Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus responsible for COVID-19, remains a major global health threat. The virus enters host cells by binding to the angiotensin-converting enzyme 2 (ACE2) receptor. Several small-molecule antiviral drugs, including molnupiravir, favipiravir, remdesivir, and nirmatrelvir have been shown to inhibit SARS-CoV-2 replication and are approved for treating SARS-CoV-2 infections. Nirmatrelvir inhibits the viral main protease (Mpro), a key enzyme for processing polyproteins in viral replication. In contrast, molnupiravir, favipiravir, and remdesivir are prodrugs that target RNA-dependent RNA polymerase (RdRp), which is crucial for genome replication and subgenomic RNA production. However, undergoing extensive metabolism profoundly impacts their therapeutic effects. Carboxylesterases (CES) are a family of enzymes that play an essential role in the metabolism of many drugs, especially prodrugs that require activation through hydrolysis. Molnupiravir is activated by carboxylesterase-2 (CES2), while remdesivir is hydrolytically activated by CES1 but inhibits CES2. Nirmatrelvir and remdesivir are oxidized by the same cytochrome P450 (CYP) enzyme. Additionally, various transporters are involved in the uptake or efflux of these drugs and/or their metabolites. It is well established that drug-metabolizing enzymes and transporters are differentially expressed depending on the cell type, and these genes exhibit significant polymorphisms. In this review, we examine how CES-related cellular and genetic factors influence the therapeutic activities of these widely used COVID-19 medications. This article highlights implications for improving product design, targeted inhibition, and personalized medicine by exploring genetic variations and their impact on drug metabolism and efficacy.
1. Introduction
The COVID-19 pandemic, caused by the SARS-CoV-2 virus, has profoundly affected healthcare systems and communications worldwide. Since 2019, the illness has been widespread, causing severe respiratory complications and significant mortality across continents [1]. The unprecedented health emergency has triggered an exponential increase in research, aiming to understand and mitigate the virus’s impact. The advent of vaccines targeting the SARS-CoV-2 spike (S) protein was a major milestone in combating COVID-19. However, the rapid mutation rate of the S protein has necessitated ongoing updates to vaccine formulations, potentially on a seasonal basis, akin to influenza vaccines.
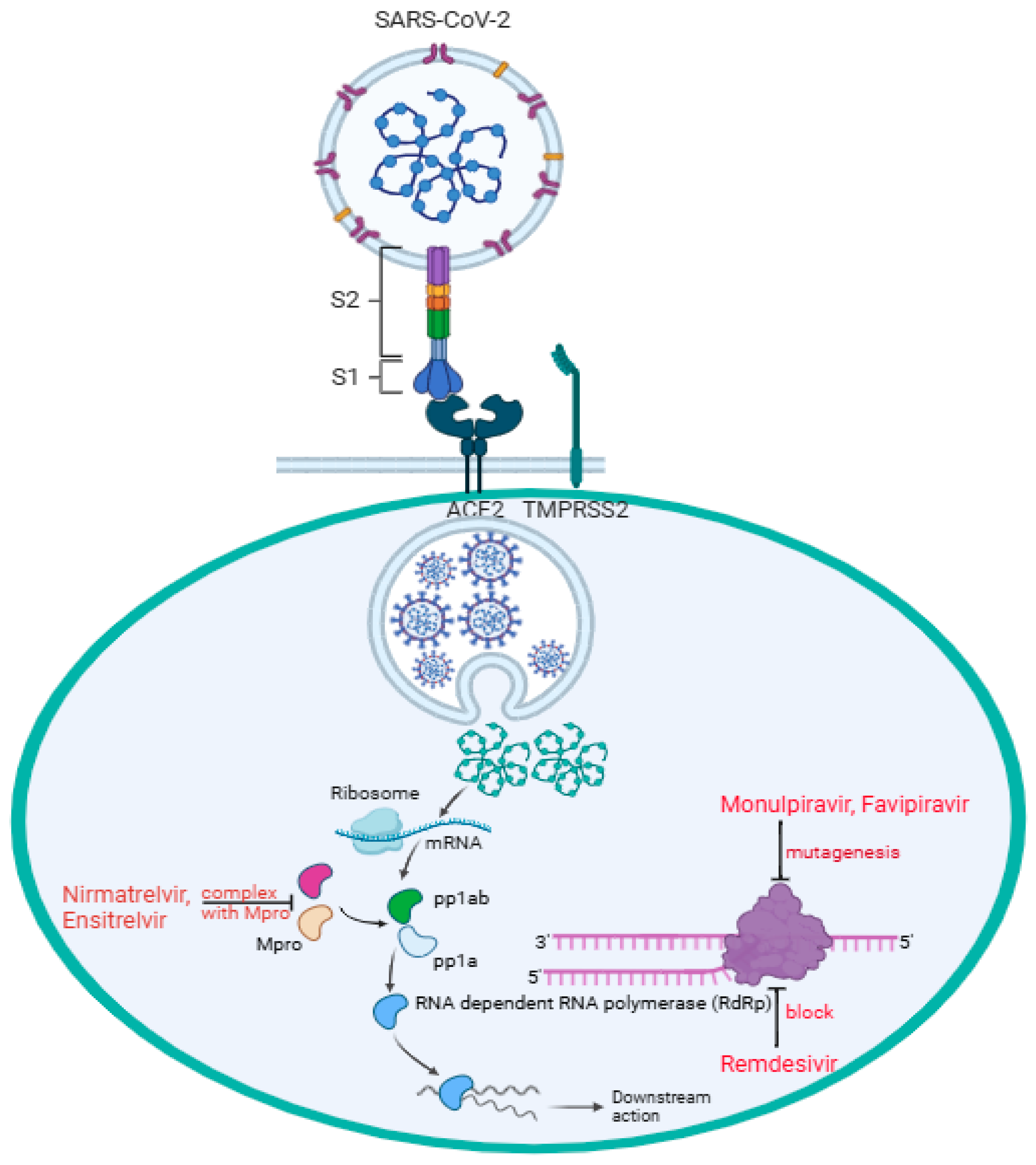
The binding of SARS-CoV-2 to the angiotensin-converting enzyme 2 (ACE2) receptor is a critical step in viral entry into the cells [2]. In Figure 1, the virus S1 subunit is binding with the host ACE2 receptor directly at the receptor-biding domain (RBD) B with the presence of the transmembrane protease, serine 2 (TMPRSS2), expressed on the surface of the respiratory epithelial cells. TMPRSS2 facilitates the activation of the S protein of SARS-CoV-2 infection, allowing the virus to fuse with the cell membrane and initiate infection [2,3]. Mutations in RBD B can enhance binding affinity and increase infectivity; thus, variants like alpha, beta, delta, and omicron have notable RBD mutations affecting transmissibility and immune escape [2,4]. In addition, the polybasic furin cleavage site (FCS) located between S1 and S2 subunits increases SARS-CoV-2 transmissibility [5,6,7]. Some studies suggest that the presence of O-linked glycans, sugar molecules attached to the S protein via oxygen-linked glycosylation, may play a role in immune evasion and host interactions [8,9]. This reflects the challenge of establishing long-lasting immunization strategies against the evolving virus. In parallel, antiviral treatments such as remdesivir, molnupiravir, and nirmatrevir have played an essential role as therapeutic interventions in managing the infectious disease by targeting viral replication. Remdesivir, molnupiravir, and nirmatrelvir have been authorized for use by the FDA and offer a treatment option that does not rely on specific viral strains. In this review, we examine how enzymatic, cellular, and genetic factors influence the therapeutic activities of remdesivir, molnupiravir, and nirmatrelvir against COVID-19.
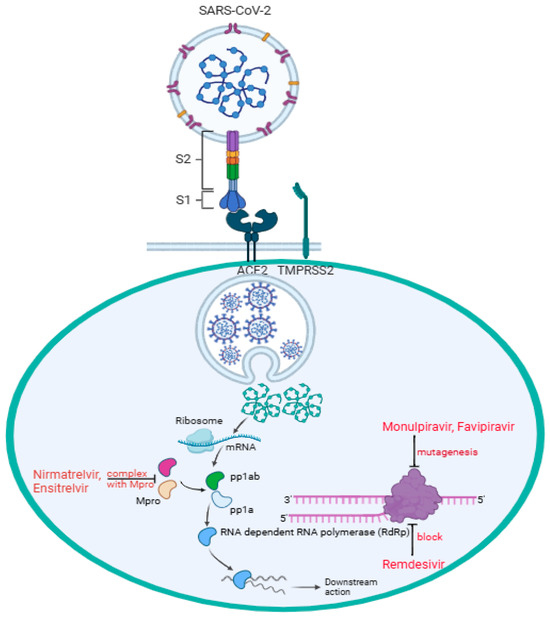
Figure 1.
