Surfactant–Particle Engineering Hybrids: Emerging Strategies for Enhancing Solubility and Oral Bioavailability of Poorly Water-Soluble Drugs
Abstract
1. Introduction
2. Surfactants in Drug Formulation
2.1. Classification and Functional Roles
2.2. Mechanistic Benefits and Limitations
3. Particle Engineering Techniques
3.1. Core Technologies
3.1.1. Micronization
3.1.2. Nanocrystals
3.1.3. Spray-Drying and Freeze-Drying
3.1.4. Amorphous Solid Dispersions (ASDs)
3.1.5. Limitations and Rationale for Integration
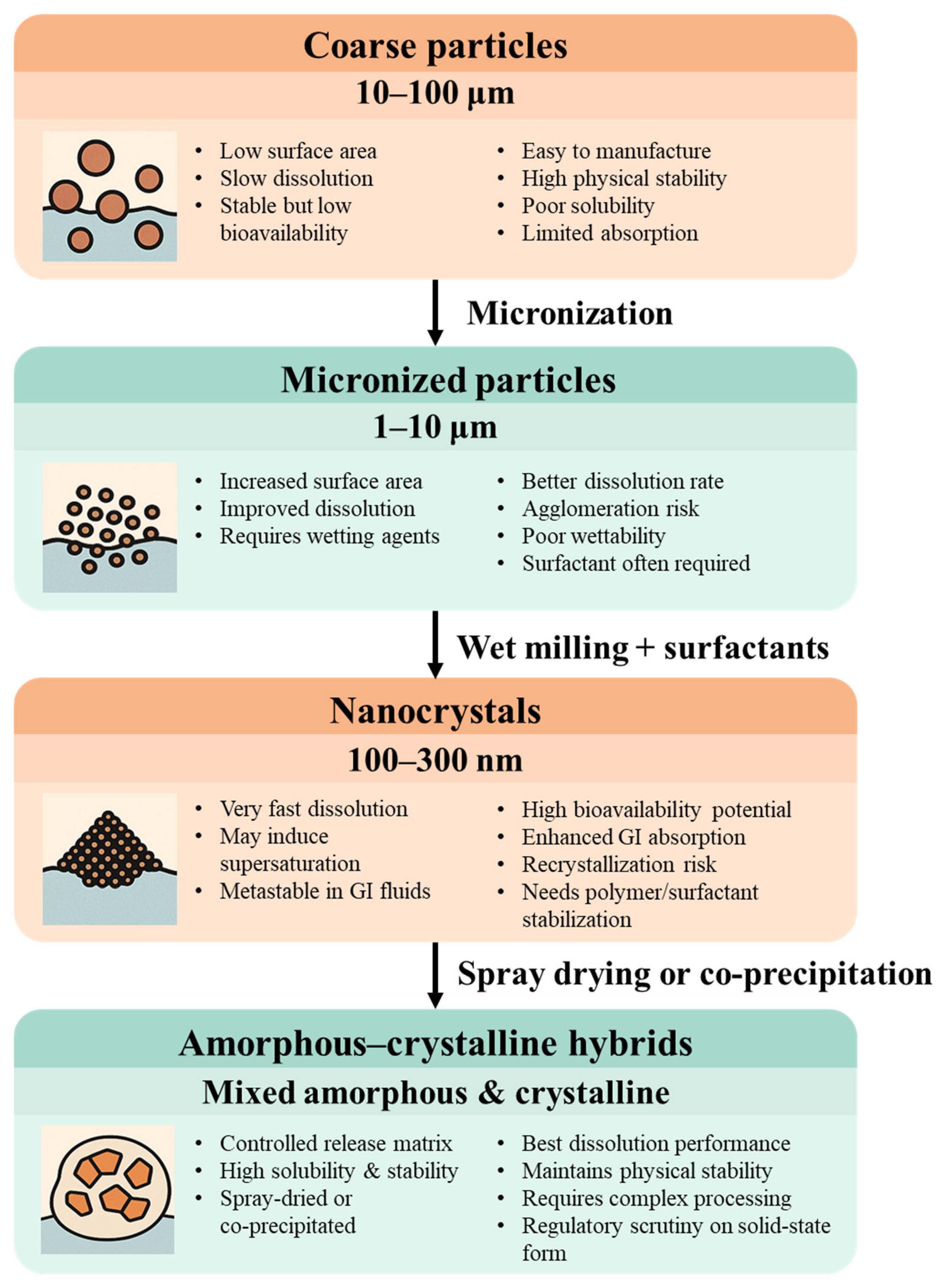
3.2. Effect of Particle Size on Dissolution and Absorption
3.2.1. Micronized Particles: Surface Area vs. Functionality
3.2.2. Nanocrystals: Supersaturation and Metastability
3.2.3. Amorphous–Crystalline Hybrids and Spray-Dried Systems
3.2.4. Shifting the Limiting Step: From Dissolution to Permeability
3.2.5. Manufacturing and Regulatory Considerations
3.2.6. Strategic Implications and Conceptual Integration
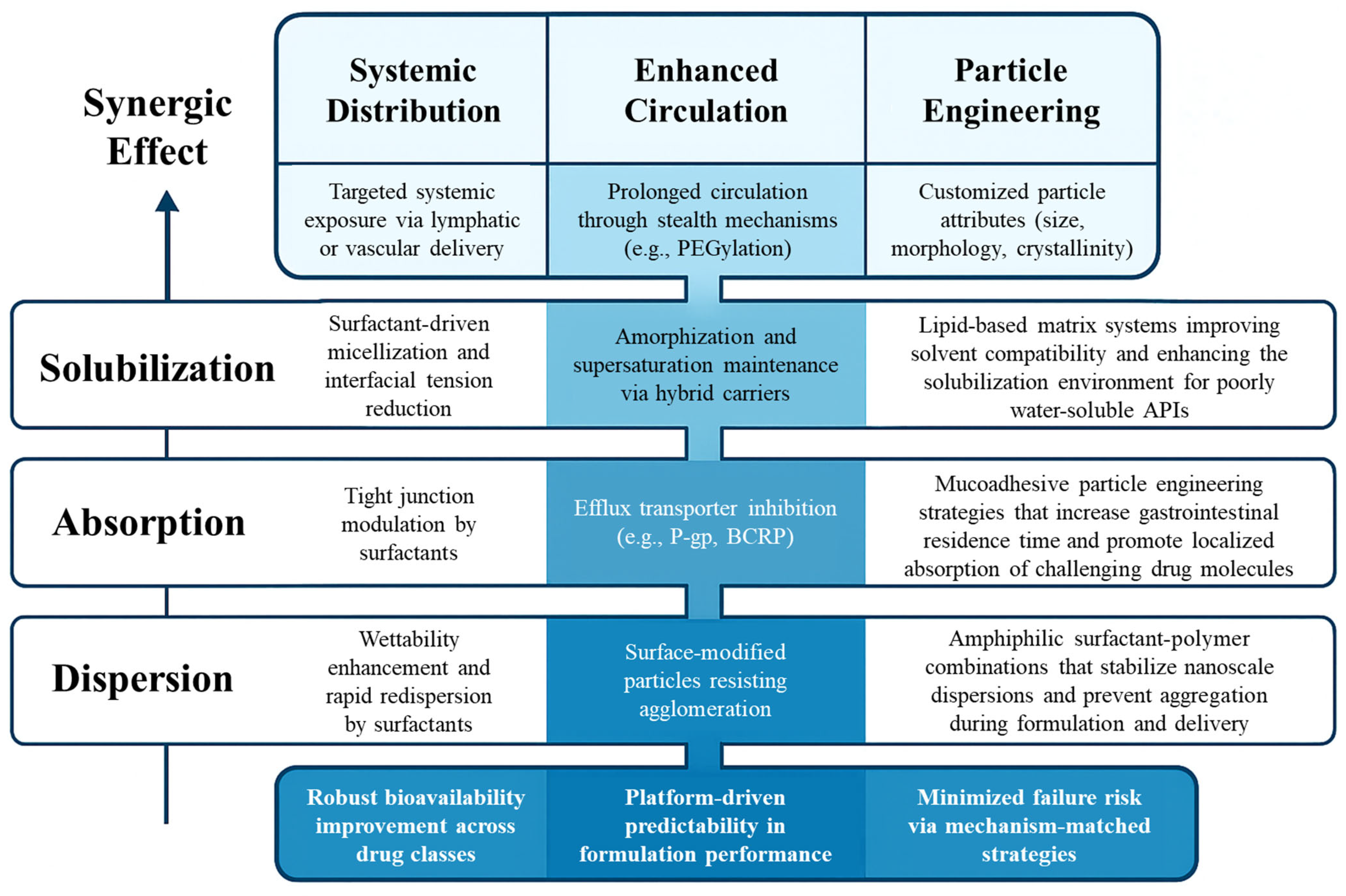
4. Hybrid Strategies: Surfactant–Particle Engineering Integration
4.1. Why Combine?
4.2. Representative Hybrid Techniques
4.2.1. Micronization
4.2.2. Surfactant-Coated Nanocrystals
4.2.3. Solid Dispersions with Surfactants
4.2.4. Self-Emulsifying Drug Delivery Systems (SEDDS) with Particle Engineering
4.2.5. Nanostructured Lipid–Surfactant Hybrid Systems
4.2.6. Spray Freeze-Drying with Surfactant Co-Precipitation
4.2.7. Comparative Evaluation of Hybrid Systems
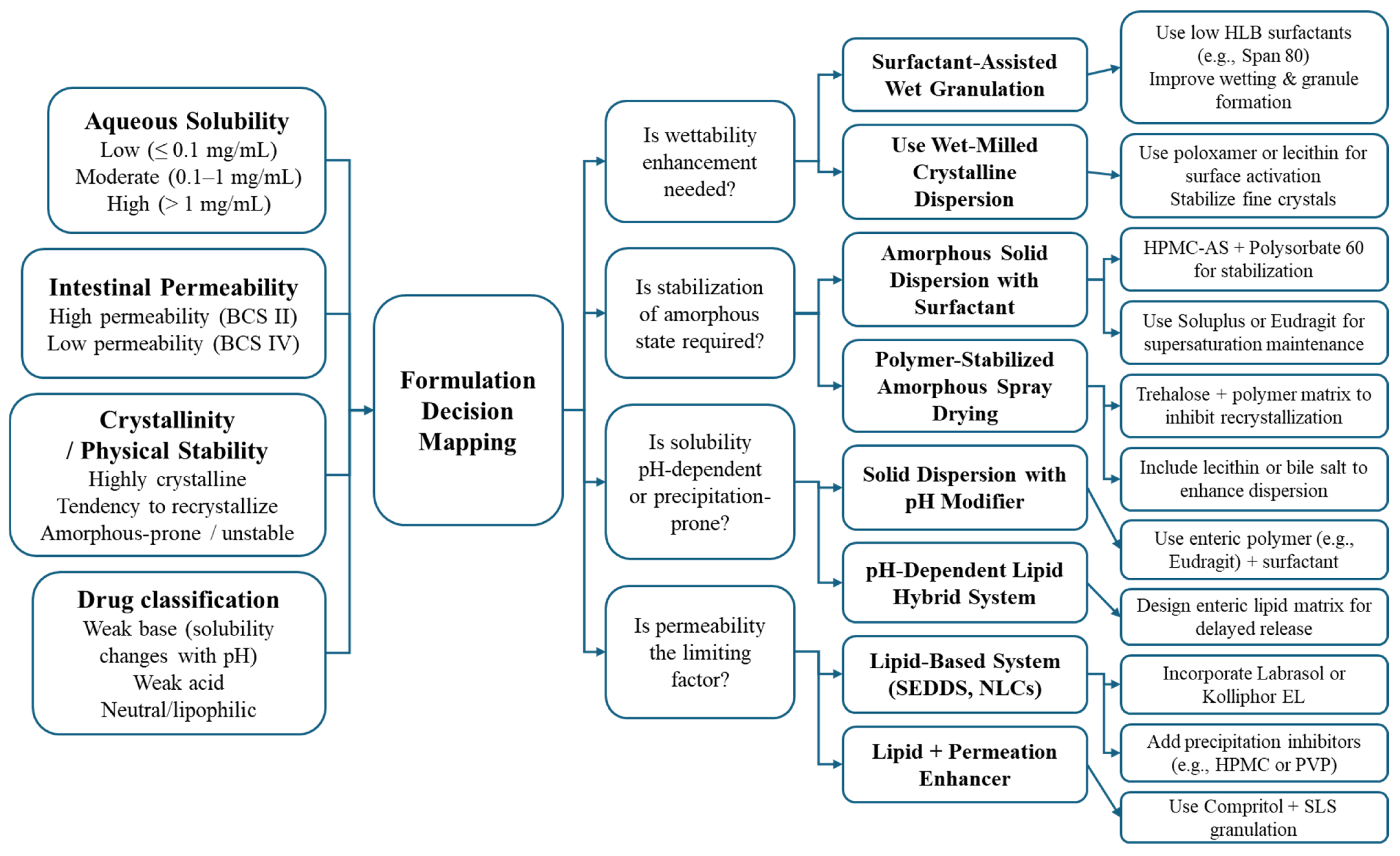
4.3. Strategy Framework for Hybrid Systems
4.3.1. Aligning Formulation Strategy with API Typology
4.3.2. Practical Formulation Constraints
4.3.3. Designing with Mechanistic Synergy in Mind
4.3.4. Toward a Decision Framework for Hybrid Selection
5. Biopharmaceutical and Industrial Perspectives
5.1. In Vitro and In Vivo Evidence
5.2. Manufacturing and Regulatory Considerations
5.3. Safety and Excipient Acceptability
6. Outlook
6.1. Strategic Advantages of Hybridization
6.2. Key Unresolved Challenges
6.3. Next-Generation Directions for Formulation Innovation
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GRAS | Generally Recognized As Safe |
| HLB | Hydrophilic-Lipophilic Balance |
| USP | United States Pharmacopeia |
| NF | National Formulary |
| FDA | Food and Drug Administration |
| PEG | Polyethylene Glycol |
| DEA | Diethanolamine |
| IV | Intravenous |
| API | Active Pharmaceutical Ingredient |
| BCS | Biopharmaceutics Classification System |
| PLGA | Poly(lactic-co-glycolic acid) |
| TPGS | D-α-Tocopheryl Polyethylene Glycol 1000 Succinate |
| SEDDS | Self-Emulsifying Drug Delivery System |
| NLCs | Nanostructured Lipid Carriers |
| QbD | Quality by Design |
| PAT | Process Analytical Technology |
| NIR | Near-Infrared Spectroscopy |
| FDA IID | FDA Inactive Ingredient Database |
| NOAEL | No-Observed-Adverse-Effect Level |
| CPPs | Critical Process Parameters |
| PXRD | Powder X-Ray Diffraction |
| DSC | Differential Scanning Calorimetry |
| ICH | International Council for Harmonisation |
| GI | Gastrointestinal |
References
- Bhalani, D.V.; Nutan, B.; Kumar, A.; Singh Chandel, A.K. Bioavailability enhancement techniques for poorly aqueous soluble drugs and therapeutics. Biomedicines 2022, 10, 2055. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Sahoo, J.; Agrawal, S.; Kumari, A. Solid dispersions: A technology for improving bioavailability. J. Anal. Pharm. Res. 2019, 8, 226–230. [Google Scholar] [CrossRef]
- Dhoble, P.; Tekade, B.; Bodke, V.; Kale, M. A systematic review of solubility enhancement techniques used for BCS Class II & IV. Int. J. Res. Pharm. Sci. 2024, 15, 1–11. [Google Scholar] [CrossRef]
- Manipriyanka, M.K.; Naidu, R.K. Advances in drug formulation technology: Enhancing bioavailability and patient compliance. J. App. Zool. Res. 2023, 44, 2125–2130. [Google Scholar] [CrossRef]
- Borgaonkar, V.B.; Jain, C.M.; Jaiswal, A.R.; Irache, P.; Yelane, A.H.; Tattu, H.P. A review on solubility enhancement technique for pharmaceutical drugs. GSC Biol. Pharm. Sci. 2024, 26, 239–253. [Google Scholar] [CrossRef]
- Williams, H.D.; Trevaskis, N.L.; Charman, S.A.; Shanker, R.M.; Charman, W.N.; Pouton, C.W.; Porter, C.J.H. Strategies to address low drug solubility in discovery and development. Pharmacol. Rev. 2013, 65, 315–499. [Google Scholar] [CrossRef]
- Kawabata, Y.; Wada, K.; Nakatani, M.; Yamada, S.; Onoue, S. Formulation design for poorly water-soluble drugs based on biopharmaceutics classification system: Basic approaches and practical applications. Int. J. Pharm. 2011, 420, 1–10. [Google Scholar] [CrossRef]
- van der Merwe, J.; Steenekamp, J.; Steyn, D.; Hamman, J. The role of functional excipients in solid oral dosage forms to overcome poor drug dissolution and bioavailability. Pharmaceutics 2020, 12, 393. [Google Scholar] [CrossRef]
- Hu, J.; Johnston, K.P.; Williams, R.O., III. Nanoparticle engineering processes for enhancing the dissolution rates of poorly water soluble drugs. Drug Dev. Ind. Pharm. 2004, 30, 233–245. [Google Scholar] [CrossRef]
- Parikh, D.M. Emerging Technologies for Particle Engineering. In Handbook of Pharmaceutical Granulation Technology, 4th ed.; CRC Press: Boca Raton, FL, USA, 2021; p. 25. [Google Scholar]
- Thomas, A.M. Pharmaceutical particle size reduction techniques: An approach to improve drug solubility, dissolution and bioavailability. World J. Pharm. Res. 2017, 6, 405–418. [Google Scholar] [CrossRef]
- Leleux, J.; Williams, R.O., III. Recent advancements in mechanical reduction methods: Particulate systems. Drug Dev. Ind. Pharm. 2014, 40, 289–300. [Google Scholar] [CrossRef]
- Jin, G.; Wang, J.; Xu, J.; Jin, Q.; Xue, J.-F.; Li, L.-H. Limitations and innovative application methods of surfactants for solubilization of poorly water-soluble drugs. Curr. Drug Deliv. 2025, 22, 1035–1046. [Google Scholar] [CrossRef] [PubMed]
- Azeez, N.A.; Venkateshwaran, K.; Kandasamy, R.; Saravanan, M.; Deepa, V.S. Importance of nano-carriers, surfactant system, and their loading mechanism to improve the absorption and bioavailability of drugs: A review. Adv. Nat. Sci. Nanosci. Nanotechnol. 2024, 15, 033002. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, Y.; Zhang, L.; Wang, Q.; Zhang, D. Stability of nanosuspensions in drug delivery. J. Control. Release 2013, 172, 1126–1141. [Google Scholar] [CrossRef] [PubMed]
- Salazar, J.; Müller, R.H.; Möschwitzer, J.P. Combinative particle size reduction technologies for the production of drug nanocrystals. J. Pharm. 2014, 2014, 265754. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.-F.; Dong, J.; Karki, S.B. Formulation Strategies and Practice Used for Drug Candidates with Water-Insoluble Properties for Toxicology, Biology, and Pharmacology Studies in Discovery Support. In Water-Insoluble Drug Formulation, 3rd ed.; Li, R., Ed.; CRC Press: Boca Raton, FL, USA, 2018; Chapter 24. [Google Scholar]
- Guo, Y.; Sun, C.C. Pharmaceutical lauryl sulfate salts: Prevalence, formation rules, and formulation implications. Mol. Pharm. 2021, 19, 390–400. [Google Scholar] [CrossRef]
