Innovations in Cancer Therapy: Endogenous Stimuli-Responsive Liposomes as Advanced Nanocarriers
Abstract
1. Introduction
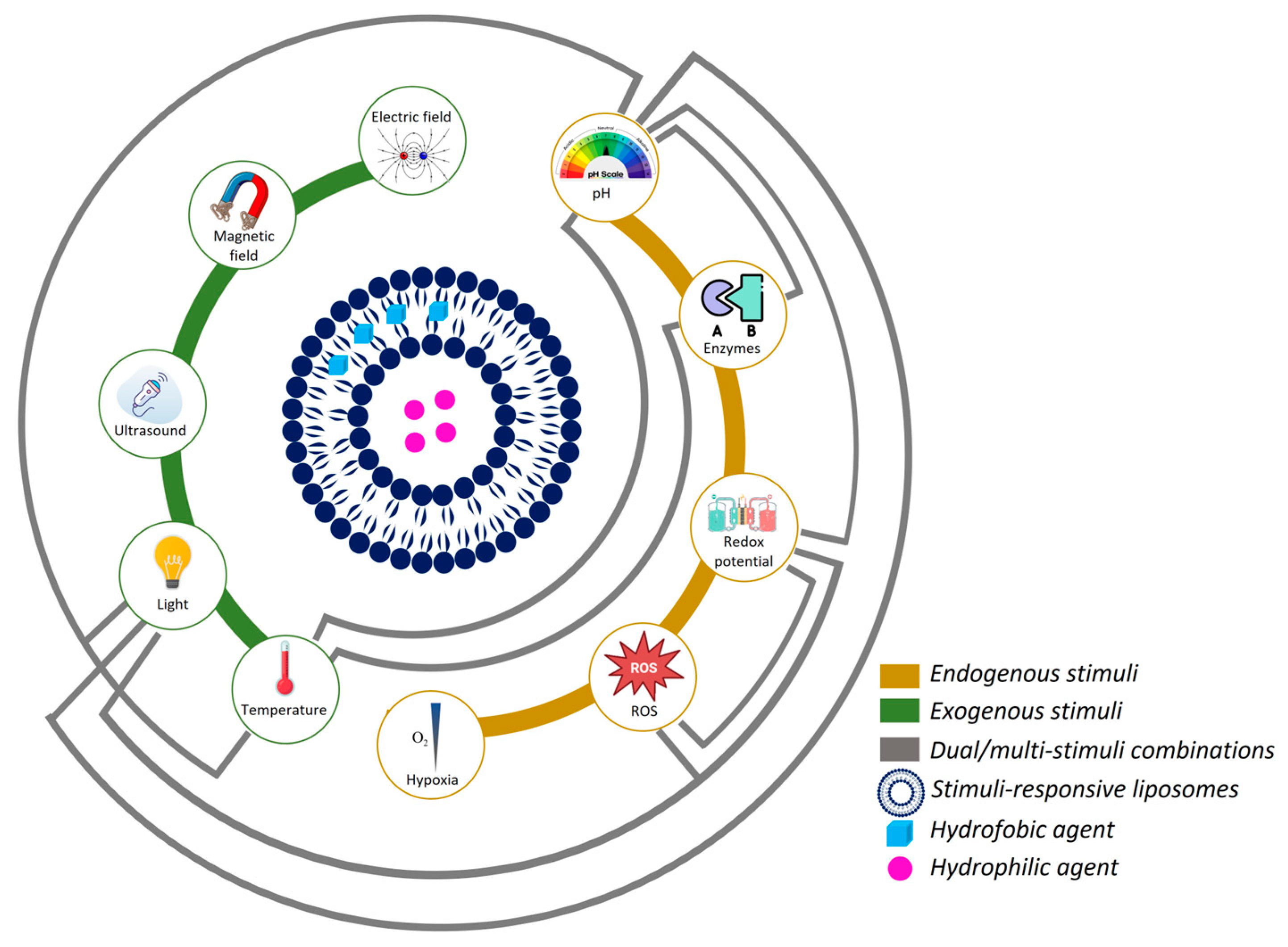
2. Stimuli-Responsive Nanocarriers
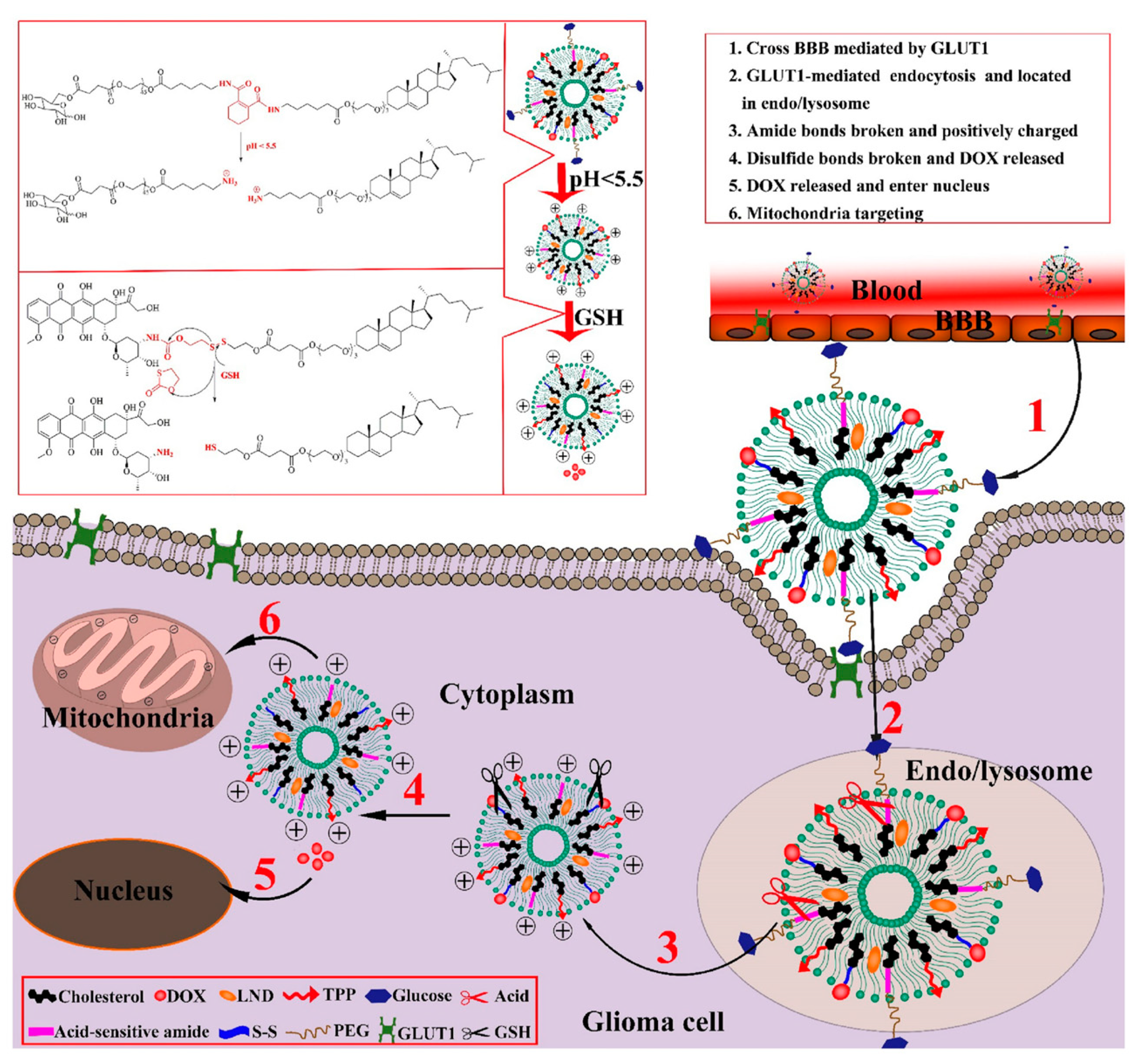
2.1. pH-Responsive Liposomes
2.2. Enzyme-Responsive Liposomes
2.3. Redox-Responsive Liposomes
2.4. ROS-Responsive Liposomes
2.5. Hypoxia-Responsive Liposomes
3. Dual/Multi-Responsive Liposomes
4. Remarks and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Niculescu, A.-G.; Grumezescu, A.M. Novel tumor-targeting nanoparticles for cancer treatment—A review. Int. J. Mol. Sci. 2022, 23, 5253. [Google Scholar] [CrossRef]
- Hossen, S.; Hossain, M.K.; Basher, M.K.; Mia, M.N.H.; Rahman, M.T.; Uddin, M.J. Smart nanocarrier-based drug delivery systems for cancer therapy and toxicity studies: A review. J. Adv. Res. 2019, 15, 1–18. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Delfi, M.; Zarrabi, A.; Bigham, A.; Sharifi, E.; Rabiee, N.; Paiva-Santos, A.C.; Kumar, A.P.; Tan, S.C.; Hushmandi, K. Stimuli-responsive liposomal nanoformulations in cancer therapy: Pre-clinical & clinical approaches. J. Control. Release 2022, 351, 50–80. [Google Scholar] [PubMed]
- AlSawaftah, N.; Pitt, W.G.; Husseini, G.A. Dual-targeting and stimuli-triggered liposomal drug delivery in cancer treatment. ACS Pharmacol. Transl. Sci. 2021, 4, 1028–1049. [Google Scholar] [CrossRef] [PubMed]
- Dymek, M.; Sikora, E. Liposomes as biocompatible and smart delivery systems–the current state. Adv. Colloid. Interface Sci. 2022, 309, 102757. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.-M.; Jeong, H.-J. Liposomes: Biomedical applications. Chonnam Med. J. 2021, 57, 27. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Lu, Y.; Qi, J.; Zhu, Q.; Chen, Z.; Wu, W. Adapting liposomes for oral drug delivery. Acta Pharm. Sin. B 2019, 9, 36–48. [Google Scholar] [CrossRef]
