Curcumin-Induced Apoptotic Cell Death in Human Glioma Cells Is Enhanced by Clusterin Deficiency
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Culture Conditions
2.2. siRNA Transfection
2.3. Cell Proliferation Assay
2.4. Curcumin Treatment
2.5. Crystal Violet Assay
2.6. Scratch Wound Migration Assay
2.7. Automated Cell Counting
2.8. Acridine Orange and Propidium Iodide Staining
2.9. Assessment of Apoptosis Using Flow Cytometry
2.10. NADPH Assay
2.11. Measurement of H2O2 Using a Fluorometric Assay
2.12. Nitric Oxide Assay
2.13. Determination of Reduced Glutathione
2.14. Protein Determination
2.15. Western Blotting Analysis
2.16. Statistical Analysis
3. Results
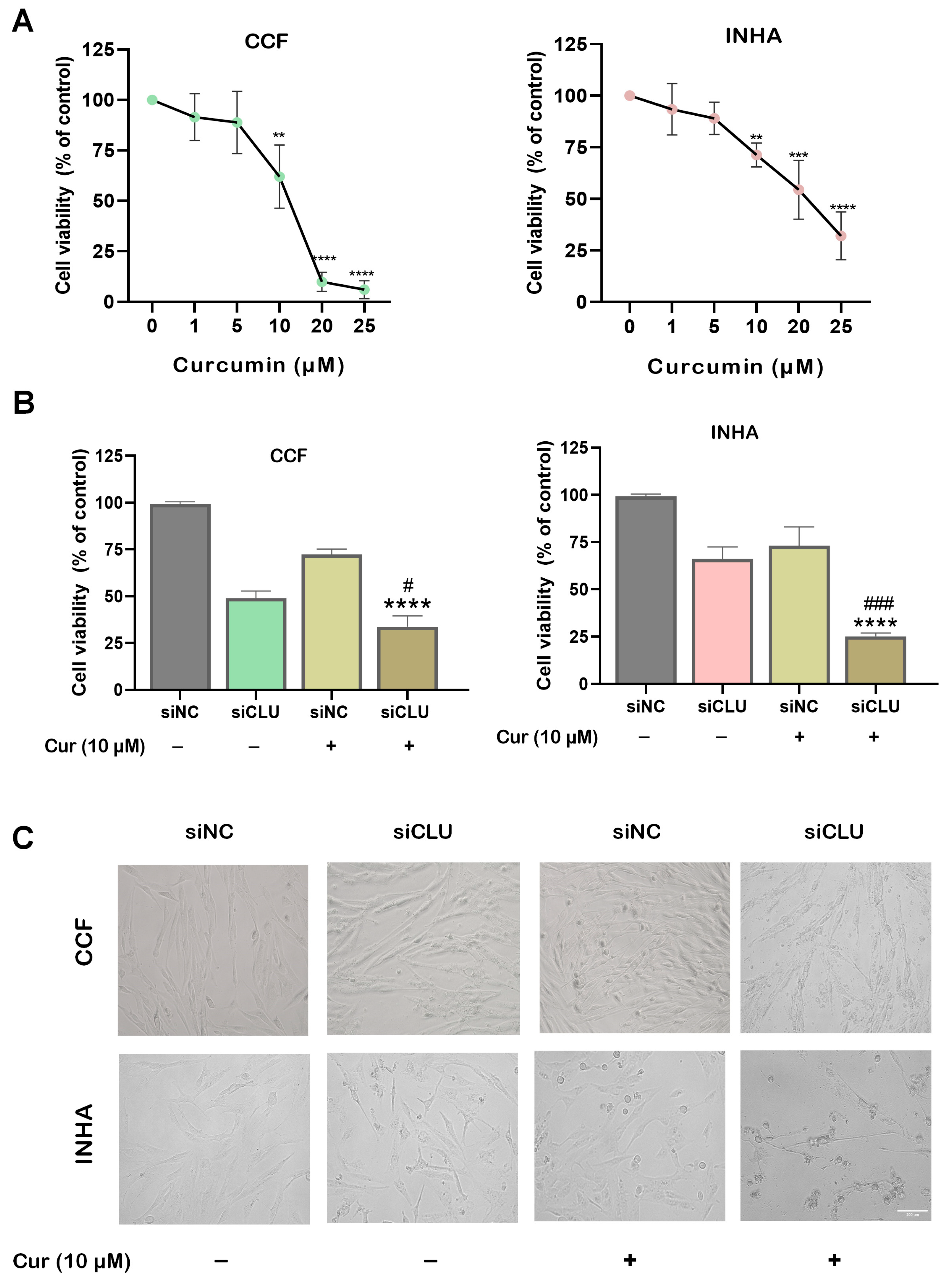
3.1. Curcumin Suppresses Cell Viability and Motility
3.2. Curcumin Mitigates ROS Production in CLU-Deficient Cells
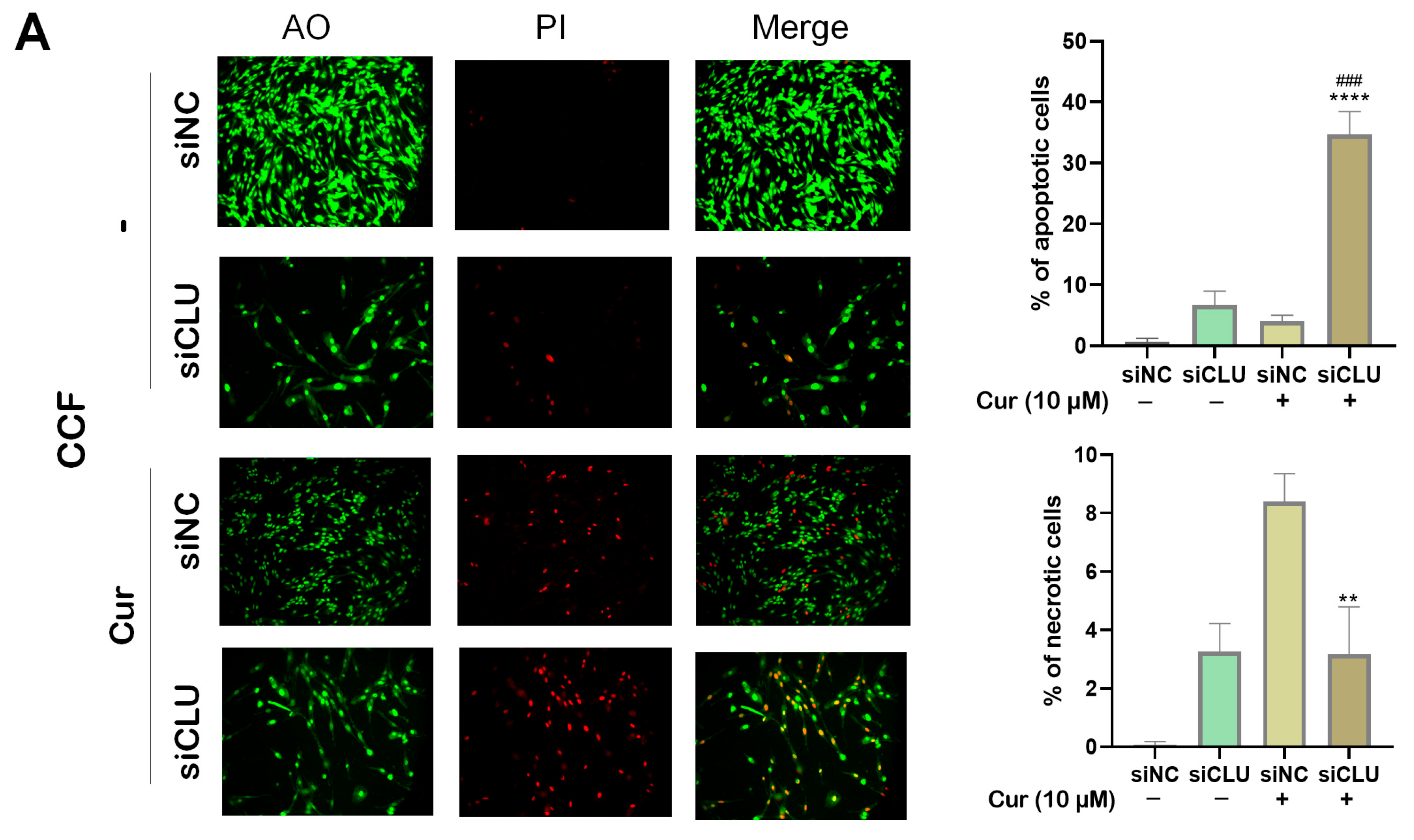
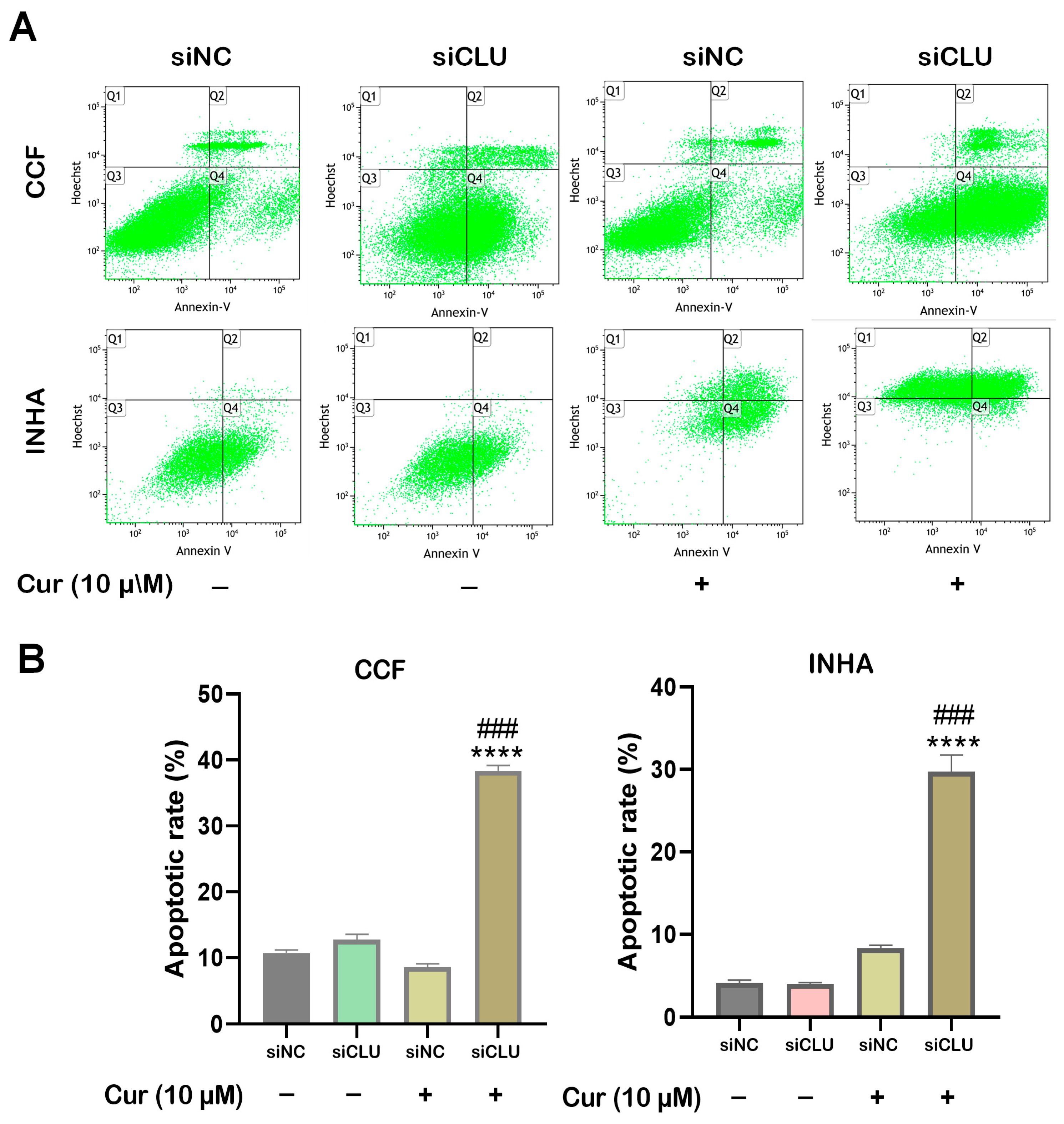
3.3. Curcumin Induces Apoptosis and Necrosis in CLU-Deficient Cells
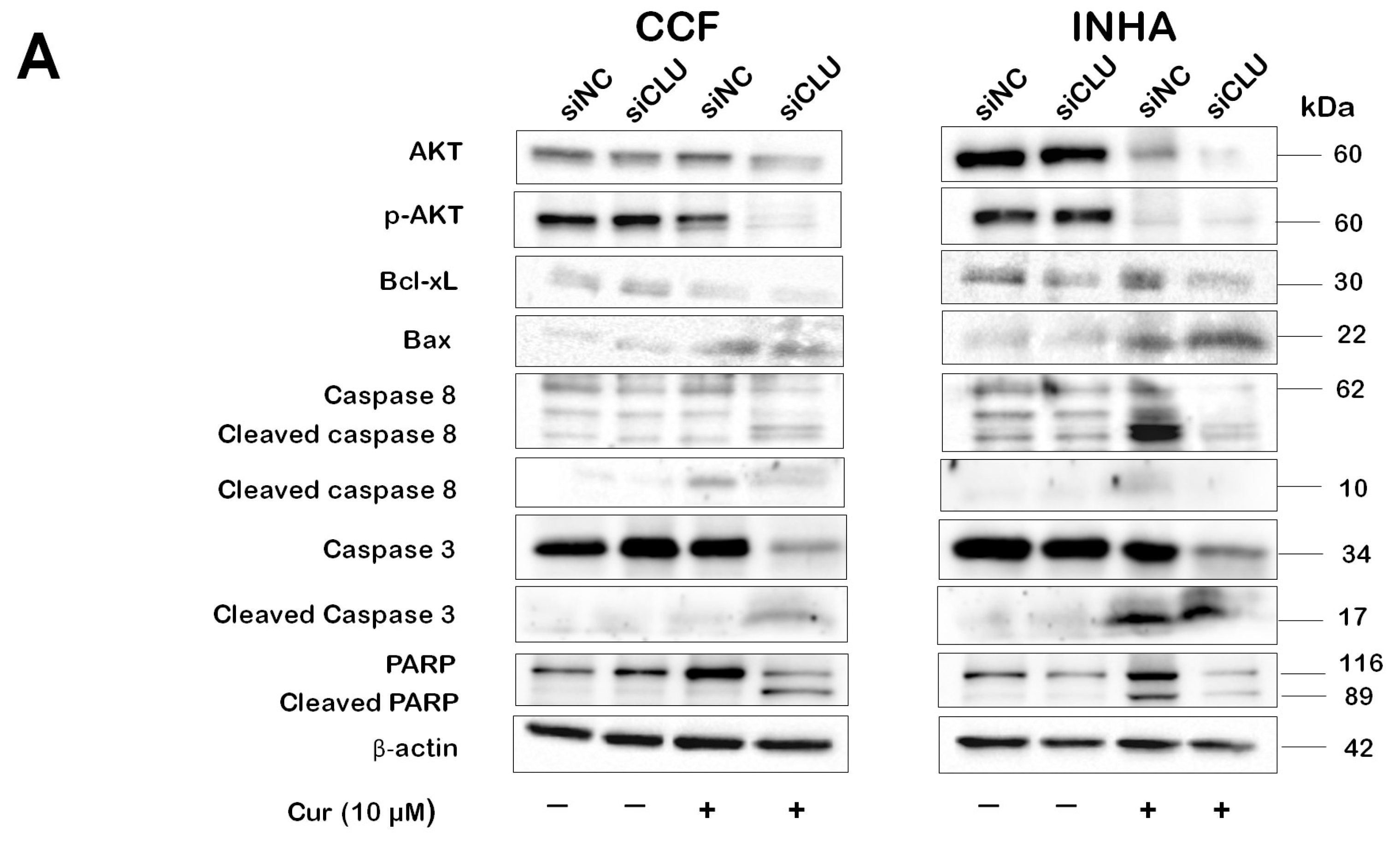
3.4. Curcumin Induces Caspase Activation in CLU-Deficient Cells
3.5. Curcumin Induces Autophagy in CLU-Deficient Cells
4. Discussion
5. Conclusions
6. Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hirtz, A.; Rech, F.; Dubois-Pot-Schneider, H.; Dumond, H. Astrocytoma: A Hormone-Sensitive Tumor? Int. J. Mol. Sci. 2020, 21, 9114. [Google Scholar] [CrossRef] [PubMed]
- Jagtap, J.C.; Parveen, D.; Shah, R.D.; Desai, A.; Bhosale, D.; Chugh, A.; Ranade, D.; Karnik, S.; Khedkar, B.; Mathur, A.; et al. Secretory prostate apoptosis response (Par)-4 sensitizes multicellular spheroids (MCS) of glioblastoma multiforme cells to tamoxifen-induced cell death. FEBS Open Bio 2015, 5, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.R.; O’Neill, B.P. Glioblastoma survival in the United States before and during the temozolomide era. J. Neurooncol. 2012, 107, 359–364. [Google Scholar] [CrossRef]
