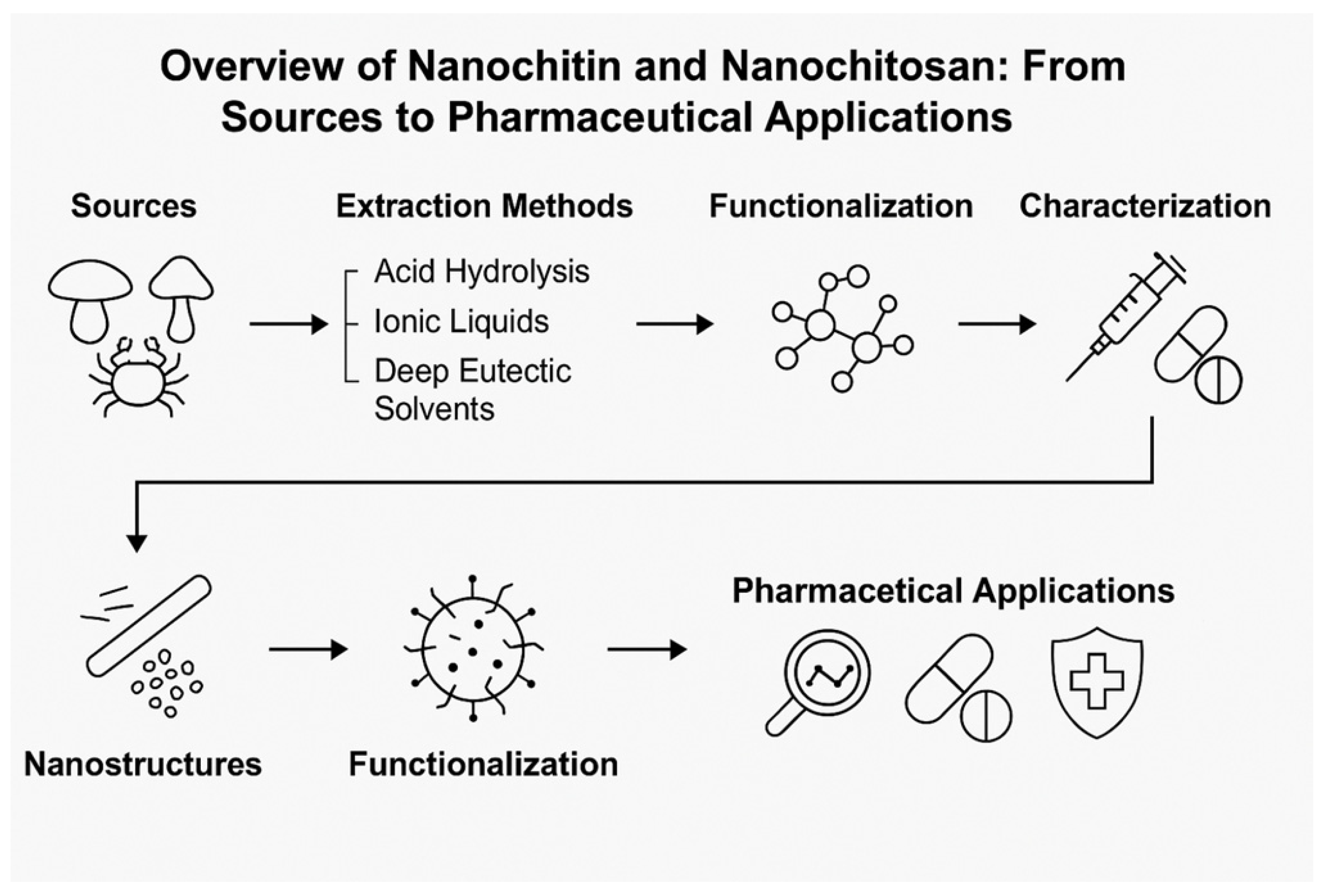
Nanochitin and Nanochitosan in Pharmaceutical Applications: Innovations, Applications, and Future Perspective
Abstract
1. Introduction
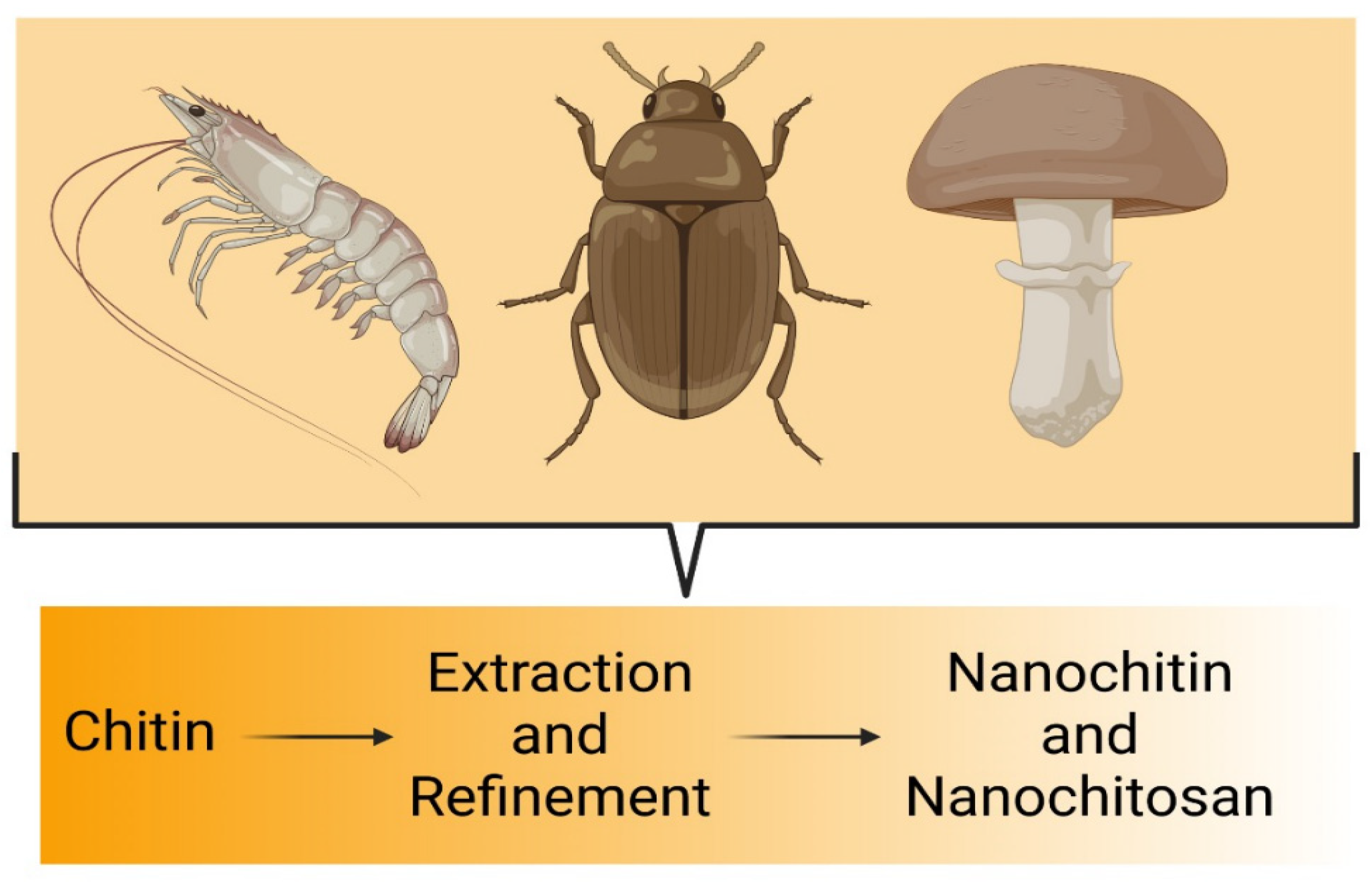
2. Chitin: A Potential Precursor for Nanochitin
2.1. Sources of Chitin
2.1.1. Insects
2.1.2. Fungi Species
2.1.3. Crustacean Shells
2.1.4. Squid and Snail
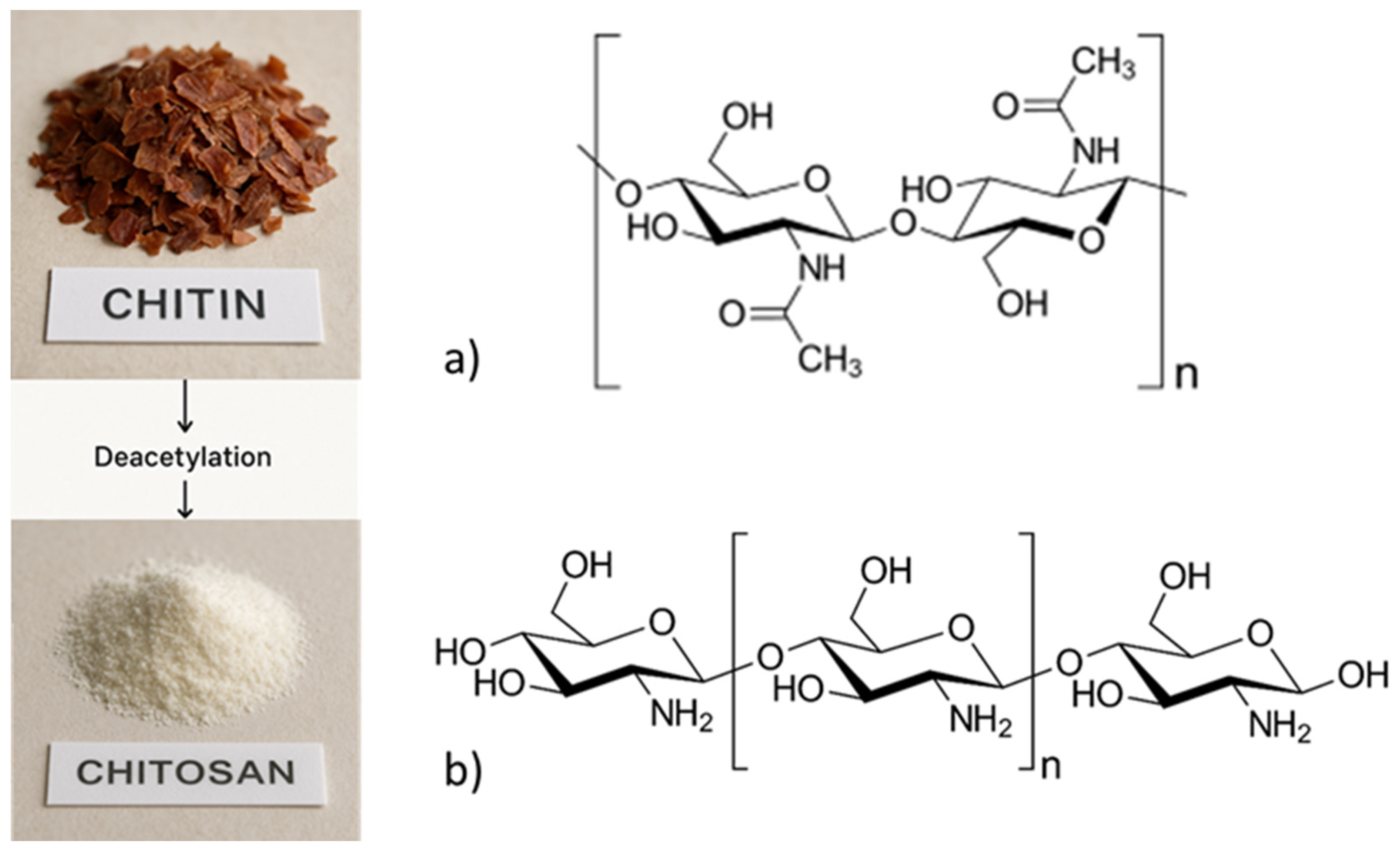
2.2. Chemical Structures of Chitin and Chitosan
- ILs, like 1-ethyl-3-methylimidazolium acetate ([EMIM][OAc]) and 1-butyl-3-methylimidazolium chloride ([BMIM]Cl), dissolve chitin/chitosan by disrupting the intermolecular hydrogen bonding network, particularly between –OH and –NH2 groups, via ion-dipole interactions [6].
- DES, such as choline chloride:lactic acid or choline chloride:urea mixtures, exhibit similar mechanisms through strong hydrogen bonding with the polymer chains [6].
2.3. Rationale for Nanoscale Forms
3. Methods of Extraction and Fabrication of Nanochitin and Nanochitosan
3.1. Extraction Methods for Nanochitin
3.2. Top-Down Methods
3.2.1. Acid Hydrolysis
3.2.2. Mechanical Disintegration
3.3. Bottom-Up Methods
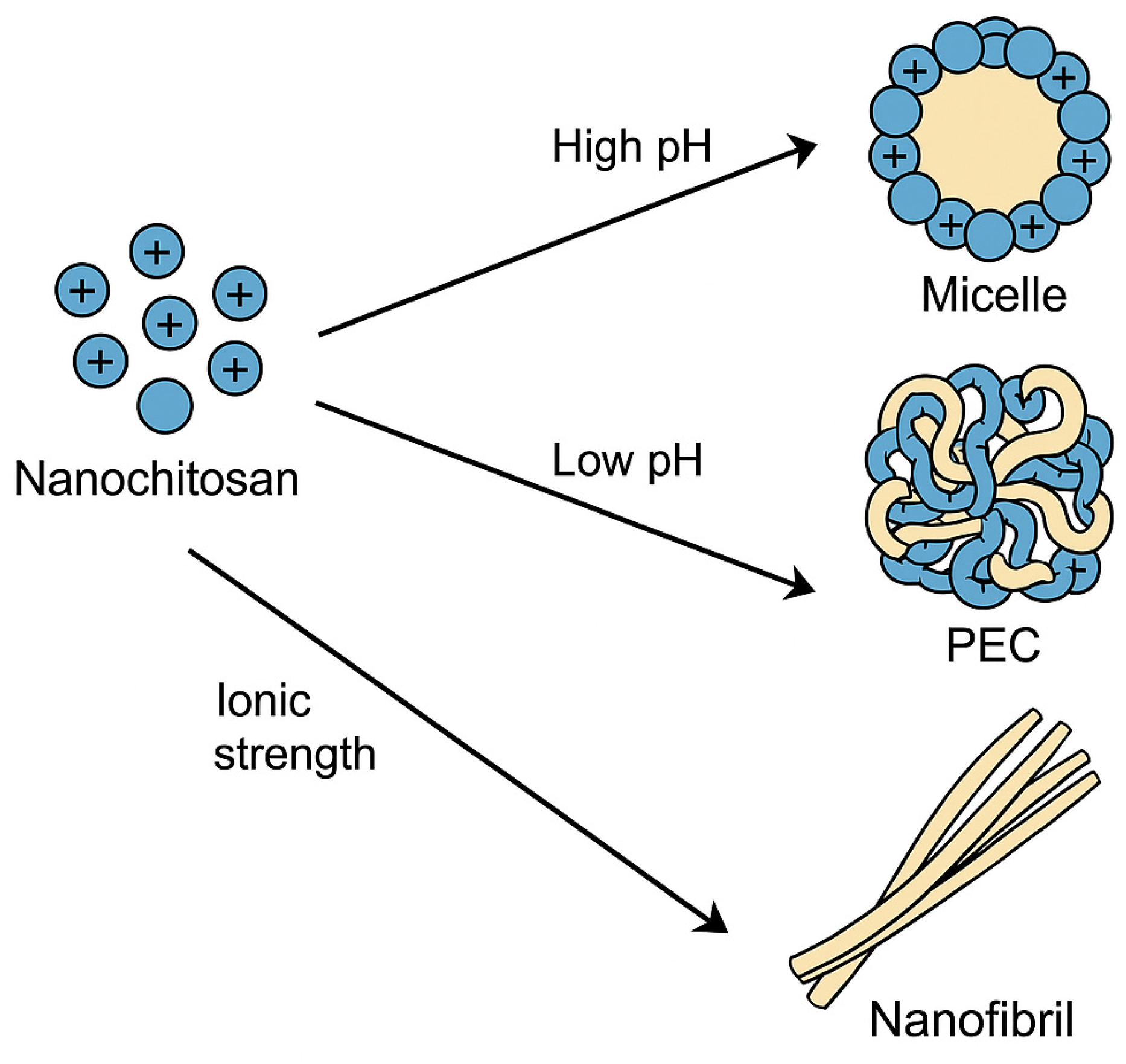
3.3.1. Self-Assembly
Nanochitosan
- Hydrophobic–Hydrophilic Balance: The ability of chitosan nanoparticles to self-assemble can be customized by adding hydrophobic portions to the gadolinium molecules. This balance helps to produce micelles that can encapsulate lipophilic therapeutic drugs, which could further improve drug delivery via non-invasive oral, nasal, pulmonary, and ocular routes [71].
