Self-Assembled Cannabigerol-Based Nanoparticles: Design, Synthesis, and Antiproliferative Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemistry
2.2. Preparation and Characterization of Nanoparticles
2.3. Cell Cultures and Cell Viability Assay
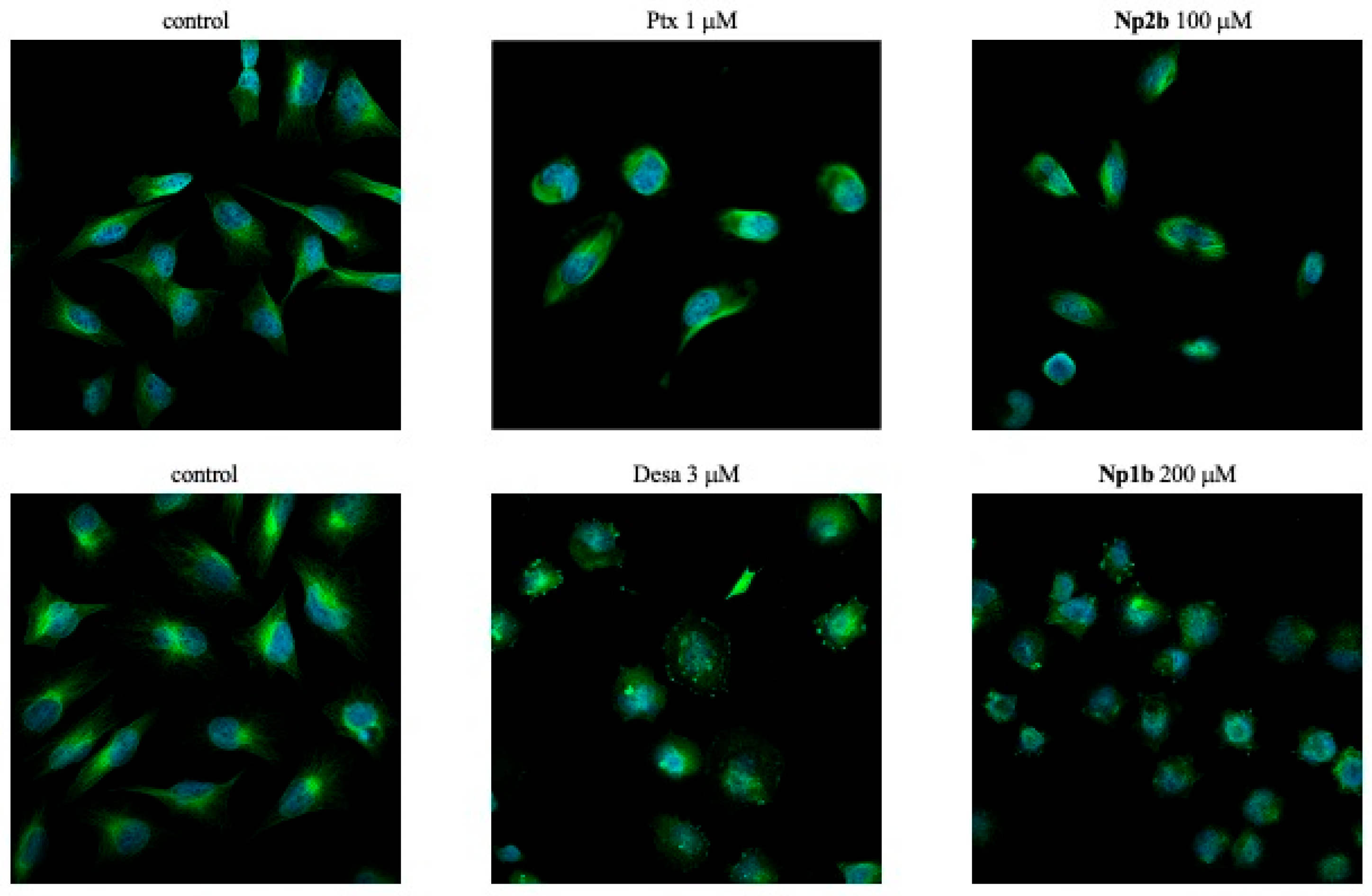
2.4. Confocal Microscopy
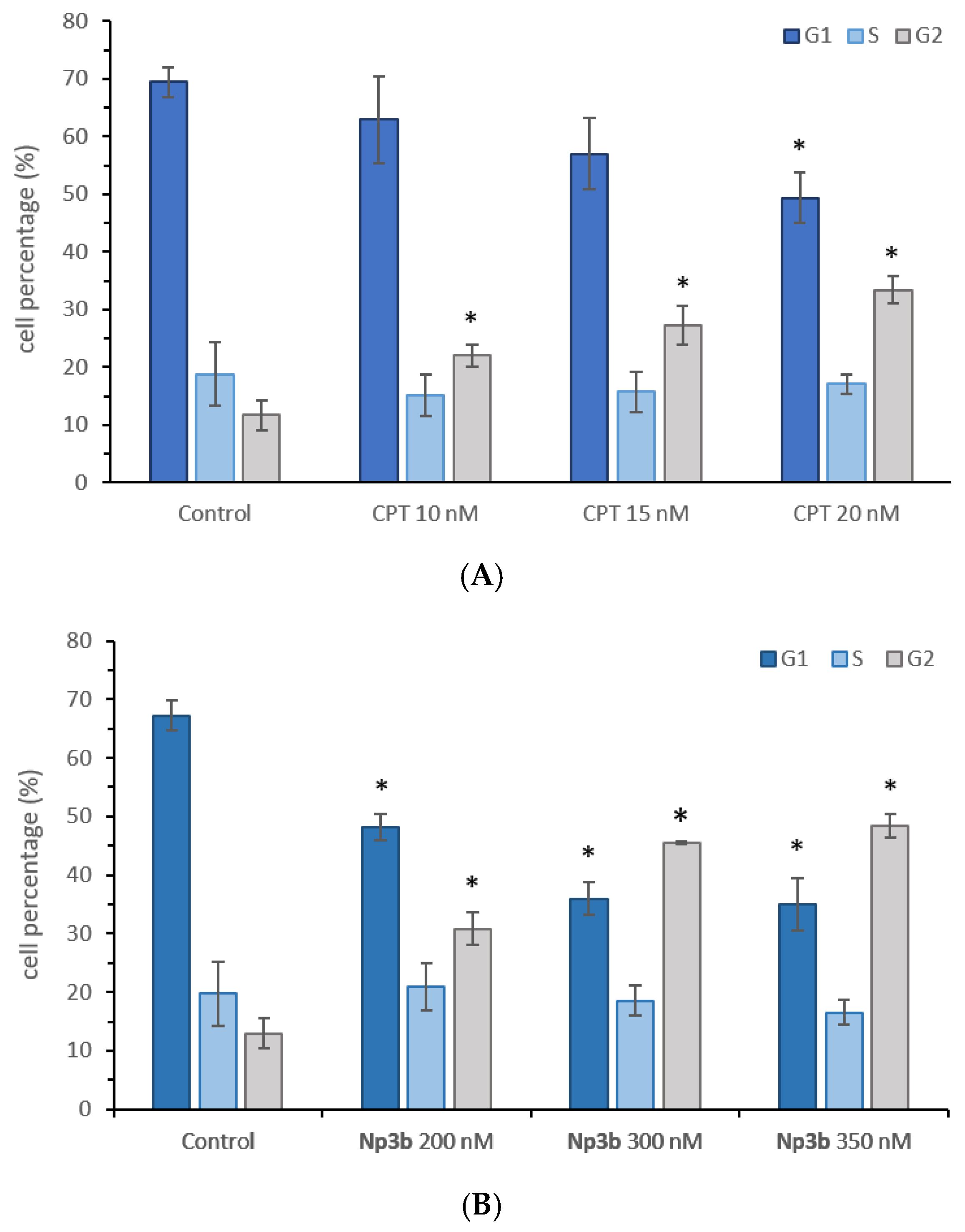
2.5. Cell Cycle Analysis
3. Results and Discussion
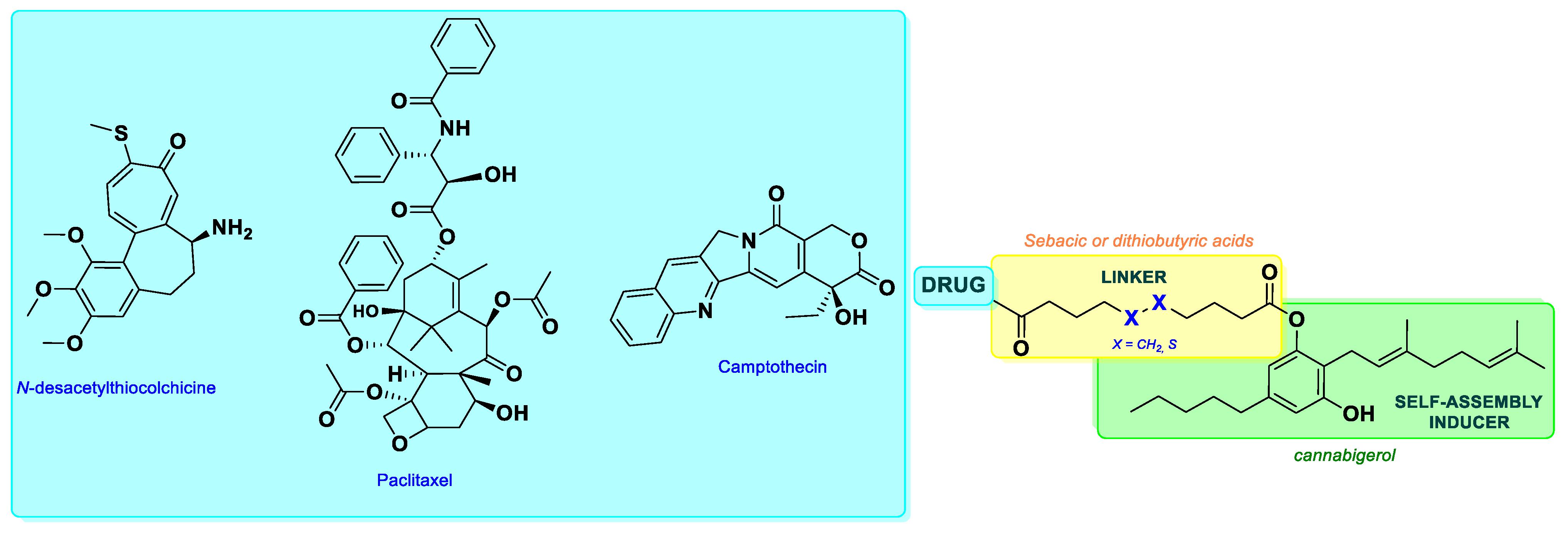
3.1. Design
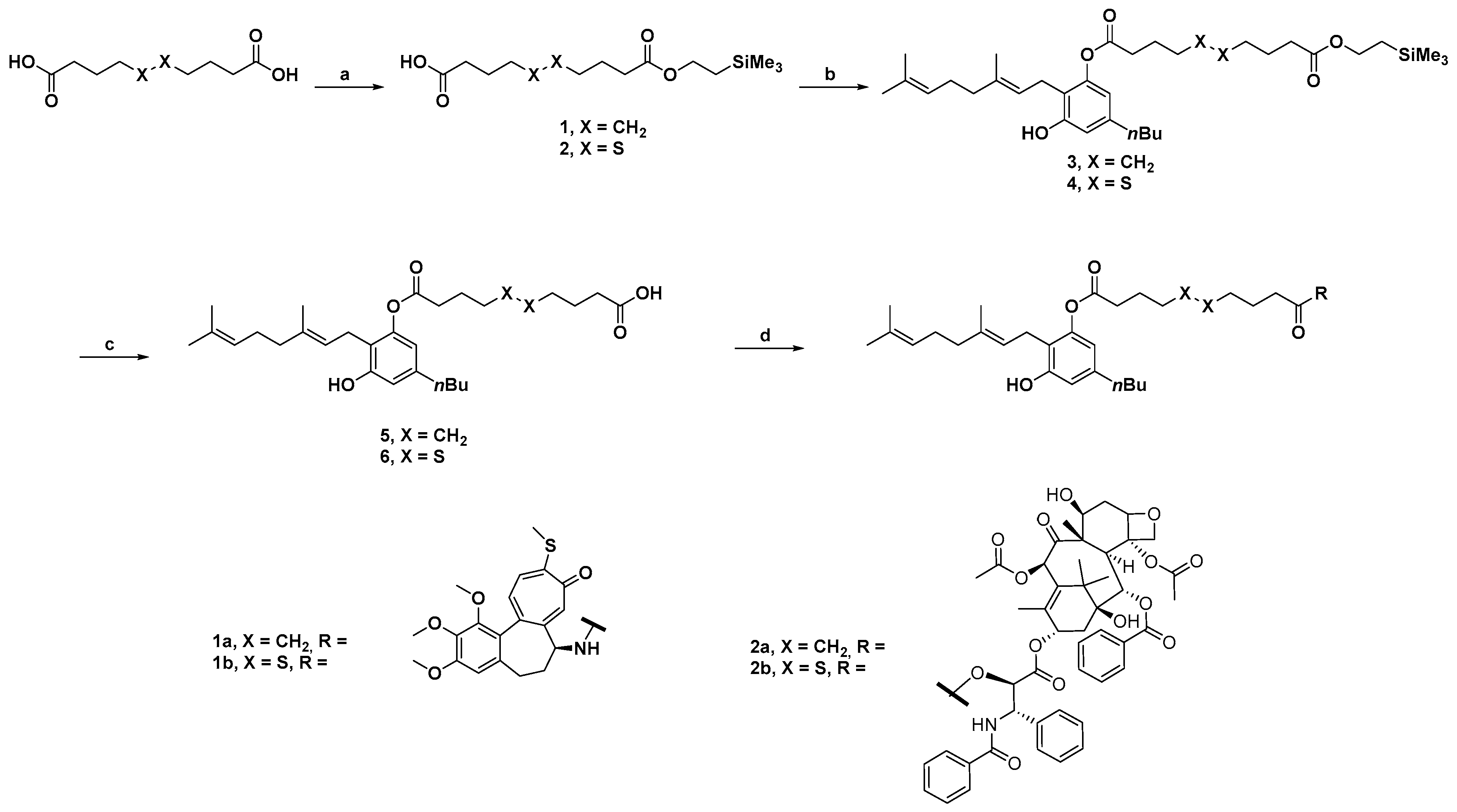
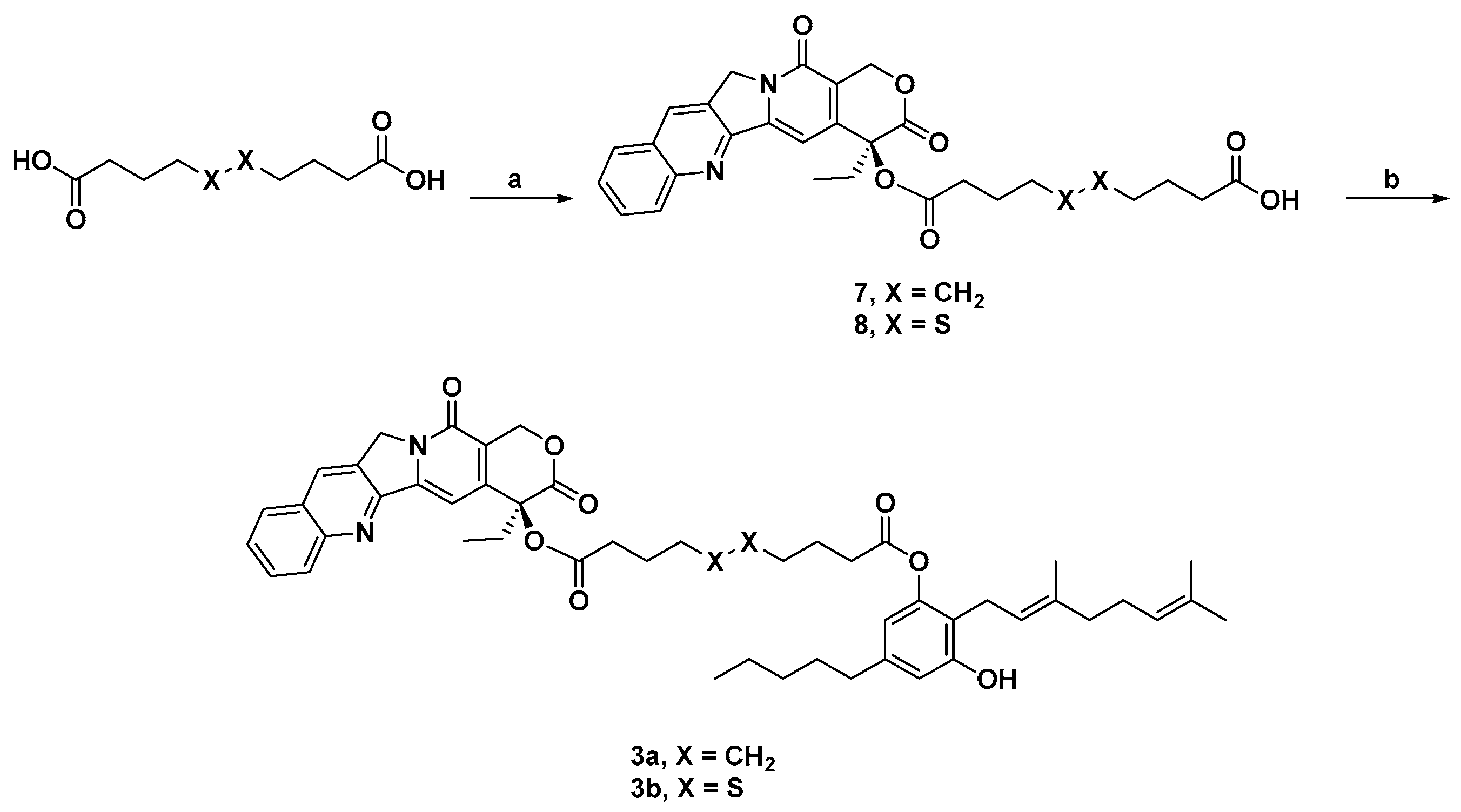
3.2. Synthesis
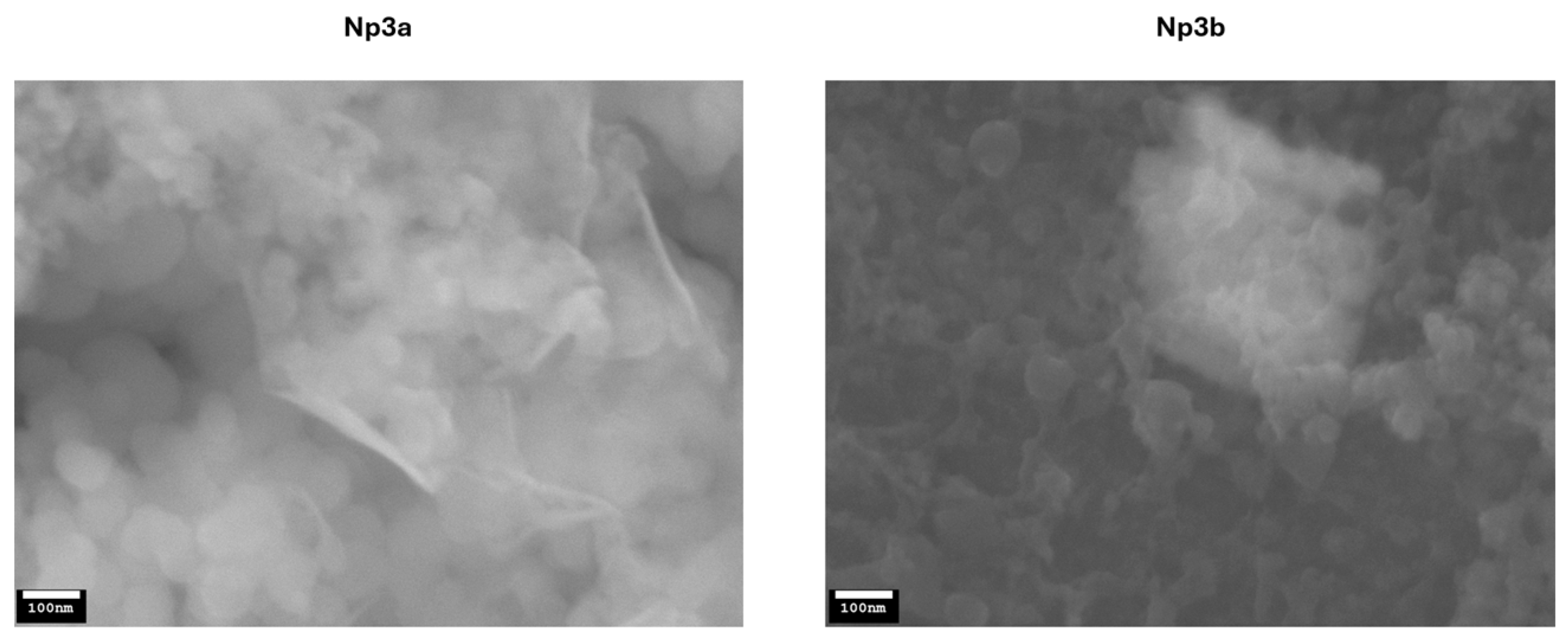
3.3. Nanoparticle Preparation and Characterization
3.4. Biological Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grzelczak, M.; Vermant, J.; Furst, E.M.; Liz-Marzán, L.M. Directed self-assembly of nanoparticles. ACS Nano 2010, 4, 3591–3605. [Google Scholar] [CrossRef]
- Fumagalli, G.; Marucci, C.; Christodoulou, M.S.; Stella, B.; Dosio, F.; Passarella, D. Self-assembly drug conjugates for anticancer treatment. Drug Discov. Today 2016, 21, 1321–1329. [Google Scholar] [CrossRef]
- Zhang, Y.; Fang, F.; Li, L.; Zhang, J. Self-Assembled Organic Nanomaterials for Drug Delivery, Bioimaging, and Cancer Therapy. ACS Biomater. Sci. Eng. 2020, 6, 4816–4833. [Google Scholar] [CrossRef]