SARS-CoV-2 enters the target cell, particularly pneumocytes, through direct interaction between the cell surface ACE2 receptor and RBD B of the virus subunit S1, while the subunit S2 facilitates fusion between the host and the viral cell membranes. Followed by viral endocytosis, viral genomic mRNA is released into the host cell cytoplasm. Two-thirds of the mRNA, mainly at the 5′ end, encoded open reading frames (ORF1a and ORF1b), which are translated into two polypeptides, pp1a and pp1ab, and the remaining one-third of the mRNA, mainly at the 3′ end, serves as a template for transcription and replication. Nirmatrelvir and ensitrelvir form a complex with Mpro, stopping pp1a and pp1ab from being processed into functional non-structural proteins (nsps) 4–16 for viral replication. Remdesivir blocks RdRp (nsp12) by mimicking adenosine (A) nucleoside incorporated into the RNA strand, preventing the virus from replication. Through RdRp, molnupiravir mimicking cytidine (C) or uridine (U) nucleosides introduces multiple mutations during replication, causing lethal mutagenesis, and effectively stopping the downstream action. Through RdRp, favipiravir mimicking guanine (G) gets incorporated into RNA strands, inducing mutations, thus causing lethal mutagenesis.
2. Background
2.1. Antiviral Drugs for the Treatment of COVID-19
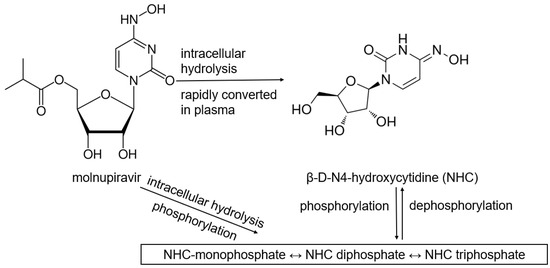
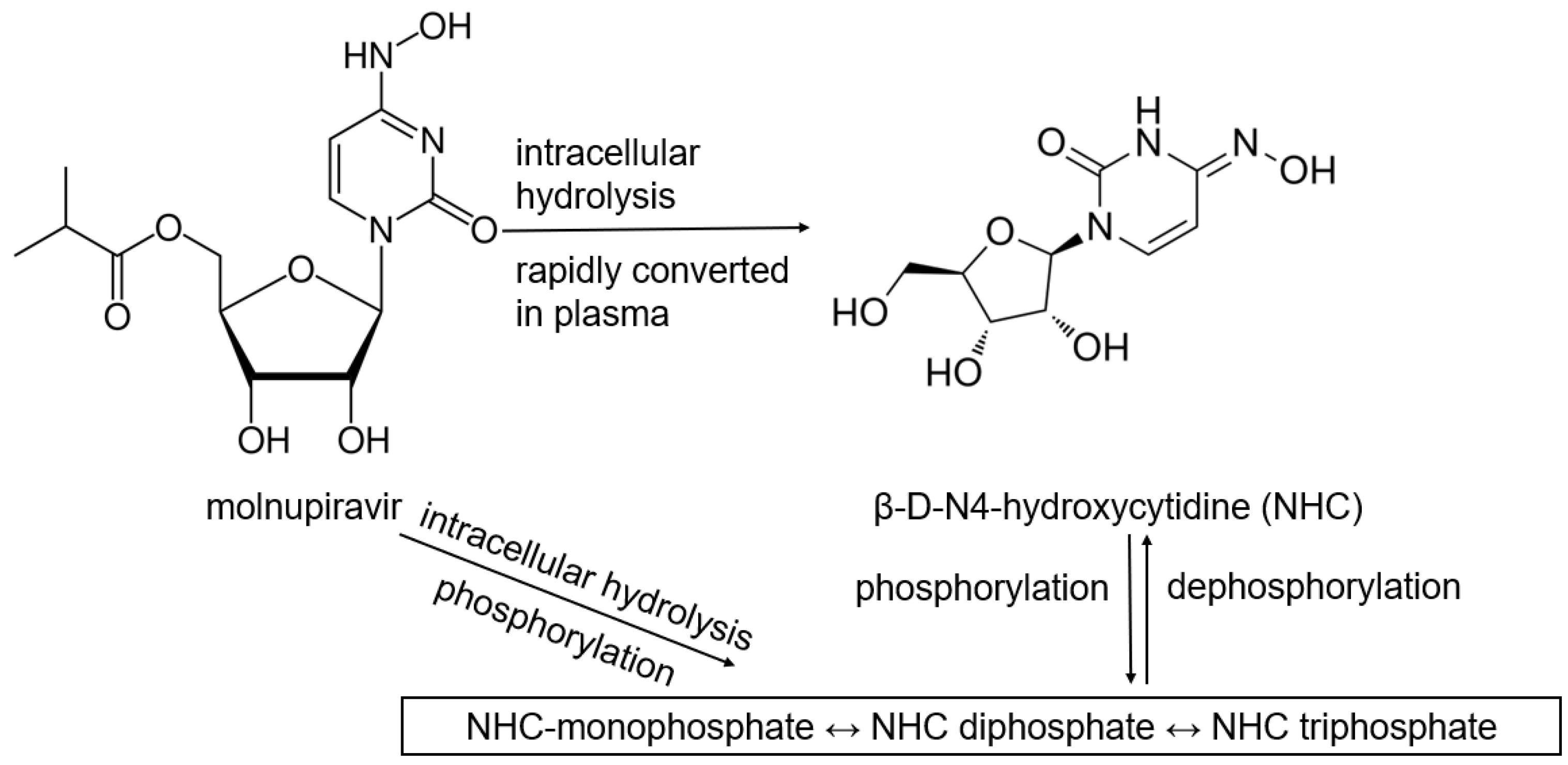
Table 1 lists antiviral drugs, including products that are required to be metabolized into their active forms. Most antiviral drugs are prodrugs since their parent drugs may not be as well absorbed, distributed, and activated at the site of infection. Another reason that prodrugs are used is that some active drugs degrade too quickly before reaching their target site of action. Moreover, some drugs are formulated to be selectively activated only within specific infected or targeted cells. Targeted activation by enzymes that are overexpressed in diseased or cancerous cells enables site-specific drug release, thus, reducing side effects [10]. Remdesivir was the first antiviral approved for COVID-19, exhibiting efficacy against SARS-CoV-2 by inhibiting the viral RNA-dependent RNA polymerase (RdRp). After being administered intravenously, remdesivir gets converted into an active nucleotide analog. Remdesivir incorporates into viral RNA chains, resulting in premature termination of viral replication [10,11,12]. Molnupiravir indirectly targets RdRp of SARS-CoV-2 by introducing a mutation into the viral genome through its active form NHC (β-D-N4-hydroxycytidine)-triphosphate, leading to lethal mutagenesis [13,14,15]. Favipiravir is a prodrug of guanosine (G) analog that inhibits RdRp and induces viral mutations, causing mutagenesis. However, favipiravir is not FDA-approved due to concerns about its inconsistent results against COVID-19 in clinical trials [14,15,16,17]. Molnupiravir, favipiravir, and remdesivir are prodrugs targeting RdRp; these nucleoside analogs typically exhibit poor cellular permeability. Phosphoramidate prodrug forms can significantly enhance their cellular uptake and metabolic activation. However, remdesivir resistance has been linked to mutations in the RdRp gene, such as E802D, which impede the drug incorporation into viral RNA [18]. Although molnupiravir has been reported to have a high barrier to drug resistance [19], mutations in RdRp, particularly within the nsp12 region, have been identified and may affect molnupiravir binding [20].
Nirmatrelvir, an active antiviral compound that inhibits proteases, on the other hand, bypasses the need for metabolic activation. Nirmatrelvir is often co-administered with ritonavir, a pharmacokinetic enhancer that can inhibit cytochrome P450 (CYP) metabolism, thereby increasing nirmatrelvir’s plasma concentration to block the viral main protease [Mpro, also known as 3-chymotrypsin-like proteases (3CLpro)]. By inhibiting Mpro, nirmatrelvir prevents the cleavage of polyproteins necessary for viral replication [21]. Recent results from the Phase 3 SCORPIO-PEP trial highlighted a breakthrough in COVID-19 treatment and prevention: Ensitrevil, a Mpro inhibitor, became the first and only oral antiviral to demonstrate significant efficacy as post-exposure prophylaxis against SARS-CoV-2 [22]. Figure 1 shows the mechanisms of remdesivir, molnupiravir, and nirmatrevil targeting the SARS-CoV-2 virus. An active drug, like nirmatrelvir, directly works against the virus or modulates the immune system without requiring metabolic activation. Resistance-conferring mutations, such as P132H and M49L occurred in the SARS-CoV-2 Mpro nsp5 domain, have been observed in vitro and in some circulating strains [23,24]. Nevertheless, nirmatrelvir may be more beneficial for COVID-19 patients who have metabolic issues such as liver failure.
