Preparation of Carrier-Free Inhalable Dry Powder of Rivaroxaban Using Two-Step Milling for Lung-Targeted Delivery
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Milled Rivaroxaban for Dry Powder Inhalation
2.3. Physicochemical Characterization
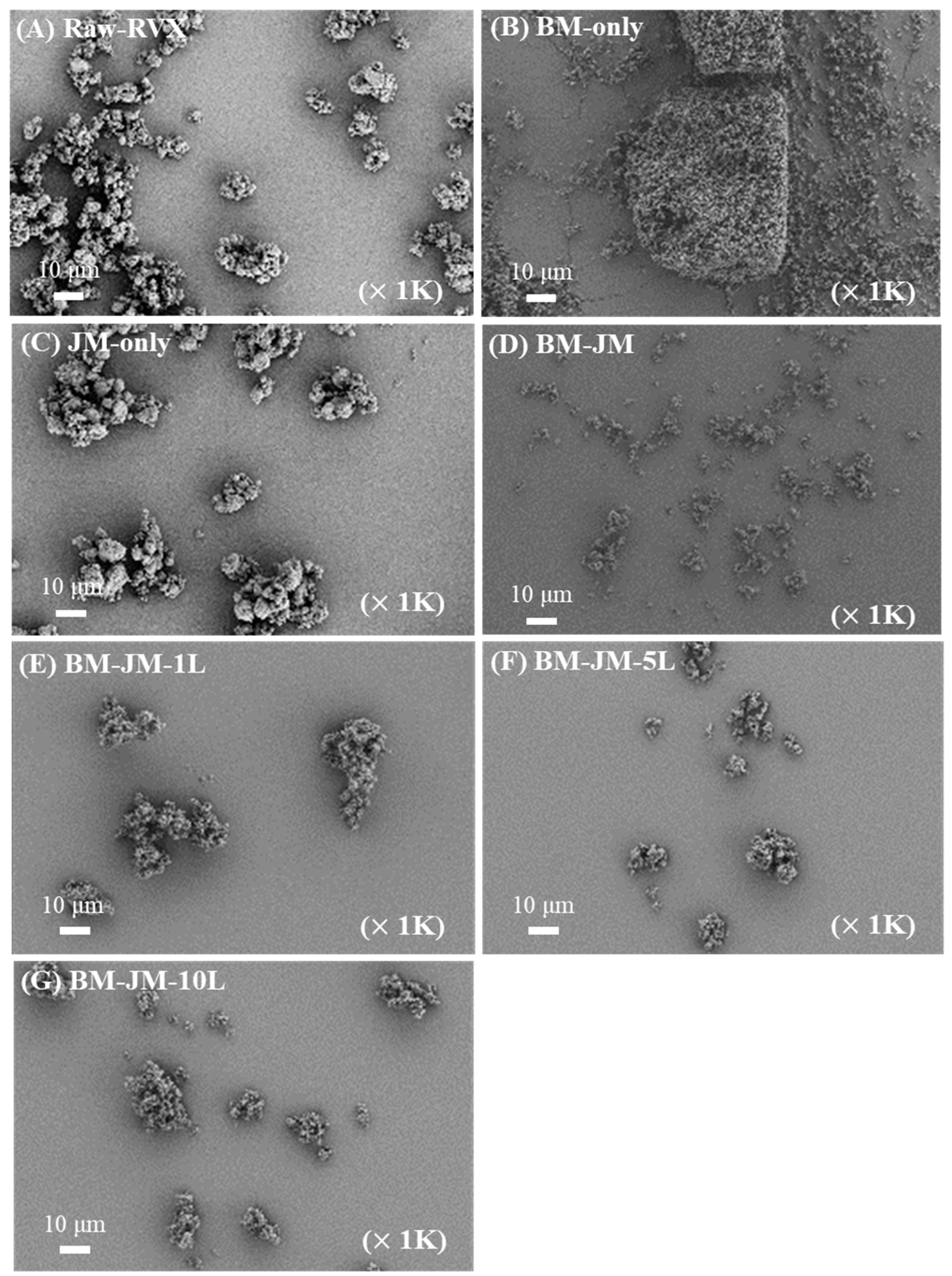
2.3.1. Morphology of Milled RVX Dry Powder
2.3.2. X-Ray Diffraction (XRD)
2.3.3. Differential Scanning Calorimetry (DSC)
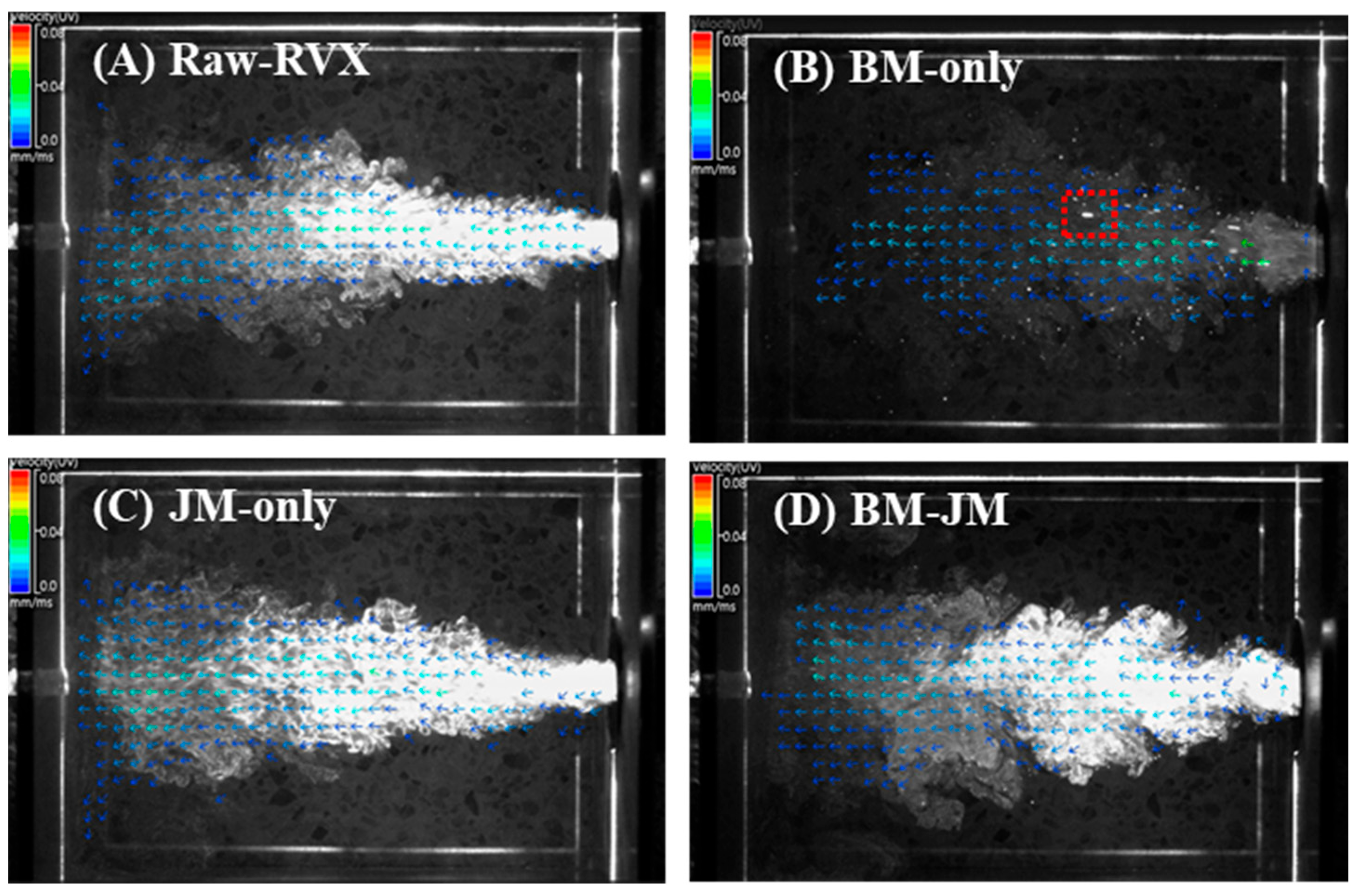
2.4. Particle Image Velocimetry
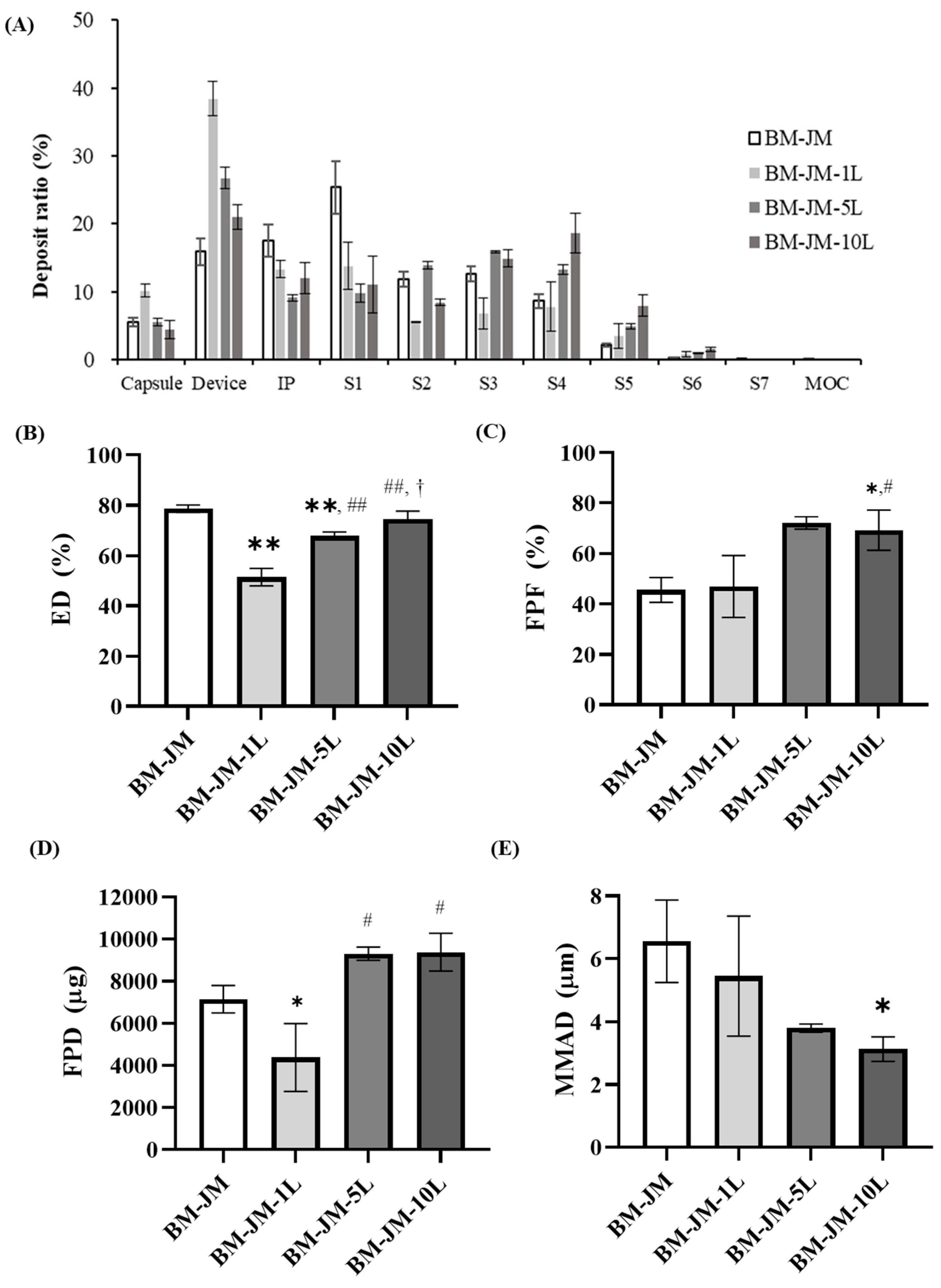
2.5. In-Vitro Aerodynamic Performance
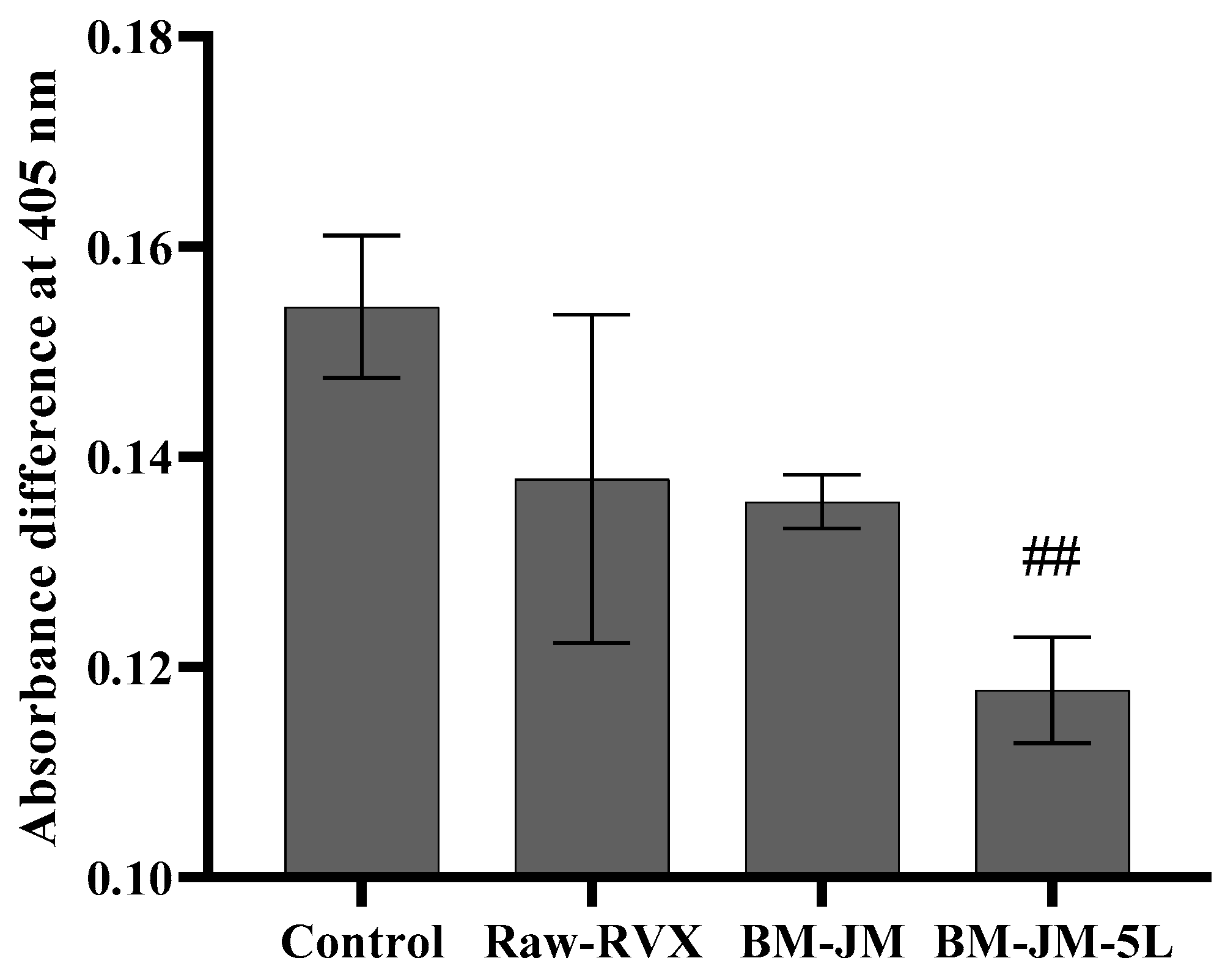
2.6. Ex-Vivo Plasma Anticoagulation Effect Tests
2.7. In-Vivo Study of Rivaroxaban
2.7.1. Pharmacokinetic Studies of Rivaroxaban
2.7.2. Lung and Heart Distribution of Rivaroxaban
2.8. Statistical Analysis
3. Results and Discussion
3.1. Preparation and Characterization of Milled Rivaroxaban Dry Powder
3.2. Comparison of Particle Dispersion and Aerodynamic Performance of RVX Formulations Prepared by Different Milling Methods
3.3. Effect of Leucine Concentration on Aerodynamic Performance in Two-Step Milling Method
3.4. Ex-Vivo Plasma Anticoagulation Effect
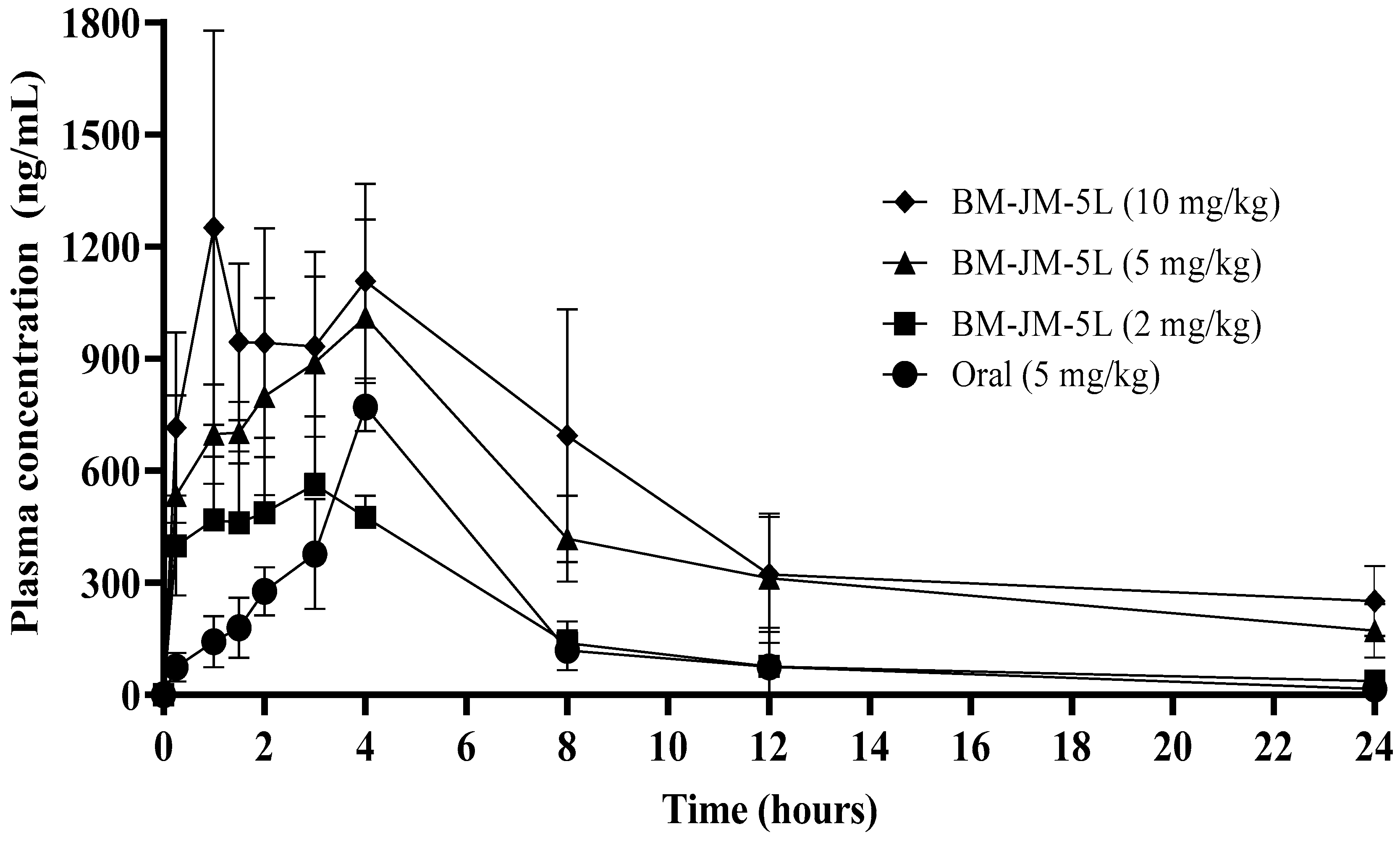
3.5. Pharmacokinetic Studies
3.6. Lung and Heart Distribution
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PE | Pulmonary embolism |
| VTE | Venous thromboembolism |
| NOACs | Non-vitamin K antagonist oral anticoagulants |
| LEU or L | L-leucine |
| FCA | Force control agent |
| BM | Bead milling |
| JM | Jet milling |
| RVX | Rivaroxaban |
| DPI | Dry powder inhalation |
| SEM | Scanning electron microscope |
| XRD | X-ray diffraction |
| DSC | Differential scanning calorimetry |
| PIV | Particle image velocimetry |
| HPLC | High-performance liquid chromatography |
| NGI | Next-generation impactor |
| ED | Emitted dose |
| FPF | Fine particle fraction |
| FPD | Fine particle dose |
| MMAD | Mass median aerodynamic diameter |
| GSD | Geometric standard deviation |
| PBS | Phosphate-buffered saline |
| PK | Pharmacokinetic |
| ANOVA | Analysis of variance |
| AUC | Area under the curve |
| Cmax | Maximum plasma concentration |
| Tmax | Time to maximum concentration |
| T1/2 | Half-life |
| CL/F | Clearance |
| BA | Bioavailability |
