Etrog Citron (Citrus medica) as a Novel Source of Antimicrobial Agents: Overview of Its Bioactive Phytochemicals and Delivery Approaches
Abstract
1. Introduction
2. Antibacterial Activity of the Extracts and Essential Oils
3. Antibacterial Activity of Phytochemicals
| Compound | Structure | Part of the Plant | References |
|---|---|---|---|
| Apigenin | 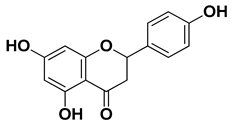 | Leaves, flowers, mesocarp, endocarp | [48] |
| Catechin | 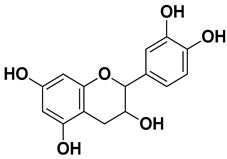 | Mesocarp, endocarp, seeds, flavedo, pulp | [49] |
| Dihydroquercetin | 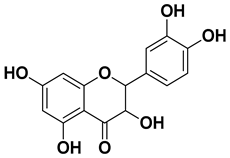 | Exocarp, endocarp, seeds | [50] |
| Epicatechin | 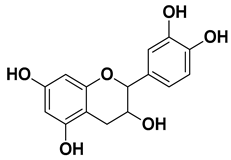 | Flavedo, pulp | [51] |
| Herbacetin | 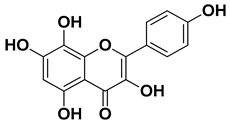 | Exocarp, mesocarp, seeds | [52] |
| Hesperidin | 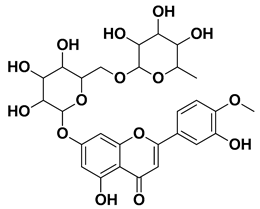 | Flavedo, exocarp, endocarp, mesocarp, seeds, flowers, leaves | [53,54] |
| Kaempferol 3-O-rutinoside | 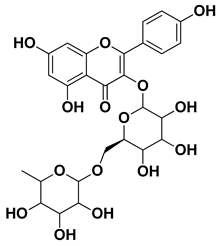 | Flavedo | [55] |
| Lonchocarpol A |  | Root, stem | [56] |
| Naringin | 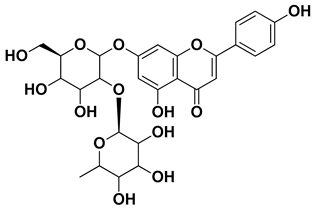 | Fructus, exocarp, mesocarp, endocarp, seeds, flavedo | [57] |
| Nobiletin | 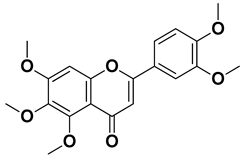 | Exocarp, mesocarp, endocarp, seeds | [22] |
| Quercitin | 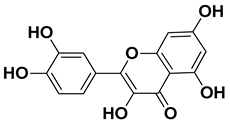 | Flowers, leaves, mesocarp, endocarp | [58] |
| Rutin (quercetin-3-rutinoside) | 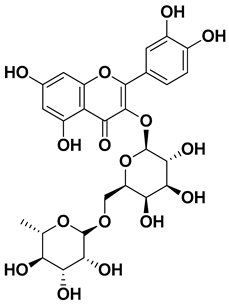 | Flavedo | [59] |
| Tangeritin | 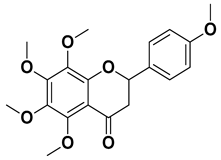 | Exocarp, mesocarp, endocarp | [22] |
| Vitexin | 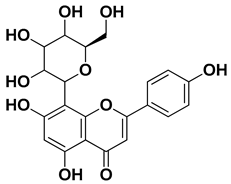 | Exocarp, endocarp, seeds | [47] |
| Compound | Structure | Part of the Plant | References |
|---|---|---|---|
| Skimmin | 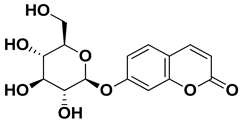 | Fresh fruit | [60] |
| Umbelliferone (7-hydroxycoumarin) | 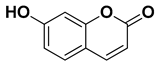 | Fresh fruit | [61] |
| Bergapten | 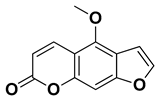 | Bark, fructus | [62] |
| Citropten (5,7-dimethoxycoumarin) |  | Fresh fruit, fructus | [62] |
| Carvacrol | 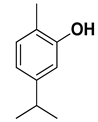 | Flavedo | [24,26,27] |
| Borneol | 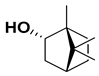 | Flavedo | [24] |
| Limonene | 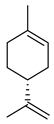 | Fructus, flavedo | [24,63,64] |
| Linalool | 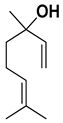 | Fresh fruit, flavedo | [24,65] |
| Nerol | 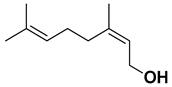 | Fresh fruit, flavedo | [24,66] |
| α-pinene |  | Exocarp, mesocarp, fresh fruit, flavedo, oil glands | [24,67] |
| β-pinene | 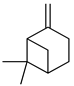 | Exocarp, mesocarp, fresh fruit, flavedo, oil glands | [24,68] |
| Sabinene | 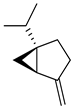 | Flavedo, oil glands | [24] |
| α-terpinene | 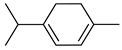 | Flavedo | [24,69] |
| Terpinen-4-ol | 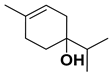 | Fructus | [24,70] |
| α-terpineole | 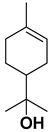 | Exocarp, mesocarp, fresh fruit, flavedo, oil glands | [24,71] |
| Geraniol | 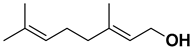 | Flavedo, fructus | [38,39,40,41] |
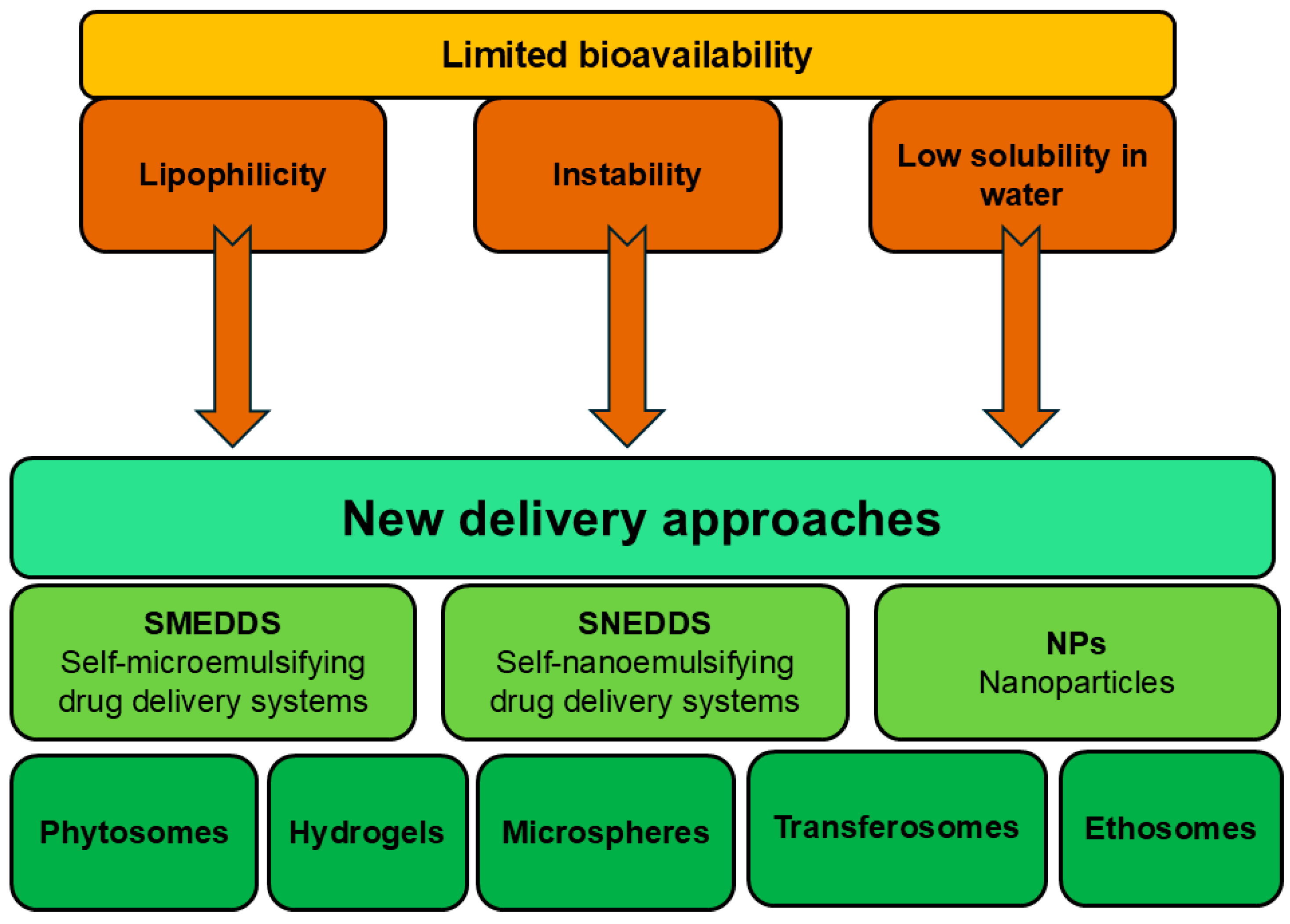
4. Applicability of Delivery Systems
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Continella, A. The Citron in Italy and Its Cultivation in Calabria. In The Citron Compendium: The Citron (Etrog) Citrus medica L.: Science and Tradition; Goldschmidt, E.E., Bar-Joseph, M., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 265–286. ISBN 978-3-031-25775-9. [Google Scholar]
- Ramadugu, C.; Keremane, M.L.; Hu, X.; Karp, D.; Federici, C.T.; Kahn, T.; Roose, M.L.; Lee, R.F. Genetic Analysis of Citron (Citrus medica L.) Using Simple Sequence Repeats and Single Nucleotide Polymorphisms. Sci. Hortic. 2015, 195, 124–137. [Google Scholar] [CrossRef]
- Chhikara, N.; Kour, R.; Jaglan, S.; Gupta, P.; Gat, Y.; Panghal, A. Citrus medica: Nutritional, Phytochemical Composition and Health Benefits—A Review. Food Funct. 2018, 9, 1978–1992. [Google Scholar] [CrossRef] [PubMed]
- Arias, B.Á.; Ramón-Laca, L. Pharmacological Properties of Citrus and Their Ancient and Medieval Uses in the Mediterranean Region. J. Ethnopharmacol. 2005, 97, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Benedetto, N.; Carlucci, V.; Faraone, I.; Lela, L.; Ponticelli, M.; Russo, D.; Mangieri, C.; Tzvetkov, N.T.; Milella, L. An Insight into Citrus medica Linn.: A Systematic Review on Phytochemical Profile and Biological Activities. Plants 2023, 12, 2267. [Google Scholar] [CrossRef]
