Soil Acidification in Nutrient-Enriched Soils Reduces the Growth, Nutrient Concentrations, and Nitrogen-Use Efficiencies of Vachellia sieberiana (DC.) Kyal. & Boatwr Saplings
Abstract
:1. Introduction
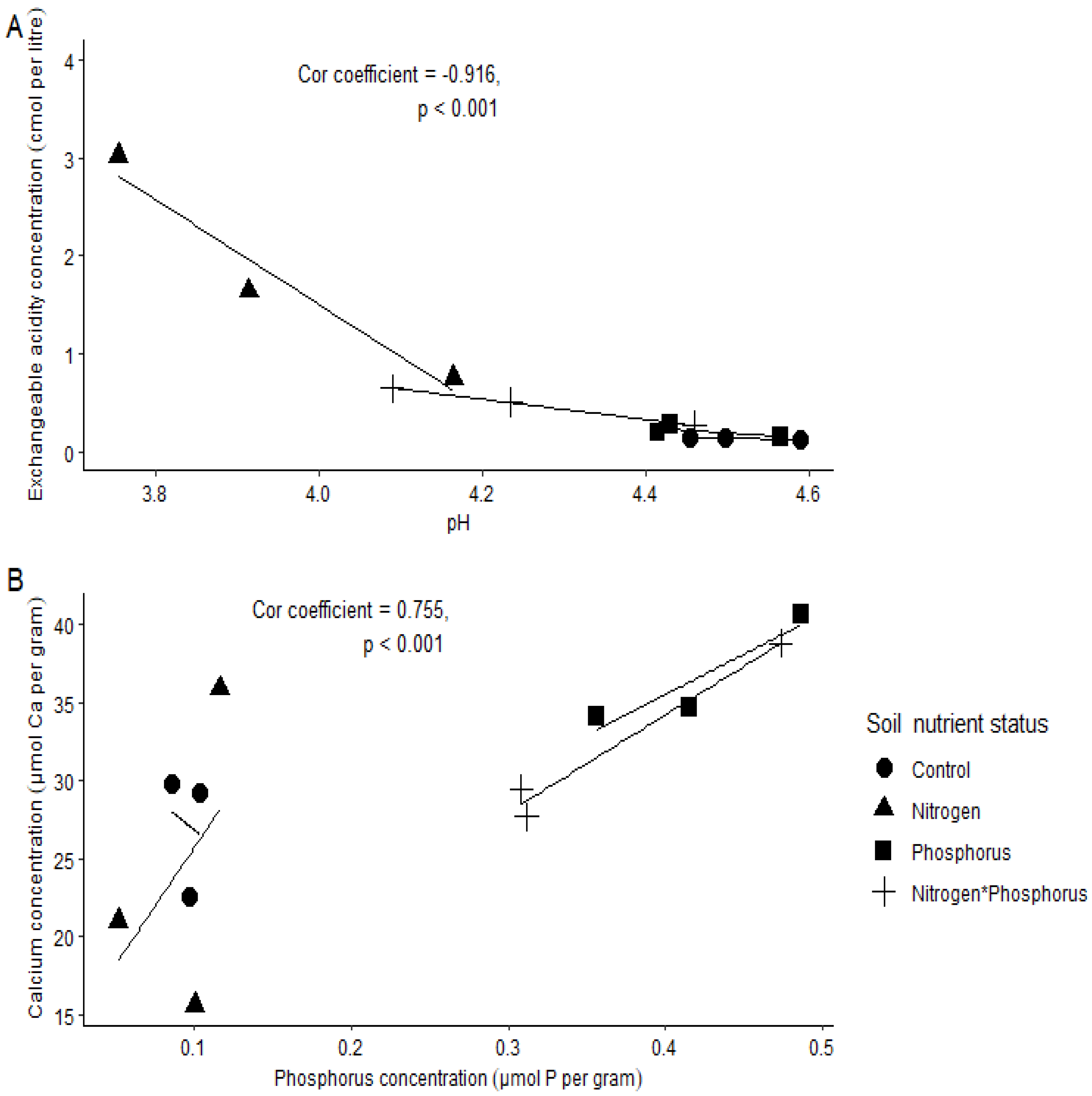
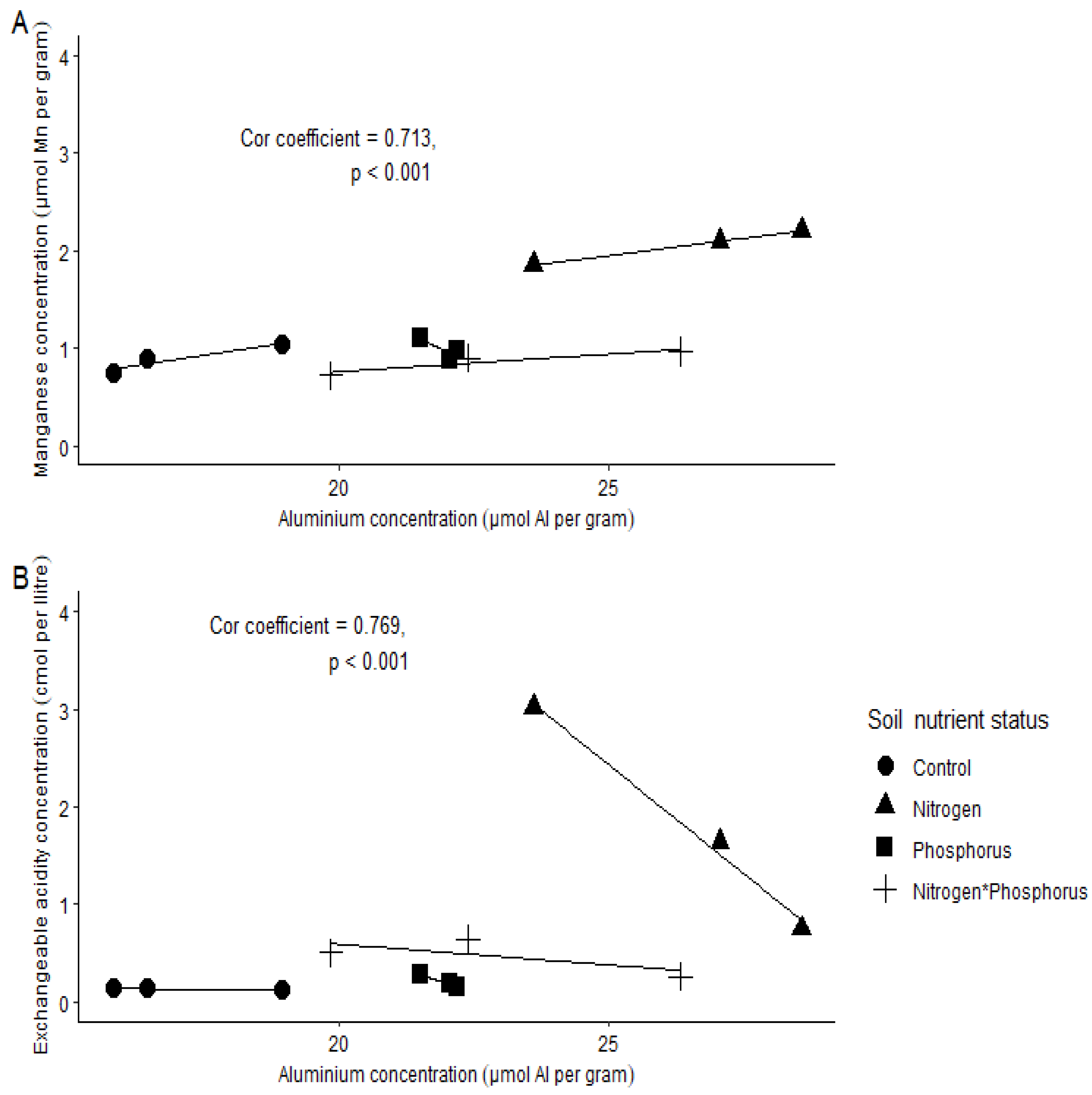
- A low soil pH will result in a high exchangeable acidity (EA); thus, there will be negative correlation between the two variables.