- Mukhamadiyarov, R.A.; Senokosova, E.A.; Krutitsky, S.S.; Voevoda, D.V.; Pyshnaya, I.A.; Ivanov, V.V.; Lewis, M.J.; Khaliulin, I. Size-dependent ability of liposomes to accumulate in the ischemic myocardium and protect the heart. J. Cardiovasc. Pharmacol. 2018, 72, 143–152. [Google Scholar] [CrossRef]
- Xiao, Y.; Shi, K.; Qu, Y.; Chu, B.; Qian, Z. Engineering nanoparticles for targeted delivery of nucleic acid therapeutics in Tumor. Mol. Ther. Methods Clin. Dev. 2019, 12, 1–18. [Google Scholar] [CrossRef] [PubMed]
- El-Hammadi, M.M.; Arias, J.L. An update on liposomes in drug delivery: A patent review (2014-2018). Expert. Opin. Ther. Pat. 2019, 29, 891–907. [Google Scholar] [CrossRef]
- Beltrán-Gracia, E.; López-Camacho, A.; Higuera-Ciapara, I.; Velázquez-Fernández, J.B.; Vallejo-Cardona, A.A. Nanomedicine review: Clinical developments in liposomal applications. Cancer Nanotechnol. 2019, 10, 1–40. [Google Scholar] [CrossRef]
- Zangabad, P.S.; Mirkiani, S.; Shahsavari, S.; Masoudi, B.; Masroor, M.; Hamed, H.; Jafari, Z.; Taghipour, Y.D.; Hashemi, H.; Karimi, M. Stimulus-responsive liposomes as smart nanoplatforms for drug delivery applications. Nanotechnol. Rev. 2018, 7, 95–122. [Google Scholar] [CrossRef]
- Dinakar, Y.H.; Karole, A.; Parvez, S.; Jain, V.; Mudavath, S.L. Organ-restricted delivery through stimuli-responsive nanocarriers for lung cancer therapy. Life Sci. 2022, 310, 121133. [Google Scholar] [CrossRef]
- Mi, P. Stimuli-responsive nanocarriers for drug delivery, tumor imaging, therapy and theranostics. Theranostics 2020, 10, 4557. [Google Scholar] [PubMed]
