Melittin-Based Nanoparticles for Cancer Therapy: Mechanisms, Applications, and Future Perspectives
Abstract
1. Introduction
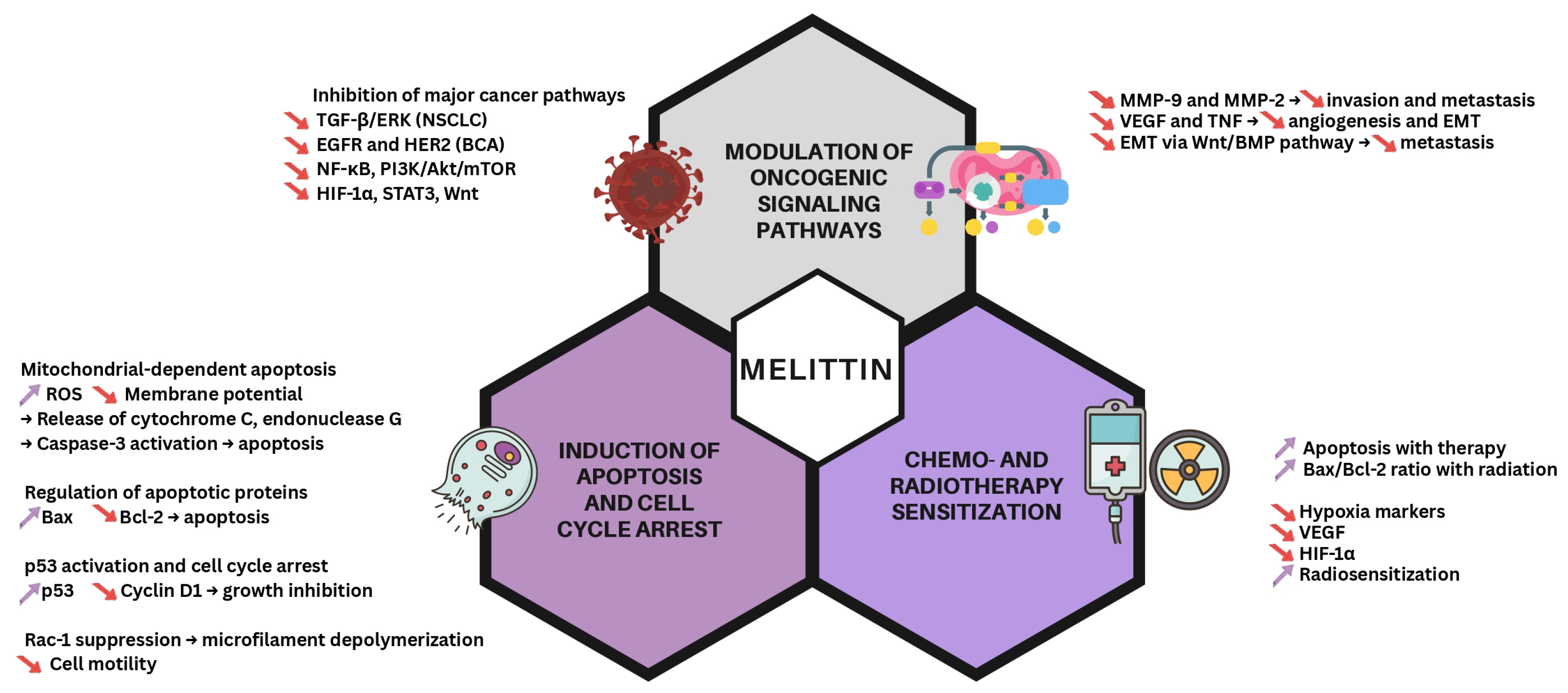
2. Mechanisms of Action of Melittin in Cancer Therapy
2.1. Its Effect on the Cell Cycle
2.2. Modulating Signaling Cascades
2.3. Chemotherapy and Radiotherapy Sensitization
3. Melittin-Based Nanoparticles: From the Shelf to the Bedside
3.1. Delivery Systems
3.2. Strategies for Enhanced Targeting and Delivery
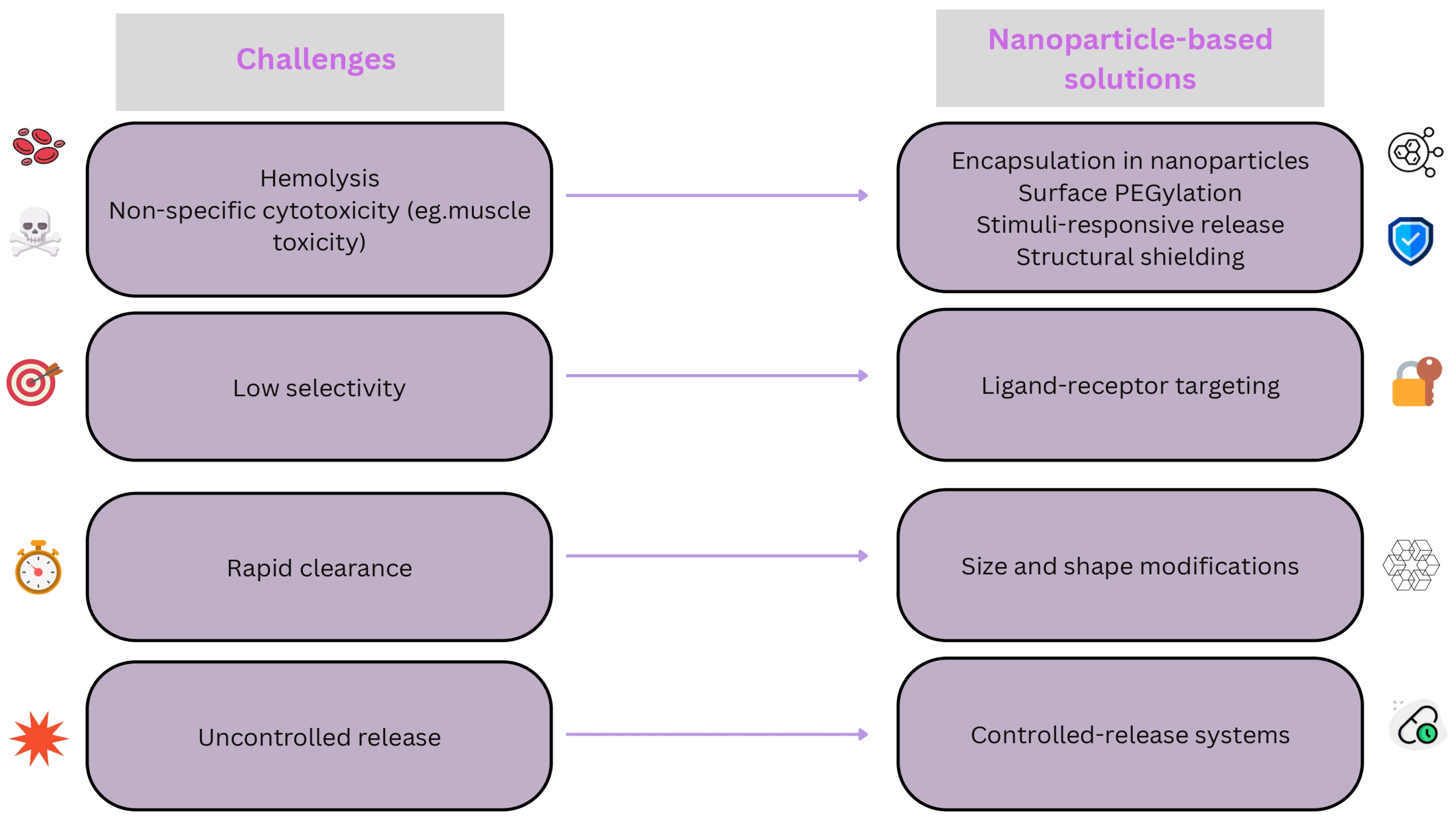
3.3. Challenges in Melittin Nanomedicine
4. Applications of Melittin-Based Nanoparticles in Cancer Therapy
4.1. A Summary of the Studies Across Cancer Types
4.1.1. Breast Cancer
4.1.2. Melanoma
4.1.3. Hepatocellular Carcinoma (HCC)
4.1.4. Ovarian Cancer
4.1.5. Colorectal Cancer
4.1.6. Prostate Cancer
4.1.7. Pancreatic Cancer
4.1.8. Lung Cancer
4.1.9. Glioblastoma
4.1.10. Osteosarcoma
4.1.11. Leukemia and Lymphoma
4.1.12. Gastric Cancer
4.1.13. Cervical Cancer
4.1.14. Bladder Cancer
4.1.15. Thyroid Cancer
4.1.16. Head and Neck Cancer
4.1.17. Esophageal Cancer
4.1.18. Endometrial Cancer
4.1.19. Other Cancers
4.2. Its Role in Overcoming Drug Resistance
4.3. Combination with Other Therapies
4.3.1. Combination with Chemotherapy
4.3.2. Combination with Immunotherapy
4.3.3. Combination with Photothermal Therapy
4.3.4. Combination with Radiotherapy
5. Future Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Akt | Protein kinase B |
| BBB | Blood–brain barrier |
| BMP | Bone morphogenetic protein |
| CRC | Colorectal cancer |
| DR | Death receptor |
| EGF | Epidermal growth factor |
| EGFR | Epidermal growth factor receptor |
| EMT | Epithelial–mesenchymal transition |
| ESCC | Esophageal squamous cell carcinoma |
| GMP | Good Manufacturing Practice |
| GSH | Glutathione |
| HA | Hyaluronic acid |
| HCC | Hepatocellular carcinoma |
| HER2 | Human epidermal growth factor receptor 2 |
| HIF-1α | Hypoxia-inducible factor 1-alpha |
| HNSCC | Head and neck squamous cell carcinoma |
| IC50 | Half-maximal inhibitory concentration |
| ICD | Immunogenic cell death |
| JAK2 | Janus kinase 2 |
| MAPK | Mitogen-activated protein kinase |
| MDR | Multidrug resistance |
| MnO2 | Manganese dioxide |
| MMP | Matrix metalloproteinase |
| mTOR | Mammalian target of rapamycin |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| NF-κB | Nuclear factor kappa B |
| NSCLC | Non-small-cell lung cancer |
| PEG | Polyethylene glycol |
| PI3K | Phosphoinositide 3-kinase |
| PLA-HA | Poly(lactic acid)–hyaluronic acid |
| P-gp | P-glycoprotein |
| PSMA | Prostate-specific membrane antigen |
| PTT | Photothermal therapy |
| RGD | Arginine–glycine–aspartic acid |
| ROS | Reactive oxygen species |
| SAMN | Silica–alginate–melittin nanoconjugate |
| siRNA | Small interfering RNA |
| SREBP1 | Sterol regulatory element-binding protein 1 |
| STAT3 | Signal transducer and activator of transcription 3 |
| TAM | Tumor-associated macrophage |
| TME | Tumor microenvironment |
| TNBC | Triple-negative breast cancer |
| TNF | Tumor necrosis factor |
| TRAIL | Tumor necrosis factor-related apoptosis-inducing ligand |
| TKIs | Tyrosine kinase inhibitors |
| VEGF | Vascular endothelial growth factor |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Anand, U.; Dey, A.; Chandel, A.K.S.; Sanyal, R.; Mishra, A.; Pandey, D.K.; De Falco, V.; Upadhyay, A.; Kandimalla, R.; Chaudhary, A.; et al. Cancer chemotherapy and beyond: Current status, drug candidates, associated risks and progress in targeted therapeutics. Genes Dis. 2023, 10, 1367–1401. [Google Scholar] [PubMed]
- Bhutia, S.K.; Maiti, T.K. Targeting tumors with peptides from natural sources. Trends Biotechnol. 2008, 26, 210–217. [Google Scholar] [CrossRef]
- Chidambaram, M.; Manavalan, R.; Kathiresan, K. Nanotherapeutics to overcome conventional cancer chemotherapy limitations. J. Pharm. Pharm. Sci. 2011, 14, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Yaacoub, C.; Wehbe, R.; Roufayel, R.; Fajloun, Z.; Coutard, B. Bee Venom and Its Two Main Components-Melittin and Phospholipase A2-As Promising Antiviral Drug Candidates. Pathogens 2023, 12, 1354. [Google Scholar] [CrossRef]
- Sheng, H.; Jianhua, W.; Xiaozhong, W.; Chenghong, L.I. Melittin: A Key Composition of Honey Bee Venom with Diverse Pharmaceutical Function. In Proceedings of the 2016 International Conference on Biological Engineering and Pharmacy (BEP 2016), Shanghai, China, 9–11 December 2016; Atlantis Press: Dordrecht, The Netherlands, 2016; pp. 193–197. [Google Scholar]
- Gajski, G.; Garaj-Vrhovac, V. Melittin: A lytic peptide with anticancer properties. Environ. Toxicol. Pharmacol. 2013, 36, 697–705. [Google Scholar] [CrossRef]