While developing antiviral drugs represents a significant scientific breakthrough for treating COVID-19, emerging research suggests their effectiveness may be variable, especially for prodrugs. For instance, in studies conducted on SARS-CoV-2 infected Vero E6 cells, the EC50 values of remdesivir were reported at different values of 0.77 μM by Wang et al. [25,26,27], 1.65 µM by Pruijssers et al. [25,26] and ranging from 0.66 to 5.63 [28]. Experiments on cell lines have revealed that the same treatment produces varying quantities of active products. These observations highlight the need for a deeper understanding of the mechanisms governing drug action both within cells and systemically.

Table 1.
List of antiviral drugs for the treatment of COVID-19.
Table 1.
List of antiviral drugs for the treatment of COVID-19.
| Drug Name | Administration Route | Prodrug Activation | Mechanism of Action | Note |
|---|---|---|---|---|
| Remdesivir (Veklury®) | Intravenously infusion | remdesivir triphosphate (RTP) | RdRp inhibitor | |
| Nirmatrelvir/Ritonavir (Paxlovid™) 1 | Oral | Nirmatrelvir is not a prodrug | Protease inhibitor | Emergency Use Authorization (EUA) in Dec. 2021, later with full approval |
| Molnupiravir (LAGEVRIO™) | Oral | NHC triphosphate | RdRp inhibitor | EUA 2 |
| Favifpiravir | Oral | favipiravir ribofuranosyl-5′-triphostphate | RdRp inhibitor | |
| Ensitrelvir | Oral | Ensitrelvir is not a prodrug | Protease inhibitor |
1 In Paxlovid™, ritonavir is used not for its antiviral activity, but to increase the concentration of nirmatrelvir by inhibiting CYP3A4, thus, prolonging nirmatrelvir’s half-life [29]. 2 LAGEVRIO™ (molnupiravir) is prescribed only if Paxlovid™ and remdesivir are not options for mild to moderate COVID-19 in patients at risk, under FDA EUA.
2.2. Enzymes Involved in Antiviral Drug Activation and Metabolism: Carboxylesterases
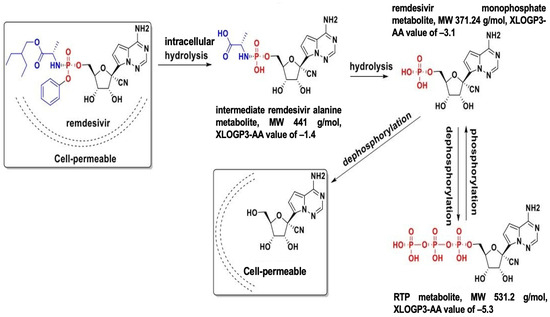
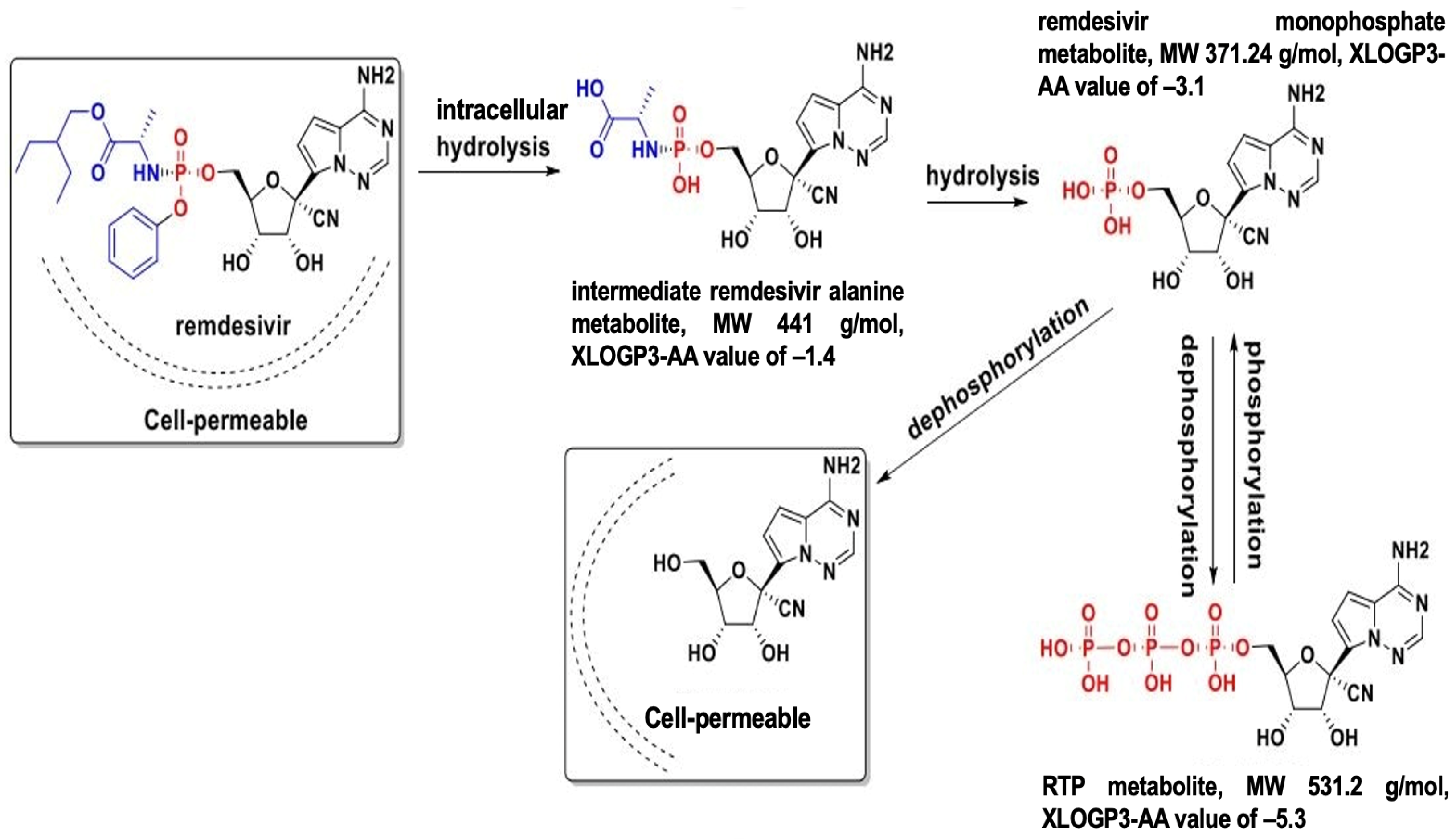
Carboxylesterases (CES) belong to an esterase enzyme family that hydrolyzes esters, amides, thioesters, and carbamates into their corresponding alcohol and carboxylic acid. CES is widely distributed throughout the body [30]. Among the CES family, CES1 and CES2 are primarily involved in the activation and metabolism of prodrugs by hydrolyzing ester bonds in prodrugs, converting them into their active metabolites, which can exert therapeutic effects [30,31]. For example, remdesivir is metabolized in the liver by CES1, converting it to remdesivir active metabolites, which are then phosphorylated into RTP inside the cells, inhibiting viral replication [10,32,33]. In contrast, molnupiravir is hydrolytically activated by CES2 [34]. Interestingly, our previous study demonstrated that remdesivir at nanomolar concentrations could inhibit CES2 through covalent modifications, while no inhibition was detected on CES1, indicating the high specificity of the inhibition [35].
Additionally, given the fact that interspecies variability in carboxylesterase activity can significantly impact prodrug activation, and some animal studies can provide valuable translational insights [36,37], this review focuses on COVID-19 treatment in human subjects to ensure relevance to human pharmacokinetics and metabolism. Moreover, a respiratory syncytial virus inhibitor (e.g., ST-2) exhibited greater metabolic stability in human blood compared to mouse and rat blood due to lower carboxylesterase activity in humans, which enhances hydrolysis resistance, thus, highlighting improved pharmacological response in humans, especially in viral infections in the lung [36].
Systemic inflammation and cytokine storms during viral infections can cause hepatic drug-metabolizing enzyme suppression [38]. Thus, we believe that CES is downregulated and CES function could be transiently reduced during SARS-CoV-2 infection. Although direct evidence for CES downregulation in the context of COVID-19 is limited, the link between the viral infection and CES dysregulation can be supported by transcriptomic analyses of liver biopsies from COVID-19 patients [39] and proteomic studies with evidence of dysregulated hepatic protein networks in COVID-19 [40,41].
3. Carboxylesterase 1 (CES1)
3.1. CES1 Expression and Substrate Specificity
Although CES1 and CES2 are two dominant enzymes involved in drug metabolism, they exhibit distinct distributions and substrate types [25,26,27,28,29,30]. CES1 is mainly expressed in the liver and gall bladder, as well as in the lungs and subsets of cells in the gastrointestinal (GI) tract. CES1 (65.52 kDa) is the most abundant enzyme in the liver and plays an important role in the metabolism of esters, thioesters, and amides. CES1 is encoded by the CES1 gene, located on chromosome 16q12.2.