- Abousalman-Rezvani, Z.; Refaat, A.; Dehghankelishadi, P.; Roghani-Mamaqani, H.; Esser, L.; Voelcker, N.H. Insights into Targeted and Stimulus-Responsive Nanocarriers for Brain Cancer Treatment. Adv. Healthc. Mater. 2024, 13, 2302902. [Google Scholar] [CrossRef]
- Qiu, Y.; Zhu, Z.; Miao, Y.; Zhang, P.; Jia, X.; Liu, Z.; Zhao, X. Polymerization of dopamine accompanying its coupling to induce self-assembly of block copolymer and application in drug delivery. Polym. Chem. 2020, 11, 2811–2821. [Google Scholar] [CrossRef]
- Zheng, K.; Chen, R.; Sun, Y.; Tan, Z.; Liu, Y.; Cheng, X.; Leng, J.; Guo, Z.; Xu, P. Cantharidin-loaded functional mesoporous titanium peroxide nanoparticles for non-small cell lung cancer targeted chemotherapy combined with high effective photodynamic therapy. Thorac. Cancer 2020, 11, 1476–1486. [Google Scholar] [CrossRef]
- Zhao, X.; Bai, J.; Yang, W. Stimuli-responsive nanocarriers for therapeutic applications in cancer. Cancer Biol. Med. 2021, 18, 319. [Google Scholar] [CrossRef]
- Saraf, S.; Jain, A.; Tiwari, A.; Verma, A.; Panda, P.K.; Jain, S.K. Advances in liposomal drug delivery to cancer: An overview. J. Drug Deliv. Sci. Technol. 2020, 56, 101549. [Google Scholar] [CrossRef]
- Aghdam, M.A.; Bagheri, R.; Mosafer, J.; Baradaran, B.; Hashemzaei, M.; Baghbanzadeh, A.; de la Guardia, M.; Mokhtarzadeh, A. Recent advances on thermosensitive and pH-sensitive liposomes employed in controlled release. J. Control. Release 2019, 315, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Rawat, P.S.; Jaiswal, A.; Khurana, A.; Bhatti, J.S.; Navik, U. Doxorubicin-induced cardiotoxicity: An update on the molecular mechanism and novel therapeutic strategies for effective management. Biomed. Pharmacother. 2021, 139, 111708. [Google Scholar] [CrossRef] [PubMed]
