Synthesis and Antiviral Activity of Nanowire Polymers Activated with Ag, Zn, and Cu Nanoclusters
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Used
2.2. Electrospinning the Filter Materials
2.3. Testing of Particle Filtration Efficiency and Breathability
2.4. Physical Characterization of Electrospun Filter Materials
2.5. Virus Strain and Virucidal Activity Determination
3. Results and Discussion
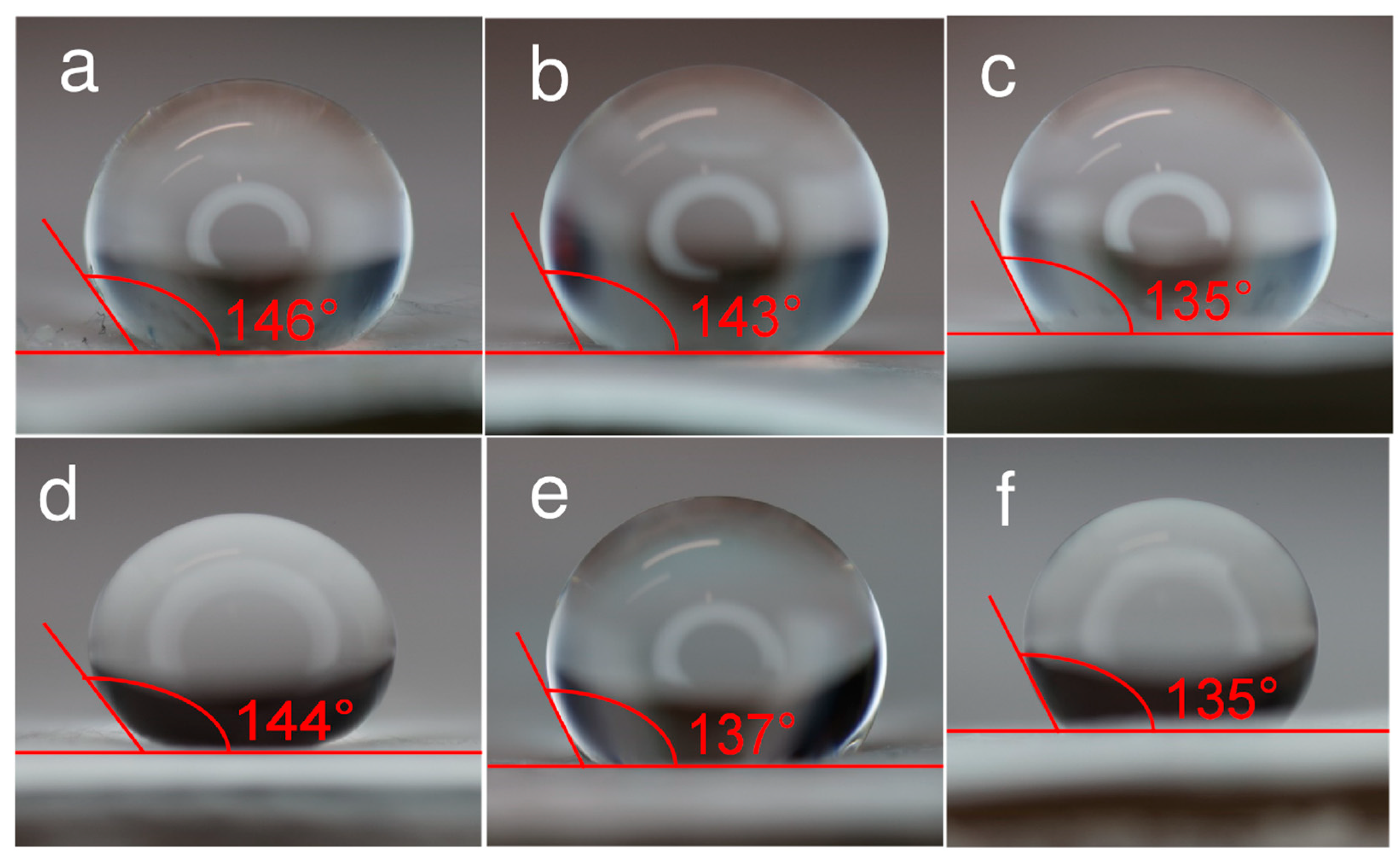
3.1. Physical Characterization Results of Electrospun Filter Materials
3.2. Particle Filtration Efficiency and Pressure Drop Values for Electrospun Filter Materials
3.3. Results of Virucidal Activity Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wibisono, Y.; Fadila, C.R.; Saiful, S.; Bilad, M.R. Facile Approaches of Polymeric Face Masks Reuse and Reinforcements for Micro-Aerosol Droplets and Viruses Filtration: A Review. Polymers 2020, 12, 2516. [Google Scholar] [CrossRef] [PubMed]
- Cook, T.M. Personal Protective Equipment during the Coronavirus Disease (COVID) 2019 Pandemic—A Narrative Review. Anaesthesia 2020, 75, 920–927. [Google Scholar] [CrossRef]
- Prather, K.A.; Wang, C.C.; Schooley, R.T. Reducing Transmission of SARS-CoV-2. Science 2020, 368, 1422–1424. [Google Scholar] [CrossRef]
- Teo, J.Y.; Kng, J.; Periaswamy, B.; Liu, S.; Lim, P.; Lee, C.E.; Tan, B.H.; Loh, X.J.; Ni, X.; Tiang, D.; et al. Exploring Reusability of Disposable Face Masks: Effects of Disinfection Methods on Filtration Efficiency, Breathability, and Fluid Resistance. Glob. Chall. 2021, 5, 2100030. [Google Scholar] [CrossRef]
- Rubio-Romero, J.C.; Pardo-Ferreira, M.d.C.; Torrecilla-García, J.A.; Calero-Castro, S. Disposable Masks: Disinfection and Sterilization for Reuse, and Non-Certified Manufacturing, in the Face of Shortages during the COVID-19 Pandemic. Saf. Sci. 2020, 129, 104830. [Google Scholar] [CrossRef] [PubMed]
- Li, A.S.H.; Sathishkumar, P.; Selahuddeen, M.L.; Mahmood, W.M.A.W.; Abidin, M.H.Z.; Wahab, R.A.; Huri, M.A.M.; Abdullah, F. Adverse Environmental Effects of Disposable Face Masks Due to the Excess Usage. Environ. Pollut. 2022, 308, 119674. [Google Scholar] [CrossRef]
- ISO 29463:2017; High Efficiency Filters and Filter Media for Removing Particles from Air—Part 1: Classification, Performance, Testing and Marking. ISO: Geneva, Switzerland, 2017.
- Zhou, Z.; You, T.; Pan, Z.; Wang, D.; Wang, H.; Wang, L.; Xu, G.; Liang, Y.; Hu, J.; Tang, M. Trichome-Like Biomimetic Air Filters via Templated Silicone Nanofilaments. Adv. Mater. 2024, 36, 2311129. [Google Scholar] [CrossRef]
