A TAT Peptide-Functionalized Liposome Delivery Phage System (TAT-Lip@PHM) for an Enhanced Eradication of Intracellular MRSA
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains and Samples
2.2. Reagents and Instruments
2.3. Isolation and Purification of Phage
2.4. Analysis of Phage Morphology and Biological Characteristics
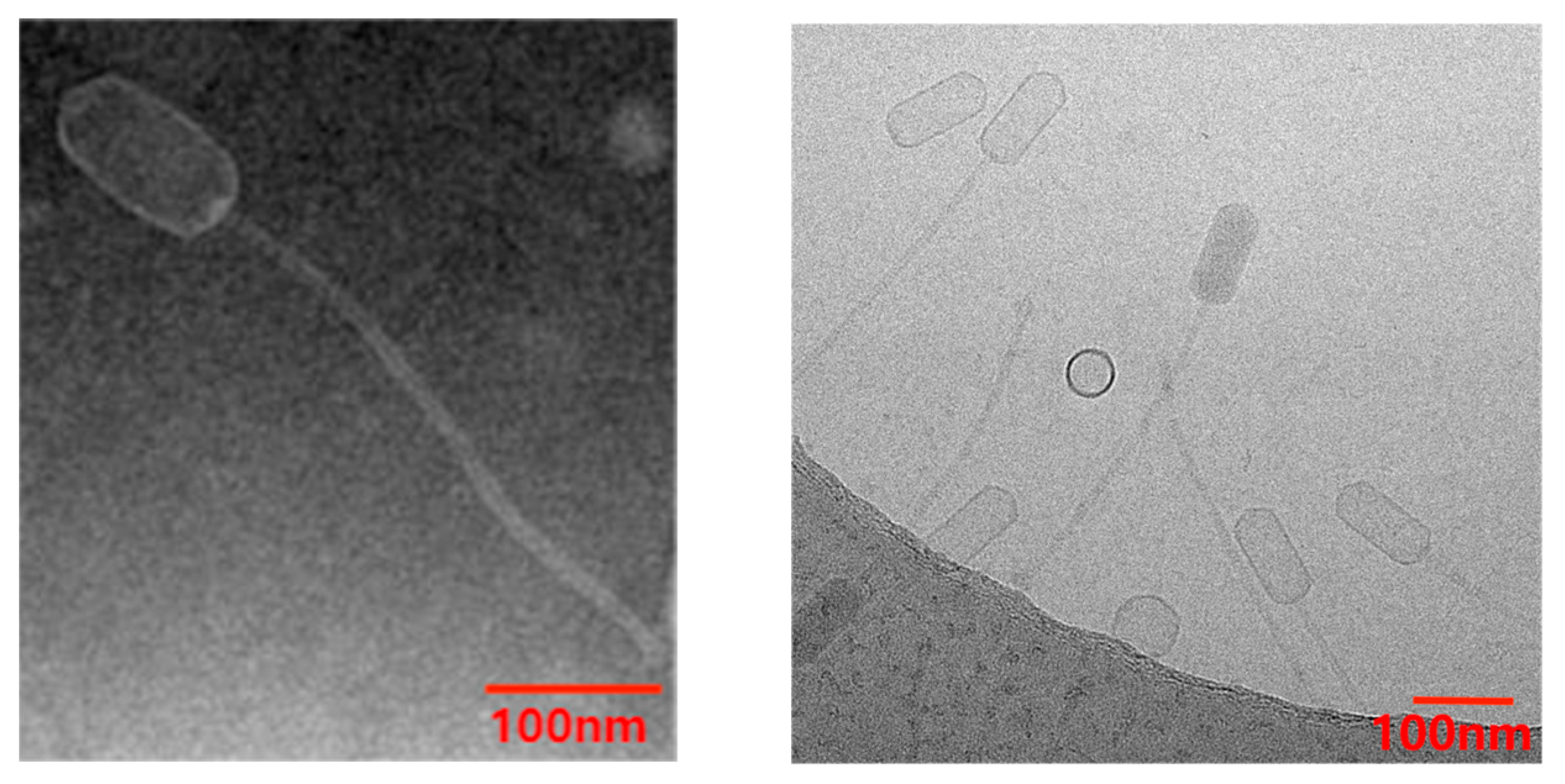
2.4.1. Morphological Observation of Phage
2.4.2. Phage Host Spectrum Test
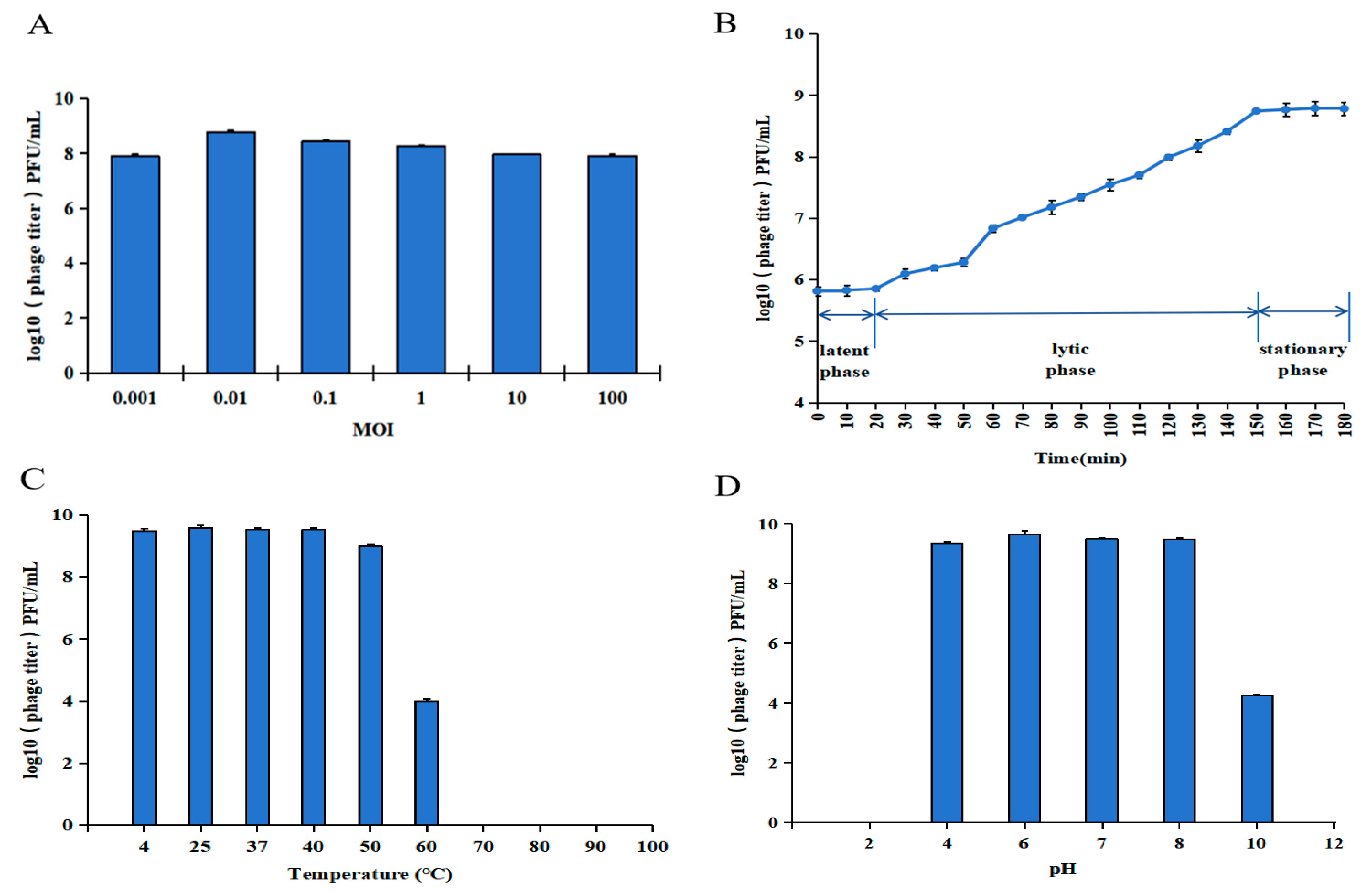
2.4.3. Determination of the Optimal Multiplicity of Infection (MOI)
2.4.4. One-Step Growth Curve Test
2.4.5. Thermal and pH Stability Assays
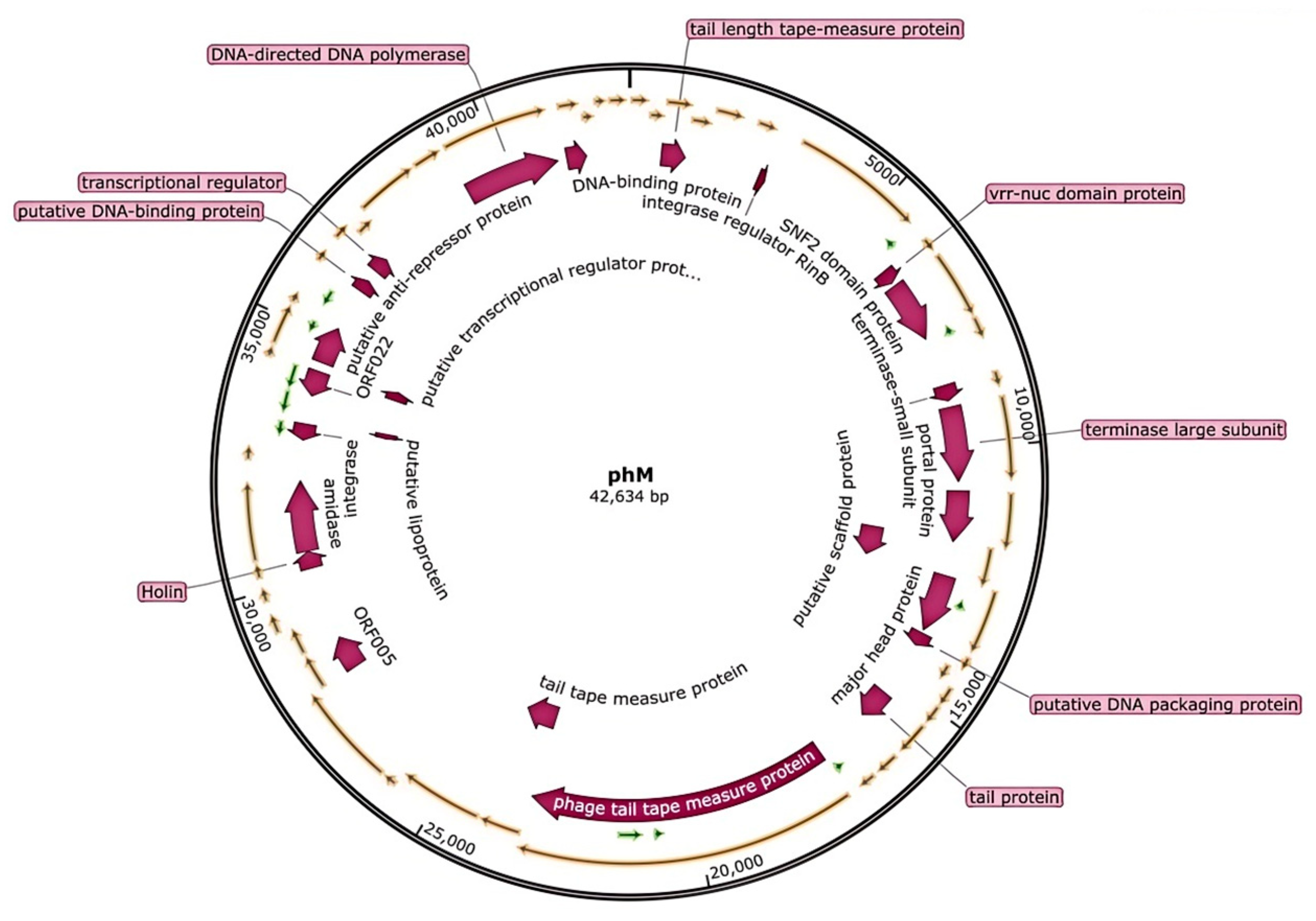
2.5. Analysis of Phage Genome
2.6. Preparation and Characterization of Lip@PHM
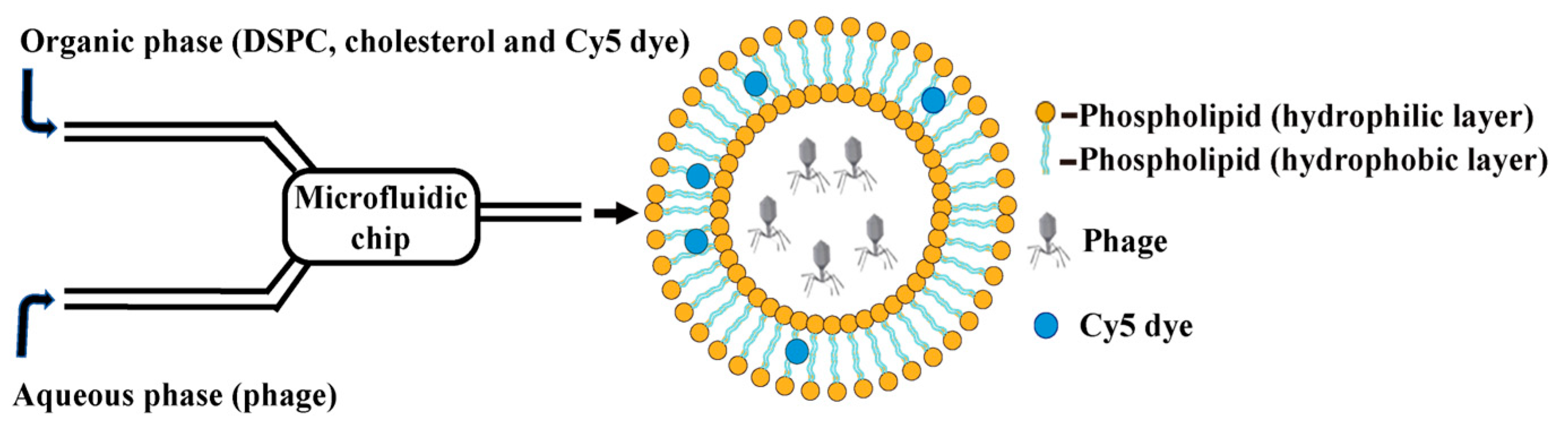
2.6.1. Preparation of Lip@PHM by Microfluidic Method
2.6.2. Purification and Characterization of Lip@PHM
2.7. Preparation and Characterization of TAT-Lip@PHM
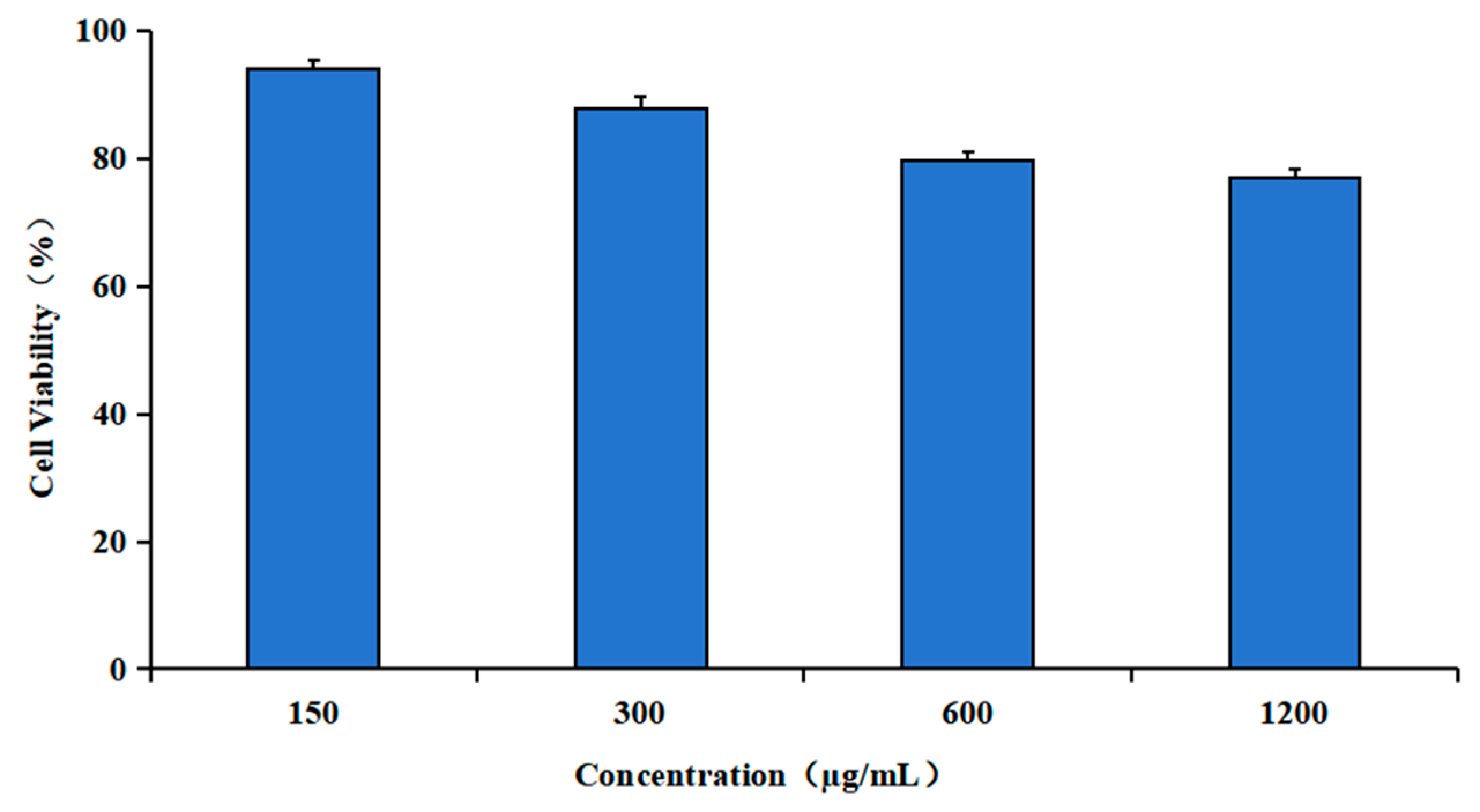
2.8. Cytotoxicity Assay of TAT-Lip@PHM
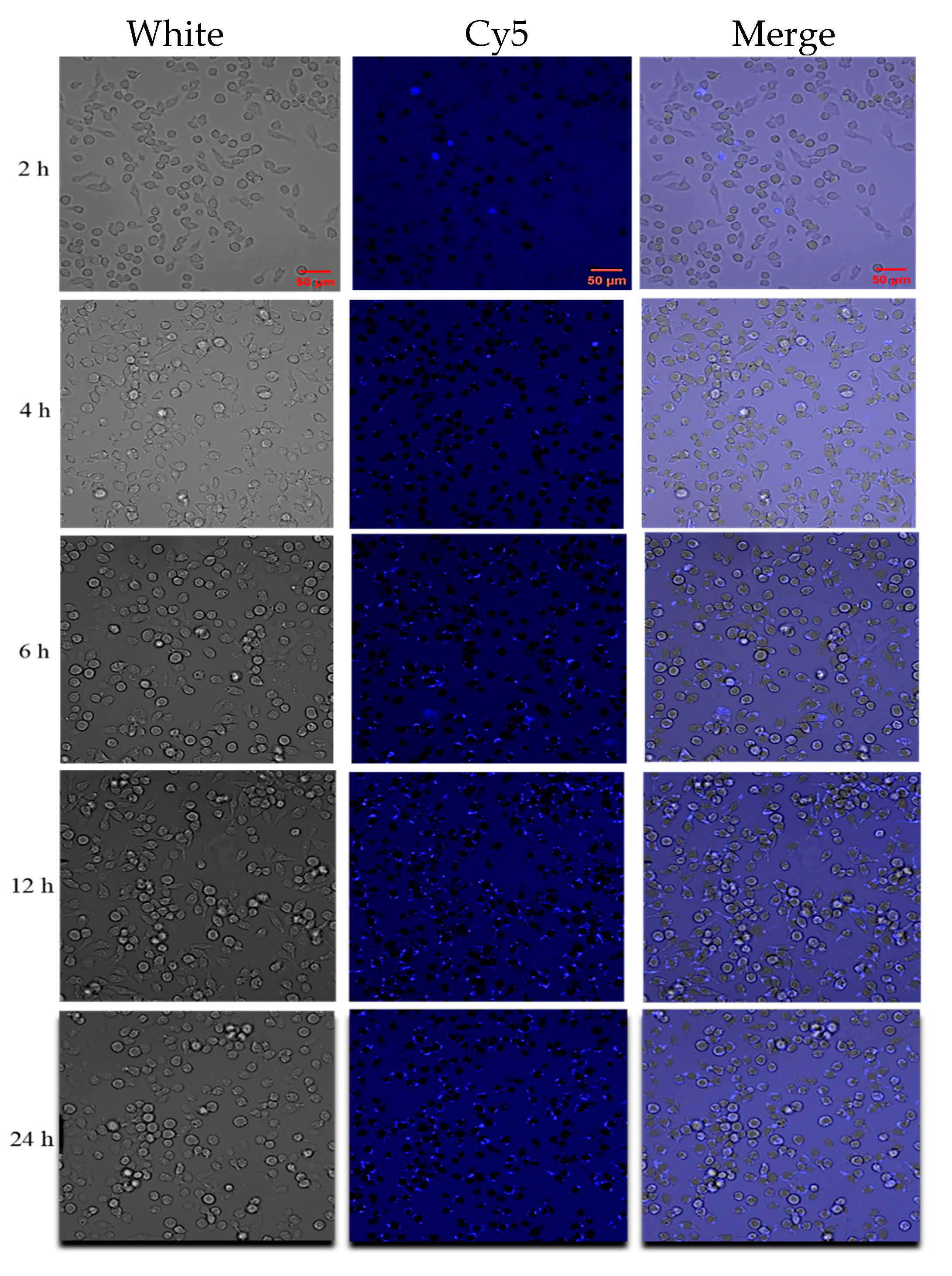
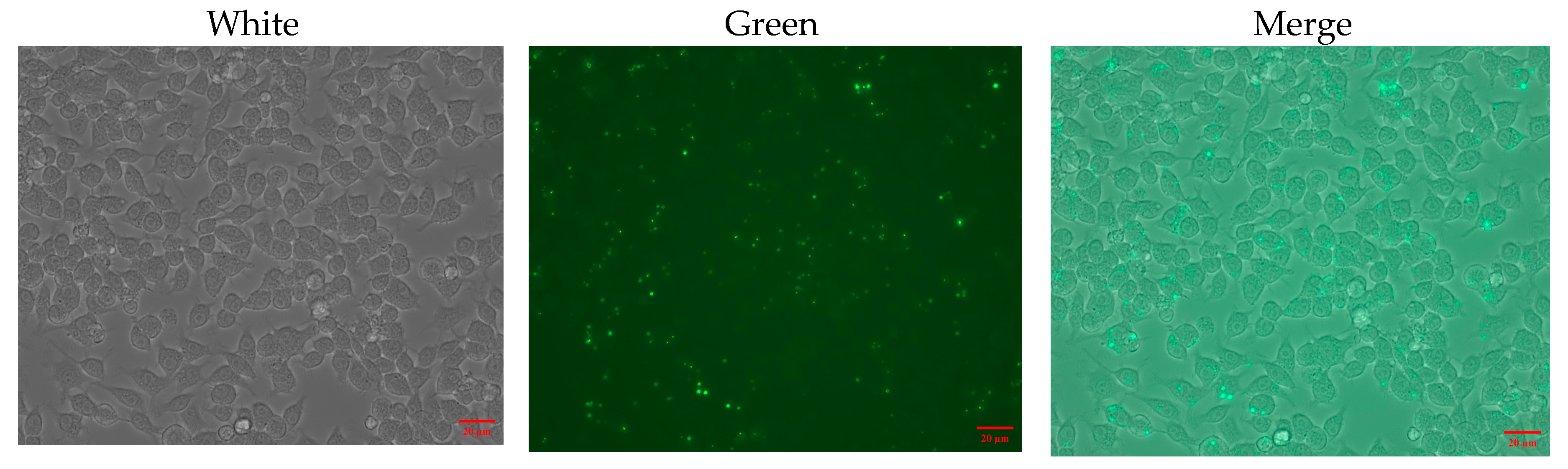
2.9. Intracellular Localization of TAT-Lip@PHM
2.10. Eradication Effect of TAT-Lip@PHM on Intracellular MRSA Infection
2.10.1. Construction of an Intracellular MRSA Infection Cell Model
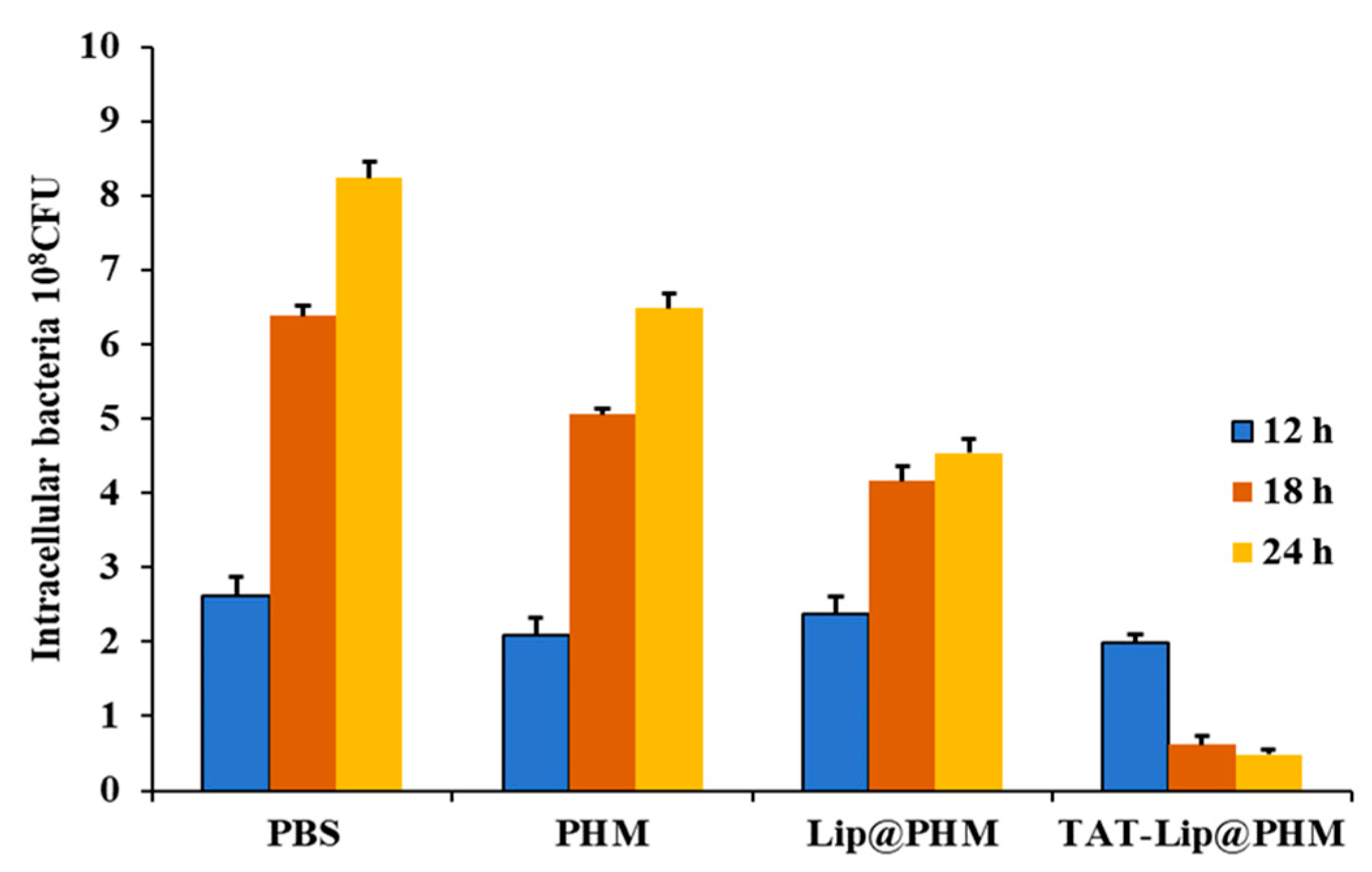
2.10.2. Evaluation of Intracellular MRSA Eradication
3. Results
3.1. Biological Characteristics of Phage vB_SauS_PHM
3.2. Genome Analysis of Bacteriophage
3.3. The Characterization Results of Lip@PHM
3.4. Characterization of TAT-Lip@PHM
3.5. The Biological Activity of TAT-Lip@PHM
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feng, W.; Li, G.; Kang, X.; Wang, R.; Liu, F.; Zhao, D.; Li, H.; Bu, F.; Yu, Y.; Moriarty, T.F. Cascade-Targeting Poly (amino acid) Nanoparticles Eliminate Intracellular Bacteria via On-Site Antibiotic Delivery. Adv. Mater. 2022, 34, 2109789. [Google Scholar] [CrossRef] [PubMed]