- Tundis, R.; Xiao, J.; Silva, A.S.; Carreiró, F.; Loizzo, M.R. Health-Promoting Properties and Potential Application in the Food Industry of Citrus medica L. and Citrus × clementina Hort. Ex Tan. Essential Oils and Their Main Constituents. Plants 2023, 12, 991. [Google Scholar] [CrossRef]
- Chang, R.Y.K.; Nang, S.C.; Chan, H.-K.; Li, J. Novel Antimicrobial Agents for Combating Antibiotic-Resistant Bacteria. Adv. Drug Deliv. Rev. 2022, 187, 114378. [Google Scholar] [CrossRef]
- Yarmolinsky, L.; Nakonechny, F.; Haddis, T.; Khalfin, B.; Dahan, A.; Ben-Shabat, S. Natural Antimicrobial Compounds as Promising Preservatives: A Look at an Old Problem from New Perspectives. Molecules 2024, 29, 5830. [Google Scholar] [CrossRef]
- Sah, A.N.; Juyal, V.; Melkani, A.B. Antimicrobial Activity of Six Different Parts of the Plant Citrus medica Linn. Pharmacogn. J. 2011, 3, 80–83. [Google Scholar] [CrossRef]
- Fratianni, F.; Cozzolino, A.; De Feo, V.; Coppola, R.; Ombra, M.N.; Nazzaro, F. Polyphenols, Antioxidant, Antibacterial, and Biofilm Inhibitory Activities of Peel and Pulp of Citrus medica L., Citrus bergamia, and Citrus medica Cv. Salò Cultivated in Southern Italy. Molecules 2019, 24, 4577. [Google Scholar] [CrossRef]
- Gao, Z.; Zhong, W.; Chen, K.; Tang, P.; Guo, J. Chemical Composition and Anti-Biofilm Activity of Essential Oil from Citrus medica L. Var. Sarcodactylis Swingle against Listeria Monocytogenes. Ind. Crops Prod. 2020, 144, 112036. [Google Scholar] [CrossRef]
- Li, Z.-H.; Cai, M.; Liu, Y.-S.; Sun, P.-L.; Luo, S.-L. Antibacterial Activity and Mechanisms of Essential Oil from Citrus medica L. Var. Sarcodactylis. Molecules 2019, 24, 1577. [Google Scholar] [CrossRef] [PubMed]
- Mitropoulou, G.; Fitsiou, E.; Spyridopoulou, K.; Tiptiri-Kourpeti, A.; Bardouki, H.; Vamvakias, M.; Panas, P.; Chlichlia, K.; Pappa, A.; Kourkoutas, Y. Citrus medica Essential Oil Exhibits Significant Antimicrobial and Antiproliferative Activity. LWT 2017, 84, 344–352. [Google Scholar] [CrossRef]
- Aliberti, L.; Caputo, L.; De Feo, V.; De Martino, L.; Nazzaro, F.; Souza, L.F. Chemical Composition and In Vitro Antimicrobial, Cytotoxic, and Central Nervous System Activities of the Essential Oils of Citrus medica L. Cv. ‘Liscia’ and C. Medica Cv. ‘Rugosa’ Cultivated in Southern Italy. Molecules 2016, 21, 1244. [Google Scholar] [CrossRef] [PubMed]
- Belletti, N.; Lanciotti, R.; Patrignani, F.; Gardini, F. Antimicrobial Efficacy of Citron Essential Oil on Spoilage and Pathogenic Microorganisms in Fruit-Based Salads. J. Food Sci. 2008, 73, M331–M338. [Google Scholar] [CrossRef]
- Wang, E.; Li, Y.; Maguy, B.L.; Lou, Z.; Wang, H.; Zhao, W.; Chen, X. Separation and Enrichment of Phenolics Improved the Antibiofilm and Antibacterial Activity of the Fractions from Citrus medica L. Var. Sarcodactylis In Vitro and In Tofu. Food Chem. 2019, 294, 533–538. [Google Scholar] [CrossRef]
- Yuan, Y.; Geng, X.; Wu, H.; Kumar, R.; Wang, J.; Xiao, J.; Tian, H. Chemical Composition, Antimicrobial Activities, and Microencapsulation by Complex Coacervation of Tea Tree Essential Oils. J. Food Process Preserv. 2022, 46, e16585. [Google Scholar] [CrossRef]
- Gabarin, A.; Yarmolinsky, L.; Budovsky, A.; Khalfin, B.; Ben-Shabat, S. Cannabis as a Source of Approved Drugs: A New Look at an Old Problem. Molecules 2023, 28, 7686. [Google Scholar] [CrossRef]
- Wang, M.; Firrman, J.; Liu, L.S.; Yam, K. A Review on Flavonoid Apigenin: Dietary Intake, ADME, Antimicrobial Effects, and Interactions with Human Gut Microbiota. BioMed Res. Int. 2019, 2019, 7010467. [Google Scholar] [CrossRef]
- Kim, H.W.; Woo, H.J.; Yang, J.Y.; Kim, J.-B.; Kim, S.-H. Hesperetin Inhibits Expression of Virulence Factors and Growth of Helicobacter Pylori. Int. J. Mol. Sci. 2021, 22, 10035. [Google Scholar] [CrossRef]
- Han, G.; Lee, D.G. Naringin Generates Three Types of Reactive Oxygen Species Contributing Differently to Apoptosis-like Death in Escherichia coli. Life Sci. 2022, 304, 120700. [Google Scholar] [CrossRef]
- Yao, X.; Zhu, X.; Pan, S.; Fang, Y.; Jiang, F.; Phillips, G.O.; Xu, X. Antimicrobial Activity of Nobiletin and Tangeretin against Pseudomonas. Food Chem. 2012, 132, 1883–1890. [Google Scholar] [CrossRef]