- Lin, X.; Huang, X.; Tian, X.; Yuan, Z.; Lu, J.; Nie, X.; Wang, P.; Lei, H.; Wang, P. Natural Small-Molecule-Based Carrier-Free Self-Assembly Library Originated from Traditional Chinese Herbal Medicine. ACS Omega 2022, 7, 43510–43521. [Google Scholar] [CrossRef]
- Whiting, P.F.; Wolff, R.F.; Deshpande, S.; Di Nisio, M.; Duffy, S.; Hernandez, A.V.; Keurentjes, J.C.; Lang, S.; Misso, K.; Ryder, S.; et al. Cannabinoids for Medical Use: A Systematic Review and Meta-analysis. JAMA 2015, 313, 2456–2473. [Google Scholar] [CrossRef]
- Onaivi, E.S.; Singh Chauhan, B.P.; Sharma, V. Challenges of cannabinoid delivery: How can nanomedicine help? Nanomedicine 2020, 15, 2023–2028. [Google Scholar] [CrossRef]
- Anokwuru, C.P.; Makolo, F.L.; Sandasi, M.; Tankeu, S.Y.; Elisha, I.L.; Agoni, C.; Combrinck, S.; Viljoen, A. Cannabigerol: A bibliometric overview and review of research on an important phytocannabinoid. Phytochem. Rev. 2022, 21, 1523–1547. [Google Scholar] [CrossRef]
- Li, S.; Li, W.; Malhi, N.K.; Huang, J.; Li, Q.; Zhou, Z.; Wang, R.; Peng, J.; Yin, T.; Wang, H. Cannabigerol (CBG): A Comprehensive Review of Its Molecular Mechanisms and Therapeutic Potential. Molecules 2024, 29, 5471. [Google Scholar] [CrossRef]
