Antibacterial Activity of Palmarosa (Cymbopogon martini (Roxb.) Will.Watson) Essential Oil and Geraniol Against Clinical Isolates from Respiratory, Skin, and Soft Tissue Infections
Abstract
1. Introduction
2. Materials and Methods
2.1. Essential Oil
2.2. Microorganisms Tested
2.3. Antibacterial Activity
2.3.1. Minimum Inhibitory Concentration (MIC)
2.3.2. Minimum Bactericidal Concentration (MBC)
2.4. Cytotoxicity
2.5. Statistical Analysis
3. Results
3.1. Antimicrobial Activity
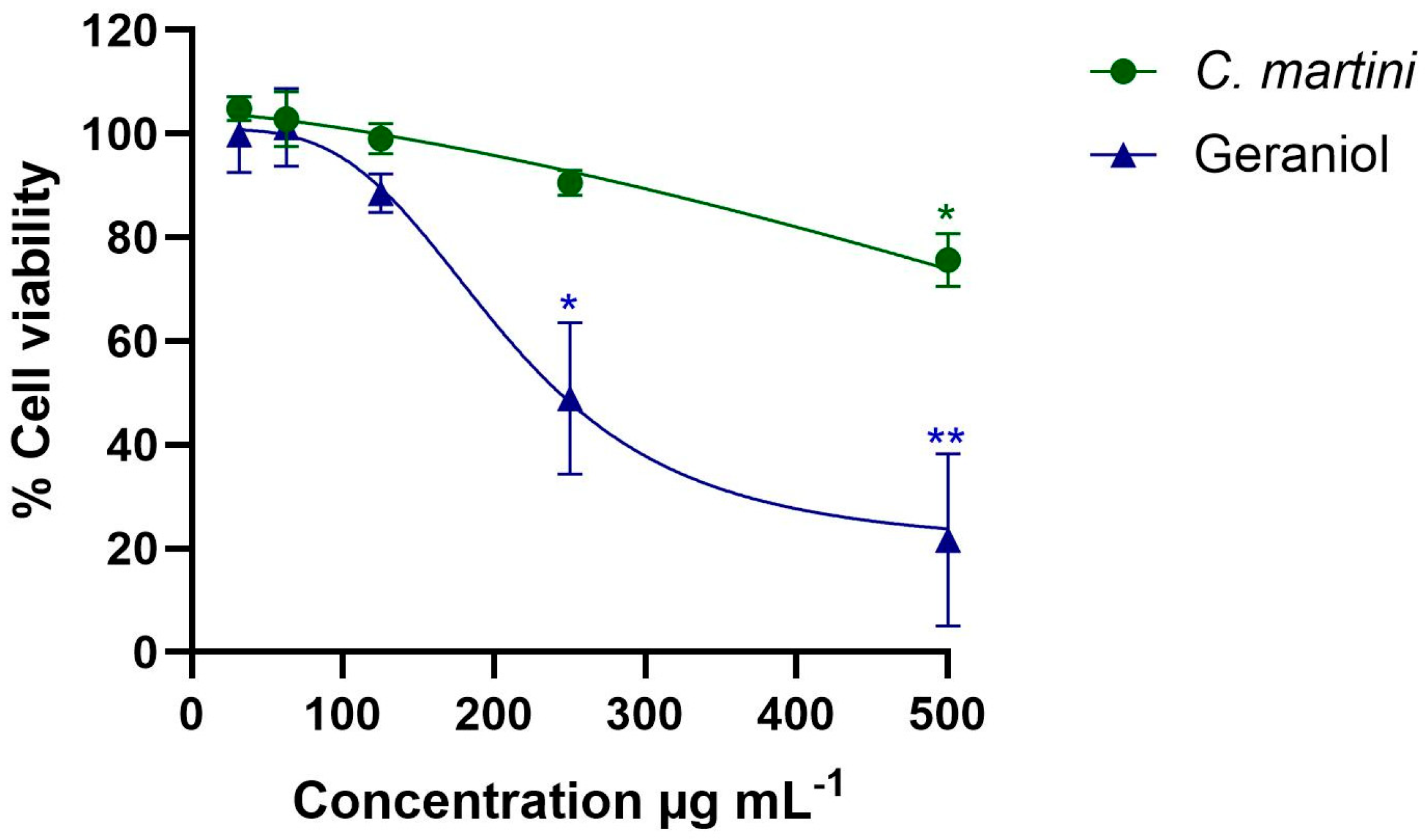
3.2. Impact on Mammalian Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| C. martini | Cymbopogon martini |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
References
- Kaspute, G.; Ivaskiene, T.; Ramanavicius, A.; Ramanavicius, S.; Prentice, U. Terpenes and Essential Oils in Pharmaceutics: Applications as Therapeutic Agents and Penetration Enhancers with Advanced Delivery Systems for Improved Stability and Bioavailability. Pharmaceutics 2025, 17, 793. [Google Scholar] [CrossRef]
- Dahan, A.; Yarmolinsky, L.; Nakonechny, F.; Semenova, O.; Khalfin, B.; Ben-Shabat, S. Etrog Citron (Citrus medica) as a Novel Source of Antimicrobial Agents: Overview of Its Bioactive Phytochemicals and Delivery Approaches. Pharmaceutics 2025, 17, 761. [Google Scholar] [CrossRef]
- Hermosilha, D.; Trigo, G.; Coelho, M.; Lehmann, I.; Melosini, M.; Serro, A.P.; Reis, C.P.; Gaspar, M.M.; Santos, S. Valorization of Thyme Combined with Phytocannabinoids as Anti-Inflammatory Agents for Skin Diseases. Pharmaceutics 2025, 17, 1291. [Google Scholar] [CrossRef]
- Sahal, G.; Donmez, H.G.; Woerdenbag, H.J.; Taner, A.; Beksac, M.S. The Potential of Thymus zygis L. (Thyme) Essential Oil Coating in Preventing Vulvovaginal Candidiasis on Intrauterine Device (IUD) Strings. Pharmaceutics 2025, 17, 1304. [Google Scholar] [CrossRef]
- El Asbahani, A.; Miladi, K.; Badri, W.; Sala, M.; Aït Addi, E.H.; Casabianca, H.; El Mousadik, A.; Hartmann, D.; Jilale, A.; Renaud, F.N.R.; et al. Essential Oils: From Extraction to Encapsulation. Int. J. Pharm. 2015, 483, 220–243. [Google Scholar] [CrossRef]
- Tibenda, J.J.; Yi, Q.; Wang, X.; Zhao, Q. Review of Phytomedicine, Phytochemistry, Ethnopharmacology, Toxicology, and Pharmacological Activities of Cymbopogon Genus. Front. Pharmacol. 2022, 13, 997918, Erratum in Front. Pharmacol. 2022, 13, 1109233. https://doi.org/10.3389/fphar.2022.1109233. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Sharma, N.; Gupta, A.K.; Chanotiya, C.S.; Lal, R.K. The Aromatic Crop Rosagrass (Cymbopogon martinii (Roxb.) Wats. Var. Motia Burk.) Its High Yielding Genotypes, Perfumery, and Pharmacological Potential: A Review. Ecol. Genet. Genom. 2024, 32, 100280. [Google Scholar] [CrossRef]