- Polyelectrolyte Complexes: In another method, complexes of polyelectrolytes are formed with polyanions, which induces the self-assembly of the chitosan nanoparticles. Recent developments highlight these interpolyelectrolyte complexes (IPECs) consisting of fully biodegradable components, which significantly enhance biocompatibility and environmental sustainability. Applications of biodegradable IPECs include controlled drug release systems, targeted tissue engineering scaffolds, and innovative biomedical formulations. Notable advantages encompass reduced environmental impact, improved biocompatibility, and enhanced biodegradability, making them suitable for medical and pharmaceutical applications. Nevertheless, these biodegradable IPECs face certain challenges, such as limited stability under specific physiological conditions, potential rapid degradation rates, and difficulties related to scalability and reproducibility in industrial production processes. Addressing these limitations is crucial for broader clinical and commercial applications. This method significantly influences drug delivery systems by enhancing biodistribution while minimizing pharmacological toxicity [71].
Nanochitin
- Hierarchical Assemblies: Nanochitin can build hierarchical structures from the nano to macro scales. These assemblies increase the toughness and resistance of the material, making them appropriate for multi-component materials [6].
- Multiscale Interactions: The nanochitin’s native architecture enables multiscale interactions, which result in dynamic and functional structures. This aspect is important for delivering advanced materials that are more tunable and multipurpose [6].
- Self-assembly is influenced by pH, solvent polarity, and ionic strength [73,74,75]. Varying pH produces the unique morphologies discovered in chitosan-sodium alginate PECs. Fibrous structures develop at low pH (3 to 7), while at high pH (approximately eight and higher), colloidal nanoparticles are produced [74].
3.3.2. Biosynthetic Approaches: Fungi and Microbial
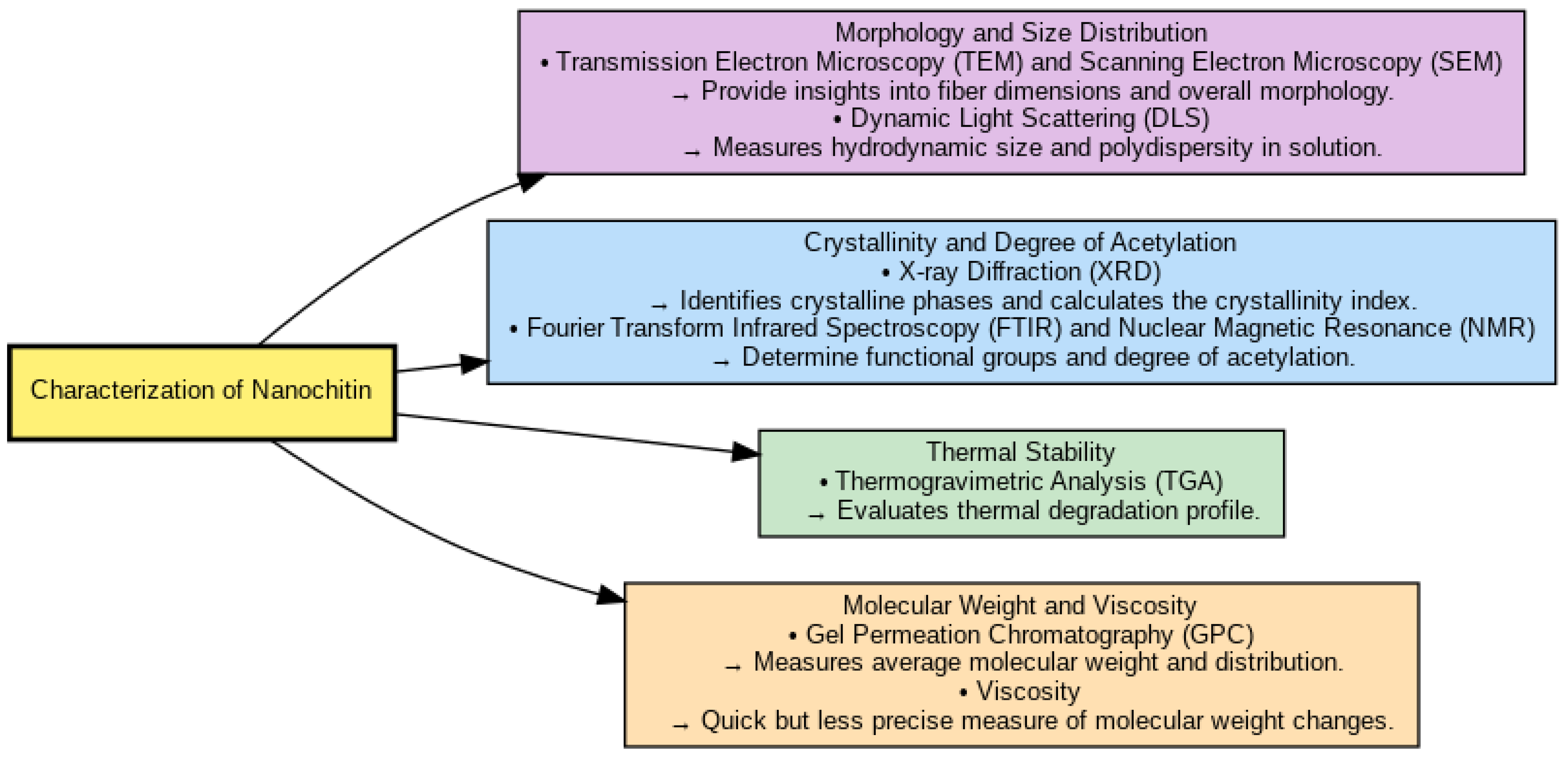
4. Characterization of Nanochitin and Nanochitosan
4.1. Morphology and Size Distribution
4.2. Crystallinity and Degree of Acetylation
4.3. Thermal Stability
4.4. Molecular Weight and Viscosity
5. Pharmaceutical Properties of Nanochitin and Nanochitosan
5.1. Biocompatibility and Biodegradability
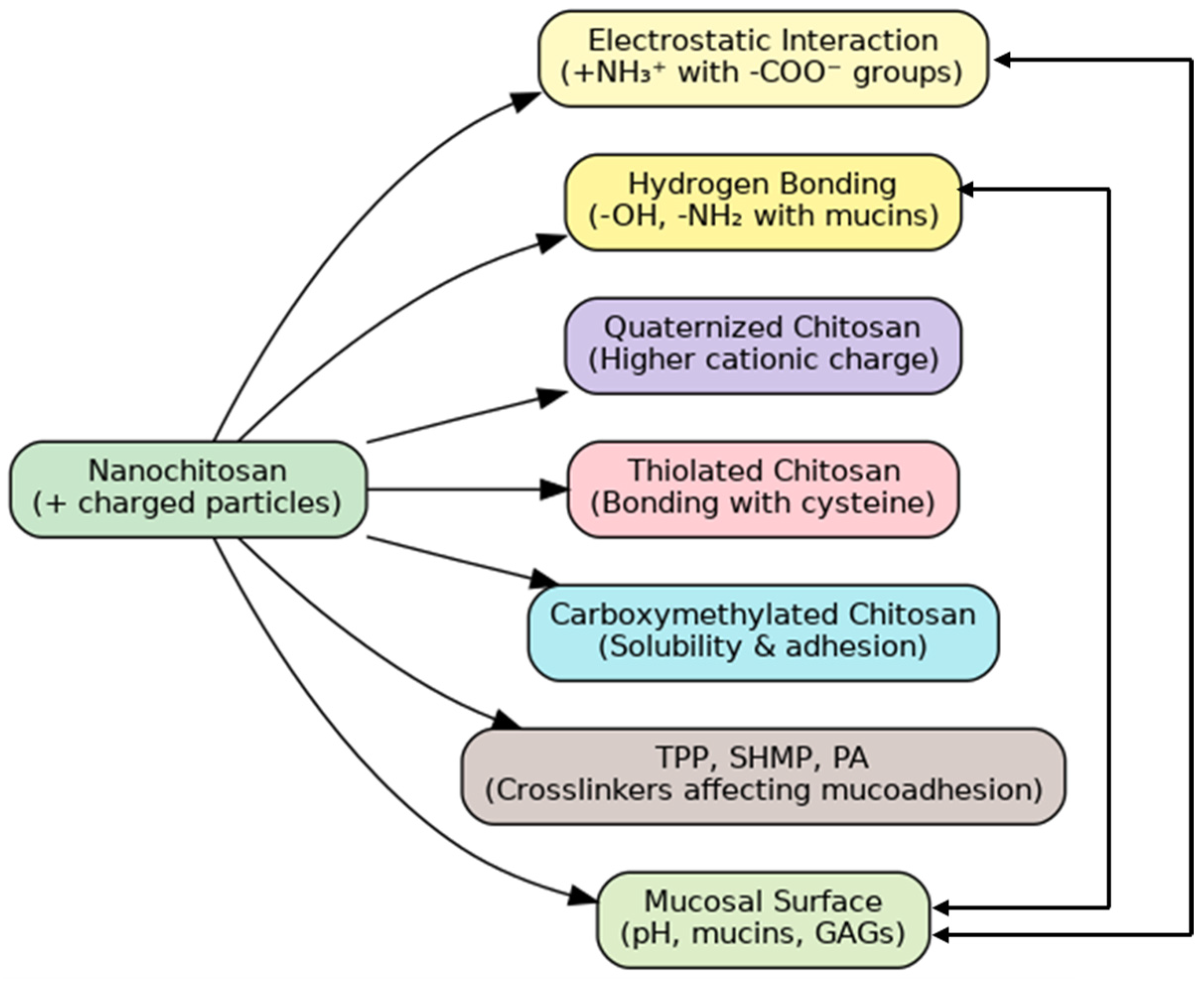
5.2. Mucoadhesive and Bioadhesive Characteristics
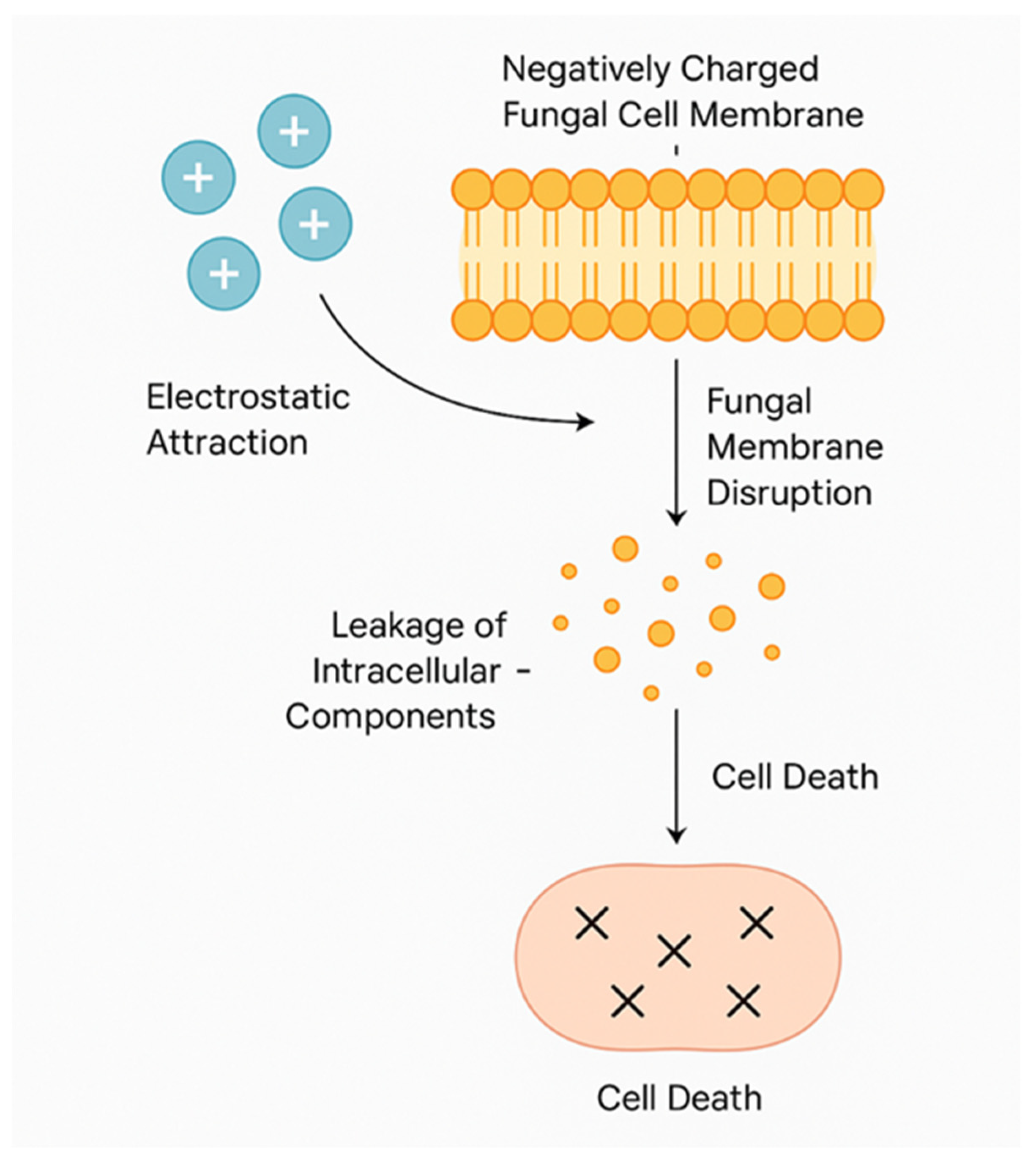
5.3. Antimicrobial, Antibacterial, and Immunomodulatory Effects
6. Nanochitin and Nanochitosan and Their Pharmaceutical Applications
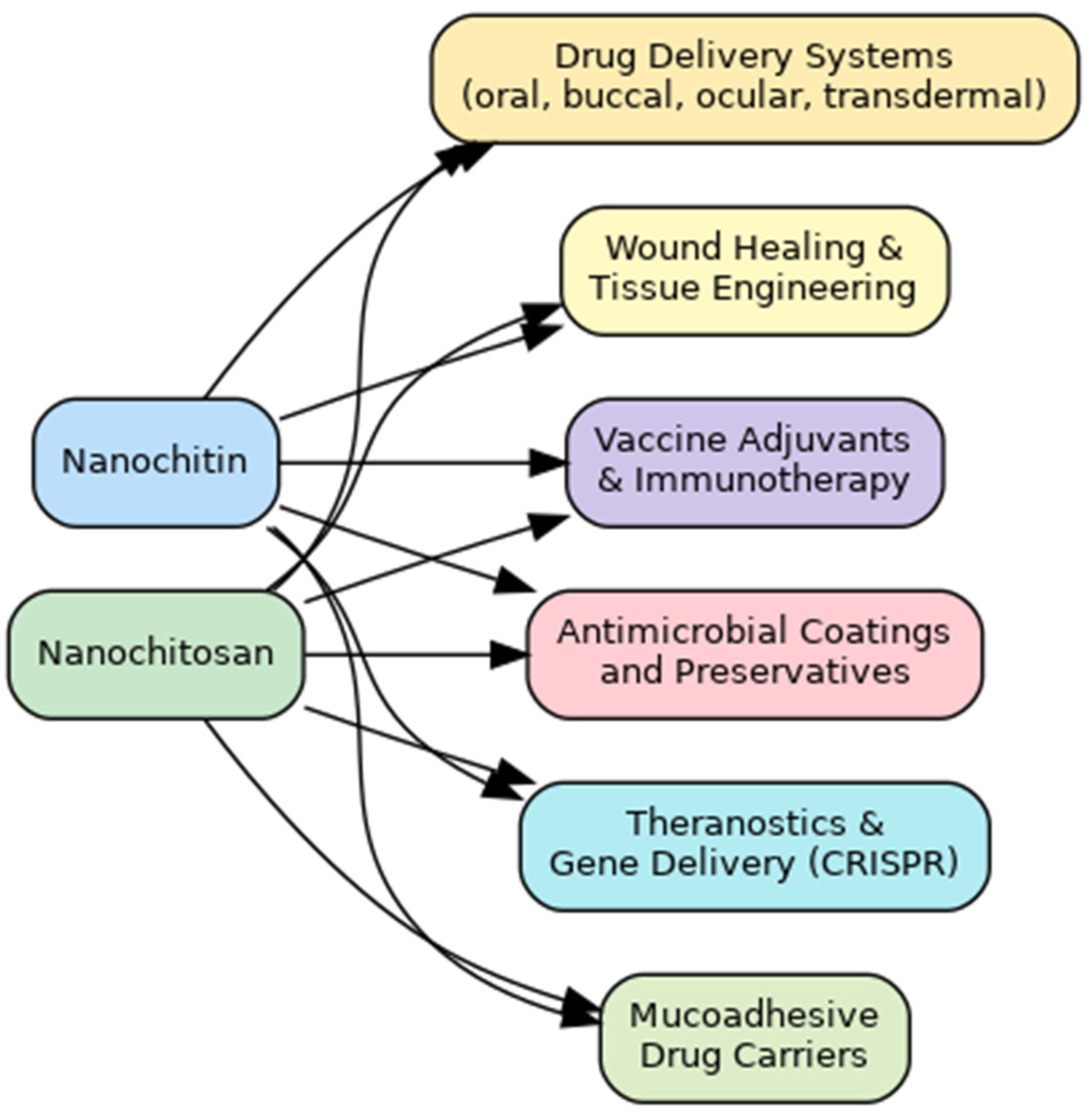
6.1. Nanochitin and Nanochitosan-Based Drug Delivery Systems
6.1.1. Oral and Buccal Delivery
6.1.2. Ocular Delivery
6.1.3. Transdermal Delivery
6.1.4. Targeted and Stimuli-Responsive Delivery
6.1.5. Additional Formulations and Dosage Forms
6.2. Tissue Regeneration and Wound Healing
6.3. Vaccine Adjuvants and Immunotherapy
6.4. Antimicrobial Formulations and Preservatives
6.5. Tissue Engineering Scaffolds
7. Toxicological and Regulatory Considerations
7.1. Toxicity Profile
7.2. Regulatory Framework
7.3. Environmental and Ethical Aspects
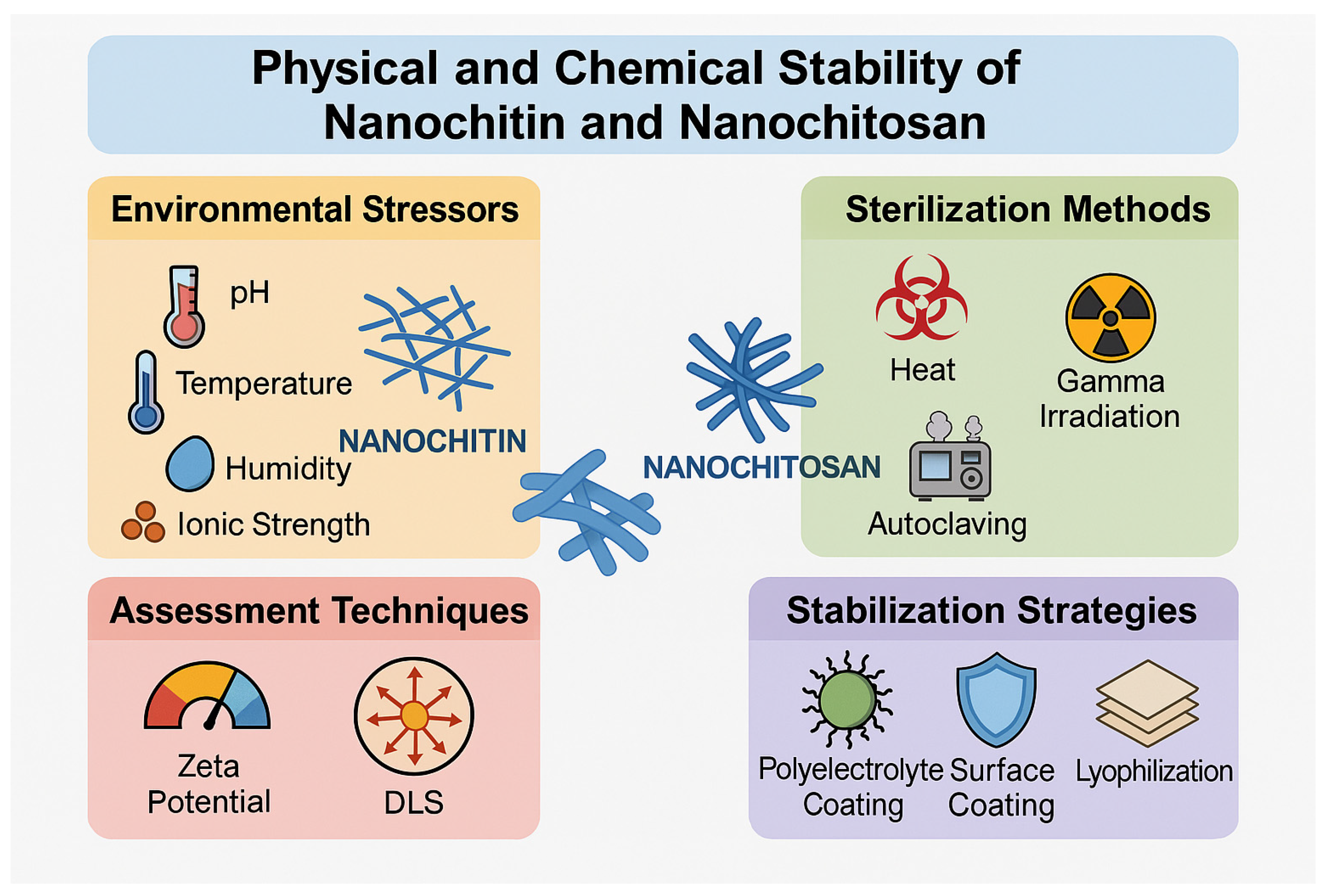
7.4. Physical and Chemical Stability
8. Looking Ahead: Issues and Perspectives
8.1. Scalability and Cost-Efficiency
8.2. Standardization and Quality Control
8.3. Multifunctional Systems
8.4. Personalized Medicine and Theranostics
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rinaudo, M. Chitin and Chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Ravi Kumar, M.N.V.; Muzzarelli, R.A.A.; Muzzarelli, C.; Sashiwa, H.; Domb, A.J. Chitosan chemistry and pharmaceutical perspectives. Chem. Rev. 2004, 104, 6017–6084. [Google Scholar] [CrossRef] [PubMed]
- Kurita, K. Controlled functionalization of the polysaccharide chitin. Prog. Polym. Sci. 2001, 26, 1921–1971. [Google Scholar] [CrossRef]
- Percot, A.; Viton, C.; Domard, A. Optimization of chitin extraction from shrimp shells. Biomacromolecules 2003, 4, 12–18. [Google Scholar] [CrossRef]
- Kasaai, M.R. Various methods for determination of the degree of N-acetylation of chitin and chitosan: A review. J. Agric. Food Chem. 2009, 57, 1667–1676. [Google Scholar] [CrossRef]
- Bai, L.; Liu, L.; Esquivel, M.; Tardy, B.; Huan, S.; Niu, X.; Liu, S.; Yang, G.; Fan, Y.; Rojas, O. Nanochitin: Chemistry, Structure, Assembly, and Applications. Chem. Rev. 2022, 122, 11604–11674. [Google Scholar] [CrossRef]
- Lee, S.; Hao, L.; Park, J.; Oh, D.; Hwang, D. Nanochitin and Nanochitosan: Chitin Nanostructure Engineering with Multiscale Properties for Biomedical and Environmental Applications. Adv. Mater. 2022, 35, 2203325. [Google Scholar] [CrossRef]
- Jin, T.; Liu, T.; Lam, E.; Moores, A. Chitin and Chitosan on the nanoscale. Nanoscale Horiz. 2021, 6, 1046–1061. [Google Scholar] [CrossRef]
- Liu, L.; Bai, L.; Tripathi, A.; Yu, J.; Wang, Z.; Borghei, M.; Fan, Y.; Rojas, O. High Axial Ratio Nanochitins for Ultrastrong and Shape-Recoverable Hydrogels and Cryogels via Ice Templating. ACS Nano 2019, 13, 2927–2935. [Google Scholar] [CrossRef]
- Joseph, B.; Mavelil Sam, R.; Balakrishnan, P.; Gopi, S.; Volova, T.; Thomas, S. Extraction of Nanochitin from Marine Resources and Fabrication of Polymer Nanocomposites: Recent Advances. Polymers 2020, 12, 1664. [Google Scholar] [CrossRef]
- Kean, T.; Thanou, M. Biodegradation, biodistribution, and toxicity of chitosan. Adv. Drug Deliv. Rev. 2010, 62, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Mohan, K.; Ganesan, A.; Muralisankar, T.; Jayakumar, R.; Sathishkumar, P.; Uthayakumar, V.; Chandirasekar, R.; Revathi, N. Recent insights into the extraction, characterization, and bioactivities of chitin and chitosan from insects. Trends Food Sci. Technol. 2020, 105, 17–42. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Merzendorfer, H.; Zhang, W.; Zhang, J.; Muthukrishnan, S. Biosynthesis, Turnover, and Functions of Chitin in Insects. Annu. Rev. Entomol. 2016, 61, 177–196. [Google Scholar] [CrossRef]
- Khayrova, A.; Lopatin, S.; Varlamov, V. Obtaining chitin, chitosan, and their melanin complexes from insects. Int. J. Biol. Macromol. 2020, 162, 318–329. [Google Scholar] [CrossRef]
- Rehman, K.; Hollah, C.; Wiesotzki, K.; Heinz, V.; Aganovic, K.; Rehman, R.; Petrusan, J.; Zheng, L.; Zhang, J.; Sohail, S.; et al. Insect-Derived Chitin and Chitosan: A Still Unexploited Resource for the Edible Insect Sector. Sustainability 2023, 15, 4864. [Google Scholar] [CrossRef]
- Nurfikari, A.; De Boer, W. Chitin Determination in Residual Streams Derived from Insect Production by LC-ECD and LC-MS/MS Methods. Front. Sustain. Food Syst. 2021, 5, 795694. [Google Scholar] [CrossRef]
- Da Silva Lucas, A.; Oreste, E.; Costa, H.; López, H.; Saad, C.; Prentice, C. Extraction, physicochemical characterization, and morphological properties of chitin and chitosan from cuticles of edible insects. Food Chem. 2020, 315, 128550. [Google Scholar] [CrossRef]
- Synowiecki, J.; Al-Khateeb, N. Production, properties, and some new applications of chitin and its derivatives. Crit. Rev. Food Sci. Nutr. 2003, 43, 145–171. [Google Scholar] [CrossRef]
- Brown, H.E.; Esher, S.K.; Alspaugh, J.A. Chitin: A “Hidden Figure” in the Fungal Cell Wall. Curr. Top. Microbiol. Immunol. 2020, 425, 83–111. [Google Scholar] [CrossRef]
- Lenardon, M.; Munro, C.; Gow, N. Chitin synthesis and fungal pathogenesis. Curr. Opin. Microbiol. 2010, 13, 416–423. [Google Scholar] [CrossRef]
- Wagener, J.; Malireddi, R.K.; Lenardon, M.D.; Köberle, M.; Vautier, S.; MacCallum, D.M.; Biedermann, T.; Schaller, M.; Netea, M.G.; Kanneganti, T.D.; et al. Fungal chitin dampens inflammation through IL-10 induction mediated by NOD2 and TLR9 activation. PLoS Pathog. 2014, 10, e1004050. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, C.; Tang, C.; He, Q.; Li, N.; Li, J. Dysbiosis of Gut Fungal Microbiota is Associated with Mucosal Inflammation in Crohn’s Disease. J. Clin. Gastroenterol. 2014, 48, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, C.; Tang, C.; Li, N.; Li, J. Molecular-Phylogenetic Characterization of the Microbiota in Ulcerated and Non-Ulcerated Regions in Patients with Crohn’s Disease. PLoS ONE 2012, 7, e34939. [Google Scholar] [CrossRef]
- Brauer, V.; Pessoni, A.; Freitas, M.; Cavalcanti-Neto, M.; Ries, L.; Almeida, F. Chitin Biosynthesis in Aspergillus Species. J. Fungi 2023, 9, 89. [Google Scholar] [CrossRef]
- Lafi, A.; Santhanam, J.; Khaithir, T.; Musa, N.; Huyop, F. Evaluation of Chitin as a Biomarker of Pathogenic Fungal Isolates. Sains Malays. 2021, 50, 735–742. [Google Scholar] [CrossRef]
- El-Feki, K.; El-Metwally, M.; Mohammed, Y.; Ahmed, T. Production of chitin from some soil fungi. J. Agric. Environ. Sci. 2022, 21, 131189. [Google Scholar] [CrossRef]
- Ghormade, V.; Pathan, E.; Deshpande, M. Can fungi compete with marine sources for chitosan production? Int. J. Biol. Macromol. 2017, 104, 1415–1421. [Google Scholar] [CrossRef]
- Kozma, M.; Acharya, B.; Bissessur, R. Chitin, Chitosan, and Nanochitin: Extraction, Synthesis, and Applications. Polymers 2022, 14, 3989. [Google Scholar] [CrossRef]
- Shimahara, K.; Takiguchi, Y. Preparation of Crustacean Chitin. Methods Enzymol. 1988, 161, 417–423. [Google Scholar] [CrossRef]
- Arbia, W.; Arbia, L.; Adour, L.; Amrane, A. Chitin Extraction from Crustacean Shells Using Biological Methods—A Review. Food Technol. Biotechnol. 2013, 51, 12–25. [Google Scholar]
- Huang, W.; Zhao, D.; Guo, N.; Xue, C.; Mao, X. Green and Facile Production of Chitin from Crustacean Shells Using a Natural Deep Eutectic Solvent. J. Agric. Food Chem. 2018, 66, 11897–11901. [Google Scholar] [CrossRef]
