Topical Percutaneous Drug Delivery for Allergic Diseases: A Novel Strategy for Site-Directed Pharmacologic Modulation
Abstract
1. Introduction
2. Pharmacologic Basis of Transdermal Therapy
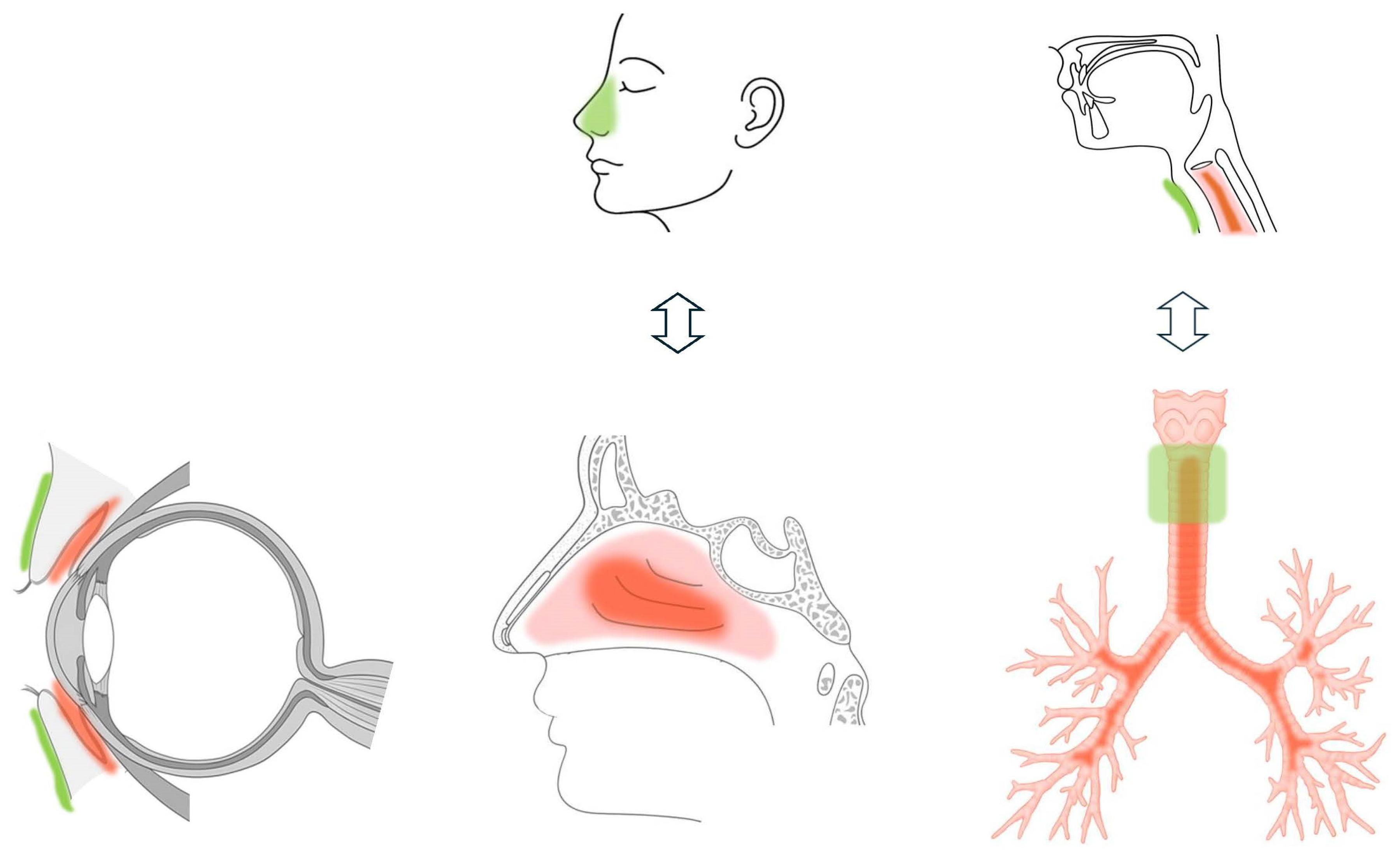
3. Application to Allergic Conjunctivitis
4. Application to Allergic Rhinitis
5. Application to Asthma-Related Cough
6. Comparative Assessment
6.1. Clinical Effectiveness of Topical Diphenhydramine Therapy on Each Site
6.2. AEs of Topical Diphenhydramine Therapy and Site-Dependent Sensitivity
| Eyelid | Nasal Ala | Pretracheal Skin | |
|---|---|---|---|
| target | conjunctiva | nasal cavity, mainly turbinates | trachea |
| Thickness (mm) | 3.35 [23] | 5–7 * | 4.6–9.2 [50,51] |
| location of the target organ | directly beneath | close to turbinates but not directly beneath | directly beneath |
| Anatomical coverage of the target organ by the application site | full | partial | partial |
| barrier function | weak [61,62] | moderate [61] | moderate [60] ** |
| drug absorption | high [25] | ND | ND |
| sensitivity to irritation | high [59] | ND | high [60] *** |
| topical percutaneous diphenhydramine delivery: efficacy | high [8] | partly [9] | less partly [10] |
| topical percutaneous diphenhydramine delivery: AEs | frequent [8] | none [9] | none [10] |
7. Future Research Directions
8. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| RCT | Randomized control Trial |
| ICS | Inhaled corticosteroids |
| NSAID | Non-steroidal anti-inflammatory drug |
| AEs | Adverse events |
| FeNO | Fractional exhaled nitric oxide |
References
- Asher, M.I.; Montefort, S.; Björkstén, B.; Lai, C.K.; Strachan, D.P.; Weiland, S.K.; Williams, H.; ISAAC Phase Three Study Group. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet 2006, 368, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Greiner, A.N.; Hellings, P.W.; Rotiroti, G.; Scadding, G.K. Allergic rhinitis. Lancet 2011, 378, 2112–2122. [Google Scholar] [CrossRef] [PubMed]
- Bousquet, J.; Anto, J.M.; Bachert, C.; Baiardini, I.; Bosnic-Anticevich, S.; Walter Canonica, G.; Melén, E.; Palomares, O.; Scadding, G.K.; Togias, A.; et al. Allergic rhinitis. Nat. Rev. Dis. Primers 2020, 6, 95. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, D.; Fukushima, A.; Uchio, E.; Shoji, J.; Namba, K.; Ebihara, N.; Takamura, E.; Fukuda, K.; Matsuda, A.; Okamoto, S.; et al. Executive summary: Japanese guidelines for allergic conjunctival diseases 2021. Allergol. Int. 2022, 71, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Berger, W.E.; Meltzer, E.O. Intranasal spray medications for maintenance therapy of allergic rhinitis. Am. J. Rhinol. Allergy 2015, 29, 273–282. [Google Scholar] [CrossRef]
- O’Brien, T.P. Allergic conjunctivitis: An update on diagnosis and management. Curr. Opin. Allergy Clin. Immunol. 2013, 13, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Global Initiative for Asthma (GINA). Global Strategy for Asthma Management and Prevention, 2024 Update; GINA: Fontana, WI, USA, 2024; Available online: https://ginasthma.org (accessed on 28 June 2025).
