Abstract
Introduction: Market access, pricing, and reimbursement of digital health technologies (DHTs) in Europe are significantly challenged by regulatory fragmentation and various assessment methodologies. Understanding the challenges and priorities of technology developers is essential for developing effective and relevant policy responses. This study explores perceived barriers and developer-driven priorities to inform the development of a harmonized health technology assessment (HTA) framework under the EDiHTA project. Methods: A mixed-methods approach was adopted, including a scoping review to identify key challenges, a survey of 20 DHT developers, and interviews and focus groups with 29 industry representatives from startups to multinational companies across 10 European countries during 2024. Results: Key challenges included a lack of transparency in reimbursement processes, fragmented HTA requirements, and misalignment between traditional evidence models and the agile development of DHTs. Developers highlighted the need to integrate real-world evidence, consider usability and implementation factors, and provide structured, lifecycle-based guidance. Financial barriers and procedural burdens were particularly significant for small and medium-sized enterprises. Conclusions: These findings highlight the need for an HTA framework that reflects the iterative nature of digital development, integrates real-world evidence, and reduces uncertainty for developers. The EDiHTA project aims to respond to these challenges by building a harmonized and flexible approach that aligns with the goals of the European HTA Regulation.
1. Introduction
The rapid evolution of digital health technologies (DHTs) presents significant opportunities for healthcare systems globally. These technologies, encompassing mobile health applications, telemedicine, and artificial intelligence (AI) solutions, have the potential to revolutionize disease management, improve patient outcomes, and enhance the efficiency of healthcare systems [1]. However, unlike pharmaceuticals and medical devices that follow well-established regulatory, health technology assessment (HTA), and reimbursement frameworks, the pathways applicable to DHTs are often not clearly defined or are still emerging in some countries. Their variety, broad range of use cases, software-driven iterative nature, and user-centricity pose significant challenges for conventional value assessment models [2,3,4]. The connection between market access, HTA, and pricing and reimbursement in relation to DHTs is evolving, with a growing emphasis on flexible and adaptive assessment methodologies that better align with the unique characteristics of DHTs. However, the methodological questions regarding the assessment of DHTs are still significant, which may limit their integration into health systems and delay access by healthcare professionals and patients.
To improve the uptake of DHTs, countries are taking a variety of approaches to integrate them into their national healthcare systems. Germany [5], France [6], and Belgium [7] have implemented comprehensive fast-track pathways coupled with funding streams that enable safe and effective DHTs to be reimbursed by national health insurance funds. Other countries like Finland [8], Spain [9], and the United Kingdom [10] have developed dedicated assessment methodologies for DHTs to inform decisions regarding implementation. Some countries, like France and the UK, utilize additional checklists for AI-enabled technologies that assess multiple additional factors in addition to the traditional HTA process [11,12]. The majority of countries still rely on the traditional HTA processes, making it more challenging for DHTs to demonstrate their value within existing frameworks due to a lack of flexibility to account for digital-specific characters like usability data, cybersecurity, or real-world performance [13].
The nature and complexity of digital health technologies, as well as the methodological limitations associated with their assessments, can contribute to uncertainties for health technology developers1 in both regulatory and HTA-informed decision making [14]. The lack of transparency in pricing and reimbursement policies further complicates the process, as many national systems do not provide clear guidelines on what constitutes sufficient evidence for coverage decisions, and pricing negotiations are confidential. As a result, some developers opt to launch their products in markets with more predictable and standardized pathways, such as the United States or China, rather than navigating Europe’s fragmented landscape [15]. Such delays in market access may limit the timely availability of innovative services for patients and health systems in the region. These challenges underscore the importance of understanding how system-level differences translate into practical barriers for developers. It also highlights the need for harmonized policies and streamlined HTA processes to facilitate market access and the reimbursement of DHTs across Europe [16,17], ensuring that technology developers can navigate this complex environment efficiently.
Recognizing these systematic challenges, the European Digital Health Technology Assessment (EDiHTA) project was launched in 2024, funded by the European Commission (Horizon Europe) for four years [18,19]. EDiHTA’s mission is to map and address current regulatory and HTA barriers by developing a harmonized, flexible, and transparent HTA framework tailored specifically to the unique characteristics of DHTs. This framework will enable the assessment of various DHTs—including artificial intelligence (AI) solutions—with different intended purposes, while also considering different maturity levels and decision-making levels. EDiHTA brings together 19 partners from 10 European countries, including policymakers, HTA bodies, developers, patient organizations, and academic institutions. The goal of the project is to harmonize different standards relevant to the assessment of DHTs, while considering the development of the framework and the perspectives of all relevant stakeholders. To this end, stakeholder involvement was embedded from the beginning, with participants contributing to the definition of key concepts, evidence requirements, and practical considerations through consultations and iterative feedback rounds.
For health technology developers, the establishment of a clear and predictable HTA pathway is crucial, as market access and reimbursement decisions directly influence their go and no-go decisions in their development strategies. Their investment decisions often hinge on whether a clear and predictable pathway exists for obtaining market access and funding at the national level [20]. It is therefore important to consider the developer perspective, as investment decisions are often influenced by whether clear assessment and reimbursement pathways are available. The successful integration of DHTs into healthcare systems requires collaboration among multiple stakeholders, including technology developers, healthcare providers, patients, policymakers/payers, and HTA agencies. Initiatives such as EDiHTA exemplify the importance of cross-sector and cross-border partnerships in addressing the challenges associated with market access and the reimbursement of DHTs. To support the design of future frameworks like EDiHTA, it is first necessary to understand the barriers, needs, and facilitators experienced by stakeholders, including developers, in navigating current assessment and reimbursement processes.
2. Objective
The aim of this research is to identify the specific challenges and requirements faced by technology developers in securing market access and reimbursement for DHTs in Europe. By identifying these barriers, this study aims to support the development of a harmonized HTA framework under the EDiHTA project that accounts for developers’ needs.
3. Methods
To identify the needs and requirements of technology developers, a threefold methodology was employed. A scoping literature review was conducted to identify and analyze the factors that act as barriers or facilitators in the decision-making processes of health technology developers. By examining existing evidence, this review identified key barriers and facilitators shaping decision-making, offering insights into what fosters sustained innovation or hinders technological advancements in healthcare. Based on the scoping review, a survey was developed and distributed among technology developers to identify the most critical factors in developing new digital health technologies from a developer’s perspective. Survey responses were followed up with focus groups and interviews with participating technology developers. Additional participants were identified and included in the interview process through a snowball sampling method, based on recommendations from stakeholders already involved.
