Linking TLR-7 Signaling to Downregulation of Placental P-Glycoprotein: Implications for Fetal Drug Exposure
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Model
2.2. Sexing Rat Embryos
2.3. Rat Placental Explants
2.4. Placental Proteomics
2.5. Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
2.6. Western Blot
2.7. Statistical Analysis
3. Results
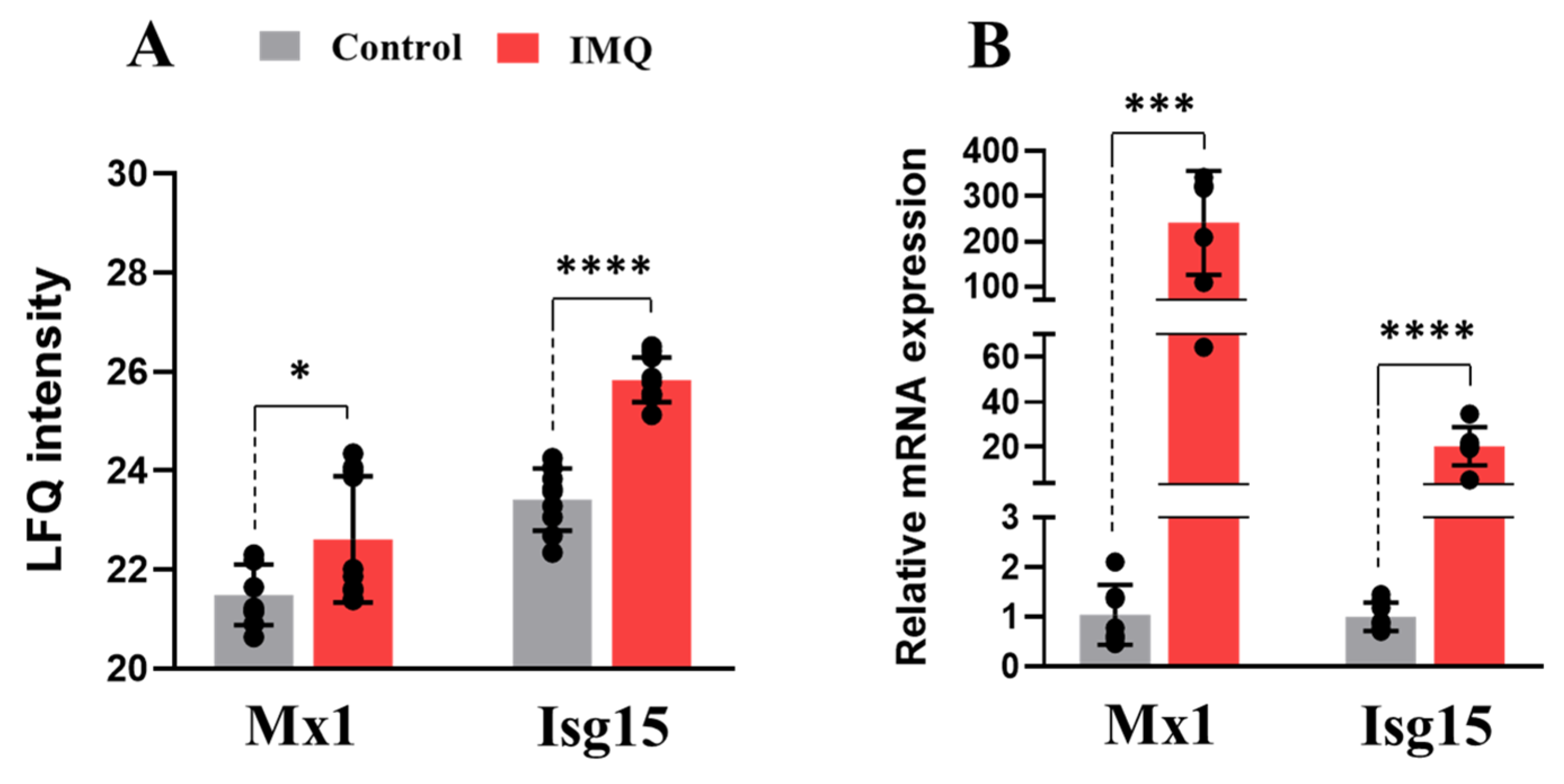
3.1. In Vivo Administration of IMQ Activates TLR-7-Mediated Inflammatory Response
3.2. IMQ Alters the Placental Proteome and Activates Inflammatory Pathways
3.3. In Vivo Administration of IMQ Reduces Expression of PGP
3.4. Ex Vivo TLR-7 Activation Is Associated with Downregulation of Mdr1b in Placental Explants
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABC | ATP-binding Cassette |
| ADs | Autoimmune diseases |
| ANAs | Antinuclear antibodies |
| AOC1 | Copper-containing amine oxidase |
| BCRP | Breast cancer resistance protein |
| CNDP2 | Cytosolic non-specific dipeptidase or carnosine dipeptidase 2 |
| DMEM | Dulbecco’s Modified Eagle Medium |
| EIF4E | Eukaryotic translation initiation factor 4E |
| FGA/FGG | Fibrinogen A |
| GD | Gestational day |
| GLUT-1 | Glucose transporter-1 |
| GPX-3 | Glutathione peroxidase-3 |
| GST-4 | Glutathione s-transferase-4 |
| HIV | Human immunodeficiency virus |
| HP | Haptoglobin |
| HPX | Hemopexin |
| HTRA1 | High-Temperature Requirement A Serine Peptidase 1 |
| I.P. | Intraperitoneal |
| IFN-α | Interferon alpha |
| IFN-γ | Interferon gamma |
| IG | Immunoglobulin |
| IL-6 | Interleukin 6 |
| IMQ | Imiquimod |
| IP-10 | Interferon gamma-induced protein 10 |
| IRF7 | Interferon regulatory factor 7 |
| ISG15 | Interferon-stimulated gene 15 |
| IUGR | Intrauterine growth restriction |
| LAMP-1 | Lysosome-associated membrane glycoprotein 1 |
| MCP-1 | Monocyte Chemoattractant Protein-1 |
| MCT-1 | Monocarboxylate transporter-1 |
| MDR | Multidrug resistance |
| MIP-1α | Macrophage Inflammatory Protein-1 alpha |
| MRP | Multidrug resistance protein |
| MX1 | Myxovirus resistance protein 1 |
| OAS1B | 2′-5′ oligoadenylate synthetase 1B |
| PGP | P-glycoprotein |
| PKR | RNA-dependent protein kinase |
| poly(I:C) | Polyinosinic-polycytidylic acid |
| PON1 | Paraoxonase 1 |
| RAB-6A | Ras-related protein Rab-6A |
| RT-qPCR | Real-time quantitative polymerase chain reaction |
| SERPING1 | Serpin Peptidase Inhibitor, Clade G, Member 1 |
| ssRNA | Single-stranded RNA |
| TLR-7 | Toll-like receptor 7 |
| TNF-α | Tumor necrosis factor alpha |
References
- Scaffidi, J.; Mol, B.; Keelan, J. The pregnant women as a drug orphan: A global survey of registered clinical trials of pharmacological interventions in pregnancy. BJOG Int. J. Obstet. Gynaecol. 2017, 124, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Shields, K.E.; Lyerly, A.D. Exclusion of pregnant women from industry-sponsored clinical trials. Obs. Gynecol. 2013, 122, 1077–1081. [Google Scholar] [CrossRef] [PubMed]