- Trung, T.; Tram, L.; Van Tan, N.; Van Hoa, N.; Minh, N.; Loc, P.; Stevens, W. Improved method for production of chitin and chitosan from shrimp shells. Carbohydr. Res. 2020, 489, 107913. [Google Scholar] [CrossRef] [PubMed]
- Hisham, F.; Akmal, M.; Ahmad, F.; Ahmad, K. Facile extraction of chitin and chitosan from shrimp shell. Mater. Today Proc. 2021, 36, 151–158. [Google Scholar] [CrossRef]
- Abdel-Rahman, R.; Hrdina, R.; Abdel-Mohsen, A.; Fouda, M.; Soliman, A.; Mohamed, F.; Mohsin, K.; Pinto, T. Chitin and chitosan from Brazilian Atlantic Coast: Isolation, characterization and antibacterial activity. Int. J. Biol. Macromol. 2015, 80, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Aneesh, P.; Anandan, R.; Kumar, L.; Ajeeshkumar, K.; Kumar, K.; Mathew, S. A step to shell biorefinery—Extraction of astaxanthin-rich oil, protein, chitin, and chitosan from shrimp processing waste. Biomass Convers. Biorefin. 2020, 13, 205–214. [Google Scholar] [CrossRef]
- Yang, H.; Gözaydın, G.; Nasaruddin, R.; Har, J.; Chen, X.; Wang, X.; Yan, N. Toward the Shell Biorefinery: Processing Crustacean Shell Waste Using Hot Water and Carbonic Acid. ACS Sustain. Chem. Eng. 2019, 7, 18002–18011. [Google Scholar] [CrossRef]
- Vallejo-Domínguez, D.; Rubio-Rosas, E.; Águila-Almanza, E.; Hernández-Cocoletzi, H.; Ramos-Cassellis, M.; Luna-Guevara, M.; Rambabu, K.; Manickam, S.; Munawaroh, H.; Show, P. Ultrasound in the deproteinization process for chitin and chitosan production. Ultrason. Sonochem. 2020, 72, 105417. [Google Scholar] [CrossRef]
- Kumari, S.; Annamareddy, S.; Abanti, S.; Rath, P. Physicochemical properties and characterization of chitosan synthesized from fish scales, crab, and shrimp shells. Int. J. Biol. Macromol. 2017, 104, 1697–1705. [Google Scholar] [CrossRef]
- Pădurețu, C.; Isopescu, R.; Rău, I.; Apetroaei, M.; Schröder, V. Influence of the parameters of chitin deacetylation process on the chitosan obtained from crab shell waste. Korean J. Chem. Eng. 2019, 36, 1890–1899. [Google Scholar] [CrossRef]
- Yadav, M.; Goswami, P.; Paritosh, K.; Kumar, M.; Pareek, N.; Vivekanand, V. Seafood waste: A source for preparation of commercially employable chitin/chitosan materials. Bioresour. Bioprocess. 2019, 6, 8. [Google Scholar] [CrossRef]
- Fernández-Marín, R.; Hernandez-Ramos, F.; Salaberria, A.; Sánchez, M.; Labidi, J.; Fernandes, S. Eco-friendly isolation and characterization of nanochitin from different origins by microwave irradiation: Optimization using response surface methodology. Int. J. Biol. Macromol. 2021, 191, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Goy, R.C.; Britto, D.; Assis, O.B.G. A review of the antimicrobial activity of chitosan. Polímeros 2009, 19, 241–247. [Google Scholar] [CrossRef]
- Minke, R.; Blackwell, J. The structure of α-chitin. J. Mol. Biol. 1978, 120, 167–181. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, N.; Garnica-González, M.; Ramírez-Hernández, J.Y.; Flores-Albino, B.; Gimeno, M. Effect of temperature on chitin and astaxanthin recoveries from shrimp waste using lactic acid bacteria. Bioresour. Technol. 2009, 100, 2849–2854. [Google Scholar] [CrossRef]
- Ahmad, S.; Ahmad, R.; Khan, M.; Kant, R.; Shahid, S.; Gautam, L.; Hasan, G.; Hassan, M. Chitin and its derivatives: Structural properties and biomedical applications. Int. J. Biol. Macromol. 2020, 164, 526–539. [Google Scholar] [CrossRef]
- Muxika, A.; Etxabide, A.; Uranga, J.; Guerrero, P.; Caba, K. Chitosan as a bioactive polymer: Processing, properties, and applications. Int. J. Biol. Macromol. 2017, 105, 1358–1368. [Google Scholar] [CrossRef]
- Brigham, C. Chitin and Chitosan: Sustainable, Medically Relevant Biomaterials. Int. J. Biotechnol. Wellness Ind. 2017, 6, 41–47. [Google Scholar] [CrossRef]
- Chen, X.; Yang, H.; Yan, N. Shell Biorefinery: Dream or Reality? Chem. Eur. J. 2016, 22, 13402–13421. [Google Scholar] [CrossRef]
- Kumar, M.N.V.R. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Pillai, C.K.S.; Paul, W.; Sharma, C.P. Chitin and chitosan polymers: Chemistry, solubility and fiber formation. Prog. Polym. Sci. 2009, 34, 641–678. [Google Scholar] [CrossRef]
- Raafat, D.; Sahl, H. Chitosan and its antimicrobial potential—A critical literature survey. Microb. Biotechnol. 2009, 2, 186–201. [Google Scholar] [CrossRef] [PubMed]
- Sorlier, P.; Denuzière, A.; Viton, C.; Domard, A. Relation between the degree of acetylation and the electrostatic properties of chitin and chitosan. Biomacromolecules 2001, 2, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.; Reist, M.; Mayer, J.M.; Felt, O.; Gurny, R. Structure and interactions in chitosan hydrogels formed by complexation or aggregation for biomedical applications. Eur. J. Pharm. Biopharm. 2004, 57, 35–52. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, R.; Ramachandran, R.; Divyarani, V.; Chennazhi, K.; Tamura, H.; Nair, S. Fabrication of chitin-chitosan/nano TiO2-composite scaffolds for tissue engineering applications. Int. J. Biol. Macromol. 2011, 48, 336–344. [Google Scholar] [CrossRef]
- Ifuku, S.; Ikuta, A.; Izawa, H.; Morimoto, M.; Saimoto, H. Control of mechanical properties of chitin nanofiber film using glycerol without losing its characteristics. Carbohydr. Polym. 2014, 101, 714–717. [Google Scholar] [CrossRef]
- Baharlouei, P.; Rahman, A. Chitin and Chitosan: Prospective Biomedical Applications in Drug Delivery, Cancer Treatment, and Wound Healing. Mar. Drugs 2022, 20, 460. [Google Scholar] [CrossRef]
- Fathi, M.; Majidi, S.; Zangabad, P.; Barar, J.; Erfan-Niya, H.; Omidi, Y. Chitosan-based multifunctional nanomedicines and theranostics for targeted therapy of cancer. Med. Res. Rev. 2018, 38, 2110–2136. [Google Scholar] [CrossRef]
- Ali, A.; Ahmed, S. A review on chitosan and its nanocomposites in drug delivery. Int. J. Biol. Macromol. 2018, 109, 273–286. [Google Scholar] [CrossRef]
- Reshmi, C.R.; Suja, P.S.; Manaf, O.; Sanu, P.P.; Sujith, A. Nanochitosan enriched poly ε-caprolactone electrospun wound dressing membranes: A fine tuning of physicochemical properties, hemocompatibility and curcumin release profile. Int. J. Biol. Macromol. 2018, 108, 1261–1272. [Google Scholar] [CrossRef]
- Pratiwi, R.; Muttaqien, S.; Gustini, N.; Difa, N.; Syahputra, G.; Rosyidah, A. Eco-friendly synthesis of chitosan and its medical application: From chitin extraction to nanoparticle preparation. ADMET DMPK 2023, 11, 435–455. [Google Scholar] [CrossRef]
- Liu, L.; Chenhuang, J.; Lu, Y.; Fan, Y.; Wang, Z. Facile preparation of nanochitins via acid assisted colloid milling in glycerol. Cellulose 2020, 27, 7655–7664. [Google Scholar] [CrossRef]
- Pereira, A.; Muniz, E.; Hsieh, Y. Chitosan-sheath and chitin-core nanowhiskers. Carbohydr. Polym. 2014, 107, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, C.; Wang, Q.; Zhao, Z. Efficient hydrolysis of chitosan in ionic liquids. Carbohydr. Polym. 2009, 78, 685–689. [Google Scholar] [CrossRef]
- Gonçalves, C.; Ferreira, N.; Lourenço, L. Production of Low Molecular Weight Chitosan and Chitooligosaccharides (COS): A Review. Polymers 2021, 13, 2466. [Google Scholar] [CrossRef]
- Zewude, D.; Izawa, H.; Ifuku, S. Optimization of Chitin Nanofiber Preparation by Ball Milling as Filler for Composite Resin. J. Compos. Sci. 2022, 6, 197. [Google Scholar] [CrossRef]
- Aklog, Y.; Nagae, T.; Izawa, H.; Morimoto, M.; Saimoto, H.; Ifuku, S. Preparation of chitin nanofibers by surface esterification of chitin with maleic anhydride and mechanical treatment. Carbohydr. Polym. 2016, 153, 55–59. [Google Scholar] [CrossRef]
- Tang, E.; Huang, M.; Lim, L. Ultrasonication of chitosan and chitosan nanoparticles. Int. J. Pharm. 2003, 265, 103–114. [Google Scholar] [CrossRef]
- Zewude, D.; Izawa, H.; Ifuku, S. Optimum Preparation Conditions for Highly Individualized Chitin Nanofibers Using Ultrasonic Generator. Polymers 2021, 13, 2501. [Google Scholar] [CrossRef]
- Zhao, H.; Feng, X.; Gao, H. Ultrasonic technique for extracting nanofibers from nature materials. Appl. Phys. Lett. 2007, 90, 073112. [Google Scholar] [CrossRef]
- Ogawa, K.; Yui, T.; Okuyama, K. Three D structures of chitosan. Int. J. Biol. Macromol. 2004, 34, 1–8. [Google Scholar] [CrossRef]
- Quiñones, J.; Peniche, H.; Peniche, C. Chitosan Based Self-Assembled Nanoparticles in Drug Delivery. Polymers 2018, 10, 235. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yang, Z.; He, Z.; Zhou, C.; Wang, C.; Chen, Y.; Liu, X.; Li, S.; Li, P. Self-assembled amphiphilic chitosan nanomicelles to enhance the solubility of quercetin for efficient delivery. Colloids Surf. B Biointerfaces 2019, 179, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Montroni, D.; Marzec, B.; Valle, F.; Nudelman, F.; Falini, G. β-Chitin Nanofibril Self-Assembly in Aqueous Environments. Biomacromolecules 2019, 20, 2421–2429. [Google Scholar] [CrossRef] [PubMed]
- Wasupalli, G.; Verma, D. Molecular interactions in self-assembled nanostructures of chitosan-sodium alginate-based polyelectrolyte complexes. Int. J. Biol. Macromol. 2018, 114, 10–17. [Google Scholar] [CrossRef]
- Dey, A.; Kamat, A.; Nayak, S.; Danino, D.; Kesselman, E.; Dandekar, P.; Jain, R. Role of proton balance in formation of self-assembled chitosan nanoparticles. Colloids Surf. B Biointerfaces 2018, 166, 127–134. [Google Scholar] [CrossRef]
- Mane, S.; Pathan, E.; Tupe, S.; Deshmukh, S.; Kale, D.; Ghormade, V.; Chaudhari, B.; Deshpande, M. Isolation and Characterization of Chitosans from Different Fungi with Special Emphasis on Zygomycetous Dimorphic Fungus Benjaminiella poitrasii: Evaluation of Its Chitosan Nanoparticles for the Inhibition of Human Pathogenic Fungi. Biomacromolecules 2022, 23, 1503–1515. [Google Scholar] [CrossRef]
- Liao, J.; Huang, H. Preparation, Characterization and Gelation of a Fungal Nano Chitin Derived from Hericium erinaceus Residue. Polymers 2022, 14, 474. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Chelliah, R.; Mubarak Ali, D.; Jeevithan, E.; Oh, D.; Kathiresan, K.; Wang, M. Fungal enzyme-mediated synthesis of chitosan nanoparticles and its biocompatibility, antioxidant and bactericidal properties. Int. J. Biol. Macromol. 2018, 118, 1542–1549. [Google Scholar] [CrossRef]