The literature review was conducted as a scoping review (ScR), following the framework outlined in the PRISMA-ScR guidelines [21]. The search strategy aimed to balance comprehensiveness with precision, capturing a broad array of studies related to digital health technology assessment and decision-making while also ensuring the inclusion of only methodologically sound, peer-reviewed, and relevant publications. The core search string was developed to include terms related to the development, decisions, assessment, evaluation, and use of digital health technologies. The search was conducted in multiple electronic databases, including PubMed, Embase, Web of Science, and Scopus, supplemented by grey literature from policy organizations, regulatory agencies, and industry reports. Studies published in English between 2012 and 2024 were included to ensure that the analysis was both current and relevant. Inclusion criteria encompassed studies addressing aspects of the assessment, implementation, and use of digital technologies involving relevant stakeholders (e.g., clinicians, patients, providers). Exclusion criteria included duplicates, studies unrelated to the objectives, case reports, letters to the editor, and articles with incomplete information. After duplicate removal, an initial screening was conducted based on titles and abstracts. Eligible studies were then subjected to full-text reviews to confirm their relevance to the defined criteria. Two junior researchers independently screened all titles and abstracts in a double-blind manner. Disagreements regarding the inclusion of studies were resolved through discussion with a senior researcher, who made the final decision in cases of persistent uncertainty. The quality of the included studies in the scoping review was assessed using the Joanna Briggs Institute (JBI) critical appraisal tools [22]. The full search strategy, including the detailed query and the application of PRISMA-ScR methods, is presented in Supplementary File S1.
The questionnaire was created to complement the results of the literature review. The survey was distributed to international health industry players and start-ups/SMEs through conferences (e.g., HIMSS Europe 2024), networking events, social media, and consortium partner networks between June and September 2024 to understand what factors influence the decisions around the development of a DHT. Closed questions were used to capture the decision-making process regarding developers’ willingness to invest in the development of a DHT, as well as the main factors that influence their decision to go to market and other strategy-related issues. No open questions or qualitative-related questions were included. In exploring the priorities of digital health technology developers, the developers shared information regarding their company and background and rated the importance of various aspects for market access and reimbursement on a Likert scale from 1 to 9. For the survey, reliability and validity checks were conducted, including a pilot test before distribution: an initial version of the questionnaire was reviewed by a panel of experts, composed of academic researchers with experience in HTA that evaluated the clarity of the questions, their relevance to the study objectives, and the comprehensiveness of the domains covered; the questionnaire was tested on a preliminary sample of developers, representative of the target population. The feedback received was used to improve the wording of the questions and ensure consistency in the Likert response scale. The final version of the questionnaire demonstrated a good level of clarity and comprehensibility, and no systematic issues were reported during its completion. The questionnaire can be found in Supplementary File S2.
Focus groups and interviews were intended to validate the results of the literature review and survey, regarding the past and present, while also imagining the future of the DHT market in the EU and how the EDiHTA framework could provide a decision-making tool for all relevant stakeholders, including developers. Developers who completed the survey were invited to participate further in interviews and focus groups between September and November 2024. Additional participants were identified using a snowball sampling approach, whereby existing contacts recommended relevant peers or colleagues. A standardized protocol was developed for both focus groups and interviews (Supplementary File S3), with open-ended questions to guide the sessions. This approach allowed flexibility for discussions to cover various aspects and types of technologies. The protocol was structured based on the International Health Technology Assessment Model (IHTAM) [23], which guided participants through a reflective and forward-looking process. In the first part of the sessions, participants were asked to “learn from the past and present” by describing the current market access and reimbursement processes and identifying limitations within current HTA systems. In the second part, participants were encouraged to “imagine the future”, visualizing an ideal HTA process while identifying potential barriers and facilitators of its realization. Once challenges and needs were identified, stakeholders were asked to decide whether existing methods could be improved or if novel methods needed to be developed to address these challenges.
A standardized data extraction form was used to capture relevant information from the scoping review, survey, and qualitative data collection. The extracted data were synthesized using a thematic analysis approach to identify recurring themes across regulatory challenges, reimbursement models, HTA methodologies, and the impact of real-world data (RWD) on market access [24]. Thematic analysis of qualitative data followed an iterative coding process to ensure consistency and reliability. Interview and focus group sessions were audio- or video-recorded to ensure accuracy and take comprehensive notes. All participants were asked to validate the summary of their input following each session to ensure the accuracy and completeness of the responses collected. Results were analyzed through a thematic analysis process.
4. Results
4.1. Literature Review
A total of 1247 studies were initially identified through the database search, which underwent a two-stage screening process. After title and abstract screening, 705 studies were excluded due to irrelevance, leaving 542 studies for full-text review. The studies were categorized into three key thematic areas: telemedicine (456 studies), eHealth (285 studies), and artificial intelligence (AI) (5 studies). However, all AI-related studies were excluded due to a lack of alignment with the study criteria, leaving a final inclusion of 17 studies for telemedicine [25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41] (Figure 1a) and 31 for eHealth [42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72] (Figure 1b). The five studies identified in the field of AI were excluded as they did not provide relevant evidence in relation to the specific objective of this study: the identification of factors acting as barriers or facilitators in the development, implementation, and market access of DHTs by developers. In particular, the AI studies analyzed were primarily focused on technical or clinical aspects (e.g., diagnostic accuracy, algorithmic performance), without delving into the regulatory, economic, or systemic context implications that influence development and commercialization decisions.
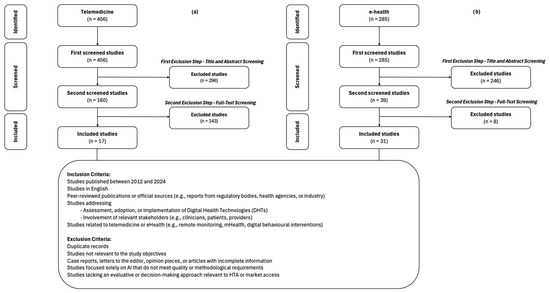
Figure 1.
PRISMA-ScR flowchart of literature review results: (a) telemedicine; (b) eHealth.
The literature review identified key barriers and facilitators influencing the development and implementation of DHTs, particularly in the areas of telemedicine and eHealth (Table 1). For telemedicine, enhanced accessibility, diagnostic accuracy comparable to in-person visits, high patient satisfaction, and cost reduction were recognized facilitators. However, several barriers remained, such as digital literacy disparities among older adults and socio-economically disadvantaged groups, data security concerns, lack of interoperability with electronic health records (EHRs), and regulatory complexity due to inconsistent legal and reimbursement frameworks across countries. Similarly, eHealth solutions served as facilitators for improved chronic disease management, enhanced patient engagement, and cost savings through reduced healthcare utilization. Yet, their long-term sustainability was challenged by limited funding models, regulatory compliance requirements, and the absence of standardized evaluation frameworks.

Table 1.
Key barriers and facilitators of eHealth (eH) and telemedicine (T) solutions during development and implementation based on the literature review.
Several key factors were identified that influence technology developers’ decisions on whether to continue or discontinue DHTs in the development phase. Regulatory and market access barriers, such as the lack of a harmonized European framework, create inconsistencies in approval and reimbursement, discouraging innovation. Financial constraints, including high compliance, certification, and proof generation costs, pose notable challenges, particularly for SMEs with limited resources. Technological issues, such as integration with existing health systems, interoperability, and cybersecurity concerns, hinder long-term adoption. Low user engagement, due to usability issues, gaps in digital literacy, and skepticism on the part of patients and healthcare providers, further affects the sustainability of DHTs.
The barriers and facilitators identified in the literature review, such as regulatory fragmentation, financial constraints, and technological integration, guided the development of the survey, allowing us to quantitatively assess the extent to which these challenges are perceived by technology developers themselves.
4.2. Survey
The survey was completed by 23 technology developers, with three incomplete responses that were excluded from the analysis. Responses were deemed incomplete if the respondent only completed part (1) General information of the survey, leaving part (2) Information regarding market access & reimbursement strategies empty. Out of the twenty technology developers with complete answers, six were categorized as mobile app developers, four as telemedicine developers, and five as AI developers. The other five developers were large industry representatives and/or part of MedTech Europe.