3.2. CES1 Pharmacogenetic Variability
Several CES1 variants have been shown to influence the efficacy of medications and clinical outcomes, highlighting the importance of individual genetic variation in drug metabolism (Table 2). The G143E CES1 variant has been observed in various clinical populations, with a frequency ranging from 2.5% to 5.8% depending on the cohort [42,43,44,45,46,47,48]. The G143E variant can affect the functionality of CES1, thus, affecting the efficacy and safety of drugs that rely on CES1 for activation/inactivation, including remdesivir. Interethnic variability in CES1 polymorphisms has significant implications in the context of a global pandemic like COVID-19 [42]. Population-based frequency data of G143E and rs2244613 is shown in Table 2. The prevalence of rs2244613 [47,49,50,51,52], as well as other genetic polymorphisms of the CES1 gene (Table 2), suggests pharmacogenomic factors related to dosing and drug responses could be significant for drugs like remdesivir. It is important to note that CES1 exhibits various genetic variability, and the variants that have been found to significantly impact CES1 enzyme function have been well-documented. Identifying the variants and their prevalence can help with dosing adjustments and tailoring drug therapy based on pharmacogenomic data, as well as providing valuable insights into the genetic variability of response to treatment within a certain population. When a drug is administered as a prodrug to the body, it is converted to its active form by CES and simultaneously transported and metabolized by P-glycoprotein (P-gp) and CYP enzymes in the liver and intestines. Therefore, CES, P-gp, and CYP genetic variants and their connections can potentially affect the metabolism and the plasma level of remdesivir. Meanwhile, CYP genetic variations can affect the metabolism of nirmatrevir and remdesivir, as both drugs undergo oxidation by CYP [53]. In clinical settings, the choice between remdesivir and Paxlovid™ should depend a lot on drug metabolism, whether the patient with COVID-19 infection has CES or CYP genetic variations, and how much CYP involvement determines how well the patient responds to the treatment.

Table 2.
List of genetic polymorphisms of CES1 affecting the metabolism of COVID-19 drug.
4. Carboxylesterase 2 (CES2)
4.1. CES2 Expression and Substrate Specificity
For patients with CYP genetic variations, remdesivir is generally a safer choice since its activation depends on CES rather than CYP, but for patients who receive remdesivir as a COVID-19 treatment, there is a risk of drug-drug interactions with other medications metabolized by CES2 since remdesivir has been shown to inhibit CES2 [35]. Due to its high potency and irreversible inhibition, caution is advised when using remdesivir alongside medications that are hydrolyzed by CES2 such as molnupiravir [34], gemcitabine prodrugs [59], irinotecan [59,60], clopidogrel [59,61], vicagrel [61], orlistat [62], and even lipid-based drug/drug delivery systems since CES2 is known for being responsible for lipid metabolism in the intestines. CES2, which is found in the liver and intestines, hydrolyzes esters that contain a large alcohol group and a small acyl group, while CES1 hydrolyzes esters that contain a small alcohol group and a large acyl group. The crystal structure of mouse CES2 was reported to have structural parallels with human CES1 in substrate regulation and release [37].
4.2. CES2 Pharmacogenetic Variability
Like CES1, CES2 also plays a key role in hydrolyzing drugs, which can affect how certain drugs are activated or deactivated in the body, thus, genetic variations in CES2 can affect how quickly a drug is metabolized, how much active drug is available in the system, and the duration of its effect (Table 3). Interestingly, CES2 is more polymorphic across Asian populations, as exemplified by studies in Japanese individuals [42,63,64]; however, further research is needed to definitively confirm a strong association between rs2241409 and reduced CES2 activity, specifically in Asian populations. In general, CES1 is considered more well-studied than CES2; therefore, the clinical relevance of CES1 and the impact of CES1 variants on prodrug activation, especially G143E, has been well demonstrated. Several assertions regarding CES variants are based on isolated in vitro studies. This review compiles available data on CES variants affecting prodrug activation and takes into consideration findings from various studies, including in vitro studies. Some are isolated in vitro studies without further evidence for consistency or reproducibility across diverse experimental models. While we aim to be comprehensive, it is important to differentiate high-confidence and preliminary evidence for accurately interpreting the implications.

Table 3.
List of genetic polymorphisms of CES2 affecting the metabolism of COVID-19 drug.
6. Implications and Conclusions
While it is well established that COVID-19 can broadly suppress xenobiotic metabolism pathways, the impact of SARS-CoV-2 infection on CES1 and CES2 activity remains underexplored, and experimental validation in hepatocytes or in vivo models needs to be performed. This review highlights the clinical significance of CES1 and CES2 genetic polymorphisms, particularly concerning remdesivir and monupiravir. Given the role of kinases in the phosphorylation of the activation of remdesivir and possibly monupiravir, systematic profiling of kinase expression and activity across cell types could help refine predictions of antiviral efficacy. Yet, there is a lack of clinical trials for prodrugs requiring multi-step activation pathways, where hydrolysis is followed by phosphorylation. CES gene variants affect drug activation, thus, raising concerns about altered drug activation, distribution, and elimination, necessitating further consideration of their influence on transmembrane transport. While CES1 polymorphisms such as G143E can impair hydrolytic activation, additional genetic mechanisms of CES induced during SARS-CoV-2 infection may further compromise drug activation. Future studies should further explore and include the regulatory mechanisms affecting CES expression during SARS-CoV-2 infection, including transcriptional control by factors such as activating transcription factors (ATF). For example, ATF3 can increase hepatic CES1 and CES2 protein levels.
Cellular metabolizing enzymes and transporters significantly impact the fate of common antiviral medications used for SARS-CoV-2 infection. In conclusion, remdesivir and molnupiravir show a complex relationship between ACE2/TMPRSS2 expression at the entry sites and the intracellular availability of CES (activation), ENT (uptake), and CYP enzymes (metabolism or inactivation), suggesting that host genetic and cellular expression profiles may influence antiviral efficacy and toxicity. The connection may extend to nirmatrevir, where the coordinated activity of the enzymes also determines therapeutic outcomes. We provide a summary table for clinicians that bridges CES with drug-specific activation pathways and interactions in COVID-19 treatment, especially for complex treatment regimens (Table S1).
CES genotyping has been explored in other therapeutic areas (Table 2 and Table 3), where functional polymorphisms have been associated with altered drug response. We suggest developing CES-based diagnostics, including genotyping as a foundational tool or clinical guidelines supporting CES1/2 genotyping for antiviral therapy. The efficacy of antiviral drugs against SARS-CoV-2 is challenged not only by host-related metabolic variability (e.g., CES polymorphisms) but also by viral resistance, which is critical for long-term treatment strategies. The interplay of viral mutations and host enzymatic activation presents a significant challenge related to inefficient CES activation and patient-specific CES profiles, especially under COVID-19 conditions.
Targeted delivery strategies, particularly those focusing on CES2-rich intestinal tissues, offer an opportunity to maximize the bioavailability and optimize the activation and metabolism of prodrugs like molnupiravir or novel remdesivir analogs with desired dosage forms and routes of administration, less frequent dosing, and reduced toxicity. Furthermore, the development of precision antiviral therapies that correspond with patient genotypes and tissue-specific expression patterns can also be made possible by shifting the focus toward cell-type-specific drug activation by integrating local enzyme expression and regulation. This strongly supports the potential of personalized medicine in antiviral therapy.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pharmaceutics17070832/s1, Table S1: CES-mediated activation of antiviral drugs and potential drug-drug interactions.
Author Contributions
Conceptualization, B.Y. and Y.S.; methodology, B.Y., Y.S., and W.E.; software, W.E. and L.D.; validation, L.D., W.E., and B.Y.; formal analysis, Y.S. and W.E.; investigation, Y.S., W.E., and L.D.; resources, B.Y.; data curation, Y.S., W.E., and L.D.; writing—original draft preparation, Y.S., B.Y., W.E., and L.D.; writing—review and editing, L.D. and B.Y.; visualization, L.D.; supervision, B.Y.; project administration, B.Y.; funding acquisition, B.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Institutes of Health (Grants: R01 AI172959).
Data Availability Statement
The authors confirm that the data supporting this study are within the article.
Acknowledgments
Figure 1 was created in BioRender. Linh, D (2025) https://app.biorender.com/illustrations/67ed778d415074e26f2593dd?slideId=a9aa00ef-fcc4-4b68-8ac4-3d933719b582 (accessed on 30 March 2025). Figure 2 is based on Figure “Proposed remdesivir metabolic pathway and chemical structures ofmetabolites” by Ananya Mandal (Remdesivir metabolite GS-441524 inhibits SARS CoV-2 in mouse model, finds study. Available online at https://www.news-medical.net/news/20201102/Remdesivir-metabolite-GS-441524-inhibits-SARS-CoV-2-in-mouse-model-finds-study.aspx, accessed on 1 April 2025).