References
- Jannati, S.; Patnaik, R.; Banerjee, Y. Beyond anticoagulation: A Comprehensive Review of Non-vitamin K oral anticoagulants (NOACs) in inflammation and protease-activated receptor signaling. Int. J. Mol. Sci. 2024, 25, 8727. [Google Scholar] [CrossRef] [PubMed]
- Harder, S. Pharmacokinetic and pharmacodynamic evaluation of rivaroxaban: Considerations for the treatment of venous thromboembolism. Thromb. J. 2014, 12, 22. [Google Scholar] [CrossRef]
- Kreutz, R. Pharmacodynamic and pharmacokinetic basics of rivaroxaban. Fundam. Clin. Pharmacol. 2012, 26, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Kitchens, C.S.; Konkle, B.A.; Kessler, C.M. Consultative Hemostasis and Thrombosis: Expert Consult-Online and Print; Elsevier Health Sciences: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Kubitza, D.; Berkowitz, S.D.; Misselwitz, F. Evidence-based development and rationale for once-daily rivaroxaban dosing regimens across multiple indications. Clin. Appl. Thromb./Hemost. 2016, 22, 412–422. [Google Scholar] [CrossRef]
- Kleinstreuer, C.; Feng, Y.; Childress, E. Drug-targeting methodologies with applications: A review. World J. Clin. Cases 2014, 2, 742–756. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.-S.; Kang, J.-H.; Kim, D.-W.; Park, C.-W. Recent developments in dry powder inhalation (DPI) formulations for lung-targeted drug delivery. J. Pharm. Investig. 2024, 54, 113–130. [Google Scholar] [CrossRef]
- Anderson, S.; Atkins, P.; Bäckman, P.; Cipolla, D.; Clark, A.; Daviskas, E.; Disse, B.; Entcheva-Dimitrov, P.; Fuller, R.; Gonda, I.; et al. Inhaled medicines: Past, present, and future. Pharmacol. Rev. 2022, 74, 48–118. [Google Scholar] [CrossRef]
- Groß, R.; Kožák, J.; Chrétien, C.; Berkenfeld, K.; Pellequer, Y.; Lamprecht, A. Fast onset of thrombolytic effect of efficiently inhalable spray-dried Rivaroxaban powder formulations. Int. J. Pharm. 2024, 667, 124912. [Google Scholar] [CrossRef]
- Rashid, A.; Muneer, S.; Mendhi, J.; Sabuj, M.Z.R.; Alhamhoom, Y.; Xiao, Y.; Wang, T.; Izake, E.L.; Islam, N. Inhaled edoxaban dry powder inhaler formulations: Development, characterization and their effects on the coagulopathy associated with COVID-19 infection. Int. J. Pharm. 2021, 608, 121122. [Google Scholar] [CrossRef]