- Sinha, S.; Biswas, D.; Mukherjee, A. Antigenotoxic and Antioxidant Activities of Palmarosa and Citronella Essential Oils. J. Ethnopharmacol. 2011, 137, 1521–1527. [Google Scholar] [CrossRef]
- Duarte, R.B.; Lima, K.R.d.; Assis-Silva, Z.M.d.; Ramos, D.G.d.S.; Monteiro, C.M.d.O.; Braga, Í.A. Acaricidal Potential of Essential Oils on Rhipicephalus linnaei: Alternatives and Prospects. Vet. Parasitol. 2024, 331, 110291. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Bai, L.; Xu, X.; Feng, B.; Cao, R.; Zhao, W.; Zhang, J.; Xing, W.; Yang, X. The Diverse Enzymatic Targets of the Essential Oils of Ilex purpurea and Cymbopogon martini and the Major Components Potentially Mitigated the Resistance Development in Tick Haemaphysalis longicornis. Pestic. Biochem. Physiol. 2025, 208, 106271. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Bhatt, D.; Singh, M.K.; Maurya, A.K.; Israr, K.M.; Chauhan, A.; Padalia, R.C.; Verma, R.S.; Bawankule, D.U. P-Menthadienols-Rich Essential Oil from Cymbopogon martini Ameliorates Skin Inflammation. Inflammopharmacology 2022, 30, 895–905. [Google Scholar] [CrossRef]
- Kumari, K.U.; Imam, M.W.; Kushwaha, S.; Khaliq, A.; Meena, A.; Chanotiya, C.S.; Yadav, N.P.; Tandon, S.; Chanda, D.; Luqman, S. Palmarosa Essential Oil Inhibits the Growth of Dandruff-Associated Microbes by Increasing ROS Production and Modulating the Efflux Pump. Microb. Pathog. 2025, 200, 107323. [Google Scholar] [CrossRef]
- Sidrim, J.J.C.; Martins, D.V.; da Rocha, M.G.; dos Santos Araújo, G.; de Aguiar Cordeiro, R.; de Melo Guedes, G.M.; de Aquino Pereira-Neto, W.; de Souza Collares Maia Castelo-Branco, D.; Rocha, M.F.G. Geraniol Inhibits Both Planktonic Cells and Biofilms of the Candida parapsilosis Species Complex: Highlight for the Improved Efficacy of Amphotericin B, Caspofungin and Fluconazole plus Geraniol. Med. Mycol. 2024, 62, myae105. [Google Scholar] [CrossRef]
- Maczka, W.; Winska, K.; Grabarczyk, M. One Hundred Faces of Geraniol. Molecules 2020, 25, 3303. [Google Scholar] [CrossRef]
- Khoshnazar, S.M.; Mohagheghi, M.; Rahimi, S.; Dabiri, S.; Shahrokhi, N.; Shafieipour, S. Geraniol Modulates Inflammatory and Antioxidant Pathways to Mitigate Intestinal Ischemia–Reperfusion Injury in Male Rats. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2025, 398, 8713–8727. [Google Scholar] [CrossRef]
- Chen, W.; Viljoen, A.M. Geraniol—A Review Update. S. Afr. J. Bot. 2022, 150, 1205–1219. [Google Scholar] [CrossRef]