- Qi, W.; Qi, W.; Xiong, D.; Long, M. Quercetin: Its Antioxidant Mechanism, Antibacterial Properties and Potential Application in Prevention and Control of Toxipathy. Molecules 2022, 27, 6545. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Dong, M.; Chen, X.; Jiang, M.; Lv, X.; Zhou, J. Antimicrobial Activity of an Endophytic Xylaria Sp.YX-28 and Identification of Its Antimicrobial Compound 7-Amino-4-Methylcoumarin. Appl. Microbiol. Biotechnol. 2008, 78, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Gabriele, B.; Fazio, A.; Dugo, P.; Costa, R.; Mondello, L. Essential Oil Composition of Citrus medica L. Cv. Diamante (Diamante Citron) Determined after Using Different Extraction Methods. J. Sep. Sci. 2009, 32, 99–108. [Google Scholar] [CrossRef]
- Dorman, H.J.D.; Deans, S.G. Antimicrobial Agents from Plants: Antibacterial Activity of Plant Volatile Oils. J. Appl. Microbiol. 2000, 88, 308–316. [Google Scholar] [CrossRef]
- Raei, P.; Pourlak, T.; Memar, M.Y.; Alizadeh, N.; Aghamali, M.; Zeinalzadeh, E.; Asgharzadeh, M.; Kafil, H.S. Thymol and Carvacrol Strongly Inhibit Biofilm Formation and Growth of Carbapenemase-Producing Gram Negative Bacilli. Cell Mol. Biol. 2017, 63, 108–112. [Google Scholar] [CrossRef]
- Aćimović, M.; Zorić, M.; Zheljazkov, V.D.; Pezo, L.; Čabarkapa, I.; Stanković Jeremić, J.; Cvetković, M. Chemical Characterization and Antibacterial Activity of Essential Oil of Medicinal Plants from Eastern Serbia. Molecules 2020, 25, 5482. [Google Scholar] [CrossRef]
- Han, Y.; Sun, Z.; Chen, W. Antimicrobial Susceptibility and Antibacterial Mechanism of Limonene against Listeria Monocytogenes. Molecules 2020, 25, 33. [Google Scholar] [CrossRef]
- Gupta, A.; Jeyakumar, E.; Lawrence, R. Strategic Approach of Multifaceted Antibacterial Mechanism of Limonene Traced in Escherichia coli. Sci. Rep. 2021, 11, 13816. [Google Scholar] [CrossRef]
- Guo, F.; Chen, Q.; Liang, Q.; Zhang, M.; Chen, W.; Chen, H.; Yun, Y.; Zhong, Q.; Chen, W. Antimicrobial Activity and Proposed Action Mechanism of Linalool Against Pseudomonas Fluorescens. Front. Microbiol. 2021, 12, 562094. [Google Scholar] [CrossRef]
- He, R.; Chen, W.; Chen, H.; Zhong, Q.; Zhang, H.; Zhang, M.; Chen, W. Antibacterial Mechanism of Linalool against L. Monocytogenes, a Metabolomic Study. Food Control 2022, 132, 108533. [Google Scholar] [CrossRef]
- Ghosh, T.; Srivastava, S.K.; Gaurav, A.; Kumar, A.; Kumar, P.; Yadav, A.S.; Pathania, R.; Navani, N.K. A Combination of Linalool, Vitamin C, and Copper Synergistically Triggers Reactive Oxygen Species and DNA Damage and Inhibits Salmonella enterica subsp. enterica Serovar Typhi and Vibrio fluvialis. Appl. Environ. Microbiol. 2019, 85, e02487-18. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ren, F.; Chen, D.; Chen, H.; Chen, W. Antibacterial Mechanism of Linalool against Pseudomonas Fragi: A Transcriptomic Study. Foods 2022, 11, 2058. [Google Scholar] [CrossRef]
- Li, Y.; He, R.; Chen, H.; Chen, D.; Chen, W. Respiratory Depression as Antibacterial Mechanism of Linalool against Pseudomonas Fragi Based on Metabolomics. Int. J. Mol. Sci. 2022, 23, 11586. [Google Scholar] [CrossRef]
- Salehi, B.; Upadhyay, S.; Erdogan Orhan, I.; Kumar Jugran, A.; Jayaweera, S.L.D.; Dias, D.A.; Sharopov, F.; Taheri, Y.; Martins, N.; Baghalpour, N.; et al. Therapeutic Potential of α- and β-Pinene: A Miracle Gift of Nature. Biomolecules 2019, 9, 738. [Google Scholar] [CrossRef]
- Aini Khairunnisa, N.; Yuandani; Raina Nasution, H.; Sari Utami, D.; Frimayanti, N.; Jantan, I.; Nirmal, N.; Wira Septama, A. A Synergistic Interaction Between Citrus hystrix Peel Essential Oil and Tetracycline and Evaluation of Their Antibacterial Mechanism of Action In Vitro and In Silico Against Escherichia coli. Chem. Biodivers. 2024, 22, e202401291. [Google Scholar] [CrossRef]
- Si, W.; Gong, J.; Tsao, R.; Zhou, T.; Yu, H.; Poppe, C.; Johnson, R.; Du, Z. Antimicrobial Activity of Essential Oils and Structurally Related Synthetic Food Additives towards Selected Pathogenic and Beneficial Gut Bacteria. J. Appl. Microbiol. 2006, 100, 296–305. [Google Scholar] [CrossRef]
- Dubreuil, J.D. Antibacterial and Antidiarrheal Activities of Plant Products against Enterotoxinogenic Escherichia coli. Toxins 2013, 5, 2009–2041. [Google Scholar] [CrossRef]