The majority of developers (n = 12/20) reported having a department or employee dedicated to evaluation, market access, or HTA within their organization. Additionally, 16 developers indicated that they investigate the HTA process of the target market before making a development decision. Out of the 31 factors grouped in seven main groups, developers prioritized clinical relevance, regulatory compliance, adaptability, and data privacy when designing and deploying DHTs. Ensuring alignment with clinical needs and patient populations was key, as was navigating regulatory landscapes for market access. Cost considerations were indicated as moderately important, with the importance of initial hardware costs rated as low but ongoing maintenance and financial sustainability, especially for AI and mobile apps, being significant. Factors related to direct costs, such as initial hardware expenses or shipping costs, received the lowest mean scores across all technology types. In contrast, domains such as regulatory compliance, usability, and stakeholder engagement consistently ranked higher, suggesting that non-financial barriers are more prominent concerns for developers. Additionally, usability, patient acceptance, and early clinician involvement are crucial for adoption, while data privacy remains of high importance in the implementation of DHTs, given Europe’s strict regulations. Aggregated results of the questionnaire as per type of technology can be found in Table 2. To contextualize the aggregated results, mobile apps in the sample included tools designed to support self-management and patient engagement in the form of mobile applications. Telemedicine technologies covered platforms for remote consultations and digital care coordination, while AI solutions ranged from diagnostic support tools to risk prediction algorithms integrated into clinical workflows.

Table 2.
Results of survey to question “how important is [below factor] in your decision to develop and implement a technology on a scale of 1 (not important) to 9 (very important)?”.
Building on these quantitative insights, the subsequent focus groups and interviews provided an opportunity to validate and expand upon the survey findings, particularly regarding developers’ experiences with regulatory inconsistency, cost burdens, and the need for clearer assessment pathways.
4.3. Interviews and Focus Groups
Several themes raised during the focus groups and one-on-one interviews mirrored survey responses, reinforcing key priorities such as regulatory clarity, the impracticality of traditional evidence models, and the financial sustainability of assessment processes. A deeper context for these trends was provided through four focus group discussions with startups and SMEs and one focus group and five one-on-one interviews with industry representatives conducted between September and November 2024, involving, altogether, 29 participants in this phase of the research. In total, this study captured input from 31 unique digital health companies across Europe (see Table 3).

Table 3.
Characteristics of developers participating in the survey and interview phase of this research.
Developers confirmed that the main issues were navigating the varied and often unclear pathways for market access and reimbursement in different European countries. While obtaining a CE-mark may be sufficient for regulatory compliance, it does not guarantee market access or reimbursement for DHTs, as each country has its own set of requirements and pathways. In most European countries, there are no established processes in place to secure funding for digital health technologies. While securing a CE-mark is a clear advantage when entering the market, even after certification, national-level reimbursement processes and evidence requirements lack transparency, particularly in countries where reimbursement frameworks specific to DHTs are still evolving. As a potential strategy, some developers take a proactive, bottom-up approach by attempting to trigger decisions from payers to adopt their technologies or pursue an alternative route by selling their solutions directly to hospitals, utilizing this purchase model to achieve market entry. In countries like Germany, France, and Belgium, where a dedicated assessment framework for DHTs is implemented and coupled with reimbursement, several types of DHTs are not eligible to apply for reimbursement through the dedicated pathways (e.g., diagnostic tools, non-patient-facing tools, solutions that improve workflow). Developers feel that markets are currently cherry-picking DHTs, when the greatest added value would be in primary and secondary prevention.
Industry representatives and startups/SMEs highlighted the lengthy process of securing reimbursement, which can take up to 18 months in some countries. The lengthy processes in a field of ever-evolving innovation place considerable strain on the resources of developers, particularly for start-ups and smaller companies. Financial concerns were a major focus, particularly the high costs of clinical validation and compliance. Developers noted that initial investments in certification and compliance with both EU-wide and local regulations were substantial. One participant developing an AI solution mentioned that after investing heavily in trials to prove the value of their technology, slow reimbursement processes (ca. 18 months) in Germany nearly halted their market entry. Unlike larger multinational corporations, which may have diversified portfolios and greater financial resilience, smaller firms often rely on limited funding rounds. If initial efforts are unsuccessful, securing further investment can be challenging, leading to potential project termination or even bankruptcy. This unfavorable regulatory environment may also incentivize companies to shift focus toward markets with simpler, more predictable regulatory requirements, such as the US or China.
Developers highlighted the need for market access strategies that account for diverse regulatory environments when entering new countries. Compliance with regulations, especially those beyond core EU mandates like the General Data Protection Regulation (GDPR) [73] and European Health Data Space (EHDS) [74], was seen as a significant barrier. Different interpretations of regulations across countries made scaling products across Europe difficult. Developers described the challenge of needing a country-by-country strategy for access and reimbursement and often managing contradictory advice from consultants due to the complex regulatory landscape, resulting in delays and increased costs.
Consistent with literature and survey results, participants highlighted the limitations of current evidence generation models and advocated for the inclusion of real-world evidence (RWE), underscoring a shared industry preference for flexible, adaptive assessment models. Developers emphasized the limitations of RCTs, which are largely influenced by pharmaceutical practices and not well-suited for DHTs. Traditional RCTs were seen as too costly and time-consuming for digital health innovations. Industry representatives validated the findings of the literature review that classic studies, while valuable for drugs, are often impractical to design and implement for DHTs. They proposed alternative methods, such as simulation environments and platform studies, which provide faster and more accurate insights into the benefits of DHTs. Developers advocated for increased incorporation of RWE to complement traditional data collection models, allowing for continuous validation and adaptability throughout a DHT’s lifecycle.
When it came to evidence requirements, developers highlighted the limitations of traditional assessment domains for several types of DHTs and suggested looking at additional aspects more in line with the characteristics of DHTs. Apart from the classic assessment factors, usability and social acceptance should be at the center of the assessment process to ensure retention and greater patient benefits. AI developers also advocated for the importance of being transparent about the datasets that are used to train AI technologies, since data obtained based on a specific population/dataset may not be transferable to another product. A suggestion was to include assessment aspects related to the characteristics of the development (e.g., transparency with data sources).
When it came to the adaptability and scalability of DHTs, flexibility in development was mentioned as a critical factor. Integration into various clinical workflows and existing systems often presents underestimated challenges for developers. Mobile app developers stressed the importance of designing technology to align with distinct clinical environments, noting that this can differ widely across European healthcare systems. Interoperability was also highlighted as a significant barrier. Developers often prioritized markets with robust digital infrastructures, which led to difficulties in scaling technologies to regions with lower digital readiness and exacerbated access inequities. It was emphasized that to test and incorporate new evidence generation methods (e.g., RWD collection), infrastructures need to have adequate infrastructure to support these methods, which is often not the case in Europe.
Strongly resonating with the results of the literature review, developers validated the need for broader stakeholder engagement throughout the HTA process, from development to post-market surveillance. This involvement would help ensure that DHTs meet real-world clinical needs and align with existing care pathways. Telemedicine developers highlighted the benefits of early clinician involvement in usability testing, which improved integration into healthcare settings and adoption rates. These perspectives not only highlight challenges across the lifecycle of digital health technologies but also point to broader limitations in current HTA practices, which are further explored in the following discussion. Key challenges and barriers for developers are summarized in Figure 2.
