Microneedle-Mediated Treatment of Obesity
Abstract
1. Introduction
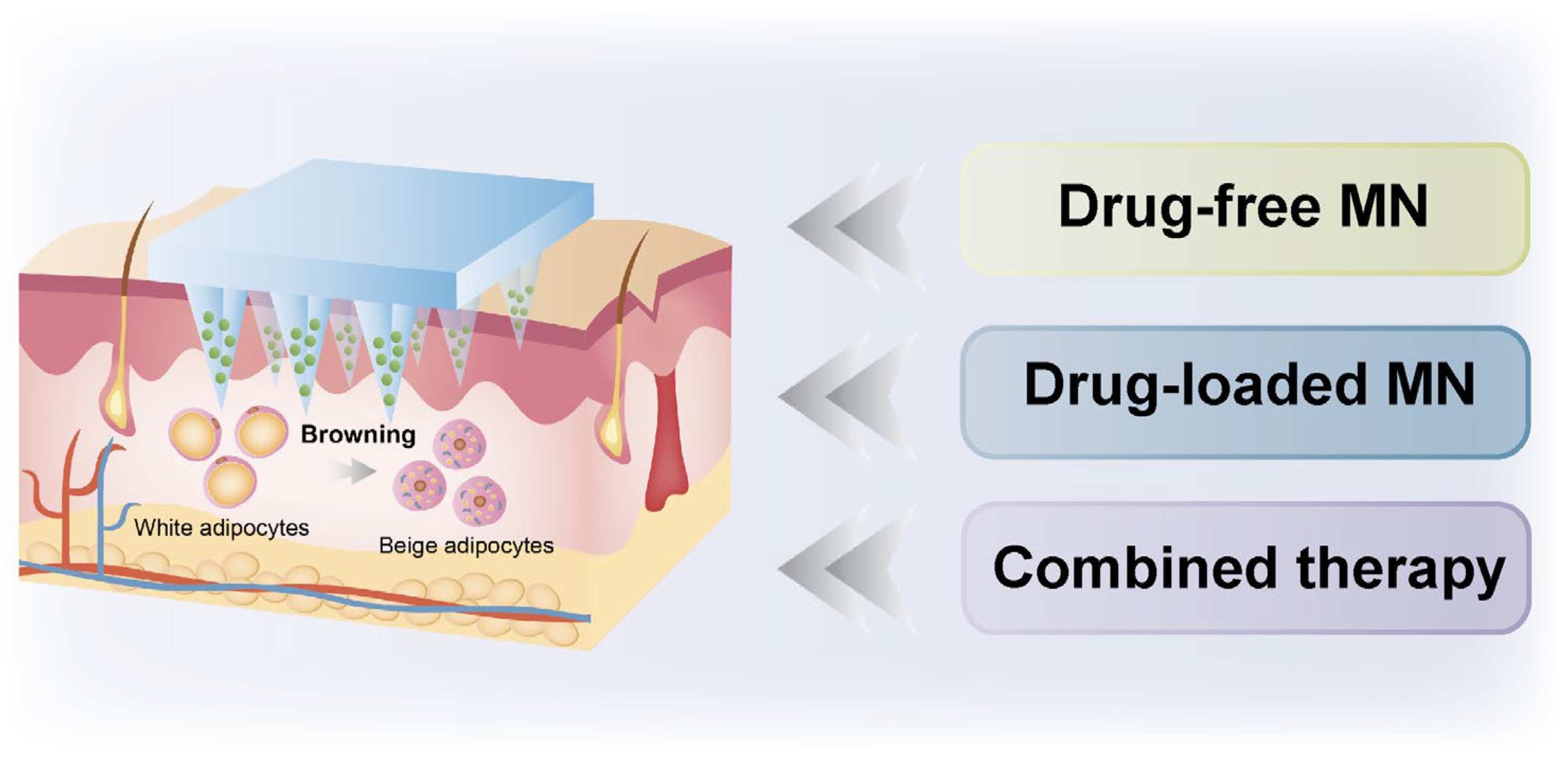
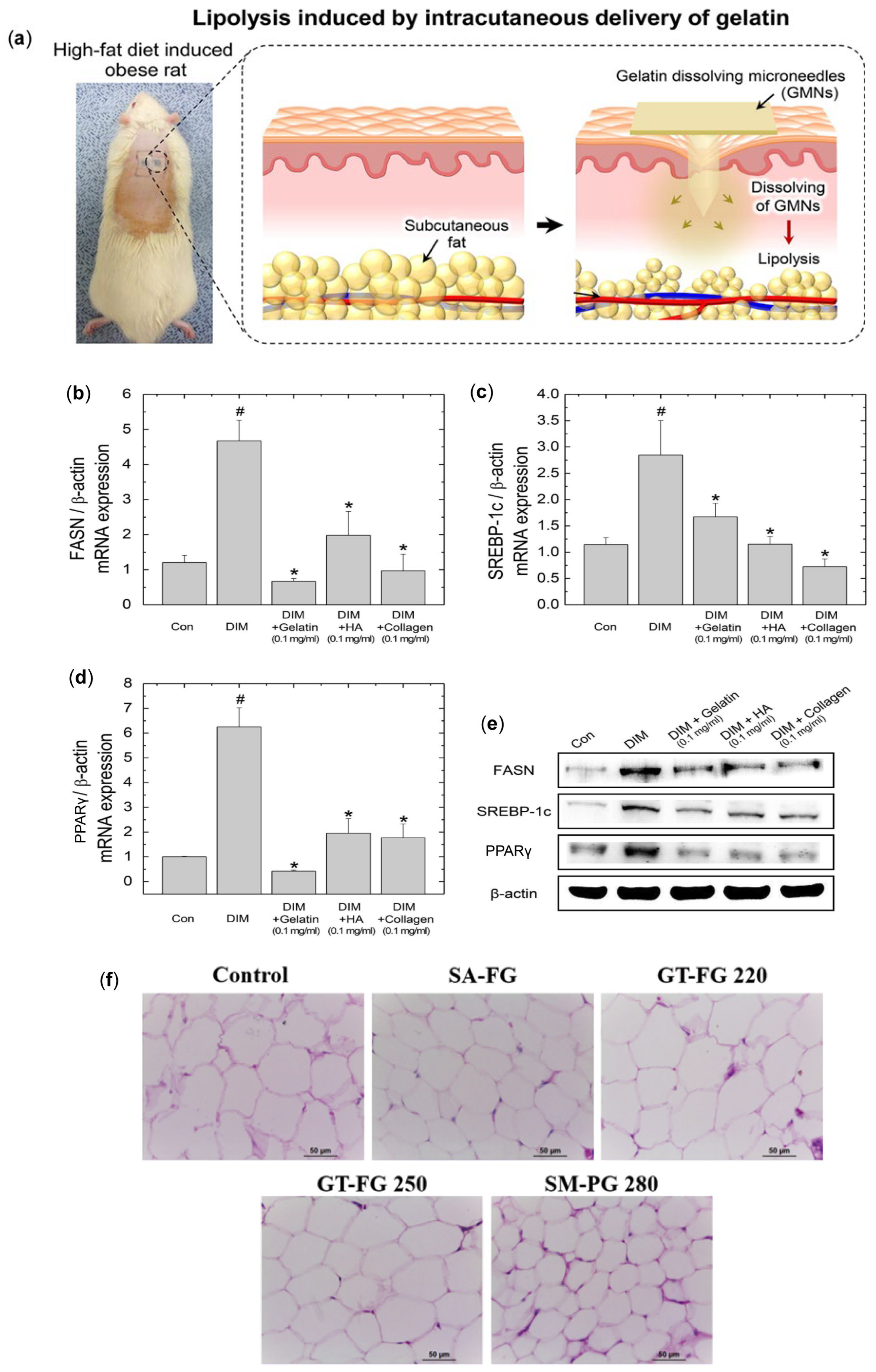
2. Drug-Free MN for Obesity Treatment
3. Drug-Loaded MN for Obesity Treatment
3.1. MNs Delivering Capsaicin for Obesity Treatment
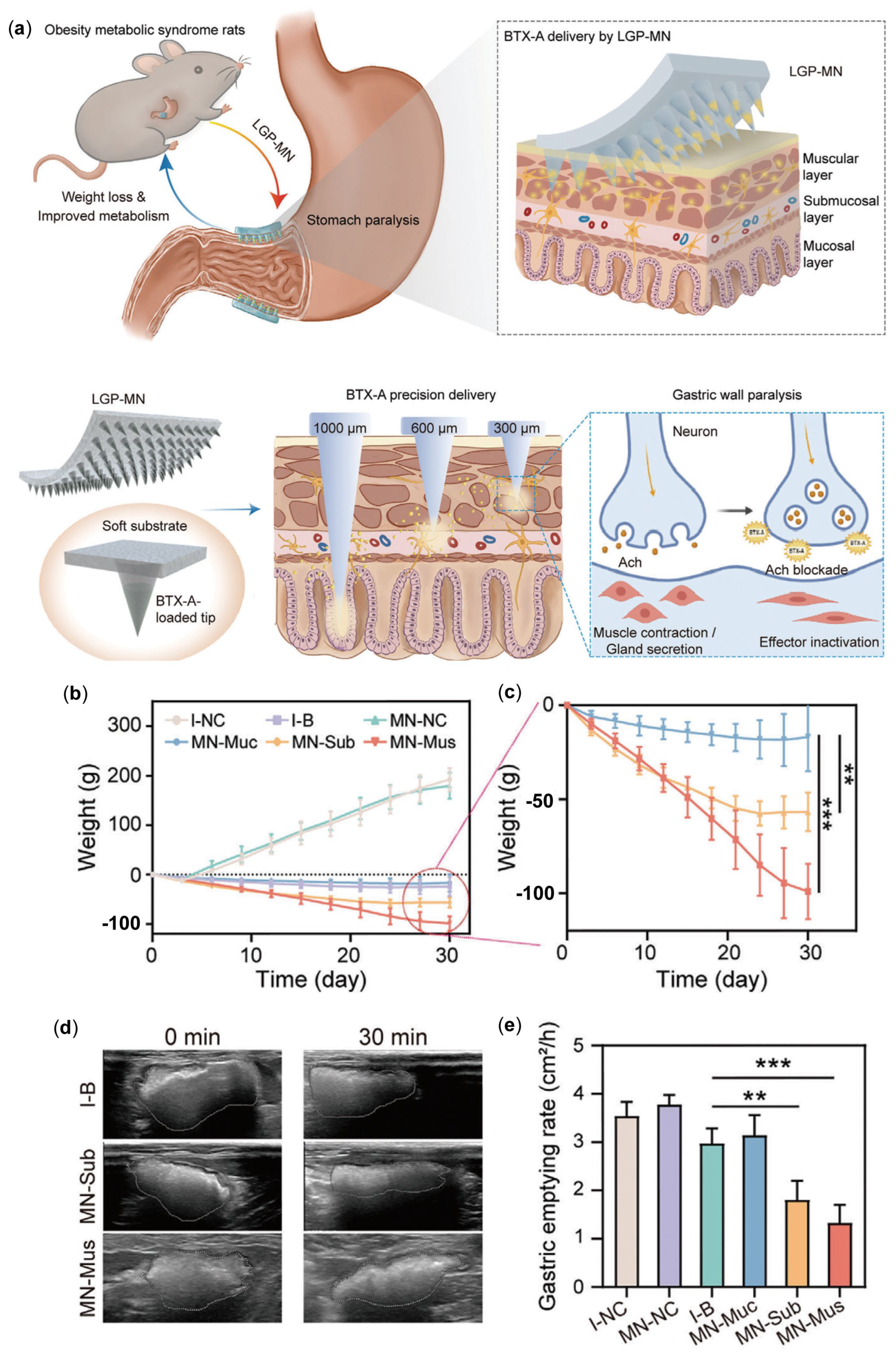
3.2. MNs Delivering Botulinum Toxin for Obesity Treatment
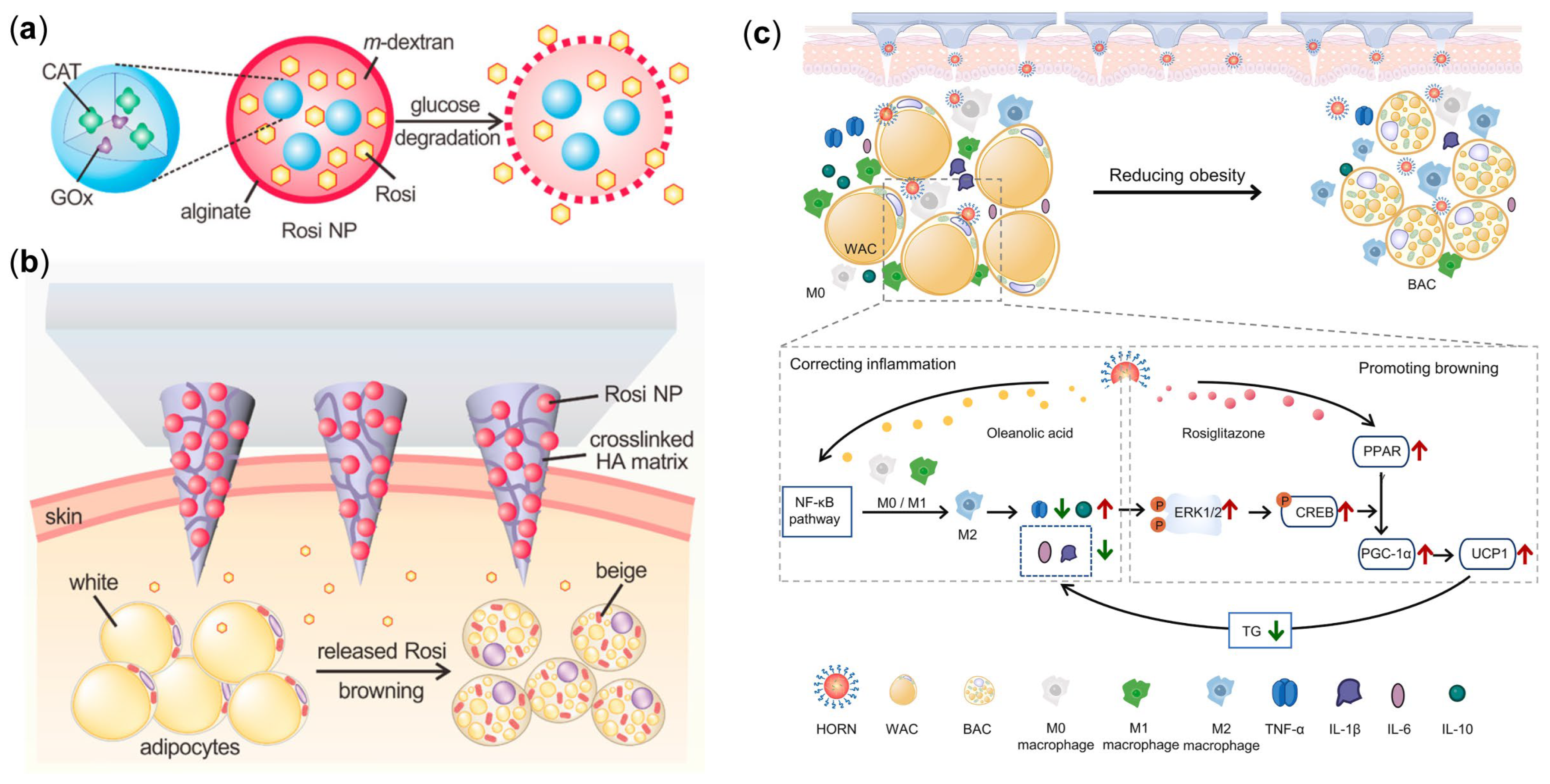
3.3. MN Delivering Rosiglitazone for Obesity Treatment
3.4. MN Delivering Liraglutide for Obesity Treatment
4. Combination Therapy
4.1. Microneedling Combined with INT for Obesity Treatment
4.2. Microneedling Combined with PTT for Obesity Treatment

5. Conclusions and Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Mahase, E. Global cost of overweight and obesity will hit $4.32tn a year by 2035, report warns. BMJ 2023, 380, 523. [Google Scholar] [CrossRef] [PubMed]
- Seidell, J.C.; Halberstadt, J. The global burden of obesity and the challenges of prevention. Ann. Nutr. Metab. 2015, 66 (Suppl. S2), 7–12. [Google Scholar] [CrossRef]
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet 2017, 390, 2627–2642. [Google Scholar] [CrossRef] [PubMed]
- Bell, J.A.; Kivimaki, M.; Hamer, M. Metabolically healthy obesity and risk of incident type 2 diabetes: A meta-analysis of prospective cohort studies. Obes. Rev. 2014, 15, 504–515. [Google Scholar] [CrossRef] [PubMed]
- Bianchini, F.; Kaaks, R.; Vainio, H. Overweight, obesity, and cancer risk. Lancet. Oncol. 2002, 3, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Alderman, A.K.; Collins, E.D.; Streu, R.; Grotting, J.C.; Sulkin, A.L.; Neligan, P.; Haeck, P.C.; Gutowski, K.A. Benchmarking outcomes in plastic surgery: National complication rates for abdominoplasty and breast augmentation. Plast. Reconstr. Surg. 2009, 124, 2127–2133. [Google Scholar] [CrossRef]
- Yoho, R.A.; Romaine, J.J.; O’Neil, D. Review of the liposuction, abdominoplasty, and face-lift mortality and morbidity risk literature. Dermatol. Surg. 2005, 31 Pt 1, 733–743; discussion 743. [Google Scholar] [CrossRef]
- Dombrowski, S.U.; Knittle, K.; Avenell, A.; Araújo-Soares, V.; Sniehotta, F.F. Long term maintenance of weight loss with non-surgical interventions in obese adults: Systematic review and meta-analyses of randomised controlled trials. BMJ 2014, 348, g2646. [Google Scholar] [CrossRef]
- Noh, Y.; Heo, C.Y. The effect of phosphatidylcholine and deoxycholate compound injections to the localized adipose tissue: An experimental study with a murine model. Arch. Plast. Surg. 2012, 39, 452–456. [Google Scholar] [CrossRef][Green Version]
- Rotunda, A.M.; Kolodney, M.S. Mesotherapy and phosphatidylcholine injections: Historical clarification and review. Dermatol. Surg. 2006, 32, 465–480. [Google Scholar] [CrossRef] [PubMed]
- Son, J.W.; Kim, S. Comprehensive review of current and upcoming anti-obesity drugs. Diabetes Metab. J. 2020, 44, 802–818. [Google Scholar] [CrossRef] [PubMed]
- Manstein, D.; Laubach, H.; Watanabe, K.; Farinelli, W.; Zurakowski, D.; Anderson, R.R. Selective cryolysis: A novel method of non-invasive fat removal. Lasers Surg. Med. 2008, 40, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Moraga, J.; Valero-Altés, T.; Riquelme, A.M.; Isarria-Marcosy, M.I.; de la Torre, J.R. Body contouring by non-invasive transdermal focused ultrasound. Lasers Surg. Med. 2007, 39, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Mulholland, R.S.; Paul, M.D.; Chalfoun, C. Noninvasive body contouring with radiofrequency, ultrasound, cryolipolysis, and low-level laser therapy. Clin. Plast. Surg. 2011, 38, 503–520. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhang, Z.; Xia, T.; Liu, C.; Sun, C. circNrxn2 promoted WAT browning via sponging miR-103 to relieve its inhibition of FGF10 in HFD mice. Mol. Ther. Nucleic Acids 2019, 17, 551–562. [Google Scholar] [CrossRef] [PubMed]