- Csicsák, D.; Szolláth, R.; Kádár, S.; Ambrus, R.; Bartos, C.; Balogh, E.; Antal, I.; Köteles, I.; Tőzsér, P.; Bárdos, V.; et al. The effect of the particle size reduction on the biorelevant solubility and dissolution of poorly soluble drugs with different acid-base character. Pharmaceutics 2023, 15, 278. [Google Scholar] [CrossRef]
- Fröhlich, E.; Mercuri, A.; Wu, S.; Salar-Behzadi, S. Measurements of deposition, lung surface area and lung fluid for simulation of inhaled compounds. Front. Pharmacol. 2016, 7, 181. [Google Scholar] [CrossRef] [PubMed]
- Ueda, K.; Moseson, D.E.; Taylor, L.S. Amorphous solubility advantage: Theoretical considerations, experimental methods, and contemporary relevance. J. Pharm. Sci. 2025, 114, 18–39. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Kunnath, K.T.; Han, X.; Deng, X.; Chen, L.; Davé, R.N. Ultra-fine dispersible powders coated with L-leucine via two-step co-milling. Adv. Powder Technol. 2018, 29, 2957–2965. [Google Scholar] [CrossRef]
- Sibum, I.; Hagedoorn, P.; de Boer, A.H.; Frijlink, H.W.; Grasmeijer, F. Challenges for pulmonary delivery of high powder doses. Int. J. Pharm. 2018, 548, 325–336. [Google Scholar] [CrossRef]
- Luner, P.E.; Zhang, Y.; Abramov, Y.A.; Carvajal, M.T. Evaluation of milling method on the surface energetics of molecular crystals using inverse gas chromatography. Cryst. Growth Des. 2012, 12, 5271–5282. [Google Scholar] [CrossRef]
- Lechanteur, A.; Evrard, B. Influence of composition and spray-drying process parameters on carrier-free DPI properties and behaviors in the lung: A review. Pharmaceutics 2020, 12, 55. [Google Scholar] [CrossRef]
- Demoly, P.; Hagedoorn, P.; de Boer, A.H.; Frijlink, H.W. The clinical relevance of dry powder inhaler performance for drug delivery. Respir. Med. 2014, 108, 1195–1203. [Google Scholar] [CrossRef]
- Raula, J.; Thielmann, F.; Naderi, M.; Lehto, V.-P.; Kauppinen, E.I. Investigations on particle surface characteristics vs. dispersion behaviour of l-leucine coated carrier-free inhalable powders. Int. J. Pharm. 2010, 385, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Healy, A.M.; Amaro, M.I.; Paluch, K.J.; Tajber, L. Dry powders for oral inhalation free of lactose carrier particles. Adv. Drug Deliv. Rev. 2014, 75, 32–52. [Google Scholar] [CrossRef]