- Issakhov, M.; Shakeel, M.; Pourafshary, P.; Aidarova, S.; Sharipova, A. Hybrid surfactant-nanoparticles assisted CO2 foam flooding for improved foam stability: A review of principles and applications. Petrol. Res. 2022, 7, 186–203. [Google Scholar] [CrossRef]
- Nimaming, N.; Sadeghpour, A.; Murray, B.S.; Sarkar, A. Hybrid particles for stabilization of food-grade Pickering emulsions: Fabrication principles and interfacial properties. Trends Food Sci. Technol. 2023, 138, 671–684. [Google Scholar] [CrossRef]
- Polarz, S.; Landsmann, S.; Klaiber, A. Hybrid surfactant systems with inorganic constituents. Angew. Chem. Int. Ed. 2014, 53, 946–954. [Google Scholar] [CrossRef]
- Ding, Y.; Zhao, T.; Fang, J.; Song, J.; Dong, H.; Liu, J.; Li, S.; Zhao, M. Recent developments in the use of nanocrystals to improve bioavailability of APIs. WIREs Nanomed. Nanobiotechnol. 2024, 16, e1958. [Google Scholar] [CrossRef]
- Lu, T.; Sun, Y.; Ding, D.; Zhang, Q.; Fan, R.; He, Z.; Wang, J. Study on enhanced dissolution of azilsartan-loaded solid dispersion, prepared by combining wet milling and spray-drying technologies. AAPS PharmSciTech 2017, 18, 473–480. [Google Scholar] [CrossRef]
- Wang, T.; Xue, J.; Hu, Q.; Zhou, M.; Chang, C.; Luo, Y. Synthetic surfactant- and cross-linker-free preparation of highly stable lipid-polymer hybrid nanoparticles as potential oral delivery vehicles. Sci. Rep. 2017, 7, 2750. [Google Scholar] [CrossRef] [PubMed]
- Wauthoz, N.; Amighi, K. Phospholipids in pulmonary drug delivery. Eur. J. Lipid Sci. Technol. 2014, 116, 1114–1128. [Google Scholar] [CrossRef]
- Wu, F.; Cristofoletti, R.; Zhao, L.; Rostami-Hodjegan, A. Scientific considerations to move towards biowaiver for biopharmaceutical classification system class III drugs: How modeling and simulation can help. Biopharm. Drug Dispos. 2021, 42, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Koehl, N.J.; Holm, R.; Kuentz, M.; Jannin, V.; Griffin, B.T. Exploring the impact of surfactant type and digestion: Highly digestible surfactants improve oral bioavailability of nilotinib. Mol. Pharm. 2020, 17, 3202–3213. [Google Scholar] [CrossRef]
- Izutsu, K.; Abe, Y.; Yoshida, H. Approaches to supply bioequivalent oral solid pharmaceutical formulations through the lifecycles of products: Four-media dissolution monitoring program in Japan. J. Drug Deliv. Sci. Technol. 2020, 56, 101378. [Google Scholar] [CrossRef]
- Xu, F.; Zhong, X.; Li, Z.; Cao, W.; Yang, Y.; Liu, M. Synergistic mechanisms between nanoparticles and surfactants: Insight into NP–surfactant interactions. Front. Energy Res. 2022, 10, 913360. [Google Scholar] [CrossRef]
- Shao, X.; Duan, F.; Hou, Y.; Zhong, X. Role of surfactant in controlling the deposition pattern of a particle-laden droplet: Fundamentals and strategies. Adv. Colloid Interface Sci. 2020, 275, 102049. [Google Scholar] [CrossRef]
- Jwalapuram, R.; Abdul Ahad, H.; Haranath, C.; Thadipatri, R.; Varshitha, C.V.H.; Kumar, Y.B. Solubility enhancement of drugs with aid of surfactants: Research done since last two decades. Int. J. Lifesci. Pharm. Res. 2020, 10, 11–16. [Google Scholar]
- Schittny, A.; Philipp-Bauer, S.; Detampel, P.; Huwyler, J.; Puchkov, M. Mechanistic insights into effect of surfactants on oral bioavailability of amorphous solid dispersions. J. Control. Release 2020, 320, 214–225. [Google Scholar] [CrossRef]
- Callender, S.P.; Mathews, J.A.; Kobernyk, K.; Wettig, S.D. Microemulsion utility in pharmaceuticals: Implications for multi-drug delivery. Int. J. Pharm. 2017, 526, 425–442. [Google Scholar] [CrossRef] [PubMed]
- ten Tije, A.J.; Verweij, J.; Loos, W.J.; Sparreboom, A. Pharmacological effects of formulation vehicles: Implications for cancer chemotherapy. Clin. Pharmacokinet. 2003, 42, 665–685. [Google Scholar] [CrossRef] [PubMed]
- Al-Ali, A.A.A.; Nielsen, R.B.; Steffansen, B.; Holm, R.; Nielsen, C.U. Nonionic surfactants modulate the transport activity of ATP-binding cassette (ABC) transporters and solute carriers (SLC): Relevance to oral drug absorption. Int. J. Pharm. 2019, 566, 410–433. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, R.; Chauhan, C.S.; Garg, A. A snapshot on polymeric micelles as a carrier for drug delivery. Curr. Nanomed. 2023, 13, 27–38. [Google Scholar] [CrossRef]
- Yang, B.; Wei, C.; Qian, F.; Li, S. Surface wettability modulated by surfactant and its effects on the drug release and absorption of fenofibrate solid dispersions. AAPS PharmSciTech 2019, 20, 234. [Google Scholar] [CrossRef]
- Singh, S.; Mishra, J.N. Formulation and evaluation of self-nanoemulsifying dual drug delivery system of celecoxib and curcumin. J. Popul. Ther. Clin. Pharmacol. 2023, 30, 726–740. [Google Scholar] [CrossRef]
- Yang, H.; Kim, H.; Jung, S.; Seo, H.; Nida, S.K.; Yoo, S.-Y.; Lee, J. Pharmaceutical strategies for stabilizing drug nanocrystals. Curr. Pharm. Des. 2018, 24, 2362–2374. [Google Scholar] [CrossRef]
- Narayan, P.; Porter, W.W., III; Brackhagen, M.; Tucker, C. Polymers and surfactants. In Pharmaceutical Amorphous Solid Dispersions; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Siegel, I.A.; Gordon, H.P. Surfactant-induced alterations of permeability of rabbit oral mucosa in vitro. Exp. Mol. Pathol. 1986, 44, 132–137. [Google Scholar] [CrossRef]
- Maher, S.; Geoghegan, C.; Brayden, D.J. Safety of surfactant excipients in oral drug formulations. Adv. Drug Deliv. Rev. 2023, 202, 115086. [Google Scholar] [CrossRef]
- Darusman, F.; Rusdiana, T.; Sopyan, I.; Rahma, H.; Hanifa, M. Recent progress in pharmaceutical excipients as P-glycoprotein inhibitors for potential improvement of oral drug bioavailability: A comprehensive overview. Pharmacia 2025, 72, 1–16. [Google Scholar] [CrossRef]
- Nguyen, T.-T.-L.; Duong, V.-A.; Maeng, H.-J. Pharmaceutical formulations with P-glycoprotein inhibitory effect as promising approaches for enhancing oral drug absorption and bioavailability. Pharmaceutics 2021, 13, 1103. [Google Scholar] [CrossRef] [PubMed]
- Ujhelyi, Z.; Vecsernyés, M.; Bácskay, I. Study of the effect of surface-active agents on living cells, used as component parts in microemulsions, based on their chemical structures and critical micelle-formative concentration (CMC). Acta Pharm. Hung. 2013, 83, 3–11. [Google Scholar] [PubMed]
- Comoglu, T.; Ozyilmaz, E.D. Pharmaceutical excipients in pediatric and geriatric drug formulations: Safety, efficacy, and regulatory perspectives. Pharm. Dev. Technol. 2025, 30, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rathod, S.; Desai, H.; Patil, R.; Sarolia, J. Non-ionic surfactants as a P-glycoprotein (P-gp) efflux inhibitor for optimal drug delivery—A concise outlook. AAPS PharmSciTech 2022, 23, 55. [Google Scholar] [CrossRef]
- Vinarov, Z.; Katev, V.; Radeva, D.; Tcholakova, S.; Denkov, N.D. Micellar solubilization of poorly water-soluble drugs: Effect of surfactant and solubilizate molecular structure. Drug Dev. Ind. Pharm. 2018, 44, 677–686. [Google Scholar] [CrossRef]
