV | Commercial Ethanolic ext | leaf | Liver from rats exposed to Bisphenol A. TBARS assay. | [87] | |||
| VIV | Ethanolic ext | leaf | Rat male genital system. TBARS assay. | [88] | |||
| VIT | Ethanolic ext fra | leaf | DPPH ORAC and FRAP assays. | [89] | |||
| Antitumor and chemoprotective | Antiproliferative activity. | VIT | BfL-II rBfL-II | seed | HT-29 (colon) and MCF-7 (breast) human cancer cells. | [90] | |
| Antiproliferative and apoptotic activities. | VIT | HY53 | leaf | Hep-G2 (liver) human cancer cells. | [91] | ||
| HY52 | leaf | HeLa (cervical) human cancer cells. | [92] | ||||
| Antitumoral activity. | VIV | Flavonoid-rich fra, purified kaempferitrin | leaf | Murine melanoma. | [93] | ||
| Cytostatic activity. | VIT | rBfL | Several cancer cell lines (e.g., melanoma non-small cell lung ovarian renal and breast) included in the NCI-60 panel. | [94] | |||
| VIT | Hydroethanolic ext | leaf | MCF-7(breast), NCI-ADR/RES (ovary with phenotype resistance to multiple drugs), 786-O (kidney), NCI-H460 (lung), OVCAR-3 (ovary), HT-29 (colon), K562 (bone marrow) human cancer cells and HaCaT (normal keratinocyte) cell line. | [70] | |||
| Cytotoxic activity. | VIT | BfL | seed | MCF-7 (breast) human cancer cells. | [95] | ||
| VIT | Hydro-alcoholic ext | leaf | FO-1 (melanoma) human cells. | [35] | |||
| Chemoprotective effects. | VIT | Aqueous ext | leaf | Bone marrow cells of Wistar rats exposed to clophosphamide. | [96] | ||
| VIT VIV | Flavonoid-rich fra, kaempferitrin | leaf | Intestinal cells (IEC-6 cells) exposed to irinotecan. Irinotecan-induced mucositis in mice. | [93] | |||
| Other biological activities | Edema inhibition (induced by snake venoms). | VIV | Aqueous ext | leaf | Edema induced by Crotalus durissus terrificus venom in mice. | [65] | |
| Inhibition of cholinesterase activity. | VIT f,p | Hydromethanolic ext | leaf | Cholinesterases (acetyl- or butyrylcholinesterase). | [78] | ||
| Protection against vacuous chewing movements induced by haloperidol. | VIV | Decoction | leaf | Orofacial dyskinesia induced by long-term treatment with haloperidol in rats. | [80] | ||
| hol | Antioxidant | VIV | Hydroalcoholic ext enriched in quercetin and myricetin. | leaf | Ethanol-induced gastric ulcer in rats. | [97] | |
| Antitumor | Antiproliferative and apoptotic activity. Protective effects against DNA damage. | VIT | Hydroalcoholic ext enriched in isorhamentin and quercetin derivatives. | leaf | Hep-G2 (liver) human cancer cells. | [98] | |
| Anti-ulcer | Anti-ulcer activity. Antidiarrheal effect. | VIV | Hydroalcoholic ext enriched in quercetin and myricetin. | leaf | Ethanol-induced gastric ulcer in rats. | [97] | |
| Anti-ulcer activity. | Aqueous ext | leaf | HCl-Ethanol-induced gastric ulcer in rats or mice. NSAIDS-Bethanecol induced gastric ulcer in mice. | [99] | |||
| Other biological activities | Anti-dengue activity. | VIT | Hydroethanolic ext | leaf | Dengue virus serotype DENV-2. | [100] | |
| mic | Analgesic | Analgesic. | VIV | Methanolic ext Quercitrin | leaf | Abdominal constrictions induced by injection of acetic acid in mice and capsaicin- and formalin-induced licking. | [39,101] |
