Efficacy and Safety of Antibody-Drug Conjugates for Lung Cancer Therapy: A Systematic Review of Randomized and Non-Randomized Clinical Trials
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Selection of Studies, Data Extraxtion and Analysis
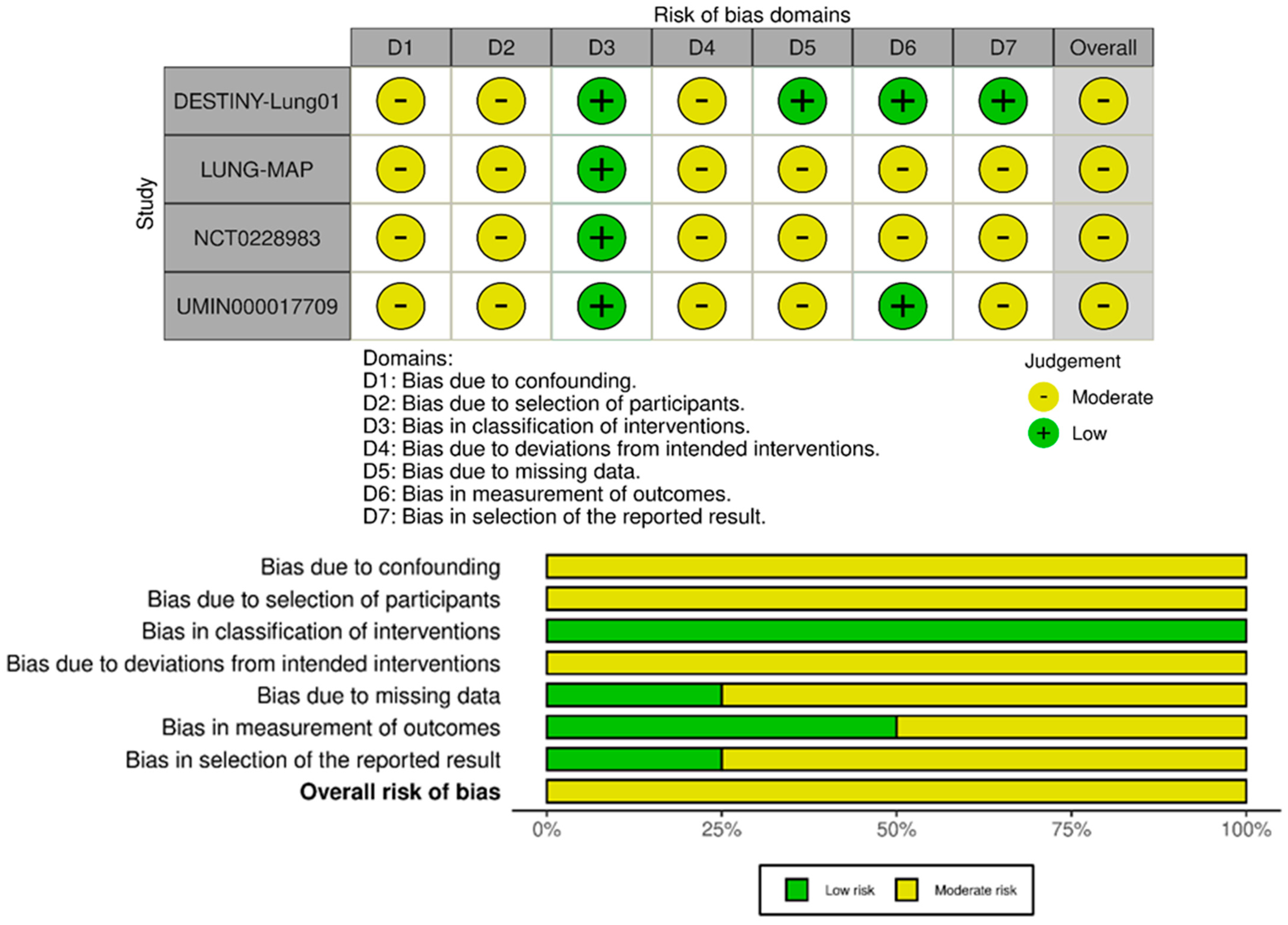
2.4. Quality Assessment of the Included Studies
2.5. Registration and Protocol
3. Results
3.1. Literature Search Results and Quality Assessment
3.2. Baseline Characteristics of the Included Studies
3.3. ADC Targeting HER2
3.3.1. Trastuzumab Deruxtecan (T-Dxd)
3.3.2. HER2-Mutant Versus HER2-Overexpressing NSCLC Populations
3.3.3. Trastuzumab Emtansine (T-DM1)
3.4. ADC Targeting TROP2
3.5. ADC Targeting HER3
3.6. ADC Targeting c-MET
4. Discussion
5. Limitations of the Study
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Postmus, P.E.; Kerr, K.M.; Oudkerk, M.; Senan, S.; Waller, D.A.; Vansteenkiste, J.; Escriu, C.; Peters, S.; ESMO Guidelines Committee. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28, iv1–iv21. [Google Scholar] [CrossRef]
- Molina, J.R.; Yang, P.; Cassivi, S.D.; Schild, S.E.; Adjei, A.A. Non-small cell lung cancer: Epidemiology, risk factors, treatment, and survivorship. Mayo Clin. Proc. 2008, 83, 584–594. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society. Targeted Drug Therapy for Non-Small Cell Lung Cancer. Available online: https://www.cancer.org/cancer/types/lung-cancer/treating-non-small-cell/targeted-therapies.html (accessed on 1 February 2025).