- Gauba, A.; Rahman, K.M. Evaluation of Antibiotic Resistance Mechanisms in Gram-Negative Bacteria. Antibiotics 2023, 12, 1590. [Google Scholar] [CrossRef]
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial Resistance: A Global Multifaceted Phenomenon. Pathog. Glob. Health 2015, 109, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Okeke, I.N.; de Kraker, M.E.A.; Van Boeckel, T.P.; Kumar, C.K.; Schmitt, H.; Gales, A.C.; Bertagnolio, S.; Sharland, M.; Laxminarayan, R. The Scope of the Antimicrobial Resistance Challenge. Lancet 2024, 403, 2426–2438. [Google Scholar] [CrossRef]
- Naghavi, M.; Vollset, S.E.; Ikuta, K.S.; Swetschinski, L.R.; Gray, A.P.; Wool, E.E.; Robles Aguilar, G.; Mestrovic, T.; Smith, G.; Han, C.; et al. Global Burden of Bacterial Antimicrobial Resistance 1990–2021: A Systematic Analysis with Forecasts to 2050. Lancet 2024, 404, 1199. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Bacterial Priority Pathogens List, 2024: Bacterial Pathogens of Public Health Importance to Guide Research, Development and Strategies to Prevent and Control Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2024. [Google Scholar]
- Zhao, J.; Fan, Y.; Cheng, Z.; Kennelly, E.J.; Long, C. Ethnobotanical Uses, Phytochemistry and Bioactivities of Cymbopogon Plants: A Review. J. Ethnopharmacol. 2024, 330, 118181. [Google Scholar] [CrossRef] [PubMed]
- Ishak, A.; Mazonakis, N.; Spernovasilis, N.; Akinosoglou, K.; Tsioutis, C. Bactericidal versus Bacteriostatic Antibacterials: Clinical Significance, Differences and Synergistic Potential in Clinical Practice. J. Antimicrob. Chemother. 2024, 80, 1. [Google Scholar] [CrossRef] [PubMed]
- Rajeswara Rao, B.R.; Rajput, D.K.; Patel, R.P.; Purnanand, S. Essential Oil Yield and Chemical Composition Changes during Leaf Ontogeny of Palmarosa (Cymbopogon martinii var. motia). Nat. Prod. Commun. 2010, 5, 1947–1950. [Google Scholar] [CrossRef]
- Smitha, G.R.; Rana, V.S. Variations in Essential Oil Yield, Geraniol and Geranyl Acetate Contents in Palmarosa (Cymbopogon martinii, Roxb. Wats. var. motia) Influenced by Inflorescence Development. Ind. Crops Prod. 2015, 66, 150–160. [Google Scholar] [CrossRef]
- Lira, M.H.P.d.; Andrade Júnior, F.P.d.; Moraes, G.F.Q.; Macena, G.d.S.; Pereira, F.d.O.; Lima, I.O. Antimicrobial Activity of Geraniol: An Integrative Review. J. Essent. Oil Res. 2020, 32, 187–197. [Google Scholar] [CrossRef]
- Krzyściak, W.; Pluskwa, K.K.; Jurczak, A.; Kościelniak, D. The Pathogenicity of the Streptococcus Genus. Eur. J. Clin. Microbiol. Infect. Dis. 2013, 32, 1361. [Google Scholar] [CrossRef] [PubMed]