| Antihyperalgesic. | VIV | Methanolic ext Quercitrin | leaf | Carrageenan- capsaicin- substance P- bradykinin- and adrenaline-induced mechanical hyperalgesia in rat paw. | [101] | ||
| Antispasmodic effect. | VIV | Methanol ext, ethyl acetate fra | leaf | Smooth muscle preparations of guineapig ileum and rat uterus. | [102] | ||
| Antioxidant | VIT | Hydroalcoholic ext | leaf | DPPH and phosphomolybdenum assays. | [103] | ||
| VIT | Aqueous and hydroethanolic ext | leaf | TRAP TEAC TBARS NO superoxide and hydroxyl radical assays. | [15] | |||
| VIT | Various ext and fra | leaf, stem | DPPH and phosphomolybdenum assays. | [104] | |||
| VIT m | Ethnolic ext and fra | leaf | DPPH ORAC and ABTS assays. | [105] | |||
| Other biological activities | Photoprotective. | VIT/ HUM | Oil-in-water emulsions/sunscreens and water-acetone or activated carbon treated-ethanol ext | leaf | In vitro sun protection factor determination and UVA protection factor assessment. Colipa test in human volunteers to assess sun protection factor. | [106] | |
| ruf | Antimicrobial | VIT | Aqueous and ethanolic ext | leaf | Strains of Candida spp. | [107] | |
| Antitumor | Apoptotic activity. | VIT | rBbKIm, modified with RGD/RGE motifs of BrTI | DU145 and PC3 (prostate) human cancer cells. | [108] | ||
| Inhibition of adhesion. | VIT | BrTI and synthetic peptide containing RGD motif | seed | B16F10 and Tm5 murine melanoma cells. | [109] | ||
| Inhibition of capillary-like tube network formation. | VIT | rBbKIm, modified with RGD/RGE motifs of BrTI | HUVEC human umbilical vein endothelial cells. | [108] | |||
| Thrombolytic activity. | VIT | Hexane ext | leaf | Human venous blood samples. Clot lysis. | [110] | ||
| Other biological activities | Kunitz-tipe proteinase inhibitors activity. | VIT | BrTI | seed | Plasma kallikrein and trypsin. | [109] | |
| Larvicide. | VIT | Metanolic ext and fra. Butane, hexane, dichloromethane, ethyl acetate | leaf | Culex quinquefasciatus. | [111] | ||
| Ung | Antimicrobial | VIT | Aqueous | leaf | Staphylococcus aureus Escherichia coli and Pseudomonas aeruginosa. | [112] | |
| VIT | Essential oils | leaf | Candida albicans Bacillus cereus Salmonella typhimurium Staphylococcus aureus and Citrobacter freundii. | [113] | |||
| Antioxidant | VIT | Ethanolic ext and fra (chloroform, ethyl acetate, hexane, hydroalcoholic) | leaf | DPPH phosphomolybdenum and lipid peroxidation (TBARS) assays. | [114] | ||
| VIT | Ethyl acetate fra | stem | Phosphomolibdenum ROS NO hydrogen peroxide and lipid peroxidation (TBARS) assays in LPS- RAW 264.7 stimulated macrophages. | [115] | |||
| Antitumor | Antiproliferative activity. | VIT | BUL | seed | HT-29 (colon) human cancer cells. | [116] | |
| Cytotoxic activity. | VIT | Essential oils | leaf | HL-60 (leukemia), MCF-7 (breast), NCI-H292 (lung) and HEP-2 (cervical) human cancer cells. | [117] | ||
| VIT | Bibenzyl | root | HL-60 (leukemia) and Hep-2 (cervical) human cancer cells. | [118] | |||
| Wound healing | VIV | Ethyl acetate fra | leaf | Surgical wound model in mice. Monolayers of A549 (lung adenocarcinoma) human epithelial cells. | [119] | ||
| Other biological activities | Inhibition of acetylcholinesterase activity. | VIT | Hexane ext | flower | Acetylcholinesterase. | [120] | |