- Zhou, Z.; You, T.; Wang, D.; Pan, Z.; Xu, G.; Liang, Y.; Tang, M. Conformal Build-Up of Functionalized Air Filters with Improved Air Cleaning and Bioprotective Traps. Adv. Funct. Mater. 2024, 34, 2306777. [Google Scholar] [CrossRef]
- Jazie, A.A.; Albaaji, A.J.; Abed, S.A. A Review on Recent Trends of Antiviral Nanoparticles and Airborne Filters: Special Insight on COVID-19 Virus. Air Qual. Atmos. Health 2021, 14, 1811–1824. [Google Scholar] [CrossRef]
- Ge, Z.; Yang, L.; Xia, J.; Fu, X.; Zhang, Y. Possible Aerosol Transmission of COVID-19 and Special Precautions in Dentistry. J. Zhejiang Univ. Sci. B 2020, 21, 361–368. [Google Scholar] [CrossRef]
- Mamun, A.; Blachowicz, T.; Sabantina, L. Electrospun Nanofiber Mats for Filtering Applications—Technology, Structure and Materials. Polymers 2021, 13, 1368. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.C.; Wada, N.I.; Grabowski, M.K.; Gurley, E.S.; Lessler, J. The Engines of SARS-CoV-2 Spread. Science 2020, 370, 406–407. [Google Scholar] [CrossRef] [PubMed]
- Babaahmadi, V.; Amid, H.; Naeimirad, M.; Ramakrishna, S. Biodegradable and Multifunctional Surgical Face Masks: A Brief Review on Demands during COVID-19 Pandemic, Recent Developments, and Future Perspectives. Sci. Total Environ. 2021, 798, 149233. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Prather, K.A.; Sznitman, J.; Jimenez, J.L.; Lakdawala, S.S.; Tufekci, Z.; Marr, L.C. Airborne Transmission of Respiratory Viruses. Science 2021, 373, eabd9149. [Google Scholar] [CrossRef]
- Du, H.; Huang, S.; Wang, J. Environmental Risks of Polymer Materials from Disposable Face Masks Linked to the COVID-19 Pandemic. Sci. Total Environ. 2022, 815, 152980. [Google Scholar] [CrossRef]
- Türkmen, B.A. Life Cycle Environmental Impacts of Disposable Medical Masks. Environ. Sci. Pollut. Res. 2022, 29, 25496–25506. [Google Scholar] [CrossRef]
- Liu, R.; Mabury, S.A. Single-Use Face Masks as a Potential Source of Synthetic Antioxidants to the Environment. Environ. Sci. Technol. Lett. 2021, 8, 651–655. [Google Scholar] [CrossRef]
- Prata, J.C.; Silva, A.L.P.; Walker, T.R.; Duarte, A.C.; Rocha-Santos, T. COVID-19 Pandemic Repercussions on the Use and Management of Plastics. Environ. Sci. Technol. 2020, 54, 7760–7765. [Google Scholar] [CrossRef]
- Chang, S.-Y.; Huang, K.-Y.; Chao, T.-L.; Kao, H.-C.; Pang, Y.-H.; Lu, L.; Chiu, C.-L.; Huang, H.-C.; Cheng, T.-J.R.; Fang, J.-M.; et al. Nanoparticle Composite TPNT1 Is Effective against SARS-CoV-2 and Influenza Viruses. Sci. Rep. 2021, 11, 8692. [Google Scholar] [CrossRef]