- Soil Al concentration will increase with increasing soil EA and Mn.
- Soil P concentration will increase with soil Ca concentration, and this phenomenon will be stronger in the P-enriched soils.
- Vachellia sieberiana saplings grown in soils with extremely low pH (below 4) will possess significantly reduced root, stem, leaf, and overall biomass compared to saplings grown in conditions with slightly higher pH (above 4).
- Sixty-seven years of N and P enrichment will significantly increase both plant N and P concentrations in V. sieberiana saplings.
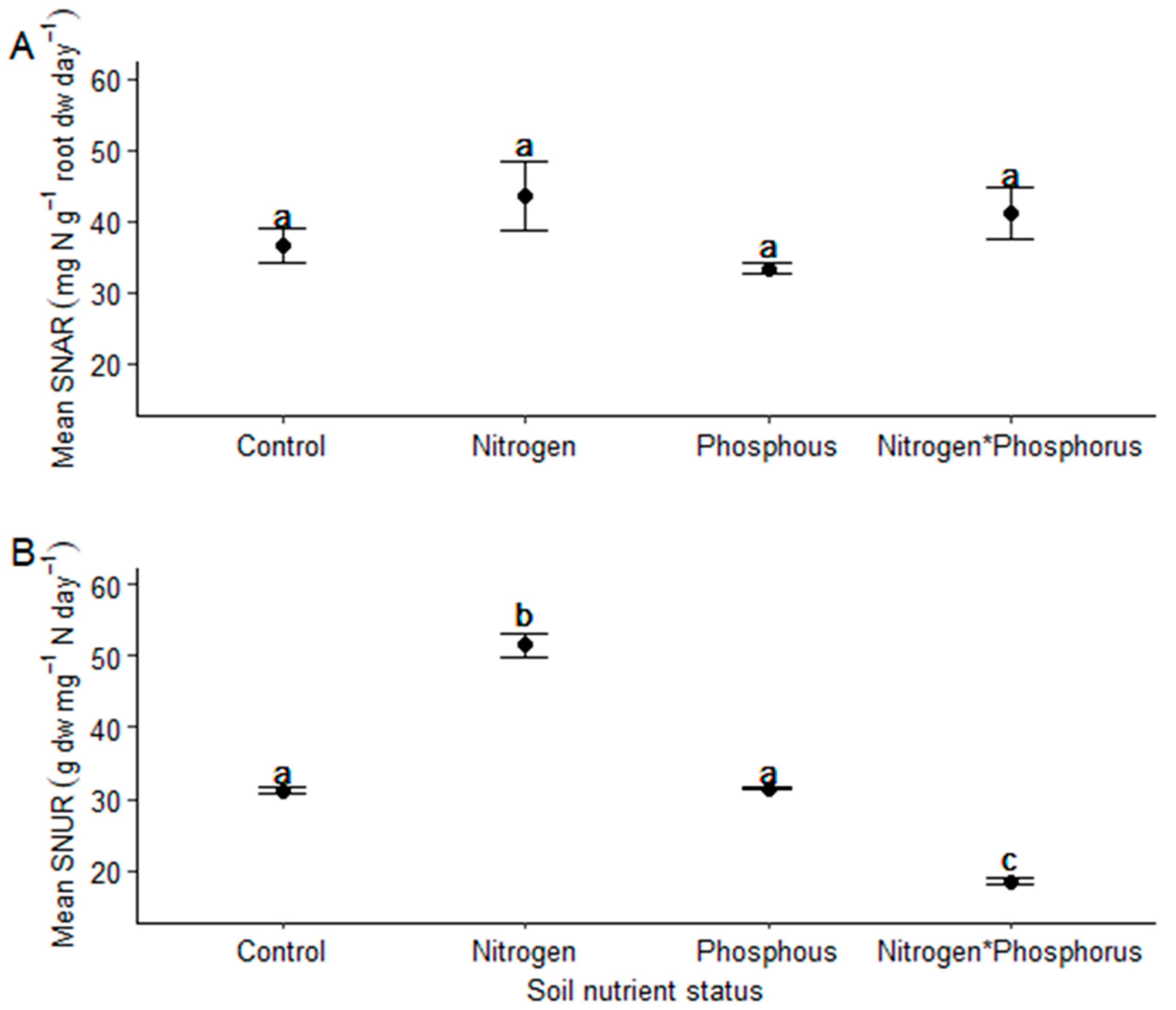
- Vachellia sieberiana saplings grown in N-enriched soils will have significantly reduced nitrogen-use efficiencies compared to those grown in soils not enriched with N.