- Morgan, E.T.; Goralski, K.B.; Piquette-Miller, M.; Renton, K.W.; Robertson, G.R.; Chaluvadi, M.R.; Charles, K.A.; Clarke, S.J.; Kacevska, M.; Liddle, C. Regulation of drug-metabolizing enzymes and transporters in infection, inflammation, and cancer. Drug Metab. Dispos. 2008, 36, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Taggi, V.; Riera Romo, M.; Piquette-Miller, M.; Meyer zu Schwabedissen, H.E.; Neuhoff, S. Transporter Regulation in Critical Protective Barriers: Focus on Brain and Placenta. Pharmaceutics 2022, 14, 1376. [Google Scholar] [CrossRef]
- Iqbal, M.; Audette, M.C.; Petropoulos, S.; Gibb, W.; Matthews, S.G. Placental drug transporters and their role in fetal protection. Placenta 2012, 33, 137–142. [Google Scholar] [CrossRef]
- Atkinson, D.E.; Sibley, C.P.; Fairbairn, L.J.; Greenwood, S.L. MDR1 P-gp expression and activity in intact human placental tissue; Upregulation by retroviral transduction. Placenta 2006, 27, 707–714. [Google Scholar] [CrossRef]
- zu Schwabedissen, H.E.M.; Grube, M.; Dreisbach, A.; Jedlitschky, G.; Meissner, K.; Linnemann, K.; Fusch, C.; Ritter, C.A.; Völker, U.; Kroemer, H.K. Epidermal growth factor-mediated activation of the map kinase cascade results in altered expression and function of ABCG2 (BCRP). Drug Metab. Dispos. 2006, 34, 524–533. [Google Scholar] [CrossRef]
- Mathias, A.A.; Hitti, J.; Unadkat, J.D. P-glycoprotein and breast cancer resistance protein expression in human placentae of various gestational ages. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2005, 289, R963–R969. [Google Scholar] [CrossRef]
- Leslie, E.M.; Deeley, R.G.; Cole, S.P. Multidrug resistance proteins: Role of P-glycoprotein, MRP1, MRP2, and BCRP (ABCG2) in tissue defense. Toxicol. Appl. Pharmacol. 2005, 204, 216–237. [Google Scholar] [CrossRef]
- Durmus, S.; Hendrikx, J.J.; Schinkel, A.H. Apical ABC transporters and cancer chemotherapeutic drug disposition. Adv. Cancer Res. 2015, 125, 1–41. [Google Scholar]
- St-Pierre, M.V.; Serrano, M.A.; Macias, R.I.R.; Dubs, U.; Hoechli, M.; Lauper, U.; Meier, P.J.; Marin, J.J.G. Expression of members of the multidrug resistance protein family in human term placenta. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2000, 279, R1495–R1503. [Google Scholar] [CrossRef] [PubMed]
- Dallmann, A.; Liu, X.I.; Burckart, G.J.; van den Anker, J.J. Drug transporters expressed in the human placenta and models for studying maternal-fetal drug transfer. J. Clin. Pharmacol. 2019, 59, S70–S81. [Google Scholar] [CrossRef] [PubMed]
- Pascolo, L.; Fernetti, C.; Pirulli, D.; Crovella, S.; Amoroso, A.; Tiribelli, C. Effects of maturation on RNA transcription and protein expression of four MRP genes in human placenta and in BeWo cells. Biochem. Biophys. Res. Commun. 2003, 303, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Petrovic, V.; Piquette-Miller, M. Impact of polyinosinic/polycytidylic acid on placental and hepatobiliary drug transporters in pregnant rats. Drug Metab. Dispos. 2010, 38, 1760–1766. [Google Scholar] [CrossRef]
- Petrovic, V.; Piquette-Miller, M. Polyinosinic/Polycytidylic Acid–Mediated Changes in Maternal and Fetal Disposition of Lopinavir in Rats. Drug Metab. Dispos. 2015, 43, 951–957. [Google Scholar] [CrossRef]