- Xia, K.; Zhou, Y.; Wang, W.; Cai, Y. Streptococcus anginosus: The Potential Role in the Progression of Gastric Cancer. J. Cancer Res. Clin. Oncol. 2025, 151, 143. [Google Scholar] [CrossRef]
- Dangol, S.; Poudel, D.K.; Ojha, P.K.; Maharjan, S.; Poudel, A.; Satyal, R.; Rokaya, A.; Timsina, S.; Dosoky, N.S.; Satyal, P.; et al. Essential Oil Composition Analysis of Cymbopogon Species from Eastern Nepal by GC-MS and Chiral GC-MS, and Antimicrobial Activity of Some Major Compounds. Molecules 2023, 28, 543. [Google Scholar] [CrossRef]
- Ramírez, N.; Cassola, F.; Gambero, A.; Sartoratto, A.; Gómez Castellanos, L.M.; Ribeiro, G.; Ferreira Rodrigues, R.A.; Duarte, M.C.T. Control of Pathogenic Bacterial Biofilm Associated with Acne and the Anti-Inflammatory Potential of an Essential Oil Blend. Microb. Pathog. 2024, 194, 106834. [Google Scholar] [CrossRef]
- Janny, S.; Bert, F.; Dondero, F.; Nicolas Chanoine, M.H.; Belghiti, J.; Mantz, J.; Paugam-Burtz, C. Fatal Escherichia coli Skin and Soft Tissue Infections in Liver Transplant Recipients: Report of Three Cases. Transpl. Infect. Dis. 2013, 15, E49–E53. [Google Scholar] [CrossRef]
- Petkovšek, Ž.; Eleršič, K.; Gubina, M.; Žgur-Bertok, D.; Erjavec, M.S. Virulence Potential of Escherichia coli Isolates from Skin and Soft Tissue Infections. J. Clin. Microbiol. 2009, 47, 1811. [Google Scholar] [CrossRef]
- Ho, W.S.; Gan, H.M.; Yap, K.P.; Balan, G.; Yeo, C.C.; Thonga, K.L. Genome Sequence of Multidrug-Resistant Escherichia coli EC302/04, Isolated from a Human Tracheal Aspirate. J. Bacteriol. 2012, 194, 6691–6692. [Google Scholar] [CrossRef]
- Filipic, B.; Kojic, M.; Vasiljevic, Z.; Sovtic, A.; Dimkic, I.; Wood, E.; Esposito, A. A Longitudinal Study of Escherichia coli Clinical Isolates from the Tracheal Aspirates of a Paediatric Patient—Strain Type Similar to Pandemic ST131. Microorganisms 2024, 12, 1990. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, A.C.; Meireles, L.M.; Lemos, M.F.; Guimarães, M.C.C.; Endringer, D.C.; Fronza, M.; Scherer, R. Antibacterial Activity of Terpenes and Terpenoids Present in Essential Oils. Molecules 2019, 24, 2471. [Google Scholar] [CrossRef] [PubMed]
- Prashara, A.; Hili, P.; Veness, R.G.; Evans, C.S. Antimicrobial Action of Palmarosa Oil (Cymbopogon martinii) on Saccharomyces Cerevisiae. Phytochemistry 2003, 63, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Chen, Q.; Liang, Q.; Zhang, M.; Chen, W.; Chen, H.; Yun, Y.; Zhong, Q.; Chen, W. Antimicrobial Activity and Proposed Action Mechanism of Linalool Against Pseudomonas fluorescens. Front. Microbiol. 2021, 12, 562094. [Google Scholar] [CrossRef]