- Sanguansermsri, D.; Sanguansermsri, P.; Buaban, K.; Kawaree, R.; Wongkattiya, N. Antimicrobial Activity and Time-Kill Kinetics of Boesenbergia Rotunda Essential Oil and Geraniol Alcohol against Oral Bacterial Pathogens. J. Appl. Pharm. Sci. 2024, 14, 215–221. [Google Scholar] [CrossRef]
- Lira, M.H.P.d.; Andrade Júnior, F.P.d.; Moraes, G.F.Q.; Macena, G.d.S.; Pereira, F.d.O.; Lima, I.O. Antimicrobial Activity of Geraniol: An Integrative Review. J. Essent. Oil Res. 2020, 32, 187–197. [Google Scholar] [CrossRef]
- Mączka, W.; Wińska, K.; Grabarczyk, M. One Hundred Faces of Geraniol. Molecules 2020, 25, 3303. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, M.J.; King, A.B.; Graham, A.C.; Onishi, H.R.; Bartizal, K.F.; Abruzzo, G.K.; Gill, C.J.; Ramjit, H.G.; Pitzenberger, S.M.; Witherup, K.M. Antibacterial Activity of Lonchocarpol A. J. Nat. Prod. 1998, 61, 640–642. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Peng, W.; Kou, X.; Chen, Z.; Zhang, L. High-Throughput Screening Identification of Apigenin That Reverses the Colistin Resistance of Mcr-1-Positive Pathogens. Microbiol. Spectr. 2024, 12, e0034124. [Google Scholar] [CrossRef] [PubMed]
- Abuelsaad, A.S.A.; Mohamed, I.; Allam, G.; Al-Solumani, A.A. Antimicrobial and Immunomodulating Activities of Hesperidin and Ellagic Acid against Diarrheic Aeromonas Hydrophila in a Murine Model. Life Sci. 2013, 93, 714–722. [Google Scholar] [CrossRef]
- Zhao, G.; Huang, Q.; Jing, X.; Huang, L.; Liu, C.; Pan, X.; Li, Z.; Li, S.; Qiu, Z.; Xin, R. Therapeutic Effect and Safety Evaluation of Naringin on Klebsiella Pneumoniae in Mice. Int. J. Mol. Sci. 2023, 24, 15940. [Google Scholar] [CrossRef]
- Das, M.C.; Samaddar, S.; Jawed, J.J.; Ghosh, C.; Acharjee, S.; Sandhu, P.; Das, A.; Daware, A.V.; De, U.C.; Majumdar, S.; et al. Vitexin Alters Staphylococcus aureus Surface Hydrophobicity to Obstruct Biofilm Formation. Microbiol. Res. 2022, 263, 127126. [Google Scholar] [CrossRef]
- Kim, S.; Woo, E.-R.; Lee, D.G. Apigenin Promotes Antibacterial Activity via Regulation of Nitric Oxide and Superoxide Anion Production. J. Basic. Microbiol. 2020, 60, 862–872. [Google Scholar] [CrossRef]
- Sinsinwar, S.; Vadivel, V. Catechin Isolated from Cashew Nut Shell Exhibits Antibacterial Activity against Clinical Isolates of MRSA through ROS-Mediated Oxidative Stress. Appl. Microbiol. Biotechnol. 2020, 104, 8279–8297. [Google Scholar] [CrossRef]
- Cai, J.; Wang, S.; Wang, Q. Antibacterial Activity of Dihydroquercetin Separated from Fructus Polygoni Orientalis against Clavibacter Michiganensis Subsp. Sepedonicus via Damaging Cell Membrane. Foods 2024, 13, 23. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Taylor, P.W.; Nagaoka, Y.; Uesato, S.; Hara, Y.; Lamb, A.J. Investigation of the Antibacterial Activity of 3-O-octanoyl-(−)-epicatechin. J. Appl. Microbiol. 2008, 105, 1461–1469. [Google Scholar] [CrossRef]
- Zhong, L.; Peng, L.; Fu, J.; Zou, L.; Zhao, G.; Zhao, J. Phytochemical, Antibacterial and Antioxidant Activity Evaluation of Rhodiola Crenulata. Molecules 2020, 25, 3664. [Google Scholar] [CrossRef] [PubMed]
- Yarmolinsky, L.; Nakonechny, F.; Budovsky, A.; Zeigerman, H.; Khalfin, B.; Sharon, E.; Yarmolinsky, L.; Ben-Shabat, S.; Nisnevitch, M. Antimicrobial and Antiviral Compounds of Phlomis viscosa Poiret. Biomedicines 2023, 11, 441. [Google Scholar] [CrossRef] [PubMed]
- Carević, T.; Kostić, M.; Nikolić, B.; Stojković, D.; Soković, M.; Ivanov, M. Hesperetin—Between the Ability to Diminish Mono- and Polymicrobial Biofilms and Toxicity. Molecules 2022, 27, 6806. [Google Scholar] [CrossRef]
- Kordbacheh, H.; Eftekhar, F.; Ebrahimi, S.N. Anti-Quorum Sensing Activity of Pistacia atlantica against Pseudomonas aeruginosa PAO1 and Identification of Its Bioactive Compounds. Microb. Pathog. 2017, 110, 390–398. [Google Scholar] [CrossRef]
- Rahman, M.M.; Gray, A.I. Antimicrobial Constituents from the Stem Bark of Feronia limonia. Phytochemistry 2002, 59, 73–77. [Google Scholar] [CrossRef]
- Wang, R.; Wu, N.; Zhan, D.; Chen, F. Naringin Exerts Antibacterial and Anti-Inflammatory Effects on Mice with Staphylococcus aureus–Induced Osteomyelitis. J. Biochem. Mol. Toxicol. 2024, 38, e23753. [Google Scholar] [CrossRef]
- Wang, S.; Yao, J.; Zhou, B.; Yang, J.; Chaudry, M.T.; Wang, M.; Xiao, F.; Li, Y.; Yin, W. Bacteriostatic Effect of Quercetin as an Antibiotic Alternative In Vivo and Its Antibacterial Mechanism In Vitro. J. Food Prot. 2018, 81, 68–78. [Google Scholar] [CrossRef]
