Development of a Performance Measurement Framework for European Health Technology Assessment: Stakeholder-Centric Key Performance Indicators Identified in a Delphi Approach by the European Access Academy
Abstract
1. Introduction
- The Population of interest includes individual patients as well as society as a whole (i.e., reflecting the perspective of all involved stakeholders and collaborators).
- The Intervention reflects the introduction of EU HTA.
- The Comparator is the plethora of previous national HTA processes across the Member States (MS).
- Finally, the Outcomes of interest included in this framework reflect the desired health system improvements.
2. Methods
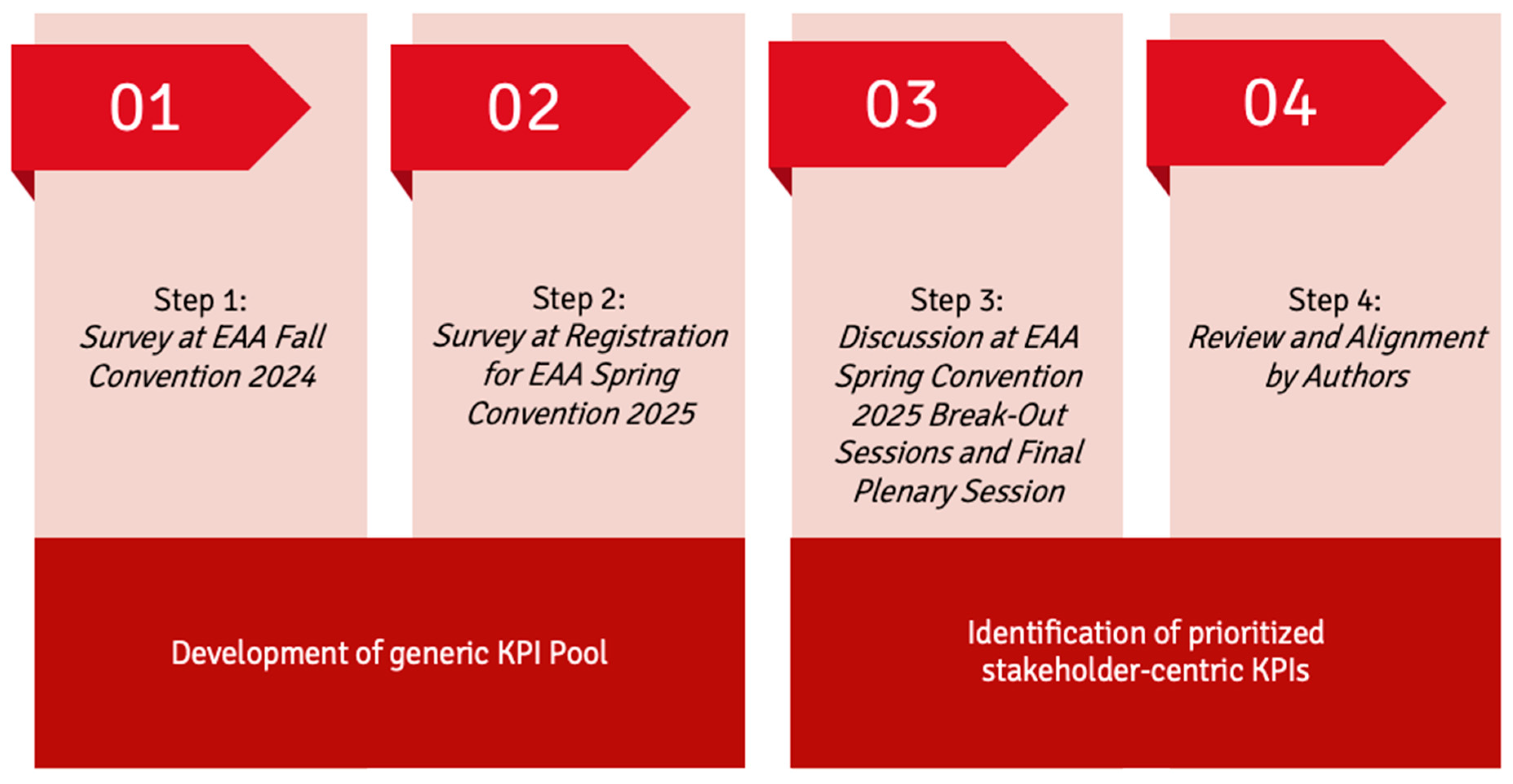
2.1. Delphi Approach—Steps 1–2: Development of Generic KPI Pool
2.2. Delphi Approach—Steps 3–4: Identification of Prioritized Stakeholder-Centric KPIs
- WG 1: Patient perspective
- WG 2: Clinician perspective
- WG 3: HTD perspective
- WG 4: System-level/member state perspective
- WG 5: Birds’ eye view (i.e., integrative perspective)
3. Results
3.1. Delphi Approach—Steps 1–2: Development of Generic KPI Pool
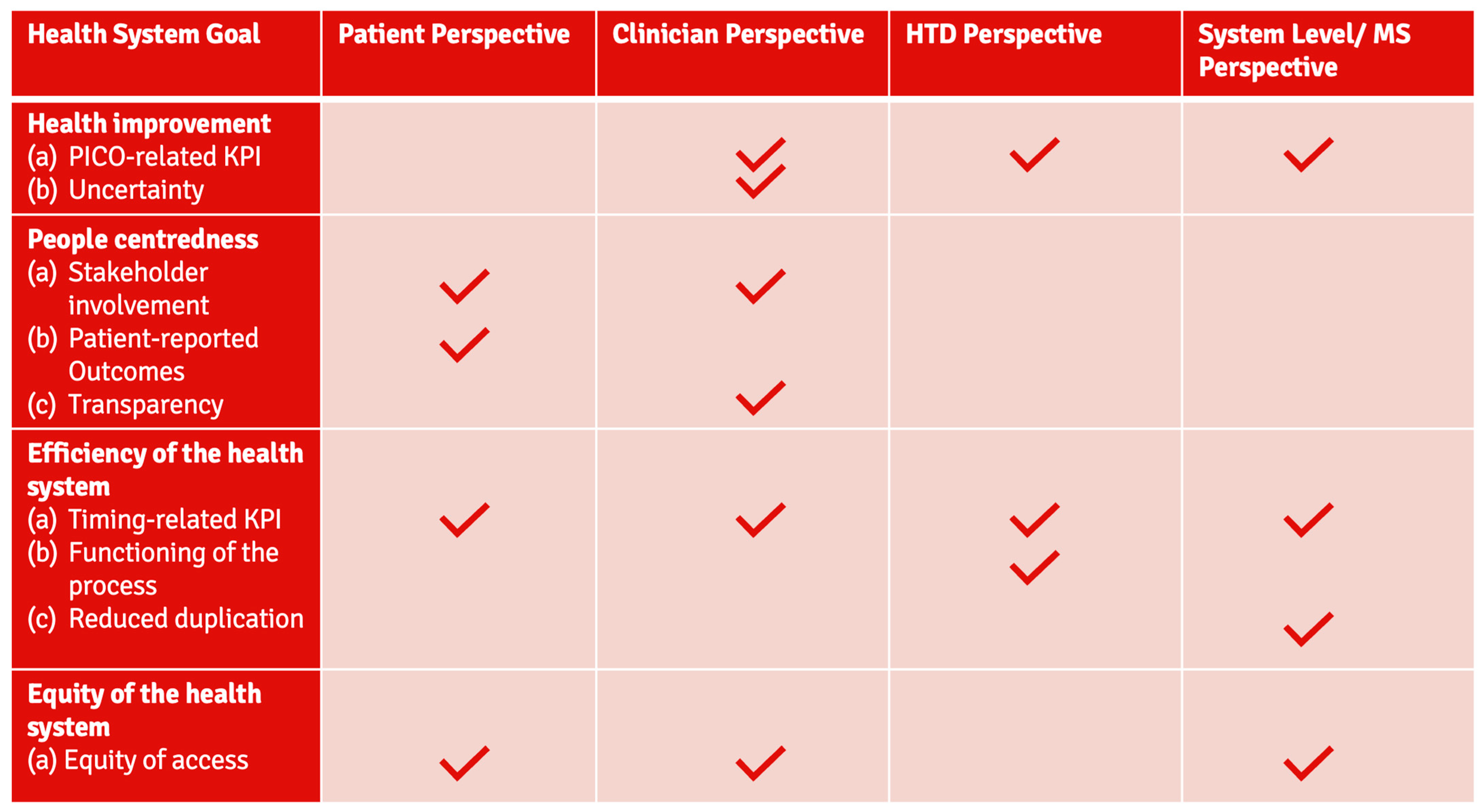
3.2. Delphi Approach—Steps 3–4: Identification of Prioritized Stakeholder-Centric KPIs
- Regarding timing: 4/4 groups,
- Regarding equity: 3/4 groups,
- PICO-related: 3/4 groups,
- Stakeholder involvement: 2/4 groups.