- Filipiuc, L.E.; Ababei, D.C.; Alexa-Stratulat, T.; Pricope, C.V.; Bild, V.; Stefanescu, R.; Stanciu, G.D.; Tamba, B.-I. Major Phytocannabinoids and Their Related Compounds: Should We Only Search for Drugs That Act on Cannabinoid Receptors? Pharmaceutics 2021, 13, 1823. [Google Scholar] [CrossRef]
- Monou, P.K.; Mamaligka, A.M.; Tzimtzimis, E.K.; Tzetzis, D.; Vergkizi-Nikolakaki, S.; Vizirianakis, I.S.; Andriotis, E.G.; Eleftheriadis, G.K.; Fatouros, D.G. Fabrication and Preliminary In Vitro Evaluation of 3D-Printed Alginate Films with Cannabidiol (CBD) and Cannabigerol (CBG) Nanoparticles for Potential Wound-Healing Applications. Pharmaceutics 2022, 14, 1637. [Google Scholar] [CrossRef]
- Borrelli, S.; Christodoulou, M.S.; Ficarra, I.; Silvani, A.; Cappelletti, G.; Cartelli, D.; Damia, G.; Ricci, F.; Zucchetti, M.; Dosio, F.; et al. New class of squalene-based releasable nanoassemblies of paclitaxel, podophyllotoxin, camptothecin and epothilone A. Eur. J. Med. Chem. 2014, 85, 179–190. [Google Scholar] [CrossRef]
- Borrelli, S.; Cartelli, D.; Secundo, F.; Fumagalli, G.; Christodoulou, M.S.; Borroni, A.; Perdicchia, D.; Dosio, F.; Milla, P.; Cappelletti, G.; et al. Self-Assembled Squalene-based Fluorescent Heteronanoparticles. ChemPlusChem 2015, 80, 47–49. [Google Scholar] [CrossRef]