- Sibuyi, N.R.S.; Moabelo, K.L.; Meyer, M.; Onani, M.O.; Dube, A.; Madiehe, A.M. Nanotechnology advances towards development of targeted-treatment for obesity. J. Nanobiotechnol. 2019, 17, 122. [Google Scholar] [CrossRef] [PubMed]
- Bartelt, A.; Heeren, J. Adipose tissue browning and metabolic health. Nat. Rev. Endocrinol. 2014, 10, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Q.; Yu, J.; Yu, S.; Wang, J.; Qiang, L.; Gu, Z. Locally induced adipose tissue browning by microneedle patch for obesity treatment. ACS Nano 2017, 11, 9223–9230. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhang, H.; Wang, Z.; Shiu, C.Y.A.; Gu, Z. Microneedle array patches integrated with nanoparticles for therapy and diagnosis. Small Struct. 2021, 2, 2000097. [Google Scholar] [CrossRef]
- Henry, S.; McAllister, D.V.; Allen, M.G.; Prausnitz, M.R. Microfabricated microneedles: A novel approach to transdermal drug delivery. J. Pharm. Sci. 1998, 87, 922–925. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Hu, W.; Ren, T.; Wang, X.; Lu, C.; Pan, X.; Wu, C.; Peng, T. Combating multidrug resistance of breast cancer with ginsenoside Rh2-irrigated nano-in-thermogel. Int. J. Pharm. 2024, 650, 123718. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhou, C.; Chen, T.; Jiang, Z.; Lu, C.; Wu, C.; Pan, X.; Huang, Z.; Peng, T. State-of-the-art strategies to enhance the mechanical properties of microneedles. Int. J. Pharm. 2024, 663, 124547. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yang, B.; Ren, T.; Wang, X.; Chen, H.; Lu, C.; Wu, C.; Pan, X.; Peng, T. Dual engine-driven bionic microneedles for early intervention and prolonged treatment of Alzheimer’s disease. J. Control. Release 2024, 367, 184–196. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, R.F.; Singh, T.R.R.; Woolfson, A.D. Microneedle-based drug delivery systems: Microfabrication, drug delivery, and safety. Drug Deliv. 2010, 17, 187–207. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, B.Z.; Wang, Q.L.; Jin, X.; Guo, X.D. Fabrication of coated polymer microneedles for transdermal drug delivery. J. Control. Release 2017, 265, 14–21. [Google Scholar] [CrossRef]
- Davis, S.P.; Martanto, W.; Allen, M.G.; Prausnitz, M.R. Hollow metal microneedles for insulin delivery to diabetic rats. IEEE Trans. Biomed. Eng. 2005, 52, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Duong, H.T.T.; Kim, N.W.; Thambi, T.; Phan, V.H.G.; Lee, M.S.; Yin, Y.; Jeong, J.H.; Lee, D.S. Microneedle arrays coated with charge reversal pH-sensitive copolymers improve antigen presenting cells-homing DNA vaccine delivery and immune responses. J. Control. Release 2018, 269, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, Y.; Xian, Y.; Singh, P.; Feng, J.; Cui, S.; Carrier, A.; Oakes, K.; Luan, T.; Zhang, X. Multifunctional graphene-oxide-reinforced dissolvable polymeric microneedles for transdermal drug delivery. ACS Appl. Mater. Interfaces 2020, 12, 352–360. [Google Scholar] [CrossRef] [PubMed]
- McAllister, D.V.; Wang, P.M.; Davis, S.P.; Park, J.H.; Canatella, P.J.; Allen, M.G.; Prausnitz, M.R. Microfabricated needles for transdermal delivery of macromolecules and nanoparticles: Fabrication methods and transport studies. Proc. Natl. Acad. Sci. USA 2003, 100, 13755–13760. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, S.P.; Murthy, N.; Prausnitz, M.R. Minimally invasive protein delivery with rapidly dissolving polymer microneedles. Adv. Mater. 2008, 20, 933–938. [Google Scholar] [CrossRef] [PubMed]