- Stupp, R.; Hegi, M.E.; Mason, W.P.; van den Bent, M.J.; Taphoorn, M.J.B.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K.; et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef]
- Iacob, G.; Dinca, E.B. Current data and strategy in glioblastoma multiforme. J. Med. Life 2009, 2, 386–393. [Google Scholar]
- Alimohammadi, E.; Bagheri, S.R.; Taheri, S.; Dayani, M.; Abdi, A. The impact of extended adjuvant temozolomide in newly diagnosed glioblastoma multiforme: A meta-analysis and systematic review. Oncol. Rev. 2020, 14, 461. [Google Scholar] [CrossRef] [PubMed]
- Minniti, G.; Amelio, D.; Amichetti, M.; Salvati, M.; Muni, R.; Bozzao, A.; Lanzetta, G.; Scarpino, S.; Arcella, A.; Enrici, R.M. Patterns of failure and comparison of different target volume delineations in patients with glioblastoma treated with conformal radiotherapy plus concomitant and adjuvant temozolomide. Radiother. Oncol. 2010, 97, 377–381. [Google Scholar] [CrossRef]
- Yu, J.T.; Tan, L. The role of clusterin in Alzheimer’s disease: Pathways, pathogenesis, and therapy. Mol. Neurobiol. 2012, 45, 314–326. [Google Scholar] [CrossRef]
- Foster, E.M.; Dangla-Valls, A.; Lovestone, S.; Ribe, E.M.; Buckley, N.J. Clusterin in Alzheimer’s Disease: Mechanisms, Genetics, and Lessons From Other Pathologies. Front. Neurosci. 2019, 13, 164. [Google Scholar] [CrossRef]