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Commission. Health Technology Assessment: Commission Welcomes the Adoption of New Rules to Improve Access to Innovative Technologies. 2021. Available online: https://ec.europa.eu/commission/presscorner/detail/en/IP_21_6771 (accessed on 5 June 2025).
- European Commission. European Health Union. Available online: https://commission.europa.eu/strategy-and-policy/priorities-2019-2024/promoting-our-european-way-life/european-health-union_en (accessed on 5 June 2025).
- Banta, D.; Kristensen, F.B.; Jonsson, E. A History of Health Technology Assessment at the European Level. Int. J. Technol. Assess. Health Care 2009, 25, 68–73. [Google Scholar] [CrossRef]
- Ruether, A.; Imaz-Iglesia, I.; Bélorgey, C.; Lo Scalzo, A.; Garrett, Z.; Guardian, M. European collaboration on health technology assessment: Looking backward and forward. Int. J. Technol. Assess. Health Care 2022, 38, e34. [Google Scholar]
- Imaz-Iglesia, I.; Wild, C. EUnetHTA’s contribution to the new legal framework for health technology assessment cooperation in Europe. Int. J. Technol. Assess. Health Care 2022, 38, e50. [Google Scholar]
- Kristensen, F.B.; Mäkelä, M.; Neikter, S.A. European network for Health Technology Assessment, EUnetHTA: Planning, development, and implementation of a sustainable European network for Health Technology Assessment. Int. J. Technol. Assess. Health Care 2009, 25, 107–116. [Google Scholar]
- European Commission. EUnetHTA21 Service Contract. Available online: https://health.ec.europa.eu/health-technology-assessment/regulation-health-technology-assessment/eunethta21-service-contract_en (accessed on 5 June 2025).
- EUnetHTA. News and Publications. Available online: https://web.archive.org/web/20240705175716/https://www.eunethta.eu/ (accessed on 5 June 2025).
- EUnetHTA. JA3 Work Package 7—National Implementation and Impact. Available online: https://web.archive.org/web/20221007033012/https://www.eunethta.eu/ja3-archive/work-package-7-national-implementation-and-impact/ (accessed on 5 June 2025).
- Garrett, Z.; Imaz-Iglesia, I.; Willemsen, A. Building a model of health technology assessment cooperation: Lessons learned from EUnetHTA joint action 3. Int. J. Technol. Assess. Health Care 2022, 38, e14. [Google Scholar] [PubMed]
- Charles River Associates. EU REA—Learnings from the First Three EUnetHTA Joint Action 3 Assessments; Final Report; Charles River Associates: Washington, DC, USA, 2018. [Google Scholar]
- European Commission. Implementation Rolling Plan: Regulation (EU) 2021/2282 on Health Technology Assessment. Available online: https://health.ec.europa.eu/document/download/397b2a2e-1793-48fd-b9f5-7b8f0b05c7dd_en (accessed on 5 June 2025).
- Urbina, I.; Adams, R.; Fernandez, J.; Willemsen, A.; Hedberg, N.; Rüther, A. Advancing cooperation in Health Technology Assessment in Europe: Insights from the EUnetHTA 21 project amidst the evolving legal landscape of European HTA. Int. J. Technol. Assess. Health Care 2024, 40, e75. [Google Scholar] [CrossRef] [PubMed]
- Brinkhuis, F.; Ruof, J.; van den Ham, H.; Gianfrate, F.; Strammiello, V.; Berntgen, M.; Pavlovic, M.; Mol, P.; Wasem, J.; Van Dyck, W.; et al. Evaluating progress towards implementation of the European HTA Regulation: Insights generated from the European Access Academy’s multi-stakeholder survey. Health Policy Technol. 2024, 13, 100930. [Google Scholar]
- European Access Academy. Open Letter to DG Santé and the Member State Coordination Group on HTA. 2024. Available online: https://irp.cdn-website.com/e52b6f19/files/uploaded/Open_Letter_Methods_EU_HTA.pdf (accessed on 5 June 2025).