- Bariya, S.H.; Gohel, M.C.; Mehta, T.A.; Sharma, O.P. Microneedles: An emerging transdermal drug delivery system. J. Pharm. Pharmacol. 2012, 64, 11–29. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Kim, J.D.; Lee, C.Y.; Her, S.; Jung, H. A high-capacity, hybrid electro-microneedle for in-situ cutaneous gene transfer. Biomaterials 2011, 32, 7705–7710. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, R.F.; Singh, T.R.; Tunney, M.M.; Morrow, D.I.; McCarron, P.A.; O’Mahony, C.; Woolfson, A.D. Microneedle arrays allow lower microbial penetration than hypodermic needles in vitro. Pharm. Res. 2009, 26, 2513–2522. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Liu, X.; Fu, Y.; Song, Y. Recent advances of microneedles for biomedical applications: Drug delivery and beyond. Acta Pharm. Sin. B 2019, 9, 469–483. [Google Scholar] [CrossRef] [PubMed]
- Halder, J.; Gupta, S.; Kumari, R.; Gupta, G.D.; Rai, V.K. Microneedle array: Applications, recent ddvances, and clinical pertinence in transdermal drug delivery. J. Pharm. Innov. 2021, 16, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yang, B.; Luan, X.; Jiang, L.; Lu, C.; Wu, C.; Pan, X.; Peng, T. State of the art in constructing gas-propelled dissolving microneedles for significantly enhanced drug-loading and delivery efficiency. Pharmaceutics 2023, 15, 1059. [Google Scholar] [CrossRef] [PubMed]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007, 2, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhang, Z.; Joseph, J.; Zhang, X.; Ferdows, B.E.; Patel, D.N.; Chen, W.; Banfi, G.; Molinaro, R.; Cosco, D.; et al. Biomaterials and nanomedicine for bone regeneration: Progress and future prospects. Exploration 2021, 1, 20210011. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Pan, X.; Wu, Q.; Guo, J.; Wang, C.; Liu, H. Manganese carbonate nanoparticles-mediated mitochondrial dysfunction for enhanced sonodynamic therapy. Exploration 2021, 1, 20210010. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, H.; Chen, Y.; Li, Z.; Liu, S.; Guan, W.; Lin, Y.; Cao, C.; Zheng, W.; Wu, J. Novel emerging nano-assisted anti-cancer strategies based on the STING pathway. Acta Mater. Medica 2023, 2, 323–341. [Google Scholar] [CrossRef]
- Nasra, S.; Bhatia, D.; Kumar, A. Recent advances in nanoparticle-based drug delivery systems for rheumatoid arthritis treatment. Nanoscale Adv. 2022, 4, 3479–3494. [Google Scholar] [CrossRef]
- Olsen, D.; Yang, C.; Bodo, M.; Chang, R.; Leigh, S.; Baez, J.; Carmichael, D.; Perälä, M.; Hämäläinen, E.R.; Jarvinen, M.; et al. Recombinant collagen and gelatin for drug delivery. Adv. Drug Deliv. Rev. 2003, 55, 1547–1567. [Google Scholar] [CrossRef] [PubMed]
- Siersbæk, R.; Nielsen, R.; Mandrup, S. Transcriptional networks and chromatin remodeling controlling adipogenesis. Trends Endocrinol. Metab. 2012, 23, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Palmer, D.G.; Rutter, G.A.; Tavaré, J.M. Insulin-stimulated fatty acid synthase gene expression does not require increased sterol response element binding protein 1 transcription in primary adipocytes. Biochem. Biophys. Res. Commun. 2002, 291, 439–443. [Google Scholar] [CrossRef] [PubMed]
- An, S.M.; Seong, K.Y.; Yim, S.G.; Hwang, Y.J.; Bae, S.H.; Yang, S.Y.; An, B.S. Intracutaneous delivery of gelatins induces lipolysis and suppresses lipogenesis of adipocytes. Acta Biomater. 2018, 67, 238–247. [Google Scholar] [CrossRef]
- An, S.M.; Kim, M.J.; Seong, K.Y.; Jeong, J.S.; Kang, H.G.; Kim, S.Y.; Kim, D.S.; Kang, D.H.; Yang, S.Y.; An, B.S. Intracutaneous delivery of gelatins reduces fat accumulation in subcutaneous adipose tissue. Toxicol. Res. 2019, 35, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Rupasinghe, H.P.; Sekhon-Loodu, S.; Mantso, T.; Panayiotidis, M.I. Phytochemicals in regulating fatty acid β-oxidation: Potential underlying mechanisms and their involvement in obesity and weight loss. Pharmacol. Ther. 2016, 165, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Riley, M.; Taylor, A.W.; Page, A. Chilli consumption and the incidence of overweight and obesity in a Chinese adult population. Int. J. Obes. 2017, 41, 1074–1079. [Google Scholar] [CrossRef] [PubMed]
