Phonophoresis through Nonsteroidal Anti-Inflammatory Drugs for Knee Osteoarthritis Treatment: Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Study Selection and Data Extraction
2.4. Methodological Quality of the Studies
2.5. Statistical Analysis
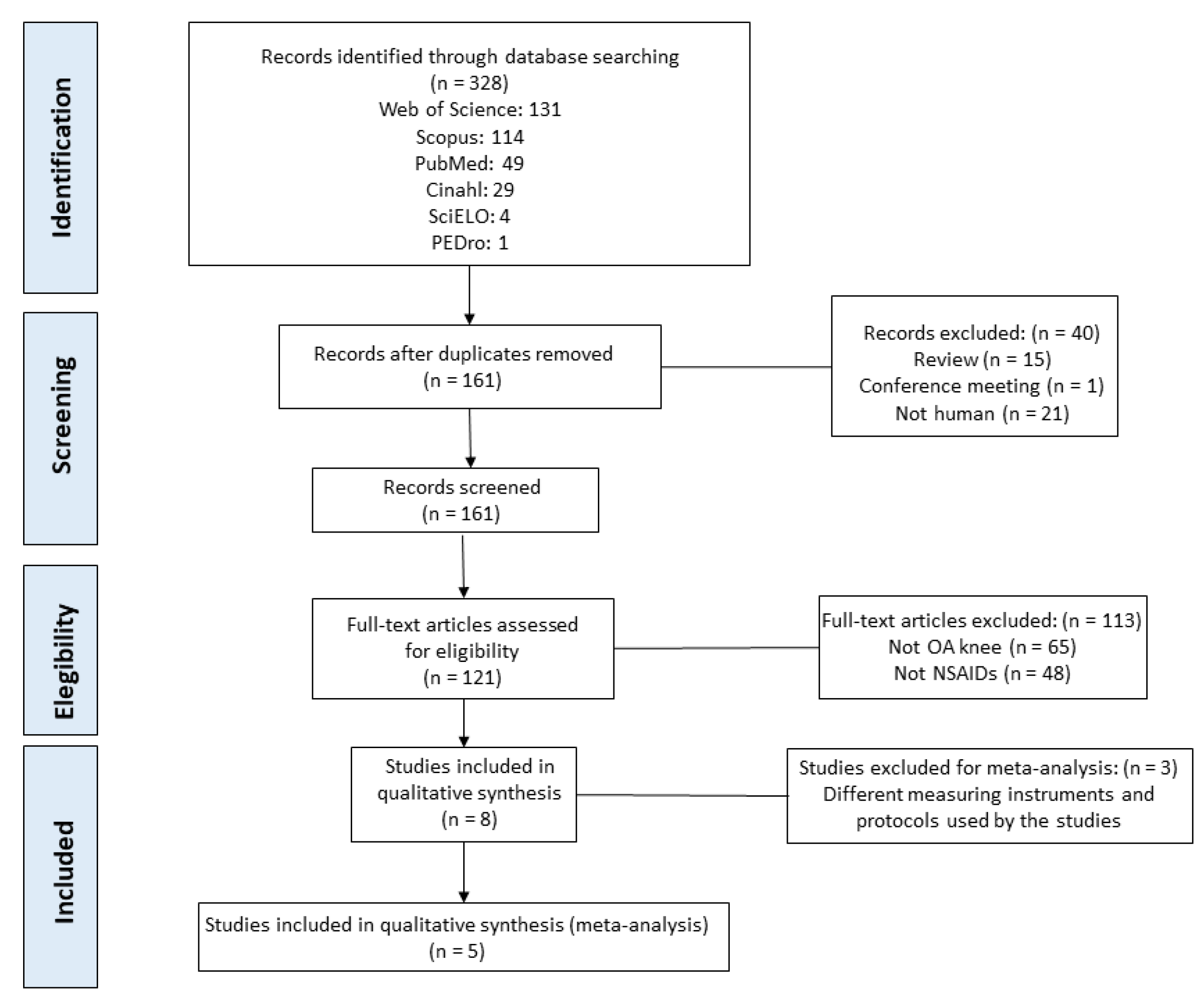
3. Results
3.1. Study Included in the Systematic Review
3.2. Study Groups Included in the Meta-Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baron, D.; Flin, C.; Porterie, J.; Despaux, J.; Vincent, P. Inyección Intraarticular Única de Ácido Hialurónico En La Artrosis de Rodilla: Estudio Multicéntrico Prospectivo Abierto (ART-ONE 75) Mediante Comparación Post-Hoc Con Placebo. Curr. Ther. Res. Clin. Exp. 2019, 90, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Monfort, J.; Pujol, J.; Contreras-Rodríguez, O.; Llorente-Onaindia, J. López-Solà, M.; Blanco-Hinojo, L.; Vergés, J.; Herrero, M.; Sánchez, L.; Ortiz, H.; et al. Effects of Chondroitin Sulfate on Brain Response to Painful Stimulation in Knee Osteoarthritis Patients. A Randomized, Double-Blind, Placebo-Controlled Functional Magnetic Resonance Imaging Study. Med. Clin. 2017, 148, 539–547. [Google Scholar] [CrossRef] [Green Version]
- Iijima, H.; Shimoura, K.; Ono, T.; Aoyama, T.T.M. Proximal Gait Adaptations in Individuals with Knee Osteoarthritis: A Systematic Review and Meta-Analysis. J. Biomech. 2019, 18, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Harding, G.T.; Hubley-Kozey, C.L.; Dunbar, M.J. Stanish, W.D.; Wilson, J.A.L. Body Mass Index Affects Knee Joint Mechanics during Gait Differently with and without Moderate Knee Osteoarthritis. Osteoarthr. Cartil. 2012, 20, 1234–1242. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.C.; Huang, H.T.; Huang, P.J.; Liu, Z.M.; Shih, C.L. Efficacy and Safety of Extracorporeal Shockwave Therapy for Treatment of Knee Osteoarthritis: A Systematic Review and Meta-Analysis. Pain Med. 2020, 21, 822–835. [Google Scholar] [CrossRef]
- Akinbo, S.R.; Aiyejusunle, C.B.; Akinyemi, O.A.; Adesegun, S.A.; Danesi, M.A. Comparison of the Therapeutic Efficacy of Phonophoresis and Iontophoresis Using Dexamethasone Sodium Phosphate in the Management of Patients with Knee Osteoarthritis. Niger. Postgrad. Med. J. 2007, 14, 190–194. [Google Scholar]
- Rocha, T.C.; Ramos, P.D.S.; Dias, A.G.; Martins, E.A. The Effects of Physical Exercise on Pain Management in Patients with Knee Osteoarthritis: A Systematic Review with Metanalysis. Pain Med. 2020, 55, 509–517. [Google Scholar] [CrossRef]
- Scaturro, D.; Vitagliani, F.; Terrana, P.; Cuntrera, D.; Falco, V.; Tomasello, S.; Mauro, G.L. Intra-Articular Hybrid Hyaluronic Acid Injection Treatment in Overweight Patients with Knee Osteoarthritis: A Single-Center, Open-Label, Prospective Study. Appl. Sci. 2021, 11, 8711. [Google Scholar] [CrossRef]