- Li, X.; Li, Z.; Meng, Y.Q.; Qiao, H.; Zhai, K.R.; Li, Z.Q.; Wei, S.L.; Li, B. Melittin kills A549 cells by targeting mitochondria and blocking mitophagy flux. Redox Rep. 2023, 28, 2284517. [Google Scholar] [CrossRef]
- Rady, I.; Siddiqui, I.A.; Rady, M.; Mukhtar, H. Melittin, a major peptide component of bee venom, and its conjugates in cancer therapy. Cancer Lett. 2017, 402, 16–31. [Google Scholar] [CrossRef]
- Wang, A.; Zheng, Y.; Zhu, W.; Yang, L.; Yang, Y.; Peng, J. Melittin-Based Nano-Delivery Systems for Cancer Therapy. Biomolecules 2022, 12, 118. [Google Scholar] [CrossRef]
- Pandey, P.; Khan, F.; Khan, M.A.; Kumar, R.; Upadhyay, T.K. An Updated Review Summarizing the Anticancer Efficacy of Melittin from Bee Venom in Several Models of Human Cancers. Nutrients 2023, 15, 3111. [Google Scholar] [CrossRef]
- Kong, G.M.; Tao, W.H.; Diao, Y.L.; Fang, P.H.; Wang, J.J.; Bo, P.; Qian, F. Melittin induces human gastric cancer cell apoptosis via activation of mitochondrial pathway. World J. Gastroenterol. 2016, 22, 3186–3195. [Google Scholar] [CrossRef] [PubMed]
- Zarrinnahad, H.; Mahmoodzadeh, A.; Hamidi, M.P.; Mahdavi, M.; Moradi, A.; Bagheri, K.P.; Shahbazzadeh, D. Apoptotic Effect of Melittin Purified from Iranian Honey Bee Venom on Human Cervical Cancer HeLa Cell Line. Int. J. Pept. Res. Ther. 2018, 24, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Lee, H.W.; Park, H.W.; Lee, H.W.; Chun, K.H. Bee venom inhibits the proliferation and migration of cervical-cancer cells in an HPV E6/E7-dependent manner. BMB Rep. 2020, 53, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Nikodijević, D.D.; Milutinović, M.G.; Cvetković, D.M.; Ćupurdija, M.Đ.; Jovanović, M.M.; Mrkić, I.V.; Jankulović-Gavrović, M.Đ.; Marković, S.D. Impact of bee venom and melittin on apoptosis and biotransformation in colorectal carcinoma cell lines. Toxin Rev. 2021, 40, 1272–1279. [Google Scholar] [CrossRef]
- Zhou, J.; Yitao, Q.; Qingyun, D.; Liming, W.; Xia, D.; Yi, L.; Sun, L. Cytotoxicity of melittin and apamin in human hepatic L02 and HepG2 cells in vitro. Toxin Rev. 2013, 32, 60–67. [Google Scholar] [CrossRef]
- Yu, R.; Wang, M.; Wang, M.; Han, L. Melittin suppresses growth and induces apoptosis of non-small-cell lung cancer cells via down-regulation of TGF-β-mediated ERK signal pathway. Braz. J. Med. Biol. Res. 2020, 54, e9017. [Google Scholar] [CrossRef]
- Duffy, C.; Sorolla, A.; Wang, E.; Golden, E.; Woodward, E.; Davern, K.; Ho, D.; Johnstone, E.; Pfleger, K.; Redfern, A.; et al. Honeybee venom and melittin suppress growth factor receptor activation in HER2-enriched and triple-negative breast cancer. NPJ Precis. Oncol. 2020, 4, 24. [Google Scholar] [CrossRef]
- Jeong, Y.J.; Choi, Y.; Shin, J.M.; Cho, H.J.; Kang, J.H.; Park, K.K.; Choe, J.Y.; Bae, Y.S.; Han, S.M.; Kim, C.H.; et al. Melittin suppresses EGF-induced cell motility and invasion by inhibiting PI3K/Akt/mTOR signaling pathway in breast cancer cells. Food Chem. Toxicol. 2014, 68, 218–225. [Google Scholar] [CrossRef]
- Aufschnaiter, A.; Kohler, V.; Khalifa, S.; Abd El-Wahed, A.; Du, M.; El-Seedi, H.; Büttner, S. Apitoxin and Its Components against Cancer, Neurodegeneration and Rheumatoid Arthritis: Limitations and Possibilities. Toxins 2020, 12, 66. [Google Scholar] [CrossRef]
- Memariani, H.; Memariani, M.; Shahidi-Dadras, M.; Nasiri, S.; Akhavan, M.M.; Moravvej, H. Melittin: From honeybees to superbugs. Appl. Microbiol. Biotechnol. 2019, 103, 3265–3276. [Google Scholar] [CrossRef]
- Huang, J.Y.; Peng, S.F.; Chueh, F.S.; Chen, P.Y.; Huang, Y.P.; Huang, W.W.; Chung, J.G. Melittin suppresses epithelial-mesenchymal transition and metastasis in human gastric cancer AGS cells via regulating Wnt/BMP associated pathway. Biosci. Biotechnol. Biochem. 2021, 85, 2250–2262. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.-N.; Kim, S.-H.; Kim, H.J.; Jeong, Y.J.; Lee, K.C. In Vitro and In Vivo Investigation of the Radiation-Sensitizing Effects of Melittin in Breast Cancer Cells. Int. J. Pept. Res. Ther. 2021, 28, 8. [Google Scholar] [CrossRef]
- Yang, X.; Zhu, H.; Ge, Y.; Liu, J.; Cai, J.; Qin, Q.; Zhan, L.; Zhang, C.; Xu, L.; Liu, Z.; et al. Melittin enhances radiosensitivity of hypoxic head and neck squamous cell carcinoma by suppressing HIF-1α. Tumour Biol. 2014, 35, 10443–10448. [Google Scholar] [CrossRef]
- Yao, Y.; Zhou, Y.; Liu, L.; Xu, Y.; Chen, Q.; Wang, Y.; Wu, S.; Deng, Y.; Zhang, J.; Shao, A. Nanoparticle-Based Drug Delivery in Cancer Therapy and Its Role in Overcoming Drug Resistance. Front. Mol. Biosci. 2020, 7, 193. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ruan, S.; Wang, Z.; Feng, N.; Zhang, Y. Hyaluronic Acid Coating Reduces the Leakage of Melittin Encapsulated in Liposomes and Increases Targeted Delivery to Melanoma Cells. Pharmaceutics 2021, 13, 1235. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.; Sylvestre, M.; Song, K.; Pun, S.H. Development of D-melittin polymeric nanoparticles for anti-cancer treatment. Biomaterials 2021, 277, 121076. [Google Scholar] [CrossRef] [PubMed]