| VIT | Essential oils | leaf | Acetylcholinesterase. | [113] | |||
| Inhibition of matrix metalloproteinases activity. | VIT | Ethyl acetate partition | stem | Matrix metalloproteinases MMP-2 and MMP-9. | [121] | ||
| Kunitz-tipe proteinase inhibitors activity. | VIT | BuXI | seed | Trypsin and kallikrein. | [122] | ||
| Larvicide. | VIT | Essential oils | leaf | Aedes aegypti. | [117] | ||
| Spp | Study Type/Treatment/ Time | Extract/ Compound | Doses/Day Tested | Study Model/Enzymes/ Method | Gender | Basal Glycemia | Effects | References |
|---|---|---|---|---|---|---|---|---|
| for * | VIV/ O/40d c | Aqueous infusion | 20 g/L | ALX diabetic rats. | M | X = 181 and 36,927 mg/dL | – No hypoglycemic activity. – No improvement in glucose tolerance. – No reduction in cholesterol levels. – No reduction in water and food intake. – No changes in body weight. | [123] |
| VIV/O/31d | Decoction | 150 g leaf/L; 352 ± 78 mL/kg | STZ diabetic rats and normal rats. | M | >500 mg/dL | + Hypoglycemic activity in diabetic rats. – No hypoglycemic activity in normal rats. + Reduction in urine glucose levels. – No changes in hepatic glycogen. – No reduction in triglycerides and cholesterol. + Reduction in urinary urea. – No reduction in food and liquid intake. – No changes in body weight. – No reduction in urinary volume. | [124] | |
| VIV/O/A | N-butanol fra | 400, 600, 800 mg/kg | ALX diabetic rats and normal rats. | M | X = 3305 mg/dL | + Hypoglycemic activity in diabetic and normal rats. – No improvement in glucose tolerance in normal rats. | [125] | |
| VIV/O/20d | Aqueous ext | 500–1000 mg/kg | STZ diabetic pregnant rats | F | >200 mg/dL | + Increment of hepatic glycogen. – No hypoglycemic activity. – No control of total lipid, triglyceride and cholesterol levels (lower mean values observed). + Reduction in uric acid concentration. – No changes in total protein and albumin levels. | [126] | |
| VIV O/A IV/A c | Methanolic ext and fra butanolic fra | 8 mg/kg | ALX diabetic rabbits. | F-M | 250–320 mg/dL | + Hypoglycemic activity. + Improvement in glucose tolerance. + Reduction in urine glucose levels. + Reduction in urine volume. | [127] | |
| VIV/ O/A VIT | Purified kaempferitrin | 100 mg/kg | ALX diabetic rats and normal rats. Soleus muscle from diabetic and normal rats. | M | 25–30 mmol/l | + Hypoglycemic activity in diabetic rats. + Stimulatory effect of glucose uptake in muscle from normal rats. – No reduction in glucosuria in normal and diabetic rats. – No changes in protein synthesis in muscle from normal and diabetic rats. | [128] | |
| VIV/O/7d | Aqueous, ethanolic and hexanic ext | 200 and 400 mg/kg | ALX diabetic rats. | M | >200 mg/dL | + Hypoglycemic activity. + Reduction in triglycerides, total cholesterol and HDL-cholesterol. – No reduction in LDL levels. | [129] | |
| VIV/O/A | Purified kaempferitrin | 50, 100, 200 mg/kg | ALX diabetic rats and normal rats. | M | 25–30 mmol/l | + Hypoglycemic activity in normal and diabetic rats. – No improvement in glucose tolerance in normal rats. | [74] | |