- Arboleya, L.R.; Figuera, E.; García, M.S.; Aragón, B. Tratamiento Sintomático de La Artrosis: Patrón de Utilización de Antiinflamatorios No Esteroides En Los Centros de Salud Españoles. Rev. Española Reumatol. 2002, 29, 300–307. [Google Scholar]
- Aliaga, L.; Baños, J.E.; de Barutell, C. Dolor y Utilización Clínica de Los Analgésicos; MCR: Barcelona, Spain, 1996; ISBN 84-7625-085-1. [Google Scholar]
- Rafanan, B.S.; Valdecañas, B.F.; Lim, B.P.; Al, E. Consensus Recommendations for Managing Osteoarthritic Pain with Topical NSAIDs in Asia-Pacific. Pain Manag. 2018, 8, 115–128. [Google Scholar] [CrossRef]
- Cagnie, B.; Vinck, E.; Rimbaut, S.; Vanderstraeten, G. Phonophoresis versus Topical Application of Ketoprofen: Comparison between Tissue and Plasma Levels. Phys. Ther. 2003, 83, 707–712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polat, B.E.; Hart, D.; Langer, R.; Blankschtein, D. Ultrasound-Mediated Transdermal Drug Delivery: Mechanisms, Scope, and Emerging Trends. J. Control. Release 2011, 152, 330–348. [Google Scholar] [CrossRef] [PubMed]
- Bosnjakovic, A.; Mishra, M.K.; Ren, W.; Al, E. Poly(Amidoamine) Dendrimer-Erythromycin Conjugates for Drug Delivery to Macrophages Involved in Periprosthetic Inflammation. Nanomedicine 2011, 7, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Dong, W.J.; Yang, G.Y.; Wang, W.; Ji, C.H.; Zhou, F.N. Dendrimer-Coupled Sonophoresis-Mediated Transdermal Drug-Delivery System for Diclofenac. Drug Des. Dev. Ther. 2015, 9, 3867–3876. [Google Scholar] [CrossRef] [Green Version]
- Rutjes, A.W.; Nüesch, E.; Sterchi, R.; Jüni, P. Therapeutic Ultrasound for Osteoarthritis of the Knee or Hip. Cochrane Database Syst. Rev. 2010, 1, CD003132. [Google Scholar] [CrossRef]
- Watson, T. Electroterapia. In Práctica Basada En La Evidencia; 12a Edició; Elsevier: Barcelona, España, 2009; ISBN 978-84-8086-444-2. [Google Scholar]
- Derry, S.; Conaghan, P.; Da Silva, J.A.; Wiffen, P.J.; Moore, R.A. Topical NSAIDs for Chronic Musculoskeletal Pain in Adults. Cochrane Database Syst. Rev. 2016, 4, CD007400. [Google Scholar] [CrossRef]
- Byl, N.N. The Use of Ultrasound as an Enhancer for Transcutaneous Drug Delivery: Phonophoresis. Phys. Ther. 1995, 75, 539–553. [Google Scholar] [CrossRef]
- Leite, C.B.S.; Coelho, J.M.; Ferreira-Nunes, R.; Al., E. Phonophoretic Application of a Glucosamine and Chondroitin Nanoemulsion for Treatment of Knee Chondropathies. Nanomedicine 2020, 15, 647–659. [Google Scholar] [CrossRef]
- Rigby, J.H.; Hagan, A.M.; Kelcher, A.R.; Ji, C. Dexamethasone Sodium Phosphate Penetration during Phonophoresis at 2 Ultrasound Frequencies. J. Athl. Train. 2020, 55, 628–635. [Google Scholar] [CrossRef] [Green Version]
- Heyadati, R.; Aminian-Far, A.; Darbani, M.; Al, E. Efficacy of Glucosamine Compounds Phonophoresis in Knee Osteoarthritis. Koomesh 2016, 18, 276–285. [Google Scholar]