- Saha, U.; De, R.; Das, B. Interactions between loaded drugs and surfactant molecules in micellar drug delivery systems: A critical review. J. Mol. Liq. 2023, 382, 121906. [Google Scholar] [CrossRef]
- Seelig, A.; Gerebtzoff, G. Enhancement of drug absorption by noncharged detergents through membrane and P-glycoprotein binding. Expert Opin. Drug Metab. Toxicol. 2006, 2, 733–752. [Google Scholar] [CrossRef]
- Zastre, J.A.; Jackson, J.K.; Wong, W.; Burt, H.M. P-Glycoprotein efflux inhibition by amphiphilic diblock copolymers: Relationship between copolymer concentration and substrate hydrophobicity. Mol. Pharm. 2008, 5, 643–653. [Google Scholar] [CrossRef]
- Glynn, A.; Igra, A.M.; Sand, S.; Ilbäck, N.G.; Hellenäs, K.E.; Rosén, J.; Aspenström-Fagerlund, B. Are additive effects of dietary surfactants on intestinal tight junction integrity an overlooked human health risk?—A mixture study on Caco-2 monolayers. Food Chem. Toxicol. 2017, 106, 314–323. [Google Scholar] [CrossRef]
- Sharma, A. Effect of residual reactive impurities in excipients on the stability of pharmaceutical products. In Innovative Dosage Forms: Design and Development at Early Stage, Methods and Principles in Medicinal Chemistry; Bachhav, Y., Ed.; Wiley-VCH: Weinheim, Germany, 2019; Chapter 3. [Google Scholar]
- Robnik, B.; Naumoska, K.; Časar, Z. A novel testing approach for oxidative degradation dependent incompatibility of amine moiety containing drugs with PEGs in solid-state. Pharmaceutics 2020, 12, 37. [Google Scholar] [CrossRef]
- Dai, W.-G.; Dong, L.C.; Li, S.; Deng, Z. Combination of Pluronic/Vitamin E TPGS as a potential inhibitor of drug precipitation. Int. J. Pharm. 2008, 355, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Brewster, M.E.; Verreck, G.; Bevernage, J.; Brouwers, J.; Van den Mooter, G.; Augustijns, P. Preclinical and Clinical Studies. In Pharmaceutical Amorphous Solid Dispersions; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Mhaske, N.S.; Raskar, P.B. Advanced formulation strategies for enhancing solubility and permeability of ritonavir: A comprehensive review. J. Adv. Zool. 2024, 45, 710–717. [Google Scholar]
- Katz, J.S.; Chou, D.K.; Christian, T.R.; Das, T.K.; Patel, M.; Singh, S.N.; Wen, Y. Emerging challenges and innovations in surfactant-mediated stabilization of biologic formulations. J. Pharm. Sci. 2022, 111, 919–932. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Moudgil, B.M. Interfacial engineering of particulate & surfactant systems for enhanced performance in industrial applications. KONA Powder Part. J. 2023, 40, 29–49. [Google Scholar] [CrossRef]
- Cun, D.; Zhang, C.; Bera, H.; Yang, M. Particle engineering principles and technologies for pharmaceutical biologics. Adv. Drug Deliv. Rev. 2021, 174, 140–167. [Google Scholar] [CrossRef]
- Han, X.; Ghoroi, C.; To, D.; Chen, Y.; Davé, R. Simultaneous micronization and surface modification for improvement of flow and dissolution of drug particles. Int. J. Pharm. 2011, 415, 185–195. [Google Scholar] [CrossRef]
- Rasenack, N.; Müller, B.W. Micron-size drug particles: Common and novel micronization techniques. Pharm. Dev. Technol. 2004, 9, 1–13. [Google Scholar] [CrossRef]
- Trementozzi, A.N.; Leung, C.-Y.; Osei-Yeboah, F.; Irdam, E.; Lin, Y.; MacPhee, J.M.; Boulas, P.; Karki, S.B.; Zawaneh, P.N. Engineered particles demonstrate improved flow properties at elevated drug loadings for direct compression manufacturing. Int. J. Pharm. 2017, 523, 133–141. [Google Scholar] [CrossRef]
- Sivanathan, G.; Rajagopal, S.; Mahadevaswamy, G.; Angamuthu, G.; Dhandapani, N.V. Pharmaceutical nanocrystals: An extensive overview. Int. J. Appl. Pharm. 2024, 16, 1–9. [Google Scholar] [CrossRef]
- Fan, M.; Geng, S.; Liu, Y.; Wang, J.; Wang, Y.; Zhong, J.; Yan, Z.; Yu, L. Nanocrystal technology as a strategy to improve drug bioavailability and antitumor efficacy for the cancer treatment. Curr. Pharm. Des. 2018, 24, 2416–2424. [Google Scholar] [CrossRef]
- Gigliobianco, M.R.; Casadidio, C.; Censi, R.; Di Martino, P. Nanocrystals of poorly soluble drugs: Drug bioavailability and physicochemical stability. Pharmaceutics 2018, 10, 134. [Google Scholar] [CrossRef]
- Feth, M.P.; Heyse, W.; Baumgartner, B.; Nagel, N.; Tappertzhofen, C.; Olpp, T.; Jurascheck, J.; Ulrich, J.; Helmdach, L.; Petzoldt, C. From laboratory to pilot plant: The solid-state process development of a highly potent cathepsin S/K inhibitor. Eur. J. Pharm. Biopharm. 2013, 83, 436–448. [Google Scholar] [CrossRef] [PubMed]
- Rezvankhah, A.; Emam-Djomeh, Z.; Askari, G. Encapsulation and delivery of bioactive compounds using spray and freeze-drying techniques: A review. Drying Technol. 2020, 38, 235–258. [Google Scholar] [CrossRef]
- Sharma, A.; Khamar, D.; Cullen, S.; Hayden, A.; Hughes, H. Innovative drying technologies for biopharmaceuticals. Int. J. Pharm. 2021, 609, 121115. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Guo, M.; Luo, M.; Cai, T. Advances in the development of amorphous solid dispersions: The role of polymeric carriers. Asian J. Pharm. Sci. 2023, 18, 100834. [Google Scholar] [CrossRef]
- Luebbert, C.; Sadowski, G. Moisture-induced phase separation and recrystallization in amorphous solid dispersions. Int. J. Pharm. 2017, 532, 635–646. [Google Scholar] [CrossRef]
- Yen, C.-W.; Kuhn, R.; Hu, C.; Zhang, W.; Chiang, P.-C.; Chen, J.Z.; Hau, J.; Estevez, A.; Nagapudi, K.; Leung, D.H. Impact of surfactant selection and incorporation on in situ nanoparticle formation from amorphous solid dispersions. Int. J. Pharm. 2021, 607, 120980. [Google Scholar] [CrossRef]
- Dizaj, S.M.; Vazifehasl, Z.; Salatin, S.; Adibkia, K.; Javadzadeh, Y. Nanosizing of drugs: Effect on dissolution rate. Res. Pharm. Sci. 2015, 10, 95–108. [Google Scholar]
- Martin, T.B.; Jayaraman, A. Tuning the wetting–dewetting and dispersion–aggregation transitions in polymer nanocomposites using composition of graft and matrix polymers. Mater. Res. Express 2016, 3, 034001. [Google Scholar] [CrossRef]
- Huang, Z.; Xiong, W.; Kunnath, K.; Bhaumik, S.; Davé, R.N. Improving blend content uniformity via dry particle coating of micronized drug powders. Eur. J. Pharm. Sci. 2017, 104, 344–355. [Google Scholar] [CrossRef]
- Karde, V.; Ghoroi, C. Influence of surface modification on wettability and surface energy characteristics of pharmaceutical excipient powders. Int. J. Pharm. 2014, 475, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Peltonen, L.; Hirvonen, J. Drug nanocrystals—Versatile option for formulation of poorly soluble materials. Int. J. Pharm. 2018, 537, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Joshi, P.; Sangamwar, A.T. Stabilizing supersaturated drug-delivery system through mechanism of nucleation and crystal growth inhibition of drugs. Ther. Deliv. 2018, 9, 873–885. [Google Scholar] [CrossRef] [PubMed]