- Fumagalli, G.; Stella, B.; Pastushenko, I.; Ricci, F.; Christodoulou, M.S.; Damia, G.; Mazza, D.; Arpicco, S.; Giannini, C.; Morosi, L.; et al. Heteronanoparticles by self-Assembly of Doxorubicin and Cyclopamine Conjugates. ACS Med. Chem. Lett. 2017, 8, 953–957. [Google Scholar] [CrossRef]
- Colombo, E.; Polito, L.; Biocotino, M.; Marzullo, P.; Hyeraci, M.; Via, L.D.; Passarella, D. New Class of Betulinic Acid-Based Nanoassemblies of Cabazitaxel, Podophyllotoxin, and Thiocolchicine. ACS Med. Chem. Lett. 2020, 11, 895–898. [Google Scholar] [CrossRef]
- Citarella, A.; Cavinato, M.; Rosini, E.; Shehi, H.; Ballabio, F.; Camilloni, C.; Fasano, V.; Silvani, A.; Passarella, D.; Pollegioni, L.; et al. Nicotinic Acid Derivatives As Novel Noncompetitive α-Amylase and α-Glucosidase Inhibitors for Type 2 Diabetes Treatment. ACS Med. Chem. Lett. 2024, 15, 1474–1481. [Google Scholar] [CrossRef]
- Antoniou, A.I.; Nordio, G.; Di Paolo, M.L.; Colombo, E.; Gaffuri, B.; Polito, L.; Amenta, A.; Seneci, P.; Dalla Via, L.; Perdicchia, D.; et al. 2-Hydroxyoleic Acid as a Self-Assembly Inducer for Anti-Cancer Drug-Centered Nanoparticles. Pharmaceuticals 2023, 16, 722. [Google Scholar] [CrossRef]
- Calinescu, A.A.; Castro, M.G. Microtubule targeting agents in glioma. Transl. Cancer Res. 2016, 5 (Suppl. S1), S54–S60. [Google Scholar] [CrossRef]