Figure 2.
Challenges and barriers for developers along the lifecycle of DHTs. The figure does not aim to represent all lifecycle phases but rather offer a high-level overview of the main stages where developers typically face challenges and barriers. Arrows indicate the progression of DHTs over the lifecycle phases.
Across all research phases, developers consistently expressed that a harmonized HTA framework, such as the one envisioned by EDiHTA, would significantly help overcome existing challenges in market access and reimbursement. Participants viewed EDiHTA as a valuable framework to streamline evidence generation, reduce regulatory uncertainty, and facilitate early engagement with stakeholders. In addition, resources like interactive guidance platforms, standardized assessment pathways, and case study repositories were highlighted as key enablers for more efficient and predictable market entry.
5. Discussion
Developing and launching digital health technologies in Europe comes with a unique set of challenges, primarily due to the fragmented nature of health systems and regulatory frameworks. Developers consistently pointed to unclear, inconsistent, and duplicative requirements between existing HTA frameworks in European countries, complicating efforts to bring innovative digital solutions to market. One of the central issues highlighted was the absence of standardized and transparent HTA requirements across countries. Participants repeatedly stressed that without alignment and clarity, administrative and financial burdens will continue to hinder innovation. In addition to fragmented and inconsistent frameworks, developers raised broader concerns about several systemic challenges, including the inconsistent application and timing of evaluations across countries, limited stakeholder involvement, and static HTA models that are misaligned with the iterative nature of digital innovation. Infrastructure shortcomings and the high compliance burden, especially for SMEs, were also seen as major issues. These findings suggest that traditional HTA processes may not be sufficiently agile to accommodate DHTs and reinforce the need for a more dynamic, modular approach, affirming the relevance of EDiHTA in addressing developers’ operational and strategic needs across Europe. Similar findings have been reported by Mathias et al. [75] and van Kessel et al. [3], who emphasized that current assessment and reimbursement processes are often misaligned with the needs of developers and the nature of DHTs. Our study adds value by being one of the few that directly involved developers, providing first-hand insights into these barriers.
Incorporating RWE into assessments was highlighted as a key priority, accommodating the rapid, iterative development cycles of digital technologies. Several developers advocated for continuous data collection during the deployment of DHTs in the post-marketing phase to inform adaptive re-assessments, citing RWE as essential for this approach. To shorten market access and reimbursement timelines, the framework should allow DHTs to be evaluated at different development stages. Early and ongoing stakeholder discussions, along with clear guidance on evidence generation methods at each lifecycle stage, could further support developers. Timely consultations throughout the HTA process must be available. Establishing a pool of experts available for consultation, alongside HTA body consultations, could help accelerate market entry and reimbursement decisions.
In terms of the format and tools of the EDiHTA framework, developers indicated practical, structured guidance formats and dynamic toolkits as offering the best added value. A flexible, centralized resource could simplify compliance navigation, minimizing trial-and-error approaches and reducing development delays. Importantly, developers emphasized the perceived benefit of such a platform being tailored to reflect evolving digital health regulations, facilitating real-time adaptation of their strategies. To foster transparency in processes, the creation of a case study repository of already conducted assessments was seen as a valuable reference for developers for study types, sample sizes, outcomes, and other aspects. This would offer deeper insights into navigating the HTA landscape and highlight successful strategies employed by similar technologies. Case studies showing successful navigation of HTA processes were seen as practical references that developers could consult. For the uptake and sustainability of the EDiHTA initiative, the framework needs to be validated through pilot programs involving key European countries to test the methodology as well as multi-stakeholder engagement. From the developers’ perspective, these tools not only assist in understanding market expectations and streamlining planning processes. As Schliess et al. [76] noted in the case of DiGA, even with structured fast-track models in place, many types of DHTs remain excluded, reinforcing the need for broader, inclusive, and flexible frameworks like EDiHTA.
Our findings illustrate that developers are not only aware of these systemic inefficiencies but are actively seeking out practical ways to overcome them. They recognize that their concerns need a broader, coordinated, cross-border response rather than individual solutions. Across different company sizes and European markets, participants welcomed the initiative and expressed interest in actively engaging with EDiHTA, provided it aligns with their day-to-day realities and emerging needs. Ultimately, developers perceive the EDiHTA framework as a potential enabler of a coordinated approach to HTA of DHTs that promotes wider, faster adoption of DHTs in the European market. These insights reinforce the relevance of the EDiHTA initiative and confirm industry readiness to utilize and engage with the framework throughout the design and implementation phases.
This study has some limitations. Although participants represented a diverse mix of company sizes and geographic contexts, the sample size was relatively small (n = 31) and may have overrepresented companies already engaged in HTA or market access discussions. While feedback was collected and reviewed across different types of technologies, no substantial differences were observed; developers shared similar concerns across modalities, particularly around evidence requirements, financial constraints, and regulatory fragmentation. It should also be acknowledged that this study did not capture the perspectives of companies that may have exited the market or gone bankrupt due to these barriers. Including such experiences could potentially reinforce the findings or reveal even more critical challenges in navigating the current system. Future studies could explore whether more granular differences emerge in specific subgroups or use cases.
6. Conclusions
The literature review and perspectives from 31 unique DHT companies (20 companies in the survey, 29 companies in the qualitative phase, and 16 companies only participating in the interview phase), spanning startups, and well-established international firms across 10 European markets shed light on the needs of developers and the perceived added value of the EDiHTA framework in response to market access and reimbursement challenges, operational barriers, and decision-making processes when developing DHTs. The findings underscore the need for a standardized HTA methodology that aligns with the realities faced by technology developers in Europe. By aligning with the unique characteristics of DHTs, such a framework can support developers in navigating complex processes, driving the adoption of innovative technologies and ultimately improving patient outcomes across Europe.
Health technology developers suggested several critical areas that they believe the EDiHTA framework should prioritize. First, the simplification, clarification, and harmonization of regulatory and Health Technology Assessment (HTA) requirements are essential to reducing administrative burdens and accelerating market access and reimbursement pathways. A structured approach to linking assessment requirements with specific technology types could further enhance transparency and efficiency. Additionally, a multi-phase assessment model that integrates real-world evidence (RWE) into evaluations is crucial. Enabling digital health solutions to be assessed at different development stages with continuous data collection for adaptive (re-)assessment would facilitate a more dynamic and evidence-driven regulatory process. Financial sustainability remains a pressing concern, particularly for startups and SMEs, emphasizing the need for tailored funding opportunities to support data collection, clinical validation, compliance, and reimbursement. Finally, promoting transparency through a public HTA case study repository could serve as a valuable reference for developers, offering insights into study designs, sample sizes, key outcomes, and best practices of already conducted HTA processes.
Although HTA is primarily targeted at decision-makers, it concerns a wide range of stakeholders as it has an influence on the access and timing of the availability of new technologies to patients. Hence, it regards those who decide upon the availability of the technology on the market (e.g., policy makers), the end-users (e.g., patients), the ones prescribing the technologies (e.g., health care professionals), organizations reimbursing the technology (e.g., social security system, private insurance companies), and those that develop and sell the technology (e.g., industry). The foundational insights retrieved in this research will guide the iterative development and conceptualization of the EDiHTA framework, ensuring its sustainability and relevance in a dynamic digital health ecosystem.