- Sensibar, J.A.; Sutkowski, D.M.; Raffo, A.; Buttyan, R.; Griswold, M.D.; Sylvester, S.R.; Kozlowski, J.M.; Lee, C. Prevention of cell death induced by tumor necrosis factor alpha in LNCaP cells by overexpression of sulfated glycoprotein-2 (clusterin). Cancer Res. 1995, 55, 2431–2437. [Google Scholar]
- Trougakos, I.P.; So, A.; Jansen, B.; Gleave, M.E.; Gonos, E.S. Silencing Expression of the Clusterin/Apolipoprotein J Gene in Human Cancer Cells Using Small Interfering RNA Induces Spontaneous Apoptosis, Reduced Growth Ability, and Cell Sensitization to Genotoxic and Oxidative Stress. Cancer Res. 2004, 64, 1834–1842. [Google Scholar] [CrossRef] [PubMed]
- Beer, T.M.; Hotte, S.J.; Saad, F.; Alekseev, B.; Matveev, V.; Fléchon, A.; Gravis, G.; Joly, F.; Chi, K.N.; Malik, Z.; et al. Custirsen (OGX-011) combined with cabazitaxel and prednisone versus cabazitaxel and prednisone alone in patients with metastatic castration-resistant prostate cancer previously treated with docetaxel (AFFINITY): A randomised, open-label, international, phase 3 trial. Lancet Oncol. 2017, 18, 1532–1542. [Google Scholar] [PubMed]
- Mitsufuji, S.; Iwagami, Y.; Kobayashi, S.; Sasaki, K.; Yamada, D.; Tomimaru, Y.; Akita, H.; Asaoka, T.; Noda, T.; Gotoh, K.; et al. Inhibition of Clusterin Represses Proliferation by Inducing Cellular Senescence in Pancreatic Cancer. Ann. Surg. Oncol. 2022, 29, 4937–4946. [Google Scholar] [CrossRef] [PubMed]
- Sultana, P.; Honc, O.; Hodny, Z.; Novotny, J. Clusterin Deficiency Promotes Cellular Senescence in Human Astrocytes. Mol. Neurobiol. 2024, 1862, 5774–5786. [Google Scholar] [CrossRef]
- Sultana, P.; Novotny, J. Clusterin: A double-edged sword in cancer and neurological disorders. EXCLI J. 2024, 23, 912–936. [Google Scholar]