- Sathiya Deepika, M.; Thangam, R.; Sakthidhasan, P.; Arun, S.; Sivasubramanian, S.; Thirumurugan, R. Combined Effect of a Natural Flavonoid Rutin from Citrus sinensis and Conventional Antibiotic Gentamicin on Pseudomonas aeruginosa Biofilm Formation. Food Control 2018, 90, 282–294. [Google Scholar] [CrossRef]
- Chu, L.L.; Pandey, R.P.; Lim, H.N.; Jung, H.J.; Thuan, N.H.; Kim, T.-S.; Sohng, J.K. Synthesis of Umbelliferone Derivatives in Escherichia coli and Their Biological Activities. J. Biol. Eng. 2017, 11, 15. [Google Scholar] [CrossRef]
- Cela-López, J.M.; Camacho Roldán, C.J.; Gómez-Lizarraga, G.; Martínez, V. A Natural Alternative Treatment for Urinary Tract Infections: Itxasol©, the Importance of the Formulation. Molecules 2021, 26, 4564. [Google Scholar] [CrossRef]
- Mohammed, H.S.; Ibrahim, M.H.; Abdel-Aziz, M.M.; Ghareeb, M.A. Anti-Helicobacter Pylori, Anti-Biofilm Activity, and Molecular Docking Study of Citropten, Bergapten, and Its Positional Isomer Isolated from Citrus sinensis L. Leaves. Heliyon 2024, 10, e25232. [Google Scholar] [CrossRef] [PubMed]
- Schroder, V.; Radu, N.; Cornea, P.C.; Coman, O.A.; Pirvu, L.C.; Mohammed, M.S.O.; Stefaniu, A.; Pintilie, L.; Bostan, M.; Caramihai, M.D.; et al. Studies Regarding the Antimicrobial Behavior of Clotrimazole and Limonene. Antibiotics 2022, 11, 1816. [Google Scholar] [CrossRef] [PubMed]
- Sreepian, A.; Popruk, S.; Nutalai, D.; Phutthanu, C.; Sreepian, P.M. Antibacterial Activities and Synergistic Interaction of Citrus Essential Oils and Limonene with Gentamicin against Clinically Isolated Methicillin-Resistant Staphylococcus aureus. Sci. World J. 2022, 2022, 8418287. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Zhang, Z.; Xu, L.; Chen, W.; Zhang, M.; Zhong, Q.; Chen, H.; Chen, W. Antibacterial Mechanism of Linalool Emulsion against Pseudomonas aeruginosa and Its Application to Cold Fresh Beef. World J. Microbiol. Biotechnol. 2022, 38, 56. [Google Scholar] [CrossRef]
- Yang, F.; Chen, L.; Zhao, D.; Guo, T.; Yu, D.; Zhang, X.; Li, P.; Chen, J. A Novel Water-Soluble Chitosan Grafted with Nerol: Synthesis, Characterization and Biological Activity. Int. J. Biol. Macromol. 2023, 232, 123498. [Google Scholar] [CrossRef]
- Karimkhani, M.M.; Mahmoud, N.; Mehdi, M.; Abdollah, J.; Mohammad Saeed, K.; Danial, D.; Jafari, S.M. Extraction and Purification of α-Pinene; a Comprehensive Review. Crit. Rev. Food Sci. Nutr. 2024, 64, 4286–4311. [Google Scholar] [CrossRef]
- Hendel, N.; Madani, S.; Djamel, S.; Soumia, S.; Edoardo, N.; Mounir, S.; Ruberto, G. Phytochemical Analysis, Antibacterial and Antifungal Effect of Lavandula dentata L. Essential Oil and Methanol Extract. Nat. Prod. Res. 2024, 38, 3498–3507. [Google Scholar] [CrossRef]
- Simirgiotis, M.J.; Burton, D.; Parra, F.; López, J.; Muñoz, P.; Escobar, H.; Parra, C. Antioxidant and Antibacterial Capacities of Origanum vulgare L. Essential Oil from the Arid Andean Region of Chile and Its Chemical Characterization by GC-MS. Metabolites 2020, 10, 414. [Google Scholar] [CrossRef]
- Cordeiro, L.; Figueiredo, P.; Souza, H.; Sousa, A.; Andrade-Júnior, F.; Medeiros, D.; Nóbrega, J.; Silva, D.; Martins, E.; Barbosa-Filho, J.; et al. Terpinen-4-Ol as an Antibacterial and Antibiofilm Agent against Staphylococcus aureus. Int. J. Mol. Sci. 2020, 21, 4531. [Google Scholar] [CrossRef]
- Al-Mijalli, S.H.; Mrabti, H.N.; Ouassou, H.; Flouchi, R.; Abdallah, E.M.; Sheikh, R.A.; Alshahrani, M.M.; Awadh, A.A.A.; Harhar, H.; Omari, N.E.; et al. Chemical Composition, Antioxidant, Anti-Diabetic, Anti-Acetylcholinesterase, Anti-Inflammatory, and Antimicrobial Properties of Arbutus unedo L. and Laurus nobilis L. Essential Oils. Life 2022, 12, 1876. [Google Scholar] [CrossRef]
- Jahangir, M.A.; Anand, C.; Muheem, A.; Gilani, S.J.; Taleuzzaman, M.; Zafar, A.; Jafar, M.; Verma, S.; Barkat, M.A. Nano Phytomedicine Based Delivery System for CNS Disease. Curr. Drug Metab. 2020, 21, 661–673. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J.; Xiao, H. Potential Biological Fate of Ingested Nanoemulsions: Influence of Particle Characteristics. Food Funct. 2012, 3, 202–220. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shabat, S.; Yarmolinsky, L.; Porat, D.; Dahan, A. Antiviral Effect of Phytochemicals from Medicinal Plants: Applications and Drug Delivery Strategies. Drug Deliv. Transl. Res. 2020, 10, 354–367. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Yadav, N.; Chaudhary, B.; Jain, V.; Balaramnavar, V.M.; Alharbi, K.S.; Alenezi, S.K.; Al-Malki, W.H.; Ghoneim, M.M.; Alshehri, S.; et al. Promises of Phytochemical Based Nano Drug Delivery Systems in the Management of Cancer. Chem. Biol. Interact. 2022, 351, 109745. [Google Scholar] [CrossRef]