- Ye, R.; Zheng, Y.; Chen, Y.; Wei, X.; Shi, S.; Chen, Y.; Zhu, W.; Wang, A.; Yang, L.; Xu, Y.; et al. Stable Loading and Delivery of Melittin with Lipid-Coated Polymeric Nanoparticles for Effective Tumor Therapy with Negligible Systemic Toxicity. ACS Appl. Mater. Interfaces 2021, 13, 55902–55912. [Google Scholar] [CrossRef]
- Amreddy, N.; Babu, A.; Munshi, A.; Ramesh, R. Dendrimers as Drug Carriers for Cancer Therapy. In Nanopharmaceuticals: Principles and Applications; Yata, V.K., Ranjan, S., Dasgupta, N., Lichtfouse, E., Eds.; Springer International Publishing: Cham, Switzerland, 2020; Volume 3, pp. 245–269. [Google Scholar]
- Dey, A.D.; Bigham, A.; Esmaeili, Y.; Ashrafizadeh, M.; Moghaddam, F.D.; Tan, S.C.; Yousefiasl, S.; Sharma, S.; Maleki, A.; Rabiee, N.; et al. Dendrimers as nanoscale vectors: Unlocking the bars of cancer therapy. Semin. Cancer Biol. 2022, 86, 396–419. [Google Scholar]
- Bober, Z.; Bartusik-Aebisher, D.; Aebisher, D. Application of Dendrimers in Anticancer Diagnostics and Therapy. Molecules 2022, 27, 3237. [Google Scholar] [CrossRef]
- Crintea, A.; Motofelea, A.C.; Șovrea, A.S.; Constantin, A.M.; Crivii, C.B.; Carpa, R.; Duțu, A.G. Dendrimers: Advancements and Potential Applications in Cancer Diagnosis and Treatment-An Overview. Pharmaceutics 2023, 15, 1406. [Google Scholar]
- Raveendran, R.; Chen, F.; Kent, B.; Stenzel, M.H. Estrone-Decorated Polyion Complex Micelles for Targeted Melittin Delivery to Hormone-Responsive Breast Cancer Cells. Biomacromolecules 2020, 21, 1222–1233. [Google Scholar] [CrossRef] [PubMed]
- Bayat, P.; Abnous, K.; Balarastaghi, S.; Taghdisi, S.M.; Saeedi, M.; Yazdian-Robati, R.; Mahmoudi, M. Aptamer AS1411-functionalized gold nanoparticle-melittin complex for targeting MCF-7 breast cancer cell line. Nanomed. J. 2022, 9, 164–169. [Google Scholar]
- Castillo, R.R.; Lozano, D.; Vallet-Regí, M. Mesoporous Silica Nanoparticles as Carriers for Therapeutic Biomolecules. Pharmaceutics 2020, 12, 432. [Google Scholar] [CrossRef] [PubMed]
- Soman, N.R.; Baldwin, S.L.; Hu, G.; Marsh, J.N.; Lanza, G.M.; Heuser, J.E.; Arbeit, J.M.; Wickline, S.A.; Schlesinger, P.H. Molecularly targeted nanocarriers deliver the cytolytic peptide melittin specifically to tumor cells in mice, reducing tumor growth. J. Clin. Investig. 2009, 119, 2830–2842. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Zhang, T.; Qin, S.; Huang, Z.; Zhou, L.; Shi, J.; Nice, E.C.; Xie, N.; Huang, C.; Shen, Z. Enhancing the therapeutic efficacy of nanoparticles for cancer treatment using versatile targeted strategies. J. Hematol. Oncol. 2022, 15, 132. [Google Scholar]
- Tarokh, Z.; Naderi-Manesh, H.; Nazari, M. Towards prostate cancer gene therapy: Development of a chlorotoxin-targeted nanovector for toxic (melittin) gene delivery. Eur. J. Pharm. Sci. 2017, 99, 209–218. [Google Scholar] [CrossRef]
- He, Q.; Chen, J.; Yan, J.; Cai, S.; Xiong, H.; Liu, Y.; Peng, D.; Mo, M.; Liu, Z. Tumor microenvironment responsive drug delivery systems. Asian J. Pharm. Sci. 2020, 15, 416–448. [Google Scholar]
- Huang, S.; Gao, Y.; Ma, L.; Jia, B.; Zhao, W.; Yao, Y.; Li, W.; Lin, T.; Wang, R.; Song, J.; et al. Design of pH-responsive antimicrobial peptide melittin analog-camptothecin conjugates for tumor therapy. Asian J. Pharm. Sci. 2024, 19, 100890. [Google Scholar] [CrossRef]
- Bei, C.; Bindu, T.; Remant, K.C.; Peisheng, X. Dual secured nano-melittin for the safe and effective eradication of cancer cells. J. Mater. Chem. B 2015, 3, 25–29. [Google Scholar]
- Jallouk, A.P.; Palekar, R.U.; Marsh, J.N.; Pan, H.; Pham, C.T.; Schlesinger, P.H.; Wickline, S.A. Delivery of a Protease-Activated Cytolytic Peptide Prodrug by Perfluorocarbon Nanoparticles. Bioconjug. Chem. 2015, 26, 1640–1650. [Google Scholar] [CrossRef]
- Knop, K.; Hoogenboom, R.; Fischer, D.; Schubert, U.S. Poly(ethylene glycol) in drug delivery: Pros and cons as well as potential alternatives. Angew. Chem. Int. Ed. Engl. 2010, 49, 6288–6308. [Google Scholar] [CrossRef] [PubMed]
- Jo, H.; Jeoung, J.; Kim, W.; Jeoung, D. Regulating Immune Responses Induced by PEGylated Messenger RNA-Lipid Nanoparticle Vaccine. Vaccines 2024, 13, 14. [Google Scholar] [CrossRef]
- Xu, X.; Ho, W.; Zhang, X.; Bertrand, N.; Farokhzad, O. Cancer nanomedicine: From targeted delivery to combination therapy. Trends Mol. Med. 2015, 21, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Jia, S.; Yu, S.; Chen, Y.; Zhang, C.; Chen, H.; Dai, Y. Recent advances in melittin-based nanoparticles for antitumor treatment: From mechanisms to targeted delivery strategies. J. Nanobiotechnol. 2023, 21, 454. [Google Scholar] [CrossRef]