- Remon, J.; Soria, J.-C.; Peters, S. Early and locally advanced non-small-cell lung cancer: An update of the ESMO Clinical Practice Guidelines focusing on diagnosis, staging, systemic and local therapy. Ann. Oncol. 2021, 32, 1637–1642. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [PubMed]
- Gerull, W.D.; Puri, V.; Kozower, B.D. The epidemiology and biology of pulmonary metastases. J. Thorac. Dis. 2021, 13, 2585–2589. [Google Scholar] [CrossRef]
- Doglioni, G.; Fernández-García, J.; Igelmann, S.; Altea-Manzano, P.; Blomme, A.; La Rovere, R.; Liu, X.-Z.; Liu, Y.; Tricot, T.; Nobis, M.; et al. Aspartate signalling drives lung metastasis via alternative translation. Nature 2025, 638, 244–250. [Google Scholar] [CrossRef]
- Eberhardt, W.E.; Mitchell, A.; Crowley, J.; Kondo, H.; Kim, Y.T.; Turrisi, A.; Goldstraw, P.; Rami-Porta, R. The IASLC Lung Cancer Staging Project: Proposals for the Revision of the M Descriptors in the Forthcoming Eighth Edition of the TNM Classification of Lung Cancer. J. Thorac. Oncol. 2015, 10, 1515–1522. [Google Scholar] [CrossRef]
- Novello, S.; Barlesi, F.; Califano, R.; Cufer, T.; Ekman, S.; Levra, M.G.; Kerr, K.; Popat, S.; Reck, M.; Senan, S.; et al. Metastatic non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2016, 27, v1–v27. [Google Scholar] [CrossRef]
- Planchard, D.; Popat, S.T.; Kerr, K.; Novello, S.; Smit, E.F.; Faivre-Finn, C.; Mok, T.S.; Reck, M.; Van Schil, P.E.; Hellmann, M.D.; et al. Updated Version Published 15 September 2020 by the ESMO Guidelines Committee Metastatic Non-Small Cell Lung Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-up. Ann. Oncol. 2018, 29, iv192–iv237. [Google Scholar] [CrossRef]
- Rodak, O.; Peris-Díaz, M.D.; Olbromski, M.; Podhorska-Okołów, M.; Dzięgiel, P. Current Landscape of Non-Small Cell Lung Cancer: Epidemiology, Histological Classification, Targeted Therapies, and Immunotherapy. Cancers 2021, 13, 4705. [Google Scholar] [CrossRef]
- Alduais, Y.; Zhang, H.; Fan, F.; Chen, J.; Chen, B. Non-small cell lung cancer (NSCLC): A review of risk factors, diagnosis, and treatment. Medicine 2023, 102, e32899. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zhang, R.; Yang, H.; Gao, Y.; Zou, Y.; Zhang, X. Antibody-drug conjugates for non-small cell lung cancer: Advantages and challenges in clinical translation. Biochem. Pharmacol. 2024, 226, 116378. [Google Scholar] [CrossRef] [PubMed]
- Filis, P.; Zerdes, I.; Soumala, T.; Matikas, A.; Foukakis, T. The ever-expanding landscape of antibody-drug conjugates (ADCs) in solid tumors: A systematic review. Crit. Rev. Oncol. Hematol. 2023, 192, 104189. [Google Scholar] [CrossRef]
- Weinstein, I.B.; American Association for the Advancement of Science. Addiction to Oncogenes-the Achilles Heal of Cancer. Science 2002, 297, 63–64. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Xiao, D.; Xie, F.; Liu, L.; Wang, Y.; Fan, S.; Zhou, X.; Li, S. Antibody–drug conjugates: Recent advances in linker chemistry. Acta Pharm. Sin. B 2021, 11, 3889–3907. [Google Scholar] [CrossRef]
- Sheyi, R.; de la Torre, B.G.; Albericio, F. Linkers: An Assurance for Controlled Delivery of Antibody-Drug Conjugate. Pharmaceutics 2022, 14, 396. [Google Scholar] [CrossRef]
- El-Sayed, M.M.; Bianco, J.R.; Li, Y.; Fabian, Z. Tumor-Agnostic Therapy—The Final Step Forward in the Cure for Human Neoplasms? Cells 2024, 13, 1071. [Google Scholar] [CrossRef]
- Westphalen, C.; Martins-Branco, D.; Beal, J.; Cardone, C.; Coleman, N.; Schram, A.; Halabi, S.; Michiels, S.; Yap, C.; André, F.; et al. The ESMO Tumour-Agnostic Classifier and Screener (ETAC-S): A tool for assessing tumour-agnostic potential of molecularly guided therapies and for steering drug development. Ann. Oncol. 2024, 35, 936–953. [Google Scholar] [CrossRef]
- Godwin, C.D.; Gale, R.P.; Walter, R.B. Gemtuzumab ozogamicin in acute myeloid leukemia. Leukemia 2017, 31, 1855–1868. [Google Scholar] [CrossRef]
- Pazo, C.D.; Nawaz, K.; Webster, R.M. The oncology market for antibody–drug conjugates. Nat. Rev. Drug Discov. 2021, 20, 583–584. [Google Scholar] [CrossRef]
- Chen, X.; Zeng, C. Pioneering the Way: The Revolutionary Potential of Antibody–Drug Conjugates in NSCLC. Curr. Treat. Options Oncol. 2024, 25, 556–584. [Google Scholar] [CrossRef] [PubMed]
- Nakada, T.; Sugihara, K.; Jikoh, T.; Abe, Y.; Agatsuma, T. The latest research and development into the antibody–drug conjugate, [fam-] trastuzumab deruxtecan (DS-8201a), for HER2 cancer therapy. Chem. Pharm. Bull. 2019, 67, 173–185. [Google Scholar] [CrossRef]
- Hoe, H.J.; Solomon, B.J. Optimizing Dosing of Trastuzumab Deruxtecan in HER2-Mutant Non–Small-Cell Lung Cancer: A Reminder That More Is Not Always Better. J. Clin. Oncol. 2023, 41, 4849–4851. [Google Scholar] [CrossRef]
- Gogia, P.; Ashraf, H.; Bhasin, S.; Xu, Y. Antibody–Drug Conjugates: A Review of Approved Drugs and Their Clinical Level of Evidence. Cancers 2023, 15, 3886. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef] [PubMed]
- Delgado, A.; Guddati, A.K. Clinical endpoints in oncology-a primer. Am. J. Cancer Res. 2021, 11, 11211131. [Google Scholar]
- US Department of Health and Human Services NI of HNCI. Common Terminology Criteria for Adverse Events (CTCAE); US Department of Health and Human Services: Washington, DC, USA, 2017.