2. Results
Soil Chemical Properties
3. Discussion
4. Materials and Methods
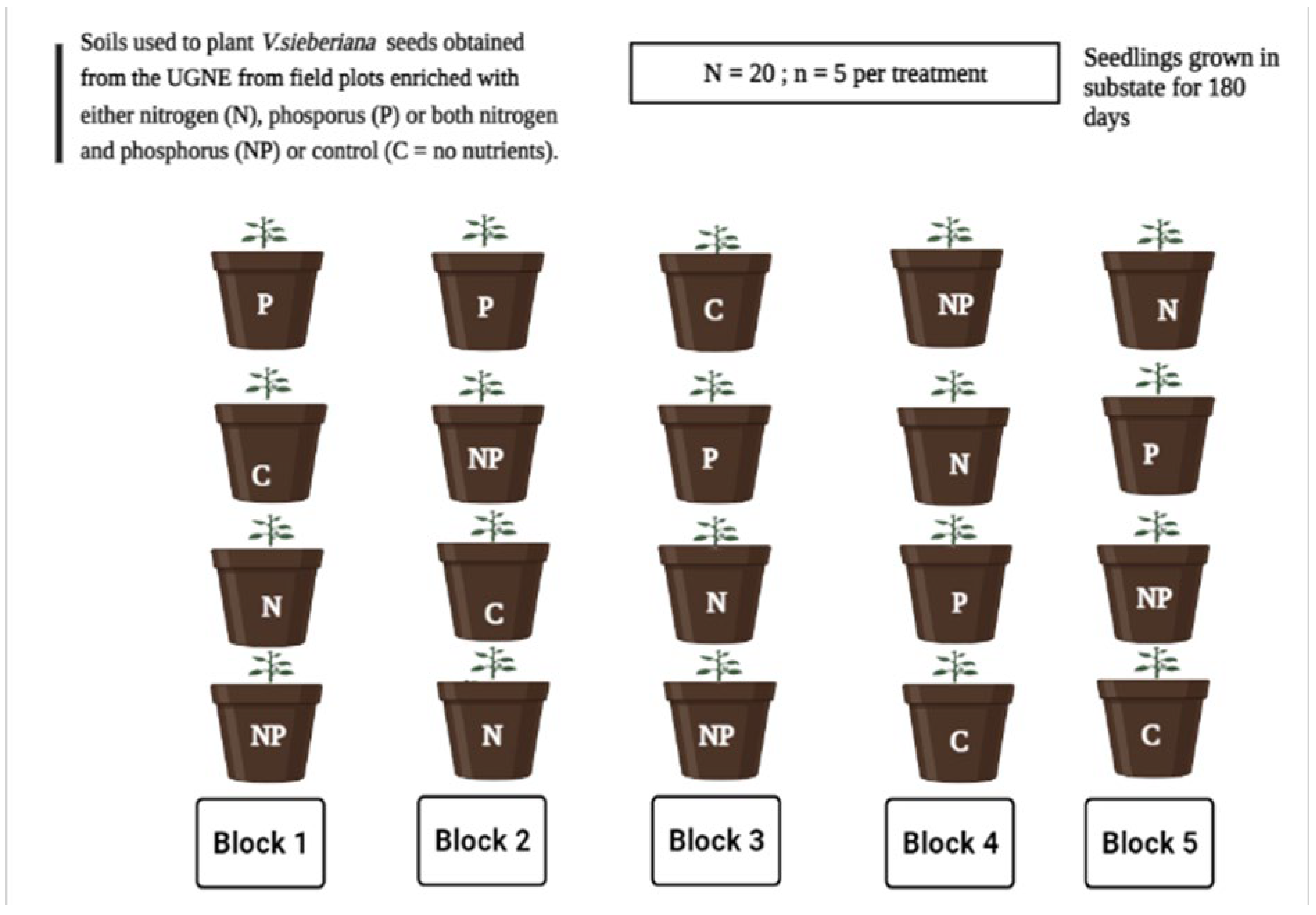
4.1. Study Species and Soil Collection Site
4.2. Experimental Site and Soil Treatment Selection
4.3. Seed Germination and Growth Conditions
4.4. Plant Preparation and Nutrient Analysis
4.5. Calculation of the Specific N Absorption Rate
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goldman, A. Threats to sustainability in African agriculture: Searching for appropriate paradigms. Hum. Ecol. 1995, 23, 291–334. [Google Scholar] [CrossRef]
- Henao, J.; Baanante, C. Estimating Rates of Nutrient Depletion in Soils of Agricultural Lands of Africa Library of Congress Cataloging-in-Publication Data Estimating Rates of Nutrient Depletion in Soils of Agricultural Lands of Africa; International Fertilizer Development Center: Muscle Shoals, AL, USA, 1999. [Google Scholar]
- Zungu, N.S.; Egbewale, S.O.; Olaniran, A.O.; Pérez-Fernández, M.; Magadlela, A. Soil nutrition, microbial composition and associated soil enzyme activities in KwaZulu-Natal grasslands and savannah ecosystems soils. Appl. Soil Ecol. 2020, 155, 103663. [Google Scholar] [CrossRef]
- Mafongoya, P.L.; Bationo, A.; Kihara, J.; Waswa, B.S. Appropriate technologies to replenish soil fertility in southern Africa. Nutr. Cycl. Agroecosyst. 2006, 76, 137–151. [Google Scholar] [CrossRef]
- Nandwa, S.M. Soil organic carbon (SOC) management for sustainable productivity of cropping and agro-forestry systems in Eastern and Southern Africa. In Managing Organic Matter in Tropical Soils: Scope and Limitations; Springer: Dordrecht, The Netherlands, 2001; pp. 143–158. [Google Scholar]
- Aprile, F.; Lorandi, R. Evaluation of Cation Exchange Capacity (CEC) in Tropical Soils Using Four Different Analytical Methods. J. Agric. Sci. 2012, 4, 278. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.B.; Sayyed, R.Z.; Trivedi, M.H.; Gobi, T.A. Phosphate solubilizing microbes: Sustainable approach for managing phosphorus deficiency in agricultural soils. Springer Plus 2013, 2, 587. [Google Scholar] [CrossRef] [Green Version]
- Afonso, S.; Arrobas, M.; Rodrigues, M. Soil and Plant Analyses to Diagnose Hop Fields Irregular Growth. J. Soil Sci. Plant Nutr. 2020, 20, 1999–2013. [Google Scholar] [CrossRef]
- Mitra, D.; Anđelković, S.; Panneerselvam, P.; Senapati, A.; Vasić, T.; Ganeshamurthy, A.N.; Chauhan, M.; Uniyal, N.; Mahakur, B.; Radha, T.K. Phosphate-Solubilizing Microbes and Biocontrol Agent for Plant Nutrition and Protection: Current Perspective. Commun. Soil Sci. Plant Anal. 2020, 51, 645–657. [Google Scholar] [CrossRef]
- Vanlauwe, B.; Hungria, M.; Kanampiu, F.; Giller, K. The role of legumes in the sustainable intensification of African smallholder agriculture: Lessons learnt and challenges for the future. Agric. Ecosyst. Environ. 2019, 284, 106583. [Google Scholar] [CrossRef]
- Richardson, A.E.; Barea, J.-M.; McNeill, A.M.; Prigent-Combaret, C. Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms. Plant Soil 2009, 321, 305–339. [Google Scholar] [CrossRef]
- Widdig, M.; Schleuss, P.-M.; Weig, A.R.; Guhr, A.; Biederman, L.A.; Borer, E.T.; Crawley, M.J.; Kirkman, K.P.; Seabloom, E.; Wragg, P.; et al. Nitrogen and Phosphorus Additions Alter the Abundance of Phosphorus-Solubilizing Bacteria and Phosphatase Activity in Grassland Soils. Front. Environ. Sci. 2019, 7, 185. [Google Scholar] [CrossRef]
- Pueyo, J.J.; Quiñones, M.A.; de la Peña, T.C.; Fedorova, E.E.; Lucas, M.M. Nitrogen and Phosphorus Interplay in Lupin Root Nodules and Cluster Roots. Front. Plant Sci. 2021, 12, 644218. [Google Scholar] [CrossRef]
- Rotaru, V.; Sinclair, T.R. Interactive influence of phosphorus and iron on nitrogen fixation by soybean. Environ. Exp. Bot. 2009, 66, 94–99. [Google Scholar] [CrossRef]
- Mitran, T.; Meena, R.S.; Lal, R.; Layek, J.; Kumar, S.; Datta, R. Role of Soil Phosphorus on Legume Production. In Legumes for Soil Health and Sustainable Management; Springer: Singapore, 2018; pp. 487–510. [Google Scholar]
- Ndabankulu, K.; Egbewale, S.O.; Tsvuura, Z.; Magadlela, A. Soil microbes and associated extracellular enzymes largely impact nutrient bioavailability in acidic and nutrient poor grassland ecosystem soils. Sci. Rep. 2022, 12, 12601. [Google Scholar] [CrossRef] [PubMed]
- Vance, C.P.; Uhde-Stone, C.; Allan, D.L. Phosphorus acquisition and use: Critical adaptations by plants for securing a nonrenewable resource. New Phytol. 2003, 157, 423–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zahran, H.H. Rhizobium-Legume Symbiosis and Nitrogen Fixation under Severe Conditions and in an Arid Climate. Microbiol. Mol. Biol. Rev. 1999, 63, 968–989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferguson, B.J.; Indrasumunar, A.; Hayashi, S.; Lin, M.-H.; Lin, Y.-H.; Reid, D.E.; Gresshoff, P.M. Molecular Analysis of Legume Nodule Development and Autoregulation. J. Integr. Plant Biol. 2010, 52, 61–76. [Google Scholar] [CrossRef]