- Unlu, A.; Nayir, E.; Dogukan Kalenderoglu, M.; Kirca, O.; Ozdogan, M. Curcumin (Turmeric) and cancer. J. BUON 2016, 21, 1050–1060. [Google Scholar]
- Cherif, H.; Bisson, D.G.; Jarzem, P.; Weber, M.; Ouellet, J.A.; Haglund, L. Curcumin and o-Vanillin Exhibit Evidence of Senolytic Activity in Human IVD Cells In Vitro. J. Clin. Med. 2019, 29, 433. [Google Scholar] [CrossRef]
- Dirsch, V.M.; Stuppner, H.; Vollmar, A.M. The Griess assay: Suitable for a bio-guided fractionation of anti-inflammatory plant extracts? Planta Med. 1998, 64, 423–426. [Google Scholar] [CrossRef]
- Lambeth, J.D. NOX enzymes and the biology of reactive oxygen. Nat. Rev. Immunol. 2004, 4, 181–189. [Google Scholar] [CrossRef]
- Bedard, K.; Krause, K.H. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef]
- Radi, R. Oxygen radicals, nitric oxide, and peroxynitrite: Redox pathways in molecular medicine. Proc. Natl. Acad. Sci. USA 2018, 115, 5839–5848. [Google Scholar] [CrossRef] [PubMed]
- Trougakos, I.P.; Gonos, E.S. Clusterin/apolipoprotein J in human aging and cancer. Int. J. Biochem. Cell Biol. 2002, 34, 1430–1448. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Chang, C.; Qi, T.; Yang, P.; Wang, Y.; Zhou, X.; Guan, F.; Li, X. Clusterin Is a Prognostic Biomarker of Lower-Grade Gliomas and Is Associated with Immune Cell Infiltration. Int. J. Mol. Sci. 2023, 24, 13413. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Bu, S. Curcumin Induces Autophagy, Apoptosis, and Cell Cycle Arrest in Human Pancreatic Cancer Cells. Evid. Based Complement. Alternat. Med. 2017, 2017, 5787218. [Google Scholar] [CrossRef]