- Gajski, G.; Domijan, A.M.; Žegura, B.; Štern, A.; Gerić, M.; Novak Jovanović, I.; Vrhovac, I.; Madunić, J.; Breljak, D.; Filipič, M.; et al. Melittin induced cytogenetic damage, oxidative stress and changes in gene expression in human peripheral blood lymphocytes. Toxicon 2016, 110, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Blondelle, S.E.; Houghten, R.A. Probing the relationships between the structure and hemolytic activity of melittin with a complete set of leucine substitution analogs. Pept. Res. 1991, 4, 12–18. [Google Scholar]
- Mingomataj, E.C.; Bakiri, A.H. Episodic hemorrhage during honeybee venom anaphylaxis: Potential mechanisms. J. Investig. Allergol. Clin. Immunol. 2012, 22, 237–244. [Google Scholar]
- Zolfagharian, H.; Mohajeri, M.; Babaie, M. Honey Bee Venom (Apis mellifera) Contains Anticoagulation Factors and Increases the Blood-clotting Time. J. Pharmacopunct. 2015, 18, 7–11. [Google Scholar] [CrossRef]
- Jallouk, A.P.; Moley, K.H.; Omurtag, K.; Hu, G.; Lanza, G.M.; Wickline, S.A.; Hood, J.L. Nanoparticle incorporation of melittin reduces sperm and vaginal epithelium cytotoxicity. PLoS ONE 2014, 9, e95411. [Google Scholar] [CrossRef]
- Lv, Y.; Chen, X.; Chen, Z.; Shang, Z.; Li, Y.; Xu, W.; Mo, Y.; Wang, X.; Xu, D.; Li, S.; et al. Melittin Tryptophan Substitution with a Fluorescent Amino Acid Reveals the Structural Basis of Selective Antitumor Effect and Subcellular Localization in Tumor Cells. Toxins 2022, 14, 428. [Google Scholar] [CrossRef]
- Perekalin, D.S.; Novikov, V.V.; Pavlov, A.A.; Ivanov, I.A.; Anisimova, N.Y.; Kopylov, A.N.; Volkov, D.S.; Seregina, I.F.; Bolshov, M.A.; Kudinov, A.R. Selective ruthenium labeling of the tryptophan residue in the bee venom Peptide melittin. Chemistry 2015, 21, 4923–4925. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Valenta, D.T.; Altman, Y.; Harvey, S.; Xie, H.; Mitragotri, S.; Smith, J.W. Polymer particle shape independently influences binding and internalization by macrophages. J. Control. Release 2010, 147, 408–412. [Google Scholar] [CrossRef]
- Jia, F.; Chen, P.; Wang, D.; Sun, Y.; Ren, M.; Wang, Y.; Cao, X.; Zhang, L.; Fang, Y.; Tan, X.; et al. Bottlebrush Polymer-Conjugated Melittin Exhibits Enhanced Antitumor Activity and Better Safety Profile. ACS Appl. Mater. Interfaces 2021, 13, 42533–42542. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Wang, J.; Wang, H.; Tan, T.; Li, J.; Wang, Z.; Sun, K.; Li, Y.; Zhang, Z. Cell-penetrating peptide-based nanovehicles potentiate lymph metastasis targeting and deep penetration for anti-metastasis therapy. Theranostics 2018, 8, 3597–3610. [Google Scholar] [CrossRef]
- Qiao, H.; Mei, J.; Yuan, K.; Zhang, K.; Zhou, F.; Tang, T.; Zhao, J. Immune-regulating strategy against rheumatoid arthritis by inducing tolerogenic dendritic cells with modified zinc peroxide nanoparticles. J. Nanobiotechnol. 2022, 20, 323. [Google Scholar] [CrossRef]
- Yu, G.; Zhang, M.; Saha, M.L.; Mao, Z.; Chen, J.; Yao, Y.; Zhou, Z.; Liu, Y.; Gao, C.; Huang, F.; et al. Antitumor Activity of a Unique Polymer That Incorporates a Fluorescent Self-Assembled Metallacycle. J. Am. Chem. Soc. 2017, 139, 15940–15949. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Jin, H.; Qian, Y.; Qi, S.; Luo, H.; Luo, Q.; Zhang, Z. Hybrid melittin cytolytic Peptide-driven ultrasmall lipid nanoparticles block melanoma growth in vivo. ACS Nano 2013, 7, 5791–5800. [Google Scholar] [CrossRef]
- Shin, M.C.; Min, K.A.; Cheong, H.; Moon, C.; Huang, Y.; He, H.; Yang, V.C. Preparation and Characterization of Gelonin-Melittin Fusion Biotoxin for Synergistically Enhanced Anti-Tumor Activity. Pharm. Res. 2016, 33, 2218–2228. [Google Scholar] [CrossRef]
- Wang, D.; Hu, L.; Su, M.; Wang, J.; Xu, T. Preparation and functional characterization of human vascular endothelial growth factor-melittin fusion protein with analysis of the antitumor activity in vitro and in vivo. Int. J. Oncol. 2015, 47, 1160–1168. [Google Scholar] [CrossRef]
- Sun, D.; Sun, M.; Zhu, W.; Wang, Z.; Li, Y.; Ma, J. The anti-cancer potency and mechanism of a novel tumor-activated fused toxin, DLM. Toxins 2015, 7, 423–438. [Google Scholar] [CrossRef]
- Rajabnejad, S.H.; Mokhtarzadeh, A.; Abnous, K.; Taghdisi, S.M.; Ramezani, M.; Razavi, B.M. Targeted delivery of melittin to cancer cells by AS1411 anti-nucleolin aptamer. Drug Dev. Ind. Pharm. 2018, 44, 982–987. [Google Scholar] [CrossRef] [PubMed]
- Yazdian-Robati, R.; Arab, A.; Ramezani, M.; Rafatpanah, H.; Bahreyni, A.; Nabavinia, M.S.; Abnous, K.; Taghdisi, S.M. Smart aptamer-modified calcium carbonate nanoparticles for controlled release and targeted delivery of epirubicin and melittin into cancer cells in vitro and in vivo. Drug Dev. Ind. Pharm. 2019, 45, 603–610. [Google Scholar] [CrossRef]