- Morozova, T.I.; Lee, V.E.; Bizmark, N.; Datta, S.S.; Prud’homme, R.K.; Nikoubashman, A.; Priestley, R.D. In silico design enables the rapid production of surface-active colloidal amphiphiles. ACS Cent. Sci. 2020, 6, 166–173. [Google Scholar] [CrossRef]
- Rahman, M.; Radgman, K.; Tarabokija, J.; Ahmad, S.; Bilgili, E. Preparation and characterization of spray-dried hybrid nanocrystal–amorphous solid dispersions (HyNASDs) for supersaturation enhancement of a slowly crystallizing drug. Nanomaterials 2023, 13, 2419. [Google Scholar] [CrossRef]
- Chaturvedi, S.; Mishra, R. Insight into delivery approaches for Biopharmaceutics Classification System Class II and IV drugs. Drug Deliv. Lett. 2020, 10, 255–277. [Google Scholar] [CrossRef]
- Hilden, J.; Burcham, C.L.; Stamatis, S.D.; Miesle, J.; Coutant, C.A. Guidance on drug substance particle size controls for solid oral dose forms. In Particles and Nanoparticles in Pharmaceutical Products: Design, Manufacturing, Behavior and Performance; Merkus, H.G., Meesters, G.M.H., Ostroa, W., Eds.; Springer: Cham, Switzerland, 2018; pp. 277–302. [Google Scholar]
- Attia, L.; Nguyen, D.; Gokhale, D.; Zheng, T.; Doyle, P.S. Surfactant–polymer complexation and competition on drug nanocrystal surfaces control crystallinity. ACS Appl. Mater. Interfaces 2024, 16, 34409–34418. [Google Scholar] [CrossRef]
- Müllertz, A.; Ogbonna, A.; Ren, S.; Rades, T. New perspectives on lipid and surfactant based drug delivery systems for oral delivery of poorly soluble drugs. J. Pharm. Pharmacol. 2010, 62, 1622–1636. [Google Scholar] [CrossRef]
- Lu, Y.; Park, K. Polymeric micelles and alternative nanonized delivery vehicles for poorly soluble drugs. Int. J. Pharm. 2013, 453, 198–214. [Google Scholar] [CrossRef]
- Simovic, S.; Barnes, T.J.; Tan, A.; Prestidge, C.A. Assembling nanoparticle coatings to improve the drug delivery performance of lipid based colloids. Nanoscale 2012, 4, 1226–1234. [Google Scholar] [CrossRef]
- Tang, J.; Sun, J.; He, Z.-G. Self-Emulsifying Drug Delivery Systems: Strategy for Improving Oral Delivery of Poorly Soluble Drugs. Curr. Drug Ther. 2007, 2, 85–93. [Google Scholar] [CrossRef]
- Bosselmann, S.; Williams, R.O., III. Route-Specific Challenges in the Delivery of Poorly Water-Soluble Drugs. In Formulating Poorly Water Soluble Drugs; Williams, R.O., III, Watts, A.B., Miller, D.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 1–26. [Google Scholar]
- Liu, Y.; Liang, Y.; Yuhong, J.; Xin, P.; Han, J.L.; Du, Y.; Yu, X.; Zhu, R.; Zhang, M.; Chen, W.; et al. Advances in nanotechnology for enhancing the solubility and bioavailability of poorly soluble drugs. Drug Des. Dev. Ther. 2024, 18, 1469–1495. [Google Scholar] [CrossRef] [PubMed]
- Yalkowsky, S.H. Perspective on improving passive human intestinal absorption. J. Pharm. Sci. 2012, 101, 3047–3050. [Google Scholar] [CrossRef] [PubMed]
- Ricarte, R.G.; Van Zee, N.J.; Li, Z.; Johnson, L.M.; Lodge, T.P.; Hillmyer, M.A. Recent advances in understanding the micro- and nanoscale phenomena of amorphous solid dispersions. Mol. Pharm. 2019, 16, 4089–4103. [Google Scholar] [CrossRef]
- Bikiaris, D.N. Solid dispersions, Part I: Recent evolutions and future opportunities in manufacturing methods for dissolution rate enhancement of poorly water-soluble drugs. Expert Opin. Drug Deliv. 2011, 8, 1501–1519. [Google Scholar] [CrossRef]
- Lee, A.Y.; Myerson, A.S. Particle Engineering: Fundamentals of Particle Formation and Crystal Growth. MRS Bull. 2006, 31, 881–886. [Google Scholar] [CrossRef]
- Aldeeb, R.A.; El-Miligi, M.F.; El-Nabarawi, M.; Tag, R.; Amin, H.M.S.; Taha, A.A. Enhancement of the solubility and dissolution rate of Telmisartan by surface solid dispersions employing superdisintegrants, hydrophilic polymers and combined carriers. Sci. Pharm. 2022, 90, 71. [Google Scholar] [CrossRef]
- de Oliveira, M.M.; Ghaly, F.M. Using fluid bed granulation to improve the dissolution of poorly water-soluble drugs. Braz. Arch. Biol. Technol. 2012, 55, 445–451. [Google Scholar] [CrossRef]
- Maheswari, P.D.; Rambhau, D.; Narasu, M.L. Micellar solubilization in the formulation development of poorly soluble naproxen. Pharmaceut. Regul. Aff. 2013, 2, 1000108. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, C.; Dun, J.; Du, L.; Hawley, M.; Sun, C.C. Mechanism for the reduced dissolution of ritonavir tablets by sodium lauryl sulfate. J. Pharm. Sci. 2019, 108, 516–524. [Google Scholar] [CrossRef]
- Shah, K.A.; Razzaq, A.; You, B.; Dormocara, A.; Iqbal, H.; Cui, J.-H. Unveiling the potential of pulmonary surfactant-based nanocarriers for protein inhalation therapy. Eur. J. Pharm. Biopharm. 2024, 205, 114574. [Google Scholar] [CrossRef] [PubMed]
- Lizoňová, D.; Hládek, F.; Chvíla, S.; Baláž, A.; Staňková, Š.; Štěpánek, F. Surface stabilization determines macrophage uptake, cytotoxicity, and bioactivity of curcumin nanocrystals. Int. J. Pharm. 2022, 626, 122133. [Google Scholar] [CrossRef] [PubMed]
- Raihan, R.; Wafa, A.; Zhakfar, A.M.; Sudhakar, C.K. Oral disintegrating films: A review. J. Nat. Sci. Rev. 2024, 2, 60–74. [Google Scholar] [CrossRef]
- Yir-Erong, B.; Bayor, M.T.; Ayensu, I.; Gbedema, S.Y.; Boateng, J.S. Oral thin films as a remedy for noncompliance in pediatric and geriatric patients. Ther. Deliv. 2019, 10, 443–464. [Google Scholar] [CrossRef]
- Ramesh, K.V.R.N.S.; Rabbani, S.A.; Talat, S.; Bhuiyan, M.K.; Marsafawy, T.E.; Islam, Q. Ternary solid dispersions employing Vitamin E TPGS for enhancing dissolution—A comparative study of surfactant combinations and preparation methods. Res. J. Pharm. Technol. 2024, 17, 2063–2070. [Google Scholar] [CrossRef]
- Yang, C.; Wu, T.; Qi, Y.; Zhang, Z. Recent advances in the application of Vitamin E TPGS for drug delivery. Theranostics 2018, 8, 464–485. [Google Scholar] [CrossRef]
- Elkhazab, A.; Sarkar, S.; Dinh, J.K.; Simpson, G.J.; Taylor, L.S. Variation in supersaturation and phase behavior of ezetimibe amorphous solid dispersions upon dissolution in different biorelevant media. Mol. Pharm. 2018, 15, 193–206. [Google Scholar] [CrossRef]
- Mandić, J.; Zvonar Pobirk, A.; Vrečer, F.; Gašperlin, M. Overview of solidification techniques for self-emulsifying drug delivery systems from industrial perspective. Int. J. Pharm. 2017, 533, 335–345. [Google Scholar] [CrossRef]
- Almeida, S.R.D.; Tippavajhula, V.K. A rundown through various methods used in the formulation of solid self-emulsifying drug delivery systems (S-SEDDS). AAPS PharmSciTech 2019, 20, 323. [Google Scholar] [CrossRef]
- Deshmukh, A.; Kulkarni, S. Solid self-microemulsifying drug delivery system of ritonavir. Drug Dev. Ind. Pharm. 2014, 40, 477–487. [Google Scholar] [CrossRef]
- Rajput, A.; Pingale, P.; Telange, D.; Chalikwar, S.; Borse, V. Lymphatic transport system to circumvent hepatic metabolism for oral delivery of lipid-based nanocarriers. J. Drug Deliv. Sci. Technol. 2021, 66, 102934. [Google Scholar] [CrossRef]