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| SARS-CoV-2 | severe acute respiratory syndrome coronavirus 2 |
| ACE2 | angiotensin-converting enzyme 2 |
| Mpro | main protease |
| 3CLpro | 3-chymotrypsin-like proteases |
| RdRp | RNA-dependent RNA polymerase |
| CES | carboxylesterases |
| CYP | cytochrome P450 |
| S | spike |
| RBD | receptor-biding domain |
| TMPRSS2 | transmembrane protease, serine 2 |
| FCS | furin cleavage site |
| GI | gastrointestinal |
| NHC | β-D-N4-hydroxycytidine |
| RTP | remdesivir triphosphate |
| EUA | Emergency Use Authorization |
| ORF | open reading frames |
| nsps | non-structure proteins |
| P-gp | P-glycoprotein |
| SNP | single nucleotide polymorphism |
| TAF | tenofovir alafenamide |
| TFV | tenofovir |
| PBMC | peripheral blood mononuclear cells |
| SLC | solute carrier |
| OATP | organic anion transporting polypeptide |
| ENT | equilibrative nucleoside transporter |
| MDCK | Madin-Darby canine kidney |
| CNT | concentrative nucleoside transporter |
References
- Pollard, C.A.; Morran, M.P.; Nestor-Kalinoski, A.L. The COVID-19 pandemic: A global health crisis. Physiol. Genomics 2020, 52, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Beyerstedt, S.; Casaro, E.B.; Rangel, É.B. COVID-19: Angiotensin-converting enzyme 2 (ACE2) expression and tissue susceptibility to SARS-CoV-2 infection. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 905–919. [Google Scholar] [CrossRef] [PubMed]
- Zang, R.; Gomez Castro, M.F.; McCune, B.T.; Zeng, Q.; Rothlauf, P.W.; Sonnek, N.M.; Liu, Z.; Brulois, K.F.; Wang, X.; Greenberg, H.B.; et al. TMPRSS2 and TMPRSS4 promote SARS-CoV-2 infection of human small intestinal enterocytes. Sci. Immunol. 2020, 5, eabc3582. [Google Scholar] [CrossRef] [PubMed]
- Xue, S.; Han, Y.; Wu, F.; Wang, Q. Mutations in the SARS-CoV-2 spike receptor binding domain and their delicate balance between ACE2 affinity and antibody evasion. Protein Cell 2024, 15, 403–418. [Google Scholar] [CrossRef]
- Lavie, M.; Dubuisson, J.; Belouzard, S. SARS-CoV-2 Spike Furin Cleavage Site and S2’ Basic Residues Modulate the Entry Process in a Host Cell-Dependent Manner. J. Virol. 2022, 96, e0047422. [Google Scholar] [CrossRef]
- Peacock, T.P.; Goldhill, D.H.; Zhou, J.; Baillon, L.; Frise, R.; Swann, O.C.; Kugathasan, R.; Penn, R.; Brown, J.C.; Sanchez-David, R.Y.; et al. The furin cleavage site in the SARS-CoV-2 spike protein is required for transmission in ferrets. Nat. Microbiol. 2021, 6, 899–909. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Wu, J.; Yu, Y.; Liu, S.; Li, T.; Li, Q.; Ding, R.; Wang, H.; Nie, J.; et al. A second functional furin site in the SARS-CoV-2 spike protein. Emerg. Microbes Infect. 2022, 11, 182–194. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, H.; Wang, H. Glycans of SARS-CoV-2 Spike Protein in Virus Infection and Antibody Production. Front. Mol. Biosci. 2021, 8, 629873. [Google Scholar] [CrossRef]
- Aloor, A.; Aradhya, R.; Venugopal, P.; Gopalakrishnan Nair, B.; Suravajhala, R. Glycosylation in SARS-CoV-2 variants: A path to infection and recovery. Biochem. Pharmacol. 2022, 206, 115335. [Google Scholar] [CrossRef]
- Markovic, M.; Ben-Shabat, S.; Dahan, A. Prodrugs for Improved Drug Delivery: Lessons Learned from Recently Developed and Marketed Products. Pharmaceutics 2020, 12, 1031. [Google Scholar] [CrossRef]
- Gordon, C.J.; Tchesnokov, E.P.; Woolner, E.; Perry, J.K.; Feng, J.Y.; Porter, D.P.; Götte, M. Remdesivir is a direct-acting antiviral that inhibits RNA-dependent RNA polymerase from severe acute respiratory syndrome coronavirus 2 with high potency. J. Biol. Chem. 2020, 295, 6785–6797. [Google Scholar] [CrossRef]
- Eastman, R.T.; Roth, J.S.; Brimacombe, K.R.; Simeonov, A.; Shen, M.; Patnaik, S.; Hall, M.D. Remdesivir: A Review of Its Discovery and Development Leading to Emergency Use Authorization for Treatment of COVID-19. ACS Cent. Sci. 2020, 6, 672–683. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Pang, Z.; Li, M.; Lou, F.; An, X.; Zhu, S.; Song, L.; Tong, Y.; Fan, H.; Fan, J. Molnupiravir and Its Antiviral Activity Against COVID-19. Front. Immunol. 2022, 13, 855496. [Google Scholar] [CrossRef]
- Hashemian, S.M.R.; Pourhanifeh, M.H.; Hamblin, M.R.; Shahrzad, M.K.; Mirzaei, H. RdRp inhibitors and COVID-19: Is molnupiravir a good option? Biomed. Pharmacother. 2022, 146, 112517. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; He, G.; Huang, W. A novel model of molnupiravir against SARS-CoV-2 replication: Accumulated RNA mutations to induce error catastrophe. Signal Transduct. Target. Ther. 2021, 6, 410. [Google Scholar] [CrossRef]
- Yasri, S.; Wiwanitki, V. Molnupiravir, favipiravir and other antiviral drugs with proposed potentials for management of COVID-19: A concern on antioxidant aspect. Int. J. Biochem. Mol. Biol. 2022, 13, 1–4. [Google Scholar] [PubMed]
- Alamer, A.; Alrashed, A.A.; Alfaifi, M.; Alosaimi, B.; AlHassar, F.; Almutairi, M.; Howaidi, J.; Almutairi, W.; Mohzari, Y.; Sulaiman, T.; et al. Effectiveness and safety of favipiravir compared to supportive care in moderately to critically ill COVID-19 patients: A retrospective study with propensity score matching sensitivity analysis. Curr. Med. Res. Opin. 2021, 37, 1085–1097. [Google Scholar] [CrossRef]
- Gandhi, S.; Klein, J.; Robertson, A.J.; Peña-Hernández, M.A.; Lin, M.J.; Roychoudhury, P.; Lu, P.; Fournier, J.; Ferguson, D.; Mohamed Bakhash, S.A.K.; et al. De novo emergence of a remdesivir resistance mutation during treatment of persistent SARS-CoV-2 infection in an immunocompromised patient: A case report. Nat. Commun. 2022, 13, 1547. [Google Scholar] [CrossRef]
- Strizki, J.M.; Gaspar, J.M.; Howe, J.A.; Hutchins, B.; Mohri, H.; Nair, M.S.; Kinek, K.C.; McKenna, P.; Goh, S.L.; Murgolo, N. Molnupiravir maintains antiviral activity against SARS-CoV-2 variants and exhibits a high barrier to the development of resistance. Antimicrob. Agents Chemother. 2024, 68, e0095323. [Google Scholar] [CrossRef]
- Kabinger, F.; Stiller, C.; Schmitzová, J.; Dienemann, C.; Kokic, G.; Hillen, H.S.; Höbartner, C.; Cramer, P. Mechanism of molnupiravir-induced SARS-CoV-2 mutagenesis. Nat. Struct. Mol. Biol. 2021, 28, 740–746. [Google Scholar] [CrossRef]
- Joyce, R.P.; Hu, V.W.; Wang, J. The history, mechanism, and perspectives of nirmatrelvir (PF-07321332): An orally bioavailable main protease inhibitor used in combination with ritonavir to reduce COVID-19-related hospitalizations. Med. Chem. Res. 2022, 31, 1637–1646. [Google Scholar] [CrossRef]