- Bahreyni, A.; Mohamud, Y.; Zhang, J.; Luo, H. Engineering a facile and versatile nanoplatform to facilitate the delivery of multiple agents for targeted breast cancer chemo-immunotherapy. Biomed. Pharmacother. 2023, 163, 114789. [Google Scholar] [CrossRef]
- Jia, H.R.; Zhu, Y.X.; Xu, K.F.; Wu, F.G. Turning Toxicants into Safe Therapeutic Drugs: Cytolytic Peptide-Photosensitizer Assemblies for Optimized In Vivo Delivery of Melittin. Adv. Healthc. Mater. 2018, 7, e1800380. [Google Scholar] [CrossRef] [PubMed]
- Motiei, M.; Aboutalebi, F.; Forouzanfar, M.; Dormiani, K.; Nasr-Esfahani, M.H.; Mirahmadi-Zare, S.Z. Smart co-delivery of miR-34a and cytotoxic peptides (LTX-315 and melittin) by chitosan based polyelectrolyte nanocarriers for specific cancer cell death induction. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 128, 112258. [Google Scholar] [CrossRef]
- Shahriari, M.; Zahiri, M.; Abnous, K.; Taghdisi, S.M.; Ramezani, M.; Alibolandi, M. Enzyme responsive drug delivery systems in cancer treatment. J. Control. Release 2019, 308, 172–189. [Google Scholar] [CrossRef]
- Duan, X.; Zou, H.; Yang, J.; Liu, S.; Xu, T.; Ding, J. Melittin-incorporated nanomedicines for enhanced cancer immunotherapy. J. Control. Release 2024, 375, 285–299. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Choi, I.; Han, I.H.; Bae, H. Enhanced Therapeutic Effect of Optimized Melittin-dKLA, a Peptide Agent Targeting M2-like Tumor-Associated Macrophages in Triple-Negative Breast Cancer. Int. J. Mol. Sci. 2022, 23, 15751. [Google Scholar] [CrossRef]
- Dabbagh Moghaddam, F.; Akbarzadeh, I.; Marzbankia, E.; Farid, M.; Khaledi, L.; Reihani, A.H.; Javidfar, M.; Mortazavi, P. Delivery of melittin-loaded niosomes for breast cancer treatment: An in vitro and in vivo evaluation of anti-cancer effect. Cancer Nanotechnol. 2021, 12, 14. [Google Scholar] [CrossRef]
- Daniluk, K.; Lange, A.; Pruchniewski, M.; Małolepszy, A.; Sawosz, E.; Jaworski, S. Delivery of Melittin as a Lytic Agent via Graphene Nanoparticles as Carriers to Breast Cancer Cells. J. Funct. Biomater. 2022, 13, 278. [Google Scholar] [CrossRef]
- Das, S.; Ghosh, S. Melittin based nano drug delivery system for cancer therapy. J. Adv. Zool. 2023, 44, 2607–2610. [Google Scholar]
- Tariq, R.; Liaqat, A.; Khalid, U.A. An Insight into the Role of Bee Venom and Melittin Against Tumor Cells: A Review of Breast Cancer therapy. Arch. Breast Cancer 2021, 8, 267–276. [Google Scholar] [CrossRef]
- Lim, H.N.; Baek, S.B.; Jung, H.J. Bee Venom and Its Peptide Component Melittin Suppress Growth and Migration of Melanoma Cells via Inhibition of PI3K/AKT/mTOR and MAPK Pathways. Molecules 2019, 24, 929. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Liu, S.; Ai, M.; Wang, Z.; Wang, D.; Li, X.; Hu, K.; Gao, X.; Yang, Y. A novel melittin nano-liposome exerted excellent anti-hepatocellular carcinoma efficacy with better biological safety. J. Hematol. Oncol. 2017, 10, 71. [Google Scholar] [CrossRef] [PubMed]
- Jo, M.; Park, M.H.; Kollipara, P.S.; An, B.J.; Song, H.S.; Han, S.B.; Kim, J.H.; Song, M.J.; Hong, J.T. Anti-cancer effect of bee venom toxin and melittin in ovarian cancer cells through induction of death receptors and inhibition of JAK2/STAT3 pathway. Toxicol. Appl. Pharmacol. 2012, 258, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Li, N.; Huang, W.; Yang, Y.; Zang, R.; Song, H.; Shi, J.; Zhu, S.; Liu, Q. Melittin suppresses ovarian cancer growth by regulating SREBP1-mediated lipid metabolism. Phytomedicine 2025, 137, 156367. [Google Scholar] [CrossRef] [PubMed]
- Si, L.; Yang, S.; Lin, R.; Gu, S.; Yan, C.; Yan, J. SiO2–alginate–melittin nano-conjugates suppress the proliferation of ovarian cancer cells: A controlled release approach leveraging alginate lyase. Cancer Nanotechnol. 2024, 15, 4. [Google Scholar] [CrossRef]
- Mills, K.A.; Quinn, J.M.; Roach, S.T.; Palisoul, M.; Nguyen, M.; Noia, H.; Guo, L.; Fazal, J.; Mutch, D.G.; Wickline, S.A.; et al. p5RHH nanoparticle-mediated delivery of AXL siRNA inhibits metastasis of ovarian and uterine cancer cells in mouse xenografts. Sci. Rep. 2019, 9, 4762. [Google Scholar] [CrossRef]
- Alonezi, S.; Tusiimire, J.; Wallace, J.; Dufton, M.J.; Parkinson, J.A.; Young, L.C.; Clements, C.J.; Park, J.K.; Jeon, J.W.; Ferro, V.A.; et al. Metabolomic Profiling of the Synergistic Effects of Melittin in Combination with Cisplatin on Ovarian Cancer Cells. Metabolites 2017, 7, 14. [Google Scholar] [CrossRef]
- Wang, K.; Tao, L.; Zhu, M.; Yu, X.; Lu, Y.; Yuan, B.; Hu, F. Melittin Inhibits Colorectal Cancer Growth and Metastasis by Ac-Tivating the Mitochondrial Apoptotic Pathway and Suppressing Epithelial-Mesenchymal Transition and Angiogenesis. Int. J. Mol. Sci. 2024, 25, 11686. [Google Scholar] [CrossRef]