- Mainka, T.; Malewicz, N.M.; Baron, R.; Enax-Krumova, E.K.; Treede, R.D.; Maier, C. Presence of hyperalgesia predicts analgesic efficacy of topically applied capsaicin 8% in patients with peripheral neuropathic pain. Eur. J. Pain 2016, 20, 116–129. [Google Scholar] [CrossRef]
- Shi, Z.; Zhang, M.; Liu, J. Chili intake is inversely associated with chronic kidney disease among adults: A population-based study. Nutrients 2019, 11, 2949. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, D.; Huang, J.; Hu, Y.; Xu, Y. Application of capsaicin as a potential new therapeutic drug in human cancers. J. Clin. Pharm. Ther. 2020, 45, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Fattori, V.; Hohmann, M.S.; Rossaneis, A.C.; Pinho-Ribeiro, F.A.; Verri, W.A. Capsaicin: Current understanding of its mechanisms and therapy of pain and other pre-clinical and clinical uses. Molecules 2016, 21, 844. [Google Scholar] [CrossRef] [PubMed]
- Stuby, J.; Gravestock, I.; Wolfram, E.; Pichierri, G.; Steurer, J.; Burgstaller, J.M. Appetite-suppressing and satiety-increasing bioactive phytochemicals: A systematic review. Nutrients 2019, 11, 2238. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, V.; Baskaran, P.; Thyagarajan, B. Troglitazone activates TRPV1 and causes deacetylation of PPARγ in 3T3-L1 cells. Biochim. Biophys. Acta. (BBA) Mol. Basis. Dis. 2019, 1865, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Joo, J.I.; Kim, D.H.; Choi, J.W.; Yun, J.W. Proteomic analysis for antiobesity potential of capsaicin on white adipose tissue in rats fed with a high fat diet. J. Proteome Res. 2010, 9, 2977–2987. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.L.; Yen, G.C. Effects of capsaicin on induction of apoptosis and inhibition of adipogenesis in 3T3-L1 cells. J. Agric. Food Chem. 2007, 55, 1730–1736. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.T.; Park, I.J.; Shin, J.I.; Lee, Y.K.; Lee, S.K.; Baik, H.W.; Ha, J.; Park, O.J. Genistein, EGCG, and capsaicin inhibit adipocyte differentiation process via activating AMP-activated protein kinase. Biochem. Biophys. Res. Commun. 2005, 338, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Baboota, R.K.; Singh, D.P.; Sarma, S.M.; Kaur, J.; Sandhir, R.; Boparai, R.K.; Kondepudi, K.K.; Bishnoi, M. Capsaicin induces "brite" phenotype in differentiating 3T3-L1 preadipocytes. PLoS ONE 2014, 9, e103093. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Chen, C.; Lan, Y.; Xiao, J.; Li, R.; Huang, J.; Huang, Q.; Cao, Y.; Ho, C.T. Capsaicin-the major bioactive ingredient of chili peppers: Bio-efficacy and delivery systems. Food Funct. 2020, 11, 2848–2860. [Google Scholar] [CrossRef] [PubMed]
- Bao, C.; Li, Z.; Liang, S.; Hu, Y.; Wang, X.; Fang, B.; Wang, P.; Chen, S.; Li, Y. Microneedle patch delivery of capsaicin-containing α-lactalbumin nanomicelles to adipocytes achieves potent anti-obesity effects. Adv. Func. Mater. 2021, 31, 2011130. [Google Scholar] [CrossRef]
- Gu, Z.; Aimetti, A.A.; Wang, Q.; Dang, T.T.; Zhang, Y.; Veiseh, O.; Cheng, H.; Langer, R.S.; Anderson, D.G. Injectable nano-network for glucose-mediated insulin delivery. ACS Nano 2013, 7, 4194–4201. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Bao, C.; Li, D.; You, L.; Du, Y.; Liu, B.; Li, X.; Ren, F.; Li, Y. The construction of enzymolyzed α-lactalbumin based micellar nanoassemblies for encapsulating various kinds of hydrophobic bioactive compounds. Food Funct. 2019, 10, 8263–8272. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, S.; Baker, M.R.; Chatterjee, S.; Kumar, H. Botulinum toxin: An update on pharmacology and newer products in development. Toxins 2021, 13, 58. [Google Scholar] [CrossRef] [PubMed]