- Karimian Pour, N.; McColl, E.R.; Piquette-Miller, M. Impact of Viral Inflammation on the Expression of Renal Drug Transporters in Pregnant Rats. Pharmaceutics 2019, 11, 624. [Google Scholar] [CrossRef]
- Baenziger, S.; Heikenwalder, M.; Johansen, P.; Schlaepfer, E.; Hofer, U.; Miller, R.C.; Diemand, S.; Honda, K.; Kundig, T.M.; Aguzzi, A. Triggering TLR7 in mice induces immune activation and lymphoid system disruption, resembling HIV-mediated pathology. Blood J. Am. Soc. Hematol. 2009, 113, 377–388. [Google Scholar] [CrossRef]
- Arias-Loste, M.T.; Cabezas, J.; Llerena, S.; Iruzubieta, P.; San-Segundo, D.; Merino, D.; Cuadrado, A.; Vaqué, J.P.; López-Hoyos, M.; Crespo, J. Successful Direct Acting Antiviral Therapy in Chronic Hepatitis C Normalizes IFNγ and IL2 Production in T Cells Together with TLR8 Expression and Functionality in Peripheral Blood Mononuclear Cells. Viruses 2021, 13, 635. [Google Scholar] [CrossRef]
- Kawasaki, T.; Kawai, T. Toll-like receptor signaling pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef]
- Bender, A.T.; Tzvetkov, E.; Pereira, A.; Wu, Y.; Kasar, S.; Przetak, M.M.; Vlach, J.; Niewold, T.B.; Jensen, M.A.; Okitsu, S.L. TLR7 and TLR8 differentially activate the IRF and NF-κB pathways in specific cell types to promote inflammation. Immunohorizons 2020, 4, 93–107. [Google Scholar] [CrossRef]
- Punnanitinont, A.; Kasperek, E.M.; Zhu, C.; Yu, G.; Miecznikowski, J.C.; Kramer, J.M. TLR7 activation of age-associated B cells mediates disease in a mouse model of primary Sjögren’s disease. J. Leukoc. Biol. 2024, 115, 497–510. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Peng, Y.; Li, H.; Zhang, X. From monogenic lupus to TLR7/MyD88-targeted therapy. Innovation 2022, 3, 100299. [Google Scholar] [CrossRef] [PubMed]
- Zucchi, D.; Elefante, E.; Schilirò, D.; Signorini, V.; Trentin, F.; Bortoluzzi, A.; Tani, C. One year in review 2022: Systemic lupus erythematosus. Clin. Exp. Rheumatol. 2022, 40, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Gomes, V.; Mesquita, A.; Capela, C. Autoimmune diseases and pregnancy: Analysis of a series of cases. BMC Res. Notes 2015, 8, 216. [Google Scholar] [CrossRef]
- Yu, W.; Hu, X.; Cao, B. Viral infections during pregnancy: The big challenge threatening maternal and fetal health. Matern.-Fetal Med. 2022, 4, 72–86. [Google Scholar] [CrossRef]
- Evers, R.; Piquette-Miller, M.; Polli, J.W.; Russel, F.G.; Sprowl, J.A.; Tohyama, K.; Ware, J.A.; de Wildt, S.N.; Xie, W.; Brouwer, K.L. Disease-associated changes in drug transporters may impact the pharmacokinetics and/or toxicity of drugs: A white paper from the International Transporter Consortium. Clin. Pharmacol. Ther. 2018, 104, 900–915. [Google Scholar] [CrossRef]
- Thomsen, L.L.; Topley, P.; Daly, M.G.; Brett, S.J.; Tite, J.P. Imiquimod and resiquimod in a mouse model: Adjuvants for DNA vaccination by particle-mediated immunotherapeutic delivery. Vaccines 2004, 22, 1799–1809. [Google Scholar] [CrossRef]
- Li, W.; Titov, A.A.; Morel, L. An update on lupus animal models. Curr. Opin. Rheumatol. 2017, 29, 434–441. [Google Scholar] [CrossRef]