- Herridge, D.F.; Robertson, M.J.; Cocks, B.; Peoples, M.B.; Holland, J.F.; Heuke, L. Low nodulation and nitrogen fixation of mungbean reduce biomass and grain yields. Aust. J. Exp. Agric. 2005, 45, 269–277. [Google Scholar] [CrossRef]
- Sulieman, S.; Tran, L.-S.P. Phosphorus homeostasis in legume nodules as an adaptive strategy to phosphorus deficiency. Plant Sci. 2015, 239, 36–43. [Google Scholar] [CrossRef]
- Werner, D.; Newton, W. Nitrogen Fixation in Agriculture, Forestry, Ecology, and the Environment, 4th ed.; Springer Science and Business Media: Berlin/Heidelberg, Germany, 2005. [Google Scholar]
- Balemi, T.; Negisho, K. Management of soil phosphorus and plant adaptation mechanisms to phosphorus stress for sustainable crop production: A review. J. Soil Sci. Plant Nutr. 2012, 12, 547–562. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.C.; Liao, H. Organic acid anions: An effective defensive weapon for plants against aluminum toxicity and phosphorus deficiency in acidic soils. J. Genet. Genom. 2016, 43, 631–638. [Google Scholar] [CrossRef]
- Mohammadi, K.; Sohrabi, Y.; Heidari, G.; Khalesro, S. Effective Factors on Biological Nitrogen Fixation. Afr. J. Agric. Res. 2012, 7, 1782–1788. [Google Scholar]
- Bekere, W. Liming Effects on Yield and Yield Attributes of Nitrogen Fertilized and Bradyrhizobia Inoculated Soybean (Glycine max L.) Grown in Acidic Soil at Jimma, South Western Ethiopia. J. Biol. Agric. Healthc. 2013, 3, 139–144. [Google Scholar]
- Zaidi, A.; Khan, M.; Ahemad, M.; Oves, M. Plant growth promotion by phosphate solubilizing bacteria. Acta Microbiol. Immunol. Hung. 2009, 56, 263–284. [Google Scholar] [CrossRef] [PubMed]
- Otuba, M.; Weih, M. Effects of Soil Substrate and Nitrogen Fertilizer on Growth Rate of Acacia senegal and Acacia sieberiana in North Eastern Uganda. Int. J. Agric. For. 2015, 5, 10–16. [Google Scholar]
- Grellier, S.; Ward, D.; Janeau, J.-L.; Podwojewski, P.; Lorentz, S.; Abbadie, L.; Valentin, C.; Barot, S. Positive versus negative environmental impacts of tree encroachment in South Africa. Acta Oecologica 2013, 53, 1–10. [Google Scholar] [CrossRef]
- Schleicher, J.; Wiegand, K.; Ward, D. Changes of woody plant interaction and spatial distribution between rocky and sandy soil areas in a semi-arid savanna, South Africa. J. Arid. Environ. 2011, 75, 270–278. [Google Scholar] [CrossRef]
- Grellier, S.; Janeau, J.-L.; Richard, P.; Florsch, N.; Ward, D.; Bariac, T.; Lorentz, S. Water uptake plasticity of savanna trees in encroached grassland: Small trees match the mature trees. Afr. J. Range Forage Sci. 2020, 38, 231–243. [Google Scholar] [CrossRef]
- Wakeling, J.L.; Cramer, M.D.; Bond, W.J. Is the lack of leguminous savanna trees in grasslands of South Africa related to nutritional constraints? Plant Soil 2010, 336, 173–182. [Google Scholar] [CrossRef]
- Reverchon, F.; Xu, Z.; Blumfield, T.; Chen, C.; Abdullah, K. Impact of global climate change and fire on the occurrence and function of understorey legumes in forest ecosystems. J. Soils Sediments 2011, 12, 150–160. [Google Scholar] [CrossRef] [Green Version]
- Salvagiotti, F.; Cassman, K.; Specht, J.; Walters, D.; Weiss, A.; Dobermann, A. Nitrogen uptake, fixation and response to fertilizer N in soybeans: A review. Field Crop. Res. 2008, 108, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Santachiara, G.; Salvagiotti, F.; Rotundo, J. Nutritional and environmental effects on biological nitrogen fixation in soybean: A meta-analysis. Field Crop. Res. 2019, 240, 106–115. [Google Scholar] [CrossRef]
- Shah, A.S.; Wakelin, S.A.; Moot, D.J.; Blond, C.; Laugraud, A.; Ridgway, H.J. Trifolium repens and T. subterraneum modify their nodule microbiome in response to soil pH. J. Appl. Microbiol. 2021, 131, 1858–1869. [Google Scholar] [CrossRef] [PubMed]
- Fowler, D.; Coyle, M.; Skiba, U.; Sutton, M.A.; Cape, J.N.; Reis, S.; Sheppard, L.J.; Jenkins, A.; Grizzetti, B.; Galloway, J.N.; et al. The global nitrogen cycle in the Twentyfirst century. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 1621. [Google Scholar]
- Steffen, W.; Richardson, K.; Rockström, J.; Cornell, S.E.; Fetzer, I.; Bennett, E.M.; Biggs, R.; Carpenter, S.R.; De Vries, W.; De Wit, C.A.; et al. Planetary boundaries: Guiding human development on a changing planet. Science 2015, 347, 6223. [Google Scholar] [CrossRef] [PubMed]