- Pirazzini, M.; Rossetto, O.; Eleopra, R.; Montecucco, C. Botulinum neurotoxins: Biology, pharmacology, and toxicology. Pharmacol. Rev. 2017, 69, 200–235. [Google Scholar] [CrossRef] [PubMed]
- Topazian, M.; Camilleri, M.; De La Mora-Levy, J.; Enders, F.B.; Foxx-Orenstein, A.E.; Levy, M.J.; Nehra, V.; Talley, N.J. Endoscopic ultrasound-guided gastric botulinum toxin injections in obese subjects: A pilot study. Obes. Surg. 2008, 18, 401–407. [Google Scholar] [CrossRef]
- Forrest, A.S.; Ordög, T.; Sanders, K.M. Neural regulation of slow-wave frequency in the murine gastric antrum. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 290, G486–G495. [Google Scholar] [CrossRef] [PubMed]
- Lammers, W.J. Inhomogeneities in the propagation of the slow wave in the stomach. Neurogastroenterol. Motil. 2015, 27, 1349–1353. [Google Scholar] [CrossRef] [PubMed]
- Elshakh, H.; El-Ejji, K.; Taheri, S. The role of endoscopic intra-gastric botulinum toxin-A for obesity treatment. Obes. Surg. 2017, 27, 2471–2478. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, Y.; Lin, L.; Li, Z.; Liu, F.; Zhu, L.; Chen, J.; Zhang, N.; Cao, X.; Ran, S.; et al. Layer-specific BTX-A delivery to the gastric muscularis achieves effective weight control and metabolic improvement. Adv. Sci. 2023, 10, e2300822. [Google Scholar] [CrossRef]
- Lehmann, J.M.; Moore, L.B.; Smith-Oliver, T.A.; Wilkison, W.O.; Willson, T.M.; Kliewer, S.A. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma (PPARγ). J. Biol. Chem. 1995, 270, 12953–12956. [Google Scholar] [CrossRef] [PubMed]
- Rosen, E.D.; Spiegelman, B.M. PPARγ: A nuclear regulator of metabolism, differentiation, and cell growth. J. Biol. Chem. 2001, 276, 37731–37734. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Xu, X.; Zhang, X.Q.; Farokhzad, O.C.; Langer, R. Preventing diet-induced obesity in mice by adipose tissue transformation and angiogenesis using targeted nanoparticles. Proc. Natl. Acad. Sci. USA 2016, 113, 5552–5557. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P.; Kajimura, S. The cellular and functional complexity of thermogenic fat. Nat. Rev. Mol. Cell. Biol. 2021, 22, 393–409. [Google Scholar] [CrossRef] [PubMed]
- Boden, G.; Cheung, P.; Mozzoli, M.; Fried, S.K. Effect of thiazolidinediones on glucose and fatty acid metabolism in patients with type 2 diabetes. Metabolism 2003, 52, 753–759. [Google Scholar] [CrossRef]
- McLaughlin, T.M.; Liu, T.; Yee, G.; Abbasi, F.; Lamendola, C.; Reaven, G.M.; Tsao, P.; Cushman, S.W.; Sherman, A. Pioglitazone increases the proportion of small cells in human abdominal subcutaneous adipose tissue. Obesity 2010, 18, 926–931. [Google Scholar] [CrossRef] [PubMed]
- Gaytan, S.L.; Beaven, E.; Gadad, S.S.; Nurunnabi, M. Progress and prospect of nanotechnology for cardiac fibrosis treatment. Interdiscip. Med. 2023, 1, e20230018. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Qi, Z.; Chao, J. Framework nucleic acids directed assembly of Au nanostructures for biomedical applications. Interdiscip. Med. 2023, 1, e20220009. [Google Scholar] [CrossRef]
- Kolonin, M.G.; Saha, P.K.; Chan, L.; Pasqualini, R.; Arap, W. Reversal of obesity by targeted ablation of adipose tissue. Nat. Med. 2004, 10, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Hossen, M.N.; Kajimoto, K.; Akita, H.; Hyodo, M.; Harashima, H. Vascular-targeted nanotherapy for obesity: Unexpected passive targeting mechanism to obese fat for the enhancement of active drug delivery. J. Control. Release 2012, 163, 101–110. [Google Scholar] [CrossRef]