- Lv, Z.D.; Liu, X.P.; Zhao, W.J.; Dong, Q.; Li, F.N.; Wang, H.B.; Kong, B. Curcumin induces apoptosis in breast cancer cells and inhibits tumor growth in vitro and in vivo. Int. J. Clin. Exp. Pathol. 2014, 7, 2818–2824. [Google Scholar]
- Wu, S.H.; Hang, L.W.; Yang, J.S.; Chen, H.Y.; Lin, H.Y.; Chiang, J.H.; Lu, C.C.; Yang, J.L.; Lai, T.Y.; Ko, Y.C.; et al. Curcumin induces apoptosis in human non-small cell lung cancer NCI-H460 cells through ER stress and caspase cascade- and mitochondria-dependent pathways. Anticancer Res. 2010, 30, 2125–2133. [Google Scholar]
- Ryskalin, L.; Biagioni, F.; Busceti, C.L.; Lazzeri, G.; Frati, A.; Fornai, F. The Multi-Faceted Effect of Curcumin in Glioblastoma from Rescuing Cell Clearance to Autophagy-Independent Effects. Molecules 2020, 25, 4839. [Google Scholar] [CrossRef]
- Wong, S.C.; Kamarudin, M.N.A.; Naidu, R. Anticancer Mechanism of Curcumin on Human Glioblastoma. Nutrients 2021, 13, 950. [Google Scholar] [CrossRef]
- Gersey, Z.C.; Rodriguez, G.A.; Barbarite, E.; Sanchez, A.; Walters, W.M.; Ohaeto, K.C.; Komotar, R.J.; Graham, R.M. Curcumin decreases malignant characteristics of glioblastoma stem cells via induction of reactive oxygen species. BMC Cancer 2017, 17, 99. [Google Scholar] [CrossRef]
- Manica, D.; Silva, G.B.D.; Silva, A.P.D.; Marafon, F.; Maciel, S.; Bagatini, M.D.; Moreno, M. Curcumin promotes apoptosis of human melanoma cells by caspase 3. Cell Biochem. Funct. 2023, 41, 1295–1304. [Google Scholar] [CrossRef]
- Georgiou-Siafis, S.K.; Tsiftsoglou, A.S. The Key Role of GSH in Keeping the Redox Balance in Mammalian Cells: Mechanisms and Significance of GSH in Detoxification via Formation of Conjugates. Antioxidants 2023, 12, 1953. [Google Scholar] [CrossRef] [PubMed]