- European Commission. Health Technology Assessment. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=legissum:4572723 (accessed on 5 June 2025).
- Glasziou, P. Health Technology Assessment. Med. Decis. Mak. 2012, 32, E20–E24. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.C.; Mossialos, E.; Papanicolas, I. Performance Measurement for Health System Improvement: Experiences, Challenges and Prospects; Background Document for the WHO European Ministerial Conference on Health Systems “Health Systems, Health and Wealth”; European Observatory on Health Systems and Policies: Brussels, Belgium, 2008. [Google Scholar]
- Papanicolas, I.; Rajan, D.; Karanikolos, M.; Soucat, A. Health System Performance Assessment: A Framework for Policy Analysis; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Niederberger, M.; Spranger, J. Delphi Technique in Health Sciences: A Map. Front. Public Health 2020, 8, 457. [Google Scholar]
- European Access Academy. LinkedIn Profile. Available online: https://www.linkedin.com/in/eaa-european-access-academy-454856234/ (accessed on 5 June 2025).
- European Access Academy. Available online: https://www.euaac.org/ (accessed on 5 June 2025).
- European Commission. EU Health Policy Platform. Available online: https://webgate.ec.europa.eu/hpf/ (accessed on 5 June 2025).
- European Access Academy. European Access Academy EAA Convention Proceedings: Approaching Performance Measurement for EU HTA; European Access Academy: Basel, Switzerland, 2025; Volume 7, pp. 1–44. [Google Scholar]
- European Access Academy. Conventions. Available online: https://www.euaac.org/conventions (accessed on 5 June 2025).
- European Access Academy. Publications. Available online: https://www.euaac.org/publications (accessed on 5 June 2025).
- Dierks, C. EU HTA Regulation revisited—Embedded Performance Criteria Measuring the Success of the EU HTA Regulation with a KPI-Based Approach. In EAA Convention Proceedings Volume 7; European Access Academy: Basel, Switzerland, 2025; pp. 24–27. [Google Scholar]
- Hogervorst, M.A.; Møllebæk, M.; Vreman, R.A.; Lu, T.A.; Wang, J.; De Bruin, M.L.; Leufkens, H.G.M.; Mantel-Teeuwisse, A.; Goettsch, W. Perspectives on How to Build Bridges between Regulation, Health Technology Assessment and Clinical Guideline Development: A Qualitative Focus Group Study with European Experts. BMJ Open 2023, 13, e072309. [Google Scholar] [CrossRef]
- Lips, P.; Timmers, L.; Bal, R.; Delnoij, D. Involvement of Patients and Medical Professionals in the Assessment of Relative Effectiveness: A Need for Closer Cooperation. Value Health 2022, 25, 1480–1488. [Google Scholar] [CrossRef]
- Schünemann, H.J.; Reinap, M.; Piggott, T. The Ecosystem of Health Decision Making: From Fragmentation to Synergy. Lancet Public Health 2022, 7, e378–e390. [Google Scholar] [CrossRef]
- European Commission. Commission Implementing Regulation (EU) 2024/1381 of 23 May 2024; European Commission: Brussels, Belgium, 2024.