- Montanari, E.; Mancini, P.; Galli, F.; Varani, M.; Santino, I.; Coviello, T.; Mosca, L.; Matricardi, P.; Rancan, F.; Di Meo, C. Biodistribution and intracellular localization of hyaluronan and its nanogels. A strategy to target intracellular S. aureus in persistent skin infections. J. Control. Release 2020, 326, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Soe, Y.M.; Bedoui, S.; Stinear, T.P.; Hachani, A. Intracellular Staphylococcus aureus and host cell death pathways. Cell. Microbiol. 2021, 23, e13317. [Google Scholar] [CrossRef] [PubMed]
- Cascioferro, S.; Carbone, D.; Parrino, B.; Pecoraro, C.; Giovannetti, E.; Cirrincione, G.; Diana, P. Therapeutic Strategies to Counteract Antibiotic Resistance in MRSA Biofilm-Associated Infections. ChemMedChem 2021, 16, 65–80. [Google Scholar] [CrossRef]
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Lehar, S.M.; Pillow, T.; Xu, M.; Staben, L.; Kajihara, K.K.; Vandlen, R.; DePalatis, L.; Raab, H.; Hazenbos, W.L.; Morisaki, J.H. Novel antibody–antibiotic conjugate eliminates intracellular S. aureus. Nature 2015, 527, 323–328. [Google Scholar] [CrossRef]
- Li, L.; Zhong, Q.; Zhao, Y.; Bao, J.; Liu, B.; Zhong, Z.; Wang, J.; Yang, L.; Zhang, T.; Cheng, M.; et al. First-in-human application of double-stranded RNA bacteriophage in the treatment of pulmonary Pseudomonas aeruginosa infection. Microb. Biotechnol. 2023, 16, 862–867. [Google Scholar] [CrossRef]
- Jamal, M.; Bukhari, S.M.A.U.S.; Andleeb, S.; Ali, M.; Raza, S.; Nawaz, M.A.; Hussain, T.; Rahman, S.U.; Shah, S.S.A. Bacteriophages: An overview of the control strategies against multiple bacterial infections in different fields. J. Basic Microbiol. 2019, 59, 123–133. [Google Scholar] [CrossRef]
- Weber-Dąbrowska, B.; Żaczek, M.; Łobocka, M.; Łusiak-Szelachowska, M.; Owczarek, B.; Orwat, F.; Łodej, N.; Skaradzińska, A.; Łaczmański, Ł.; Martynowski, D.; et al. Characteristics of Environmental Klebsiella pneumoniae and Klebsiella oxytoca Bacteriophages and Their Therapeutic Applications. Pharmaceutics 2023, 15, 434. [Google Scholar] [CrossRef]