- Ogura, K.; Brasselet, C.; Cabrera-Barjas, G.; Hamidi, M.; Shavandi, A.; Dols-Lafargue, M.; Sawamura, N.; Delattre, C. Production of Fungal Nanochitosan Using High-Pressure Water Jet System for Biomedical Applications. Materials 2022, 15, 1375. [Google Scholar] [CrossRef]
- Hermosilla, E.; Díaz, M.; Vera, J.; Contreras, M.; Leal, K.; Salazar, R.; Barrientos, L.; Tortella, G.; Rubilar, O. Synthesis of Antimicrobial Chitosan-Silver Nanoparticles Mediated by Reusable Chitosan Fungal Beads. Int. J. Mol. Sci. 2023, 24, 2318. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A. New Techniques for Optimization of Surface Area and Porosity in Nanochitins and Nanochitosans. In Chitosan for Biomaterials II. Advances in Polymer Science; Jayakumar, R., Prabaharan, M., Muzzarelli, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; Volume 244, pp. 167–186. [Google Scholar] [CrossRef]
- Rashki, S.; Asgarpour, K.; Tarrahimofrad, H.; Hashemipour, M.; Ebrahimi, M.; Fathizadeh, H.; Khorshidi, A.; Khan, H.; Marzhoseyni, Z.; Salavati-Niasari, M.; et al. Chitosan-based nanoparticles against bacterial infections. Carbohydr. Polym. 2021, 251, 117108. [Google Scholar] [CrossRef] [PubMed]
- Chouhan, D.; Mandal, P. Applications of chitosan and chitosan-based metallic nanoparticles in agrosciences—A review. Int. J. Biol. Macromol. 2020, 151, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Santiago, T.; Bonatto, C.; Rossato, M.; Lopes, C.; Lopes, C.; Mizubuti, E.; Silva, L. Green synthesis of silver nanoparticles using tomato leaf extract and their entrapment in chitosan nanoparticles to control bacterial wilt. J. Sci. Food Agric. 2019, 99, 4248–4259. [Google Scholar] [CrossRef]
- Wongpreecha, J.; Polpanich, D.; Suteewong, T.; Kaewsaneha, C.; Tangboriboonrat, P. One-pot, large-scale green synthesis of silver nanoparticles-chitosan with enhanced antibacterial activity and low cytotoxicity. Carbohydr. Polym. 2018, 199, 641–648. [Google Scholar] [CrossRef]
- Chen, S.X.; Han, Y.; Wei, Y.; Guo, Q.; Yang, S.; Zhang, Y.; Liao, W.; Gao, Y. Effect of chitosan molecular weight on zein-chitosan nanocomplexes: Formation, characterization, and the delivery of quercetagetin. Int. J. Biol. Macromol. 2020, 161, 1294–1305. [Google Scholar] [CrossRef]
- Alves, H.; Gasparrini, L.; Silva, F.; Caciano, L.; De Muniz, G.; Ballester, E.; Cremonez, P.; Arantes, M. Alternative methods for the pilot-scale production and characterization of chitosan nanoparticles. Environ. Sci. Pollut. Res. 2021, 28, 10977–10987. [Google Scholar] [CrossRef]
- Maleki, A.; Firouzi-Haji, R.; Hajizadeh, Z. Magnetic guanidinylated chitosan nanobiocomposite: A green catalyst for the synthesis of 1,4-dihydropyridines. Int. J. Biol. Macromol. 2018, 116, 320–326. [Google Scholar] [CrossRef]
- Brugnerotto, J.; Lizardi, J.; Goycoolea, F.; Argüelles-Monal, W.; Desbrières, J.; Rinaudo, M. An infrared investigation in relation with chitin and chitosan characterization. Polymer 2001, 42, 3569–3580. [Google Scholar] [CrossRef]
- Pereira, A.; Muniz, E.; Hsieh, Y. ¹H NMR and ¹H-¹³C HSQC surface characterization of chitosan-chitin sheath-core nanowhiskers. Carbohydr. Polym. 2015, 123, 46–52. [Google Scholar] [CrossRef]
- Kasaai, M. Determination of the degree of N-acetylation for chitin and chitosan by various NMR spectroscopy techniques: A review. Carbohydr. Polym. 2010, 79, 801–810. [Google Scholar] [CrossRef]
- Chee, P.; Sathasivam, T.; Tan, Y.; Wu, W.; Leow, Y.; Lim, Q.; Yew, P.; Zhu, Q.; Kai, D. Nanochitin for sustainable and advanced manufacturing. Nanoscale 2024, 16, 364–376. [Google Scholar] [CrossRef] [PubMed]
- Corazzari, I.; Nisticò, R.; Turci, F.; Faga, M.; Franzoso, F.; Tabasso, S.; Magnacca, G. Advanced physico-chemical characterization of chitosan by means of TGA coupled on-line with FTIR and GCMS: Thermal degradation and water adsorption capacity. Polym. Degrad. Stab. 2015, 112, 1–9. [Google Scholar] [CrossRef]
- Sugashini, S.; Gomathi, T.; Devi, R.; Sudha, P.; Rambabu, K.; Banat, F. Nanochitosan/carboxymethyl cellulose/TiO2 biocomposite for visible-light-induced photocatalytic degradation of crystal violet dye. Environ. Res. 2021, 200, 112047. [Google Scholar] [CrossRef]
- Fiamingo, A.; Delezuk, J.; Trombotto, S.; David, L.; Campana-Filho, S. Extensively deacetylated high molecular weight chitosan from the multistep ultrasound-assisted deacetylation of beta-chitin. Ultrason. Sonochem. 2016, 32, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Weißpflog, J.; Vehlow, D.; Müller, M.; Kohn, B.; Scheler, U.; Boye, S.; Schwarz, S. Characterization of chitosan with different degree of deacetylation and equal viscosity in dissolved and solid state—Insights by various complimentary methods. Int. J. Biol. Macromol. 2021, 168, 555–567. [Google Scholar] [CrossRef]
- González-Espinosa, Y.; Sabagh, B.; Moldenhauer, E.; Clarke, P.; Goycoolea, F. Characterisation of Chitosan molecular weight distribution by multi-detection asymmetric flow-field flow fractionation (AF4) and SEC. Int. J. Biol. Macromol. 2019, 136, 911–919. [Google Scholar] [CrossRef]
- Rigaux, G.; Gheran, C.; Callewaert, M.; Cadiou, C.; Voicu, S.; Dinischiotu, A.; Andry, M.; Elst, V.; Laurent, S.; Muller, R.; et al. Characterization of Gd loaded chitosan-TPP nanohydrogels by a multi-technique approach combining dynamic light scattering (DLS), asymetrical flow-field-flow-fractionation (AF4) and atomic force microscopy (AFM) and design of positive contrast agents for molecular resonance imaging (MRI). Nanotechnology 2017, 28, 055701. [Google Scholar] [CrossRef]
- Kang, Y.; Wu, X.; Ji, X.; Bo, S.; Liu, Y. Strategy to improve the characterization of chitosan by size exclusion chromatography coupled with multi-angle laser light scattering. Carbohydr. Polym. 2019, 202, 99–105. [Google Scholar] [CrossRef]
- Birolli, W.; Delezuk, J.; Campana-Filho, S. Ultrasound-assisted conversion of alpha-chitin into chitosan. Appl. Acoust. 2016, 103, 239–242. [Google Scholar] [CrossRef]
- Weska, R.; Moura, J.; De Moraes Batista, L.; Rizzi, J.; Pinto, L. Optimization of deacetylation in the production of chitosan from shrimp wastes: Use of response surface methodology. J. Food Eng. 2007, 80, 749–753. [Google Scholar] [CrossRef]
- Karakuş, S. Preparation and rheological characterization of Chitosan-Gelatine@ZnO-Si nanoparticles. Int. J. Biol. Macromol. 2019, 138, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Tsaih, M. Effect of temperature on the intrinsic viscosity and conformation of chitosans in dilute HCl solution. Int. J. Biol. Macromol. 1998, 23, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Abuzaytoun, R. Chitin, chitosan, and co-products: Chemistry, production, applications, and health effects. Adv. Food Nutr. Res. 2005, 49, 93–135. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, S.; Cuie, Y.; Winnik, F.; Shi, Q.; Lavigne, P.; Benderdour, M.; Fernandes, J.C. Chitosan-DNA nanoparticles as non-viral vectors in gene therapy: Strategies to improve transfection efficacy. Int. J. Biol. Macromol. 2013, 50, 111–119. [Google Scholar] [CrossRef]
- Agnihotri, S.A.; Mallikarjuna, N.N.; Aminabhavi, T.M. Recent advances on chitosan-based micro- and nanoparticles in drug delivery. J. Control. Release 2004, 100, 5–28. [Google Scholar] [CrossRef]
- Ways, T.; Lau, W.; Khutoryanskiy, V. Chitosan and Its Derivatives for Application in Mucoadhesive Drug Delivery Systems. Polymers 2018, 10, 267. [Google Scholar] [CrossRef]
- Kim, E.; Kim, D.; Lee, J.; Lee, H. Mucoadhesive chitosan-gum arabic nanoparticles enhance the absorption and antioxidant activity of quercetin in the intestinal cellular environment. J. Agric. Food Chem. 2019, 67, 4682–4691. [Google Scholar] [CrossRef]
- Sang, Z.; Qian, J.; Han, J.; Deng, X.; Shen, J.; Li, G.; Xie, Y. Comparison of three water-soluble polyphosphate tripolyphosphate, phytic acid, and sodium hexametaphosphate as crosslinking agents in chitosan nanoparticle formulation. Carbohydr. Polym. 2020, 230, 115577. [Google Scholar] [CrossRef]
- Abruzzo, A.; Giordani, B.; Miti, A.; Vitali, B.; Zuccheri, G.; Cerchiara, T.; Luppi, B.; Bigucci, F. Mucoadhesive and mucopenetrating chitosan nanoparticles for glycopeptide antibiotic administration. Int. J. Pharm. 2021, 604, 120874. [Google Scholar] [CrossRef]
- Hejjaji, E.; Smith, A.; Morris, G. Evaluation of the mucoadhesive properties of chitosan nanoparticles prepared using different chitosan to tripolyphosphate (CS:TPP) ratios. Int. J. Biol. Macromol. 2018, 120, 1610–1617. [Google Scholar] [CrossRef]
- Coutinho, A.; Lima, S.; Afonso, C.; Reis, S. Mucoadhesive and pH responsive fucoidan-chitosan nanoparticles for the oral delivery of methotrexate. Int. J. Biol. Macromol. 2020, 158, 1286–1297. [Google Scholar] [CrossRef] [PubMed]
- Stie, M.; Gätke, J.; Wan, F.; Chronakis, I.; Jacobsen, J.; Nielsen, H. Swelling of mucoadhesive electrospun chitosan/polyethylene oxide nanofibers facilitates adhesion to the sublingual mucosa. Carbohydr. Polym. 2020, 242, 116428. [Google Scholar] [CrossRef] [PubMed]
- Denora, N.; Lopedota, A.; Perrone, M.; Laquintana, V.; Iacobazzi, R.; Milella, A.; Fanizza, E.; Depalo, N.; Cutrignelli, A.; Lopalco, A.; et al. Spray-dried mucoadhesives for intravesical drug delivery using N-acetylcysteine- and glutathione-glycol chitosan conjugates. Acta Biomater. 2016, 43, 170–184. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Cabe, M.; Nowak, H.; Langert, K. Chitosan/poly(lactic-co-glycolic) acid Nanoparticle Formulations with Finely Tuned Size Distributions for Enhanced Mucoadhesion. Pharmaceutics 2022, 14, 95. [Google Scholar] [CrossRef]