- Yang, S.K.; Yusoff, K.; Ajat, M.; Wee, C.Y.; Yap, P.S.X.; Lim, S.H.E.; Lai, K.S. Combinatorial Antimicrobial Efficacy and Mechanism of Linalool Against Clinically Relevant Klebsiella pneumoniae. Front. Microbiol. 2021, 12, 635016. [Google Scholar] [CrossRef]
- Celuppi, L.C.M.; Capelezzo, A.P.; Cima, L.B.; Zeferino, R.C.F.; Carniel, T.A.; Zanetti, M.; de Mello, J.M.M.; Fiori, M.A.; Riella, H.G. Microbiological, Thermal and Mechanical Performance of Cellulose Acetate Films with Geranyl Acetate. Int. J. Biol. Macromol. 2023, 228, 517–527. [Google Scholar] [CrossRef]
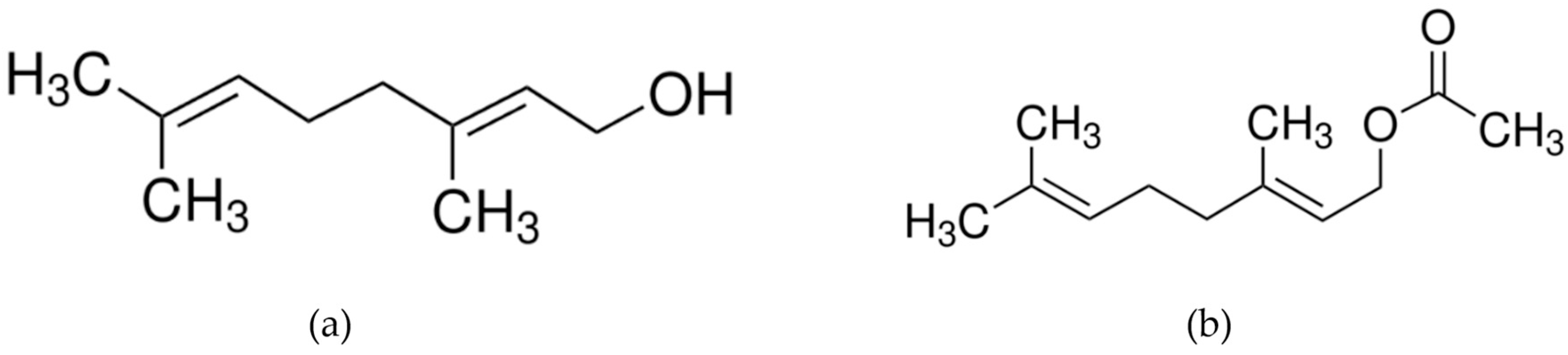
| Phytochemical Profile | ||||
|---|---|---|---|---|
| Peak | Retention Time | Constituent | Cas Number | % |
| 1 | 15.56 | 6-methyl-5-hepten-2-one | 110-93-3 | 0.05 |
| 2 | 15.70 | Beta-myrcene | 123-35-3 | 0.19 |
| 3 | 16.26 | Alpha-phellandrene | 99-83-2 | 0.01 |
| 4 | 17.07 | Limonene | 138-86-3 | 0.10 |
| 5 | 17.13 | Cis-beta-ocimene | 3338-55-4 | 0.39 |
| 6 | 17.48 | Trans-beta-ocimene | 3779-61-1 | 1.51 |
| 7 | 19.15 | Linalool | 78-70-6 | 2.71 |
| 8 | 22.88 | Nerol | 106-25-2 | 0.21 |
| 9 | 23.30 | Neral | 106-26-3 | 0.13 |
| 10 | 23.85 | Geraniol | 106-24-1 | 82.83 |
| 11 | 24.18 | Geranial | 141-27-5 | 0.25 |
| 12 | 24.90 | Neryl formate | 2142-94-1 | 0.06 |
| 13 | 26.60 | Neryl acetate | 141-12-8 | 0.02 |
| 14 | 27.04 | Geranyl acetate | 105-87-3 | 7.21 |
| 15 | 27.37 | Ylangene | 14912-44-8 | 0.01 |
| 16 | 27.57 | Beta-elemene | 141-12-8 | 0.09 |
| 17 | 28.52 | Beta-caryophyllene | 87-44-5 | 1.89 |
| 18 | 29.42 | Alpha-humulene | 6753-98-6 | 0.11 |
| 19 | 31.46 | Geranyl butyrate | 106-29-6 | 0.16 |
| 20 | 31.67 | Trans-nerolidol | 40716-66-3 | 0.12 |
| 21 | 32.59 | Caryophyllene oxide | 1139-30-6 | 0.19 |
| 22 | 35.23 | Farnesol | 4602-84-0 | 0.82 |
| 23 | 35.87 | Geranyl caproate | 4602-84-0 | 0.62 |
| 24 | 37.66 | Farnesyl acetate | 4128-17-0 | 0.07 |
| 25 | 39.90 | Geranyl caprylate | 51532-26-4 | 0.13 |
| Total | 99.88 | |||
| Classification of identified compounds | ||||
| Terpene class | Percentage (%) | |||
| Monoterpene hydrocarbons: β-myrcene, α-phellandrene, limonene, cis-β-ocimene, trans-β-ocimene | 2.20 | |||
| Oxygenated monoterpenoids: linalool, nerol, neral, geraniol, geranial, neryl formate, neryl acetate, geranyl acetate, geranyl butyrate, geranyl caproate, geranyl caprylate | 94.33 | |||
| Oxygenated sesquiterpenoids: trans-nerolidol, caryophyllene oxide, farnesol, farnesyl acetate | 1.20 | |||
| Others: 6-methyl-5-hepten-2-one | 0.05 | |||
| Total identified compounds | 99.88 | |||
| Antibacterial Activity | |||||
|---|---|---|---|---|---|