- Toopchizadeg, V.; Javadi, R.; Sadat, B.E. Therapeutic Efficacy of Dexamethasone Phonophoresis on Symptomatic Knee Osteoarthritis in Elderly Women. Int. J. Women’s Health Reprod. Sci. 2014, 2, 168–177. [Google Scholar] [CrossRef] [Green Version]
- Haupenthal, D.P.D.S.; Dias, F.M.; Zaccaron, R.P.; Al, E. Effects of Phonophoresis with Ibuprofen Associated with Gold Nanoparticles in Animal Model of Traumatic Muscle Injury. Eur. J. Pharm. Sci. 2020, 143, 105120. [Google Scholar] [CrossRef] [PubMed]
- Abbas, S.A.; Khalaf, M.M.; El-Eishi, N.H. The Effect of Aloevera Phonophoresis on Skin Thickness in Patients with Psoriasis: A Randomized Controlled Trail. Eur. Asian J. Bio Sci. 2020, 14, 129–134. [Google Scholar]
- Altan, L.; Kasapoglu Aksoy, M.; Kösegil Öztürk, E. Efficacy of Diclofenac & Thiocolchioside Gel Phonophoresis Comparison with Ultrasound Therapy on Acute Low Back Pain; a Prospective, Double-Blind, Randomized Clinical Study. Ultrasonics 2019, 91, 2012–2205. [Google Scholar] [CrossRef]
- Tantawy, S.A.; Elgohary, H.M.I.; Kamel, D.M. Trans-Perineal Pumpkin Seed Oil Phonophoresis as an Adjunctive Treatment for Chronic Nonbacterial Prostatitis. Res. Rep. Urol. 2018, 10, 95–101. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Zhu, S.; Zenghui Lv, Z.; Kan, S.; Wu, Q.; Song, W.; Ning, G.; Feng, S. Effects of Therapeutic Ultrasound for Knee Osteoarthritis: A Systematic Review and Meta-Analysis. Clin. Rehabil. 2019, 33, 1863–1875. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.; Bossuyt, P.; Boutron, I.; Hoffmann, T.; Mulrow, C.D.; Moher, D. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. Syst. Rev. 2020, 10, 89. [Google Scholar] [CrossRef]
- Cumpston, M.S.; McKenzie, J.E.; Thomas, J.; Brennan, S.E. Current Practice in Systematic Reviews Including the “PICO for Each Synthesis” and Methods Other than Meta-Analysis: Protocol for a Cross-Sectional Study. F1000Research 2020, 9, 678. [Google Scholar] [CrossRef]
- Maher, C.G.; Sherrington, C.; Herbert, R.D.; Moseley, A.M.; Elkins, M. Reliability of the PEDro Scale for Rating Quality of Randomized Controlled Trials. Phys. Ther. 2003, 83, 713–721. [Google Scholar] [CrossRef] [Green Version]
- Foley, N.C.; Teasell, R.W.; Bhogal, S.K.; Speechley, M.R. Stroke Rehabilitation Evidence-Based Review: Methodology. Top Stroke Rehabil. 2003, 10, 1–7. [Google Scholar] [CrossRef]
- Altman, R.; Asch, E.; Bloch, D.; Bole, G.; Borenstein, D.; Brandt, K.; Christy, W.; Cooke, T.D.; Greenwald, R.; Hochberg, M.; et al. Development of Criteria for the Classification and Reporting of Osteoarthritis: Classification of Osteoarthritis of the Knee. Arthritis Rheum. 1986, 29, 1039–1049. [Google Scholar] [CrossRef] [PubMed]
- Akinbo, S.; Owoeye, O.; Adesegun, S. Comparison of the Therapeutic Efficacy of Diclofenac Sodium and Methyl Salicylate Phonophoresis in the Management of Knee Osteoarthritis. Turk. J. Rheumatol. 2011, 26, 111–119. [Google Scholar] [CrossRef] [Green Version]