- Jin, J.; Lee, D.; Im, H.G.; Han, Y.C.; Jeong, E.G.; Rolandi, M.; Choi, K.C.; Bae, B.S. Chitin Nanofiber Transparent Paper for Flexible Green Electronics. Adv. Mater. 2016, 28, 5169–5175. [Google Scholar] [CrossRef]
- Divya, K.; Vijayan, S.; George, T.; Jisha, M. Antimicrobial properties of chitosan nanoparticles: Mode of action and factors affecting activity. Fibers Polym. 2017, 18, 221–230. [Google Scholar] [CrossRef]
- Garrido-Maestu, A.; Ma, Z.; Paik, S.; Chen, N.; Ko, S.; Tong, Z.; Jeong, K. Engineering of chitosan-derived nanoparticles to enhance antimicrobial activity against foodborne pathogen Escherichia coli O157:H7. Carbohydr. Polym. 2018, 197, 623–630. [Google Scholar] [CrossRef]
- Alqahtani, F.; Aleanizy, F.; Tahir, E.; Alowais, H.; Binkelaib, A.; Alwathlan, B.; Al-Bdrawy, A.; Hakansson, A.; Alsarra, I. Capsule Independent Antimicrobial Activity Induced by Nanochitosan against Streptococcus pneumoniae. Polymers 2021, 13, 2924. [Google Scholar] [CrossRef]
- Marangon, C.; Martins, V.; Ling, M.; Melo, C.; Plepis, A.; Meyer, R.; Nitschke, M. Combination of rhamnolipid and chitosan in nanoparticles boosts their antimicrobial efficacy. ACS Appl. Mater. Interfaces 2020, 12, 55016–55027. [Google Scholar] [CrossRef]
- Yu, H.; Ma, Z.; Meng, S.; Qiao, S.; Zeng, X.; Tong, Z.; Jeong, K. A novel nanohybrid antimicrobial based on chitosan nanoparticles and antimicrobial peptide microcin J25 with low toxicity. Carbohydr. Polym. 2021, 253, 117309. [Google Scholar] [CrossRef]
- Perinelli, D.; Fagioli, L.; Campana, R.; Lam, J.; Baffone, W.; Palmieri, G.; Casettari, L.; Bonacucina, G. Chitosan-based nanosystems and their exploited antimicrobial activity. Eur. J. Pharm. Sci. 2018, 117, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Ng, W.; Shen, S.; Kim, S.; Tan, R. Scalable ionic gelation synthesis of chitosan nanoparticles for drug delivery in static mixers. Carbohydr. Polym. 2013, 94, 940–945. [Google Scholar] [CrossRef] [PubMed]
- Van Bavel, N.; Issler, T.; Pang, L.; Anikovskiy, M.; Prenner, E.J. A Simple Method for Synthesis of Chitosan Nanoparticles with Ionic Gelation and Homogenization. Molecules 2023, 28, 4328. [Google Scholar] [CrossRef] [PubMed]
- Alehosseini, E.; Tabarestani, H.; Kharazmi, M.; Jafari, S. Physicochemical, Thermal, and Morphological Properties of Chitosan Nanoparticles Produced by Ionic Gelation. Foods 2022, 11, 3841. [Google Scholar] [CrossRef] [PubMed]
- Saroha, A.; Verma, R.; Mittal, V.; Kaushik, D. Fabrication, Optimization and In Vitro Cytotoxicity Evaluation of Dasatinib Monohydrate-Loaded Nanoparticles. Int. J. Appl. Pharm. 2024, 16, 134–142. [Google Scholar] [CrossRef]
- Milusheva, R.; Nurgaliev, I.; Rashidova, S. Features of the Interaction and Formation of Nanostructured Chitosan Systems during Ionotropic Gelation. Prog. Chem. Appl. Chitin Deriv. 2024, 29, 138–144. [Google Scholar] [CrossRef]
- Mao, S.; Sun, W.; Kissel, T. Chitosan-based formulations for delivery of DNA and siRNA. Adv. Drug Deliv. Rev. 2010, 62, 12–27. [Google Scholar] [CrossRef]
- Veloso, S.; Marta, E.; Rodrigues, P.; Moura, C.; Amorim, C.; Amaral, V.; Correa-Duarte, M.; Castanheira, E. Chitosan/Alginate Nanogels Containing Multicore Magnetic Nanoparticles for Delivery of Doxorubicin. Pharmaceutics 2023, 15, 2194. [Google Scholar] [CrossRef]
- Nagella, S.; Choi, S.; Park, S.; Ha, C.; Jung, Y.; Chitumalla, R.; Jang, J.; Yoon, J.; Chung, I. Depolymerized Chitosan-g-[Poly(MMA-co-HEMA-cl-EGDMA)] Based Nanogels for Controlled Local Release of Bupivacaine. Int. J. Mol. Sci. 2023, 24, 16470. [Google Scholar] [CrossRef]
- Penchev, H.; Paneva, D.; Manolova, N.; Rashkov, I. Electrospun hybrid nanofibers based on chitosan or N-carboxyethylchitosan and silver nanoparticles. Macromol. Biosci. 2009, 9, 884–894. [Google Scholar] [CrossRef]
- Lee, S.; Heo, D.; Moon, J.; Ko, W.; Lee, J.; Bae, M.; Park, S.; Kim, J.; Lee, D.; Kim, E.; et al. Electrospun chitosan nanofibers with controlled levels of silver nanoparticles. Preparation, characterization, and antibacterial activity. Carbohydr. Polym. 2014, 111, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Annur, D.; Wang, Z.; Liao, J.; Kuo, C. Plasma-Synthesized Silver Nanoparticles on Electrospun Chitosan Nanofiber Surfaces for Antibacterial Applications. Biomacromolecules 2015, 16, 3248–3255. [Google Scholar] [CrossRef] [PubMed]
- Kalantari, K.; Afifi, A.; Jahangirian, H.; Webster, T. Biomedical applications of chitosan electrospun nanofibers as a green polymer—Review. Carbohydr. Polym. 2019, 207, 588–600. [Google Scholar] [CrossRef]
- Li, Q.; Dong, M.; Li, R.; Cui, Y.; Xie, G.; Wang, X.; Long, Y. Enhancement of Cr (VI) removal efficiency via adsorption/photocatalysis synergy using electrospun chitosan/g-C3N4/TiO2 nanofibers. Carbohydr. Polym. 2021, 253, 117200. [Google Scholar] [CrossRef]
- Honary, S.; Zahir, F. Effect of zeta potential on the properties of nano-drug delivery systems—A review. Trop. J. Pharm. Res. 2013, 12, 255–264. [Google Scholar] [CrossRef]
- Pinheiro, A.; Bourbon, A.; Cerqueira, M.; Maricato, É.; Nunes, C.; Coimbra, M.; Vicente, A. Chitosan/fucoidan multilayer nanocapsules as a vehicle for controlled release of bioactive compounds. Carbohydr. Polym. 2015, 115, 1–9. [Google Scholar] [CrossRef]
- Hu, Q.; Luo, Y. Chitosan-based nanocarriers for encapsulation and delivery of curcumin: A review. Int. J. Biol. Macromol. 2021, 179, 524–535. [Google Scholar] [CrossRef]
- Shao, Y.; Wu, C.; Wu, T.; Li, Y.; Chen, S.; Yuan, C.; Hu, Y. Eugenol-chitosan nanoemulsions by ultrasound-mediated emulsification: Formulation, characterization and antimicrobial activity. Carbohydr. Polym. 2018, 193, 144–152. [Google Scholar] [CrossRef]
- Wang, F.; Li, J.; Tang, X.; Huang, K.; Chen, L. Polyelectrolyte three-layer chitosan/dextran sulfate/chitosan nanoparticles for dual drug delivery. Colloids Surf. B Biointerfaces 2020, 190, 110925. [Google Scholar] [CrossRef]
- Sydow, S.; De Cassan, D.; Hänsch, R.; Gengenbach, T.; Easton, C.; Thissen, H.; Menzel, H. Layer-by-layer deposition of chitosan nanoparticles as drug-release coatings for PCL nanofibers. Biomater. Sci. 2019, 7, 233–246. [Google Scholar] [CrossRef]
- Ramasamy, T.; Tran, T.; Choi, J.; Cho, H.; Kim, J.; Yong, C.; Choi, H.; Kim, J. Layer-by-layer coated lipid-polymer hybrid nanoparticles designed for use in anticancer drug delivery. Carbohydr. Polym. 2014, 102, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Yuan, Q.; Yang, X. Preparation and characterization of metal-chitosan nanocomposites. Colloids Surf. B Biointerfaces 2004, 39, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Pitto-Barry, A.; Habtemariam, A.; Romero-Canelón, I.; Sadler, P.; Barry, N. Nanoparticles of chitosan conjugated to organo-ruthenium complexes. Inorg. Chem. Front. 2016, 3, 1058–1064. [Google Scholar] [CrossRef]
- Azzam, E.; Eshaq, G.; Rabie, A.; Bakr, A.; Abd-Elaal, A.; Metwally, A.; Tawfik, S. Preparation and characterization of chitosan-clay nanocomposites for the removal of Cu (II) from aqueous solution. Int. J. Biol. Macromol. 2016, 89, 507–517. [Google Scholar] [CrossRef]
- Wu, W.; Shen, J.; Banerjee, P.; Zhou, S. Chitosan-based responsive hybrid nanogels for integration of optical pH-sensing, tumor cell imaging, and controlled drug delivery. Biomaterials 2010, 31, 8371–8381. [Google Scholar] [CrossRef]
- Pellá, M.; Simão, A.; Lima-Tenório, M.; Tenório-Neto, E.; Scariot, D.; Nakamura, C.; Rubira, A. Chitosan hybrid microgels for oral drug delivery. Carbohydr. Polym. 2020, 239, 116236. [Google Scholar] [CrossRef]
- Elzayat, A.; Landfester, K.; Muñoz-Espí, R. Chitosan/Silica Hybrid Nanogels by Inverse Nanoemulsion for Encapsulating Hydrophilic Substances. Macromol. Mater. Eng. 2024, 309, 2400151. [Google Scholar] [CrossRef]
- Dash, M.; Chiellini, F.; Ottenbrite, R.M.; Chiellini, E. Chitosan—A versatile semi-synthetic polymer in biomedical applications. Prog. Polym. Sci. 2011, 36, 981–1014. [Google Scholar] [CrossRef]
- Lang, X.; Wang, T.; Sun, M.; Chen, X.; Liu, Y. Advances and applications of chitosan-based nanomaterials as oral delivery carriers: A review. Int. J. Biol. Macromol. 2020, 155, 1419–1432. [Google Scholar] [CrossRef]
- Guadarrama-Escobar, O.; Serrano-Castañeda, P.; Anguíano-Almazán, E.; Vázquez-Durán, A.; Peña-Juárez, M.; Vera-Graziano, R.; Morales-Florido, M.; Rodríguez-Pérez, B.; Rodríguez-Cruz, I.; Miranda-Calderón, J.; et al. Chitosan Nanoparticles as Oral Drug Carriers. Int. J. Mol. Sci. 2023, 24, 4289. [Google Scholar] [CrossRef]
- Lim, L.; Hadinoto, K. High-Payload Buccal Delivery System of Amorphous Curcumin–Chitosan Nanoparticle Complex in Hydroxypropyl Methylcellulose and Starch Films. Int. J. Mol. Sci. 2021, 22, 9399. [Google Scholar] [CrossRef]
- Luo, Y.; Teng, Z.; Li, Y.; Wang, Q. Solid lipid nanoparticles for oral drug delivery: Chitosan coating improves stability, controlled delivery, mucoadhesion and cellular uptake. Carbohydr. Polym. 2015, 122, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Zamboulis, A.; Nanaki, S.; Michailidou, G.; Koumentakou, I.; Lazaridou, M.; Ainali, N.; Xanthopoulou, E.; Bikiaris, D. Chitosan and its Derivatives for Ocular Delivery Formulations: Recent Advances and Developments. Polymers 2020, 12, 1519. [Google Scholar] [CrossRef]
- Silva, M.; Calado, R.; Marto, J.; Bettencourt, A.; Almeida, A.; Gonçalves, L. Chitosan Nanoparticles as a Mucoadhesive Drug Delivery System for Ocular Administration. Mar. Drugs 2017, 15, 370. [Google Scholar] [CrossRef]
- De Salamanca, E.; Diebold, Y.; Calonge, M.; García-Vázquez, C.; Callejo, S.; Vila, A.; Alonso, M. Chitosan nanoparticles as a potential drug delivery system for the ocular surface: Toxicity, uptake mechanism and in vivo tolerance. Investig. Ophthalmol. Vis. Sci. 2006, 47, 1416–1425. [Google Scholar] [CrossRef]
- Mukherjee, S.; Karati, D.; Singh, S.; Prajapati, B. Chitosan-based Nanomedicine in the Management of Age-related Macular Degeneration: A Review. Curr. Nanomed. 2024, 14, 13–27. [Google Scholar] [CrossRef]