- Janani, B.; Vijayakumar, M.; Priya, K.; Kim, J.H.; Prabakaran, D.S.; Shahid, M.; Al-Ghamdi, S.; Alsaidan, M.; Othman Bahakim, N.; Hassan Abdelzaher, M.; et al. EGFR-Based Targeted Therapy for Colorectal Cancer-Promises and Challenges. Vaccines 2022, 10, 499. [Google Scholar] [CrossRef] [PubMed]
- Abdelaal, I.R.; Siddiqui, I.A.; Adhami, V.M.; Abdelaal, H.F.; Mohamed, H.; Rady, M.; Mukhtar, H. Abstract 2689: Nanoformulated mellitin from bee venom exhibits excellent anti-prostate cancer efficacy. Cancer Res. 2018, 78, 2689. [Google Scholar] [CrossRef]
- Badawi, J.K. Bee Venom Components as Therapeutic Tools against Prostate Cancer. Toxins 2021, 13, 337. [Google Scholar] [CrossRef]
- Fahmy, U.A.; Badr-Eldin, S.M.; Aldawsari, H.M.; Alhakamy, N.A.; Ahmed, O.A.A.; Radwan, M.F.; Eid, B.G.; Sayed, S.R.M.; El Sherbiny, G.A.; Abualsunun, W. Potentiality of raloxifene loaded melittin functionalized lipidic nanovesicles against pancreatic cancer cells. Drug Deliv. 2022, 29, 1863–1877. [Google Scholar] [CrossRef] [PubMed]
- Alhakamy, N.A.; Badr-Eldin, S.M.; Alharbi, W.S.; Alfaleh, M.A.; Al-Hejaili, O.D.; Aldawsari, H.M.; Eid, B.G.; Bakhaidar, R.; Drago, F.; Caraci, F.; et al. Development of an Icariin-Loaded Bilosome-Melittin Formulation with Improved Anticancer Activity against Cancerous Pancreatic Cells. Pharmaceuticals 2021, 14, 1309. [Google Scholar] [CrossRef]
- Ma, J.; Li, X.; Wang, C. The Application of Nanomaterials in the Treatment of Pancreatic-Related Diseases. Int. J. Mol. Sci. 2024, 25, 13158. [Google Scholar] [CrossRef]
- Ibrahim, H.M.; Alessa, J.; Khalil, H.B.; Bekhet, G.A.; Khalifa, A. Synergistic Anti-Cancer Activity of Melittin and Erlotinib in Non-Small Cell Lung Cancer. Int. J. Mol. Sci. 2025, 26, 2903. [Google Scholar] [CrossRef] [PubMed]
- Sattayawat, P.; Kaewkod, T.; Thongyim, S.; Chiawpanit, C.; Wutti-In, Y.; Thepmalee, C.; Tragoolpua, Y.; Disayathanoowat, T.; Panya, A. A Comparative Study of Melittins from Apis florea and Apis mellifera as Cytotoxic Agents Against Non-Small Cell Lung Cancer (NSCLC) Cells and Their Combination with Gefitinib. Int. J. Mol. Sci. 2025, 26, 2498. [Google Scholar] [CrossRef]
- Lee, C.; Jeong, H.; Bae, Y.; Shin, K.; Kang, S.; Kim, H.; Oh, J.; Bae, H. Targeting of M2-like tumor-associated macrophages with a melittin-based pro-apoptotic peptide. J. Immunother. Cancer 2019, 7, 147. [Google Scholar] [CrossRef]
- Crintea, A.; Constantin, A.M.; Motofelea, A.C.; Crivii, C.B.; Velescu, M.A.; Coșeriu, R.L.; Ilyés, T.; Crăciun, A.M.; Silaghi, C.N. Targeted EGFR Nanotherapy in Non-Small Cell Lung Cancer. J. Funct. Biomater. 2023, 14, 466. [Google Scholar] [CrossRef]
- Rahman, M.A.; Jalouli, M.; Yadab, M.K.; Al-Zharani, M. Progress in Drug Delivery Systems Based on Nanoparticles for Improved Glioblastoma Therapy: Addressing Challenges and Investigating Opportunities. Cancers 2025, 17, 701. [Google Scholar] [CrossRef]
- Małek, A.; Strzemski, M.; Kapka-Skrzypczak, L.; Kurzepa, J. Anticancer Activity of Melittin-Containing Bee Venom Fraction Against Glioblastoma Cells In Vitro. Int. J. Mol. Sci. 2025, 26, 2376. [Google Scholar] [CrossRef] [PubMed]
- Ertilav, K.; Nazıroğlu, M. Honey bee venom melittin increases the oxidant activity of cisplatin and kills human glioblastoma cells by stimulating the TRPM2 channel. Toxicon 2023, 222, 106993. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Chen, Y.; Zhuo, C.; Hu, M.; Zhang, C.; Cai, H.; Li, X.; Chen, H.; Yu, X. Aptamer-modified melittin micelles efficiently inhibit osteosarcoma deterioration by inducing immunogenic cell death. Colloids Surf. B Biointerfaces 2025, 249, 114512. [Google Scholar] [CrossRef]
- Pedro, G.; Brasileiro, F.; Ferreira, R.S., Jr.; Bráz, A.M.M.; Laufer-Amorim, R. Melittin inhibits proliferation, migration, and invasion in osteosarcoma cell lines using 2D and 3D models. J. Venom. Anim. Toxins Incl. Trop. Dis. 2025, 31, e20240053. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, D.; Xie, X.; Li, Y.; Fan, T. Melittin inhibits lung metastasis of human osteosarcoma: Evidence of wnt/β-catenin signaling pathway participation. Toxicon 2021, 198, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Kreinest, T.; Volkmer, I.; Staege, M.S. Melittin Increases Cisplatin Sensitivity and Kills KM-H2 and L-428 Hodgkin Lymphoma Cells. Int. J. Mol. Sci. 2020, 22, 343. [Google Scholar] [CrossRef]
- Vinhas, R.; Mendes, R.; Fernandes, A.R.; Baptista, P.V. Nanoparticles-Emerging Potential for Managing Leukemia and Lymphoma. Front. Bioeng. Biotechnol. 2017, 5, 79. [Google Scholar] [CrossRef]
- Hou, K.K.; Pan, H.; Ratner, L.; Schlesinger, P.H.; Wickline, S.A. Mechanisms of nanoparticle-mediated siRNA transfection by melittin-derived peptides. ACS Nano 2013, 7, 8605–8615. [Google Scholar] [CrossRef]