- Kellgren, J.H.; Lawrence, J.S. Radiological Assessment of Osteo-Arthrosis. Ann. Rheum. Dis. 1957, 16, 494–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlsson, A.M. Assessment of Chronic Pain. I. Aspects of the Reliability and Validity of the Visual Analogue Scale. Pain 1983, 16, 87–101. [Google Scholar] [CrossRef]
- Basaran, S.; Guzel, R.; Seydaoglu, G.; Guler-Uysal, F. Validity, Reliability, and Comparison of the WOMAC Osteoarthritis Index and Lequesne Algofunctional Index in Turkish Patients with Hip or Knee Osteoarthritis. Clin. Rheumatol. 2010, 29, 749–756. [Google Scholar] [CrossRef]
- Bellamy, N.; Buchanan, W.W.; Goldsmith, C.H.; Campbell, J.; Stitt, L.W. Validation Study of WOMAC: A Health Status Instrument for Measuring Clinically Important Patient Relevant Outcomes to Antirheumatic Drug Therapy in Patients with Osteoarthritis of the Hip or Knee. J. Rheumatol. 1998, 15, 1833–1840. [Google Scholar]
- Monisha, R.; Manikumar, M.; Krishnakumar, A. Evaluating the Effectiveness of Phonophoresis by Piroxicam and Dimethyl Sulfoxide for Women’s with Osteoarthritis Knee Joint. Asian J. Pharm. Clin. Res. 2018, 11, 329–331. [Google Scholar] [CrossRef]
- Jun, Z.; Qingfu, W.; Ji, W.; Xinchao, S.; Qingxue, Q.; Haoyun, Z.; Song, L.; Lili, Y.; Dong aa Zhao Jun, Z.; Dong, Z. Therapeutic Effects of Low-Frequency Phonophoresis with a Chinese Herbal Medicine versus Sodium Diclofenac for Treatment of Knee Os-Teoarthritis: A Double-Blind, Randomized, Placebo-Controlled Clini-Cal Trial. J. Tradit. Chin. Med. 2015, 35, 613–617. [Google Scholar] [CrossRef] [Green Version]
- Lequesne, M.G.; Mery, C.; Samson, M.; Gerard, P. Indexes of Severity for Osteoarthritis of the Hip and Knee: Validation-Value in Comparition with Other Assessment Tests. Scand J. Rheumatol. Suppl. 1987, 65, 85–89. [Google Scholar] [CrossRef]
- Bruce, B.; Fries, J.F. The Health Assessment Questionnaire (HAQ). Clin. Exp. Rheumatol. 2005, 23, 14–18. [Google Scholar]
- Boyaci, A.; Tutoglu, A.; Boyaci, N.; Aridici, R.; Koca, I. Comparison of the Efficacy of Ketoprofen Phonophoresis, Ultrasound, and Short-Wave Diathermy. Rheumatol. Int. 2013, 33, 2811–2818. [Google Scholar] [CrossRef] [PubMed]
- Belindayi, I.C.; Gokcen, N.; Basaran, S. Comparative Short-Term Effectiveness of Ibuprofen Gel and Cream Phonophoresis in Patients with Knee Osteoarthritis Enhanced Reader. Rheumatol. Int. 2018, 38, 1927–1932. [Google Scholar] [CrossRef]
- Boonhong, J.; Suntornpiyapan, P. Ultrasound Combined Transcutaneous Electrical Nerve Stimulation (UltraTENS) versus Phonophoresis of Piroxicam (PhP) in Symptomatic Knee Osteoarthritis: A Randomized Double-Blind, Controlled Trial. J. Back Musculoskelet Rehabil. 2018, 1, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Luksurapan, W.; Boonhong, J. Effects of Phonophoresis of Piroxicam and Ultrasound on Symptomatic Knee Osteoarthritis. Arch Phys. Med. Rehabil. 2013, 94, 250–255. [Google Scholar] [CrossRef]