- Yokogawa, M.; Takaishi, M.; Nakajima, K.; Kamijima, R.; Fujimoto, C.; Kataoka, S.; Terada, Y.; Sano, S. Epicutaneous application of Toll-like receptor 7 agonists leads to systemic autoimmunity in wild-type mice: A new model of systemic lupus erythematosus. J. Arthritis Rheumatol. 2014, 66, 694–706. [Google Scholar] [CrossRef]
- McColl, E.R.; Henderson, J.T.; Piquette-Miller, M. Dysregulation of Amino Acid Transporters in a Rat Model of TLR7-Mediated Maternal Immune Activation. Pharmaceutics 2023, 15, 1857. [Google Scholar] [CrossRef]
- McColl, A.; Thomson, C.A.; Nerurkar, L.; Graham, G.J.; Cavanagh, J. TLR7-mediated skin inflammation remotely triggers chemokine expression and leukocyte accumulation in the brain. J. Neuroinflamm. 2016, 13, 102. [Google Scholar] [CrossRef] [PubMed]
- Miyajima, A.; Sunouchi, M.; Mitsunaga, K.; Yamakoshi, Y.; Nakazawa, K.; Usami, M. Sexing of postimplantation rat embryos in stored twodimensional electrophoresis (2-DE) samples by polymerase chain reaction (PCR) of an Sry sequence. J. Toxicol. Sci. 2009, 34, 681–685. [Google Scholar] [CrossRef] [PubMed]
- Boles, J.L.; Ross, M.G.; Beloosesky, R.; Desai, M.; Belkacemi, L. Placental-mediated increased cytokine response to lipopolysaccharides: A potential mechanism for enhanced inflammation susceptibility of the preterm fetus. J. Inflamm. Res. 2012, 5, 67–75. [Google Scholar] [CrossRef] [PubMed][Green Version]
- George, E.M.; Cockrell, K.; Adair, T.H.; Granger, J.P. Regulation of sFlt-1 and VEGF secretion by adenosine under hypoxic conditions in rat placental villous explants. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2010, 299, R1629–R1633. [Google Scholar] [CrossRef]
- Lehmann, S.M.; Rosenberger, K.; Kruger, C.; Habbel, P.; Derkow, K.; Kaul, D.; Rybak, A.; Brandt, C.; Schott, E.; Wulczyn, F.G.; et al. Extracellularly delivered single-stranded viral RNA causes neurodegeneration dependent on TLR7. J. Immunol. 2012, 189, 1448–1458. [Google Scholar] [CrossRef]
- Heil, F.; Hemmi, H.; Hochrein, H.; Ampenberger, F.; Kirschning, C.; Akira, S.; Lipford, G.; Wagner, H.; Bauer, S. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science 2004, 303, 1526–1529. [Google Scholar] [CrossRef]
- Naud, J.; Michaud, J.; Beauchemin, S.; Hebert, M.J.; Roger, M.; Lefrancois, S.; Leblond, F.A.; Pichette, V. Effects of chronic renal failure on kidney drug transporters and cytochrome P450 in rats. Drug Metab. Dispos. 2011, 39, 1363–1369. [Google Scholar] [CrossRef]
- Pfeifer, E.; Parrott, J.; Lee, G.T.; Domalakes, E.; Zhou, H.; He, L.; Mason, C.W. Regulation of human placental drug transporters in HCV infection and their influence on direct acting antiviral medications. Placenta 2018, 69, 32–39. [Google Scholar] [CrossRef]
- McColl, E.R.; Piquette-Miller, M. Viral model of maternal immune activation alters placental AMPK and mTORC1 signaling in rats. Placenta 2021, 112, 36–44. [Google Scholar] [CrossRef]