- Balcão, V.M.; Belline, B.G.; Silva, E.C.; Almeida, P.F.F.B.; Baldo, D.Â.; Amorim, L.R.P.; Oliveira Júnior, J.M.; Vila, M.M.D.C.; Del Fiol, F.S. Isolation and Molecular Characterization of Two Novel Lytic Bacteriophages for the Biocontrol of Escherichia coli in Uterine Infections: In Vitro and Ex Vivo Preliminary Studies in Veterinary Medicine. Pharmaceutics 2022, 14, 2344. [Google Scholar] [CrossRef]
- Kevin, S.; Wolfgang, P.; Alex, K.; HansPeter, H. Synergy between Phage Sb-1 and Oxacillin against Methicillin-Resistant Staphylococcus aureus. Antibiotics 2021, 10, 849. [Google Scholar] [CrossRef] [PubMed]
- Fei, T.; Xiaoyu, X.; Songsong, Z.; Guiwei, L.; Ruichong, W.; Lanlan, Z.; Xiaona, W.; Han, Z.; Jiaxuan, L.; Yijing, L.; et al. Efficacy Assessment of Phage Therapy in Treating Staphylococcus aureus-Induced Mastitis in Mice. Viruses 2022, 14, 620. [Google Scholar] [CrossRef]
- Jonathan, S.; Wenqian, W.E.; Dustin, G.; Joseph, F.; Bri’Anna, H.; Theodore, M.; Doub, J.B. Successful Use of Salvage Bacteriophage Therapy for a Recalcitrant MRSA Knee and Hip Prosthetic Joint Infection. Pharmaceuticals 2022, 15, 177. [Google Scholar] [CrossRef]
- Doub, J.B.; Ng, V.Y.; Myounghee, L.; Andrew, C.; Alina, L.; Silvia, W.; Benjamin, C. Salphage: Salvage Bacteriophage Therapy for Recalcitrant MRSA Prosthetic Joint Infection. Antibiotics 2022, 11, 616. [Google Scholar] [CrossRef] [PubMed]
- Uyttebroek, S.; Chen, B.; Onsea, J.; Ruythooren, F.; Debaveye, Y.; Devolder, D.; Spriet, I.; Depypere, M.; Wagemans, J.; Lavigne, R.; et al. Safety and efficacy of phage therapy in difficult-to-treat infections: A systematic review. Lancet Infect. Dis. 2022, 22, e208–e220. [Google Scholar] [CrossRef]
- Colom, J.; Cano-Sarabia, M.; Otero, J.; Cortés, P.; Maspoch, D.; Llagostera, M. Liposome-Encapsulated Bacteriophages for Enhanced Oral Phage Therapy against Salmonella spp. Appl. Environ. Microbiol. 2015, 81, 4841–4849. [Google Scholar] [CrossRef]
- Poerio, N.; Olimpieri, T.; De Angelis, H.L.; De Santis, F.; Thaller, M.C.; D’Andrea, M.M.; Fraziano, M. Fighting MDR-Klebsiella pneumoniae Infections by a Combined Host- and Pathogen-Directed Therapeutic Approach. Front. Immunol. 2022, 13, 835417. [Google Scholar] [CrossRef] [PubMed]