- Jastrząb, A.; Jarocka-Karpowicz, I.; Skrzydlewska, E. The Origin and Biomedical Relevance of Cannabigerol. Int. J. Mol. Sci. 2022, 23, 7929. [Google Scholar] [CrossRef]
- Perez, E.; Fernandez, J.R.; Fitzgerald, C.; Rouzard, K.; Tamura, M.; Savile, C. In Vitro and Clinical Evaluation of Cannabigerol (CBG) Produced via Yeast Biosynthesis: A Cannabinoid with a Broad Range of Anti-Inflammatory and Skin Health-Boosting Properties. Molecules 2022, 27, 491. [Google Scholar] [CrossRef]
- Amenta, A.; Comi, S.; Kravicz, M.; Sesana, S.; Antoniou, A.; Passarella, D.; Seneci, P.; Pellegrino, S.; Re, F. A novel, glutathione-activated prodrug of pimasertib loaded in liposomes for targeted cancer therapy. RSC Med. Chem. 2024, 16, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.Z.; Duncan, R.E. Regulatory Effects of Cannabidiol on Mitochondrial Functions: A Review. Cells 2021, 10, 1251. [Google Scholar] [CrossRef]
- Lah, T.T.; Novak, M.; Pena Almidon, M.A.; Marinelli, O.; Žvar Baškovič, B.; Majc, B.; Mlinar, M.; Bošnjak, R.; Breznik, B.; Zomer, R.; et al. Cannabigerol Is a Potential Therapeutic Agent in a Novel Combined Therapy for Glioblastoma. Cells 2021, 10, 340. [Google Scholar] [CrossRef]