| VIV/O/A | Aqueous infusion | 1 g/kg/0.5 mL wat er | Rats and mice exposed to Tityus serrulatus scorpion venom. | M | + Hypoglycemic activity in treated rats. – No hypoglycemic activity in untreated rats. + Delay in glycogenolysis (but not avoidance). – Decrease of serum levels of insulin induced by venom. – Enhanced venom lethality in mice. | [130] | ||
| VIV/O/A | Aqueous ext | 10% w/v | Normoglycemic mice. | M | + Hypoglycemic activity. | [131] | ||
| VIV/O/20d | Aqueous ext | 500–1000 mg/kg, 2 doses | STZ diabetic pregnant rats and normal rats. | F | >300 mg/dL | – No hypoglycemic activity in diabetic and normal rats. – No improvement of various maternal reproductive outcomes in diabetic rats. | [76] | |
| VIV/O/7d | Spray-dried, oven-dried, wet granulated ext | 200 mg/kg | STZ diabetic rats. | M | >200 mg/kg body wt | + Hypoglycemic activity. – No prevention of decrease in liver glycogen. | [132] | |
| VIV/ O/14d c | Aqueous-ethanol ext | 120 mg/kg | ALX diabetic rats. | F-M | 200–300 mg/dL | + Hypoglycemic activity. | [68] | |
| VIV/O/35d | Spouted bed-dried hydroalcoholic ext | 0.125 g/L and 0.25 g/L, 2 doses of 1 mL | STZ diabetic rats. | M | X = 514 mg/dL | – No hypoglycemic activity. – No reduction in urinary glucose. – No reduction in cholesterol, triglycerides and HDL-cholesterol levels. – No reduction in water and food intake. – No changes in body weight. – No reduction in urine volume, urinary urea and proteinuria. – Hepatic toxicity: increament of aspartate and alanine aminotransferase activities. | [133] | |
| VIV/O/20d | Aqueous ext | 800 mg/kg | Nonobese diabetic (NOD) mice. Isolated salivary glands. | F | >300 mg/dL | + Hypoglycemic activity. + Weight recovery. + Reduction in urine pH and proteinuria. – No improvement in salivary glands tissue recovery. | [134] | |
| VIV/ O/21d p | Aqueous infusion | 1 mg/mL (313 mg/kg) | STZ diabetic mice. | M | >300 mg/dL | – No hypoglycemic activity. – No changes in liver/body weight ratio. | [84] | |
| VIV | Aqueous infusion | 5 mg/mL medium | Drosophila melanogaster fed on high-sucrose diet. | - | + Reduction of hemolymph glucose levels. + Reduction of the hemolymph levels of triacylglycerols. + Improvement of the effects induced by diet intake (developmental time, survival, body weight). | [85] | ||
| VIT c | Butanol ext | 0.001–0.07 mg/mg protein | Isolated gastric glands of ALX diabetic and normal rabbits. | F-M | 200–260 mg/dL | + Stimulatory effect of glucose uptake in normal and diabetic glands. | [135] | |
| VIT f VIT p | Hydromethanolic ext | α-glucosidase activity. | + Inhibition of activity in B. forficata. – No inhibition of activity in B. forficata subsp. pruinosa. | [78] | ||||
| VIT | Purified kaempferitrin | 0.001, 0.0, 0.1, 1, 10, 100, 1000 ηM | Soleus muscle from ALX diabetic and normal rats. | M | + Stimulatory effect of glucose uptake in muscle from normal rats. + Increase in glycogen content in muscle from diabetic rats. + Stimulation glycogen synthesis in muscle from normal rats. + Increment in protein synthesis in muscle from normal rats. | [136] | ||
| VIT | Hydromethanolic ext | α-glucosidase activity. | ± Weak inhibition of activity. | [83] | ||||
| VIT | Hexane ext Ethanolic ext | α-glycosidase, α-amylase and lipase activity. method. | + Inhibition of enzyme activity. + Antiglycation activity. | [86] | ||||