- Tang, S.; Zheng, J. Antibacterial Activity of Silver Nanoparticles: Structural Effects. Adv. Healthc. Mater. 2018, 7, 1701503. [Google Scholar] [CrossRef]
- Mura, P.; Cirri, M.; Mennini, N.; Casella, G.; Maestrelli, F. Polymeric Mucoadhesive Tablets for Topical or Systemic Buccal Delivery of Clonazepam: Effect of Cyclodextrin Complexation. Carbohydr. Polym. 2016, 152, 755–763. [Google Scholar] [CrossRef]
- Veider, F.; Akkuş-Dağdeviren, Z.B.; Knoll, P.; Bernkop-Schnürch, A. Design of Nanostructured Lipid Carriers and Solid Lipid Nanoparticles for Enhanced Cellular Uptake. Int. J. Pharm. 2022, 624, 122014. [Google Scholar] [CrossRef]
- Doktorovová, S.; Kovačević, A.B.; Garcia, M.L.; Souto, E.B. Preclinical Safety of Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: Current Evidence from In Vitro and In Vivo Evaluation. Eur. J. Pharm. Biopharm. 2016, 108, 235–252. [Google Scholar] [CrossRef]
- Jang, K.-I.; Lee, H. Stability of Chitosan Nanoparticles for l -Ascorbic Acid during Heat Treatment in Aqueous Solution. J. Agric. Food Chem. 2008, 56, 1936–1941. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, M.; Ju, R.; Mujumdar, A.S.; Deng, D. Recent Advances in Essential Oil Complex Coacervation by Efficient Physical Field Technology: A Review of Enhancing Efficient and Quality Attributes. Crit. Rev. Food Sci. Nutr. 2024, 64, 3384–3406. [Google Scholar] [CrossRef]
- Ward, K.R.; Matejtschuk, P. The Principles of Freeze-Drying and Application of Analytical Technologies. In Cryopreservation and Freeze-Drying Protocols; Wolkers, W.F., Oldenhof, H., Eds.; Springer: New York, NY, USA, 2021; pp. 99–127. ISBN 978-1-0716-0783-1. [Google Scholar]
- Mohammed, N.K.; Tan, C.P.; Manap, Y.A.; Muhialdin, B.J.; Hussin, A.S.M. Spray Drying for the Encapsulation of Oils—A Review. Molecules 2020, 25, 3873. [Google Scholar] [CrossRef] [PubMed]
- Borro, B.C.; Malmsten, M. Complexation between Antimicrobial Peptides and Polyelectrolytes. Adv. Colloid. Interface Sci. 2019, 270, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Memar, M.Y.; Dalir Abdolahinia, E.; Yekani, M.; Kouhsoltani, M.; Sharifi, S.; Maleki Dizaj, S. Preparation of Rutin-Loaded Mesoporous Silica Nanoparticles and Evaluation of Its Physicochemical, Anticancer, and Antibacterial Properties. Mol. Biol. Rep. 2023, 50, 203–213. [Google Scholar] [CrossRef]
- Patil, A.G.; Jobanputra, A.H. Rutin-Chitosan Nanoparticles: Fabrication, Characterization and Application in Dental Disorders. Polym. Plast. Technol. Eng. 2015, 54, 202–208. [Google Scholar] [CrossRef]
- Alshahrani, S.M. Development and Optimization of Oral Nanoemulsion of Rutin for Enhancing Its Dissolution Rate, Permeability, and Oral Bioavailability. Pharm. Dev. Technol. 2022, 27, 588–597. [Google Scholar] [CrossRef]
- Sharma, S.; Rabbani, S.A.; Narang, J.K.; Hyder Pottoo, F.; Ali, J.; Kumar, S.; Baboota, S. Role of Rutin Nanoemulsion in Ameliorating Oxidative Stress: Pharmacokinetic and Pharmacodynamics Studies. Chem. Phys. Lipids 2020, 228, 104890. [Google Scholar] [CrossRef]
- Dammak, I.; de Carvalho, R.A.; Trindade, C.S.F.; Lourenço, R.V.; do Amaral Sobral, P.J. Properties of Active Gelatin Films Incorporated with Rutin-Loaded Nanoemulsions. Int. J. Biol. Macromol. 2017, 98, 39–49. [Google Scholar] [CrossRef]
- Hassane Hamadou, A.; Zhang, J.; Chao, C.; Xu, B. Stability of Rutin Using Pectin-Chitosan Dual Coating Nanoliposomes. LWT 2022, 170, 114084. [Google Scholar] [CrossRef]
- Sengupta, P.; Das, D.; Bhattacharya, S.; Sur, R.; Bose, A.; Sen, K. A PH-Driven Method for Liposomal Encapsulation of Dietary Flavonoid Rutin: Sustained Release and Enhanced Bioefficacy. Food Biosci. 2023, 52, 102392. [Google Scholar] [CrossRef]
- Ravi, G.S.; Charyulu, R.N.; Dubey, A.; Prabhu, P.; Hebbar, S.; Mathias, A.C. Nano-Lipid Complex of Rutin: Development, Characterisation and In Vivo Investigation of Hepatoprotective, Antioxidant Activity and Bioavailability Study in Rats. AAPS PharmSciTech 2018, 19, 3631–3649. [Google Scholar] [CrossRef]