- Borrelli, F.; Pagano, E.; Romano, B.; Panzera, S.; Maiello, F.; Coppola, D.; De Petrocellis, L.; Buono, L.; Orlando, P.; Izzo, A.A. Colon carcinogenesis is inhibited by the TRPM8 antagonist cannabigerol, a Cannabis-derived non-psychotropic cannabinoid. Carcinogenesis 2014, 35, 2787–2797. [Google Scholar] [CrossRef]
- Calapai, F.; Cardia, L.; Esposito, E.; Ammendolia, I.; Mondello, C.; Lo Giudice, R.; Gangemi, S.; Calapai, G.; Mannucci, C. Pharmacological Aspects and Biological Effects of Cannabigerol and Its Synthetic Derivatives. Evid. Based Complement. Alternat. Med. 2022, 2022, 3336516. [Google Scholar] [CrossRef]
- Colvin, E.K.; Hudson, A.L.; Anderson, L.L.; Kumar, R.P.; McGregor, I.S.; Howell, V.M.; Arnold, J.C. An Examination of the Anti-Cancer Properties of Plant Cannabinoids in Preclinical Models of Mesothelioma. Cancers 2022, 14, 3813. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Deng, F.; Zheng, N.; Liang, Y.; Zhang, H.; He, B.; Dai, W.; Wang, X.; Zhang, Q. The effect of linkers on the self-assembling and anti-tumor efficacy of disulfide-linked doxorubicin drug-drug conjugate nanoparticles. J. Control. Release 2018, 279, 136–146. [Google Scholar] [CrossRef]
| NPs (C: 100 µM) | Hydrodynamic Diameter (nm) | Polydispersity Index (PI) | ζ-Potential (mV) |