| VIT | Ethanolic ext fra | α-glycosidase, α-amylase and lipase activity. BSA/FRU, BSA/MGO and Arg/MGO methods. | + Inhibition of enzyme activity. + Antiglycation activity. | [89] | ||||
| HUM/O/10m | Aqueous infusion (tea) | A dessert spoon of grounded leaves in water, 3 doses | Type 2 diabetes volunteers (n = 26) and diabetic control group (n = 29). | F-M | 148.70 mg/dL 154.35 mg/dL | – No hypoglycemic activity. – No reduction in glycated hemoglobin values. – No changes in body mass index values. + No changes in serum creatinine and cortisol concentration. | [137] | |
| HUM/ O/3m p | Aqueous infusion, containing rutin and trigonelline | 0.15% w/v (containing rutin 2.80 μg/mL and trigonelline 2.87 μg/mL), 3 doses | Type 2 diabetes volunteers (n = 11) and prediabetic volunteers (n = 4). | F-M | X = 155.57 md/dL | + Reduction in glycated hemoglobin values. – No hypoglycemic activity. – Increase in diuresis. – No correlation between weight and glycemia. | [138] | |
| HUM/O/3m | Aqueous infusion, containing rutin and trigonelline (tea) | 0.4% w/v (containing rutin 1.02 mg and trigonelline 4.30 mg, in 200 mL), 2 doses | Type 2 diabetes mellitus volunteers (n = 25). | nd | X = 268 md/dL (post-prandial) | + Reduction in glycated hemoglobin values. – No reduction in postprandial glycemia. + Reduction in triglycerides and total cholesterol levels (not clinically significant). – No changes in body weight. | [139] | |
| hol | VIV/O/21d | Aqueous ext | 400 mg/kg | STZ diabetic rats and normal rats. | F | >300 mg/dL | – No hypoglycemic activity in normal and diabetic rats. – No improvement in glucose tolerance in normal and diabetic rats. + Reduction in HDL-cholesterol levels in diabetic mice. – No reduction triglycerides, cholesterol and VLDL levels. – No reduction in water and food intake. + Reduction in total protein levels. – Hepatic toxicity: reduction in body weight and increment of aspartate and alanine aminotransferase activities. | [140] |
| VIV/O/14d VIT/ VIV | Ethanolic ext | 400 mg/kg | STZ diabetic mice and normal mice. | nd | >250 mg/dL | + Hypoglycemic activity in diabetic mice. + Improvement in glucose tolerance in diabetic mice. + Increment of hepatic glycogen. – No changes in muscle glycogen. + Activation of gene and protein expression of enzymes involved in liver and muscle glycogenesis and glucose uptake in the muscle. + Inhibition of gene and protein expression of liver gluconeogenesis enzymes. + Inhibition of α-glucosidases (α-amilase and maltase) activity in vitro and in vivo. | [141] |
| Spp | Chemical Constituents | References |
|---|---|---|
| che | Flavonoids: Present but not characterized Other compounds: α-copaene, α-guaiene, α-gurjunene, α-humulene, α-muurolol, α-pinene, α-terpineol, α-ylangene, β-colacorene, β-elemene, β-gurjunene, β-pinene, δ-cadinene, δ-elemene, λ-cadinene, λ-eudesmol, λ-muurolene, 1-epi-cubenol, 2,3-dihydro-farnesol, allo-aromadendrene, aromadendrene, bicyclogermacrene, bulnesol, camphene, caryophyllene, cubenol, (E)-bisabol-11-ol, (E)-caryophyllene, elemol, germacrene D, globulol, humulene epoxide II, limonene, maaliol, myrcene, phytol, sabinene, spathulenol, terpinen-4-ol, trans-b-guaiene, trans-isolongifolanone, tricyclene, viridiflorene, viridiflorol | [61,142] |