- Mehta, P.P.; Pawar, A.P.; Mahadik, K.R.; Kadam, S.S.; Dhapte-Pawar, V. Dry powder coating techniques and role of force controlling agents in aerosol. In Polymer Coatings: Technology and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2020; pp. 41–74. [Google Scholar]
- Park, H.; Ha, E.-S.; Kim, M.-S. Surface modification strategies for high-dose dry powder inhalers. J. Pharm. Investig. 2021, 51, 635–668. [Google Scholar] [CrossRef]
- Feng, A.; Boraey, M.; Gwin, M.; Finlay, P.; Kuehl, P.; Vehring, R. Mechanistic models facilitate efficient development of leucine containing microparticles for pulmonary drug delivery. Int. J. Pharm. 2011, 409, 156–163. [Google Scholar] [CrossRef]
- Jong, T.; Li, J.; Morton, D.A.; Zhou, Q.T.; Larson, I. Investigation of the changes in aerosolization behavior between the jet-milled and spray-dried colistin powders through surface energy characterization. J. Pharm. Sci. 2016, 105, 1156–1163. [Google Scholar] [CrossRef] [PubMed]
- Mangal, S.; Meiser, F.; Tan, G.; Gengenbach, T.; Denman, J.; Rowles, M.R.; Larson, I.; Morton, D.A. Relationship between surface concentration of l-leucine and bulk powder properties in spray dried formulations. Eur. J. Pharm. Biopharm. 2015, 94, 160–169. [Google Scholar] [CrossRef]
- Begat, P.; Price, R.; Harris, H.; Morton, D.A.; Staniforth, J.N. The influence of force control agents on the cohesive-adhesive balance in dry powder inhaler formulations. KONA Powder Part. J. 2005, 23, 109–121. [Google Scholar] [CrossRef]
- Kwon, Y.-B.; Kang, J.-H.; Han, C.-S.; Kim, D.-W.; Park, C.-W. The effect of particle size and surface roughness of spray-dried bosentan microparticles on aerodynamic performance for dry powder inhalation. Pharmaceutics 2020, 12, 765. [Google Scholar] [CrossRef]
- Çelebier, M.; Reçber, T.; Koçak, E.; Altınöz, S.; Kır, S. Determination of Rivaroxaban in human plasma by solid-phase extraction–high performance liquid chromatography. J. Chromatogr. Sci. 2016, 54, 216–220. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wolberg, A.S.; Gabriel, D.A.; Hoffman, M. Analyzing fibrin clot structure using a microplate reader. Blood Coagul. Fibrinolysis 2002, 13, 533–539. [Google Scholar] [CrossRef]