- De Gaetano, F.; Cristiano, M.C.; Venuti, V.; Crupi, V.; Majolino, D.; Paladini, G.; Acri, G.; Testagrossa, B.; Irrera, A.; Paolino, D.; et al. Rutin-Loaded Solid Lipid Nanoparticles: Characterization and In Vitro Evaluation. Molecules 2021, 26, 1039. [Google Scholar] [CrossRef] [PubMed]
- Dammak, I.; do Amaral Sobral, P.J. Formulation and Stability Characterization of Rutin-Loaded Oil-in-Water Emulsions. Food Bioproc. Tech. 2017, 10, 926–939. [Google Scholar] [CrossRef]
- Mel, M.M.R.D.; Gunathilake, K.D.P.P.; Fernando, C.A.N. Formulation of Microencapsulated Rutin and Evaluation of Bioactivity and Stability upon In Vitro Digestive and Dialysis Conditions. Int. J. Biol. Macromol. 2020, 159, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Lou, Z.; Chen, J.; Yu, F.; Wang, H.; Kou, X.; Ma, C.; Zhu, S. The Antioxidant, Antibacterial, Antibiofilm Activity of Essential Oil from Citrus medica L. Var. Sarcodactylis and Its Nanoemulsion. LWT 2017, 80, 371–377. [Google Scholar] [CrossRef]
- Murphy, E.A.; Majeti, B.K.; Barnes, L.A.; Makale, M.; Weis, S.M.; Lutu-Fuga, K.; Wrasidlo, W.; Cheresh, D.A.; Carson, D.A. Nanoparticle-Mediated Drug Delivery to Tumor Vasculature Suppresses Metastasis. Proc. Natl. Acad. Sci. USA 2008, 105, 9343–9348. [Google Scholar] [CrossRef]
- Hu, C.-M.J.; Aryal, S.; Zhang, L. Nanoparticle-Assisted Combination Therapies for Effective Cancer Treatment. Ther. Deliv. 2010, 1, 323–334. [Google Scholar] [CrossRef]
- Acharya, C.; Mishra, S.; Chaurasia, S.K.; Pandey, B.K.; Dhar, R.; Pandey, J.K. Synthesis of Metallic Nanoparticles Using Biometabolites: Mechanisms and Applications. BioMetals 2025, 38, 21–54. [Google Scholar] [CrossRef]
- Kubavat, K.; Trivedi, P.; Ansari, H.; Kongor, A.; Panchal, M.; Jain, V.; Sindhav, G. Green Synthesis of Silver Nanoparticles Using Dietary Antioxidant Rutin and Its Biological Contour. Beni-Suef Univ. J. Basic. Appl. Sci. 2022, 11, 115. [Google Scholar] [CrossRef]
- Shanmuganathan, R.; Sathishkumar, G.; Brindhadevi, K.; Pugazhendhi, A. Fabrication of Naringenin Functionalized-Ag/RGO Nanocomposites for Potential Bactericidal Effects. J. Mater. Res. Technol. 2020, 9, 7013–7019. [Google Scholar] [CrossRef]
- Mizielińska, M.; Nawrotek, P.; Stachurska, X.; Ordon, M.; Bartkowiak, A. Packaging Covered with Antiviral and Antibacterial Coatings Based on ZnO Nanoparticles Supplemented with Geraniol and Carvacrol. Int. J. Mol. Sci. 2021, 22, 1717. [Google Scholar] [CrossRef]
- Chen, P.; Chen, F.; Guo, Z.L.; Lei, J.; Zhou, B. Recent Advancement in Bioeffect, Metabolism, Stability, and Delivery Systems of Apigenin, a Natural Flavonoid Compound: Challenges and Perspectives. Front. Nutr. 2023, 10, 1221227. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Cao, Y.; Wang, M.; Kong, B.; Liu, Q.; Wang, H.; Wang, H. Zein-Quercetin Covalent Nanoparticles Encapsulating Oregano Essential Oil: Improved Stability, Antioxidant, and Antibacterial Properties. Process Biochem. 2025, 149, 248–259. [Google Scholar] [CrossRef]
- Keawchaoon, L.; Yoksan, R. Preparation, Characterization and In Vitro Release Study of Carvacrol-Loaded Chitosan Nanoparticles. Colloids Surf. B Biointerfaces 2011, 84, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Shakeri, F.; Shakeri, S.; Hojjatoleslami, M. Preparation and Characterization of Carvacrol Loaded Polyhydroxybutyrate Nanoparticles by Nanoprecipitation and Dialysis Methods. J. Food Sci. 2014, 79, N697–N705. [Google Scholar] [CrossRef]
- Martínez-Sanz, M.; Bilbao-Sainz, C.; Du, W.X.; Chiou, B.-S.; Williams, T.; Wood, D.F.; Imam, S.H.; Orts, W.J.; López-Rubio, A.; Lagarón, J.M. Antimicrobial Poly(Lactic Acid)-Based Nanofibres Developed by Solution Blow Spinning. J. Nanosci. Nanotechnol. 2015, 15, 616–627. [Google Scholar] [CrossRef]
- Tenci, M.; Rossi, S.; Aguzzi, C.; Carazo, E.; Sandri, G.; Bonferoni, M.C.; Grisoli, P.; Viseras, C.; Caramella, C.M.; Ferrari, F. Carvacrol/Clay Hybrids Loaded into In Situ Gelling Films. Int. J. Pharm. 2017, 531, 676–688. [Google Scholar] [CrossRef]
- Landis, R.F.; Gupta, A.; Lee, Y.; Wang, L.; Golba, B.; Couillaud, B.; Ridolfo, R.; Das, R.; Rotello, V.M. Cross-Linked Polymer-Stabilized Nanocomposites for the Treatment of Bacterial Biofilms. ACS Nano 2017, 11, 946–952. [Google Scholar] [CrossRef]