- Hossen, N.; Kajimoto, K.; Akita, H.; Hyodo, M.; Harashima, H. A comparative study between nanoparticle-targeted therapeutics and bioconjugates as obesity medication. J. Control. Release 2013, 171, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Hossen, M.N.; Kajimoto, K.; Akita, H.; Hyodo, M.; Ishitsuka, T.; Harashima, H. Therapeutic assessment of cytochrome C for the prevention of obesity through endothelial cell-targeted nanoparticulate system. Mol. Ther. 2013, 21, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Won, Y.W.; Adhikary, P.P.; Lim, K.S.; Kim, H.J.; Kim, J.K.; Kim, Y.H. Oligopeptide complex for targeted non-viral gene delivery to adipocytes. Nat. Mater. 2014, 13, 1157–1164. [Google Scholar] [CrossRef] [PubMed]
- Salameh, A.; Daquinag, A.C.; Staquicini, D.I.; An, Z.; Hajjar, K.A.; Pasqualini, R.; Arap, W.; Kolonin, M.G. Prohibitin/annexin 2 interaction regulates fatty acid transport in adipose tissue. JCI Insight 2016, 1, e86351. [Google Scholar] [CrossRef] [PubMed]
- Hiradate, R.; Khalil, I.A.; Matsuda, A.; Sasaki, M.; Hida, K.; Harashima, H. A novel dual-targeted rosiglitazone-loaded nanoparticle for the prevention of diet-induced obesity via the browning of white adipose tissue. J. Control. Release 2021, 329, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Mudhakir, D.; Akita, H.; Khalil, I.A.; Futaki, S.; Harashima, H. Pharmacokinetic analysis of the tissue distribution of octaarginine modified liposomes in mice. Drug Metab. Pharmacokinet. 2005, 20, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wang, J.; Sun, L.; Xia, F.; Li, W.; Yuan, L.; Liu, C.; Li, P.; Bao, C.; Wang, M.; et al. A quick paster type of soluble nanoparticle microneedle patch for the treatment of obesity. Biomaterials 2024, 311, 122687. [Google Scholar] [CrossRef] [PubMed]
- Bailey, C.J.; Flatt, P.R.; Conlon, J.M. An update on peptide-based therapies for type 2 diabetes and obesity. Peptides 2023, 161, 170939. [Google Scholar] [CrossRef] [PubMed]
- Freibott, C.E.; Phillips, K.T.; Anderson, B.J.; Stewart, C.; Liebschutz, J.M.; Stein, M.D. Under the skin: The relationship between subcutaneous injection and skin infections among people who inject drugs. J. Addict. Med. 2022, 16, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Zhang, F.; Huang, Y.; Hu, W.; Chen, Y.; Jiang, L.; Pan, X.; Wu, C.; Lu, C.; Peng, T. Site-specifically launched microneedles for the combined treatment of psoriasis-diabetic comorbidity. ACS Appl. Mater. Interfaces 2023, 15, 46613–46625. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Peng, T.; Huang, Y.; Ren, T.; Chen, H.; Chen, Y.; Feng, D.; Wu, C.; Pan, X. Hyaluronidase-powered microneedles for significantly enhanced transdermal delivery efficiency. J. Control. Release 2023, 353, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.; Chen, Y.; Luan, X.; Hu, W.; Wu, W.; Guo, B.; Lu, C.; Wu, C.; Pan, X. Microneedle technology for enhanced topical treatment of skin infections. Bioact. Mater. 2025, 45, 274–300. [Google Scholar] [CrossRef] [PubMed]
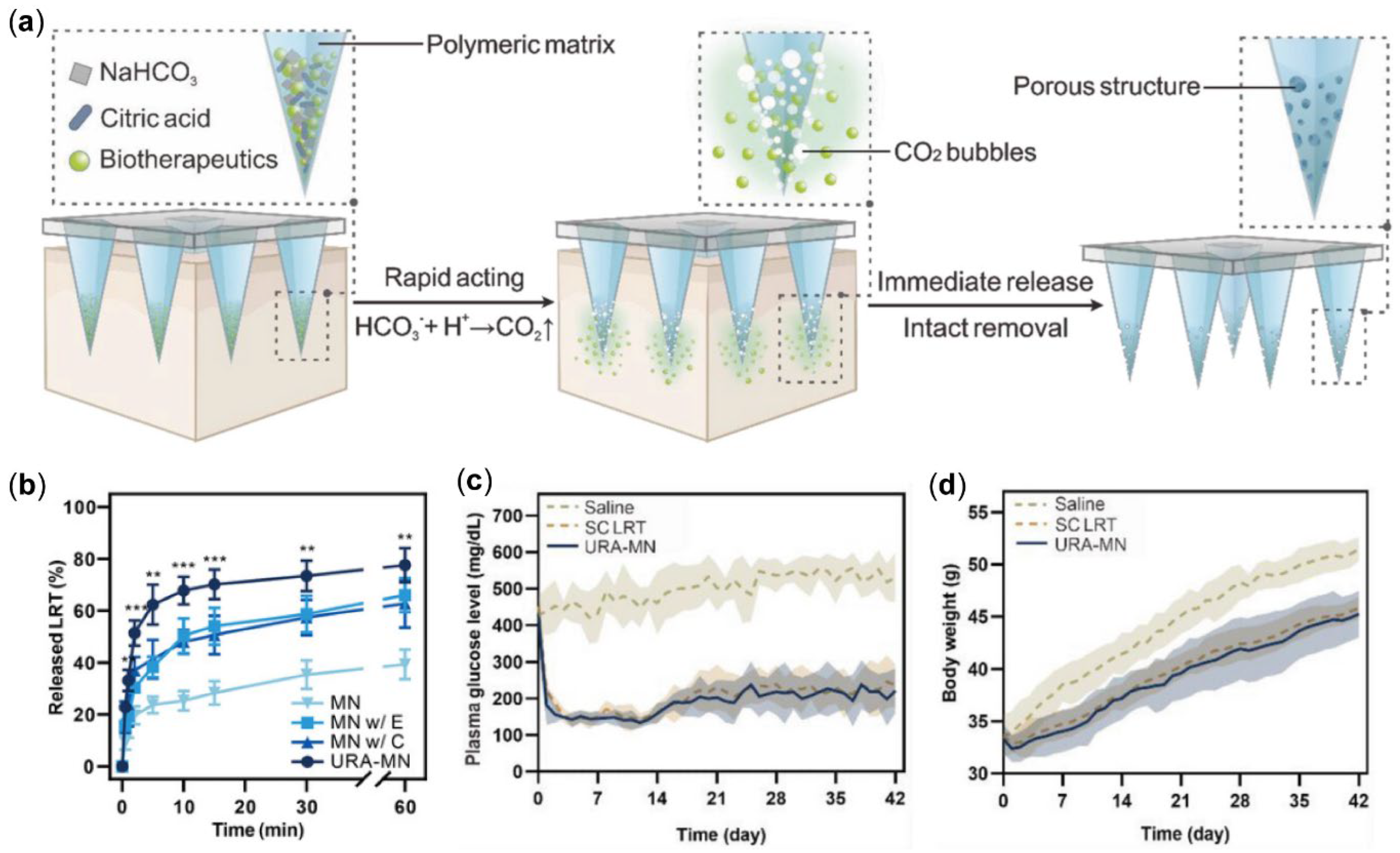
- You, J.; Yang, C.; Han, J.; Wang, H.; Zhang, W.; Zhang, Y.; Lu, Z.; Wang, S.; Cai, R.; Li, H.; et al. Ultrarapid-acting microneedles for immediate delivery of biotherapeutics. Adv. Mater. 2023, 35, e2304582. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, M.; Heath, B. Iontophoresis and electroporation-assisted microneedles: Advancements and therapeutic potentials in transdermal drug delivery. Drug Deliv. Transl. Res. 2024. [Google Scholar] [CrossRef] [PubMed]