- Wang, X.; Luo, Y.; Yang, Y.; Zheng, B.; Yan, F.; Wei, F.; Friis, T.E.; Crawford, R.W.; Xiao, Y. Alteration of clot architecture using bone substitute biomaterials (beta-tricalcium phosphate) significantly delays the early bone healing process. J. Mater. Chem. B 2018, 6, 8204–8213. [Google Scholar] [CrossRef]
- Shi, H.; Zhao, F.; Chen, H.; Zhou, Q.; Geng, P.; Zhou, Y.; Wu, H.; Chong, J.; Wang, F.; Dai, D.; et al. Naringenin has an inhibitory effect on rivaroxaban in rats both in vitro and in vivo. Pharmacol. Res. Perspect. 2021, 9, e00782. [Google Scholar] [CrossRef]
- Chong, J.; Chen, H.; Dai, D.; Wang, S.; Zhou, Q.; Liu, J.; Lü, Y.; Wu, H.; Du, M.; Chen, F.; et al. Effects of ticagrelor on the pharmacokinetics of rivaroxaban in rats. Pharm. Biol. 2020, 58, 630–635. [Google Scholar] [CrossRef]
- Lee, H.C.; Kim, D.Y.; Choi, M.-J.; Jin, S.G.; Choi, Y.S. A simple and efficient method to determine Rivaroxaban in rat plasma using liquid-liquid extraction and LC-MRM. Mass Spectrom. Lett. 2019, 10, 66–70. [Google Scholar]
- Sun, Y.; Qin, L.; Liu, C.; Su, J.; Zhang, X.; Yu, D.; Guo, C.; Lu, H.; Li, L.; Xiong, W.; et al. Exploring the influence of drug content on DPI powder properties and potential prediction of pulmonary drug deposition. Int. J. Pharm. 2020, 575, 119000. [Google Scholar] [CrossRef]
- Tamura, G.; Sakae, H.; Fujino, S. In vitro evaluation of dry powder inhaler devices of corticosteroid preparations. Allergol. Int. 2012, 61, 149–154. [Google Scholar] [CrossRef]
- Joni, I.M.; Purwanto, A.; Iskandar, F.; Okuyama, K. Dispersion stability enhancement of titania nanoparticles in organic solvent using a bead mill process. Ind. Eng. Chem. Res. 2009, 48, 6916–6922. [Google Scholar] [CrossRef]
- Mangal, S.; Park, H.; Nour, R.; Shetty, N.; Cavallaro, A.; Zemlyanov, D.; Thalberg, K.; Puri, V.; Nicholas, M.; Narang, A.S.; et al. Correlations between surface composition and aerosolization of jet-milled dry powder inhaler formulations with pharmaceutical lubricants. Int. J. Pharm. 2019, 568, 118504. [Google Scholar] [CrossRef] [PubMed]
- Raula, J.; Vartiainen, V.; Kauppinen, E.I.; Bimbo, L.M.; Hirvonen, J. Aerosolization, Drug Permeation and Cellular Interaction of Leucine Coated Combination DPI powders. J. Aerosol Med. Pulm. Drug Deliv. 2016, 29, A6. [Google Scholar]