- Kim, S.; Chung, J.; Lee, S.H.; Yoon, J.H.; Kweon, D.-H.; Chung, W.-J. Tannic Acid-Functionalized HEPA Filter Materials for Influenza Virus Capture. Sci. Rep. 2021, 11, 979. [Google Scholar] [CrossRef]
- Mallakpour, S.; Azadi, E.; Hussain, C.M. Fabrication of Air Filters with Advanced Filtration Performance for Removal of Viral Aerosols and Control the Spread of COVID-19. Adv. Colloid Interface Sci. 2022, 303, 102653. [Google Scholar] [CrossRef]
- Lee, J.; Bae, J.; Youn, D.-Y.; Ahn, J.; Hwang, W.-T.; Bae, H.; Bae, P.K.; Kim, I.-D. Violacein-Embedded Nanofiber Filters with Antiviral and Antibacterial Activities. Chem. Eng. J. 2022, 444, 136460. [Google Scholar] [CrossRef] [PubMed]
- Thomberg, T.; Bulgarin, H.; Lust, A.; Nerut, J.; Koppel, M.; Romann, T.; Palm, R.; Månsson, M.; March, N.M.F.; Junninen, H.; et al. The Anti SARS-CoV-2 Activity of Nanofibrous Filter Materials Activated with Metal Clusters. Atmos. Environ. X 2023, 17, 100212. [Google Scholar] [CrossRef]
- Thomberg, T.; Ramah, P.; Lust, A.; Nerut, J.; Koppel, M.; Romann, T.; Palm, R.; Månsson, M.; March, N.M.F.; Junninen, H.; et al. Preparation of Nanofibrous Materials Activated with Metal Clusters for Active and Long-Lasting Air Filters. Sep. Purif. Technol. 2022, 288, 120697. [Google Scholar] [CrossRef]
- Bulgarin, H.; Thomberg, T.; Lust, A.; Nerut, J.; Koppel, M.; Romann, T.; Palm, R.; Månsson, M.; Vana, M.; Junninen, H.; et al. Enhanced and Copper Concentration Dependent Virucidal Effect against SARS-CoV-2 of Electrospun Poly(Vinylidene Difluoride) Filter Materials. iScience 2024, 27, 109835. [Google Scholar] [CrossRef]
- Lu, T.; Cui, J.; Qu, Q.; Wang, Y.; Zhang, J.; Xiong, R.; Ma, W.; Huang, C. Multistructured Electrospun Nanofibers for Air Filtration: A Review. ACS Appl. Mater. Interfaces 2021, 13, 23293–23313. [Google Scholar] [CrossRef] [PubMed]
- Senthil, R.; Sumathi, V.; Tamilselvi, A.; Kavukcu, S.B.; Aruni, A.W. Functionalized Electrospun Nanofibers for High Efficiency Removal of Particulate Matter. Sci. Rep. 2022, 12, 8411. [Google Scholar] [CrossRef] [PubMed]
- Karabulut, F.N.H.; Höfler, G.; Ashok Chand, N.; Beckermann, G.W. Electrospun Nanofibre Filtration Media to Protect against Biological or Nonbiological Airborne Particles. Polymers 2021, 13, 3257. [Google Scholar] [CrossRef]
- Tõnurist, K.; Jänes, A.; Thomberg, T.; Kurig, H.; Lust, E. Influence of Mesoporous Separator Properties on the Parameters of Electrical Double-Layer Capacitor Single Cells. J. Electrochem. Soc. 2009, 156, A334–A342. [Google Scholar] [CrossRef]