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Schünemann, H.J. What is “quality of evidence” and why is it important to clinicians? BMJ 2008, 336, 995–998. [Google Scholar] [CrossRef]
- Brignardello-Petersen, R.; Guyatt, G.H. Assessing the certainty of the evidence in systematic reviews: Importance, process, and use. Am. J. Epidemiol. 2025, 00, 1–6. [Google Scholar] [CrossRef]
- Goto, K.; Goto, Y.; Kubo, T.; Ninomiya, K.; Kim, S.-W.; Planchard, D.; Ahn, M.-J.; Smit, E.F.; de Langen, A.J.; Pérol, M.; et al. Trastuzumab Deruxtecan in Patients With HER2-Mutant Metastatic Non–Small-Cell Lung Cancer: Primary Results From the Randomized, Phase II DESTINY-Lung02 Trial. J. Clin. Oncol. 2023, 41, 4852–4863. [Google Scholar] [CrossRef] [PubMed]
- Ahn, M.-J.; Tanaka, K.; Paz-Ares, L.; Cornelissen, R.; Girard, N.; Pons-Tostivint, E.; Baz, D.V.; Sugawara, S.; Cobo, M.; Pérol, M.; et al. Datopotamab Deruxtecan Versus Docetaxel for Previously Treated Advanced or Metastatic Non–Small Cell Lung Cancer: The Randomized, Open-Label Phase III TROPION-Lung01 Study. J. Clin. Oncol. 2025, 43, 260–272. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.A.; Yang, J.C.H.; Hayashi, H.; Goto, Y.; Felip, E.; Reck, M.; Vigliotti, M.; Dong, Q.; Cantero, F.; Fan, P.D.; et al. HERTHENA-Lung01: A phase II study of patritumab deruxtecan (HER3-DXd) in previously treated metastatic EGFR-mutated NSCLC. Future Oncol. 2023, 19, 1319–1329. [Google Scholar] [CrossRef] [PubMed]
- Smit, E.F.; Felip, E.; Uprety, D.; Nagasaka, M.; Nakagawa, K.; Rodríguez, L.P.-A.; Pacheco, J.M.; Li, B.T.; Planchard, D.; Baik, C.; et al. Trastuzumab deruxtecan in patients with metastatic non-small-cell lung cancer (DESTINY-Lung01): Primary results of the HER2-overexpressing cohorts from a single-arm, phase 2 trial. Lancet Oncol. 2024, 25, 439–454. [Google Scholar] [CrossRef]
- Hotta, K.; Aoe, K.; Kozuki, T.; Ohashi, K.; Ninomiya, K.; Ichihara, E.; Kubo, T.; Ninomiya, T.; Chikamori, K.; Harada, D.; et al. A Phase II Study of Trastuzumab Emtansine in HER2-Positive Non–Small Cell Lung Cancer. J. Thorac. Oncol. 2018, 13, 273–279. [Google Scholar] [CrossRef]
- Waqar, S.N.; Redman, M.W.; Arnold, S.M.; Hirsch, F.R.; Mack, P.C.; Schwartz, L.H.; Gandara, D.R.; Stinchcombe, T.E.; Leighl, N.B.; Ramalingam, S.S.; et al. A Phase II Study of Telisotuzumab Vedotin in Patients With c–MET-positive Stage IV or Recurrent Squamous Cell Lung Cancer (LUNG-MAP Sub-study S1400K, NCT03574753). Clin. Lung Cancer 2020, 22, 170–177. [Google Scholar] [CrossRef]
- Peters, S.; Stahel, R.A.; Bubendorf, L.; Bonomi, P.; Villegas, A.; Kowalski, D.M.; Baik, C.S.; Isla, D.; Carpeno, J.D.C.; Garrido, P.; et al. Trastuzumab Emtansine (T-DM1) in Patients with Previously Treated HER2-Overexpressing Metastatic Non–Small Cell Lung Cancer: Efficacy, Safety, and Biomarkers. Clin. Cancer Res. 2019, 25, 64–72. [Google Scholar] [CrossRef]
- Uy, N.F.; Merkhofer, C.M.; Baik, C.S. HER2 in Non-Small Cell Lung Cancer: A Review of Emerging Therapies. Cancers 2022, 14, 4155. [Google Scholar] [CrossRef]