- Kamimura, M.; Ohnishi, T.; Terada, H.; Mouri, A.; Naoi, T.; Todo, H.; Sugibayashi, K. Application of diphenydramine 0ointment to the eyelids for allergic conjunctivitis. Iberoam. J. Med. 2021, 3, 44–50. [Google Scholar] [CrossRef]
- Kamimura, M.; Inui, T.; Mouri, A.; Todo, H.; Sugibayashi, K.; Asano, K. A Pilot Study of Transdermal Application of Diphenhydramine to the Nasal Ala in Patients with Allergic Rhinitis and Asthma. Tokai J. Exp. Clin. Med. 2022, 47, 170–176. [Google Scholar] [PubMed]
- Kamimura, M.; Mouri, A.; Takayama, K.; Mizutani, T.; Hamamoto, Y.; Iikura, M.; Furihata, K.; Ishii, H.; Sugibayashi, K. Transdermal Application of Steroid to Cervical Trachea for the Cough in Patients with Bronchial Asthma and Cough Variant Asthma-A Pilot Study. J. Allergy Ther. 2013, 4, 152. [Google Scholar] [CrossRef]
- Fujishima, H.; Shoji, J. Safety and efficacy of a novel 0.5% epinastine topical eyelid cream in allergic conjunctivitis: A phase 3 trial. Jpn. J. Ophthalmol. 2024, 68, 651–659. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Prausnitz, M.R.; Langer, R. Transdermal drug delivery. Nat. Biotechnol. 2008, 26, 1261–1268. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hasegawa, T.; Kawazome, A.; Yanagimoto, G.; Hayashi, T.; Seki, T.; Akimoto, M.; Todo, H.; Sugibayashi, K. Analysis of skin disposition of flurbiprofen after topical application using dual agar gel discs-inserted rats. Biol. Pharm. Bull. 2007, 30, 2135–2140. [Google Scholar] [CrossRef] [PubMed]
- Efe, T.; Sagnak, E.; Roessler, P.P.; Getgood, A.; Patzer, T.; Fuchs-Winkelmann, S.; Peterlein, C.D.; Schofer, M.D. Penetration of topical diclofenac sodium 4% spray gel into the synovial tissue and synovial fluid of the knee: A randomised clinical trial. Knee Surg. Sports Traumatol. Arthrosc. 2014, 22, 345–350. [Google Scholar] [CrossRef]
- Seefried, L.; Blyth, M.; Maheshwari, R.; McDonnell, S.M.; Frappin, G.; Hagen, M.; Maybaum, N.; Moreira, S.; Pandit, H. Penetration of topical diclofenac into synovial tissue and fluid of osteoarthritic knees: A multicenter, randomized, placebo-controlled, pharmacokinetic study. Ther. Adv. Musculoskelet. Dis. 2020, 12, 1759720X20943088. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Singh, P.; Roberts, M.S. Skin permeability and local tissue concentrations of nonsteroidal anti-inflammatory drugs after topical application. J. Pharmacol. Exp. Ther. 1994, 268, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Roberts, M.S. Deep tissue penetration of bases and steroids after dermal application in rat. J. Pharm. Pharmacol. 1994, 46, 956–964. [Google Scholar] [CrossRef]
- Shirinsky, I.; Shirinsky, V. H1-antihistamines are associated with lower prevalence of radiographic knee osteoarthritis: A cross-sectional analysis of the Osteoarthritis Initiative data. Arthritis Res. Ther. 2018, 20, 116. [Google Scholar] [CrossRef]
- Apiliogullari, S.; Keles, B.; Apiliogullari, B.; Balasar, M.; Yilmaz, H.; Duman, A. Comparison of diphenhydramine and lidocaine for prevention of pain after injection of propofol: A double-blind, placebo-controlled, randomized study. Eur. J. Anaesthesiol. 2007, 24, 235–238. [Google Scholar] [CrossRef]