- Zylberberg, C.; Matosevic, S. Pharmaceutical liposomal drug delivery: A review of new delivery systems and a look at the regulatory landscape. Drug Deliv. 2016, 23, 3319–3329. [Google Scholar] [CrossRef]
- Kaur, S.; Kumari, A.; Kumari Negi, A.; Galav, V.; Thakur, S.; Agrawal, M.; Sharma, V. Nanotechnology based approaches in phage therapy: Overcoming the pharmacological barriers. Front. Pharmacol. 2021, 12, 699054. [Google Scholar] [CrossRef]
- Cinquerrui, S.; Mancuso, F.; Malik, D.J. Nanoencapsulation of bacteriophages in liposomes prepared using microfluidic hydrodynamic flow focusing. Front. Microbiol. 2018, 9, 409783. [Google Scholar] [CrossRef]
- Frankel, A.D.; Pabo, C.O. Cellular uptake of the tat protein from human immunodeficiency virus. Cell 1988, 55, 1189–1193. [Google Scholar] [CrossRef] [PubMed]
- Shan, S.; Jia, S.; Lawson, T.; Yan, L.; Lin, M.; Liu, Y. The use of TAT peptide-functionalized graphene as a highly nuclear-targeting carrier system for suppression of choroidal melanoma. Int. J. Mol. Sci. 2019, 20, 4454. [Google Scholar] [CrossRef]
- Choong, H.F.; Yap, B.K. Cell-Penetrating Peptides: Correlation between Peptide-Lipid Interaction and Penetration Efficiency. ChemPhysChem 2021, 22, 493–498. [Google Scholar] [CrossRef]
- Parrasia, S.; Szabò, I.; Zoratti, M.; Biasutto, L. Peptides as Pharmacological Carriers to the Brain: Promises, Shortcomings and Challenges. Mol. Pharm. 2022, 19, 3700–3729. [Google Scholar] [CrossRef]
- Wang, Z.; Kong, L.; Liu, Y.; Fu, Q.; Cui, Z.; Wang, J.; Ma, J.; Wang, H.; Yan, Y.; Sun, J. A Phage Lysin Fused to a Cell-Penetrating Peptide Kills Intracellular Methicillin-Resistant Staphylococcus aureus in Keratinocytes and Has Potential as a Treatment for Skin Infections in Mice. Appl. Environ. Microbiol. 2018, 84, e00380-18. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Tan, X.; Liu, Z.-Q.; Dou, L.; Liu, D.; Pan, Y.-J.; Ma, Y.-F.; Yu, J.-L. Engineered phage with cell-penetrating peptides for intracellular bacterial infections. mSystems 2023, 8, e00646-23. [Google Scholar] [CrossRef] [PubMed]