- Wu, K.; Wang, X.; Anderson, M.; Tran, S. Advancements in Nanosystems for Ocular Drug Delivery: A Focus on Pediatric Retinoblastoma. Molecules 2024, 29, 2263. [Google Scholar] [CrossRef]
- Dai, T.; Tanaka, M.; Huang, Y.Y.; Hamblin, M.R.; Chitosan, X. Chitosan preparations for wounds and burns: Antimicrobial and wound-healing effects. Expert Rev. Anti Infect. Ther. 2011, 9, 857–879. [Google Scholar] [CrossRef]
- Lee, J.; Oh, H.; Kim, S.; Lee, J.; Shin, Y.; Choi, W. A Novel Chitosan Nanosponge as a Vehicle for Transepidermal Drug Delivery. Pharmaceutics 2021, 13, 1329. [Google Scholar] [CrossRef]
- Samiotaki, C.; Koumentakou, I.; Christodoulou, E.; Bikiaris, N.; Vlachou, M.; Karavas, E.; Tourlouki, K.; Kehagias, N.; Barmpalexis, P. Fabrication of PLA-Based Nanoneedle Patches Loaded with Transcutol-Modified Chitosan Nanoparticles for the Transdermal Delivery of Levofloxacin. Molecules 2024, 29, 4289. [Google Scholar] [CrossRef]
- Cui, X.; Guan, X.; Zhong, S.; Chen, J.; Zhu, H.; Li, Z.; Xu, F.; Chen, P.; Wang, H. Multi-stimuli responsive smart chitosan-based microcapsules for targeted drug delivery and triggered drug release. Ultrason. Sonochem. 2017, 38, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Yao, W.; Sun, W.; Guo, T.; Lv, H.; Wang, X.; Ying, H.; Wang, Y.; Wang, P. A self-targeting and controllable drug delivery system constituting mesoporous silica nanoparticles fabricated with a multi-stimuli responsive chitosan-based thin film layer. Int. J. Biol. Macromol. 2019, 122, 1090–1099. [Google Scholar] [CrossRef] [PubMed]
- Sabourian, P.; Ji, J.; Lotocki, V.; Moquin, A.; Hanna, R.; Frounchi, M.; Maysinger, D.; Kakkar, A. Facile design of autogenous stimuli-responsive chitosan/hyaluronic acid nanoparticles for efficient small molecules to protein delivery. J. Mater. Chem. B 2020, 8, 5891–5902. [Google Scholar] [CrossRef] [PubMed]
- Sinani, G.; Sessevmez, M.; Gök, K.; Özgümüş, S.; Alpar, O.; Cevher, E. Modified chitosan-based nanoadjuvants enhance the immunogenicity of protein antigens after mucosal vaccination. Int. J. Pharm. 2019, 565, 214–223. [Google Scholar] [CrossRef]
- Lin, Y.; Sun, B.; Jin, Z.; Zhao, K. Enhanced Immune Responses to Mucosa by Functionalized Chitosan-Based Composite Nanoparticles as a Vaccine Adjuvant for Intranasal Delivery. ACS Appl. Mater. Interfaces 2022, 14, 16615–16630. [Google Scholar] [CrossRef]
- Muzzarelli, R.; Mehtedi, E.; Mattioli-Belmonte, M. Emerging Biomedical Applications of Nano-Chitins and Nano-Chitosans Obtained via Advanced Eco-Friendly Technologies from Marine Resources. Mar. Drugs 2014, 12, 5468–5502. [Google Scholar] [CrossRef]
- Kim, Y.; Zharkinbekov, Z.; Raziyeva, K.; Tabyldiyeva, L.; Berikova, K.; Zhumagul, D.; Temirkhanova, K.; Saparov, A. Chitosan-Based Biomaterials for Tissue Regeneration. Pharmaceutics 2023, 15, 807. [Google Scholar] [CrossRef]
- Rodriguez-Vazquez, M.; Vega-Ruiz, B.; Ramos-Zúñiga, R.; Saldana-Koppel, D.A.; Quinones-Olvera, L.F. Chitosan and its potential use as a scaffold for tissue engineering in regenerative medicine. BioMed Res. Int. 2015, 2015, 821279. [Google Scholar] [CrossRef]
- AbdelAllah, N.; Gaber, Y.; Abou-Taleb, H.; Rashed, M.; Azmy, A.; Abdelghani, S. Alginate-coated chitosan nanoparticles act as effective adjuvant for hepatitis A vaccine in mice. Int. J. Biol. Macromol. 2020, 154, 674–685. [Google Scholar] [CrossRef]
- Li, X.; Min, M.; Du, N.; Gu, Y.; Hode, T.; Naylor, M.; Chen, D.; Nordquist, R.; Chen, W. Chitin, Chitosan, and Glycated Chitosan Regulate Immune Responses: The Novel Adjuvants for Cancer Vaccine. Clin. Dev. Immunol. 2013, 2013, 387023. [Google Scholar] [CrossRef]
- Tan, J.; Ding, B.; Teng, B.; Lin, J. Understanding Structure–Function Relationships of Nanoadjuvants for Enhanced Cancer Vaccine Efficacy. Adv. Funct. Mater. 2022, 32, 2111670. [Google Scholar] [CrossRef]
- Khattak, S.; Wahid, F.; Liu, L.; Jia, S.; Chu, L.; Xie, Y.; Li, Z.; Zhong, C. Applications of cellulose and chitin/chitosan derivatives and composites as antibacterial materials: Current state and perspectives. Appl. Microbiol. Biotechnol. 2019, 103, 1989–2006. [Google Scholar] [CrossRef]
- Liu, M.; Zheng, H.; Chen, J.; Li, S.; Huang, J.; Zhou, C. Chitosan-chitin nanocrystal composite scaffolds for tissue engineering. Carbohydr. Polym. 2016, 152, 832–840. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, R.; Ramachandran, R.; Kumar, P.; Divyarani, V.; Srinivasan, S.; Chennazhi, K.; Tamura, H.; Nair, S. Fabrication of chitin-chitosan/nano ZrO2 composite scaffolds for tissue engineering applications. Int. J. Biol. Macromol. 2011, 49, 274–280. [Google Scholar] [CrossRef]
- Jiang, J.; Oguzlu, H.; Jiang, F. 3D printing of lightweight, super-strong yet flexible all-cellulose structure. Chem. Eng. J. 2021, 405, 126668. [Google Scholar] [CrossRef]
- Raftery, R.; Tierney, E.; Curtin, C.; Cryan, S.; O’Brien, F. Development of a gene-activated scaffold platform for tissue engineering applications using chitosan-pDNA nanoparticles on collagen-based scaffolds. J. Control. Release 2015, 210, 84–94. [Google Scholar] [CrossRef]
- Elsabahy, M.; Hamad, M. Design and Preclinical Evaluation of Chitosan/Kaolin Nanocomposites with Enhanced Hemostatic Efficiency. Mar. Drugs 2021, 19, 50. [Google Scholar] [CrossRef]
- Khan, M.; Mujahid, M. A review on recent advances in chitosan-based composite for hemostatic dressings. Int. J. Biol. Macromol. 2019, 124, 138–147. [Google Scholar] [CrossRef]
- Grotenhuis, T.; Van Grunsven, P.; Heutz, W.; Tan, E. Prehospital use of hemostatic dressings in emergency medical services in the Netherlands: A prospective study of 66 cases. Injury 2016, 47, 1007–1011. [Google Scholar] [CrossRef]
- Rall, J.; Cox, J.; Songer, A.; Cestero, R.; Ross, J. Comparison of novel hemostatic dressings with QuikClot combat gauze in a standardized swine model of uncontrolled hemorrhage. J. Trauma Acute Care Surg. 2013, 75, S150–S156. [Google Scholar] [CrossRef]
- Gerling, K.; Kersey, A.; Lauria, A.; Mares, J.; Hutzler, J.; White, P.; Abel, B.; Burmeister, D.; Propper, B.; White, J. Evaluation of novel hemostatic agents in a coagulopathic swine model of junctional hemorrhage. J. Trauma Acute Care Surg. 2023, 95, S144–S151. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, R.B.; Reynolds, B.Z.; Shiver, S.A.; Lerner, E.B.; Greenfield, E.M.; Solis, R.A.; McManus, J.G. Comparison of Two Packable Hemostatic Gauze Dressings in a Porcine Hemorrhage Model. Prehosp. Emerg. Care 2011, 15, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Lucchesi, L.; Teach, J.; Gregory, K.; Buckley, L.; Real, K. Comparison of Hemostatic Efficacy of ChitoGauze® and Combat Gauze in a Lethal Femoral Arterial Injury in Swine Model. In Proceedings of the Advanced Technology Applications for Combat Casualty Care (ATACCC 2009), St. Pete Beach, FL, USA, 10–12 August 2009; HemCon Medical Technologies Inc.: Portland, OR, USA, 2009. [Google Scholar]
- Al-Kassas, R.; Wen, J.; Cheng, A.; Kim, A.; Liu, S.; Yu, J. Transdermal delivery of propranolol hydrochloride through chitosan nanoparticles dispersed in mucoadhesive gel. Carbohydr. Polym. 2016, 153, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Khezri, F.; Lakshmi, C.; Bukka, R.; Nidhi, M.; Nargund, S. Pharmacokinetic Study and Brain Tissue Analysis of Zolmitriptan Loaded Chitosan Nanoparticles in Rats by LC-MS Method. Int. J. Biol. Macromol. 2019, 138, 1139–1149. [Google Scholar] [CrossRef]
- Bonferoni, M.; Rossi, S.; Sandri, G.; Ferrari, F.; Gavini, E.; Rassu, G.; Giunchedi, P. Nanoemulsions for “Nose-to-Brain” Drug Delivery. Pharmaceutics 2019, 11, 84. [Google Scholar] [CrossRef]
- Chatzitaki, A.; Jesus, S.; Karavasili, C.; Andreadis, D.; Fatouros, D.; Borges, O. Chitosan-coated PLGA nanoparticles for the nasal delivery of ropinirole hydrochloride: In vitro and ex vivo evaluation of efficacy and safety. Int. J. Pharm. 2020, 591, 119776. [Google Scholar] [CrossRef]
- Alameh, M.; Lavertu, M.; Tran-Khanh, N.; Chang, C.; Lesage, F.; Bail, M.; Darras, V.; Chevrier, A.; Buschmann, M. siRNA Delivery with Chitosan: Influence of Chitosan Molecular Weight, Degree of Deacetylation, and Amine to Phosphate Ratio on in Vitro Silencing Efficiency, Hemocompatibility, Biodistribution, and in Vivo Efficacy. Biomacromolecules 2018, 19, 112–131. [Google Scholar] [CrossRef]
- Yu, Y.; Xu, S.; Yu, S.; Li, J.; Tan, G.; Li, S.; Pan, W. A Hybrid Genipin-Cross-Linked Hydrogel/Nanostructured Lipid Carrier for Ocular Drug Delivery: Cellular, Ex Vivo, and In Vivo Evaluation. ACS Biomater. Sci. Eng. 2020, 6, 1543–1552. [Google Scholar] [CrossRef]
- Albarqi, H.; Garg, A.; Ahmad, M.; Alqahtani, A.; Walbi, I.; Ahmad, J. Recent Progress in Chitosan-Based Nanomedicine for Its Ocular Application in Glaucoma. Pharmaceutics 2023, 15, 681. [Google Scholar] [CrossRef]
- Silva, B.; Gonçalves, L.; Braz, B.; Delgado, E. Topical Administration of a Nanoformulation of Chitosan-Hyaluronic Acid-Epoetin Beta in a Rat Model of Glaucoma. Pharmaceuticals 2023, 16, 164. [Google Scholar] [CrossRef]
- Hassanen, E.; Khalaf, A.; Tohamy, A.; Mohammed, E.; Farroh, K. Toxicopathological and immunological studies on different concentrations of chitosan-coated silver nanoparticles in rats. Int. J. Nanomed. 2019, 14, 4723–4739. [Google Scholar] [CrossRef] [PubMed]
- Aluani, D.; Tzankova, V.; Kondeva-Burdina, M.; Yordanov, Y.; Nikolova, E.; Odzhakov, F.; Apostolov, A.; Markova, T.; Yoncheva, K. Evaluation of biocompatibility and antioxidant efficiency of chitosan-alginate nanoparticles loaded with quercetin. Int. J. Biol. Macromol. 2017, 103, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Frigaard, J.; Jensen, J.; Galtung, H.; Hiorth, M. The Potential of Chitosan in Nanomedicine: An Overview of the Cytotoxicity of Chitosan Based Nanoparticles. Front. Pharmacol. 2022, 13, 880377. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration (FDA). Guidance for Industry: Considering whether an FDA-regulated product involves the application of nanotechnology. Biotechnol. Law Rep. 2011, 30, 613–616. [Google Scholar] [CrossRef]