| for | Flavonoids: aromadendrin, catechin, epicatechin, eriodictyol, gallocatechin, hispidulin, isoquercitrin, isorhamnetin-3-O-glucoside, isorhamnetin-3-O-rhamnosyl rutinoside, isorhamnetin-3-O-rutinoside, kaempferol, kaempferol-3-(2/3/4-di-rhamnosyl) glucoside, kaempferol-3-O-(2-rhamnosyl) glucoside-7-O-rhamnoside, kaempferol-3-O-(2-rhamnosyl) rutinoside, kaempferol-3-O-(2-rhamnosyl) rutinoside-7-O-rhamnoside, kaempferol 3-O-(4-O-p-coumaroyl) glucoside, kaempferol-3-O-(α)-glucoside-(1′′′6′′)-rhamnoside-7-O-(α)-rhamnoside, kaempferol-3-O-[α-L-rhamnopyranosyl-(1→6)-β-D-glucopyranosyl]-7-O-α-L-rhamnopyranoside, kaempferol-3-O-dirhamnoside, kaempferol-3-O-glucoside, kaempferol-3-O-rhamnoside, kaempferol-3-O-rhamnosyl rutinoside, kaempferol-3-O-robinoside, kaempferol-3-O-rutinoside, kaempferol-3-O-rutinoside-7-O-rhamnoside, kaempferol-3-rhamnoside, kaempferol-7-O-glucoside, kaempferol-7-O-(α)-rhamnoside, kaempferol-7-O-α-L-rhamnopyranoside, kaempferol-37-O-(α)-dirhamnoside (kaempferitrin), kaempferol-arabinoside- rhamnoside, liquiritigenin, luteolin-C-hexoside, myricetin, myricetin-3-O-arabinopiranoside, myricetin-3-O-galactoside, myricetin-3-O-rhamnoside, myricetin-O-(O-galloyl)-hexoside, myricetin-O-(O-galloyl)-hexoside epigallocatech-(48) epicatechin, naringenin, naringin, quercetin, quercetin-O-arabinoside, quercetin-3-arabinoside, quercetin-3-O-(2-rhamnosyl)rutinoside-7-O-rhamnoside, quercetin-3-O-α-L-pyranoside, quercetin-3-O-[α-L-rhamnopyranosyl-(1→6)-β-D-glucopyranosyl]-7-O-α-L-rhamnopyranoside, quercetin-3-O-hexoside (isoquercetin), quercetin-3-O-galactoside, quercetin-3-O-rhamnoside (quercitrin), quercetin-3-O-rhamnosyl rutinoside, quercetin-3-O-rutinoside-7-O-rhamnoside, quercetin-3-O-rutinoside (rutin), quercetin 3-rutinoside-7-rhamnoside, quercetin-37-di-O-α-L-rhamnopyranoside, quercetin-37-di-O-rhamnoside, quercetin-O-hexoside, quercetin-O-(O-galloyl)-hexoside, quercitrin, taxifolin-3-O-rhamnoside Other compounds: 2,4,6-trihydroxy-octadecadienoic acid, α-bisabolol, α-bulnesene, α-cadinol, α-copaene, α-humulene, α-pinene, β-caryophyllene, β-elemene, β-ocimene, β-pinene, ß-sitosterol, γ-elemene, λ-elemene, benzyltartaric acid, bicyclogermacrene, caffeic acid, caryophyllene oxide, chlorogenic acid, copaene isomer, dihydroxyhexadecanoic acid, eicosane, epi-α-muurolol, ellagic acid, epigallocatechin-(4,8)epicatechin, eriodictyol, ferulic acid, gallic acid, germacrene, globulol, hispidulin p-coumaric acid, hydroxy-octadecatrienoic acid, isophytol, protocatechuic acid, rosmarinic acid, sabinene, salicylic acid, sinapic acid, spathulenol, syringic acid, trans-caffeic acid, trihydroxyphenanthren-2-glycoside, umbelliferone, vanillic acid, (Z)-β-farnesene, (Z,E)-farnesol, (Z,Z)-farnesol | [35,69,78,80,82,83,85,88,89,125,131,143,144,145,146,147] |
| subsp. pruinosa Flavonoids: isorhamnetin-3-O-rutinoside, kaempferol, kaempferol-3-(2/3/4-di-rhamnosyl) glucoside, kaempferol-3-O-(2-rhamnosyl) rutinoside, kaempferol-3-O-robinoside, kaempferol-3-O-rutinoside, kaempferol-37-dirhamnoside (kaempferitrin), myricetin-3-O-arabinopiranoside, quercetin, quercetin-3-O-rutinoside (rutin), quercetin-3-O-(2-rhamnosyl) rutinoside, quercetin-3-O-(2/3/4-di-rhamnosyl) glucoside, quercetin-37-di-O-rhamnoside Other compounds: trigonelline | [78,81,84,138,139,148,149,150] | |