- Peñuelas, J.; Poulter, B.; Sardans, J.; Ciais, P.; Van Der Velde, M.; Bopp, L.; Boucher, O.; Godderis, Y.; Hinsinger, P.; Llusia, J.; et al. Human-induced nitrogen–phosphorus imbalances alter natural and managed ecosystems across the globe. Nat. Commun. 2013, 4, 2934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bauer, J.T.; Kleczewski, N.; Bever, J.D.; Clay, K.; Reynolds, H.L. Nitrogen-fixing bacteria, arbuscular mycorrhizal fungi, and the productivity and structure of prairie grassland communities. Oecologia 2012, 170, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Suding, K.N.; Collins, S.L.; Gough, L.; Clark, C.; Cleland, E.E.; Gross, K.L.; Milchunas, D.G.; Pennings, S. Functional- and abundance-based mechanisms explain diversity loss due to N fertilization. Proc. Natl. Acad. Sci. USA 2005, 102, 4387–4392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tilman, D. Resources: A Graphical-Mechanistic Approach to Competition and Predation. Am. Nat. 1980, 116, 362–393. [Google Scholar] [CrossRef]
- Augusto, L.; Delerue, F.; Gallet-Budynek, A.; Achat, D.L. Global assessment of limitation to symbiotic nitrogen fixation by phosphorus availability in terrestrial ecosystems using a meta-analysis approach. Glob. Biogeochem. Cycles 2013, 27, 804–815. [Google Scholar] [CrossRef]
- Divito, G.A.; Sadras, V.O. How do phosphorus, potassium and sulphur affect plant growth and biological nitrogen fixation in crop and pasture legumes? A meta-analysis. Field Crop. Res. 2014, 156, 161–171. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Cassman, K.; Cleveland, C.; Crews, T.; Field, C.B.; Grimm, N.B.; Howarth, R.W.; Marino, R.; Martinelli, L.; Rastetter, E.B.; et al. Towards an ecological understanding of biological nitrogen fixation. Biogeochemistry 2002, 57–58, 1–45. [Google Scholar] [CrossRef]
- Vance, C.P. ymbiotic Nitrogen Fixation and Phosphorus Acquisition. Plant Nutrition in a World of Declining Renewable Resources. Plant Physiol. 2001, 127, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Fay, P.A.; Prober, S.M.; Harpole, W.S.; Knops, J.M.; Bakker, J.D.; Borer, E.T.; Lind, E.M.; MacDougall, A.S.; Seabloom, E.W.; Wragg, P.D.; et al. Grassland productivity limited by multiple nutrients. Nat. Plants 2015, 1, 15080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harpole, W.S.; Ngai, J.T.; Cleland, E.E.; Seabloom, E.W.; Borer, E.T.; Bracken, M.E.; Elser, J.J.; Gruner, D.S.; Hillebrand, H.; Shurin, J.B.; et al. Nutrient co-limitation of primary producer communities. Ecol. Lett. 2011, 14, 852–862. [Google Scholar] [CrossRef]
- Morris, C.D.; Fynn, R.W.S. The Ukulinga long-term grassland trials: Reaping the fruits of meticulous, patient research. Bull. Grassl. Soc. S. Afr. 2001, 11, 7–22. [Google Scholar]
- Schroder, J.L.; Raun, W.R.; Penn, C.J.; Payton, M.E. Soil Acidifi cation from Long-Term Use of Nitrogen Fertilizers on Winter Wheat. Soil Fertil. Plant Nutr. Plant Nutr. 2011, 75, 957–964. [Google Scholar]
- Cai, Z.; Wang, B.; Xu, M.; Zhang, H.; He, X.; Zhang, L.; Gao, S. Intensified soil acidification from chemical N fertilization and prevention by manure in an 18-year field experiment in the red soil of southern China. J. Soils Sediments 2014, 15, 260–270. [Google Scholar] [CrossRef]
- von Uexkull, H.; Mutert, E. Global extent, development and economic impact of acid soils. Plant Soil 1995, 171, 1–15. [Google Scholar] [CrossRef]
- Liu, J.; Ma, X.; Duan, Z.; Jiang, J.; Reichstein, M.; Jung, M. Impact of temporal precipitation variability on ecosystem productivity. WIREs Water 2020, 7. [Google Scholar] [CrossRef]
- Zhang, Y.; He, X.; Liang, H.; Zhao, J.; Zhang, Y.; Xu, C.; Shi, X. Long-term tobacco plantation induces soil acidification and soil base cation loss. Environ. Sci. Pollut. Res. 2015, 23, 5442–5450. [Google Scholar] [CrossRef]
- Aber, J.; McDowell, W.; Nadelhoffer, K.; Magill, A.; Berntson, G.; Kamakea, M.; McNulty, S.; Currie, W.; Rustad, L.; Fernandez, I. Nitrogen saturation in temperate forest ecosystems: Hypotheses revisited. BioScience 1998, 48, 921–934. [Google Scholar] [CrossRef] [Green Version]
- Hue, N.V.; Vega, S.; Silva, J.A. Manganese Toxicity in a Hawaiian Oxisol Affected by Soil pH and Organic Amendments. Soil Sci. Soc. Am. J. 2001, 65, 153–160. [Google Scholar] [CrossRef] [Green Version]
- Moir, J.; Moot, D. Medium-term soil pH and exchangeable aluminium response to liming at three high country locations. Proc. N. Z. Grassl. Assoc. 2014, 76, 41–46. [Google Scholar] [CrossRef]
- Dai, K.H.; Richter, D.D. A re-examination of exchangeable acidity as extracted by potassium chloride and potassium fluoride. Commun. Soil Sci. Plant Anal. 2000, 31, 115–139. [Google Scholar] [CrossRef]
- Onwuka, M.I.; Ozurumba, U.V.; Nkwocha, O.S. Changes in Soil pH and Exchangeable Acidity of Selected Parent Materials as Influenced by Amendments in South East of Nigeria. J. Geosci. Environ. Prot. 2016, 4, 80–88. [Google Scholar] [CrossRef] [Green Version]
- Cai, J.; Weiner, J.; Wang, R.; Luo, W.; Zhang, Y.; Liu, H.; Xu, Z.; Li, H.; Zhang, Y.; Jiang, Y. Effects of nitrogen and water addition on trace element stoichiometry in five grassland species. J. Plant Res. 2017, 130, 659–668. [Google Scholar] [CrossRef]