- Tõnurist, K.; Thomberg, T.; Jänes, A.; Lust, E. Specific Performance of Supercapacitors at Lower Temperatures Based on Different Separator Materials. J. Electrochem. Soc. 2013, 160, A449–A457. [Google Scholar] [CrossRef]
- Tõnurist, K.; Thomberg, T.; Jänes, A.; Kink, I.; Lust, E. Specific Performance of Electrical Double Layer Capacitors Based on Different Separator Materials in Room Temperature Ionic Liquid. Electrochem. Commun. 2012, 22, 77–80. [Google Scholar] [CrossRef]
- Tõnurist, K.; Vaas, I.; Thomberg, T.; Jänes, A.; Kurig, H.; Romann, T.; Lust, E. Application of Multistep Electrospinning Method for Preparation of Electrical Double-Layer Capacitor Half-Cells. Electrochim. Acta 2014, 119, 72–77. [Google Scholar] [CrossRef]
- Tõnurist, K. Influence of Electrospun Separator Materials Properties on Electrochemical Performance of Electrical Double-Layer Capacitors. Ph.D. Thesis, University of Tartu, Tartu, Estonia, 2013. [Google Scholar]
- Ramakrishna, S. An Introduction to Electrospinning and Nanofibers; World Scientific: Singapore, 2005; ISBN 978-981-256-761-1. [Google Scholar]
- Kubo, A.-L.; Rausalu, K.; Savest, N.; Žusinaite, E.; Vasiliev, G.; Viirsalu, M.; Plamus, T.; Krumme, A.; Merits, A.; Bondarenko, O. Antibacterial and Antiviral Effects of Ag, Cu and Zn Metals, Respective Nanoparticles and Filter Materials Thereof against Coronavirus SARS-CoV-2 and Influenza A Virus. Pharmaceutics 2022, 14, 2549. [Google Scholar] [CrossRef]
- Laidmäe, I.; Meos, A.; Kjærvik, I.A.; Ingebrigtsen, S.G.; Škalko-Basnet, N.; Kirsimäe, K.; Romann, T.; Joost, U.; Kisand, V.; Kogermann, K. Electrospun Amphiphilic Nanofibers as Templates for In Situ Preparation of Chloramphenicol-Loaded Liposomes. Pharmaceutics 2021, 13, 1742. [Google Scholar] [CrossRef]
- Ivask, A.; Juganson, K.; Bondarenko, O.; Mortimer, M.; Aruoja, V.; Kasemets, K.; Blinova, I.; Heinlaan, M.; Slaveykova, V.; Kahru, A. Mechanisms of Toxic Action of Ag, ZnO and CuO Nanoparticles to Selected Ecotoxicological Test Organisms and Mammalian Cells in Vitro: A Comparative Review. Nanotoxicology 2014, 8, 57–71. [Google Scholar] [CrossRef]
- Jeremiah, S.S.; Miyakawa, K.; Morita, T.; Yamaoka, Y.; Ryo, A. Potent Antiviral Effect of Silver Nanoparticles on SARS-CoV-2. Biochem. Biophys. Res. Commun. 2020, 533, 195–200. [Google Scholar] [CrossRef]
- Galdiero, S.; Falanga, A.; Vitiello, M.; Cantisani, M.; Marra, V.; Galdiero, M. Silver Nanoparticles as Potential Antiviral Agents. Molecules 2011, 16, 8894–8918. [Google Scholar] [CrossRef]