- Holder, C.F.; Schaak, R.E. Tutorial on powder X-ray diffraction for characterizing nanoscale materials. ACS Nano 2019, 13, 7359–7365. [Google Scholar] [CrossRef]
- Kim, Y.H.; Li, D.D.; Park, S.; Yi, D.S.; Yeoh, G.H.; Abbas, A. Computational investigation of particle penetration and deposition pattern in a realistic respiratory tract model from different types of dry powder inhalers. Int. J. Pharm. 2022, 612, 121293. [Google Scholar] [CrossRef]
- dos Reis, L.G.; Chaugule, V.; Fletcher, D.F.; Young, P.M.; Traini, D.; Soria, J. In-vitro and particle image velocimetry studies of dry powder inhalers. Int. J. Pharm. 2021, 592, 119966. [Google Scholar] [CrossRef]
- Sun, Y. Supercritical fluid particle design of DPI formulations. Curr. Pharm. Des. 2015, 21, 2516–2542. [Google Scholar] [CrossRef]
- Kwok, P.C.L.; Chan, H.K. Electrostatics of Pharmaceutical Inhalation Aerosols. J. Pharm. Pharmacol. 2009, 61, 1587–1599. [Google Scholar] [CrossRef] [PubMed]
- Kaialy, W. A review of factors affecting electrostatic charging of pharmaceuticals and adhesive mixtures for inhalation. Int. J. Pharm. 2016, 503, 262–276. [Google Scholar] [CrossRef] [PubMed]
- Nikolaeva, L.; Belov, G.; Lyapina, L.; Grigorjeva, M.; Obergan, T. The study of anticoagulant properties of leucine and combined activities of its complexes with high-molecular-weight heparin. Adv. Chem. Res. 2015, 23, 215–236. [Google Scholar]
- Nikolaeva, L.S.; Belov, G.V.; Rulev, Y.A.; Semenov, A.N. Thermodynamic characteristics of the heparin-leucine-CaCl 2 system in a diluted physiological solution. Russ. J. Phys. Chem. A 2013, 87, 437–443. [Google Scholar] [CrossRef]
- Eedara, B.B. Slow Dissolving Inhalable Dry Powders for Treatment of Pulmonary Tuberculosis. Doctoral Dissertation, University of Otago, Dunedin, New Zealand, 2019. [Google Scholar]
- Eedara, B.B.; Tucker, I.G.; Zujovic, Z.D.; Rades, T.; Price, J.R.; Das, S.C. Crystalline adduct of moxifloxacin with trans-cinnamic acid to reduce the aqueous solubility and dissolution rate for improved residence time in the lungs. Eur. J. Pharm. Sci. 2019, 136, 104961. [Google Scholar] [CrossRef]