- Viazis, S.; Akhtar, M.; Feirtag, J.; Brabban, A.D.; Diez-Gonzalez, F. Isolation and characterization of lytic bacteriophages against enterohaemorrhagic Escherichia coli. J. Appl. Microbiol. 2011, 110, 1323–1331. [Google Scholar] [CrossRef]
- Ding, T.; Sun, H.; Pan, Q.; Zhao, F.; Zhang, Z.; Ren, H. Isolation and characterization of Vibrio parahaemolyticus bacteriophage vB_VpaS_PG07. Virus Res. 2020, 286, 198080. [Google Scholar] [CrossRef]
- Adriaenssens, E.; Brister, J.R. How to Name and Classify Your Phage: An Informal Guide. Viruses 2017, 9, 70. [Google Scholar] [CrossRef]
- Leung, S.S.; Morales, S.; Britton, W.; Kutter, E.; Chan, H.-K. Microfluidic-assisted bacteriophage encapsulation into liposomes. Int. J. Pharm. 2018, 545, 176–182. [Google Scholar] [CrossRef]
- Che, J.; Okeke, C.I.; Hu, Z.-B.; Xu, J. DSPE-PEG: A distinctive component in drug delivery system. Curr. Pharm. Des. 2015, 21, 1598–1605. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Leptihn, S.; Dong, K.; Loh, B.; Zhang, Y.; Stefan, M.I.; Li, M.; Guo, X.; Cui, Z. JD419, a Staphylococcus aureus Phage with a Unique Morphology and Broad Host Range. Front. Microbiol. 2021, 12, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.; Zhao, H.; Zhang, R.; Ding, Y.; Gao, C.; He, Y.; Wang, R. Bacteria-mimetic nanomedicine for targeted eradication of intracellular MRSA. J. Control. Release 2023, 357, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Xue, H.Y.; Buttaro, B.A.; Tran, N.T.; Wong, H.L. Enhanced eradication of intracellular and biofilm-residing methicillin-resistant Staphylococcus aureus (MRSA) reservoirs with hybrid nanoparticles delivering rifampicin. Int. J. Pharm. 2020, 589, 119784. [Google Scholar] [CrossRef]
- Pei, Y.; Mohamed, M.F.; Seleem, M.N.; Yeo, Y. Particle engineering for intracellular delivery of vancomycin to methicillin-resistant Staphylococcus aureus (MRSA)-infected macrophages. J. Control. Release 2017, 267, 133–143. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, W.; Bian, C.; Hou, X.; Deng, B.; McComb, D.W.; Chen, X.; Dong, Y. Antibiotic-Derived Lipid Nanoparticles to Treat Intracellular Staphylococcus aureus. ACS Appl. Bio Mater. 2019, 2, 1270–1277. [Google Scholar] [CrossRef]