- Ohmagari, N.; Yotsuyanagi, H.; Doi, Y.; Yamato, M.; Imamura, T.; Sakaguchi, H.; Yamanaka, H.; Imaoka, R.; Fukushi, A.; Ichihashi, G.; et al. Efficacy and Safety of Ensitrelvir for Asymptomatic or Mild COVID-19: An Exploratory Analysis of a Multicenter, Randomized, Phase 2b/3 Clinical Trial. Influenza Other Respir. Viruses 2024, 18, e13338. [Google Scholar] [CrossRef]
- Sacco, M.D.; Hu, Y.; Gongora, M.V.; Meilleur, F.; Kemp, M.T.; Zhang, X.; Wang, J.; Chen, Y. The P132H mutation in the main protease of Omicron SARS-CoV-2 decreases thermal stability without compromising catalysis or small-molecule drug inhibition. Cell Res. 2022, 32, 498–500. [Google Scholar] [CrossRef] [PubMed]
- Bouzidi, H.S.; Driouich, J.S.; Klitting, R.; Bernadin, O.; Piorkowski, G.; Amaral, R.; Fraisse, L.; Mowbray, C.E.; Scandale, I.; Escudié, F.; et al. Generation and evaluation of protease inhibitor-resistant SARS-CoV-2 strains. Antivir. Res. 2024, 222, 105814. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Cao, R.; Zhang, L.; Yang, X.; Liu, J.; Xu, M.; Shi, Z.; Hu, Z.; Zhong, W.; Xiao, G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020, 30, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Pruijssers, A.J.; George, A.S.; Schäfer, A.; Leist, S.R.; Gralinksi, L.E.; Dinnon, K.H., III; Yount, B.L.; Agostini, M.L.; Stevens, L.J.; Chappell, J.D.; et al. Remdesivir Inhibits SARS-CoV-2 in Human Lung Cells and Chimeric SARS-CoV Expressing the SARS-CoV-2 RNA Polymerase in Mice. Cell Rep. 2020, 32, 107940. [Google Scholar] [CrossRef]
- Ko, W.C.; Rolain, J.M.; Lee, N.Y.; Chen, P.L.; Huang, C.T.; Lee, P.I.; Hsueh, P.R. Arguments in favour of remdesivir for treating SARS-CoV-2 infections. Int. J. Antimicrob. Agents 2020, 55, 105933. [Google Scholar] [CrossRef]
- Macip, G.; Garcia-Segura, P.; Mestres-Truyol, J.; Saldivar-Espinoza, B.; Pujadas, G.; Garcia-Vallvé, S. A Review of the Current Landscape of SARS-CoV-2 Main Protease Inhibitors: Have We Hit the Bullseye Yet? Int. J. Mol. Sci. 2021, 23, 259. [Google Scholar] [CrossRef]
- Marzolini, C.; Kuritzkes, D.R.; Marra, F.; Boyle, A.; Gibbons, S.; Flexner, C.; Pozniak, A.; Boffito, M.; Waters, L.; Burger, D.; et al. Recommendations for the Management of Drug-Drug Interactions Between the COVID-19 Antiviral Nirmatrelvir/Ritonavir (Paxlovid) and Comedications. Clin. Pharmacol. Ther. 2022, 112, 1191–1200. [Google Scholar] [CrossRef]
- Yan, B. Carboxylesterases. Part II: Enzyme Systems Involved in Drug Metabolism and Interactions in Animals and Humans. In Encyclopedia of Drug Metabolism and Interactions, 1st ed.; Lyubimov, A.V., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012. [Google Scholar] [CrossRef]
- Wang, D.; Zou, L.; Jin, Q.; Hou, J.; Ge, G.; Yang, L. Human carboxylesterases: A comprehensive review. Acta Pharm. Sin. B 2018, 8, 699–712. [Google Scholar] [CrossRef]
- Shen, Y.; Eades, W.; Yan, B. The COVID-19 Medicine Remdesivir Is Therapeutically Activated by Carboxylesterase-1, and Excessive Hydrolysis Increases Cytotoxicity. Hepatol. Commun. 2021, 5, 1622–1623. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Liclican, A.; Xu, Y.; Pitts, J.; Niu, C.; Zhang, J.; Kim, C.; Zhao, X.; Soohoo, D.; Babusis, D.; et al. Key Metabolic Enzymes Involved in Remdesivir Activation in Human Lung Cells. Antimicrob. Agents Chemother. 2021, 65, e0060221. [Google Scholar] [CrossRef]
- Shen, Y.; Eades, W.; Liu, W.; Yan, B. The COVID-19 Oral Drug Molnupiravir Is a CES2 Substrate: Potential Drug-Drug Interactions and Impact of CES2 Genetic Polymorphism In Vitro. Drug Metab. Dispos. 2022, 50, 1151–1160. [Google Scholar] [CrossRef]
- Shen, Y.; Eades, W.; Yan, B. Remdesivir potently inhibits carboxylesterase-2 through covalent modifications: Signifying strong drug-drug interactions. Fundam. Clin. Pharmacol. 2021, 35, 432–434. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, A.S.; Okhina, A.A.; Shtro, A.A.; Klabukov, A.M.; Galochkina, A.V.; Nikolaeva, Y.V.; Petukhova, G.D.; Yarovaya, O.I.; Rogachev, A.D.; Baev, D.S.; et al. Biostability, in vivo antiviral activity against respiratory syncytial virus, and pharmacokinetic profiles of (-)-borneol esters. Eur. J. Pharmacol. 2025, 996, 177567. [Google Scholar] [CrossRef] [PubMed]
- Eisner, H.; Riegler-Berket, L.; Gamez, C.F.R.; Sagmeister, T.; Chalhoub, G.; Darnhofer, B.; Jazleena, P.J.; Birner-Gruenberger, R.; Pavkov-Keller, T.; Haemmerle, G.; et al. The Crystal Structure of Mouse Ces2c, a Potential Ortholog of Human CES2, Shows Structural Similarities in Substrate Regulation and Product Release to Human CES1. Int. J. Mol. Sci. 2022, 23, 13101. [Google Scholar] [CrossRef]
- Elens, L.; Langman, L.J.; Hesselink, D.A.; van Gelder, T. The Impact of COVID-19 on Drug Metabolism and Pharmacokinetics. Clin. Pharmacokinet. 2020, 59, 1357–1365. [Google Scholar]
- Gene Expression Omnibus (GEO) Database. GSE150316. 2020. Available online: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE150316 (accessed on 31 May 2025).
- Su, Y.; Chen, D.; Yuan, D.; Lausted, C.; Choi, J.; Dai, C.L.; Voillet, V.; Duvvuri, V.R.; Scherler, K.; Troisch, P.; et al. Multi-Omics Resolves a Sharp Disease-State Shift between Mild and Moderate COVID-19. Nature 2020, 588, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Nie, X.; Qian, L.; Sun, R.; Huang, B.; Dong, X.; Xiao, Q.; Zhang, Q.; Lu, T.; Yue, L.; Chen, S.; et al. Multi-Omics Analyses Reveal Systemic Insights into COVID-19 Pathophysiology. Cell 2021, 184, 3165–3179. [Google Scholar]
- Liu, Y.; Li, J.; Zhu, H.J. Regulation of carboxylesterases and its impact on pharmacokinetics and pharmacodynamics: An up-to-date review. Expert Opin. Drug Metab. Toxicol. 2024, 20, 377–397. [Google Scholar] [CrossRef]
- Lewis, J.P.; Horenstein, R.B.; Ryan, K.; O’Connell, J.R.; Gibson, Q.; Mitchell, B.D.; Tanner, K.; Chai, S.; Bliden, K.P.; Tantry, U.S.; et al. The functional G143E variant of carboxylesterase 1 is associated with increased clopidogrel active metabolite levels and greater clopidogrel response. Pharmacogenet Genom. 2013, 23, 1–8. [Google Scholar] [CrossRef]
- Wang, X.; Her, L.; Xiao, J.; Shi, J.; Wu, A.H.; Bleske, B.E.; Zhu, H.J. Impact of carboxylesterase 1 genetic polymorphism on trandolapril activation in human liver and the pharmacokinetics and pharmacodynamics in healthy volunteers. Clin. Transl. Sci. 2021, 14, 1380–1389. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Shi, J.; Thompson, B.R.; Smith, D.E.; Zhang, T.; Zhu, H.J. Physiologically-Based Pharmacokinetic Modeling to Predict Methylphenidate Exposure Affected by Interplay Among Carboxylesterase 1 Pharmacogenetics, Drug-Drug Interactions, and Sex. J. Pharm. Sci. 2022, 111, 2606–2613. [Google Scholar] [CrossRef] [PubMed]