| Bacterial Strain | Isolated From | C. martini | Geraniol | ||
| MIC | MBC | MIC | MBC | ||
| Gram-positive | |||||
| Streptococcus agalactiae | Diabetic foot ulcer | 125 | 250 | 300 | 400 |
| Streptococcus anginosus | Surgical wound | 125 | 250 | 500 | 500 |
| Streptococcus dysgalactiae | Otic swab | 250 | 500 | 300 | 400 |
| Streptococcus pyogenes | Pharyngeal sample | 250 | 250 | >1000 | >1000 |
| Streptococcus pyogenes | Otic swab | 250 | 250 | >1000 | >1000 |
| Staphylococcus aureus | Non-surgical wound | 300 | 400 | 500 | 600 |
| Staphylococcus lugdunensis | Otic swab | 300 | 400 | 500 | 500 |
| Gram-negative | |||||
| Pseudomonas aeruginosa | Ulcer | >1000 | >1000 | >1000 | >1000 |
| Pseudomonas aeruginosa | Non-surgical wound | >1000 | >1000 | >1000 | >1000 |
| Pseudomonas aeruginosa | Sputum | >1000 | >1000 | >1000 | >1000 |
| Morganella morganii | Non-surgical wound | 350 | 500 | 500 | 500 |
| Escherichia coli | Surgical wound | 400 | 500 | 500 | 550 |
| Escherichia coli | Tracheal aspirate | 350 | 450 | 500 | 500 |
| Moraxella catarrhalis | Otic swab | 250 | 250 | 300 | 300 |
| Achromobacter xylosoxidans | Otic swab | 900 | >1000 | 500 | 500 |
| Serratia marcescens | Sputum | 350 | 400 | 500 | 500 |
| Klebsiella oxytoca | Sputum | 450 | 450 | 300 | 300 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Cebollada, P.; Alvarado, E.; Seral, C.; López, V. Antibacterial Activity of Palmarosa (Cymbopogon martini (Roxb.) Will.Watson) Essential Oil and Geraniol Against Clinical Isolates from Respiratory, Skin, and Soft Tissue Infections. Pharmaceutics 2026, 18, 39. https://doi.org/10.3390/pharmaceutics18010039
Cebollada P, Alvarado E, Seral C, López V. Antibacterial Activity of Palmarosa (Cymbopogon martini (Roxb.) Will.Watson) Essential Oil and Geraniol Against Clinical Isolates from Respiratory, Skin, and Soft Tissue Infections. Pharmaceutics. 2026; 18(1):39. https://doi.org/10.3390/pharmaceutics18010039
Chicago/Turabian StyleCebollada, Pilar, Elena Alvarado, Cristina Seral, and Víctor López. 2026. "Antibacterial Activity of Palmarosa (Cymbopogon martini (Roxb.) Will.Watson) Essential Oil and Geraniol Against Clinical Isolates from Respiratory, Skin, and Soft Tissue Infections" Pharmaceutics 18, no. 1: 39. https://doi.org/10.3390/pharmaceutics18010039
APA StyleCebollada, P., Alvarado, E., Seral, C., & López, V. (2026). Antibacterial Activity of Palmarosa (Cymbopogon martini (Roxb.) Will.Watson) Essential Oil and Geraniol Against Clinical Isolates from Respiratory, Skin, and Soft Tissue Infections. Pharmaceutics, 18(1), 39. https://doi.org/10.3390/pharmaceutics18010039