|---|---|---|---|
| 1a | 66.6 ± 1.72 | 0.11 ±0.048 | −26.76 ± 1.09 |
| 1b | 73.5 ± 0.94 | 0.13 ± 0.025 | −29.2 ± 1.48 |
| 2a | 61.03 ± 1.32 | 0.13 ± 0.054 | −26.33 ± 1.35 |
| 2b | 88.3 ± 2.25 | 0.05 ± 0.012 | −29.5 ± 1.47 |
| 3a | 68.3 ± 1.17 | 0.11 ± 0.058 | −29.53 ± 0.46 |
| 3b | 73.5 ± 1.25 | 0.11 ± 0.059 | −31.82 ± 1.52 |
| Compound | GI50 (µM) a | |||
|---|---|---|---|---|
| LN229 | HT-29 | MSTO-211H | MeT-5A | |
| Desa | 0.021 ± 0.001 | 0.015 ± 0.002 | 0.012 ± 0.001 | 0.014 ± 0.004 |
| PTX | 0.0042 ± 0.0007 | 0.0028 ± 0.0002 | 0.0033 ± 0.0006 | 0.0044 ± 0.0001 |
| CPT | 0.0079 ± 0.0001 | 0.0079 ± 0.0008 | 0.0033 ± 0.0010 | 0.0078 ± 0.0002 |
| CBG | 5.9 ± 1.3 | 9.7 ± 0.2 | 16 ± 2 | 16 ± 2 |
| Np1a | 1.0 ± 0.2 | 0.80 ± 0.18 | 1.0 ± 0.2 | 0.88 ±0.05 |
| Np1b | 1.6 ± 0.6 | 1.0 ± 0.1 | 1.7 ± 0.1 | 1.6 ±0.1 |
| Np2a | 3.7 ± 0.6 | 1.9 ± 0.5 | 6.2 ± 1.7 | 3.4 ± 0.5 |
| Np2b | 0.20 ± 0.04 | 0.16 ± 0.03 | 0.33 ± 0.11 | 0.40 ± 0.07 |
| Np3a | 13 ± 1 | 2.7 ± 0.2 | 4.2 ± 0.8 | 5.2 ± 0.5 |
| Np3b | 0.16 ± 0.01 | 0.21± 0.01 | 0.050 ± 0.003 | 0.27 ± 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amenta, A.; Nordio, G.; Piazzola, F.; Di Paolo, M.L.; Milani, F.; Giacomini, M.; Citarella, A.; Ciriello, U.; Paladino, G.; Pellegrino, S.; et al. Self-Assembled Cannabigerol-Based Nanoparticles: Design, Synthesis, and Antiproliferative Activity. Pharmaceutics 2025, 17, 636. https://doi.org/10.3390/pharmaceutics17050636