- Mao, Q.; Lu, X.; Zhou, K.; Chen, H.; Zhu, X.; Mori, T.; Mo, J. Effects of long-term nitrogen and phosphorus additions on soil acidification in an N-rich tropical forest. Geoderma 2017, 285, 57–63. [Google Scholar] [CrossRef]
- Elser, J.J.; Bracken, M.E.; Cleland, E.E.; Gruner, D.S.; Harpole, W.S.; Hillebrand, H.; Ngai, J.T.; Seabloom, E.W.; Shurin, J.B.; Smith, J.E. Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol. Lett. 2007, 10, 1135–1142. [Google Scholar] [CrossRef] [Green Version]
- Magadlela, A.; Lembede, Z.; Egbewale, S.O.; Olaniran, A.O. The metabolic potential of soil microorganisms and enzymes in phosphorus-deficient KwaZulu-Natal grassland ecosystem soils. Appl. Soil Ecol. 2022, 181, 104647. [Google Scholar] [CrossRef]
- Somavilla, A.; Caner, L.; da Silva, I.C.B.; Bastos, M.C.; Moro, L.; Schaefer, G.L.; Gatiboni, L.C.; dos Santos, D.R. Chemical pattern of vegetation and topsoil of rangeland fertilized over 21 years with phosphorus sources and limestone. Soil Tillage Res. 2021, 205, 104759. [Google Scholar] [CrossRef]
- Tate, K.R.; Salcedo, I. Phosphorus control of soil organic matter accumulation and cycling. Biogeochemistry 1988, 5, 99–107. [Google Scholar] [CrossRef]
- Zhan, X.; Zhang, L.; Zhou, B.; Zhu, P.; Zhang, S.; Xu, M. Changes in Olsen Phosphorus Concentration and Its Response to Phosphorus Balance in Black Soils under Different Long-Term Fertilization Patterns. PLoS ONE 2015, 10, e0131713. [Google Scholar] [CrossRef] [PubMed]
- Kisinyo, P.O.; Opala, P.A.; Gudu, S.O.; Othieno, C.O.; Okalebo, J.R.; Palapala, V.; Otinga, A.N. Recent advances towards understanding and managing Kenyan acid soils for improved crop production. Afr. J. Agric. Res. 2014, 9, 2397–2408. [Google Scholar] [CrossRef] [Green Version]
- Keller, A.B.; Walter, C.A.; Blumenthal, D.M.; Borer, E.T.; Collins, S.L.; DeLancey, L.C.; Fay, P.A.; Hofmockel, K.S.; Knops, J.M.H.; Leakey, A.D.B.; et al. Stronger fertilization effects on aboveground versus belowground plant properties across nine U.S. grasslands. Ecology 2022, e3891. [Google Scholar] [CrossRef]
- Tian, J.; Wei, K.; Condron, L.M.; Chen, Z.; Xu, Z.; Chen, L. Impact of land use and nutrient addition on phosphatase activities and their relationships with organic phosphorus turnover in semi-arid grassland soils. Biol. Fertil. Soils 2016, 52, 675–683. [Google Scholar] [CrossRef]
- Bekele, A.; Kibret, K.; Bedadi, B.; Yli-Halla, M.; Balemi, T. Effects of Lime, Vermicompost, and Chemical P Fertilizer on Selected Properties of Acid Soils of Ebantu District, Western Highlands of Ethiopia. Appl. Environ. Soil Sci. 2018, 2018, 8178305. [Google Scholar] [CrossRef]
- Ceulemans, T.; Merckx, R.; Hens, M.; Honnay, O. Plant species loss from European semi-natural grasslands following nutrient enrichment-is it nitrogen or is it phosphorus? Glob. Ecol. Biogeogr. 2012, 22, 73–82. [Google Scholar] [CrossRef]
- Harpole, W.S.; Sullivan, L.L.; Lind, E.M.; Firn, J.; Adler, P.B.; Borer, E.T.; Chase, J.; Fay, P.A.; Hautier, Y.; Hillebrand, H.; et al. Addition of multiple limiting resources reduces grassland diversity. Nature 2016, 537, 93–96. [Google Scholar] [CrossRef] [Green Version]
- Almeida, J.P.F.; Hartwig, U.A.; Frehner, M.; Nösberger, J.; Lüscher, A. Evidence that P deficiency induces N feedback regulation of symbiotic N2 fixation in white clover (Trifolium repens L.). J. Exp. Bot. 2000, 51, 1289–1297. [Google Scholar] [CrossRef] [Green Version]
- Lynch, J.P.; Ho, M.D.; Low Phosphorus. Rhizoeconomics: Carbon costs of phosphorus acquisition. Plant Soil 2005, 269, 45–56. [Google Scholar] [CrossRef]
- Harrison, M.T.; Edwards, E.J.; Farquhar, G.D.; Nicotra, A.B.; Evans, J.R. Nitrogen in cell walls of sclerophyllous leaves accounts for little of the variation in photosynthetic nitrogen-use efficiency. Plant Cell Environ. 2009, 32, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Sithole, N.; Tsvuura, Z.; Kirkman, K.; Magadlela, A. Altering Nitrogen Sources Affects Growth Carbon Costs in Vachellia nilotica Growing in Nutrient-Deficient Grassland Soils. Plants 2021, 10, 1762. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.W.; Williams, M.L.; Sistani, K.R. N 2 fixation by soybean-Bradyrhizobium combinations under acidity, low P and high AI stresses. Plant Soil 1991, 131, 293–300. [Google Scholar] [CrossRef]
- Bhatti, A.A.; Haq, S.; Bhat, R.A. Microbial Pathogenesis Actinomycetes benefaction role in soil and plant health. Microb. Pathog. 2017, 111, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Boonkerd, N. Symbiotic Association between Frankia and Actinorhizal Plants. In Nitrogen Fixation with Non-Legumes; Springer: Dordrecht, The Netherlands, 1998; pp. 327–331. [Google Scholar] [CrossRef]