- Meric-Bernstam, F.; Makker, V.; Oaknin, A.; Oh, D.-Y.; Banerjee, S.; González-Martín, A.; Jung, K.H.; Ługowska, I.; Manso, L.; Manzano, A.; et al. Efficacy and Safety of Trastuzumab Deruxtecan in Patients With HER2-Expressing Solid Tumors: Primary Results From the DESTINY-PanTumor02 Phase II Trial. J. Clin. Oncol. 2024, 42, 47–58. [Google Scholar] [CrossRef]
- Raghav, K.; Siena, S.; Takashima, A.; Kato, T.; Eynde, M.V.D.; Pietrantonio, F.; Komatsu, Y.; Kawakami, H.; Peeters, M.; Andre, T.; et al. Trastuzumab deruxtecan in patients with HER2-positive advanced colorectal cancer (DESTINY-CRC02): Primary results from a multicentre, randomised, phase 2 trial. Lancet Oncol. 2024, 25, 1147–1162. [Google Scholar] [CrossRef]
- Von Minckwitz, G.; Huang, C.-S.; Mano, M.S.; Loibl, S.; Mamounas, E.P.; Untch, M.; Wolmark, N.; Rastogi, P.; Schneeweiss, A.; Redondo, A.; et al. Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N. Engl. J. Med. 2019, 380, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Tsao, L.-C.; Wang, J.S.; Ma, X.; Sodhi, S.; Ragusa, J.V.; Liu, B.; McBane, J.; Wang, T.; Wei, J.; Liu, C.-X.; et al. Effective extracellular payload release and immunomodulatory interactions govern the therapeutic effect of trastuzumab deruxtecan (T-DXd). Nat. Commun. 2025, 16, 3167. [Google Scholar] [CrossRef]
- Coleman, N.; Yap, T.A.; Heymach, J.V.; Meric-Bernstam, F.; Le, X. Antibody-drug conjugates in lung cancer: Dawn of a new era? NPJ Precis. Oncol. 2023, 7, 5. [Google Scholar] [CrossRef]
- Goldenberg, D.M.; Stein, R.; Sharkey, R.M. The emergence of trophoblast cell-surface antigen 2 (TROP-2) as a novel cancer target. Oncotarget 2018, 9, 28989–29006. [Google Scholar] [CrossRef] [PubMed]
- Guerra, E.; Trerotola, M.; Alberti, S. Targeting Trop-2 as a Cancer Driver. J. Clin. Oncol. 2023, 41, 4688–4692. [Google Scholar] [CrossRef] [PubMed]
- Mito, R.; Matsubara, E.; Komohara, Y.; Shinchi, Y.; Sato, K.; Yoshii, D.; Ohnishi, K.; Fujiwara, Y.; Tomita, Y.; Ikeda, K.; et al. Clinical impact of TROP2 in non-small lung cancers and its correlation with abnormal p53 nuclear accumulation. Pathol. Int. 2020, 70, 287–294. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhou, H.; Liu, J.; Ha, W.; Xia, X.; Li, J.; Chao, T.; Xiong, H. Progress and Innovative Combination Therapies in Trop-2-Targeted ADCs. Pharmaceuticals 2024, 17, 652. [Google Scholar] [CrossRef]
- Santi, D.V.; Cabel, L.; Bidard, F.-C. Does sacituzumab-govitecan act as a conventional antibody drug conjugate (ADC), a prodrug of SN-38 or both? Ann. Transl. Med. 2021, 9, 1113. [Google Scholar] [CrossRef]
- Gilead Sciences, Inc. U.S. FDA Grants Breakthrough Therapy Designation to Trodelvy® (sacituzumab govitecan-hziy) for Second-Line Treatment of Extensive-Stage Small Cell Lung Cancer. 2024. Available online: https://www.gilead.com/news/news-details/2024/us-fda-grants-breakthrough-therapy-designation-to-trodelvy-sacituzumab-govitecan-hziy-for-second-line-treatment-of-extensive-stage-small-cell-lung-cancer (accessed on 17 December 2024).