Amenta A, Nordio G, Piazzola F, Di Paolo ML, Milani F, Giacomini M, Citarella A, Ciriello U, Paladino G, Pellegrino S, et al. Self-Assembled Cannabigerol-Based Nanoparticles: Design, Synthesis, and Antiproliferative Activity. Pharmaceutics. 2025; 17(5):636. https://doi.org/10.3390/pharmaceutics17050636
Chicago/Turabian StyleAmenta, Arianna, Giulia Nordio, Francesco Piazzola, Maria Luisa Di Paolo, Fabio Milani, Martina Giacomini, Andrea Citarella, Umberto Ciriello, Giuseppe Paladino, Sara Pellegrino, and et al. 2025. "Self-Assembled Cannabigerol-Based Nanoparticles: Design, Synthesis, and Antiproliferative Activity" Pharmaceutics 17, no. 5: 636. https://doi.org/10.3390/pharmaceutics17050636
APA StyleAmenta, A., Nordio, G., Piazzola, F., Di Paolo, M. L., Milani, F., Giacomini, M., Citarella, A., Ciriello, U., Paladino, G., Pellegrino, S., Silvestri, F., Fasano, V., Dalla Via, L., & Passarella, D. (2025). Self-Assembled Cannabigerol-Based Nanoparticles: Design, Synthesis, and Antiproliferative Activity. Pharmaceutics, 17(5), 636. https://doi.org/10.3390/pharmaceutics17050636