- Engel, J.B.; Heckler, C.; Tondo, E.C.; Daroit, D.J.; da Silva Malheiros, P. Antimicrobial Activity of Free and Liposome-Encapsulated Thymol and Carvacrol against Salmonella and Staphylococcus aureus Adhered to Stainless Steel. Int. J. Food Microbiol. 2017, 252, 18–23. [Google Scholar] [CrossRef]
- Ayres Cacciatore, F.; Dalmás, M.; Maders, C.; Ataíde Isaía, H.; Brandelli, A.; da Silva Malheiros, P. Carvacrol Encapsulation into Nanostructures: Characterization and Antimicrobial Activity against Foodborne Pathogens Adhered to Stainless Steel. Food Res. Int. 2020, 133, 109143. [Google Scholar] [CrossRef]
- Castro, D.O.; Tabary, N.; Martel, B.; Gandini, A.; Belgacem, N.; Bras, J. Effect of Different Carboxylic Acids in Cyclodextrin Functionalization of Cellulose Nanocrystals for Prolonged Release of Carvacrol. Mater. Sci. Eng. C 2016, 69, 1018–1025. [Google Scholar] [CrossRef]
- Nostro, A.; Scaffaro, R.; Botta, L.; Filocamo, A.; Marino, A.; Bisignano, G. Effect of Temperature on the Release of Carvacrol and Cinnamaldehyde Incorporated into Polymeric Systems to Control Growth and Biofilms of Escherichia coli and Staphylococcus aureus. Biofouling 2015, 31, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Bertuola, M.; Grillo, C.A.; Pissinis, D.E.; Prieto, E.D.; Fernández Lorenzo de Mele, M. Is the Biocompatibility of Copper with Polymerized Natural Coating Dependent on the Potential Selected for the Electropolymerization Process? Colloids Surf. B Biointerfaces 2017, 159, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Bertuola, M.; Fagali, N.; Fernández Lorenzo de Mele, M. Detection of Carvacrol in Essential Oils by Electrochemical Polymerization. Heliyon 2020, 6, e03714. [Google Scholar] [CrossRef]
- Jabir, M.S.; Taha, A.A.; Sahib, U.I. Linalool Loaded on Glutathione-Modified Gold Nanoparticles: A Drug Delivery System for a Successful Antimicrobial Therapy. Artif. Cells Nanomed. Biotechnol. 2018, 46, 345–355. [Google Scholar] [CrossRef]
- Zarei, Z.; Kharaziha, M.; Karimzadeh, F.; Khadem, E. Synthesis and Biological Applications of Nanocomposite Hydrogels Based on the Methacrylation of Hydroxypropyl Methylcellulose and Lignin Loaded with Alpha-Pinene. Carbohydr. Polym. 2024, 346, 122642. [Google Scholar] [CrossRef]
- Nemoda, M.; Veljković, F.; Nikolić, B.; Brkić, S.; Marković, D.; Momčilović, M.; Lal, M.; Živković, L.; Marinković, J. Geraniol In Vitro and Geraniol-Based Emulsion Ex Vivo Potential against Four-Species Streptococcus spp. Biofilm Relevant for Dentistry. Ind. Crops Prod. 2024, 222, 119827. [Google Scholar] [CrossRef]
- Balta, I.; Brinzan, L.; Ch Stratakos, A.; Linton, M.; Kelly, C.; Pinkerton, L.; Corcionivoschi, N. Geraniol and Linalool Loaded Nanoemulsions and Their Antimicrobial Activity. Bull. UASVM Anim. Sci. Biotechnol. 2017, 74, 2. [Google Scholar] [CrossRef][Green Version]
- Sinsinwar, S.; Vadivel, V. Development and Characterization of Catechin-in-Cyclodextrin-in-Phospholipid Liposome to Eradicate MRSA-Mediated Surgical Site Infection: Investigation of Their Anti-Infective Efficacy through In Vitro and In Vivo Studies. Int. J. Pharm. 2021, 609, 121130. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dahan, A.; Yarmolinsky, L.; Nakonechny, F.; Semenova, O.; Khalfin, B.; Ben-Shabat, S. Etrog Citron (Citrus medica) as a Novel Source of Antimicrobial Agents: Overview of Its Bioactive Phytochemicals and Delivery Approaches. Pharmaceutics 2025, 17, 761. https://doi.org/10.3390/pharmaceutics17060761
Dahan A, Yarmolinsky L, Nakonechny F, Semenova O, Khalfin B, Ben-Shabat S. Etrog Citron (Citrus medica) as a Novel Source of Antimicrobial Agents: Overview of Its Bioactive Phytochemicals and Delivery Approaches. Pharmaceutics. 2025; 17(6):761. https://doi.org/10.3390/pharmaceutics17060761
Chicago/Turabian StyleDahan, Arik, Ludmila Yarmolinsky, Faina Nakonechny, Olga Semenova, Boris Khalfin, and Shimon Ben-Shabat. 2025. "Etrog Citron (Citrus medica) as a Novel Source of Antimicrobial Agents: Overview of Its Bioactive Phytochemicals and Delivery Approaches" Pharmaceutics 17, no. 6: 761. https://doi.org/10.3390/pharmaceutics17060761
APA StyleDahan, A., Yarmolinsky, L., Nakonechny, F., Semenova, O., Khalfin, B., & Ben-Shabat, S. (2025). Etrog Citron (Citrus medica) as a Novel Source of Antimicrobial Agents: Overview of Its Bioactive Phytochemicals and Delivery Approaches. Pharmaceutics, 17(6), 761. https://doi.org/10.3390/pharmaceutics17060761