- Dowlati, A.; Chiang, A.C.; Cervantes, A.; Babu, S.; Hamilton, E.; Wong, S.F.; Tazbirkova, A.; Sullivan, I.G.; van Marcke, C.; Italiano, A.; et al. Phase 2 Open-Label Study of Sacituzumab Govitecan as Second-Line Therapy in Patients With Extensive-Stage SCLC: Results From TROPiCS-03. J. Thorac. Oncol. 2025. [Google Scholar] [CrossRef]
- Bardia, A.; Hurvitz, S.A.; Tolaney, S.M.; Loirat, D.; Punie, K.; Oliveira, M.; Brufsky, A.; Sardesai, S.D.; Kalinsky, K.; Zelnak, A.B.; et al. Sacituzumab Govitecan in Metastatic Triple-Negative Breast Cancer. N. Engl. J. Med. 2021, 384, 1529–1541. [Google Scholar] [CrossRef]
- Rugo, H.S.; Bardia, A.; Tolaney, S.M.; Arteaga, C.; Cortes, J.; Sohn, J.; Marmé, F.; Hong, Q.; Delaney, R.J.; Hafeez, A.; et al. TROPiCS-02: A Phase III Study Investigating Sacituzumab Govitecan in the Treatment of HR+/HER2- Metastatic Breast Cancer. Futur. Oncol. 2020, 16, 705–715. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Bordeau, B.M.; Balthasar, J.P. Mechanisms of ADC Toxicity and Strategies to Increase ADC Tolerability. Cancers 2023, 15, 713. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Liu, K.; Wang, K.; Zhu, H. Treatment-related adverse events of antibody–drug conjugates in clinical trials: A systematic review and meta-analysis. Cancer 2022, 129, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Liu, X.; Wu, L.; Jiang, Y.; Zhang, Y.; Wang, Y. Interstitial lung disease with antibody–drug conjugates: A real-world pharmacovigilance study based on the FAERS database during the period 2014–2023. Ther. Adv. Respir. Dis. 2024, 18, 17534666241299935. [Google Scholar] [CrossRef]
- Chai, M.; Li, L.; Wu, H.; Liu, Y.; Yi, Z.; Yu, H. Lung toxicity induced by anti-HER2 antibody—Drug conjugates for breast cancer. Crit. Rev. Oncol./Hematol. 2024, 195, 104274. [Google Scholar] [CrossRef] [PubMed]
- Masters, J.C.; Nickens, D.J.; Xuan, D.; Shazer, R.L.; Amantea, M. Clinical toxicity of antibody drug conjugates: A meta-analysis of payloads. Investig. New Drugs 2017, 36, 121–135. [Google Scholar] [CrossRef]
- Pedersini, R.; Buffoni, M.; Petrelli, F.; Ghidini, A.; di Mauro, P.; Amoroso, V.; Parati, M.C.; Laini, L.; Cosentini, D.; Schivardi, G.; et al. Gastrointestinal Toxicity of Antibody Drug Conjugates (ADCs) in Metastatic Breast Cancer: A Pooled Analysis. Clin. Breast Cancer 2024, 24, 411–420. [Google Scholar] [CrossRef]
- Bardia, A.; Jhaveri, K.; Im, S.A.; Pernas, S.; De Laurentiis, M.; Wang, S.; Jañez, N.M.; Borges, G.; Cescon, D.W.; Hattori, M.; et al. Datopotamab Deruxtecan Versus Chemotherapy in Previously Treated Inoperable/Metastatic Hormone Receptor–Positive Human Epidermal Growth Factor Receptor 2–Negative Breast Cancer: Primary Results From TROPION-Breast01. J. Clin. Oncol. 2025, 43, 285–296. [Google Scholar] [CrossRef]


| Study Identifier (NCT/ADC) | Year | Study Phase/ Allocation/Interventional Model/Status | Study Arms and Dosages | Number of Patients | Average Age (Years ± SD) | Male Patients n (%) |
|---|---|---|---|---|---|---|
| DESTINY-Lung01 [36] (NCT03505710/Trastuzumab deruxtecan) | 2024 | II/Not randomized/Parallel assignment/Completed | HER2 overexpressing NSCLC: trastuzumab deruxtecan 6.4 (cohort 1) or 5.4 mg/kg (cohort 1A), Q3W | 181 | 60.8 ± 10.9 | 83 (45.9) |
| DESTINY-Lung02 [33] (NCT04644237/Trastuzumab deruxtecan) | 2024 | II/ Randomized/Parallel assignment/Active, not rectruiting | HER2-mutated metastatic NSCLC: trastuzumab deruxtecan 6.4 or 5.4 mg/kg, Q3W | 152 | 59.7 ± 11.7 | 53 (34.9) |
| HERTHENA-Lung01 [35] (NCT04619004/Patritumab deruxtecan) | 2024 | II/ Randomized/Parallel assignment/Active not recruiting | Cohort A (dose escalation): patritumab deruxtecan up-titration, Q3W Cohort B (dose expansion): patritumab deruxtecan 5.6 mg/kg, Q3W. | 277 | 62.2 ± 9.95 | 115 (41.5) |
| TROPION-Lung01 [34] (NCT04656652/Datopotamab deruxtecan) | 2024 | III/Randomized/Parallel assignment/Active not recruiting | Dato-DXd arm: Dato-DXd 6 mg/kg, Q3W. Docetaxel arm: docetaxel 75 mg/m2, Q3W | 590 | 63.5 | 393 (66.6) |