- Oktayoǧlu, P.; Gür, A.; Yardimeden, I.; Çaǧlayan, M.; Çevik, F.; Bozkurt, M.; Em, S.; Uçar, D.; Nas, K. Comparison of the Efficacy of Phonophoresis and Conventional Ultrasound Therapy in Patients with Primary Knee Osteoarthritis. Erciyes Tip Derg. 2014, 36, 11–18. [Google Scholar] [CrossRef] [Green Version]
- Seah, B.C.; Teo, B. Recent Advances in Ultrasound-Based Transdermal Drug Delivery. Int. J. Nanomed. 2018, 13, 7749–7763. [Google Scholar] [CrossRef]
- Rodríguez, J. Electroterapia En Fisioterapia, 3rd ed.; Panamericana: Madrid, Spain, 2014; ISBN 978-84-9835-758-5. [Google Scholar]
- Ita, K. Recent Progress in Transdermal Sonophoresis. Pharm. Dev. Technol. 2017, 22, 458–466. [Google Scholar] [CrossRef]
- Aldwaikat, M.; Alarjah, M. Investigating the Sonophoresis Effect on the Permeation of Diclofenac Sodium Using 3D Skin Equivalent. Ultrason. Sonochem 2015, 22, 580–587. [Google Scholar] [CrossRef]
- Yin, L.; Qin, F.; Zhou, Y.; Qi, X. Enhancing Percutaneous Permeability of Sinomenine Hydrochloride Using Dual-Frequency Sonophoresis. J. Drug Deliv. Sci. Technol. 2016, 36, 62–67. [Google Scholar] [CrossRef]
- Goh, S.-L.; Persson, M.S.; Stocks, J.; Hou, Y.; Lin, J.; Hall, M.C.; Doherty, M.; Zhang, W. Efficacy and Potential Determinants of Exercise Therapy in Knee and Hip Osteoarthritis: A Systematic Review and Meta-Analysis. Ann. Phys. Rehabil. Med. 2019, 62, 356–365. [Google Scholar] [CrossRef]
- Solís, U.; Prada, D.M.; Molinero, C.; de Armas, A.; García, V.; Hernández, A. Rasgos Demográficos de La Osteoartritis de Rodilla. Rev. Cuba Reumatol. 2015, 17, 32–39. [Google Scholar]
- Farreras, P.; Rozman, C. Medicina Interna (Vol. 1); Editorial: Barcelona, Spain, 1982; ISBN 84-7102-981-2. [Google Scholar]
- Denegar, C.R.; Schimizzi, M.E.; Dougherty, D.R.; Al, E. Responses to Superficial Heating and Cooling Differ in Men and Women with Knee Osteoarthritis. Physiother. Theory Pract. 2012, 28, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Paradowski, P.T.; Bergman, S.; Sunden-Lundius, A.; Lohmander, L.S.; Roos, E.M. Knee Complaints Vary with Age and Gender in the Adult Population. Population-Based Reference Data for the Knee Injury and Osteoarthritis Outcome Score (KOOS). BMC Musculoskelet Disord. 2006, 7, 38. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Physical Status: The Use and Interpretation of Anthropometry. Report of the WHO Expert Committee. In Report of the WHO Expert Committee; WHO Technical Report Series 854; WHO: Geneva, Switzerland, 1995. [Google Scholar]
- Rodríguez, F. Evidence for the Use of Topical Non-Steroidal Anti-Inflammatory Drugs. Rev. Clin. Med. Fam. 2013, 6, 152–159. [Google Scholar]