- Magomedova, L.; Tiefenbach, J.; Zilberman, E.; Le Billan, F.; Voisin, V.; Saikali, M.; Boivin, V.; Robitaille, M.; Gueroussov, S.; Irimia, M. ARGLU1 is a transcriptional coactivator and splicing regulator important for stress hormone signaling and development. Nucleic Acids Res. 2019, 47, 2856–2870. [Google Scholar] [CrossRef]
- Pollinzi, A.; Mirdamadi, K.; Pour, N.K.; Asthana-Nijjar, R.; Lee, D.; Nevo, O.; Piquette-Miller, M. Decreased expression of P-glycoprotein in the placenta of women with autoimmune disease. Drug Metab. Dispos. 2025, 53, 100031. [Google Scholar] [CrossRef] [PubMed]
- Engel, A.L.; Holt, G.E.; Lu, H. The pharmacokinetics of Toll-like receptor agonists and the impact on the immune system. Expert. Rev. Clin. Pharmacol. 2011, 4, 275–289. [Google Scholar] [CrossRef] [PubMed]
- Keppler, M.; Straß, S.; Geiger, S.; Fischer, T.; Späth, N.; Weinstein, T.; Schwamborn, A.; Guezguez, J.; Guse, J.-H.; Laufer, S. Imidazoquinolines with improved pharmacokinetic properties induce a high IFNα to TNFα ratio in vitro and in vivo. Front. Immunol. 2023, 14, 1168252. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Saadaoui, M.; Al Khodor, S. Infections and pregnancy: Effects on maternal and child health. Front. Cell. Infect. Microbiol. 2022, 12, 873253. [Google Scholar] [CrossRef]
- Sappenfield, E.; Jamieson, D.J.; Kourtis, A.P. Pregnancy and susceptibility to infectious diseases. Infect. Dis. Obs. Gynecol. 2013, 2013, 752852. [Google Scholar] [CrossRef]
- Missig, G.; Robbins, J.O.; Mokler, E.L.; McCullough, K.M.; Bilbo, S.D.; McDougle, C.J.; Carlezon, W.A., Jr. Sex-dependent neurobiological features of prenatal immune activation via TLR7. Mol. Psychiatry 2020, 25, 2330–2341. [Google Scholar] [CrossRef]
- Collier, A.Y.; Smith, L.A.; Karumanchi, S.A. Review of the immune mechanisms of preeclampsia and the potential of immune modulating therapy. Hum. Immunol. 2021, 82, 362–370. [Google Scholar] [CrossRef]
- Psianou, K.; Panagoulias, I.; Papanastasiou, A.D.; de Lastic, A.-L.; Rodi, M.; Spantidea, P.I.; Degn, S.E.; Georgiou, P.; Mouzaki, A. Clinical and immunological parameters of Sjögren’s syndrome. Autoimmun. Rev. 2018, 17, 1053–1064. [Google Scholar] [CrossRef]
- Rabaan, A.A.; Al-Ahmed, S.H.; Muhammad, J.; Khan, A.; Sule, A.A.; Tirupathi, R.; Mutair, A.A.; Alhumaid, S.; Al-Omari, A.; Dhawan, M.; et al. Role of Inflammatory Cytokines in COVID-19 Patients: A Review on Molecular Mechanisms, Immune Functions, Immunopathology and Immunomodulatory Drugs to Counter Cytokine Storm. Vaccines 2021, 9, 436. [Google Scholar] [CrossRef]
- Akase, I.E.; Musa, B.O.P.; Obiako, R.O.; Ahmad Elfulatiy, A.; Mohammed, A.A. Immune Dysfunction in HIV: A Possible Role for Pro- and Anti-Inflammatory Cytokines in HIV Staging. J. Immunol. Res. 2017, 2017, 4128398. [Google Scholar] [CrossRef]
- Lundstrom, K.; Hromić-Jahjefendić, A.; Bilajac, E.; Aljabali, A.A.; Baralić, K.; Sabri, N.A.; Shehata, E.M.; Raslan, M.; Ferreira, A.C.B.; Orlandi, L. COVID-19 signalome: Pathways for SARS-CoV-2 infection and impact on COVID-19 associated comorbidity. Cell. Signal. 2023, 101, 110495. [Google Scholar] [CrossRef] [PubMed]