| LUNG-MAP SUB-STUDY [38] (NCT03574753/Telisotuzumab vedotin) | 2021 | II/N/A/Single group assignment/Completed | ABBV-399 2.7 mg/kg, Q3W, in ICI-naïve (Cohort 1) and ICI-refractory (Cohort 2) c-MET-positive recurrent NSCLC | 23 | 65.3 | 13 (56.5) |
| (NCT0228983/ Trastuzumab emtansine) [39] | 2019 | II/Not randomized/Single group assignment/Completed | HER2-overexpressing IHC2+ (Cohort 1) or IHC3+ (Cohort 2): trastuzumab emtansine 3.6 mg/kg, Q3W | 49 | 62.4 ± 9.6 | 29 (59.2) |
| (UMIN000017709/Trastuzumab emtansine) [37] | 2018 | II/Not randomized/Single group assignment/Terminated | HER2-mutated NSCLC: trastuzumab emtansine 3.6 mg/kg, Q3W | 15 | 67 | 7 (47) |
| ADC | Target (mAb/Payload) | Linker | DAR | FDA/EMA Authorization Details (Year of Approval, Therapeutic Indications and Dosage) |
|---|---|---|---|---|
| Fam-trastuzumab deruxtecan (T-DXd) | HER2/TOPO I | GGFG (cleavable) | 8 | 2019/2021 HER2-positive breast cancer It is indicated as monotherapy for the treatment of adult patients with unresectable or metastatic HER2-positive breast cancer who have received one or more prior anti-HER2-based regimens. Dosage: 5.4 mg/kg, Q3W. HER2-low breast cancer It is indicated as monotherapy for the treatment of adult patients with unresectable or metastatic HER2-low breast cancer who have received prior chemotherapy in metastatic setting or developed disease recurrence during or within 6 months of completing adjuvant chemotherapy. Dosage: 5.4 mg/kg, Q3W. Lung cancer It is indicated as monotherapy for the treatment of adult patients with advanced NSCLC whose tumors have an activating HER2 (ERBB2) mutation and who require systemic therapy following platinum-based chemotherapy with or without immunotherapy. Dosage: 5.4 mg/kg, Q3W. Gastric cancer It is indicated as monotherapy for the treatment of adult patients with advanced HER2-positive gastric or GEJ adenocarcinoma who have received a prior trastuzumab-based regimen. Dosage: 6.4 mg/kg, Q3W. |
| Ado-trastuzumab emtansine (T-DM1) | HER2/microtubule | MCC (non- cleavable) | 3.5 | 2013 Early Breast Cancer It is indicated, as a single agent, for the adjuvant treatment of adult patients with HER2-positive early breast cancer who have residual invasive disease, in the breast and/or lymph nodes, after neoadjuvant taxane-based and HER2-targeted therapy. Dosage 3.6 mg/kg, Q3W. Metastatic Breast Cancer It is indicated, as a single agent, for the treatment of adult patients with HER2-positive, unresectable locally advanced or metastatic breast cancer who previously received trastuzumab and a taxane, separately or in combination. Dosage 3.6 mg/kg, Q3W. |
| Datopotamab deruxtecan (Dato-DXd) | TROP2/TOPO I | GGFG (cleavable) | 4 | 2024 (FDA only) Advanced breast cancer It is indicated for the treatment of adult patients with unresectable or metastatic HR–positive, HER2-negative breast cancer who have received prior endocrine-based therapy and chemotherapy. Dosage 6 mg/kg, Q3W. |
| Patritumab deruxtecan | HER3/TOPO I | GGFG (cleavable) | 8 | N/A |
| Telisotuzumab vedotin (ABBV-399) | c-MET/microtubule | VC (cleavable) | 3.1 | N/A |
| Study Identifier | Study Arms and Dosages | Efficacy Outcomes | Safety Outcomes |
|---|---|---|---|
| DESTINY-Lung01 [36] (NCT03505710/ Trastuzumab deruxtecan) | HER2 overexpressing NSCLC: trastuzumab deruxtecan 6.4 (cohort 1) or 5.4 mg/kg (cohort 1A), Q3W | Primary endpoints ORR: 26.5% (95% CI: 15.0–41.1) for cohort 1; 34.1% (95% CI: 20.1–50.6) for cohort 1A. Secondary endpoints DCR: 69.4% (95% CI: 54.6–81.8) for cohort 1; 78.0% (95% CI: 62.4–89.4) for cohort 1A. Median DOR: 5.8 months (95% CI: 4.3-NE) for cohort 1; 6.2 months (95% CI: 4.2–9.8) for cohort 1A. Median PFS: 5.7 months (95% CI: 2.8–7.2) for cohort 1; 6.7 months (95% CI: 4.2–8.4) for cohort 1A. Median OS: 12.4 months (95% CI: 7.8–17.2) for cohort 1; 11.2 months (95% CI: 8.4-NE) for cohort 1A. | Grade ≥ 3 TEAEs: 53% in cohort 1; 22% in cohort 1A. Adjudicated ILD: 20% in cohort 1 (grade 1/2: 14%, grade 5: 6%); 5% in cohort 1A (grade 2: 2%, grade 5: 2%). Common TEAEs: nausea (59% in cohort 1; 73% in cohort 1A), fatigue (59% in cohort 1; 71% in cohort 1A), decreased appetite (45% in cohort 1; 46% in cohort 1A). |