- Richy, F.; Scarpignato, C.; Lanas, A.; Reginster, J.Y. Efficacy and Safety of Piroxicam Revisited. A Global Meta-Analysis of Randomised Clinical Trials. Pharmacol. Res. 2009, 60, 254–263. [Google Scholar] [CrossRef]
- Dogruyol, S.; Kocak, A.O.; Akbas, I.; Menekse, T.S.; Gur, S.T.A.; Dogruyol, T.; Cakir, Z. Comparison of Ibuprofen and Piroxicam Gel in the Treatment of Trauma Pain: A Randomized Double-Blind Trial of Geriatric Population. Am. J. Emerg. Med. 2020, 38, 2110–2115. [Google Scholar] [CrossRef]
- Cage, S.A.; Rupp, K.A.; Castel, J.C.; Saliba, E.N.; Hertel, J.; Saliba, S.A. Relative Acoustic Transmission of Topical Preparations Used with Therapeutic Ultrasound. Arch Phys. Med. Rehabil. 2013, 94, 2126–2130. [Google Scholar] [CrossRef]
- Meshali, M.; Abdel-Aleem, H.; Sakr, F.; Nazzal, S.; El-Malah, Y. Effect of Gel Composition and Phonophoresis on the Transdermal Delivery of Ibuprofen: In Vitro and in Vivo Evaluation. Pharm. Dev. Technol. 2011, 16, 93–101. [Google Scholar] [CrossRef]
- Vance, C.G.; Rakel, B.A.; Blodgett, N.P.; De Santana, J.M.; Amendola, A.; Zimmerman, M.B.; Al, E. Effects of Transcutaneous Electri- 481 Cal Nerve Stimulation on Pain, Pain Sensitivity, and Function in 482 People with Knee Osteoarthritis: A Randomized Controlled Trial. Phys. Ther. 2012, 92, 898–910. [Google Scholar] [CrossRef] [Green Version]
- Klaiman, M.D.; Shrader, J.A.; Danoff, J.V.; Hicks, J.E.; Pesce, W.J.; Ferland, J. Phonophoresis versus Ultrasound in the Treatment of Common Musculoskeletal Conditions. Med. Sci. Sports Exerc. 1998, 30, 1349–1355. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2008, 6, e1000097. [Google Scholar] [CrossRef]




| Author | Sample | Intervention | Ultrasonic Parameters | Measuring Instruments | Results |
|---|---|---|---|---|---|
| Belindayi et al. [44] (2018). Randomized, single-blind, comparative study. | n = 61 (54 women and 7 men) Average age: 57.9 years Average BMI: 30.1 kg/m2 | Group Php +Ibuprofen 5% (gel) (n = 30). Group Php +Ibuprofen 5% (cream) (n = 31). 5 days/2 weeks. Applied to the most symptomatic knee. | Continuous mode, frequency: 1 MHz, intensity: 1 W/cm2, and application time: 5 min. |
| Both groups improve significantly, with greater improvement in the Php + Ibuprofen (gel) group (p < 0.001). |
| Boonhong et al. [45] (2018). Comparative, randomized, controlled, double-blind trial. | N = 61 (55 women and 6 men) Average age: 63.4 years Average BMI: 24.2 kg/m2 Average duration of symptoms: 4.4 to 7.6 months KL: Grade I-II-III | Group Php + Pyroxicam 0.5% (gel) + simulated TENS (n = 30). Group Ultrasound + nonsimulated TENS (n = 31). 5 days/2 weeks. Applied to the most symptomatic knee. | Continuous mode, frequency: 1 MHz, intensity: 1 W/cm2, and application time: 10 min. |
| Both interventions were effective. Observing greater improvement in the Php group with Piroxicam, although without statistically significant differences (p < 0.001). |