- Wauthoz, N.; Rosière, R.; Amighi, K. Inhaled cytotoxic chemotherapy: Clinical challenges, recent developments, and future prospects. Expert Opin. Drug Deliv. 2021, 18, 333–354. [Google Scholar] [CrossRef]
- Olsson, B.; Bondesson, E.; Borgström, L.; Edsbäcker, S.; Eirefelt, S.; Ekelund, K.; Gustavsson, L.; Hegelund-Myrbäck, T. Pulmonary Drug Metabolism, Clearance, and Absorption. In Controlled Pulmonary Drug Delivery; Smyth, H.D.C., Hickey, A.J., Eds.; Springer: New York, NY, USA, 2011; pp. 21–50. [Google Scholar] [CrossRef]
- Tena, A.F.; Clarà, P.C. Deposition of inhaled particles in the lungs. Arch. Bronconeumol. 2012, 48, 240–246. [Google Scholar] [CrossRef]
- Abouhussein, D.M.; Bahaa El Din Mahmoud, D.; Mohammad, F.E. Design of a liquid nano-sized drug delivery system with enhanced solubility of rivaroxaban for venous thromboembolism management in paediatric patients and emergency cases. J. Liposome Res. 2019, 29, 399–412. [Google Scholar] [CrossRef]


| Formulation | |||
|---|---|---|---|
| Code | Component | Process | |
| Rivaroxaban | L-leucine | ||
| BM only | 3 | - | Bead milling |
| JM-only | - | Jet milling | |
| BM-JM | - | Bead and Jet milling | |
| BM-JM-1L | 0.03 | ||
| BM-JM-5L | 0.15 | ||
| BM-JM-10L | 0.3 | ||
| Milling condition | |||
| Bead milling | Jet milling | ||
| Bead | Zirconium beads 1 mm | Grinding pressure | 0.45 MPa |
| Speed | 650 rpm | Pushing pressure | 0.50 MPa |
| Cycle | 5 cycles Rotation time 12 min Interval time 3 min | Feed rate | 40 Hz |
| Drying | 50 °C, 12 hours | ||
| Formulations | Dv 10 (μm) | Dv 50 (μm) | Dv 90 (μm) | Span |
|---|---|---|---|---|
| Raw-RVX | 3.67 ± 0.00 ##,††,!! | 8.27 ± 0.05 ##,††,!! | 16.74 ± 0.40 ##,††,!! | 1.58 ± 0.03 ##.††,!! |
| BM-only | 4.82 ± 0.16 **,††,!! | 45.9 ± 0.36 **,††, !! | 86.88 ± 0.13 **,††, !! | 1.78 ± 0.01 ††,!! |
| JM-only | 2.16 ± 0.01 **,##,!! | 6.12 ± 0.04 **,##,!! | 13.94 ± 0.20 **,##,†† | 1.92 ± 0.02 **,##,!! |
| BM-JM | 0.89 ± 0.01 **,##,†† | 2.84 ± 0.08 **,##,†† | 21.46 ± 1.75 **,##,†† | 7.21 ± 0.39 **,##,†† |
| BM-JM-1L | 0.58 ± 0.01 **,##,†† | 1.22 ± 0.01 **,##,†† | 7.39 ± 0.20 **,##,††,!! | 5.57 ± 0.15 **,##,††,!! |
| BM-JM-5L | 0.87 ± 0.01 **,##,†† | 2.58 ± 0.01 **,##,†† | 9.25 ± 0.08 **,##,††,!! | 3.23 ± 0.03 **,##,††,!! |
| BM-JM-10L | 0.89 ± 0.00 **,##,††,!! | 2.82 ± 0.01 **,##,††,!! | 7.56 ± 0.08 **,##,††,!! | 2.35 ± 0.02 **,##,!! |