| DESTINY-Lung02 [33] (NCT04644237/ Trastuzumab deruxtecan) | HER2-mutated metastatic NSCLC: trastuzumab deruxtecan 6.4 or 5.4 mg/kg, Q3W | Primary endpoints ORR: 49.0% (95% CI: 39.0–59.1) for 5.4 mg/kg; 56.0% (95% CI: 41.3–70.0) for 6.4 mg/kg. Median DOR: 16.8 months (95% CI: 6.4-NE) for 5.4 mg/kg; NE (95% CI: 8.3-NE) for 6.4 mg/kg. Secondary endpoints Median PFS: 9.9 months (95% CI: 7.4-NE) for 5.4 mg/kg; 15.4 months (95% CI: 8.3-NE) for 6.4 mg/kg. Median OS: 19.5 months (95% CI: 13.6-NE) for 5.4 mg/kg; NE (95% CI: 12.1-NE) for 6.4 mg/kg | Grade ≥ 3 TEAEs: 38.6% for 5.4 mg/kg; 58.0% for 6.4 mg/kg. Adjudicated ILD: 12.9% (Grade ≥ 3: 2.0%; Grade 5: 1.0%) for 5.4 mg/kg; 28.0% (Grade ≥ 3: 2.0%; Grade 5: 2.0%) for 6.4 mg/kg. Common TEAEs: nausea (67.3%), neutropenia (42.6%), fatigue (44.6%), decreased appetite (39.6%) for 5.4 mg/kg; nausea (82.0%), neutropenia (56.0%), fatigue (50.0%), decreased appetite (50.0%) for 6.4 mg/kg |
| HERTHENA-Lung01 [35] (NCT04619004/ Patritumab deruxtecan) | Cohort A (dose escalation): patritumab deruxtecan up-titration, Q3W Cohort B (dose expansion): patritumab deruxtecan 5.6 mg/kg, Q3W. | Primary endpoints ORR: 39% (95% CI: 26–52) in Cohort A; ~30–40% (consistent with Cohort A) in Cohort B. Secondary endpoints Median DOR: 6.9 months (95% CI: 3.1-NE) in Cohort A; similar in Cohort B. Median PFS: 8.2 months (95% CI: 4.4–8.3) in Cohort A; consistent in Cohort B. Median OS: NE (95% CI: 9.4-NE) in both cohorts. DCR: 72% for Cohort A; ~72% (consistent with Cohort A) in Cohort B | Grade ≥ 3 TEAEs: 63% in Cohort A; similar frequency in Cohort B. Adjudicated ILD: 7% in Cohort A (Grade 1/2: 5%, Grade 3: 2%); similar frequency and severity in Cohort B. Common TEAEs: thrombocytopenia (30%), neutropenia (19%), fatigue (14%) in both cohorts. |
| TROPION-Lung01 [34] (NCT04656652/ Datopotamab deruxtecan) | Dato-DXd arm: Dato-DXd 6 mg/kg, Q3W. Docetaxel arm: docetaxel 75 mg/m2, Q3W | Primary endpoints Median PFS: 4.4 months (95% CI: 4.2–5.6) for Dato-DXd vs. 3.7 months (95% CI: 2.9–4.2) for docetaxel (HR: 0.75, p = 0.004). Median OS: 12.9 months (95% CI: 11.0–13.9) for Dato-DXd vs. 11.8 months (95% CI: 10.1–12.8) for docetaxel (HR: 0.94, p = 0.530). Secondary endpoints ORR: 26.4% (95% CI: 21.5–31.8) for Dato-DXd vs. 12.8% (95% CI: 9.3–17.1) for docetaxel. Median DOR: 7.1 months (95% CI: 5.6–10.9) for Dato-DXd vs. 5.6 months (95% CI: 5.4–8.1) for docetaxel | Grade ≥ 3 TEAEs: 25.6% for Dato-DXd vs. 42.1% for docetaxel. Adjudicated ILD: 8.8% (grade ≥ 3: 3.7%; grade 5: 2.4%) for Dato-DXd vs. 4.1% (grade ≥ 3: 1.4%; grade 5: 0.3%) for docetaxel. Common TEAEs: stomatitis (47.5%), nausea (34.0%), decreased appetite (22.9%) for Dato-DXd; alopecia (34.8%), neutropenia (26.2%), anemia (20.7%) for docetaxel |
| LUNG-MAP SUB-STUDY [38] (NCT03574753/ Telisotuzumab vedotin) | ABBV-399 2.7 mg/kg, Q3W, in ICI-naïve (Cohort 1) and ICI-refractory (Cohort 2) c-MET-positive recurrent NSCLC | Primary endpoints ORR: 13% (95% CI: 1–37%). Median DOR: 12.7+ months for CR, 2.3 months for PR. Secondary endpoints DCR: 53% (95% CI: 27–79%) in Cohort 1; 50% (95% CI: 21–79%) in Cohort 2. Median PFS: 3.5 months (95% CI: 1.4–4.2) in Cohort 1; 2.0 months (95% CI: 0.9–3.0) in Cohort 2. Median OS: 5.8 months (95% CI: 3.5–9.7) in Cohort 1; 5.5 months (95% CI: 3.7–8.9) in Cohort 2. | Grade ≥ 3 TEAEs: 17% in both cohorts. Grade 5 events: 1 (4%) bronchopulmonary hemorrhage in Cohort 1; 2 (9%) pneumonitis in Cohort 2. Common TEAEs: fatigue (9%), hypophosphatemia (9%), nausea (4%), and peripheral sensory neuropathy (4%). |
| (NCT0228983/ Trastuzumab emtansine) [37] | HER2-overexpressing IHC2+ (Cohort 1) or IHC3+ (Cohort 2): trastuzumab emtansine 3.6 mg/kg, Q3W | Primary endpoints ORR: 0% (95% CI: 0.0–11.9) in Cohort 1; 20% (95% CI: 5.7–43.7) in Cohort 2. Secondary endpoints Median PFS: 2.6 months (95% CI: 1.4–2.8) in Cohort 1; consistent in Cohort 2. Median OS: 12.2 months (95% CI: 3.8–23.3) in Cohort 1; 15.3 months (95% CI: 4.1–NE) in Cohort 2. DCR: 72% for Cohort A; ~72% (consistent with Cohort A) in Cohort B | Grade ≥ 3 TEAEs: 35% in both cohorts. Common TEAEs: fatigue (6.9%), infusion-related reactions (6.9%). No grade 4 or 5 events observed in Cohort 1; thrombocytopenia (5%), fatigue (10%). One grade 4 seizure in a patient with brain metastases in Cohort 2. |