- Khan, A.; Jilani, G.; Akhtar, M.; Naqvi, S.; Rasheed, M. Phosphorus Solubilizing Bacteria: Occurrence, Mechanisms and their Role in Crop Production. J. Agric. Biol. Sci. 2009, 1, 48–58. [Google Scholar]
- Tsvetkova, G.; Georgiev, G. Growth, nodulation and dinitrogen fixation related to the changes of phosphate fractions of nodules and leaves of phosphorus starved soybean (Glycine max L. Merr) plants. Comptes Rendus De L’academie Bulg. Des Sci. 2003, 56, 9–47. [Google Scholar]
- Weisany, W.; Raei, Y.; Allahverdipoor, K.H. Role of Some of Mineral Nutrients in Biological Nitrogen Fixation. Bull. Environ. Pharmacol. Life Sci. 2013, 2, 77–84. [Google Scholar]
- Batterman, S.A.; Wurzburger, N.; Hedin, L.O. Nitrogen and phosphorus interact to control tropical symbiotic N2 fixation: A test in inga punctata. J. Ecol. 2013, 101, 1400–1408. [Google Scholar] [CrossRef]
- Afzal, F.; Khan, T.; Khan, A.; Khan, S.; Raza, H.; Ihsan, A. Nutrient deficiencies under stress in legumes: An overview. In Legumes under Environmental Stress: Yield, Improvement and Adaptations; Wiley: Hoboken, NJ, USA, 2015; pp. 55–65. [Google Scholar]
- Sithole, N.; Pérez-fernández, M.; Magadlela, A. Nutritional status of soils from KwaZulu-Natal modulate symbiotic interactions and plant performance in Vigna unguiculata L. (Walp). Appl. Soil Ecol. 2019, 142, 1–7. [Google Scholar] [CrossRef]
- Ward, D.; Kirkman, K.; Tsvuura, Z. An African grassland responds similarly to long-term fertilization to the Park Grass experiment. PLoS ONE 2017, 12, e0177208. [Google Scholar] [CrossRef] [Green Version]
- Ward, D.; Kirkman, K.P.; Tsvuura, Z.; Morris, C.; Fynn, R.W. Are there common assembly rules for different grasslands? Comparisons of long-term data from a subtropical grassland with temperate grasslands. J. Vegetat. Sci. 2020, 31, 780–791. [Google Scholar] [CrossRef]
- Soil Classification Working Group. Soil Classification: A Taxonomic System for South Africa; Department of Agricultural Development: Pretoria, South Africa, 1991. [Google Scholar]
- Zama, N.; Magadlela, A.; Mkhize, N.; Tedder, M.; Kirkman, K. Assessing long-term nutrient and lime enrichment effects on a subtropical South African grassland. Afr. J. Range Forage Sci. 2022, 1–13. [Google Scholar] [CrossRef]
- Fynn, R.W.; O’Connor, T. Determinants of community organization of a South African mesic grassland. J. Veg. Sci. 2005, 16, 93–102. [Google Scholar] [CrossRef]
- Tsvuura, Z.; Kirkman, K.P. Yield and species composition of a mesic grassland savanna in South Africa are influenced by long-term nutrient addition. Austral Ecol. 2013, 38, 959–970. [Google Scholar] [CrossRef]
- Coetzee, P.E.; Ceronio, G.M.; Preez, C.C.d. Effect of phosphorus and nitrogen sources on essential nutrient concentration and uptake by maize (Zea mays L.) during early growth and development. S. Afr. J. Plant Soil 2017, 34, 55–64. [Google Scholar] [CrossRef]
- Xia, J.; Wan, S. Independent effects of warming and nitrogen addition on plant phenology in the Inner Mongolian steppe. Ann. Bot. 2013, 111, 1207–1217. [Google Scholar] [CrossRef]
- Du, C.; Jing, J.; Shen, Y.; Liu, H.; Gao, Y. Short-term grazing exclusion improved topsoil conditions and plant characteristics in degraded alpine grasslands. Ecol. Indic. 2019, 108, 105680. [Google Scholar] [CrossRef]
- Fageria, N.K.; Moreira, A. The role of mineral nutrition on root growth of crop plants. In Advances in Agronomy, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2011; Volume 110, pp. 251–331. [Google Scholar]
- Paal, J.; Degtjarenko, P.; Suija, A.; Liira, J. Vegetation responses to long-term alkaline cement dust pollution in Pinus sylvestris-dominated boreal forests-niche breadth along the soil pH gradient. Appl. Veg. Sci. 2012, 16, 248–259. [Google Scholar] [CrossRef]
- Li, K.-W.; Lu, H.-L.; Nkoh, J.N.; Hong, Z.-N.; Xu, R.-K. Aluminum mobilization as influenced by soil organic matter during soil and mineral acidification: A constant pH study. Geoderma 2022, 418, 115853. [Google Scholar] [CrossRef]
- Nielsen, K.L.; Eshel, A.; Lynch, J.P. The effect of phosphorus availability on the carbon economy of contrasting common bean (Phaseolus vulgaris L.) genotypes. J. Exp. Bot. 2001, 52, 329–339. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R: Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Le, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Kassambara, A.; Mundt, F. Package ‘factoextra’. Extract and Visualize the Results of Multivariate Data Analyses; R: Foundation for Statistical Computing: Vienna, Austria, 2017; Volume 76, pp. 1–74. [Google Scholar]
- Harrell, F.E. Package ‘Hmisc’; Harrel Miscellaneous, R package version 4.7.2; R: Foundation for Statistical Computing: Vienna, Austria, 2022; pp. 1–455. Available online: https://hbiostat.org/R/Hmisc/ (accessed on 11 November 2022).