- Utreja, P.; Karnik, I.; Youssef, A.A.A.; Narala, N.; Elkanayati, R.M.; Baisa, S.; Alshammari, N.D.; Banda, S.; Vemula, S.K.; Repka, M.A. Self-emulsifying drug delivery systems (SEDDS): Transition from liquid to solid—A comprehensive review of formulation, characterization, applications, and future trends. Pharmaceutics 2025, 17, 63. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.S.; Sharma, S.; Bhatnagar, P.; Banik, B.K. Advancements in nanostructured lipid carriers and its patent innovations. Curr. Nanomed. 2024; in press. [Google Scholar] [CrossRef]
- Doost, A.S.; Kassozi, V.; Grootaert, C.; Claeys, M.; Dewettinck, K.; Van Camp, J.; Van der Meeren, P. Self-assembly, functionality, and in-vitro properties of quercetin loaded nanoparticles based on shellac-almond gum biological macromolecules. Int. J. Biol. Macromol. 2019, 129, 1024–1033. [Google Scholar] [CrossRef]
- Al-Jubori, A.A.; Sulaiman, G.M.; Tawfeeq, A.T. Antioxidant activities of resveratrol loaded Poloxamer 407: An in vitro and in vivo study. J. Appl. Sci. Nanotechnol. 2021, 1, 1–12. [Google Scholar] [CrossRef]
- Gaynanova, G.; Vasileva, L.; Kashapov, R.; Kuznetsova, D.; Kushnazarova, R.; Tyryshkina, A.; Vasilieva, E.; Petrov, K.; Zakharova, L.; Sinyashin, O. Self-assembling drug formulations with tunable permeability and biodegradability. Molecules 2021, 26, 6786. [Google Scholar] [CrossRef]
- Mann, A.K.P.; Schenck, L.; Koynov, A.; Jin, X.; Marota, M.; Dalton, C. Producing amorphous solid dispersions via co-precipitation and spray drying: Impact to physicochemical and biopharmaceutical properties. J. Pharm. Sci. 2018, 107, 183–191. [Google Scholar] [CrossRef]
- Moon, C.; Watts, A.B.; Lu, X.; Su, Y.; Williams, R.O., III. Enhanced aerosolization of high potency nanoaggregates of voriconazole by dry powder inhalation. Mol. Pharm. 2019, 16, 1799–1812. [Google Scholar] [CrossRef]
- Semba, K.; Kadota, K.; Kämäräinen, T.; Nakayama, Y.; Hatanaka, Y.; Uchiyama, H.; Arima-Osonoi, H.; Sugiyama, K.; Tozuka, Y. Tailored sugar-mediated porous particle structures for improved dispersion of drug nanoparticles in spray-freeze-drying. Langmuir 2024, 40, 14440–14454. [Google Scholar] [CrossRef]
- Sartzi, M.I.; Drettas, D.; Stramarkou, M.; Krokida, M. A comprehensive review of the latest trends in spray freeze drying and comparative insights with conventional technologies. Pharmaceutics 2024, 16, 1533. [Google Scholar] [CrossRef]
- Kontogiannis, O.; Selianitis, D.; Lagopati, N.; Pippa, N.; Pispas, S.; Gazouli, M. Surfactant and block copolymer nanostructures: From design and development to nanomedicine preclinical studies. Pharmaceutics 2023, 15, 501. [Google Scholar] [CrossRef] [PubMed]
- Meruva, S.; Thool, P.; Gong, Y.; Karki, S.; Bowen, W.; Kumar, S. Role of wetting agents and disintegrants in development of danazol nanocrystalline tablets. Int. J. Pharm. 2020, 577, 119026. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, T.M.; Shi, H.; Pietryka, J.; Hoplag, S.W.; Medek, A. Investigation of polymer/surfactant interactions and their impact on itraconazole solubility and precipitation kinetics for developing spray-dried amorphous solid dispersions. Mol. Pharm. 2018, 15, 962–974. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.C.; Carbone, C.; Souto, E.B. Beyond liposomes: Recent advances on lipid based nanostructures for poorly soluble/poorly permeable drug delivery. Prog. Lipid Res. 2017, 68, 1–11. [Google Scholar] [CrossRef]
- Pandey, V.; Kohli, S. Lipids and surfactants: The inside story of lipid-based drug delivery systems. Crit. Rev. Ther. Drug Carrier Syst. 2018, 35, 99–155. [Google Scholar] [CrossRef]
- Schenck, L.; Neri, C.; Jia, X.; Canfield, N.; Li, F.; Shah, V. A co-processed API approach for a shear sensitive compound affording improved chemical stability and streamlined drug product processing. J. Pharm. Sci. 2021, 110, 3238–3245. [Google Scholar] [CrossRef]
- Thabet, Y.; Klingmann, V.; Breitkreutz, J. Drug formulations: Standards and novel strategies for drug administration in pediatrics. J. Clin. Pharmacol. 2018, 58, S26–S35. [Google Scholar] [CrossRef]
- Baghel, S.; Cathcart, H.; O’Reilly, N.J. Investigation into the solid-state properties and dissolution profile of spray-dried ternary amorphous solid dispersions: A rational step toward the design and development of a multicomponent amorphous system. Mol. Pharm. 2018, 15, 3796–3812. [Google Scholar] [CrossRef]
- Yang, R.; Zhang, G.G.Z.; Zemlyanov, D.Y.; Purohit, H.S.; Taylor, L.S. Drug release from surfactant-containing amorphous solid dispersions: Mechanism and role of surfactant in release enhancement. Pharm. Res. 2023, 40, 2817–2845. [Google Scholar] [CrossRef]
- Rajan, N.; Kanaujiya, S. Unlocking the potential of drug delivery systems: A comprehensive review of formulation strategies and technologies in the field of pharmaceutics. Curr. Drug Ther. 2024, 19, 661–677. [Google Scholar] [CrossRef]
- Saminen, R.; Chimakurthy, J.; Konidala, S. Emerging role of biopharmaceutical classification and biopharmaceutical drug disposition system in dosage form development: A systematic review. Turk. J. Pharm. Sci. 2022, 19, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, T.; Sarmento, B.; Costa, P. Solid dispersions as strategy to improve oral bioavailability of poor water soluble drugs. Drug Discov. Today 2007, 12, 1068–1075. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Khemtong, C.; Yang, X.; Chang, X.; Gao, J. Nanonization strategies for poorly water-soluble drugs. Drug Discov. Today 2011, 16, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Imono, M.; Uchiyama, H.; Yoshida, S.; Miyazaki, S.; Tamura, N.; Tsutsumimoto, H.; Kadota, K.; Tozuka, Y. The elucidation of key factors for oral absorption enhancement of nanocrystal formulations: In vitro–in vivo correlation of nanocrystals. Eur. J. Pharm. Biopharm. 2020, 146, 84–92. [Google Scholar] [CrossRef]
- Janssens, S.; Nagels, S.; Novoa de Armas, H.; D’Autry, W.; Van Schepdael, A.; Van den Mooter, G. Formulation and characterization of ternary solid dispersions made up of itraconazole and two excipients, TPGS 1000 and PVPVA 64, that were selected based on a supersaturation screening study. Eur. J. Pharm. Biopharm. 2008, 69, 158–166. [Google Scholar] [CrossRef]
- Ramachandran, G.; Sudheesh, M.S. Role of permeability on the biopredictive dissolution of amorphous solid dispersions. AAPS PharmSciTech 2021, 22, 243. [Google Scholar] [CrossRef]
- Dhake, P.R.; Kumbhar, S.T.; Gaikwad, V.L. Biowaiver based on biopharmaceutics classification system: Considerations and requirements. Pharm. Sci. Adv. 2024, 2, 100020. [Google Scholar] [CrossRef]
- Gite, S.; Kakade, P.; Patravale, V. Surface engineering of fenofibrate nanocrystals using nano-by-design multivariate integration: A biopharmaceutical and pharmacokinetic perspective. Curr. Drug Deliv. 2021, 18, 1314–1329. [Google Scholar] [CrossRef]
- Patil, H.; Tiwari, R.V.; Repka, M.A. Hot-melt extrusion: From theory to application in pharmaceutical formulation. AAPS PharmSciTech 2016, 17, 20–42. [Google Scholar] [CrossRef]