| Monisha et al. [39] (2018). Double-blind, randomized, controlled trial. | N = 50 women Age range: 40–70 years KL: Grade II-III | Group Php + Piroxicam (gel) + coupling gel (ratio 4:10). Php + Dimethyl Sulfoxide Group. Ultrasonic Group. 5 days/2 weeks. Applied on both knees. | Continuous mode, frequency: 1 MHz, intensity: 1 W/cm2, and application time: 10 min. |
| Treatment with Php + Piroxicam (gel) provides better pain relief than ultrasound in mild to moderate osteoarthritis of the knee (p < 0.00). |
| Jun et al. [40] (2015). Randomized, double-blind, placebo-controlled trial. | N = 96 (77 women and 19 men) Average age: 60 years Average BMI: 30.8 kg/m2 Average duration of symptoms: 7.2 months | Group Php + Chinese medicine substance (n = 38). Group Php +Diclofenac sodium (gel) 10 mg (n = 39). Placebo group (n = 19). | Low-frequency Php (40 kHz), application time: 30 min/session. |
| Significant improvement in pain and physical function in the Php treatment groups versus the placebo group. No significant differences were observed between the two groups with respect to the improvement of stiffness and range of motion (p < 0.05). |
| Oktayoglu et al. [47] (2014). Randomized controlled study. | N= 40 (30 women and 10 men) Average age: 54.8 years Average BMI: 29.9 kg/m2 Mean duration of symptoms: 4.6 years KL: Grade II-III-IV | Group Php + Diclofenac diethylammonium 1.16% (gel). Ultrasonic Group. 5 days/2 weeks. Applied on both knees. | Continuous mode, frequency: 1 MHz, intensity: 1.5 W/cm2, and application time: 10 min. |
| Both treatments are effective. Greater statistically significant improvement in pain on walking in the Php + Diclofenac diethylammonium (gel) group (p < 0.05). |
| Boyaci et al. [43] (2013). Randomized, single-blind, comparative study. | N= 101 women Average age: 51.9 years Mean BMI: 32.8 kg/m2 Average duration of symptoms: 3.5 years KL: Grade II-III | Group Php + Ketoprofen (gel) 100 mg (n = 33). Ultrasound Group (n = 33). Short wave group (n = 35). Previously hot compresses 20 min in all the groups. 5 days/2 weeks. Applied on both knees. | Frequency: 1 MHz, intensity: 1.5 W/cm2, and application time: 8 min. |
| The three treatment modalities were effective, with no significant differences between them (p < 0.001). |
| Luksurapan et al. [46] (2013). Randomized, controlled, double-blind trial. | N= 46 (45 women and 1 man) Average age: 58.9 years Mean BMI: 26.3 kg/m2 Mean duration of symptoms: 3.3 years KL: Grade I-II-III | Group Php + Pyroxicam 0.5% (gel) (n = 23). Ultrasound Group (n = 23). 5 days/2 weeks. Applied to the most symptomatic knee. | Continuous mode, frequency: 1 MHz, intensity: 1 W/cm2, and application time: 10 min. |
| Greater efficacy for pain and function in the Php +Piroxicam group. No statistically significant differences between the two groups (p < 0.001). |