- Her, L.H.; Wang, X.; Shi, J.; Choi, H.J.; Jung, S.M.; Smith, L.S.; Wu, A.H.; Bleske, B.E.; Zhu, H.J. Effect of CES1 genetic variation on enalapril steady-state pharmacokinetics and pharmacodynamics in healthy subjects. Br. J. Clin. Pharmacol. 2021, 87, 4691–4700. [Google Scholar] [CrossRef] [PubMed]
- Ikonnikova, A.; Rodina, T.; Dmitriev, A.; Melnikov, E.; Kazakov, R.; Nasedkina, T. The Influence of the CES1 Genotype on the Pharmacokinetics of Enalapril in Patients with Arterial Hypertension. J. Pers. Med. 2022, 12, 580. [Google Scholar] [CrossRef]
- Nemoda, Z.; Angyal, N.; Tarnok, Z.; Gadoros, J.; Sasvari-Szekely, M. Carboxylesterase 1 gene polymorphism and methylphenidate response in ADHD. Neuropharmacology 2009, 57, 731–733. [Google Scholar] [CrossRef]
- de With, M.; van Doorn, L.; Maasland, D.C.; Mulder, T.A.M.; Oomen-de Hoop, E.; Mostert, B.; Homs, M.Y.V.; El Bouazzaoui, S.; Mathijssen, R.H.J.; van Schaik, R.H.N.; et al. Capecitabine-induced hand-foot syndrome: A pharmacogenetic study beyond DPYD. Biomed. Pharmacother. 2023, 159, 114232. [Google Scholar] [CrossRef]
- Ji, Q.; Zhang, C.; Xu, Q.; Wang, Z.; Li, X.; Lv, Q. The impact of ABCB1 and CES1 polymorphisms on dabigatran pharmacokinetics and pharmacodynamics in patients with atrial fibrillation. Br. J. Clin. Pharmacol. 2021, 87, 2247–2255. [Google Scholar] [CrossRef]
- Shnayder, N.A.; Petrova, M.M.; Shesternya, P.A.; Savinova, A.V.; Bochanova, E.N.; Zimnitskaya, O.V.; Pozhilenkova, E.A.; Nasyrova, R.F. Using Pharmacogenetics of Direct Oral Anticoagulants to Predict Changes in Their Pharmacokinetics and the Risk of Adverse Drug Reactions. Biomedicines 2021, 9, 451. [Google Scholar] [CrossRef]
- Rodríguez-Lopez, A.; Ochoa, D.; Soria-Chacartegui, P.; Martín-Vilchez, S.; Navares-Gómez, M.; González-Iglesias, E.; Luquero-Bueno, S.; Román, M.; Mejía-Abril, G.; Abad-Santos, F. An Investigational Study on the Role of CYP2D6, CYP3A4 and UGTs Genetic Variation on Fesoterodine Pharmacokinetics in Young Healthy Volunteers. Pharmaceuticals 2024, 17, 1236. [Google Scholar] [CrossRef]
- Yan, D.; Yan, B. Viral target and metabolism-based rationale for combined use of recently authorized small molecule COVID-19 medicines: Molnupiravir, nirmatrelvir, and remdesivir. Fundam. Clin. Pharmacol. 2023, 37, 726–738. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Lee, S.; Lee, H.; Cho, J.Y.; Yoon, S.H.; Jang, I.J.; Yu, K.S.; Lim, K.S. The novel carboxylesterase 1 variant c.662A>G may decrease the bioactivation of oseltamivir in humans. PLoS ONE 2017, 12, e0176320. [Google Scholar] [CrossRef]
- Gu, Z.C.; Ma, X.W.; Zheng, X.Y.; Shen, L.; Shi, F.H.; Li, H. Left Atrial Appendage Thrombus Formation in a Patient on Dabigatran Therapy Associated With ABCB1 and CES-1 Genetic Defect. Front. Pharmacol. 2018, 9, 491. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.T.; Beery, N.; Taran, A.; Stevens, T.; Henzler, C.; Badalamenti, J.; Regal, R.; McCarty, C.A. Associations between CES1 variants and dosing and adverse effects in children taking methylphenidate. Front. Pediatr. 2023, 10, 958622. [Google Scholar] [CrossRef] [PubMed]
- Hodges, L.M.; Markova, S.M.; Chinn, L.W.; Gow, J.M.; Kroetz, D.L.; Klein, T.E.; Altman, R.B. Very important pharmacogene summary: ABCB1 (MDR1, P-glycoprotein). Pharmacogenet Genom. 2011, 21, 152–161. [Google Scholar] [CrossRef]
- Laizure, S.C.; Parker, R.B.; Herring, V.L.; Hu, Z.Y. Identification of carboxylesterase-dependent dabigatran etexilate hydrolysis. Drug Metab. Dispos. 2014, 42, 201–206. [Google Scholar] [CrossRef]
- Ning, R.; Wang, X.P.; Zhan, Y.R.; Qi, Q.; Huang, X.F.; Hu, G.; Guo, Q.L.; Liu, W.; Yang, J. Gambogic acid potentiates clopidogrel-induced apoptosis and attenuates irinotecan-induced apoptosis through down-regulating human carboxylesterase 1 and -2. Xenobiotica 2016, 46, 816–824. [Google Scholar] [CrossRef]
- Eades, W.; Liu, W.; Shen, Y.; Shi, Z.; Yan, B. Covalent CES2 Inhibitors Protect against Reduced Formation of Intestinal Organoids by the Anticancer Drug Irinotecan. Curr. Drug Metab. 2022, 23, 1000–1010. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Z.; Tian, X.; Cai, W. Predicting the Effects of CYP2C19 and Carboxylesterases on Vicagrel, a Novel P2Y12 Antagonist, by Physiologically Based Pharmacokinetic/Pharmacodynamic Modeling Approach. Front. Pharmacol. 2020, 11, 591854. [Google Scholar] [CrossRef]
- Xiao, D.; Shi, D.; Yang, D.; Barthel, B.; Koch, T.H.; Yan, B. Carboxylesterase-2 is a highly sensitive target of the antiobesity agent orlistat with profound implications in the activation of anticancer prodrugs. Biochem. Pharmacol. 2013, 85, 439–447. [Google Scholar] [CrossRef]
- Fujiyama, N.; Miura, M.; Satoh, S.; Inoue, K.; Kagaya, H.; Saito, M.; Habuchi, T.; Suzuki, T. Influence of carboxylesterase 2 genetic polymorphisms on mycophenolic acid pharmacokinetics in Japanese renal transplant recipients. Xenobiotica 2009, 39, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.R.; Sai, K.; Tanaka-Kagawa, T.; Jinno, H.; Ozawa, S.; Kaniwa, N.; Saito, Y.; Akasawa, A.; Matsumoto, K.; Saito, H.; et al. Haplotypes and a novel defective allele of CES2 found in a Japanese population. Drug Metab. Dispos. 2007, 35, 1865–1872. [Google Scholar] [CrossRef] [PubMed]
- Cura, Y.; Sánchez-Martín, A.; Márquez-Pete, N.; González-Flores, E.; Martínez-Martínez, F.; Pérez-Ramírez, C.; Jiménez-Morales, A. Association of Single-Nucleotide Polymorphisms in Capecitabine Bioactivation Pathway with Adjuvant Therapy Safety in Colorectal Cancer Patients. Pharmaceutics 2023, 15, 2548. [Google Scholar] [CrossRef]
- Maslarinou, A.; Manolopoulos, V.G.; Ragia, G. Pharmacogenomic-guided dosing of fluoropyrimidines beyond DPYD: Time for a polygenic algorithm? Front. Pharmacol. 2023, 14, 1184523. [Google Scholar] [CrossRef]
- Schiel, M.A. Human Carboxylesterase 2 Splice Variants: Expression, Activity, and Role in the Metabolism of Irinotecan and Capecitabine. Ph.D. Thesis, Department of Biochemistry & Molecular Biology, Indiana University, Bloomington, IN, USA, February 2009. [Google Scholar]
- Tang, M.; Mukundan, M.; Yang, J.; Charpentier, N.; LeCluyse, E.L.; Black, C.; Yang, D.; Shi, D.; Yan, B. Antiplatelet agents aspirin and clopidogrel are hydrolyzed by distinct carboxylesterases, and clopidogrel is transesterificated in the presence of ethyl alcohol. Pharmacol. Exp. Ther. 2006, 319, 1467–1476. [Google Scholar] [CrossRef] [PubMed]
- Kubo, T.; Kim, S.R.; Sai, K.; Saito, Y.; Nakajima, T.; Matsumoto, K.; Saito, H.; Shirao, K.; Yamamoto, N.; Minami, H.; et al. Functional characterization of three naturally occurring single nucleotide polymorphisms in the CES2 gene encoding carboxylesterase 2 (HCE-2). Drug Metab. Dispos. 2005, 33, 1482–1487. [Google Scholar] [CrossRef]