- Mahmoodzadeh, A.; Zarrinnahad, H.; Bagheri, K.P.; Moradia, A.; Shahbazzadeh, D. First report on the isolation of melittin from Iranian honey bee venom and evaluation of its toxicity on gastric cancer AGS cells. J. Chin. Med. Assoc. 2015, 78, 574–583. [Google Scholar] [CrossRef]
- Yan, R.; Dai, W.; Wu, R.; Huang, H.; Shu, M. Therapeutic targeting m6A-guided miR-146a-5p signaling contributes to the melittin-induced selective suppression of bladder cancer. Cancer Lett. 2022, 534, 215615. [Google Scholar] [CrossRef] [PubMed]
- İlhan, H.; Kabakcı, D.; Seçme, M. Cytotoxic effects of bee venom-loaded ZIF-8 nanoparticles on thyroid cancer cells: A promising strategy for targeted therapy. Med. Oncol. 2024, 42, 32. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Yang, X.; Liu, J.; Ge, Y.; Qin, Q.; Lu, J.; Zhan, L.; Liu, Z.; Zhang, H.; Chen, X.; et al. Melittin radiosensitizes esophageal squamous cell carcinoma with induction of apoptosis in vitro and in vivo. Tumour Biol. 2014, 35, 8699–8705. [Google Scholar] [CrossRef]
- Ke, M.; Dong, J.; Wang, Y.; Zhang, J.; Zhang, M.; Wu, Z.; Lv, Y.; Wu, R. MEL-pep, an analog of melittin, disrupts cell membranes and reverses 5-fluorouracil resistance in human hepatocellular carcinoma cells. Int. J. Biochem. Cell Biol. 2018, 101, 39–48. [Google Scholar] [CrossRef]
- Cui, Z.; Zhou, Z.; Sun, Z.; Duan, J.; Liu, R.; Qi, C.; Yan, C. Melittin and phospholipase A2: Promising anti-cancer candidates from bee venom. Biomed. Pharmacother. 2024, 179, 117385. [Google Scholar]
- Tang, S.; Zhou, L.; He, H.; Cui, L.; Ren, Z.; Tai, Y.; Xie, Z.; Cao, Y.; Meng, D.; Liu, Q.; et al. MnO2-melittin nanoparticles serve as an effective anti-tumor immunotherapy by enhancing systemic immune response. Biomaterials 2022, 288, 121706. [Google Scholar] [CrossRef] [PubMed]
- Graham, W.; Torbett-Dougherty, M.; Islam, A.; Soleimani, S.; Bruce-Tagoe, T.A.; Johnson, J.A. Magnetic Nanoparticles and Drug Delivery Systems for Anti-Cancer Applications: A Review. Nanomaterials 2025, 15, 285. [Google Scholar] [CrossRef]
- Zhou, J.; Wan, C.; Cheng, J.; Huang, H.; Lovell, J.F.; Jin, H. Delivery Strategies for Melittin-Based Cancer Therapy. ACS Appl. Mater. Interfaces 2021, 13, 17158–17173. [Google Scholar] [CrossRef]
- Rocha, M.M.; Dariva, I.; Zornoff, G.C.; De Laurentis, G.S.; Mendes, G.C.; Santana, M.G.; de Miguel, G.C.; Ferreira, R.S.; Sciani, J.M.; Priolli, D.G. A new therapeutic approach for bone metastasis in colorectal cancer: Intratumoral melittin. J. Venom. Anim. Toxins Incl. Trop. Dis. 2022, 28, e20210067. [Google Scholar] [CrossRef]
- Zhu, J.; Lee, H.; Huang, R.; Zhou, J.; Zhang, J.; Yang, X.; Zhou, W.; Jiang, W.; Chen, S. Harnessing nanotechnology for cancer treatment. Front. Bioeng. Biotechnol. 2025, 12, 1514890. [Google Scholar]
- Jafari, Z.; Sadeghi, S.; Dehaghi, M.M.; Bigham, A.; Honarmand, S.; Tavasoli, A.; Hoseini, M.H.M.; Varma, R.S. Immunomodulatory activities and biomedical applications of melittin and its recent advances. Arch. Pharm. 2024, 357, e2300569. [Google Scholar] [CrossRef] [PubMed]
- Đorđević, S.; Gonzalez, M.M.; Conejos-Sánchez, I.; Carreira, B.; Pozzi, S.; Acúrcio, R.C.; Satchi-Fainaro, R.; Florindo, H.F.; Vicent, M.J. Current hurdles to the translation of nanomedicines from bench to the clinic. Drug Deliv. Transl. Res. 2022, 12, 500–525. [Google Scholar] [PubMed]
- Lee, Y.; Kim, S.G.; Kim, I.S.; Lee, H.D. Standardization of the Manufacturing Process of Bee Venom Pharmacopuncture Containing Melittin as the Active Ingredient. Evid. Based Complement. Alternat. Med. 2018, 2018, 2353280. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Gómez, F.D.; Monferrer, D.; Penon, O.; Rivera-Gil, P. Regulatory pathways and guidelines for nanotechnology-enabled health products: A comparative review of EU and US frameworks. Front. Med. 2025, 12, 1544393. [Google Scholar] [CrossRef]
- Barenholz, Y. Doxil®—The first FDA-approved nano-drug: Lessons learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef]
- Desai, N. Nanoparticle Albumin-Bound Paclitaxel (Abraxane®). In Albumin in Medicine: Pathological and Clinical Applications; Otagiri, M., Chuang, V.T.G., Eds.; Springer: Singapore, 2016; pp. 101–119. [Google Scholar]
- Wang, Y.; Bucher, E.; Rocha, H.; Jadhao, V.; Metzcar, J.; Heiland, R.; Frieboes, H.B.; Macklin, P. Drug-loaded nanoparticles for cancer therapy: A high-throughput multicellular agent-based modeling study. bioRxiv 2024. [Google Scholar] [CrossRef]
- Vizirianakis, I.S.; Mystridis, G.A.; Avgoustakis, K.; Fatouros, D.G.; Spanakis, M. Enabling personalized cancer medicine decisions: The challenging pharmacological approach of PBPK models for nanomedicine and pharmacogenomics (Review). Oncol. Rep. 2016, 35, 1891–1904. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, M.A.; Fallica, A.N.; Virzì, N.; Kesharwani, P.; Pittalà, V.; Greish, K. The Promise of Nanotechnology in Personalized Medicine. J. Pers. Med. 2022, 12, 673. [Google Scholar]