- Roustit, M.; Blaise, S.; Cracowski, J.L. Trials and tribulations of skin iontophoresis in therapeutics. Br. J. Clin. Pharmacol. 2014, 77, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Roustit, M.; Blaise, S.; Cracowski, J.L. Sodium nitroprusside iontophoresis on the finger pad does not consistently increase skin blood flow in healthy controls and patients with systemic sclerosis. Microvasc. Res. 2009, 77, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Pariser, D.M.; Ballard, A. Iontophoresis for palmar and plantar hyperhidrosis. Dermatol. Clin. 2014, 32, 491–494. [Google Scholar] [CrossRef] [PubMed]
- Dhote, V.; Bhatnagar, P.; Mishra, P.K.; Mahajan, S.C.; Mishra, D.K. Iontophoresis: A potential emergence of a transdermal drug delivery system. Sci. Pharm. 2012, 80, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Jeong, W.Y.; Kwon, M.; Choi, H.E.; Kim, K.S. Recent advances in transdermal drug delivery systems: A review. Biomater. Res. 2021, 25, 24. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Liu, F.; Liu, G.; Li, H.; Xu, Y.; Sun, S. Research progress of physical transdermal enhancement techniques in tumor therapy. Chem. Commun. 2023, 59, 3339–3359. [Google Scholar] [CrossRef]
- Gao, Y.; Du, L.; Li, Q.; Li, Q.; Zhu, L.; Yang, M.; Wang, X.; Zhao, B.; Ma, S. How physical techniques improve the transdermal permeation of therapeutics: A review. Medicine 2022, 101, e29314. [Google Scholar] [CrossRef] [PubMed]
- Alimardani, V.; Abolmaali, S.S.; Yousefi, G.; Rahiminezhad, Z.; Abedi, M.; Tamaddon, A.; Ahadian, S. Microneedle arrays combined with nanomedicine approaches for transdermaldelivery of therapeutics. J. Clin. Med. 2021, 10, 181. [Google Scholar] [CrossRef] [PubMed]
- Vora, D.; Garimella, H.T.; German, C.L.; Banga, A.K. Microneedle and iontophoresis mediated delivery of methotrexate into and across healthy and psoriatic skin. Int. J. Pharm. 2022, 618, 121693. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Zhang, Z.; Xu, C. Transdermal delivery of dextran using conductive microneedles assisted by iontophoresis. J. Mater. Chem. B 2022, 10, 8075–8081. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, M.; Fan, Z.; Dawson, J.A.; Wang, S. Transdermal delivery of metformin using dissolving microneedles and iontophoresis patches for browning subcutaneous adipose tissue. Pharmaceutics 2022, 14, 879. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Ye, R.; Gong, X.; Yang, J.; Liu, B.; Xu, Y.; Nie, G.; Xie, X.; Jiang, L. Iontophoresis-driven microneedle patch for the active transdermal delivery of vaccine macromolecules. Microsyst. Nanoeng. 2023, 9, 35. [Google Scholar] [CrossRef] [PubMed]
- Petchsangsai, M.; Rojanarata, T.; Opanasopit, P.; Ngawhirunpat, T. The combination of microneedles with electroporation and sonophoresis to enhance hydrophilic macromolecule skin penetration. Biol. Pharm. Bull. 2014, 37, 1373–1382. [Google Scholar] [CrossRef] [PubMed]
- Schoellhammer, C.M.; Blankschtein, D.; Langer, R. Skin permeabilization for transdermal drug delivery: Recent advances and future prospects. Expert Opin. Drug. Deliv. 2014, 11, 393–407. [Google Scholar] [CrossRef] [PubMed]
- El-Kaream, S.A.A.; Hussein, N.G.A.; El-Kholey, S.M.; Elhelbawy, A. Microneedle combined with iontophoresis and electroporation for assisted transdermal delivery of goniothalamus macrophyllus for enhancement sonophotodynamic activated cancer therapy. Sci. Rep. 2024, 14, 7962. [Google Scholar] [CrossRef] [PubMed]
- Parrilla, M.; Detamornrat, U.; Domínguez-Robles, J.; Tunca, S.; Donnelly, R.F.; De Wael, K. Wearable microneedle-based array patches for continuous electrochemical monitoring and drug delivery: Toward a closed-loop system for methotrexate treatment. ACS Sens. 2023, 8, 4161–4170. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Fang, X.; Kong, J. Engineered microneedles for interstitial fluid cell-free DNA capture and sensing using iontophoretic dual-extraction wearable patch. Adv. Funct. Mater. 2020, 30, 2000591. [Google Scholar] [CrossRef]