- Farid, M.; Faber, T.; Dietrich, D.; Lamprecht, A. Cell membrane fusing liposomes for cytoplasmic delivery in brain endothelial cells. Colloids Surf. B Biointerfaces 2020, 194, 111193. [Google Scholar] [CrossRef] [PubMed]
- Dos Reis, S.B.; de Oliveira Silva, J.; Garcia-Fossa, F.; Leite, E.A.; Malachias, A.; Pound-Lana, G.; Mosqueira, V.C.F.; Oliveira, M.C.; de Barros, A.L.B.; de Jesus, M.B. Mechanistic insights into the intracellular release of doxorubicin from pH-sensitive liposomes. Biomed. Pharmacother. 2021, 134, 110952. [Google Scholar] [CrossRef]
- Yan, L.; Crayton, S.H.; Thawani, J.P.; Amirshaghaghi, A.; Tsourkas, A.; Cheng, Z. A pH-responsive drug-delivery platform based on glycol chitosan–coated liposomes. Small 2015, 11, 4870. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, M.; Yuba, E.; Hayashi, H.; Harada, A.; Kono, K. Hyaluronic acid-based pH-sensitive polymer-modified liposomes for cell-specific intracellular drug delivery systems. Bioconjug Chem. 2018, 29, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Shi, K.; Li, J.; Cao, Z.; Yang, P.; Qiu, Y.; Yang, B.; Wang, Y.; Long, Y.; Liu, Y.; Zhang, Q. A pH-responsive cell-penetrating peptide-modified liposomes with ACTIVE recognizing of integrin αvβ3 for the treatment of melanoma. J. Control. Release 2015, 217, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Li, L.; He, X.; Yi, Q.; He, B.; Cao, J.; Pan, W.; Gu, Z. Overcoming drug-resistant lung cancer by paclitaxel loaded dual-functional liposomes with mitochondria targeting and pH-response. Biomaterials 2015, 52, 126–139. [Google Scholar] [CrossRef]
- Yoshizaki, Y.; Yuba, E.; Sakaguchi, N.; Koiwai, K.; Harada, A.; Kono, K. Potentiation of pH-sensitive polymer-modified liposomes with cationic lipid inclusion as antigen delivery carriers for cancer immunotherapy. Biomaterials 2014, 35, 8186–8196. [Google Scholar] [CrossRef]