- Heydari, S.; Masoumi, N.; Esmaeeli, E.; Ayyoubzadeh, S.M.; Ghorbani-Bidkorpeh, F.; Ahmadi, M. Artificial intelligence in nanotechnology for treatment of diseases. J. Drug Target. 2024, 32, 1247–1266. [Google Scholar] [CrossRef]
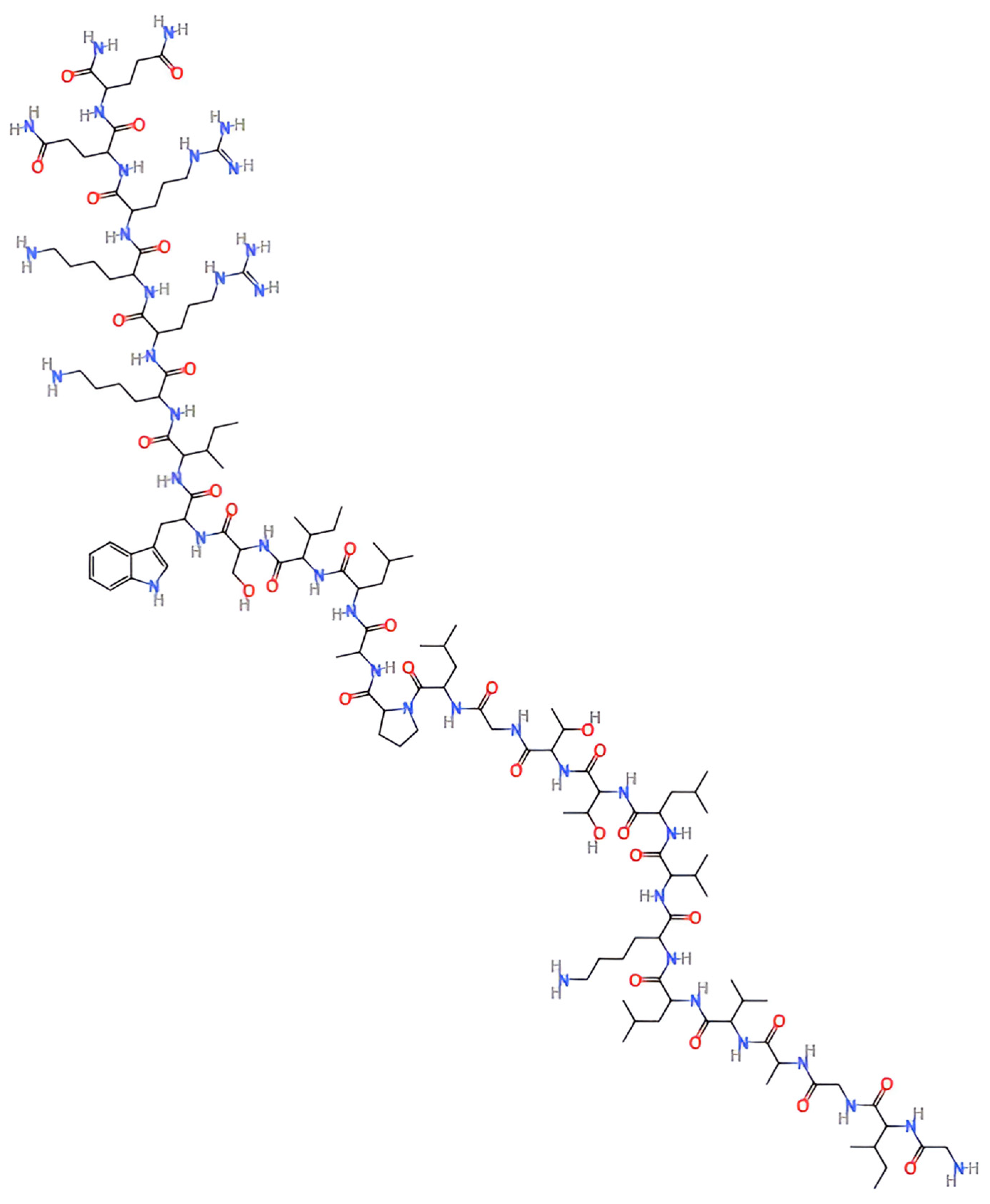

| Cancer Type | Nanoparticle System | Key Findings | Key Studies |
|---|---|---|---|
| Breast Cancer | Aptamer-functionalized gold nanoparticles | Enhanced targeting and cytotoxicity against MCF-7 cells | [34] |
| D-melittin polymeric nanoparticles | Reduced immunogenicity and improved tumor suppression in TNBC models | [27] | |
| Melittin-loaded niosomes | Inhibited migration/invasion and reduced tumor growth in 4T1 and SKBR3 models | [71] | |
| Melanoma | Hyaluronic-acid-coated liposomes | Enhanced uptake and cytotoxicity in CD44+ B16F10 melanoma cells | [26] |
| Hybrid melittin lipid nanoparticles | 82.8% tumor growth inhibition in B16F10 melanoma mice | [59] | |
| Hepatocellular Carcinoma | Poloxamer-stabilized nanoliposomes | Suppressed tumor growth in vitro and in vivo; reduced inflammation and allergic responses | [76] |
| Ovarian Cancer | Silica–alginate–melittin nanoconjugates | Inhibited the proliferation, migration, and invasion of SKOV3 cells with sustained release | [79] |
| p5RHH-siRNA nanoparticles | Suppressed invasion, migration, and metastasis in vitro and in vivo | [80] | |
| Colorectal Cancer | Redox-sensitive glycol chitosan complexes | Induced apoptosis, suppressed EMT/metastasis via mitochondrial pathways and MMP downregulation | [82] |
| Prostate Cancer | Perfluorocarbon nanoemulsions | Induced apoptosis and inhibited the PI3K/Akt pathway; reduced the tumor volume without hemolysis | [84] |
| Pancreatic Cancer | Melittin-functionalized lipidic vesicles + raloxifene | Induced apoptosis and cell cycle arrest in PANC1 cells | [86] |
| Lung Cancer | Melittin-dKLA nanofibers | Targeted M2-like TAMs and reduced tumor growth in vivo | [91] |
| Glioblastoma | Lipodisks + c(RGDyK) + paclitaxel + melittin | Synergistic cytotoxicity and reduced tumor burden in intracranial models | [10] |
| Osteosarcoma | Aptamer-modified melittin micelles | Induced immunogenic cell death and inhibited tumor progression in vivo | [96] |
| Leukemia and Lymphoma | Free melittin | Induced apoptosis via the mitochondrial and NF-κB/MAPK14 pathways in multiple leukemia and lymphoma cell lines | [46,99] |
| Gastric Cancer | Purified melittin | Dose- and time-dependent inhibition oft he proliferation of AGS cells | [102] |
| Cervical Cancer | Purified melittin | Induced apoptosis and suppressed proliferation in HeLa cells | [13] |
| Bladder Cancer | Purified melittin | Suppressed proliferation via the miR-146a-5p/NUMB/NOTCH2 axis; effective reduction in tumor growth | [103] |
| Thyroid Cancer | Bee-venom-loaded ZIF-8 nanoparticles | Cytotoxic to TT cells via apoptosis-related gene modulation | [104] |
| Head and Neck Cancer | Free melittin | Enhanced radiosensitivity by suppressing HIF-1α in hypoxic HNSCC cells | [24] |
| Esophageal Cancer | Free melittin | Radiosensitization and apoptosis induction in vivo (ESCC models) | [105] |
| Endometrial Cancer | p5RHH-siRNA nanoparticles | Inhibited metastasis in uterine cancer models | [80] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizkallah, J.; Charbel, N.; Yassine, A.; El Masri, A.; Raffoul, C.; El Sardouk, O.; Ghezzawi, M.; Abou Nasr, T.; Kreidieh, F. Melittin-Based Nanoparticles for Cancer Therapy: Mechanisms, Applications, and Future Perspectives. Pharmaceutics 2025, 17, 1019. https://doi.org/10.3390/pharmaceutics17081019
Rizkallah J, Charbel N, Yassine A, El Masri A, Raffoul C, El Sardouk O, Ghezzawi M, Abou Nasr T, Kreidieh F. Melittin-Based Nanoparticles for Cancer Therapy: Mechanisms, Applications, and Future Perspectives. Pharmaceutics. 2025; 17(8):1019. https://doi.org/10.3390/pharmaceutics17081019
Chicago/Turabian StyleRizkallah, Joe, Nicole Charbel, Abdallah Yassine, Amal El Masri, Chris Raffoul, Omar El Sardouk, Malak Ghezzawi, Therese Abou Nasr, and Firas Kreidieh. 2025. "Melittin-Based Nanoparticles for Cancer Therapy: Mechanisms, Applications, and Future Perspectives" Pharmaceutics 17, no. 8: 1019. https://doi.org/10.3390/pharmaceutics17081019
APA StyleRizkallah, J., Charbel, N., Yassine, A., El Masri, A., Raffoul, C., El Sardouk, O., Ghezzawi, M., Abou Nasr, T., & Kreidieh, F. (2025). Melittin-Based Nanoparticles for Cancer Therapy: Mechanisms, Applications, and Future Perspectives. Pharmaceutics, 17(8), 1019. https://doi.org/10.3390/pharmaceutics17081019