While enabling innovation is a key driver, the framework will also need to support balanced, evidence-informed decision-making that reflects the perspectives of other stakeholders, including policymakers, payers, HTA bodies, clinicians, and patients. By addressing these multiple dimensions, EDiHTA can contribute to a more structured, transparent, and predictable environment for digital health technologies in Europe. Relevant stakeholder groups, including health technology developers, are part of the development phase as well and will be consulted in different phases of the EDiHTA project to review and evaluate designs.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jmahp13030046/s1, File S1. Search String for Literature Review. File S2. Survey. File S3. Focus Group and Interview Protocol
Author Contributions
Conceptualization, F.M., E.T., M.B.; methodology, E.T., F.M. and M.B.; validation, R.D.B., D.S., A.C.; formal analysis, F.M., M.B., I.S., E.M.C., D.A.; investigation, F.M., E.T., M.B., A.L., I.S., E.M.C., D.A.; writing—original draft preparation, F.M.; writing—review and editing, F.M., M.B., E.T., R.D.B., D.S.; visualization, F.M. and M.B.; supervision, E.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the European Union under grant agreement no. 101136424. The views and opinions expressed are, however, those of the authors only and do not necessarily reflect those of the European Union or the European Health and Digital Executive Agency (HADEA). Neither the European Union nor the granting authority can be held responsible for them. The UK participant is supported by UKRI grant no. 10106869 (National Institute for Health and Care Excellence). The Swiss participant is supported by the Swiss State Secretariat for Education, Research, and Innovation (SERI). No additional funding was received for the preparation of this manuscript.
Institutional Review Board Statement
The study was conducted under the EU-funded the EDiHTA Research Project in which ethical requirements that are assessed by the sponsor (EU) prior to the project’s initiation. No patients, medical treatments, or clinical interventions were involved and no risk were posed to the participants.
Informed Consent Statement
Verbal informed consent was provided by all participants, after being informed about the study content, the scientific reasons justifying the research, how the data will be used and that their anonymity/confidentiality is guaranteed in line with international ethical standards, Declaration of Helsinki and GDPR.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| DHT | Digital Health Technology |
| HTA | Health Technology Assessment |
| HTA-R | Health Technology Assessment Regulation |
| EHDS | European Health Data Space |
| GDPR | General Data Protection Regulation |
| RWE | Real-World Evidence |
| RCT | Randomized Controlled Trial |
| EHR | Electronic Health Record |
Note
| 1 | The developer is intended to be a manufacturer * or provider ** (if the DHT is an AI system), according to EU regulations. * Regulation (EU) 2019/1020: Art.3(8) ‘manufacturer’ means any natural or legal person who manufactures a product or has a product designed or manufactured and markets that product under its name or trademark. ** Regulation (EU) 2024/1689: Art.3(3) ‘provider’ means a natural or legal person, public authority, agency, or other body that develops an AI system or a general-purpose AI model or that has an AI system or a general-purpose AI model developed and places it on the market or puts the AI system into service under its own name or trademark, whether for payment or free of charge.) |
References
- WHO Recommendations on Digital Interventions for Health System Strengthening. Available online: https://www.who.int/publications/i/item/9789241550505 (accessed on 21 March 2025).
- Zrubka, Z.; Champion, A.; Holtorf, A.-P.; Bidino, R.D.; Earla, J.R.; Boltyenkov, A.T.; Tabata-Kelly, M.; Asche, C.; Burrell, A. The PICOTS-ComTeC Framework for Defining Digital Health Interventions: An ISPOR Special Interest Group Report. Value Health 2024, 27, 383–396. [Google Scholar] [CrossRef]
- van Kessel, R.; Srivastava, D.; Kyriopoulos, I.; Monti, G.; Novillo-Ortiz, D.; Milman, R.; Zhang-Czabanowski, W.W.; Nasi, G.; Stern, A.D.; Wharton, G.; et al. Digital Health Reimbursement Strategies of 8 European Countries and Israel: Scoping Review and Policy Mapping. JMIR mHealth uHealth 2023, 11, e49003. [Google Scholar] [CrossRef] [PubMed]
- Drummond, M.; Tarricone, R.; Torbica, A. Assessing the Added Value of Health Technologies: Reconciling Different Perspectives. Value Health 2013, 16, S7–S13. [Google Scholar] [CrossRef]
- The Fast-Track Process for Digital Health Applications (DiGA) According to Section 139e SGB V: A Guide for Manufacturers, Service Providers and Users. Available online: https://www.bfarm.de/SharedDocs/Downloads/EN/MedicalDevices/DiGA_Guide.html (accessed on 21 March 2025).
- HAS. LPPR: Dépôt d’un Dossier Auprès de la Commission Nationale D’évaluation des Dispositifs Médicaux et des Technologies de Santé (CNEDiMTS); HAS: 2024. Available online: https://www.has-sante.fr/upload/docs/application/pdf/2016-01/guide_fabricant_2016_01_11_cnedimts_vd.pdf (accessed on 21 March 2025).
- RIZIV. Mobiele Medische Toepassingen: Uw Aanvraag Indienen. Available online: https://www.riziv.fgov.be/nl/professionals/andere-professionals/fabrikanten-en-verdelers-van-medische-hulpmiddelen/mobiele-medische-toepassingen-uw-aanvraag-indienen (accessed on 20 March 2025).
- Haverinen, J.; Keränen, N.; Falkenbach, P.; Maijala, A.; Kolehmainen, T.; Reponen, J. Digi-HTA: Health Technology Assessment Framework for Digital Healthcare Services. Finn. J. eHealth eWelfare 2019, 11, 326–341. [Google Scholar] [CrossRef]
- Segur-Ferrer, J.; Moltó-Puigmartí, C.; Pastells-Peiró, R.; Vivanco-Hidalgo, R.M. Health Technology Assessment Framework: Adaptation for Digital Health Technology Assessment: User Guide; Studies and research; Ministry of Health: Madrid, Spain; Agency for Health Quality and Assessment of Catalonia: Barcelona, Spain, 2024. [Google Scholar]
- NICE. Evidence Standards Framework for Digital Health Technologies; Corporate Document; NICE: 2024. Available online: https://www.nice.org.uk/corporate/ecd7/resources/evidence-standards-framework-for-digital-health-technologies-pdf-1124017457605 (accessed on 21 March 2025).
- Batten, L.; Needham-Taylor, A.; England, C.; Jarrom, D. OP08 Health Technology Assessments of Artificial Intelligence: Special Considerations and Development of A Checklist. Int. J. Technol. Assess. Health Care 2025, 40, S4. [Google Scholar] [CrossRef]
- HAS. LPPR: Dossier Submission to the Medical Device and Health Technology Evaluation Committee (CNEDiMTS); HAS: 2020. Available online: https://www.has-sante.fr/upload/docs/application/pdf/2022-02/guide_dm_vf_english_publi_appendix5.pdf (accessed on 21 March 2025).