- Mehta, M.; Dhanjal, D.S.; Paudel, K.R.; Singh, B.; Gupta, G.; Rajeshkumar, S.; Thangavelu, L.; Tambuwala, M.M.; Bakshi, H.A.; Chellappan, D.K. Cellular signalling pathways mediating the pathogenesis of chronic inflammatory respiratory diseases: An update. Inflammopharmacology 2020, 28, 795–817. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Jiao, Y.; Zhang, X. Immunometabolic pathways and its therapeutic implication in autoimmune diseases. Clin. Rev. Allergy Immunol. 2021, 60, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Keller, C.W.; Adamopoulos, I.E.; Lünemann, J.D. Autophagy pathways in autoimmune diseases. J. Autoimmun. 2023, 136, 103030. [Google Scholar] [CrossRef]
- Madda, R.; Lin, S.-C.; Sun, W.-H.; Huang, S.-L. Plasma proteomic analysis of systemic lupus erythematosus patients using liquid chromatography/tandem mass spectrometry with label-free quantification. PeerJ 2018, 6, e4730. [Google Scholar] [CrossRef]
- Babačić, H.; Christ, W.; Araújo, J.E.; Mermelekas, G.; Sharma, N.; Tynell, J.; García, M.; Varnaite, R.; Asgeirsson, H.; Glans, H. Comprehensive proteomics and meta-analysis of COVID-19 host response. Nat. Commun. 2023, 14, 5921. [Google Scholar] [CrossRef]
- Liu, X.; Cao, Y.; Fu, H.; Wei, J.; Chen, J.; Hu, J.; Liu, B. Proteomics analysis of serum from COVID-19 patients. ACS Omega 2021, 6, 7951–7958. [Google Scholar] [CrossRef]
- Zhang, F.; Zhou, P.; Wang, L.; Liao, X.; Liu, X.; Ke, C.; Wen, S.; Shu, Y. Polymorphisms of IFN signaling genes and FOXP4 influence the severity of COVID-19. BMC Infect. Dis. 2024, 24, 270. [Google Scholar] [CrossRef]
- Yuan, Y.; Ma, H.; Ye, Z.; Jing, W.; Jiang, Z. Interferon-stimulated gene 15 expression in systemic lupus erythematosus: Diagnostic value and association with lymphocytopenia. Z. Rheumatol. 2018, 77, 256–262. [Google Scholar] [CrossRef]
- Shimizu, Y.; Yasuda, S.; Kimura, T.; Nishio, S.; Kono, M.; Ohmura, K.; Shimamura, S.; Kono, M.; Fujieda, Y.; Kato, M. Interferon-inducible Mx1 protein is highly expressed in renal tissues from treatment-naïve lupus nephritis, but not in those under immunosuppressive treatment. Mod. Rheumatol. 2018, 28, 661–669. [Google Scholar] [CrossRef]
- Sarkar, L.; Liu, G.; Gack, M.U. ISG15: Its roles in SARS-CoV-2 and other viral infections. Trends Microbiol. 2023, 31, 1262–1275. [Google Scholar] [CrossRef]
- Mirzalieva, O.; Juncker, M.; Schwartzenburg, J.; Desai, S. ISG15 and ISGylation in human diseases. Cells 2022, 11, 538. [Google Scholar] [CrossRef] [PubMed]
- Boroujeni, M.E.; Simani, L.; Bluyssen, H.A.; Samadikhah, H.R.; Zamanlui Benisi, S.; Hassani, S.; Akbari Dilmaghani, N.; Fathi, M.; Vakili, K.; Mahmoudiasl, G.-R. Inflammatory response leads to neuronal death in human post-mortem cerebral cortex in patients with COVID-19. ACS Chem. Neurosci. 2021, 12, 2143–2150. [Google Scholar] [CrossRef] [PubMed]
- Goralski, K.B.; Hartmann, G.; Piquette-Miller, M.; Renton, K.W. Downregulation of mdr1a expression in the brain and liver during CNS inflammation alters the in vivo disposition of digoxin. Br. J. Pharmacol. 2003, 139, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.J.; Cheng, X.; Weaver, Y.M.; Klaassen, C.D. Tissue distribution, gender-divergent expression, ontogeny, and chemical induction of multidrug resistance transporter genes (Mdr1a, Mdr1b, Mdr2) in mice. Drug Metab. Dispos. 2009, 37, 203–210. [Google Scholar] [CrossRef]