- Fujita, T.; Ohue, M.; Fujii, Y.; Jotoku, T.; Miyauchi, A.; Takagi, Y.; Tsuchiya, M.; Endo, Y. Prompt analgesic effect of antihistaminic diphenhydramine ointment on bone-joint-muscle pain as assessed by skin impedance. Pharmacology 2013, 92, 158–166. [Google Scholar] [CrossRef]
- Freedman, R.B.; Jones, S.K.; Lin, A.; Robin, A.L.; Muir, K.W. Influence of parental health literacy and dosing responsibility on pediatric glaucoma medication adherence. Arch. Ophthalmol. 2012, 130, 306–311. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Uchino, M.; Yokoi, N.; Shimazaki, J.; Hori, Y.; Tsubota, K.; on behalf of the Japan Dry Eye Society. Adherence to Eye Drops Usage in Dry Eye Patients and Reasons for Non-Compliance: A Web-Based Survey. J. Clin. Med. 2022, 11, 367. [Google Scholar] [CrossRef]
- Vasanthapuram, V.H.; Saha, P.; Mohamed, A.; Naik, M.N. Ultrasound biomicroscopic features of the normal lower eyelid. Orbit 2021, 40, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Tsubota, K.; Monden, Y.; Yagi, Y.; Goto, E.; Shimmura, S. New treatment of dry eye: The effect of calcium ointment through eyelid skin delivery. Br. J. Ophthalmol. 1999, 83, 767–770. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- See, G.L.; Sagesaka, A.; Sugasawa, S.; Todo, H.; Sugibayashi, K. Eyelid skin as a potential site for drug delivery to conjunctiva and ocular tissues. Int. J. Pharm. 2017, 533, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Kimura, C.; Tojo, K. Development of a stick-type transdermal eyelid delivery system of ketotifen fumarate for ophthalmic diseases. Chem. Pharm. Bull. 2007, 55, 1002–1005. [Google Scholar] [CrossRef] [PubMed]
- See, G.L.; Arce, F., Jr.; Itakura, S.; Todo, H.; Sugibayashi, K. Prolonged distribution of tranilast in the eyes after topical application onto eyelid skin. Chem. Pharm. Bull. 2020, 68, 779–783. [Google Scholar] [CrossRef]
- Mochizuki, T.; Hata, T.; Mori, N.; Yamazaki, T.; Noto, T.; Mano, H. Trans-eyelid distribution of epinastine to the conjunctiva following eyelid application in rabbits. Jpn. J. Ophthalmol. 2024, 68, 594–602. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ogura, N.; Fujisawa, K.; Kato, M. Epinastine Cream: A Novel Once-Daily Therapeutic Agent for Allergic Conjunctivitis. J. Ocul. Pharmacol. Ther. 2024, 40, 173–180. [Google Scholar] [CrossRef]
- Ohta, K.; Bousquet, P.J.; Aizawa, H.; Akiyama, K.; Adachi, M.; Ichinose, M.; Ebisawa, M.; Tamura, G.; Nagai, A.; Nishima, S.; et al. Prevalence and impact of rhinitis in asthma. SACRA, a cross-sectional nation-wide study in Japan. Allergy 2011, 66, 1287–1295. [Google Scholar] [CrossRef] [PubMed]
- Meltzer, E.O.; Blaiss, M.; Fairchild, C.J. Comprehensive report of olopatadine 0.6% nasal spray as treatment for children with seasonal allergic rhinitis. Allergy Asthma Proc. 2011, 32, 213–220. [Google Scholar] [CrossRef]