- Mezei, F.; Horváth, K.; Pálfi, M.; Lovas, K.; Ádám, I.; Túri, G. International Practices in Health Technology Assessment and Public Financing of Digital Health Technologies: Recommendations for Hungary. Front. Public Health 2023, 11, 1197949. [Google Scholar] [CrossRef]
- Hogervorst, M.A.; Vreman, R.; Heikkinen, I.; Bagchi, I.; Gutierrez-Ibarluzea, I.; Ryll, B.; Eichler, H.-G.; Petelos, E.; Tunis, S.; Sapede, C.; et al. Uncertainty Management in Regulatory and Health Technology Assessment Decision-Making on Drugs: Guidance of the HTAi-DIA Working Group. Int. J. Technol. Assess. Health Care 2023, 39, e40. [Google Scholar] [CrossRef]
- EIT Health. Digital Medical Devices: Paths to European Harmonisation Summary Report; EIT Health: Munich, Germany, 2023; p. 27. [Google Scholar]
- Ruether, A.; Imaz-Iglesia, I.; Bélorgey, C.; Lo Scalzo, A.; Garrett, Z.; Guardian, M. European Collaboration on Health Technology Assessment: Looking Backward and Forward. Int. J. Technol. Assess. Health Care 2022, 38, e34. [Google Scholar] [CrossRef]
- Yeung, A.W.K.; Torkamani, A.; Butte, A.J.; Glicksberg, B.S.; Schuller, B.; Rodriguez, B.; Ting, D.S.W.; Bates, D.; Schaden, E.; Peng, H.; et al. The Promise of Digital Healthcare Technologies. Front. Public Health 2023, 11, 1196596. [Google Scholar] [CrossRef]
- Tsiasiotis, E.; Bidino, R.D.; Sacchini, D.; Cicchetti, A. PP39 Co-Creation Of A European Digital Health Technology Assessment Framework: The EDiHTA EU-Funded Project. Int. J. Technol. Assess. Health Care 2024, 40, S70. [Google Scholar] [CrossRef]
- EDiHTA. Available online: https://edihta-project.eu/ (accessed on 14 February 2025).
- Mantovani, A.; Leopaldi, C.; Nighswander, C.M.; Di Bidino, R. Access and Reimbursement Pathways for Digital Health Solutions and in Vitro Diagnostic Devices: Current Scenario and Challenges. Front. Med. Technol. 2023, 5, 1101476. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Moola, S.; Munn, Z.; Sears, K.; Sfetcu, R.; Currie, M.; Lisy, K.; Tufanaru, C.; Qureshi, R.; Mattis, P.; Mu, P. Conducting Systematic Reviews of Association (Etiology): The Joanna Briggs Institute’s Approach. Available online: https://pubmed.ncbi.nlm.nih.gov/26262566/ (accessed on 1 June 2025).
- Jiu, L.; Hogervorst, M.A.; Vreman, R.A.; Mantel-Teeuwisse, A.K.; Goettsch, W.G. Understanding Innovation of Health Technology Assessment Methods: The IHTAM Framework. Int. J. Technol. Assess. Health Care 2022, 38, e16. [Google Scholar] [CrossRef]
- Naeem, M.; Ozuem, W.; Howell, K.; Ranfagni, S. A Step-by-Step Process of Thematic Analysis to Develop a Conceptual Model in Qualitative Research. Int. J. Qual. Methods 2023, 22, 16094069231205789. [Google Scholar] [CrossRef]
- Ahmed, A.; Mutahar, M.; Daghrery, A.A.; Albar, N.H.; Alhadidi, I.Q.I.; Asiri, A.M.; Boreak, N.; Alshahrani, A.A.S.; Shariff, M.; Shubayr, M.A.; et al. A Systematic Review of Publications on Perceptions and Management of Chronic Medical Conditions Using Telemedicine Remote Consultations by Primary Healthcare Professionals April 2020 to December 2021 During the COVID-19 Pandemic. Med. Sci. Monit. 2024, 30, e943383-e1. [Google Scholar] [CrossRef]
- Appleton, R.; Barnett, P.; Vera San Juan, N.; Tuudah, E.; Lyons, N.; Parker, J.; Roxburgh, E.; Spyridonidis, S.; Tamworth, M.; Worden, M.; et al. Implementation Strategies for Telemental Health: A Systematic Review. BMC Health Serv. Res. 2023, 23, 78. [Google Scholar] [CrossRef]
- Braga Ferreira, L.; Lanna de Almeida, R.; Arantes, A.; Abdulazeem, H.; Weerasekara, I.; Ferreira, L.S.D.N.; Fonseca de Almeida Messias, L.; Siuves Ferreira Couto, L.; Parreiras Martins, M.A.; Suelen Antunes, N.; et al. Telemedicine-Based Management of Oral Anticoagulation Therapy: Systematic Review and Meta-Analysis. J. Med. Internet Res. 2023, 25, e45922. [Google Scholar] [CrossRef]
- Rettinger, L.; Kuhn, S. Barriers to Video Call–Based Telehealth in Allied Health Professions and Nursing: Scoping Review and Mapping Process. J. Med. Internet Res. 2023, 25, e46715. [Google Scholar] [CrossRef]
- Amiri, P.; Niazkhani, Z.; Pirnejad, H.; ShojaeiBaghini, M.; Bahaadinbeigy, K. Objectives, Outcomes, Facilitators, and Barriers of Telemedicine Systems for Patients with Alzheimer’s Disease and Their Caregivers and Care Providers: A Systematic Review. Arch. Iran. Med. 2022, 25, 564–573. [Google Scholar] [CrossRef]
- Lewinski, A.A.; Walsh, C.; Rushton, S.; Soliman, D.; Carlson, S.M.; Luedke, M.W.; Halpern, D.J.; Crowley, M.J.; Shaw, R.J.; Sharpe, J.A.; et al. Telehealth for the Longitudinal Management of Chronic Conditions: Systematic Review. J. Med. Internet Res. 2022, 24, e37100. [Google Scholar] [CrossRef] [PubMed]
- Appleton, R.; Williams, J.; Juan, N.V.S.; Needle, J.J.; Schlief, M.; Jordan, H.; Rains, L.S.; Goulding, L.; Badhan, M.; Roxburgh, E.; et al. Implementation, Adoption, and Perceptions of Telemental Health During the COVID-19 Pandemic: Systematic Review. J. Med. Internet Res. 2021, 23, e31746. [Google Scholar] [CrossRef] [PubMed]
- Bailey, J.E.; Gurgol, C.; Pan, E.; Njie, S.; Emmett, S.; Gatwood, J.; Gauthier, L.; Rosas, L.G.; Kearney, S.M.; Robler, S.K.; et al. Early Patient-Centered Outcomes Research Experience with the Use of Telehealth to Address Disparities: Scoping Review. J. Med. Internet Res. 2021, 23, e28503. [Google Scholar] [CrossRef]
- Chan, R.J.; Crichton, M.; Crawford-Williams, F.; Agbejule, O.A.; Yu, K.; Hart, N.H.; Alves, F.d.A.; Ashbury, F.D.; Eng, L.; Fitch, M.; et al. The Efficacy, Challenges, and Facilitators of Telemedicine in Post-Treatment Cancer Survivorship Care: An Overview of Systematic Reviews. Ann. Oncol. 2021, 32, 1552–1570. [Google Scholar] [CrossRef]