- Simin, k.; Dehghan, G.; Rashtbari, S.; Yekta, R.; Sheibani, N. Synergistic inhibition of catalase activity by food colorants sunset yellow and curcumin: An experimental and MLSD simulation approach. Chem. Biol. Interact. 2019, 311, 108746. [Google Scholar]
- Harris, I.S.; DeNicola, G.M. The Complex Interplay between Antioxidants and ROS in Cancer. Trends Cell Biol. 2020, 30, 440–451. [Google Scholar] [CrossRef]
- Maiti, P.; Scott, J.; Sengupta, D.; Al-Gharaibeh, A.; Dunbar, G.L. Curcumin and Solid Lipid Curcumin Particles Induce Autophagy, but Inhibit Mitophagy and the PI3K-Akt/mTOR Pathway in Cultured Glioblastoma Cells. Int. J. Mol. Sci. 2019, 20, 399. [Google Scholar] [CrossRef]
- Kuttikrishnan, S.; Siveen, K.S.; Prabhu, K.S.; Khan, A.Q.; Ahmed, E.I.; Akhtar, S.; Ali, T.A.; Merhi, M.; Dermime, S.; Steinhoff, M.; et al. Curcumin Induces Apoptotic Cell Death via Inhibition of PI3-Kinase/AKT Pathway in B-Precursor Acute Lymphoblastic Leukemia. Front. Oncol. 2019, 9, 484. [Google Scholar] [CrossRef]
- Datta, S.R.; Brunet, A.; Greenberg, M.E. Cellular survival: A play in three Akts. Genes Dev. 1999, 13, 2905–2927. [Google Scholar] [CrossRef] [PubMed]
- Van Opdenbosch, N.; Lamkanfi, M. Caspases in Cell Death, Inflammation, and Disease. Immunity 2019, 50, 1352–1364. [Google Scholar] [CrossRef]
- Scaffidi, C.; Fulda, S.; Srinivasan, A.; Friesen, C.; Li, F.; Tomaselli, K.J.; Debatin, K.M.; Krammer, P.H.; Peter, M.E. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 1998, 17, 1675–1687. [Google Scholar] [CrossRef] [PubMed]
- Tanida, I.; Minematsu-Ikeguchi, N.; Ueno, T.; Kominami, E. Lysosomal turnover, but not a cellular level, of endogenous LC3 is a marker for autophagy. Autophagy 2005, 1, 84–91. [Google Scholar] [CrossRef]