| (UMIN000017709/ Trastuzumab emtansine) [39] | HER2-mutated NSCLC: trastuzumab emtansine 3.6 mg/kg, Q3W | Primary endpoints ORR: 6.7% (90% CI: 0.2–32.0). Secondary endpoints Median PFS: 2.0 months (90% CI: 1.2–4.0). Median OS: 10.9 months (90% CI: 4.4–12.0). | Grade ≥ 3 TEAEs: thrombocytopenia (40%), hepatotoxicity (20%). Adjudicated ILD: grade 2 interstitial pneumonia in 1 patient (6.7%). No treatment-related deaths observed. |
| ADC | Key Efficacy Outcomes | Grade ≥ 3 TEAEs (%) | ILD Incidence (%) | Other Common Events (Incidence %) | Key Considerations for Safety Management |
|---|---|---|---|---|---|
| Trastuzumab deruxtecan (T-DXd) | ORR: 49.0–56.0%. PFS: 9.9–15.4 months | 38.6–58.0% | 12.9–28.0% | Nausea (67.3–82.0%), neutropenia (42.6–56.0%), fatigue (44.6–50.0%) | Regular ILD monitoring, hematologic checks, antiemetic support |
| Trastuzumab emtansine (T-DM1) | ORR: 6.7–20.0%. PFS: 2.0–2.7 months | 35–40% | 6.7% | Thrombocytopenia (40%), hepatotoxicity (20%), fatigue (10%) | Frequent liver function and platelet monitoring |
| Datopotamab deruxtecan (Dato-DXd) | ORR: 26.4%. PFS: 4.4 months | 25.6% | 8.8% | Stomatitis (47.5%), nausea (34.0%), decreased appetite (22.9%) | Lower toxicity than docetaxel, requires stomatitis prevention |
| Patritumab deruxtecan | ORR: 39.0%. PFS: 8.2 months | 63% | 7% | Thrombocytopenia (30%), neutropenia (19%), fatigue (14%) | High hematologic toxicity, close blood count monitoring |
| Telisotuzumab vedotin | ORR: 9%. PFS: 3.5 months | 17% | - | Fatigue (9%), peripheral neuropathy (4%), nausea (4%) | Lower toxicity, but modest efficacy; requires neuropathy management |
| Outcome | No. of Studies (Participants) | Relative Effect (95% CI) | Certainty of Evidence (GRADE) | Notes |
|---|---|---|---|---|
| Efficacy: ORR | 6 studies (1248 participants) | ORR: 20–56% | Moderate | Downgraded for heterogeneity in ADC mechanisms and varied patient populations. |
| Efficacy: PFS | 6 studies (1248 participants) | HR: 0.75–1.2 | Moderate | Downgraded for imprecision in small subgroup analyses and differences in treatment regimens. |
| Efficacy: OS | 4 studies (987 participants) | HR: 0.85–1.3 | Moderate | Downgraded for risk of bias (open-label designs) and variability in comparator arms. |
| Safety: TEAEs ≥ Grade 3 | 6 studies (1248 participants) | 17–63% | High | Consistent reporting across studies; manageable toxicities, mainly fatigue and neutropenia. |
| Safety: ILD incidence | 4 studies (987 participants) | 3–12% | Moderate | Downgraded for imprecision in ILD grading and variations in adjudication criteria. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallina, M.; Carollo, A.; Gallina, A.; Cutaia, S.; Rizzo, S.; Provenzani, A. Efficacy and Safety of Antibody-Drug Conjugates for Lung Cancer Therapy: A Systematic Review of Randomized and Non-Randomized Clinical Trials. Pharmaceutics 2025, 17, 608. https://doi.org/10.3390/pharmaceutics17050608
Gallina M, Carollo A, Gallina A, Cutaia S, Rizzo S, Provenzani A. Efficacy and Safety of Antibody-Drug Conjugates for Lung Cancer Therapy: A Systematic Review of Randomized and Non-Randomized Clinical Trials. Pharmaceutics. 2025; 17(5):608. https://doi.org/10.3390/pharmaceutics17050608
Chicago/Turabian StyleGallina, Matteo, Anna Carollo, Anna Gallina, Sofia Cutaia, Sergio Rizzo, and Alessio Provenzani. 2025. "Efficacy and Safety of Antibody-Drug Conjugates for Lung Cancer Therapy: A Systematic Review of Randomized and Non-Randomized Clinical Trials" Pharmaceutics 17, no. 5: 608. https://doi.org/10.3390/pharmaceutics17050608
APA StyleGallina, M., Carollo, A., Gallina, A., Cutaia, S., Rizzo, S., & Provenzani, A. (2025). Efficacy and Safety of Antibody-Drug Conjugates for Lung Cancer Therapy: A Systematic Review of Randomized and Non-Randomized Clinical Trials. Pharmaceutics, 17(5), 608. https://doi.org/10.3390/pharmaceutics17050608