- ISO/TC 229; Nanotechnologies. International Organization for Standardization: Geneva, Switzerland, 2021. Available online: https://www.iso.org/committee/381983.html (accessed on 24 April 2025).
- Varma, A.J.; Deshpande, S.V.; Kennedy, J.F. Metal complexation by chitosan and its derivatives: A review. Carbohydr. Polym. 2004, 55, 77–93. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajjadi, M.; Iravani, S.; Varma, R. Starch, cellulose, pectin, gum, alginate, chitin and chitosan derived (nano) materials for sustainable water treatment: A review. Carbohydr. Polym. 2021, 251, 116986. [Google Scholar] [CrossRef]
- Muzzarelli, R. Nanochitins and Nanochitosans, Paving the Way to Eco-Friendly and Energy-Saving Exploitation of Marine Resources. In Comprehensive Nanoscience and Technology; Elsevier: Amsterdam, The Netherlands, 2012; Volume 10, pp. 153–164. [Google Scholar] [CrossRef]
- Knidri, H.; Dahmani, J.; Addaou, A.; Laajeb, A.; Lahsini, A. Rapid and efficient extraction of chitin and chitosan for scale-up production: Effect of process parameters on deacetylation degree and molecular weight. Int. J. Biol. Macromol. 2019, 139, 735–746. [Google Scholar] [CrossRef]
- Bertel-Pérez, F.; Cogollo-Cárcamo, G.; González-Delgado, Á. Assessing Exergy Efficiency in Computer-Aided Modeled Large-Scale Production of Chitosan Microbeads Modified with Thiourea and Magnetite Nanoparticles. Sustainability 2023, 15, 14443. [Google Scholar] [CrossRef]
- FAO. Joint FAO/WHO Expert Committee on Food Additives: Safety evaluation of certain food additives and contaminants. In WHO Food Additives Series; World Health Organization: Geneva, Switzerland, 2011; Series 64; pp. 3–6. [Google Scholar]
- Marques, C.; Som, C.; Schmutz, M.; Borges, O.; Borchard, G. How the Lack of Chitosan Characterization Precludes Implementation of the Safe-by-Design Concept. Front. Bioeng. Biotechnol. 2020, 8, 165. [Google Scholar] [CrossRef]
- Kumi, M.; Wang, T.; Ejeromedoghene, O.; Wang, J.; Li, P.; Huang, W. Exploring the Potentials of Chitin and Chitosan-Based Bioinks for 3D-Printing of Flexible Electronics: The Future of Sustainable Bioelectronics. Small Methods 2024, 8, e2301341. [Google Scholar] [CrossRef]
- Maturavongsadit, P.; Narayanan, L.; Chansoria, P.; Shirwaiker, R.; Benhabbour, S. Cell-Laden Nanocellulose/Chitosan-Based Bioinks for 3D Bioprinting and Enhanced Osteogenic Cell Differentiation. ACS Appl. Bio Mater. 2021, 4, 2342–2353. [Google Scholar] [CrossRef]
- Parak, A.; Pradeep, P.; Du Toit, L.; Kumar, P.; Choonara, Y.; Pillay, V. Functionalizing bioinks for 3D bioprinting applications. Drug Discov. Today 2019, 24, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Hamedi, H.; Moradi, S.; Hudson, S.; Tonelli, A. Chitosan based hydrogels and their applications for drug delivery in wound dressings: A review. Carbohydr. Polym. 2018, 199, 445–460. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Tan, S.; Gao, J.; Wang, L. Sequential release of drugs forms a dual-delivery system based on pH-responsive nanofibrous mats towards wound care. J. Mater. Chem. B 2020, 8, 1136–1147. [Google Scholar] [CrossRef]
- Givens, B.; Naguib, Y.; Geary, S.; Devor, E.; Salem, A. Nanoparticle-Based Delivery of CRISPR/Cas9 Genome-Editing Therapeutics. AAPS J. 2018, 20, 108. [Google Scholar] [CrossRef]
- Caprifico, A.; Foot, P.; Polycarpou, E.; Calabrese, G. Advances in Chitosan-Based CRISPR/Cas9 Delivery Systems. Pharmaceutics 2022, 14, 1840. [Google Scholar] [CrossRef]
- Kazemian, P.; Yu, S.; Thomson, S.; Birkenshaw, A.; Leavitt, B.; Ross, C. Lipid-Nanoparticle-Based Delivery of CRISPR/Cas9 Genome-Editing Components. Mol. Pharm. 2022, 19, 1669–1686. [Google Scholar] [CrossRef]
- Aghamiri, S.; Talaei, S.; Ghavidel, A.; Zandsalimi, F.; Masoumi, S.; Hafshejani, N.; Jajarmi, V. Nanoparticles-mediated CRISPR/Cas9 delivery: Recent advances in cancer treatment. J. Drug Deliv. Sci. Technol. 2020, 56, 101533. [Google Scholar] [CrossRef]
- Jhaveri, J.; Raichura, Z.; Khan, T.; Momin, M.; Omri, A. Chitosan Nanoparticles—Insight into Properties, Functionalization and Applications in Drug Delivery and Theranostics. Molecules 2021, 26, 7272. [Google Scholar] [CrossRef]
- Kojima, R.; Aubel, D.; Fussenegger, M. Novel theranostic agents for next-generation personalized medicine: Small molecules, nanoparticles, and engineered mammalian cells. Curr. Opin. Chem. Biol. 2015, 28, 29–38. [Google Scholar] [CrossRef]

| Species of Fungi | Information | Reference |
|---|---|---|
| Aspergillus species | Chitin synthesis in Aspergillus is well-documented, with up to eight synthase-encoding genes identified. This highlights its significant role in fungal growth and interaction with the environment. | [24] |
| Candida albicans | This species exhibits high chitin content, making it a notable producer among pathogenic fungi. | [25] |
| Cryptococcus gattii y Aspergillus niger | Both species are recognized for their high chitin production, like Candida albicans. | [25] |
| Fusarium species | Fusarium KYM3 is noted for its high chitin yield, making it a prominent producer among soil fungi. | [26] |
| Penicillium species | Although Penicillium KYM6 is noted for lower chitin production compared to Fusarium, it is still a significant source. | [26] |
| Zygomycetous fungi | Mucor rouxii and Rhizopus oryzae are extensively studied for chitin and chitosan production. | [27] |
| Basidiomycetes | Includes fungi, like Agaricus bisporus, which are known for their chitin content and are used in various applications. | [27] |
| Solvent | Chitin | Chitosan | References |
|---|---|---|---|
| Dilute Acetic Acid | Insoluble | Soluble | [1,5] |
| Concentrated Acetic acid | Partially soluble | Soluble | [52] |
| Water | Insoluble | Insoluble | [1,6] |
| Hexafluoroisopropanol | Partially | Soluble | [11,28] |
| Ionic Liquids | Soluble | Soluble | [6,53] |
| Deep Eutectic Solvents | Soluble | Soluble | [28,33] |
| Lactic Acid | Partially | Soluble | [6,44] |
| Formic Acid | Partially | Soluble | [1,18] |
| Citric Acid | Partially | Soluble | [12,17] |
| Malic acid | Insoluble | Slightly soluble | [52] |
| Glycolic acid | Insoluble | Slightly soluble | [52] |
| EDTA and chelators | Facilitates swelling | Facilitates partial dissolution | [32,33] |
| Derivative | Modification | Properties | Applications |
|---|---|---|---|
| N-Carboxymethyl Chitosan (NCMC) | Introduction of carboxymethyl groups (-CH2-COOH) to the amino groups. | Improved water solubility, biocompatibility, and chelating ability. | Wound healing, controlled drug delivery. |
| Chitosan Sulfates | Sulfation of hydroxyl or amino groups to introduce sulfate groups (-OSO3H). | Enhanced anticoagulant and antiviral activity. | Anticoagulant materials, antiviral agents. |
| Quaternized Chitosan (QCS) | Introduction of quaternary ammonium groups (-N⁺(CH3)3) to the amino groups. | Increased water solubility, strong cationic nature, enhanced antimicrobial activity. | Antimicrobial coatings, gene delivery systems. |
| Thiolated Chitosan | Introduction of thiol groups (-SH) to the chitosan backbone. | Improved mucoadhesive properties and enhanced drug permeation. | Mucoadhesive drug delivery systems, wound healing materials. |
| Chitosan Oligosaccharides (COS) | Enzymatic or chemical hydrolysis to produce low molecular weight oligomers. | Enhanced solubility, bioavailability, and antioxidant activity. | Nutraceuticals, plant growth promoters, antifungal agents. |
| Grafted Chitosan | Grafting of synthetic polymers (e.g., polyethylene glycol) onto the chitosan backbone. | Tunable mechanical and thermal properties improved hydrophilicity or hydrophobicity. | Tissue engineering scaffolds, controlled drug delivery systems. |
| Property Enhanced | Description | Applications |
|---|---|---|
| Mechanical Properties | Due to increased surface area and intermolecular interactions, nanosizing improves tensile strength, elasticity, and durability. | Nanofibers and nanocomposites for tissue engineering, wound dressings, and biodegradable films. |
| Colloidal Stability | Nanoparticles exhibit better dispersion and stability in aqueous solutions, preventing aggregation. | Drug delivery systems, water treatment, and food packaging. |
| Biocompatibility and Biological Interactions | Nanoparticles enhance interactions with cells and tissues, improving biocompatibility and promoting bioactivity. | Tissue engineering, wound healing, and regenerative medicine. |
| Drug-Loading Capacity and Release Profiles | The high surface area of nanosized particles allows for higher drug-loading capacity and controlled release kinetics. | Cancer therapy, antimicrobial delivery, and sustained-release formulations. |
| Nanomaterial | Description | Applications | References |
|---|---|---|---|
| Ionic Gelation Nanoparticles | Formed by electrostatic interaction between acidic chitosan solution and polyanions (e.g., sodium tripolyphosphate, TPP). Produce stable nanosized particles. | Controlled drug release in oral or parenteral systems. Protection of proteins and peptides against enzymatic degradation. | [123,124,125,126,127] |
| Chitosan Nanogels | Three-dimensional polymeric networks at the nanoscale that retain significant amounts of water or physiological fluids. Can be synthesized via chemical or physical gelation (e.g., pH or ionic changes). | Stimuli-responsive release (pH, temperature, etc.). Potential for ocular, dermal, and transmucosal formulations owing to high biocompatibility. Nanogels loaded with doxorubicin have shown sustained drug release, which can be enhanced under specific conditions, like near-infrared laser irradiation and acidic pH. | [128,129,130] |