- Singh, N.D.; Banga, A.K. Controlled delivery of ropinirole hydrochloride through skin using modulated iontophoresis and microneedles. J. Drug Target. 2013, 21, 354–366. [Google Scholar] [CrossRef] [PubMed]
- Junaid, M.S.A.; Banga, A.K. Transdermal delivery of baclofen using iontophoresis and microneedles. AAPS PharmSciTech 2022, 23, 84. [Google Scholar] [CrossRef] [PubMed]
- Dandekar, A.A.; Kale, M.; Garimella, H.T.; Banga, A.K. Effect of compromised skin barrier on delivery of diclofenac sodium from brand and generic formulations via microneedles and iontophoresis. Int. J. Pharm. 2022, 628, 122271. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, R.F.; Garland, M.J.; Alkilani, A.Z. Microneedle-iontophoresis combinations for enhanced transdermal drug delivery. Methods Mol. Biol. 2014, 1141, 121–132. [Google Scholar] [PubMed]
- Arshad, M.S.; Hussain, S.; Zafar, S.; Rana, S.J.; Chohan, T.A.; Hamza, M.; Nazari, K.; Ahmad, Z. Transcutaneous delivery of dexamethasone sodium phosphate via microneedle-assisted iontophoretic enhancement—A potential therapeutic option for inflammatory disorders. Pharm. Res. 2024, 41, 1183–1199. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhou, Y.; Yang, D.; Gao, X.; Wen, T.; Fu, J.; Wen, X.; Quan, G.; Pan, X.; Wu, C. Intelligent and spatiotemporal drug release based on multifunctional nanoparticle-integrated dissolving microneedle system for synergetic chemo-photothermal therapy to eradicate melanoma. Acta Biomater. 2021, 135, 164–178. [Google Scholar] [CrossRef] [PubMed]
- Lian, H.; Guan, P.; Tan, H.; Zhang, X.; Meng, Z. Near-infrared light triggered multi-hit therapeutic nanosystem for tumor specific photothermal effect amplified signal pathway regulation and ferroptosis. Bioact. Mater. 2022, 9, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Lan, G.; Ni, K.; Lin, W. Nanoscale metal-organic frameworks for phototherapy of cancer. Coord. Chem. Rev. 2019, 379, 65–81. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Luo, M.; Jia, Y.; Gao, T.; Deng, L.; Gong, T.; Zhang, Z.; Cao, X.; Fu, Y. Adipocyte-targeted delivery of rosiglitazone with localized photothermal therapy for the treatment of diet-induced obesity in mice. Acta Biomater. 2024, 181, 317–332. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, J.; Tian, J.; Li, H. A polydopamine nanomedicine used in photothermal therapy for liver cancer knocks down the anti-cancer target NEDD8-E3 ligase ROC1 (RBX1). J. Nanobiotechnol. 2021, 19, 323. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Liu, Y.; Lin, W.; Lian, H.; Meng, Z. A microneedle patch realizes weight loss through photothermal induction of fat browning. Biomater. Sci. 2024, 12, 1726–1737. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, H.; Hu, W.; Zhou, C.; Jian, J.; Xu, J.; Lu, C.; Quan, G.; Wu, C.; Pan, X.; Peng, T. Microneedle-Mediated Treatment of Obesity. Pharmaceutics 2025, 17, 248. https://doi.org/10.3390/pharmaceutics17020248
Pan H, Hu W, Zhou C, Jian J, Xu J, Lu C, Quan G, Wu C, Pan X, Peng T. Microneedle-Mediated Treatment of Obesity. Pharmaceutics. 2025; 17(2):248. https://doi.org/10.3390/pharmaceutics17020248
Chicago/Turabian StylePan, Huanhuan, Wanshan Hu, Chunxian Zhou, Jubo Jian, Jing Xu, Chao Lu, Guilan Quan, Chuanbin Wu, Xin Pan, and Tingting Peng. 2025. "Microneedle-Mediated Treatment of Obesity" Pharmaceutics 17, no. 2: 248. https://doi.org/10.3390/pharmaceutics17020248
APA StylePan, H., Hu, W., Zhou, C., Jian, J., Xu, J., Lu, C., Quan, G., Wu, C., Pan, X., & Peng, T. (2025). Microneedle-Mediated Treatment of Obesity. Pharmaceutics, 17(2), 248. https://doi.org/10.3390/pharmaceutics17020248