- Qamar, H.; Rehman, S.; Chauhan, D.K.; Tiwari, A.K.; Upmanyu, V. Green Synthesis, Characterization and Antimicrobial Activity of Copper Oxide Nanomaterial Derived from Momordica Charantia. Int. J. Nanomed. 2020, 15, 2541–2553. [Google Scholar] [CrossRef] [PubMed]
- Aallaei, M.; Molaakbari, E.; Mostafavi, P.; Salarizadeh, N.; Maleksah, R.E.; Afzali, D. Investigation of Cu Metal Nanoparticles with Different Morphologies to Inhibit SARS-CoV-2 Main Protease and Spike Glycoprotein Using Molecular Docking and Dynamics Simulation. J. Mol. Struct. 2022, 1253, 132301. [Google Scholar] [CrossRef]
- Govind, V.; Bharadwaj, S.; Sai Ganesh, M.R.; Vishnu, J.; Shankar, K.V.; Shankar, B.; Rajesh, R. Antiviral Properties of Copper and Its Alloys to Inactivate Covid-19 Virus: A Review. Biometals 2021, 34, 1217–1235. [Google Scholar] [CrossRef]
- Rani, I.; Goyal, A.; Bhatnagar, M.; Manhas, S.; Goel, P.; Pal, A.; Prasad, R. Potential Molecular Mechanisms of Zinc- and Copper-Mediated Antiviral Activity on COVID-19. Nutr. Res. 2021, 92, 109–128. [Google Scholar] [CrossRef]
- Bahrami, A.; Arabestani, M.R.; Taheri, M.; Farmany, A.; Norozzadeh, F.; Hosseini, S.M.; Nozari, H.; Nouri, F. Exploring the Role of Heavy Metals and Their Derivatives on the Pathophysiology of COVID-19. Biol. Trace Elem. Res. 2022, 200, 2639–2650. [Google Scholar] [CrossRef] [PubMed]
- Maduray, K.; Parboosing, R. Metal Nanoparticles: A Promising Treatment for Viral and Arboviral Infections. Biol. Trace Elem. Res. 2021, 199, 3159–3176. [Google Scholar] [CrossRef] [PubMed]
- Toledo, G.G.D.; Toledo, V.H.; Lanfredi, A.J.C.; Escote, M.; Champi, A.; Silva, M.C.C.D.; Nantes-Cardoso, I.L. Promising Nanostructured Materials against Enveloped Virus. An. Acad. Bras. Ciênc. 2020, 92, e20200718. [Google Scholar] [CrossRef] [PubMed]
- de Jesus Silva, A.J.; Contreras, M.M.; Nascimento, C.R.; da Costa, M.F. Kinetics of Thermal Degradation and Lifetime Study of Poly(Vinylidene Fluoride) (PVDF) Subjected to Bioethanol Fuel Accelerated Aging. Heliyon 2020, 6, e04573. [Google Scholar] [CrossRef]
- Balaram, V. Microwave Plasma Atomic Emission Spectrometry (MP-AES) and Its Applications—A Critical Review. Microchem. J. 2020, 159, 105483. [Google Scholar] [CrossRef]
- Hotaling, N.A.; Bharti, K.; Kriel, H.; Simon, C.G. DiameterJ: A Validated Open Source Nanofiber Diameter Measurement Tool. Biomaterials 2015, 61, 327–338. [Google Scholar] [CrossRef]
- EN 14683:2019+AC:2019; Medical Face Masks—Requirements and Test Methods. UNE: Madrid, Spain, 2019. Available online: https://www.en-standard.eu/une-en-14683-2019-ac-2019-medical-face-masks-requirements-and-test-methods/ (accessed on 2 July 2025).