| Electrospun Nanofibers | Obtained through electrospinning of chitosan-based solutions (often blended with PVA, PLA, or other polymers). Produce membranes with high porosity and surface area. AgNPs are incorporated through in situ synthesis in the spinning solution, which ensures uniform dispersion within the nanofibers. | Wound dressings featuring antibacterial and hemostatic properties. Tissue engineering scaffolds promote cell adhesion and proliferation. They are particularly effective in wound dressings, where they can absorb exudates and inhibit microbial growth. Adding AgNPs enhances their antibacterial activity, making them suitable for treating infections caused by bacteria, such as Pseudomonas aeruginosa and methicillin-resistant Staphylococcus aureus (MRSA). Adding materials, like g-C3N4/TiO2, can enhance the photocatalytic properties of chitosan nanofibers, improving their efficiency in pollutant removal under visible light. | [131,132,133,134,135] |
| Nanocapsules and Nanoemulsions | Colloidal systems in which chitosan acts as an emulsifier or coating. These systems leverage the unique properties of chitosan, a natural biopolymer, to improve the encapsulation and delivery of various substances, including curcumin and peptides. Often oil-in-water (O/W) emulsions at the nanoscale. | Enhanced solubility for lipophilic drugs. Targeted delivery (via surface modifications) for improved therapeutic outcomes. Chitosan nanoemulsions and nanocapsules are used in the food and pharmaceutical industries to improve the solubility, stability, and bioavailability of hydrophobic compounds, like curcumin and eugenol. | [136,137,138,139] |
| Layer-by-Layer (LbL) Polyelectrolyte Nanoparticles | Built by alternately depositing cationic (chitosan) and anionic (e.g., alginate, carrageenan) layers. Yield multilayered nanoparticles or coatings with tunable properties. | Sequential or pulsatile drug release. Fine control over surface properties and responsiveness (e.g., pH, ionic strength). Chitosan/dextran sulfate/chitosan (CS/DEX/CS) nanoparticles have been developed for dual drug delivery. They demonstrate controlled release profiles and enhance cytotoxic effects against cancer cells. Similarly, chitosan nanoparticles have been used to create drug-release coatings on PCL nanofibers, showing potential for therapeutic protein delivery. | [140,141,142] |
| Chitosan–Metal Nanocomposites | Incorporate metallic nanoparticles (e.g., silver, gold, zinc oxide) dispersed in the chitosan matrix. Combine the bioactivity of chitosan with the antimicrobial or catalytic properties of metals. Typical metals used include silver, gold, platinum, and palladium. Silver nanoparticles are generally more significant than others, leading to different morphologies in the resulting films. | Enhanced antimicrobial performance for medical device coatings and wound dressings. Potential in biosensors or catalytic applications due to combined biocompatibility and metallic functionality. | [143,144,145] |
| “Nanogel-in-Microsphere” Hybrid Systems | Hybrid structures where chitosan nanoparticles are encapsulated within microparticles made of another polymer, or vice versa. Enable multiple modes of drug release. | Dual drug release (for hydrophilic and hydrophobic molecules). Applications in vaccines and gene therapy (protection and controlled release of DNA or RNA). Chitosan-based hybrid nanogels with covalent crosslinking show excellent stability and reversible pH response, combining multiple functions into a single nano-object for biomedical applications. | [146,147] |
| Drug–Nanochitosan Conjugates | Covalent or electrostatic conjugates between nanochitosan and drugs, proteins, enzymes, or antibodies. Often include ligands for tissue or cell-specific targeting. | Targeted therapy via site-specific delivery to cells or organs. High intracellular efficacy for delivering oligonucleotides, siRNA, etc. | [2,50,148] |
| Nanochitin Cryogels | Highly porous, sponge-like networks formed via freeze-drying of nanochitin hydrogels. | Wound healing, tissue regeneration, scaffolds for 3D cell culture. | [6,9] |
| Nanofibrillar Films | Thin films are composed of aligned nanochitin fibrils with high mechanical strength and water retention. | Antimicrobial wound dressings, drug release matrices, packaging for biomedical products. | [65,149] |
| β-Chitin Nanowhiskers | Rod-shaped nanostructures from squid pens with enhanced surface area and reactivity. | Controlled drug delivery, mucoadhesive systems, injectable depots. | [6,75] |
| Commercial Name | Formulation | Indication | Product Type | Observations | References |
|---|---|---|---|---|---|
| ChitoTech Hemostatic Dressing | Hemostatic product based on chitosan. Utilizes nano/micro-scale chitosan particles with high adsorption capacity. | Rapid control of bleeding in acute or traumatic wounds. Infection prevention. | Medical device (hemostatic dressing) | It is commercially available for emergencies and hospital use. The manufacturer highlights “nano/micro chitosan” to enhance adhesion and hemostasis. It is offered in various sizes for different wound types. | [178,179] |
| ChitoGauze® (Tricol Biomedical/HemCon) | Gauze impregnated with submicron chitosan. Designed to adhere to bleeding sites and enhance coagulation. | Emergency hemorrhage control in trauma. Used in both military and civilian settings for external bleeding. | Medical device (hemostatic dressing) | FDA-cleared in the U.S. via 510 (k) as a hemostatic dressing. Although “nano” is not explicitly labeled, the chitosan is reportedly present in reduced particle sizes for increased contact area and faster action. | [180,181,182,183,184] |
| Chitoderm® Gel | Gel containing “nanochitosan” or “oligochitosan” to improve penetration and antimicrobial effect. | Treatment of minor burns, diabetic foot ulcers, and pressure sores. Promotes wound healing. | Medical device/wound care product | Marketed in certain regions (primarily Asia/Eastern Europe) as a topical gel. It claims superior performance due to higher surface area from nanochitosan, yet there is limited public data on exact particle size. | [185] |
| Nasal Sprays with nanochitosan (various brands) | Aqueous solution with chitosan nanoparticles or microcapsules to improve mucosal adhesion in the nasal cavity. | Alleviates nasal congestion and allergic rhinitis or serves as a protective barrier. It may enhance the absorption of nasal drugs or supplements. | Medical device/nutraceutical (depending on jurisdiction) | The exact composition and concentration vary by manufacturer. Often registered as “medical devices” (CE) or supplements. Reported to have good tolerance and prolonged residence on the nasal mucosa. | [186,187,188] |
| “Nano-Chitosan Fat Binder” Supplements (various) | Capsules or powders claimed to contain nano-sized (or submicron) chitosan for enhanced fat-binding capacity. | Weight management and dietary cholesterol reduction. Marketed as adjuncts to weight-control regimens. | Nutritional supplement/nutraceutical | Available under different brand names, often without extensively validating proper nanoscale dimensions. Loosely regulated; classified as “dietary supplements” in some countries. Efficacy and bioavailability may vary widely across products. | [136] |
| mRNA/siRNA Delivery Formulations (early clinical) | - Vesicles or chitosan nanoparticles modified to encapsulate nucleic acids (RNA, siRNA, mRNA). | Gene therapy and next-generation vaccination. Protects genetic material from enzymatic degradation and improves cellular uptake. | Experimental formulations in preclinical/early clinical trials | Not yet widely commercialized. Smaller biotech companies are exploring pulmonary, nasal, or oral administration prototypes. Require rigorous regulatory approval to be recognized as “drugs.” | [128,189] |
| Ocular Implants/Gels (Prototypes) | Gels and microcapsules containing nanochitosan to prolong ocular drug release. | Treatment of ocular diseases (glaucoma, infections, etc.) with sustained drug release. | Preclinical/clinical research formulations | It is still in the testing phase; it is not broadly available on the market. It is claimed that nanochitosan ensures good corneal bioadhesion and improves drug bioavailability. | [106,190,191,192] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vega-Baudrit, J.R.; Lopretti, M.; Montes de Oca, G.; Camacho, M.; Batista, D.; Corrales, Y.; Araya, A.; Bahloul, B.; Corvis, Y.; Castillo-Henríquez, L. Nanochitin and Nanochitosan in Pharmaceutical Applications: Innovations, Applications, and Future Perspective. Pharmaceutics 2025, 17, 576. https://doi.org/10.3390/pharmaceutics17050576
Vega-Baudrit JR, Lopretti M, Montes de Oca G, Camacho M, Batista D, Corrales Y, Araya A, Bahloul B, Corvis Y, Castillo-Henríquez L. Nanochitin and Nanochitosan in Pharmaceutical Applications: Innovations, Applications, and Future Perspective. Pharmaceutics. 2025; 17(5):576. https://doi.org/10.3390/pharmaceutics17050576
Chicago/Turabian StyleVega-Baudrit, José Roberto, Mary Lopretti, Gabriela Montes de Oca, Melissa Camacho, Diego Batista, Yendry Corrales, Andrea Araya, Badr Bahloul, Yohann Corvis, and Luis Castillo-Henríquez. 2025. "Nanochitin and Nanochitosan in Pharmaceutical Applications: Innovations, Applications, and Future Perspective" Pharmaceutics 17, no. 5: 576. https://doi.org/10.3390/pharmaceutics17050576
APA StyleVega-Baudrit, J. R., Lopretti, M., Montes de Oca, G., Camacho, M., Batista, D., Corrales, Y., Araya, A., Bahloul, B., Corvis, Y., & Castillo-Henríquez, L. (2025). Nanochitin and Nanochitosan in Pharmaceutical Applications: Innovations, Applications, and Future Perspective. Pharmaceutics, 17(5), 576. https://doi.org/10.3390/pharmaceutics17050576