- Kovács, A.; Berkó, S.; Csányi, E.; Csóka, I. Development of nanostructured lipid carriers containing salicylic acid for dermal use based on the Quality by Design method. Eur. J. Pharm. Sci. 2017, 99, 246–257. [Google Scholar] [CrossRef]
- Panda, R.; Lankalapalli, S. Design of experiments and optimization of amorphous solid dispersion of a BCS Class IV anti-platelet drug through factorial design. Int. J. Appl. Pharm. 2023, 15, 353–364. [Google Scholar] [CrossRef]
- Fonteyne, M.; Vercruysse, J.; Córdoba Díaz, D.; Gildemyn, D.; Vervaet, C.; Remon, J.P.; De Beer, T. Real-time assessment of critical quality attributes of a continuous granulation process. Pharm. Dev. Technol. 2013, 18, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Dumortier, G.; Grossiord, J.L.; Agnely, F.; Chaumeil, J.C. A review of Poloxamer 407 pharmaceutical and pharmacological characteristics. Pharm. Res. 2006, 23, 2709–2728. [Google Scholar] [CrossRef] [PubMed]
- Kriegel, C.; Festag, M.; Kishore, R.S.K.; Roethlisberger, D.; Schmitt, G. Pediatric safety of polysorbates in drug formulations. Children 2020, 7, 1. [Google Scholar] [CrossRef]
- Maghrebi, S.; Prestidge, C.A.; Joyce, P. An update on polymer-lipid hybrid systems for improving oral drug delivery. Expert Opin. Drug Deliv. 2019, 16, 507–524. [Google Scholar] [CrossRef]
- Gelderblom, H.; Verweij, J.; Nooter, K.; Sparreboom, A. Cremophor EL: The drawbacks and advantages of vehicle selection for drug formulation. Eur. J. Cancer 2001, 37, 1590–1598. [Google Scholar] [CrossRef]
- Darkoh, C.; Brown, E.L.; Kaplan, H.B.; DuPont, H.L. Bile salt inhibition of host cell damage by Clostridium difficile toxins. PLoS ONE 2013, 8, e79631. [Google Scholar] [CrossRef]
- Buckley, L.A.; Salunke, S.; Thompson, K.; Baer, G.; Fegley, D.; Turner, M.A. Challenges and strategies to facilitate formulation development of pediatric drug products: Safety qualification of excipients. Int. J. Pharm. 2018, 536, 563–569. [Google Scholar] [CrossRef]
- Montharu, J.; Le Guellec, S.; Kittel, B.; Rabemampianina, Y.; Guillemain, J.; Gauthier, F.; Diot, P.; de Monte, M. Evaluation of lung tolerance of ethanol, propylene glycol, and sorbitan monooleate as solvents in medical aerosols. J. Aerosol Med. Pulm. Drug Deliv. 2010, 23, 41–46. [Google Scholar] [CrossRef]
- Joyce, P.; Dening, T.J.; Meola, T.R.; Schultz, H.B.; Holm, R.; Thomas, N.; Prestidge, C.A. Solidification to improve the biopharmaceutical performance of SEDDS: Opportunities and challenges. Adv. Drug Deliv. Rev. 2019, 142, 102–117. [Google Scholar] [CrossRef]
- Kipp, J.E. The role of solid nanoparticle technology in the parenteral delivery of poorly water-soluble drugs. Int. J. Pharm. 2004, 284, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Rai, S.; Acharya-Siwakoti, E.; Kafle, A.; Devkota, H.P.; Bhattarai, A. Plant-derived saponins: A review of their surfactant properties and applications. Sci 2021, 3, 44. [Google Scholar] [CrossRef]
- Reichert, C.L.; Salminen, H.; Weiss, J. Quillaja saponin characteristics and functional properties. Annu. Rev. Food Sci. Technol. 2019, 10, 43–73. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Jiang, W.; Chen, Z.; Huang, Y.; Mao, J.; Zheng, W.; Hu, Y.; Shi, J. Advance in peptide-based drug development: Delivery platforms, therapeutics and vaccines. Signal Transduct. Target. Ther. 2025, 10, 74. [Google Scholar] [CrossRef]
- Gan, Y.; Baak, J.P.A.; Chen, T.; Ye, H.; Liao, W.; Lv, H.; Wen, C.; Zheng, S. Supersaturation and precipitation applicated in drug delivery systems: Development strategies and evaluation approaches. Molecules 2023, 28, 2212. [Google Scholar] [CrossRef]
- Chen, M.-L. Lipid excipients and delivery systems for pharmaceutical development: A regulatory perspective. Adv. Drug Deliv. Rev. 2008, 60, 768–777. [Google Scholar] [CrossRef]
- Yang, S.; Hu, X.; Zhu, J.; Zheng, B.; Bi, W.; Wang, X.; Wu, J.; Mi, Z.; Wu, Y. Aspects and implementation of pharmaceutical quality by design from conceptual frameworks to industrial applications. Pharmaceutics 2025, 17, 623. [Google Scholar] [CrossRef]
- Dangeti, A.; Bynagari, D.G.; Vydani, K. Revolutionizing drug formulation: Harnessing artificial intelligence and machine learning for enhanced stability, formulation optimization, and accelerated development. Int. J. Pharm. Sci. Med. 2023, 8, 18–29. [Google Scholar] [CrossRef]
- Rezaeizadeh, M.; Razavi, S.M.; Muzzio, F.J. Current state of machine learning implementation in pharmaceutical process modeling for oral solid dosage forms. Int. J. Pharm. 2026, 689, 126479. [Google Scholar] [CrossRef]
- Fonteyne, M.; Soares, S.; Vercruysse, J.; Peeters, E.; Burggraeve, A.; Vervaet, C.; Remon, J.P.; Sandler, N.; De Beer, T. Prediction of quality attributes of continuously produced granules using complementary PAT tools. Eur. J. Pharm. Biopharm. 2012, 82, 429–436. [Google Scholar] [CrossRef]
- Atre, P.; Rizvi, S.A.A. Advancements in pharmaceutical lyophilization: Integrating QbD, AI, and novel formulation strategies. Biologics 2025, 5, 35. [Google Scholar] [CrossRef]
- Ortega-Zúñiga, C.A.; Román-Ospino, A.D.; Gupta, S.; Omar, T.; Baranwal, Y.; Sanchez-Paternina, A.; Zhou, Q.; Jing, J.; Muzzio, F.J. Real-time monitoring of small changes in powder blends and ejected tablets in a low-dose formulation with 1% w/w of active pharmaceutical ingredient using Raman and near-infrared spatially resolved spectroscopy within a tablet press. Int. J. Pharm. 2025, 625, 125165. [Google Scholar] [CrossRef]
- Hartmanshenn, C.; Scherholz, M.; Androulakis, I.P. Physiologically-based pharmacokinetic models: Approaches for enabling personalized medicine. J. Pharmacokinet. Pharmacodyn. 2016, 43, 481–504. [Google Scholar] [CrossRef]
- Dabke, A.; Ghosh, S.; Dabke, P.; Sawant, K.; Khopade, A. Revisiting the in-vitro and in-vivo considerations for in-silico modelling of complex injectable drug products. J. Control. Release 2023, 360, 185–211. [Google Scholar] [CrossRef]
- Zheng, R.; Yu, C.; Yao, D.; Cai, M.; Zhang, L.; Ye, F.; Huang, X. Engineering stimuli-responsive materials for precision medicine. Small 2025, 21, 2406439. [Google Scholar] [CrossRef]


| Surfactant | HLB Value | Physicochemical Properties | Regulatory Classification |
|---|---|---|---|
| Polysorbate 80 (Tween 80) | 15.0 | Non-ionic, high solubility in water, emulsifier | GRAS, USP/NF listed, widely accepted in oral/parenteral |
| Sodium Lauryl Sulfate (SLS) | 40.0 | Anionic, strong foaming agent, detergent properties | FDA-approved within limits, used in oral/dermal |
| Poloxamer 188 | 29.0 | Non-ionic block copolymer, thermoresponsive | GRAS, used in oral and injectable formulations |
| Poloxamer 407 | 22.0 | Thermoreversible gelation, non-ionic surfactant | GRAS, used in gels and ophthalmics |
| Cremophor EL | 14.0 | Non-ionic, derived from castor oil, solubilizer | FDA inactive ingredients list, parenteral use caution |
| PEG 400 | 11.5 | Low-molecular PEG, solvent and solubilizer | GRAS, oral and parenteral solvent |
| Span 20 | 8.6 | Lipophilic surfactant, emulsifier | FDA listed, cosmetic and pharma |