| B. candicans Flavonoids: kaempferol-3-O-β-D-glucopyranosyl-(6→1)-β-L-rhamnopyranosyl-7-O-α-L-rhamnopryranoside, kaempferol-37-O-α-L-dirhamnoside (kaempferitrin), quercetin-37-O-α-L-dirhamnoside, quercetin-3-O-β-D-glucopyranosyl-(6→1)-β-L-rhamnopyranosyl-7-O-α-L-rhamnopyranoside | [68,135] | |
| hol | Flavonoids: 3-O-substituted flavonol, isorhamnetin, kaempferol-O-pentoside, luteolin, luteolin-deoxyhexose, myricetin-O-deoxyhexoside, myricetin-O-hexoside, myricetin-O-pentoside, quercetin, quercetin-3-O-deoxyhexoside, quercetin-3-O-hexoside, quercetin-O-deoxyhexoside, quercetin-O-hexoside, quercetin-O-pentoside, quercetin-O-xilopyranoside | [97,98,100,141] |
| mic | Flavonoids: catechin, kaempferol-3-O-rhamnoside, myricitrin, quercetin-3-rhamnoside (quercitrin), vitexin (apigenin 8-C-glucoside) Other compounds: gallic acid, hexatriacontane, methyl gallate | [15,39,151] |
| var.massambabensis Flavonoids: astragalin-2″6″-O-digallate, kaempferol-3-O-rhamnoside | [105] | |
| ruf | Other compounds: α-amorphene, α-cadinol, α-fenchene, α-gurjenene, α-pinene, γ-cadinene, δ-cadinene, allo-aromadendrene, aromadendrene, bicyclogermacrene, cis-a-bisabolene, germacrene, globulol, lepidozenol, spathulenol, sinularene, viridiflorol | [145] |
| ung | Flavonoids: Fisetinidol, liquiritigenin, naringenin, quercetin, quercetin arabinofuranoside, quercitrin Other compounds: 2′-hydroxy-3,5-dimethoxy-4-methylbibenzyl, 2′-hydroxy-3,5-dimethoxybibenzyl, 3-O-methyl-D-pinitol, 6,9-guaiadiene, 8-α-11-elemenedio, α-cadinol, α-calacorene, α-copaene, α-cubebene, α-guaiene, α-humulene, β-bourbonene, β-caryophyllene, β-copaene, β-elemene, β-selinene, γ-cadinene, γ-ermacrene, γ-muurolene, allo-aromadendrene, betulinic acid, caryophyllene, caryophyllene oxide, cubenol, cyclosativene, (E)-caryophyllene, eleagnine, eriodictyol, glutinol, guibourtinidol, harmane, humulene epoxide, humulene epoxide II, junenol, pacharin, sitosterol, spathulenol, stigmasterol, taraxerol, taraxerone | [115,117,118,119,152,153] |
| uru | Flavonoids: kaempferol-3-rhamnoside, kaempferol-galloyl-rhamnoside, quercetin-3-rhamnoside (quercitrin), quercetin-galloyl-rhamnoside | [150] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fortunato, R.H.; Nores, M.J. “Cow’s Hoof” (Bauhinia L., Leguminosae): A Review on Pharmacological Properties of Austral South American Species. Plants 2023, 12, 31. https://doi.org/10.3390/plants12010031
Fortunato RH, Nores MJ. “Cow’s Hoof” (Bauhinia L., Leguminosae): A Review on Pharmacological Properties of Austral South American Species. Plants. 2023; 12(1):31. https://doi.org/10.3390/plants12010031
Chicago/Turabian StyleFortunato, Renée Hersilia, and María Jimena Nores. 2023. "“Cow’s Hoof” (Bauhinia L., Leguminosae): A Review on Pharmacological Properties of Austral South American Species" Plants 12, no. 1: 31. https://doi.org/10.3390/plants12010031
APA StyleFortunato, R. H., & Nores, M. J. (2023). “Cow’s Hoof” (Bauhinia L., Leguminosae): A Review on Pharmacological Properties of Austral South American Species. Plants, 12(1), 31. https://doi.org/10.3390/plants12010031