- Berger, W.E.; Ratner, P.H.; Casale, T.B.; Meltzer, E.O.; Wall, G.M. Safety and efficacy of olopatadine hydrochloride nasal spray 0.6% in pediatric subjects with allergic rhinitis. Allergy Asthma Proc. 2009, 30, 612–623. [Google Scholar] [CrossRef] [PubMed]
- Roland, P.S.; Marple, B.F.; Wall, G.M. Olopatadine nasal spray for the treatment of allergic rhinitis. Expert Rev. Clin. Immunol. 2010, 6, 197–204. [Google Scholar] [CrossRef]
- Wu, E.L.; Harris, W.C.; Babcock, C.M.; Alexander, B.H.; Riley, C.A.; McCoul, E.D. Epistaxis Risk Associated with Intranasal Corticosteroid Sprays: A Systematic Review and Meta-analysis. Otolaryngol. Head Neck Surg. 2019, 161, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Storaas, T.; Andersson, M.; Persson, C.G.; Steinsvåg, S.K.; Marko-Varga, G.; Greiff, L. Effects of benzalkonium chloride on innate immunity physiology of the human nasal mucosa in vivo. Laryngoscope 2000, 110, 1543–1547. [Google Scholar] [CrossRef] [PubMed]
- Graf, P. Adverse effects of benzalkonium chloride on the nasal mucosa: Allergic rhinitis and rhinitis medicamentosa. Clin. Ther. 1999, 21, 1749–1755. [Google Scholar] [CrossRef] [PubMed]
- Nappi, E.; Paoletti, G.; Malvezzi, L.; Ferri, S.; Racca, F.; Messina, M.R.; Puggioni, F.; Heffler, E.; Canonica, G.W. Comorbid allergic rhinitis and asthma: Important clinical considerations. Expert Rev. Clin. Immunol. 2022, 18, 747–758. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, N.; Houser, S.M. Surgery for allergic rhinitis. Int. Forum Allergy Rhinol. 2014, 4 (Suppl. 2), S79–S83. [Google Scholar] [CrossRef] [PubMed]
- Hyun, D.W.; Min, H.J.; Kim, M.S.; Whon, T.W.; Shin, N.R.; Kim, P.S.; Kim, H.S.; Lee, J.Y.; Kang, W.; Choi, A.M.K.; et al. Dysbiosis of Inferior Turbinate Microbiota Is Associated with High Total IgE Levels in Patients with Allergic Rhinitis. Infect. Immun. 2018, 86, e00934-17. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hamizan, A.W.; Christensen, J.M.; Ebenzer, J.; Oakley, G.; Tattersall, J.; Sacks, R.; Harvey, R.J. Middle turbinate edema as a diagnostic marker of inhalant allergy. Int. Forum Allergy Rhinol. 2017, 7, 37–42. [Google Scholar] [CrossRef]
- Matsumoto, H.; Niimi, A.; Takemura, M.; Ueda, T.; Yamaguchi, M.; Matsuoka, H.; Jinnai, M.; Chin, K.; Mishima, M. Cough variant asthma: Potential pathophysiology and management. Allergol. Int. 2006, 55, 59–65. [Google Scholar] [CrossRef][Green Version]
- Niimi, A.; Ohbayashi, H.; Sagara, H.; Yamauchi, K.; Akiyama, K.; Takahashi, K.; Inoue, H.; Wakayama, T.; Kobayashi, H.; Hasegawa, M.; et al. Cough variant and cough-predominant asthma are major causes of persistent cough: A multicenter study in Japan. J. Asthma 2013, 50, 932–937. [Google Scholar] [CrossRef]
- Tanaka, H.; Yamada, G.; Saikai, T.; Hashimoto, M.; Tanaka, S.; Suzuki, K.; Fujii, M.; Takahashi, H.; Abe, S. Increased airway vascularity in newly diagnosed asthma using a high-magnification bronchovideoscope. Am. J. Respir. Crit. Care Med. 2003, 168, 1495–1499. [Google Scholar] [CrossRef] [PubMed]