- Langdon, A.; Crook, N.; Dantas, G. The effects of antibiotics on the microbiome throughout development and alternative approaches for therapeutic modulation. Genome Med. 2016, 8, 39. [Google Scholar] [CrossRef]
- Altamirano, F.L.G.; Barr, J.J. Phage Therapy in the Postantibiotic Era. Clin. Microbiol. Rev. 2019, 32, e00066-18. [Google Scholar] [CrossRef]
- Fabijan, A.P.; Lin, R.C.Y.; Ho, J.; Maddocks, S.; Ben Zakour, N.L.; Iredell, J.R.; Khalid, A.; Venturini, C.; Chard, R.; Morales, S.; et al. Publisher Correction: Safety of bacteriophage therapy in severe Staphylococcus aureus infection. Nat. Microbiol. 2020, 5, 652. [Google Scholar] [CrossRef]
- Gindin, M.; Febvre, H.P.; Rao, S.; Wallace, T.C.; Weir, T.L. Bacteriophage for Gastrointestinal Health (PHAGE) Study: Evaluating the Safety and Tolerability of Supplemental Bacteriophage Consumption. J. Am. Coll. Nutr. 2019, 38, 68–75. [Google Scholar] [CrossRef]
- Khan, A.; Rao, T.S.; Joshi, H.M. Phage therapy in the Covid-19 era: Advantages over antibiotics. Curr. Res. Microb. Sci. 2022, 3, 100115. [Google Scholar] [CrossRef] [PubMed]
- Doss, J.; Culbertson, K.; Hahn, D.; Camacho, J.; Barekzi, N. A Review of Phage Therapy against Bacterial Pathogens of Aquatic and Terrestrial Organisms. Viruses 2017, 9, 50. [Google Scholar] [CrossRef] [PubMed]
- Gharieb, R.M.A.; Saad, M.F.; Mohamed, A.S.; Tartor, Y.H. Characterization of two novel lytic bacteriophages for reducing biofilms of zoonotic multidrug-resistant Staphylococcus aureus and controlling their growth in milk. LWT 2020, 124, 109145. [Google Scholar] [CrossRef]
- Guo, M.; Zhang, Y.; Wu, L.; Xiong, Y.; Xia, L.; Cheng, Y.; Ma, J.; Wang, H.; Sun, J.; Wang, Z.; et al. Development and mouse model evaluation of a new phage cocktail intended as an alternative to antibiotics for treatment of Staphylococcus aureus-induced bovine mastitis. J. Dairy Sci. 2024, 107, 5974–5987. [Google Scholar] [CrossRef]