- Spearman, C. The proof and measurement of association between two things. Int. J. Epidemiol. 2010, 39, 1137–1150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kassambara, A. Package ‘dtwSat’ R topics documented, rstatix: Pipe-Friendly Framework for Basic Statistical Tests, R package version 0.7.0; R: Foundation for Statistical Computing: Vienna, Austria, 2021; pp. 1–90. [Google Scholar]
- Schloerke, B.; Crowley, J.; Cook, D. Package ‘GGally, GGally: Extension to ggplot2’, R package version 2.1.2; R: Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Fox, J.; Friendly, M.; Monette, G.; Chalmers, P. Package ‘heplots’: Visualizing Hypothesis Tests in Multivariate Linear Models; R: Foundation for Statistical Computing: Vienna, Austria, 2021; pp. 3–9. [Google Scholar]
- Bolan, N.S.; Hedley, M.J. Role of Carbon, Nitrogen, and Sulfur Cycles in Soil Acidification. In Handbook of Soil Acidity; Rengel, Z., Ed.; Marcel Dekker Inc.: New York, NY, USA, 2003; pp. 9–56. [Google Scholar]
- Suding, K.N.; Goldberg, D.E.; Hartman, K.M. Relationships Among Species Traits: Separating Levels of Response and Identifying Linkages to Abundance. Ecology 2003, 84, 1–16. [Google Scholar] [CrossRef]



| Soil Parameter | Control | Nitrogen | Phosphorus | Nitrogen * Phosphorus |
|---|---|---|---|---|
| pH (KCl) | 4.347 ± 0.147 | 3.945 ± 0.119 | 4.455 ± 0.089 | 4.261 ± 0.107 |
| Exchangeable acidity | 0.998 ± 0.809 | 1.799 ± 0.659 | 0.218 ± 0.031 | 0.471 ±0.112 |
| Aluminium | 17.071 ± 0.954 | 26.409 ± 409 | 21.914 ± 0.207 | 22.854 ± 1.883 |
| Calcium | 27.224 ± 2.327 | 24.156 ± 6.071 | 36.519 ± 2.101 | 32.003 ± 3.445 |
| Iron | 1.328 ± 0.059 | 1.586 ± 0.104 | 2.064 ± 0.322 | 1.444 ± 0.148 |
| Magnesium | 17.188 ± 0.336 | 11.838 ± 2.824 | 12.025 ± 0.999 | 10.446 ± 1.361 |
| Manganese | 0.892 ± 0.084 | 2.051 ± 0.105 | 0.988 ± 0.062 | 0.863 ± 0.074 |
| Phosphorus | 0.084 ± 0.012 | 0.090 ± 0.020 | 0.451 ± 0.042 | 0.364 ± 0.054 |
| Nitrogen | 0.002 ± 0.522 | 0.029 ± 0.002 | 0.024 ± 0.001 | 0.023 ± 0.001 |
| Zinc | 0.017 ± 0.006 | 0.013 ± 0.001 | 0.048 ± 0.003 | 0.017 ± 0.005 |
| Plant Traits | ||||
|---|---|---|---|---|
| Parameter | Control | Nitrogen | Phosphorus | Nitrogen * Phosphorus |
| Roots (g) | 4.379 ± 0.623 b | 2.269 ± 0.464 a | 5.828 ± 0.587 b | 5.815 ± 0.368 b |
| Leaves (g) | 0.769 ± 0.070 ab | 0.696 ± 0.079 a | 1.211 ± 0.125 bc | 1.282 ± 0.176 c |
| Stems (g) | 2.972 ± 0.866 ab | 2.367 ± 0.578 a | 5.909 ±1.400 b | 2.954 ± 0.240 ab |
| Total biomass (g) | 8.119 ± 0.604 ab | 5.331 ± 1.034 a | 12.949 ± 1.482 b | 10.051 ± 0.553 b |
| Plant mineral Nutrition | ||||
| Parameter | Control | Nitrogen | Phosphorus | Nitrogen * Phosphorus |
| Total plant P (µmol P. g−1) | 25.319 ± 2.779 a | 26.699 ± 1.139 a | 37.193 ± 2.433 b | 41.306 ± 1.963 b |
| Total plant N (mmol Ng−1) | 48.017 ± 3.046 b | 31.137 ± 2.697 a | 56.851 ± 6.259 b | 48.171 ± 0.972 b |
| Standard corrected 15N/14N | 6.021 ± 0.886 a | 4.562 ± 0.505 a | 3.896 ± 1.142 a | 5.405 ± 0.283 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zama, N.; Kirkman, K.; Mkhize, N.; Tedder, M.; Magadlela, A. Soil Acidification in Nutrient-Enriched Soils Reduces the Growth, Nutrient Concentrations, and Nitrogen-Use Efficiencies of Vachellia sieberiana (DC.) Kyal. & Boatwr Saplings. Plants 2022, 11, 3564. https://doi.org/10.3390/plants11243564
Zama N, Kirkman K, Mkhize N, Tedder M, Magadlela A. Soil Acidification in Nutrient-Enriched Soils Reduces the Growth, Nutrient Concentrations, and Nitrogen-Use Efficiencies of Vachellia sieberiana (DC.) Kyal. & Boatwr Saplings. Plants. 2022; 11(24):3564. https://doi.org/10.3390/plants11243564
Chicago/Turabian StyleZama, Naledi, Kevin Kirkman, Ntuthuko Mkhize, Michelle Tedder, and Anathi Magadlela. 2022. "Soil Acidification in Nutrient-Enriched Soils Reduces the Growth, Nutrient Concentrations, and Nitrogen-Use Efficiencies of Vachellia sieberiana (DC.) Kyal. & Boatwr Saplings" Plants 11, no. 24: 3564. https://doi.org/10.3390/plants11243564
APA StyleZama, N., Kirkman, K., Mkhize, N., Tedder, M., & Magadlela, A. (2022). Soil Acidification in Nutrient-Enriched Soils Reduces the Growth, Nutrient Concentrations, and Nitrogen-Use Efficiencies of Vachellia sieberiana (DC.) Kyal. & Boatwr Saplings. Plants, 11(24), 3564. https://doi.org/10.3390/plants11243564