| Span 60 | 4.7 | Waxy emulsifier, low solubility | Generally recognized as safe |
| Span 80 | 4.3 | Lipophilic, emulsifier for oils | GRAS, widely used emulsifier |
| Lecithin | 7.0 | Phospholipid, natural emulsifier | Natural origin, safe for oral use |
| Brij 35 | 16.9 | Polyoxyethylene ether, solubilizer | Pharmaceutical excipient, widely accepted |
| Brij 58 | 15.7 | Polyoxyethylene ether, mild surfactant | Safe non-ionic for topical and oral use |
| Pluronic F68 | 29.0 | Thermoresponsive, low toxicity | Used in IV and oral formulations |
| Pluronic F127 | 22.0 | Micelle forming, thermoresponsive | Pharmaceutical grade, injectable |
| Polysorbate 20 (Tween 20) | 16.7 | Non-ionic, emulsifier, used in food and pharma | GRAS, oral and topical |
| Polysorbate 60 (Tween 60) | 14.9 | Emulsifier, stabilizer | GRAS, used in foods and drugs |
| Sodium deoxycholate | 18.0 | Bile salt, solubilizing agent | FDA approved bile salt |
| Sodium taurocholate | 38.0 | Bile salt, stabilizer | Oral formulation enhancer, FDA listed |
| Sodium cholate | 18.0 | Bile salt, enhances solubilization | Oral solubilizer, pharmacopoeial grade |
| Sodium glycocholate | 23.1 | Bile salt, amphipathic | Generally safe, bile origin |
| Lauryl glucoside | 13.4 | Non-ionic, biodegradable | Used in natural cosmetics |
| Caprylocaproyl polyoxylglycerides | 12.0 | Medium-chain PEG glyceride | Pharmacopoeial excipient, GRAS |
| PEG-6 caprylic/capric glycerides | 12.0 | Low toxicity, used in lipid-based formulations | GRAS, oral formulations |
| Myristyl lactate | 7.5 | Ester of lactic acid, skin conditioning | Cosmetic and topical approved |
| PEG-40 hydrogenated castor oil | 14.0 | Castor oil derivative, emulsifier | GRAS, oral and parenteral |
| PEG-100 stearate | 18.8 | Stearic acid ester, solubilizer | GRAS, safe emulsifier |
| PEG-32 stearate | 15.2 | Stearic acid PEG ester, co-emulsifier | Pharma and cosmetic use |
| PEG-20 methyl glucose sesquistearate | 14.9 | Non-ionic emulsifier, skin compatible | Low toxicity, GRAS listed |
| PEG-60 almond glycerides | 11.5 | Emollient, mild solubilizer | Mild, cosmetic and pharma |
| PEG-75 lanolin | 10.5 | Lanolin derivative, solubilizer | Widely accepted cosmetic emulsifier |
| Cocamidopropyl betaine | 10.0 | Zwitterionic, gentle on skin | GRAS, topical use |
| Cocamide DEA (Diethanolamine) | 11.0 | Coconut oil-derived, foam booster | FDA approved topical use |
| Oleth-10 | 12.5 | Ether-based, mild surfactant | Used in pharma and cosmetics |
| Oleth-20 | 15.0 | Polyethylene glycol ether, solubilizer | Listed as inactive ingredient |
| Decyl glucoside | 13.6 | Sugar-based, mild non-ionic surfactant | Approved for dermal use |
| Lauryl glucoside | 13.4 | Glucoside-based, non-ionic, low irritation | GRAS, used in baby products |
| Caprylyl glycol | 11.5 | Multifunctional solubilizer | Safe in pharma and dermal |
| Glyceryl oleate | 4.3 | Non-ionic emulsifier | Cosmetic emulsifier |
| Sorbitan trioleate | 1.8 | Oil-soluble emulsifier | GRAS emulsifier |
| Sorbitan monopalmitate | 6.7 | Waxy, low HLB emulsifier | Food additive and emulsifier |
| Ethoxydiglycol | 9.8 | Hydrophilic solvent | Cosmetic excipient |
| Sucrose laurate | 21.0 | Sucrose ester, non-ionic | GRAS surfactant |
| Sodium lauroyl lactylate | 7.2 | Mild surfactant, food grade | Used in food and pharma |
| PEG-12 dimethicone | 11.0 | Silicone-based surfactant | GRAS listed |
| PEG-7 glyceryl cocoate | 14.0 | Coconut-derived solubilizer | Cosmetic solvent/emulsifier |
| Capmul MCM | 14.0 | Medium chain mono/diglycerides | Topical and oral accepted |
| Labrasol | 9.0 | PEG caprylic/capric glyceride | Oral lipid carrier |
| Labrafil M 2125 CS | 4.0 | Lipophilic PEG derivative | Oral bioavailability enhancer |
| Transcutol P | 4.5 | Solvent, enhancer | GRAS listed |
| Technique | Particle Size Range | Thermodynamic Stability | Scalability | Major Limitations | Target API Type | References |
|---|---|---|---|---|---|---|
| Micronization (Jet Milling) | 1–10 µm | Low | High | Poor flow, electrostatic charge | Hydrophobic APIs | [60,61,62] |
| Nanocrystallization (Top-down) | <1 µm | Moderate | Moderate | Ostwald ripening, sedimentation | Poorly soluble APIs | [64,65,66] |
| Nanocrystallization (Bottom-up) | <1 µm | Moderate | Moderate | Reproducibility, scale-up | BCS Class II APIs | [64,65] |
| Spray-Drying | 1–5 µm | Moderate | High | Thermal stress, solvent residue | Thermostable APIs | [68] |
| Freeze-Drying (Lyophilization) | 1–10 µm | High | Low | High cost, long duration | Thermolabile APIs | [68,69] |
| Amorphous Solid Dispersion (Hot melt) | Amorphous | Moderate | Moderate | Thermal degradation | BCS II/IV | [70] |
| Amorphous Solid Dispersion (Spray dried) | Amorphous | Moderate | High | Recrystallization risk | BCS II drugs | [70,71] |
| Antisolvent Precipitation | 50–500 nm | Low | Moderate | Solvent removal | Hydrophobic APIs | [64] |
| Supercritical Fluid Processing | 0.1–5 µm | High | Low | Expensive equipment | Heat-sensitive APIs | [60] |
| Fluidized Bed Coating | 1–100 µm | High | High | Layer uniformity | Taste masking, controlled release | [63] |
| Spray Chilling/Cooling | 10–100 µm | High | High | Particle aggregation | Lipid-based APIs | [60] |
| Cryomilling | 1–5 µm | Moderate | Low | Cold handling required | Heat-sensitive APIs | [67] |
| Hybrid Platform | Key API Examples | Surfactant Types | Engineering Technique | Performance Advantage | References |
|---|---|---|---|---|---|
| Surfactant-assisted wet granulation | Telmisartan, Ritonavir, Naproxen | Polysorbate 80, PEG 400, Sodium lauryl sulfate (SLS) | High-shear wet granulation using surfactant-containing binder solution | Improves powder wettability and granule compressibility; enhances drug release and disintegration; enables low surfactant dose | [95,96,97,98] |
| Surfactant-coated nanocrystals | Curcumin, Silymarin, PLGA-loaded Dexamethasone | Poloxamer 407, Sodium deoxycholate, TPGS | Wet milling or high-pressure homogenization followed by surfactant adsorption | Enhances redispersibility, colloidal stability, and membrane interaction; suitable for mucosal delivery and pediatric forms | [99,100] |
| Solid dispersions with surfactants | Itraconazole, Ritonavir, Tadalafil | Cremophor RH40, Solutol HS15, Vitamin E TPGS | Hot-melt extrusion or spray-drying to form amorphous ternary systems | Maintains supersaturation; reduces recrystallization; improves physical stability and bioavailability in fed/fasted states | [103,104,105] |
| Solidified SEDDS | Ritonavir, Cyclosporine, Tacrolimus | Poloxamer 188, Tween 80, Labrasol | Spray-drying of SEDDS onto porous carriers like Neusilin or Aerosil | Improves storage stability and redispersibility; enhances lymphatic transport; suitable for high log P drugs | [106,107,108,109,110] |