- Garfan, S.; Alamoodi, A.H.; Zaidan, B.B.; Al-Zobbi, M.; Hamid, R.A.; Alwan, J.K.; Ahmaro, I.Y.Y.; Khalid, E.T.; Jumaah, F.M.; Albahri, O.S.; et al. Telehealth Utilization during the Covid-19 Pandemic: A Systematic Review. Comput. Biol. Med. 2021, 138, 104878. [Google Scholar] [CrossRef]
- Khoshrounejad, F.; Hamednia, M.; Mehrjerd, A.; Pichaghsaz, S.; Jamalirad, H.; Sargolzaei, M.; Hoseini, B.; Aalaei, S. Telehealth-Based Services During the COVID-19 Pandemic: A Systematic Review of Features and Challenges. Front. Public Health 2021, 9, 711762. [Google Scholar] [CrossRef]
- Yi, J.S.; Pittman, C.; Price, C.L.; Nieman, C.L.; Oh, E.S. Telemedicine and Dementia Care: A Systematic Review of Barriers and Facilitators. J. Am. Med. Dir. Assoc. 2021, 22, 1396–1402.e18. [Google Scholar] [CrossRef]
- Aquilanti, L.; Santarelli, A.; Mascitti, M.; Procaccini, M.; Rappelli, G. Dental Care Access and the Elderly: What Is the Role of Teledentistry? A Systematic Review. Int. J. Environ. Res. Public Health 2020, 17, 9053. [Google Scholar] [CrossRef]
- DeNicola, N.; Grossman, D.; Marko, K.; Sonalkar, S.; Butler Tobah, Y.S.; Ganju, N.; Witkop, C.T.; Henderson, J.T.; Butler, J.L.; Lowery, C. Telehealth Interventions to Improve Obstetric and Gynecologic Health Outcomes: A Systematic Review. Obstet. Gynecol. 2020, 135, 371. [Google Scholar] [CrossRef]
- Banbury, A.; Nancarrow, S.; Dart, J.; Gray, L.; Parkinson, L. Telehealth Interventions Delivering Home-Based Support Group Videoconferencing: Systematic Review. J. Med. Internet Res. 2018, 20, e8090. [Google Scholar] [CrossRef] [PubMed]
- Edwards, D.; Noyes, J.; Lowes, L.; Haf Spencer, L.; Gregory, J.W. An Ongoing Struggle: A Mixed-Method Systematic Review of Interventions, Barriers and Facilitators to Achieving Optimal Self-Care by Children and Young People with Type 1 Diabetes in Educational Settings. BMC Pediatr. 2014, 14, 228. [Google Scholar] [CrossRef] [PubMed]
- French, B.; Day, E.; Watkins, C.; McLoughlin, A.; Fitzgerald, J.; Leathley, M.; Davies, P.; Emsley, H.; Ford, G.; Jenkinson, D.; et al. The Challenges of Implementing a Telestroke Network: A Systematic Review and Case Study. BMC Med. Inform. Decis. Mak. 2013, 13, 125. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V.R.; Metting, E.; Schauble, J.; Seddighi, H.; Beumeler, L.; Gallo, V. eHealth Tools to Assess the Neurological Function for Research, in Absence of the Neurologist—A Systematic Review, Part I (Software). J. Neurol. 2024, 271, 211–230. [Google Scholar] [CrossRef]
- Gryglewicz, K.; Orr, V.L.; McNeil, M.J.; Taliaferro, L.A.; Hines, S.; Duffy, T.L.; Wisniewski, P.J. Translating Suicide Safety Planning Components Into the Design of mHealth App Features: Systematic Review. JMIR Ment. Health 2024, 11, e52763. [Google Scholar] [CrossRef]
- Jiang, S.; Ng, J.Y.Y.; Chong, K.H.; Peng, B.; Ha, A.S. Effects of eHealth Interventions on 24-Hour Movement Behaviors Among Preschoolers: Systematic Review and Meta-Analysis. J. Med. Internet Res. 2024, 26, e52905. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Huang, X.; Wang, Z.; Liu, Y.; Huang, L.; Luo, X. Embodied Conversational Agents for Chronic Diseases: Scoping Review. J. Med. Internet Res. 2024, 26, e47134. [Google Scholar] [CrossRef]
- Gasteiger, N.; Dowding, D.; Norman, G.; McGarrigle, L.; Eost-Telling, C.; Jones, D.; Vercell, A.; Ali, S.M.; O’Connor, S. Conducting a Systematic Review and Evaluation of Commercially Available Mobile Applications (Apps) on a Health-Related Topic: The TECH Approach and a Step-by-Step Methodological Guide. BMJ Open 2023, 13, e073283. [Google Scholar] [CrossRef]
- Goodman, A.; Mahoney, R.; Spurling, G.; Lawler, S. Influencing Factors to mHealth Uptake with Indigenous Populations: Qualitative Systematic Review. JMIR mHealth uHealth 2023, 11, e45162. [Google Scholar] [CrossRef]
- van der Kamp, M.R.; Hengeveld, V.S.; Brusse-Keizer, M.G.J.; Thio, B.J.; Tabak, M. eHealth Technologies for Monitoring Pediatric Asthma at Home: Scoping Review. J. Med. Internet Res. 2023, 25, e45896. [Google Scholar] [CrossRef]
- Baines, R.; Bradwell, H.; Edwards, K.; Stevens, S.; Prime, S.; Tredinnick-Rowe, J.; Sibley, M.; Chatterjee, A. Meaningful Patient and Public Involvement in Digital Health Innovation, Implementation and Evaluation: A Systematic Review. Health Expect. 2022, 25, 1232–1245. [Google Scholar] [CrossRef]
- Bear, H.A.; Nunes, L.A.; DeJesus, J.; Liverpool, S.; Moltrecht, B.; Neelakantan, L.; Harriss, E.; Watkins, E.; Fazel, M. Determination of Markers of Successful Implementation of Mental Health Apps for Young People: Systematic Review. J. Med. Internet Res. 2022, 24, e40347. [Google Scholar] [CrossRef] [PubMed]
- Butler, S.; Sculley, D.; Santos, D.; Fellas, A.; Gironès, X.; Singh-Grewal, D.; Coda, A. Effectiveness of eHealth and mHealth Interventions Supporting Children and Young People Living with Juvenile Idiopathic Arthritis: Systematic Review and Meta-Analysis. J. Med. Internet Res. 2022, 24, e30457. [Google Scholar] [CrossRef]
- Gillam, J.; Davies, N.; Aworinde, J.; Yorganci, E.; Anderson, J.E.; Evans, C. Implementation of eHealth to Support Assessment and Decision-Making for Residents with Dementia in Long-Term Care: Systematic Review. J. Med. Internet Res. 2022, 24, e29837. [Google Scholar] [CrossRef]
- Jakob, R.; Harperink, S.; Rudolf, A.M.; Fleisch, E.; Haug, S.; Mair, J.L.; Salamanca-Sanabria, A.; Kowatsch, T. Factors Influencing Adherence to mHealth Apps for Prevention or Management of Noncommunicable Diseases: Systematic Review. J. Med. Internet Res. 2022, 24, e35371. [Google Scholar] [CrossRef]
- Bezabih, A.M.; Gerling, K.; Abebe, W.; Abeele, V.V. Behavioral Theories and Motivational Features Underlying eHealth Interventions for Adolescent Antiretroviral Adherence: Systematic Review. JMIR mHealth uHealth 2021, 9, e25129. [Google Scholar] [CrossRef]