- Kamimura, M.; Mouri, A.; Takayama, K.; Mizutani, T.; Hamamoto, Y.; Iikura, M.; Furihata, K. Cough challenge tests involving mechanical stimulation of the cervical trachea in patients with cough as a leading symptom. Respirology 2010, 15, 1244–1251. [Google Scholar] [CrossRef] [PubMed]
- Kamimura, M.; Izumi, S.; Hamamoto, Y.; Morita, A.; Toyota, E.; Kobayashi, N.; Kudo, K. Superiority of nebulized corticosteroids over dry powder inhalers in certain patients with cough variant asthma or cough-predominant asthma. Allergol. Int. 2012, 61, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Pavord, I.D. Cough and asthma. Pulm. Pharmacol. Ther. 2004, 17, 399–402. [Google Scholar] [CrossRef] [PubMed]
- Nelson, H.S. Prospects for antihistamines in the treatment of asthma. J. Allergy Clin. Immunol. 2003, 112 (Suppl. 4), S96–S100. [Google Scholar] [CrossRef] [PubMed]
- Buhl, R. Local oropharyngeal side effects of ICS in patients with asthma. Allergy 2006, 61, 518–526. [Google Scholar] [CrossRef]
- Bhalla, R.K.; Taylor, W.; Jones, A.S.; Roland, N.J. The inflammation produced by corticosteroid inhalers in the pharynx in asthmatics. Clin. Otolaryngol. 2008, 33, 581–586. [Google Scholar] [CrossRef]
- Bermede, O.; Sarıcaoğlu, M.C.; Baytaş, V.; Hasde, A.İ.; İnan, M.B.; Akar, A.R. Percutaneous ultrasound-guided versus bronchoscopy-guided dilatational tracheostomy after median sternotomy: A case-control study. Turk. J. Thorac. Cardiovasc. Surg. 2021, 29, 457–464. [Google Scholar] [CrossRef]
- Öztürk, M.; Uysal, E.; Bayramoğlu, Z.İ.; Özlü, M.Y.; Erdur, Ö. Measurement of the subglottic diameter of the trachea and its distance from the skin by ultrasonography in children. Int. J. Pediatr. Otorhinolaryngol. 2021, 150, 110946. [Google Scholar] [CrossRef]
- Ishii, H.; Suzuki, T.; Todo, H.; Kamimura, M.; Sugibayashi, K. Iontophoresis-facilitated delivery of prednisolone through throat skin to the trachea after topical application of its succinate salt. Pharm. Res. 2011, 28, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Al-Shouk, A.A.A.-H.M.; Tatar, İ. The blood supply of the inferior nasal concha (turbinate): A cadaveric anatomical study. Anat. Sci. Int. 2021, 96, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Neskey, D.; Eloy, J.A.; Casiano, R.R. Nasal, septal, and turbinate anatomy and embryology. Otolaryngol. Clin. N. Am. 2009, 42, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Funayama, E.; Yamamoto, Y.; Furukawa, H.; Murao, N.; Shichinohe, R.; Yamao, T.; Hayashi, T.; Oyama, A. Full-Thickness Entire Nasal Alar Reconstruction Using a Forehead Flap in Asians: No Cartilaginous Infrastructural Lining Is Necessary. J. Craniofac. Surg. 2017, 28, 734–737. [Google Scholar] [CrossRef]
- Löffler, H.; Happle, R. Profile of irritant patch testing with detergents: Sodium lauryl sulfate, sodium laureth sulfate and alkyl polyglucoside. Contact Dermat. 2003, 48, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Marrakchi, S.; Maibach, H.I. Sodium lauryl sulfate-induced irritation in the human face: Regional and age-related differences. Ski. Pharmacol. Physiol. 2006, 19, 177–180. [Google Scholar] [CrossRef]