- Bin, L.; Takashi, T.; Kenji, T.; Tze Fang, W.; Mari, T.; Akiko, K.; Satoru, N.; Nobuo, Y. Curcumin Induces Cross-Regulation Between Autophagy and Apoptosis in Uterine Leiomyosarcoma Cells. Int. J. Gynecol. Cancer 2013, 23, 803–808. [Google Scholar] [CrossRef]
- Yang, J.; Ning, J.; Peng, L.; He, D. Effect of curcumin on Bcl-2 and Bax expression in nude mice prostate cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 9272–9278. [Google Scholar] [PubMed]
- Bava, S.V.; Puliappadamba, V.T.; Deepti, A.; Nair, A.; Karunagaran, D.; Anto, R.J. Sensitization of Taxol-induced Apoptosis by Curcumin Involves Down-regulation of Nuclear Factor-κB and the Serine/Threonine Kinase Akt and Is Independent of Tubulin Polymerization. J. Biol. Chem. 2005, 280, 6301–6308. [Google Scholar] [CrossRef] [PubMed]
- Wahnou, H.; El Kebbaj, R.; Liagre, B.; Sol, V.; Limami, Y.; Duval, R.E. Curcumin-Based Nanoparticles: Advancements and Challenges in Tumor Therapy. Pharmaceutics 2025, 17, 114. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Sundaram, C.; Malani, N.; Ichikawa, H. Curcumin: The Indian solid gold. Adv. Exp. Med. Biol. 2007, 595, 1–75. [Google Scholar]
- Rodriguez, G.A.; Shah, A.H.; Gersey, Z.C.; Shah, S.S.; Bregy, A.; Komotar, R.J.; Graham, R.M. Investigating the therapeutic role and molecular biology of curcumin as a treatment for glioblastoma. Ther. Adv. Med. Oncol. 2016, 8, 248–260. [Google Scholar] [CrossRef]
- Can Karaca, A.; Rezaei, A.; Qamar, M.; Assadpour, E.; Esatbeyoglu, T.; Jafari, S.M. Lipid-based nanodelivery systems of curcumin: Recent advances, approaches, and applications. Food Chem. 2025, 463, 141193. [Google Scholar] [CrossRef]