| Akinbo et al. [34] (2011). Randomized controlled study. | N= 40 (34 women and 6 men) Average age: 57.5 years Average BMI: 31 kg/m2 | Php Group + Diclofenac sodium 1% (gel) (n = 14). Group Php + Methyl Salicylate 15% (gel) (n = 14). Ultrasound group (n = 12). Previous application of heat (15 min) and ergometer for all groups. 5 days/2 weeks. Applied on painful knee or right knee if bilateral. | Continuous mode, frequency: 1 MHz, intensity: 1 W/cm2, and application time: 5 min. |
| All groups obtained improvement except in stiffness. The Php + Diclofenac Sodium group had a statistically significant improvement over the other two groups (p < 0.05). |
| Author | C1 | C2 | C3 | C4 | C5 | C6 | C7 | C8 | C9 | C10 | C11 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Belindayi et al. [44] (2018) | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 8/10 |
| Boonhong et al. [45] (2018) | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 9/10 |
| Monisha et al. [39] (2018) | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 6/10 |
| Jun et al. [40] (2015) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 9/10 |
| Oktayoglu et al. [47] (2014) | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 7/10 |
| Boyaci et al. [43] (2013) | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 7/10 |
| Luksurapan et al. [46] (2013) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10/10 |
| Akinbo et al. [34] (2011) | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 6/10 |
| Title 1 | Begg | Egger |
|---|---|---|
| VAS REST | 0.3082 | 0.0018 |
| VAS MOVEMENT | 1.0000 | - |
| WOMAC (PAIN) | 0.2207 | 0.0439 |
| WOMAC (STIFFNESS) | 0.7341 | 0.8171 |
| WOMAC (PHYSICAL FUNCTION) | 0.3082 | 0.0700 |
| WOMAC TOTAL | 0.3082 | 0.1599 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martin-Vega, F.J.; Lucena-Anton, D.; Galán-Mercant, A.; Perez-Cabezas, V.; Luque-Moreno, C.; Vinolo-Gil, M.J.; Gonzalez-Medina, G. Phonophoresis through Nonsteroidal Anti-Inflammatory Drugs for Knee Osteoarthritis Treatment: Systematic Review and Meta-Analysis. Biomedicines 2022, 10, 3254. https://doi.org/10.3390/biomedicines10123254
Martin-Vega FJ, Lucena-Anton D, Galán-Mercant A, Perez-Cabezas V, Luque-Moreno C, Vinolo-Gil MJ, Gonzalez-Medina G. Phonophoresis through Nonsteroidal Anti-Inflammatory Drugs for Knee Osteoarthritis Treatment: Systematic Review and Meta-Analysis. Biomedicines. 2022; 10(12):3254. https://doi.org/10.3390/biomedicines10123254
Chicago/Turabian StyleMartin-Vega, Francisco Javier, David Lucena-Anton, Alejandro Galán-Mercant, Veronica Perez-Cabezas, Carlos Luque-Moreno, Maria Jesus Vinolo-Gil, and Gloria Gonzalez-Medina. 2022. "Phonophoresis through Nonsteroidal Anti-Inflammatory Drugs for Knee Osteoarthritis Treatment: Systematic Review and Meta-Analysis" Biomedicines 10, no. 12: 3254. https://doi.org/10.3390/biomedicines10123254