- Coskey, R.J. Contact dermatitis caused by diphenhydramine hydrochloride. J. Am. Acad. Dermatol. 1983, 8, 204–206. [Google Scholar] [CrossRef] [PubMed]
- Rubegni, G.; Padula, T.; Calabrese, L.; D’Onghia, M.; Tognetti, L.; Cinotti, E.; Lazzeri, L.; Ermini, G.; Cartocci, A.; Tosi, G.M. Eyelid Contact Dermatitis: 25-Year Single-Center Retrospective Study. J. Clin. Med. 2025, 14, 823. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Leroy, T.; Geveaux, C.; Crucq, J.; Douven, L.F.; Neste, D.V. The face and neck: Regional variation in skin barrier function and reactivity. Ski. Res. Technol. 1998, 4, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Tagami, H. Location-related differences in structure and function of the stratum corneum with special emphasis on those of the facial skin. Int. J. Cosmet. Sci. 2008, 30, 413–434. [Google Scholar] [CrossRef] [PubMed]
- Pratchyapruit, W.; Kikuchi, K.; Gritiyarangasan, P.; Aiba, S.; Tagami, H. Functional analyses of the eyelid skin constituting the most soft and smooth area on the face: Contribution of its remarkably large superficial corneocytes to effective water-holding capacity of the stratum corneum. Ski. Res. Technol. 2007, 13, 169–175. [Google Scholar] [CrossRef] [PubMed]
- See, G.L.; Sagesaka, A.; Todo, H.; Wierzba, K.; Sugibayashi, K. PK and tissue distribution of pilocarpine after application to eyelid skin of rats. J. Pharm. Sci. 2019, 108, 2942–2948. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Bu, F.; Wu, D.; Jiang, P.; He, Q.; Yang, D.; Zhu, X.; Wang, Y.; Xiang, X. Physiologically Based Pharmacokinetic Modeling and Clinical Extrapolation for Topical Application of Pilocarpine on Eyelids: A Comprehensive Study. J. Pharm. Sci. 2024, 113, 2861–2870. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Lim, S.; Kwak, S.S.; Kim, J. Advancements in Skin-Mediated Drug Delivery: Mechanisms, Techniques, and Applications. Adv. Healthc. Mater. 2024, 13, e2302375. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamimura, M.; Todo, H.; Sugibayashi, K.; Asano, K. Topical Percutaneous Drug Delivery for Allergic Diseases: A Novel Strategy for Site-Directed Pharmacologic Modulation. Pharmaceutics 2025, 17, 867. https://doi.org/10.3390/pharmaceutics17070867
Kamimura M, Todo H, Sugibayashi K, Asano K. Topical Percutaneous Drug Delivery for Allergic Diseases: A Novel Strategy for Site-Directed Pharmacologic Modulation. Pharmaceutics. 2025; 17(7):867. https://doi.org/10.3390/pharmaceutics17070867
Chicago/Turabian StyleKamimura, Mitsuhiro, Hiroaki Todo, Kenji Sugibayashi, and Koichiro Asano. 2025. "Topical Percutaneous Drug Delivery for Allergic Diseases: A Novel Strategy for Site-Directed Pharmacologic Modulation" Pharmaceutics 17, no. 7: 867. https://doi.org/10.3390/pharmaceutics17070867
APA StyleKamimura, M., Todo, H., Sugibayashi, K., & Asano, K. (2025). Topical Percutaneous Drug Delivery for Allergic Diseases: A Novel Strategy for Site-Directed Pharmacologic Modulation. Pharmaceutics, 17(7), 867. https://doi.org/10.3390/pharmaceutics17070867