- Ganaie, M.Y.; Qureshi, S.; Kashoo, Z.; Wani, S.A.; Hussain, M.I.; Kumar, R.; Maqbool, R.; Sikander, P.; Banday, M.S.; Malla, W.A.; et al. Isolation and characterization of two lytic bacteriophages against Staphylococcus aureus from India: Newer therapeutic agents against Bovine mastitis. Vet. Res. Commun. 2018, 42, 289–295. [Google Scholar] [CrossRef]
- Jiang, Y.; Xu, Q.; Jiang, L.; Zheng, R. Isolation and Characterization of a Lytic Staphylococcus aureus Phage WV against Staphylococcus aureus Biofilm. Intervirology 2021, 64, 169–177. [Google Scholar] [CrossRef]
- Yan, W.; Banerjee, P.; Xu, M.; Mukhopadhyay, S.; Ip, M.; Carrigy, N.B.; Lechuga-Ballesteros, D.; To, K.K.W.; Leung, S.S.Y. Formulation strategies for bacteriophages to target intracellular bacterial pathogens. Adv. Drug Deliv. Rev. 2021, 176, 113864. [Google Scholar] [CrossRef]
- Bispo, M.; Santos Sílvio, B.; Melo Luís, D.R.; Azeredo, J.; van Dijl, J.M. Targeted Antimicrobial Photodynamic Therapy of Biofilm-Embedded and Intracellular Staphylococci with a Phage Endolysin’s Cell Binding Domain. Microbiol. Spectr. 2022, 10, e0146621. [Google Scholar] [CrossRef]
- Miernikiewicz, P.; Dąbrowska, K. Endocytosis of Bacteriophages. Curr. Opin. Virol. 2022, 52, 229–235. [Google Scholar] [CrossRef]
- Kerkis, A.; Hayashi, M.A.F.; Yamane, T.; Kerkis, I. Properties of cell penetrating peptides (CPPs). IUBMB Life 2006, 58, 7–13. [Google Scholar] [CrossRef]
- Zong, W.; Shao, X.; Li, J.; Cai, Z.; Zhang, X. Towards a biomimetic cellular structure and physical morphology with liposome-encapsulated agarose sol systems. Int. J. Biol. Macromol. 2024, 264, 130418. [Google Scholar] [CrossRef] [PubMed]
- Zong, W.; Shao, X.; Chai, Y.; Wang, X.; Han, S.; Chu, H.; Zhu, C.; Zhang, X. Liposomes encapsulating artificial cytosol as drug delivery system. Biophys. Chem. 2022, 281, 106728. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, A.; Zhang, S.; Kim, J.; Xia, J.; Zhang, F.; Wang, D.; Wang, Q.; Wang, J. Paclitaxel-loaded ginsenoside Rg3 liposomes for drug-resistant cancer therapy by dual targeting of the tumor microenvironment and cancer cells. J. Adv. Res. 2023, 49, 159–173. [Google Scholar] [CrossRef] [PubMed]