- Borghouts, J.; Eikey, E.; Mark, G.; Leon, C.D.; Schueller, S.M.; Schneider, M.; Stadnick, N.; Zheng, K.; Mukamel, D.; Sorkin, D.H. Barriers to and Facilitators of User Engagement with Digital Mental Health Interventions: Systematic Review. J. Med. Internet Res. 2021, 23, e24387. [Google Scholar] [CrossRef] [PubMed]
- Feldman, N.; Back, D.; Boland, R.; Torous, J. A Systematic Review of mHealth Application Interventions for Peripartum Mood Disorders: Trends and Evidence in Academia and Industry. Arch. Womens Ment. Health 2021, 24, 881–892. [Google Scholar] [CrossRef] [PubMed]
- Feroz, A.S.; Ali, N.A.; Khoja, A.; Asad, A.; Saleem, S. Using Mobile Phones to Improve Young People Sexual and Reproductive Health in Low and Middle-Income Countries: A Systematic Review to Identify Barriers, Facilitators, and Range of mHealth Solutions. Reprod. Health 2021, 18, 9. [Google Scholar] [CrossRef] [PubMed]
- Wronikowska, M.W.; Malycha, J.; Morgan, L.J.; Westgate, V.; Petrinic, T.; Young, J.D.; Watkinson, P.J. Systematic Review of Applied Usability Metrics within Usability Evaluation Methods for Hospital Electronic Healthcare Record Systems. J. Eval. Clin. Pract. 2021, 27, 1403–1416. [Google Scholar] [CrossRef]
- Bassi, A.; Arfin, S.; John, O.; Jha, V. An Overview of Mobile Applications (Apps) to Support the Coronavirus Disease 2019 Response in India. Indian J. Med. Res. 2020, 151, 468. [Google Scholar] [CrossRef]
- Gandrup, J.; Ali, S.M.; McBeth, J.; van der Veer, S.N.; Dixon, W.G. Remote Symptom Monitoring Integrated into Electronic Health Records: A Systematic Review. J. Am. Med. Inform. Assoc. 2020, 27, 1752–1763. [Google Scholar] [CrossRef]
- Jonker, L.T.; Haveman, M.E.; de Bock, G.H.; van Leeuwen, B.L.; Lahr, M.M.H. Feasibility of Perioperative eHealth Interventions for Older Surgical Patients: A Systematic Review. J. Am. Med. Dir. Assoc. 2020, 21, 1844–1851.e2. [Google Scholar] [CrossRef]
- Leonardsen, A.-C.L.; Hardeland, C.; Helgesen, A.K.; Grøndahl, V.A. Patient Experiences with Technology Enabled Care across Healthcare Settings- a Systematic Review. BMC Health Serv. Res. 2020, 20, 779. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, K.M.; Tigershtrom, A.; Badr, H.; Benvengo, S.; Hernandez, M.; Ritterband, L.M. Dyadic Psychosocial eHealth Interventions: Systematic Scoping Review. J. Med. Internet Res. 2020, 22, e15509. [Google Scholar] [CrossRef] [PubMed]
- Tighe, S.A.; Ball, K.; Kensing, F.; Kayser, L.; Rawstorn, J.C.; Maddison, R. Toward a Digital Platform for the Self-Management of Noncommunicable Disease: Systematic Review of Platform-Like Interventions. J. Med. Internet Res. 2020, 22, e16774. [Google Scholar] [CrossRef]
- Varsi, C.; Nes, L.S.; Kristjansdottir, O.B.; Kelders, S.M.; Stenberg, U.; Zangi, H.A.; Børøsund, E.; Weiss, K.E.; Stubhaug, A.; Asbjørnsen, R.A.; et al. Implementation Strategies to Enhance the Implementation of eHealth Programs for Patients with Chronic Illnesses: Realist Systematic Review. J. Med. Internet Res. 2019, 21, e14255. [Google Scholar] [CrossRef]
- Allsop, M.J.; Powell, R.A.; Namisango, E. The State of mHealth Development and Use by Palliative Care Services in Sub-Saharan Africa: A Systematic Review of the Literature. BMJ Support. Palliat. Care 2018, 8, 155–163. [Google Scholar] [CrossRef]
- Bry, L.J.; Chou, T.; Miguel, E.; Comer, J.S. Consumer Smartphone Apps Marketed for Child and Adolescent Anxiety: A Systematic Review and Content Analysis. Behav. Ther. 2018, 49, 249–261. [Google Scholar] [CrossRef]
- Gehring, N.D.; McGrath, P.; Wozney, L.; Soleimani, A.; Bennett, K.; Hartling, L.; Huguet, A.; Dyson, M.P.; Newton, A.S. Pediatric eMental Healthcare Technologies: A Systematic Review of Implementation Foci in Research Studies, and Government and Organizational Documents. Implement. Sci. 2017, 12, 76. [Google Scholar] [CrossRef]
- Aranda-Jan, C.B.; Mohutsiwa-Dibe, N.; Loukanova, S. Systematic Review on What Works, What Does Not Work and Why of Implementation of Mobile Health (mHealth) Projects in Africa. BMC Public Health 2014, 14, 188. [Google Scholar] [CrossRef]
- Arnhold, M.; Quade, M.; Kirch, W. Mobile Applications for Diabetics: A Systematic Review and Expert-Based Usability Evaluation Considering the Special Requirements of Diabetes Patients Age 50 Years or Older. J. Med. Internet Res. 2014, 16, e2968. [Google Scholar] [CrossRef] [PubMed]
- de la Vega, R.; Miró, J. mHealth: A Strategic Field without a Solid Scientific Soul. A Systematic Review of Pain-Related Apps. PLoS ONE 2014, 9, e101312. [Google Scholar] [CrossRef] [PubMed]
- Baron, J.; McBain, H.; Newman, S. The Impact of Mobile Monitoring Technologies on Glycosylated Hemoglobin in Diabetes: A Systematic Review. J. Diabetes Sci. Technol. 2012, 6, 1185–1196. [Google Scholar] [CrossRef] [PubMed]
- General Data Protection Regulation (GDPR)—Legal Text. Available online: https://gdpr-info.eu/ (accessed on 20 May 2025).
- Regulation-EU-2025/327—EN-EUR-Lex. Available online: https://eur-lex.europa.eu/eli/reg/2025/327/oj/eng (accessed on 20 May 2025).
- Mathias, R.; McCulloch, P.; Chalkidou, A.; Gilbert, S. Digital Health Technologies Need Regulation and Reimbursement That Enable Flexible Interactions and Groupings. NPJ Digit. Med. 2024, 7, 148. [Google Scholar] [CrossRef] [PubMed]
- Schliess, F.; Affini Dicenzo, T.; Gaus, N.; Bourez, J.-M.; Stegbauer, C.; Szecsenyi, J.; Jacobsen, M.; Müller-Wieland, D.; Kulzer, B.; Heinemann, L. The German Fast Track Toward Reimbursement of Digital Health Applications: Opportunities and Challenges for Manufacturers, Healthcare Providers, and People with Diabetes. J. Diabetes Sci. Technol. 2024, 18, 470–476. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Market Access Society. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).