- Hartmann, G.; Kim, H.; Piquette-Miller, M. Regulation of the hepatic multidrug resistance gene expression by endotoxin and inflammatory cytokines in mice. Int. Immunopharmacol. 2001, 1, 189–199. [Google Scholar] [CrossRef]
- Tang, W.; Yi, C.; Kalitsky, J.; Piquette-Miller, M. Endotoxin downregulates hepatic expression of P-glycoprotein and MRP2 in 2-acetylaminofluorene-treated rats. Mol. Cell Biol. Res. Commun. 2000, 4, 90–97. [Google Scholar] [CrossRef]
- Schulze, S.; Reinhardt, S.; Freese, C.; Schmitt, U.; Endres, K. Identification of trichlormethiazide as a Mdr1a/b gene expression enhancer via a dual secretion-based promoter assay. Pharmacol. Res. Perspect. 2015, 3, e00109. [Google Scholar] [CrossRef]
- Ghoneim, R.H.; Kojovic, D.; Piquette-Miller, M. Impact of endotoxin on the expression of drug transporters in the placenta of HIV-1 transgenic (HIV-Tg) rats. Eur. J. Pharm. Sci. 2017, 102, 94–102. [Google Scholar] [CrossRef]
- Kawase, A.; Norikane, S.; Okada, A.; Adachi, M.; Kato, Y.; Iwaki, M. Distinct alterations in ATP-binding cassette transporter expression in liver, kidney, small intestine, and brain in adjuvant-induced arthritic rats. J. Pharm. Sci. 2014, 103, 2556–2564. [Google Scholar] [CrossRef]
- Murray, C.; Griffin, E.W.; O’Loughlin, E.; Lyons, A.; Sherwin, E.; Ahmed, S.; Stevenson, N.J.; Harkin, A.; Cunningham, C. Interdependent and independent roles of type I interferons and IL-6 in innate immune, neuroinflammatory and sickness behaviour responses to systemic poly I:C. Brain Behav. Immun. 2015, 48, 274–286. [Google Scholar] [CrossRef]
- Lu, Y.C.; Yeh, W.C.; Ohashi, P.S. LPS/TLR4 signal transduction pathway. Cytokine 2008, 42, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Field, A.K.; Tytell, A.A.; Lampson, G.P.; Hilleman, M.R. Inducers of interferon and host resistance. II. Multistranded synthetic polynucleotide complexes. Proc. Natl. Acad. Sci. USA 1967, 58, 1004–1010. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Chen, Z.; Kato, N.; Gale, M., Jr.; Lemon, S.M. Distinct poly(I-C) and virus-activated signaling pathways leading to interferon-beta production in hepatocytes. J. Biol. Chem. 2005, 280, 16739–16747. [Google Scholar] [CrossRef] [PubMed]
- Sukhai, M.; Yong, A.; Kalitsky, J.; Piquette-Miller, M. Inflammation and interleukin-6 mediate reductions in the hepatic expression and transcription of the mdr1a and mdr1b Genes. Mol. Cell Biol. Res. Commun. 2000, 4, 248–256. [Google Scholar] [CrossRef]
- Sukhai, M.; Yong, A.; Pak, A.; Piquette-Miller, M. Decreased expression of P-glycoprotein in interleukin-1beta and interleukin-6 treated rat hepatocytes. Inflamm. Res. 2001, 50, 362–370. [Google Scholar] [CrossRef]
- Kang, Y.; Perry, R.R. Effect of alpha-interferon on P-glycoprotein expression and function and on verapamil modulation of doxorubicin resistance. Cancer Res. 1994, 54, 2952–2958. [Google Scholar]