- Gsteiger, S.; Bucher, H.C.; Ryan, J.; Ruof, J. Technology Assessment vs. Technology Appraisal—How to Strengthen the Science/Value Dichotomy with EU HTA? J. Mark. Access Health Policy 2024, 12, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Schuster, V. EU HTA Regulation and Joint Clinical Assessment—Threat or Opportunity? J. Mark. Access Health Policy 2024, 12, 100–104. [Google Scholar]
- Desmet, T.; Julian, E.; Van Dyck, W.; Simoens, S.; Huys, I.; Giuliani, R.; Toumi, M.; Dierks, C.; Cardone, A.; Houÿez, F.; et al. An Inclusive Civil Society Dialogue for Successful Implementation of the EU HTA Regulation: Call to Action to Ensure Appropriate Involvement of Stakeholders and Collaborators. J. Mark. Access Health Policy 2024, 12, 21–34. [Google Scholar] [CrossRef]
- Julian, E.; Belleman, T.; Garcia, M.J.; Rutten-van Mölken, M.; Doeswijk, R.; Giuliani, R.; Wörmann, B.J.; Widmer, D.; Tilleul, P.; Tilleul, R.C.; et al. EU HTA: Avoiding Error and Finding the Right Balance: Insights Generated by the European Access Academy. J. Mark. Access Health Policy 2025, 13, 6. [Google Scholar] [CrossRef] [PubMed]
- Bigdeli, M.; Jacobs, B.; Tomson, G.; Laing, R.; Ghaffar, A.; Dujardin, B.; Van Damme, W. Access to Medicines from a Health System Perspective. Health Policy Plan. 2013, 28, 692–704. [Google Scholar] [PubMed]
- Perehudoff, K.; Durán, C.; Demchenko, I.; Mazzanti, V.; Parwani, P.; Suleman, F. Impact of the European Union on Access to Medicines in Low- and Middle-Income Countries: A Scoping Review. Ser. Rev. 2021, 9, 100219. [Google Scholar] [CrossRef]
- Vogler, S.; Paris, V.; Ferrario, A. How Can Pricing and Reimbursement Policies Improve Affordable Access to Medicines? Lessons Learned from European Countries. Appl. Health Econ. Health Policy 2017, 15, 307–321. [Google Scholar]
- Vogler, S.; Österle, A.; Mayer, S. Inequalities in Medicine Use in Central Eastern Europe: An Empirical Investigation of Socioeconomic Determinants in Eight Countries. Int. J. Equity Health 2015, 14, 124. [Google Scholar] [CrossRef]
- Millar, R.; Morton, A.; Bufali, M.V.; Kubitschke, L.; Engels, S.; Dabak, S.V.; Isaranuwatchai, W.; Chalkidou, K.; Teerawattananon, Y. Assessing the Performance of Health Technology Assessment (HTA) Agencies: Developing a Multi-Country, Multi-Stakeholder, and Multi-Dimensional Framework to Explore Mechanisms of Impact. Cost Eff. Resour. Alloc. 2021, 19, 37. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, R.S.; Norton, D.P. Strategic learning & the balanced scorecard. Strategy Leadersh. 1996, 24, 18–24. [Google Scholar]
- Witter, S.; Toonen, J.; Meessen, B. Performance-Based Financing as a Health System Reform: Mapping the Key Dimensions for Monitoring and Evaluation. BMC Health Serv. Res. 2013, 13, 367. [Google Scholar] [CrossRef] [PubMed]
- Dawkins, B.; Renwick, C.; Ensor, T.; Shinkins, B.; Jayne, D.; Meads, D. What Factors Affect Patients’ Ability to Access Healthcare? An Overview of Systematic Reviews. Trop. Med. Int. Health 2021, 26, 1177–1188. [Google Scholar] [CrossRef]
- Abbas, N.; Hasan, S.S.; Curley, L.; Babar, Z.U. Access to Medicines—A Systematic Review of the Literature. Res. Soc. Adm. Pharm. 2020, 16, 1166–1176. [Google Scholar] [CrossRef]
- Lublóy, Á. Factors Affecting the Uptake of New Medicines: A Systematic Literature Review. BMC Health Serv. Res. 2014, 14, 469. [Google Scholar] [CrossRef]
| Outcomes Delphi-Step 1–2 (Pre-Convention) |
|---|
| Positive developments in health care system performance |
| European scope (PICO) reflecting the most clinically relevant population(s), comparator(s), and patient-relevant outcomes, that are manageable in size |
| Recognition of innovative/disruptive treatments |
| Stakeholder involvement and feedback (integration of input from right patient and clinical experts, etc.) |
| Utilization/adoption of JCA Assessment Report in national procedures and reduction in complementary data requests at member state level |
| Reduced time to patient access on national level |
| Earlier initiation of national decision-making processes and more transparent and predictable timelines |