- Mokdad, R.; Seguin, C.; Fournel, S.; Frisch, B.; Heurtault, B.; Hadjsadok, A. Anti-inflammatory effects of free and liposome-encapsulated Algerian thermal waters in RAW 264.7 macrophages. Int. J. Pharm. 2022, 614, 121452. [Google Scholar] [CrossRef]
| Sample | Hydrodynamic Diameter (nm) | Polydispersity Index (PDI) | Zeta Potential (mV) |
|---|---|---|---|
| Phage vB_SauS_PHM | 234.3 ± 7.5 | 0.217 ± 0.005 | −13.7 ± 0.9 |
| Mixture of lipids and phages without microfluidic assembly | double peaks | —— | −10.4 ± 0.9 |
| Lip@PHM before purification | 577 ± 5.3 | 0.197 ± 0.019 | −2.1 ± 0.6 |
| Lip@PHM after purification | 627.7 ± 5.0 | 0.136 ± 0.009 | −0.7 ± 0.1 |
| TAT-Lip@PHM | 637.7 ± 5.7 | 0.146 ± 0.010 | 3.9 ± 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, K.; Lu, X.; Guo, X.; Yang, Y.; Liu, W.; Song, H.; Zhao, R. A TAT Peptide-Functionalized Liposome Delivery Phage System (TAT-Lip@PHM) for an Enhanced Eradication of Intracellular MRSA. Pharmaceutics 2025, 17, 743. https://doi.org/10.3390/pharmaceutics17060743
Liu K, Lu X, Guo X, Yang Y, Liu W, Song H, Zhao R. A TAT Peptide-Functionalized Liposome Delivery Phage System (TAT-Lip@PHM) for an Enhanced Eradication of Intracellular MRSA. Pharmaceutics. 2025; 17(6):743. https://doi.org/10.3390/pharmaceutics17060743
Chicago/Turabian StyleLiu, Kaixin, Xin Lu, Xudong Guo, Yi Yang, Wanying Liu, Hongbin Song, and Rongtao Zhao. 2025. "A TAT Peptide-Functionalized Liposome Delivery Phage System (TAT-Lip@PHM) for an Enhanced Eradication of Intracellular MRSA" Pharmaceutics 17, no. 6: 743. https://doi.org/10.3390/pharmaceutics17060743
APA StyleLiu, K., Lu, X., Guo, X., Yang, Y., Liu, W., Song, H., & Zhao, R. (2025). A TAT Peptide-Functionalized Liposome Delivery Phage System (TAT-Lip@PHM) for an Enhanced Eradication of Intracellular MRSA. Pharmaceutics, 17(6), 743. https://doi.org/10.3390/pharmaceutics17060743