- Akazawa, Y.; Kawaguchi, H.; Funahashi, M.; Watanabe, Y.; Yamaoka, K.; Hashida, M.; Takakura, Y. Effect of interferons on P-glycoprotein-mediated rhodamine-123 efflux in cultured rat hepatocytes. J. Pharm. Sci. 2002, 91, 2110–2115. [Google Scholar] [CrossRef]
- Le, H.T.; Franklin, M.R. Selective induction of phase II drug metabolizing enzyme activities by quinolines and isoquinolines. Chem.-Biol. Interact. 1997, 103, 167–178. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Gong, W.; Yang, M.Y.; Yang, Y.; Li, Z.P.; Wang, Y.L.; Zhang, Z.Q. Auto-induction effect of chloroxoquinoline on the cytochrome P450 enzymes of rats associated with CYP 3A and 1A. PLoS ONE 2015, 10, e0138875. [Google Scholar] [CrossRef]
- Bharate, J.B.; Wani, A.; Sharma, S.; Reja, S.I.; Kumar, M.; Vishwakarma, R.A.; Kumar, A.; Bharate, S.B. Synthesis, and the antioxidant, neuroprotective and P-glycoprotein induction activity of 4-arylquinoline-2-carboxylates. Org. Biomol. Chem. 2014, 12, 6267–6277. [Google Scholar] [CrossRef] [PubMed]
- Lye, P.; Bloise, E.; Javam, M.; Gibb, W.; Lye, S.J.; Matthews, S.G. Impact of bacterial and viral challenge on multidrug resistance in first- and third-trimester human placenta. Am. J. Pathol. 2015, 185, 1666–1675. [Google Scholar] [PubMed]
- Girardin, F.; Manuel, O.; Marzolini, C.; Buclin, T. Evaluating the risk of drug-drug interactions with pharmacokinetic boosters: The case of ritonavir-enhanced nirmatrelvir to prevent severe COVID-19. Clin. Microbiol. Infect. 2022, 28, 1044–1046. [Google Scholar] [CrossRef] [PubMed]
- Cianfriglia, M.; Dupuis, M.L.; Molinari, A.; Verdoliva, A.; Costi, R.; Galluzzo, C.M.; Andreotti, M.; Cara, A.; Di Santo, R.; Palmisano, L. HIV-1 integrase inhibitors are substrates for the multidrug transporter MDR1-P-glycoprotein. Retrovirology 2007, 4, 17. [Google Scholar] [CrossRef]
- Weiss, J.; Bajraktari-Sylejmani, G.; Haefeli, W.E. Interaction of Hydroxychloroquine with Pharmacokinetically Important Drug Transporters. Pharmaceutics 2020, 12, 919. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riera-Romo, M.; McColl, E.R.; Piquette-Miller, M. Linking TLR-7 Signaling to Downregulation of Placental P-Glycoprotein: Implications for Fetal Drug Exposure. Pharmaceutics 2025, 17, 741. https://doi.org/10.3390/pharmaceutics17060741
Riera-Romo M, McColl ER, Piquette-Miller M. Linking TLR-7 Signaling to Downregulation of Placental P-Glycoprotein: Implications for Fetal Drug Exposure. Pharmaceutics. 2025; 17(6):741. https://doi.org/10.3390/pharmaceutics17060741
Chicago/Turabian StyleRiera-Romo, Mario, Eliza R McColl, and Micheline Piquette-Miller. 2025. "Linking TLR-7 Signaling to Downregulation of Placental P-Glycoprotein: Implications for Fetal Drug Exposure" Pharmaceutics 17, no. 6: 741. https://doi.org/10.3390/pharmaceutics17060741
APA StyleRiera-Romo, M., McColl, E. R., & Piquette-Miller, M. (2025). Linking TLR-7 Signaling to Downregulation of Placental P-Glycoprotein: Implications for Fetal Drug Exposure. Pharmaceutics, 17(6), 741. https://doi.org/10.3390/pharmaceutics17060741