- Birkus, G.; Bam, R.A.; Willkom, M.; Frey, C.R.; Tsai, L.; Stray, K.M.; Yant, S.R.; Cihlar, T. Intracellular Activation of Tenofovir Alafenamide and the Effect of Viral and Host Protease Inhibitors. Antimicrob. Agents Chemother. 2015, 60, 316–322. [Google Scholar] [CrossRef]
- Li, Y.; Cao, L.; Li, G.; Cong, F.; Li, Y.; Sun, J.; Luo, Y.; Chen, G.; Li, G.; Wang, P.; et al. Remdesivir Metabolite GS-441524 Effectively Inhibits SARS-CoV-2 Infection in Mouse Models. J. Med. Chem. 2022, 65, 2785–2793. [Google Scholar] [CrossRef]
- Cox, R.M.; Wolf, J.D.; Lieber, C.M.; Sourimant, J.; Lin, M.J.; Babusis, D.; DuPont, V.; Chan, J.; Barrett, K.T.; Lye, D.; et al. Oral prodrug of remdesivir parent GS-441524 is efficacious against SARS-CoV-2 in ferrets. Nat. Commun. 2021, 12, 6415. [Google Scholar] [CrossRef]
- Gandhi, Z.; Mansuri, Z.; Bansod, S. Potential Interactions of Remdesivir with Pulmonary Drugs: A COVID-19 Perspective. SN Compr. Clin. Med. 2020, 2, 1707–1708. [Google Scholar] [CrossRef]
- Yang, K. What Do We Know About Remdesivir Drug Interactions? Clin. Transl. Sci. 2020, 13, 842–844. [Google Scholar] [CrossRef] [PubMed]
- Deb, S.; Reeves, A.A.; Hopefl, R.; Bejusca, R. ADME and Pharmacokinetic Properties of Remdesivir: Its Drug Interaction Potential. Pharmaceuticals 2021, 14, 655. [Google Scholar] [CrossRef] [PubMed]
- Leegwater, E.; Moes, D.J.A.R.; Bosma, L.B.E.; Ottens, T.H.; van der Meer, I.M.; van Nieuwkoop, C.; Wilms, E.B. Population Pharmacokinetics of Remdesivir and GS-441524 in Hospitalized COVID-19 Patients. Antimicrob. Agents Chemother. 2022, 66, e0025422. [Google Scholar] [CrossRef] [PubMed]
- Choe, P.G.; Jeong, S.I.; Kang, C.K.; Yang, L.; Lee, S.; Cho, J.Y.; Han, S.S.; Kim, D.K.; Lee, S.M.; Park, W.B.; et al. Exploration for the effect of renal function and renal replacement therapy on pharmacokinetics of remdesivir and GS-441524 in patients with COVID-19: A limited case series. Clin. Transl. Sci. 2022, 15, 732–740. [Google Scholar] [CrossRef]
- Xu, Y.; Barauskas, O.; Kim, C.; Babusis, D.; Murakami, E.; Kornyeyev, D.; Lee, G.; Stepan, G.; Perron, M.; Bannister, R.; et al. Off-Target In Vitro Profiling Demonstrates that Remdesivir Is a Highly Selective Antiviral Agent. Antimicrob. Agents Chemother. 2021, 65, e02237-20. [Google Scholar] [CrossRef]
- Hu, B.; Huang, S.; Yin, L. The cytokine storm and COVID-19. J. Med. Virol. 2021, 93, 250–256. [Google Scholar] [CrossRef]
- Miller, S.R.; McGrath, M.E.; Zorn, K.M.; Ekins, S.; Wright, S.H.; Cherrington, N.J. Remdesivir and EIDD-1931 Interact with Human Equilibrative Nucleoside Transporters 1 and 2: Implications for Reaching SARS-CoV-2 Viral Sanctuary Sites. Mol. Pharmacol. 2021, 100, 548–557. [Google Scholar] [CrossRef]
- Wang, A.Q.; Hagen, N.R.; Padilha, E.C.; Yang, M.; Shah, P.; Chen, C.Z.; Huang, W.; Terse, P.; Sanderson, P.; Zheng, W.; et al. Preclinical Pharmacokinetics and In Vitro Properties of GS-441524, a Potential Oral Drug Candidate for COVID-19 Treatment. Front. Pharmacol. 2022, 13, 918083. [Google Scholar] [CrossRef]
- Akinci, E.; Cha, M.; Lin, L.; Yeo, G.; Hamilton, M.C.; Donahue, C.J.; Bermudez-Cabrera, H.C.; Zanetti, L.C.; Chen, M.; Barkal, S.A.; et al. Elucidation of remdesivir cytotoxicity pathways through genome-wide CRISPR-Cas9 screening and transcriptomics. bioRxiv 2020. [Google Scholar] [CrossRef]
- Painter, W.P.; Holman, W.; Bush, J.A.; Almazedi, F.; Malik, H.; Eraut, N.C.J.E.; Morin, M.J.; Szewczyk, L.J.; Painter, G.R. Human Safety, Tolerability, and Pharmacokinetics of Molnupiravir, a Novel Broad-Spectrum Oral Antiviral Agent with Activity Against SARS-CoV-2. Antimicrob. Agents Chemother. 2021, 65, e02428-20. [Google Scholar] [CrossRef]
- Khiali, S.; Khani, E.; B Rouy, S.; Entezari-Maleki, T. Comprehensive review on molnupiravir in COVID-19: A novel promising antiviral to combat the pandemic. Future Microbiol. 2022, 17, 377–391. [Google Scholar] [CrossRef]
- Rossi, Á.D.; de Araújo, J.L.F.; de Almeida, T.B.; Ribeiro-Alves, M.; de Almeida Velozo, C.; Almeida, J.M.; de Carvalho Leitão, I.; Ferreira, S.N.; da Silva Oliveira, J.; Alves, H.J.; et al. Association between ACE2 and TMPRSS2 nasopharyngeal expression and COVID-19 respiratory distress. Sci. Rep. 2021, 11, 9658. [Google Scholar] [CrossRef] [PubMed]
- Li, L.Q.; Huang, T.; Wang, Y.Q.; Wang, Z.P.; Liang, Y.; Huang, T.B.; Zhang, H.Y.; Sun, W.; Wang, Y. COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J. Med. Virol. 2020, 92, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Harmer, D.; Gilbert, M.; Borman, R.; Clark, K.L. Quantitative mRNA expression profiling of ACE 2, a novel homologue of angiotensin converting enzyme. FEBS Lett. 2002, 532, 107–110. [Google Scholar] [CrossRef]
- Lin, L.; Zeng, F.; Mai, L.; Gao, M.; Fang, Z.; Wu, B.; Huang, S.; Shi, H.; He, J.; Liu, Y.; et al. Expression of ACE2, TMPRSS2, and SARS-CoV-2 nucleocapsid protein in gastrointestinal tissues from COVID-19 patients and association with gastrointestinal symptoms. Am. J. Med. Sci. 2023, 366, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Mady Traore, M.D.; Li, R.; Yuan, H.; He, M.; Wen, B.; Gao, W.; Jonsson, C.B.; Fitzpatrick, E.A.; Sun, D. Optimization of the Prodrug Moiety of Remdesivir to Improve Lung Exposure/Selectivity and Enhance Anti-SARS-CoV-2 Activity. J. Med. Chem. 2022, 65, 12044–12054. [Google Scholar] [CrossRef]
- Bakos, É.; Temesszentandrási-Ambrus, C.; Özvegy-Laczka, C.; Gáborik, Z.; Sarkadi, B.; Telbisz, Á. Interactions of the Anti-SARS-CoV-2 Agents Molnupiravir and Nirmatrelvir/Paxlovid with Human Drug Transporters. Int. J. Mol. Sci. 2023, 24, 11237. [Google Scholar] [CrossRef]
- Ravi, N.; Cortade, D.L.; Ng, E.; Wang, S.X. Diagnostics for SARS-CoV-2 detection: A comprehensive review of the FDA-EUA COVID-19 testing landscape. Biosens. Bioelectron. 2020, 165, 112454. [Google Scholar] [CrossRef]
- Loos, N.H.C.; Beijnen, J.H.; Schinkel, A.H. The inhibitory and inducing effects of ritonavir on hepatic and intestinal CYP3A and other drug-handling proteins. Biomed. Pharmacother. 2023, 162, 114636. [Google Scholar] [CrossRef]
- Loos, N.H.C.; Beijnen, J.H.; Schinkel, A.H. The Mechanism-Based Inactivation of CYP3A4 by Ritonavir: What Mechanism? Int. J. Mol. Sci. 2022, 23, 9866. [Google Scholar] [CrossRef]
- Ratain, M.J.; Greenblatt, D.J. Drug Interactions With a Short Course of Nirmatrelvir and Ritonavir: Prescribers and Patients Beware. J. Clin. Pharmacol. 2022, 62, 925–927. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.K.H.; Lau, J.J.; Au, I.C.H.; Lau, K.T.K.; Hung, I.F.N.; Peiris, M.; Leung, G.M.; Wu, J.T. Optimal timing of nirmatrelvir/ritonavir treatment after COVID-19 symptom onset or diagnosis: Target trial emulation. Nat. Commun. 2023, 14, 8377. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).