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Rouquerol, J.; Rouquerol, F.; Sing, K.S.W. Adsorption by Powders and Porous Solids: Principles, Methodology and Applications, 1st ed.; Academic Press: San Diego, CA, USA, 1998; ISBN 978-0-12-598920-6. [Google Scholar]
- Webb, P.A.; Orr, C.; Camp, R.W.; Olivier, J.P.; Yunes, Y.S. Analytical Methods in Fine Particle Technology, 1st ed.; Micromeritics Instrument: Norcross, GA, USA, 1997; ISBN 978-0-9656783-0-8. [Google Scholar]
- Plötze, M.; Niemz, P. Porosity and Pore Size Distribution of Different Wood Types as Determined by Mercury Intrusion Porosimetry. Eur. J. Wood Prod. 2011, 69, 649–657. [Google Scholar] [CrossRef]
- Wang, S.; Yang, P.; Dai, D.; Xue, K.; Li, D. A Study on Micro-Pore Characteristics of Clay Due to Freeze-Thaw and Compression by Mercury Intrusion Porosimetry. Front. Earth Sci. 2020, 7, 344. [Google Scholar] [CrossRef]
- Cseri, T.; Békássy, S.; Kenessey, G.; Liptay, G.; Figueras, F. Characterization of Metal Nitrates and Clay Supported Metal Nitrates by Thermal Analysis. Thermochim. Acta 1996, 288, 137–154. [Google Scholar] [CrossRef]
- Holder, C.F.; Schaak, R.E. Tutorial on Powder X-Ray Diffraction for Characterizing Nanoscale Materials. ACS Nano 2019, 13, 7359–7365. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Lei, T.; Sun, D.; Lin, L. A Critical Analysis of the α, β and γ Phases in Poly(Vinylidene Fluoride) Using FTIR. RSC Adv. 2017, 7, 15382–15389. [Google Scholar] [CrossRef]
- Wu, T.; Zhou, B.; Zhu, T.; Shi, J.; Xu, Z.; Hu, C.; Wang, J. Facile and Low-Cost Approach towards a PVDF Ultrafiltration Membrane with Enhanced Hydrophilicity and Antifouling Performance via Graphene Oxide/Water-Bath Coagulation. RSC Adv. 2015, 5, 7880–7889. [Google Scholar] [CrossRef]
- ISO 21702:2019(En); Measurement of Antiviral Activity on Plastics and Other Non-Porous Surfaces. ISO: Geneva, Switzerland, 2019. Available online: https://www.iso.org/obp/ui/en/#iso:std:iso:21702:ed-1:v1:en (accessed on 2 July 2025).