| Lipid–surfactant hybrid systems (NLCs) | Quercetin, Resveratrol, Paclitaxel | Polysorbate 80, TPGS, Cremophor EL | Lipid phase preparation followed by ultrasonication and surfactant coating | Enhances permeability and tissue targeting via surfactant-mediated surface modulation; allows dual drug loading | [111,112,113,114] |
| Spray freeze-drying with surfactants | Voriconazole, Salmon Calcitonin, Influenza vaccines | SLS, Lecithin, Tween 20 | Atomization into liquid nitrogen and lyophilization for porous hybrid microparticles | Enables pulmonary or mucosal delivery of fragile biomolecules; offers rapid dissolution and thermal protection | [115,116,117,118] |
| Hybrid System | API Load Suitability | Manufacturing Feasibility | Stability Challenges | Safety Considerations |
|---|---|---|---|---|
| Micronization | Moderate | High (conventional equipment) | Moisture sensitivity | Low |
| Nanocrystals | High | Medium (coating required) | Aggregation, Ostwald ripening | Moderate (surfactant exposure) |
| Solid Dispersions | Low–Moderate | High (HME/spray-drying) | Recrystallization, surfactant migration | Low–Moderate |
| SEDDS Hybrids | High | Medium (adsorption/spray-drying) | Reconstitution failure, phase separation | Moderate (surface migration) |
| NLC Hybrids | Moderate | Medium (emulsification) | Surfactant desorption, polymorphism | Moderate–High (immunogenicity) |
| Spray Freeze-Drying | Low | Low (cryogenic process) | Moisture uptake, oxidative degradation | Low–Moderate (route dependent) |
| Hybrid Type | Drug Release Mechanism | Mechanistic Highlights |
|---|---|---|
| Surfactant-coated Nanocrystals | Surface erosion and diffusion | Surfactants stabilize nanocrystals and enhance wetting; enables rapid dissolution. |
| Amorphous Solid Dispersions with Surfactants | Supersaturation followed by precipitation inhibition | Surfactants maintain supersaturation and prevent recrystallization. |
| SEDDS Hybrids | Emulsification followed by lipid digestion | Surfactants aid in droplet formation; supports lymphatic uptake. |
| NLC Hybrids | Diffusion through lipid matrix | Lipid matrix controls release; surfactants enhance permeability and mucosal uptake. |
| Spray Freeze-Dried Hybrids | Burst release followed by diffusion | Highly porous structure enables fast disintegration and immediate drug release. |
| Aspect | Key Considerations | Examples/Tools | References |
|---|---|---|---|
| Scalability | Process must tolerate industrial-scale loads, maintain particle size and homogeneity. | Wet granulation, spray-drying, hot-melt extrusion (HME); scalability trials. | [137] |
| Critical Quality Attributes (CQAs) | Drug content uniformity, particle size distribution, surfactant level, polymorphic form. | Design of Experiments (DoE), QbD risk assessment matrices. | [138,139] |
| Process Monitoring | Real-time monitoring of particle morphology, surfactant distribution, crystallinity. | PAT tools: in-line NIR, Raman spectroscopy, focused beam reflectance measurement. | [140] |
| Regulatory Approval | Use of excipients with prior precedence; evaluation of surfactant toxicology and stability. | FDA IID surfactant listing, GRAS status, excipient compatibility studies. | [141,142] |
| Toxicological Limits | Surfactant use limits in chronic or pediatric settings; GI and hypersensitivity issues. | Risk assessments, NOAEL (No-Observed-Adverse-Effect Level) studies, pediatric extrapolation guidelines. | [142] |
| Bioequivalence Challenges | Surfactant-lipid hybrids may alter absorption; nonlinear kinetics; lymphatic uptake. | Clinical endpoint studies, biowaivers, dissolution profile matching. | [143] |
| Manufacturing Robustness | Batch-to-batch reproducibility; control of CPPs such as drying temperature, extrusion speed. | HME barrel temperature monitoring, fluid bed granulation sensors. | [137,139] |
| Formulation Stability | Hygroscopicity, surfactant migration, recrystallization on storage. | Stability chambers, PXRD (Powder X-Ray Diffraction)/DSC (Differential Scanning Calorimetry) analysis, ICH (International Council for Harmonisation) Q1A(R2) compliance. | [140,141] |
| Surfactant (Type) | Key Safety Concerns | Mechanism of Concern | Populations at Risk | Regulatory Remarks | References |
|---|---|---|---|---|---|
| Cremophor EL (non-ionic) | Hypersensitivity, nephrotoxicity | Histamine release, renal accumulation | General population, especially IV use | Phased out in some injectable products, replaced by safer excipients | [144] |
| Vitamin E TPGS (amphiphilic) | Generally safe, but accumulation risk at high dose | Biliary clearance saturation | Pediatric, hepatic impairment | Used widely in oral formulations, QbD-preferred | [144,148] |
| Poloxamers (188, 407) (non-ionic) | GI discomfort, systemic absorption in neonates | Altered membrane fluidity | Neonates, elderly | Listed in FDA IID; max allowable concentrations vary | [144,147] |
| Sodium taurocholate (bile salt) | Epithelial erosion, tight junction damage | Permeation enhancer disrupting barrier integrity | Chronic use, GI-compromised | Thresholds undefined in humans, known toxic in animals | [145] |
| Span 80 (Sorbitan monooleate) | Hepatic accumulation in long-term exposure | Lipid accumulation | Chronic oral administration | Low-dose recommended in sustained-release platforms | [147] |
| Polysorbate 80 (non-ionic) | Oxidative degradation, hypersensitivity | Auto-oxidation, histamine release | Parenteral, elderly | GRAS by FDA, but global variability in limits (EU, JP, CN) | [144,150] |
| Lecithin (natural phospholipid) | Variable purity, allergenicity | Source-dependent variability | Immunocompromised, allergic patients | Natural, but batch variability limits scalability | [151] |
| Saponins (natural) | Hemolysis, immune stimulation | Membrane disruption, adjuvant effect | Not safe for IV, caution in oral | Still under exploration, no global monograph yet | [152] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Kim, K.-S.; Cho, H.J.; Din, F.U.; Cho, J.H.; Choi, H.-G. Surfactant–Particle Engineering Hybrids: Emerging Strategies for Enhancing Solubility and Oral Bioavailability of Poorly Water-Soluble Drugs. Pharmaceutics 2026, 18, 37. https://doi.org/10.3390/pharmaceutics18010037
Kim K-S, Cho HJ, Din FU, Cho JH, Choi H-G. Surfactant–Particle Engineering Hybrids: Emerging Strategies for Enhancing Solubility and Oral Bioavailability of Poorly Water-Soluble Drugs. Pharmaceutics. 2026; 18(1):37. https://doi.org/10.3390/pharmaceutics18010037
Chicago/Turabian StyleKim, Kyeong-Soo, Hyuk Jun Cho, Fakhar Ud Din, Jung Hyun Cho, and Han-Gon Choi. 2026. "Surfactant–Particle Engineering Hybrids: Emerging Strategies for Enhancing Solubility and Oral Bioavailability of Poorly Water-Soluble Drugs" Pharmaceutics 18, no. 1: 37. https://doi.org/10.3390/pharmaceutics18010037
APA StyleKim, K.-S., Cho, H. J., Din, F. U., Cho, J. H., & Choi, H.-G. (2026). Surfactant–Particle Engineering Hybrids: Emerging Strategies for Enhancing Solubility and Oral Bioavailability of Poorly Water-Soluble Drugs. Pharmaceutics, 18(1), 37. https://doi.org/10.3390/pharmaceutics18010037