| Properties | Raw-RVX | BM-Only | JM-Only | BM-JM | |
|---|---|---|---|---|---|
| PIV | Arrival time (ms) | 161.67 ± 46.12 | 75.83 ± 10.10 | 158.67 ± 91.11 | 146.67 ± 25.9 |
| Mean speed (mm/ms) | 0.95 ± 0.08 ## | 0.77 ± 0.08 **,†† | 1.05 ± 0.02 ## | 1.00 ± 0.05 ## | |
| Maximum speed (mm/ms) | 3.00 ± 0.06 # | 6.22 ± 0.09 *,†† | 2.93 ± 0.08 ## | 2.82 ± 0.11 ## | |
| NGI | ED (%) | - | 69.69 ± 19.91 | 84.02 ± 6.17 | 78.60 ± 1.48 |
| FPF (%) | - | 15.69 ± 1.91 †† | 27.71 ± 2.49 ## | 45.55 ± 4.90 ##,†† | |
| MMAD (μm) | - | N/A | 7.71 ± 0.03 | 6.56 ± 1.31 | |
| GSD | - | N/A | 1.66 ± 0.02 | 2.61 ± 0.18 | |
| Parameters | Oral (5 mg/kg) | BM-JM-5L (2 mg/kg) | BM-JM-5L (5 mg/kg) | BM-JM-5L (10 mg/kg) |
|---|---|---|---|---|
| t1/2 (h) | 3.85 ± 1.29 | 6.27 ± 1.82 | 8.76 ± 1.54 | 10.46 ± 3.29 |
| Tmax (h) | 4.00 ± 0.01 | 2.80 ± 0.45 | 3.80 ± 0. 45 | 3.80 ± 2.68 |
| Cmax (ng/mL) | 770.26 ± 64.46 | 566.61 ± 134.22 | 1029.48 ± 256.54 | 1334.64 ± 465.97 |
| AUC0–24 (ng·hr/mL) | 4006.47 ± 1124.41 | 3945.45 ± 666.26 | 10,272.88 ± 2516.56 ** | 12,880.85 ± 565.1 **,†† |
| CL/F (L/h/kg) | 1.31 ± 0.28 | 0.45 ± 0.07 | 0.42 ± 0.11 | 0.30 ± 0.04 |
| Relative BA | - | 2.46 | 2.56 | 1.61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.-J.; Son, J.; Han, C.-S.; Park, C.-W. Preparation of Carrier-Free Inhalable Dry Powder of Rivaroxaban Using Two-Step Milling for Lung-Targeted Delivery. Pharmaceutics 2025, 17, 634. https://doi.org/10.3390/pharmaceutics17050634
Kim Y-J, Son J, Han C-S, Park C-W. Preparation of Carrier-Free Inhalable Dry Powder of Rivaroxaban Using Two-Step Milling for Lung-Targeted Delivery. Pharmaceutics. 2025; 17(5):634. https://doi.org/10.3390/pharmaceutics17050634
Chicago/Turabian StyleKim, Young-Jin, Jaewoon Son, Chang-Soo Han, and Chun-Woong Park. 2025. "Preparation of Carrier-Free Inhalable Dry Powder of Rivaroxaban Using Two-Step Milling for Lung-Targeted Delivery" Pharmaceutics 17, no. 5: 634. https://doi.org/10.3390/pharmaceutics17050634
APA StyleKim, Y.-J., Son, J., Han, C.-S., & Park, C.-W. (2025). Preparation of Carrier-Free Inhalable Dry Powder of Rivaroxaban Using Two-Step Milling for Lung-Targeted Delivery. Pharmaceutics, 17(5), 634. https://doi.org/10.3390/pharmaceutics17050634