- Rihn, S.J.; Merits, A.; Bakshi, S.; Turnbull, M.L.; Wickenhagen, A.; Alexander, A.J.T.; Baillie, C.; Brennan, B.; Brown, F.; Brunker, K.; et al. A Plasmid DNA-Launched SARS-CoV-2 Reverse Genetics System and Coronavirus Toolkit for COVID-19 Research. PLOS Biol. 2021, 19, e3001091. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, S.; Srisombat, L.O.; Lee, T.R.; Advincula, R.C. Gold-Nanoparticle- and Gold-Nanoshell-Induced Polymorphism in Poly(Vinylidene Fluoride). Macromol. Mater. Eng. 2011, 296, 178–184. [Google Scholar] [CrossRef]
- Mandal, D.; Kim, K.J.; Lee, J.S. Simple Synthesis of Palladium Nanoparticles, β-Phase Formation, and the Control of Chain and Dipole Orientations in Palladium-Doped Poly(Vinylidene Fluoride) Thin Films. Langmuir 2012, 28, 10310–10317. [Google Scholar] [CrossRef]
- Kim, H.B.; Lee, W.J.; Choi, S.C.; Lee, K.B.; Lee, M.-H. Filter Quality Factors of Fibrous Filters with Different Fiber Diameter. Aerosol Sci. Technol. 2021, 55, 154–166. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, S.; Yin, X.; Yu, J.; Ding, B. Slip-Effect Functional Air Filter for Efficient Purification of PM2.5. Sci. Rep. 2016, 6, 35472. [Google Scholar] [CrossRef]
- Delumeau, L.-V.; Asgarimoghaddam, H.; Alkie, T.; Jones, A.J.B.; Lum, S.; Mistry, K.; Aucoin, M.G.; DeWitte-Orr, S.; Musselman, K.P. Effectiveness of Antiviral Metal and Metal Oxide Thin-Film Coatings against Human Coronavirus 229E. APL Mater. 2021, 9, 111114. [Google Scholar] [CrossRef]
- Richardson, H.W. Copper Compounds. In Ullmann’s Encyclopedia of Industrial Chemistry; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2000; ISBN 978-3-527-30673-2. [Google Scholar]




| Solution | Nitrogen Sorption | Mercury Intrusion | |||
|---|---|---|---|---|---|
| SBET (m2 g−1) | Vtot (cm3 g−1) | SHg (m2 g−1) | VHg (cm3 g−1) | Porosity (%) | |
| 28 wt% PVDF + DMA | 9.6 | 0.015 | 23.5 | 3.9 | 28 |
| 28 wt% PVDF + DMA + 2.0 wt% AgNO3 | 4.5 | 0.0075 | 22.5 | 4.7 | 26 |
| 28 wt% PVDF + DMA + 2.0 wt% ZnCl2 | 4.4 | 0.0067 | 34.0 | 4.1 | 27 |
| 28 wt% PVDF + DMA + 0.25 wt% Cu(NO3)2·2.5H2O | 3.6 | 0.0057 | 10.5 | 4.3 | 26 |
| 28 wt% PVDF + DMA + 0.75 wt% Cu(NO3)2·2.5H2O | 4.3 | 0.0060 | 7.5 | 3.8 | 29 |
| 28 wt% PVDF + DMA + 2.0 wt% Cu(NO3)2·2.5H2O | 4.3 | 0.0057 | 8.0 | 3.9 | 27 |
| 28 wt% PVDF + DMA + 3.5 wt% Cu(NO3)2·2.5H2O | 2.5 | 0.0035 | 11.0 | 3.0 | 32 |
| Solution | Filter Thickness (μm) | Eeff, 100 nm (%) | Eeff, 300 nm (%) | Eeff, 3000 nm (%) | Pressure Drop (Pa cm−2) |
|---|---|---|---|---|---|
| 28 wt% PVDF + DMA | 55 ± 5 | 65 ± 2 | 76 ± 0.1 | 99.5 ± 0.1 | 53 ± 6 |