- Fu, Y.; Du, Q.; Cui, T.; Lu, Y.; Niu, G. A pan-cancer analysis reveals role of clusterin (CLU) in carcinogenesis and prognosis of human tumors. Front. Genet. 2022, 13, 1056184. [Google Scholar] [CrossRef]
- Shiota, M.; Zardan, A.; Takeuchi, A.; Kumano, M.; Beraldi, E.; Naito, S.; Zoubeidi, A.; Gleave, M.E. Clusterin mediates TGF-β-induced epithelial-mesenchymal transition and metastasis via Twist1 in prostate cancer cells. Cancer Res. 2012, 72, 5261–5272. [Google Scholar] [CrossRef]
- Mamun, M.A.A.; Zheng, Y.-C.; Wang, N.; Wang, B.; Zhang, Y.; Pang, J.-R.; Shen, D.-D.; Liu, H.-M.; Gao, Y. Decoding CLU (Clusterin): Conquering cancer treatment resistance and immunological barriers. Int. Immunopharmacol. 2024, 137, 112355. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sultana, P.; Novotny, J. Curcumin-Induced Apoptotic Cell Death in Human Glioma Cells Is Enhanced by Clusterin Deficiency. Pharmaceutics 2025, 17, 679. https://doi.org/10.3390/pharmaceutics17060679
Sultana P, Novotny J. Curcumin-Induced Apoptotic Cell Death in Human Glioma Cells Is Enhanced by Clusterin Deficiency. Pharmaceutics. 2025; 17(6):679. https://doi.org/10.3390/pharmaceutics17060679
Chicago/Turabian StyleSultana, Pinky, and Jiri Novotny. 2025. "Curcumin-Induced Apoptotic Cell Death in Human Glioma Cells Is Enhanced by Clusterin Deficiency" Pharmaceutics 17, no. 6: 679. https://doi.org/10.3390/pharmaceutics17060679
APA StyleSultana, P., & Novotny, J. (2025). Curcumin-Induced Apoptotic Cell Death in Human Glioma Cells Is Enhanced by Clusterin Deficiency. Pharmaceutics, 17(6), 679. https://doi.org/10.3390/pharmaceutics17060679