| Reduced type I and type II errors (i.e., ensure that beneficial medicines are reaching patients) |
| Low number/low percentage of declined JSC requests |
| Inclusive/broad member state participation (e.g., as (co-)assessors) |
| Homogeneity and acceptance of state-of-the-art methodology across EU |
| JCA efficiency review (number of JCAs/time/costs/processes) |
| Equal/homogenous quality of patient access across EU |
| Equal access to innovative health technologies for patients in EU vs. US |
| Patient Perspective | Clinician Perspective | HTD Perspective | System-Level/MS Perspective | |
|---|---|---|---|---|
| Common KPIs | - Time to patient access § | - Time to patient access § | - Shorter duration of the national decision-making process | - Time to patient access § |
| - Equity of patient access (scope: availability across MS) § | - Equity of patient access (scope: availability across MS) § | - Equity of patient access (scope: availability across MS contingent on budgetary capacities) § | ||
| - PICO-related KPI (scope: i) Reduction in the number of PICOs over time for a specific disease; and (ii) reflecting clinical standards in MS | - PICO related KPI (scope: optimization) | - PICO related KPI (scope: exhaustiveness); - Learning and training the system | ||
| - Successful and meaningful patient involvement | - Successful and meaningful clinician involvement | |||
| Individual KPIs | - Utilization of patient-centric/relevant outcome measures | - Addressing uncertainty (scope: clarity on strength/convincing outcomes) | - Functioning JSC process (scope: addressing existing demand and developmental timelines) | - Reduce duplication of effort (scope: utilization of JCA report and reduction in workload) |
| - Transparency | - Functioning JCA process (scope: workability/realistic response timelines/efficient communication) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Published by MDPI on behalf of the Market Access Society. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Julian, E.; Xander, N.S.H.; Boumaki, K.; Garcia, M.J.; Jahimovica, E.; Mosset-Keane, J.; Otto, M.H.; Pavlovic, M.; Scroccaro, G.; Strammiello, V.; et al. Development of a Performance Measurement Framework for European Health Technology Assessment: Stakeholder-Centric Key Performance Indicators Identified in a Delphi Approach by the European Access Academy. J. Mark. Access Health Policy 2026, 14, 5. https://doi.org/10.3390/jmahp14010005
Julian E, Xander NSH, Boumaki K, Garcia MJ, Jahimovica E, Mosset-Keane J, Otto MH, Pavlovic M, Scroccaro G, Strammiello V, et al. Development of a Performance Measurement Framework for European Health Technology Assessment: Stakeholder-Centric Key Performance Indicators Identified in a Delphi Approach by the European Access Academy. Journal of Market Access & Health Policy. 2026; 14(1):5. https://doi.org/10.3390/jmahp14010005
Chicago/Turabian StyleJulian, Elaine, Nicolas S. H. Xander, Konstantina Boumaki, Maria João Garcia, Evelina Jahimovica, Joséphine Mosset-Keane, Monica Hildegard Otto, Mira Pavlovic, Giovanna Scroccaro, Valentina Strammiello, and et al. 2026. "Development of a Performance Measurement Framework for European Health Technology Assessment: Stakeholder-Centric Key Performance Indicators Identified in a Delphi Approach by the European Access Academy" Journal of Market Access & Health Policy 14, no. 1: 5. https://doi.org/10.3390/jmahp14010005
APA StyleJulian, E., Xander, N. S. H., Boumaki, K., Garcia, M. J., Jahimovica, E., Mosset-Keane, J., Otto, M. H., Pavlovic, M., Scroccaro, G., Strammiello, V., Bernardini, R., Capri, S., Casado-Arroyo, R., Desmet, T., Van Dyck, W., Fricke, F.-U., Gianfrate, F., Solà-Morales, O., Wasem, J., ... Ruof, J. (2026). Development of a Performance Measurement Framework for European Health Technology Assessment: Stakeholder-Centric Key Performance Indicators Identified in a Delphi Approach by the European Access Academy. Journal of Market Access & Health Policy, 14(1), 5. https://doi.org/10.3390/jmahp14010005