| 85 ± 5 | 84 ± 4 | 92 ± 2.0 | 99.9 ± 0.1 | 51 ± 5 | |
| 130 ± 8 | 96 ± 1 | 99 ± 0.2 | 99.9 ± 0.0 | 144 ± 15 | |
| 28 wt% PVDF + DMA + 2.0 wt% AgNO3 | 100 ± 10 | 100 ± 0.0 | 99 ± 0.2 | 99.9 ± 0.1 | 685 ± 60 |
| 28 wt% PVDF + DMA + 0.25 wt% ZnCl2 | 40 ± 5 | 99.7 ± 0.1 | 98 ± 0.3 | 99.9 ± 0.1 | 105 ± 10 |
| 75 ± 5 | 100 ± 0.0 | 99.8 ± 0.1 | 99.9 ± 0.1 | 205 ± 15 | |
| 160 ± 10 | 100 ± 0.0 | 99.8 ± 0.1 | 99.9 ± 0.1 | 335 ± 15 | |
| 28 wt% PVDF + DMA + 0.75 wt% ZnCl2 | 70 ± 5 | 99.9 ± 0.1 | 98.9 ± 0.2 | 99.7 ± 0.1 | 115 ± 15 |
| 125 ± 10 | 100 ± 0.0 | 99.3 ± 0.1 | 99.9 ± 0.1 | 335 ± 15 | |
| 28 wt% PVDF + DMA + 2.0 wt% ZnCl2 | 30 ± 5 | 98.9 ± 0.1 | 99 ± 0.2 | 99.9 ± 0.1 | 62 ± 9.5 |
| 28 wt% PVDF + DMA + 0.75 wt% Cu(NO3)2·2.5H2O | 25 ± 3 | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 | 130 ± 2 |
| 100 ± 10 | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 | 455 ± 10 | |
| 200 ± 20 | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 | 820 ± 50 | |
| 28 wt% PVDF + DMA + 2.0 wt% Cu(NO3)2·2.5H2O | 35 ± 4 | 98.5 ± 0.5 | 99.7 ± 0.2 | 100 ± 0.3 | 105 ± 2 |
| 90 ± 8 | 100 ± 0.0 | 100 ± 0.1 | 100 ± 0.0 | 390 ± 85 | |
| 145 ± 10 | 99.9 ± 0.1 | 99.9 ± 0.1 | 99.9 ± 0.1 | 695 ± 75 | |
| 28 wt% PVDF + DMA + 3.5 wt% Cu(NO3)2·2.5H2O | 30 ± 5 | 95.5 ± 4.0 | 100 ± 0.0 | 100 ± 0.0 | 85 ± 15 |
| 110 ± 15 | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 | 145 ± 15 | |
| 200 ± 20 | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 | 400 ± 95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thomberg, T.; Bulgarin, H.; Lust, A.; Nerut, J.; Romann, T.; Lust, E. Synthesis and Antiviral Activity of Nanowire Polymers Activated with Ag, Zn, and Cu Nanoclusters. Pharmaceutics 2025, 17, 887. https://doi.org/10.3390/pharmaceutics17070887
Thomberg T, Bulgarin H, Lust A, Nerut J, Romann T, Lust E. Synthesis and Antiviral Activity of Nanowire Polymers Activated with Ag, Zn, and Cu Nanoclusters. Pharmaceutics. 2025; 17(7):887. https://doi.org/10.3390/pharmaceutics17070887
Chicago/Turabian StyleThomberg, Thomas, Hanna Bulgarin, Andres Lust, Jaak Nerut, Tavo Romann, and Enn Lust. 2025. "Synthesis and Antiviral Activity of Nanowire Polymers Activated with Ag, Zn, and Cu Nanoclusters" Pharmaceutics 17, no. 7: 887. https://doi.org/10.3390/pharmaceutics17070887
APA StyleThomberg, T., Bulgarin, H., Lust, A., Nerut, J., Romann, T., & Lust, E. (2025). Synthesis and Antiviral Activity of Nanowire Polymers Activated with Ag, Zn, and Cu Nanoclusters. Pharmaceutics, 17(7), 887. https://doi.org/10.3390/pharmaceutics17070887