- Xuh, H. Design and evaluation of pH-sensitive liposomes constructed by poly (2-ethyl-2-oxazoline)-cholesterol hemisuccinate for doxorubicin delivery. Eur. J. Pharm. Biopharm. 2015, 91, 66. [Google Scholar]
- Yuba, E.; Tajima, N.; Yoshizaki, Y.; Harada, A.; Hayashi, H.; Kono, K. Dextran derivative-based pH-sensitive liposomes for cancer immunotherapy. Biomaterials 2014, 35, 3091–3101. [Google Scholar] [CrossRef] [PubMed]
- Rayamajhi, S.; Marchitto, J.; Nguyen, T.D.T.; Marasini, R.; Celia, C.; Aryal, S. pH-responsive cationic liposome for endosomal escape mediated drug delivery. Colloids Surf. B Biointerfaces 2020, 188, 110804. [Google Scholar] [CrossRef]
- Paliwal, S.R.; Paliwal, R.; Agrawal, G.P.; Vyas, S.P. Hyaluronic acid modified pH-sensitive liposomes for targeted intracellular delivery of doxorubicin. J. Liposome Res. 2016, 26, 276–287. [Google Scholar] [CrossRef]
- Silva, J.O.; Fernandes, R.S.; Lopes, S.C.A.; Cardoso, V.N.; Leite, E.A.; Cassali, G.D.; Marzola, M.C.; Rubello, D.; Oliveira, M.C.; de Barros, A.L.B. pH-sensitive, long-circulating liposomes as an alternative tool to deliver doxorubicin into tumors: A feasibility animal study. Mol. Imaging Biol. 2016, 18, 898–904. [Google Scholar] [CrossRef]
- Cao, H.; Dan, Z.; He, X.; Zhang, Z.; Yu, H.; Yin, Q.; Li, Y. Liposomes coated with isolated macrophage membrane can target lung metastasis of breast cancer. ACS Nano 2016, 10, 7738–7748. [Google Scholar] [CrossRef]
- Xu, H.; Paxton, J.W.; Wu, Z. Development of long-circulating pH-sensitive liposomes to circumvent gemcitabine resistance in pancreatic cancer cells. Pharm. Res. 2016, 33, 1628–1637. [Google Scholar] [CrossRef]
- Zhao, Y.; Ren, W.; Zhong, T.; Zhang, S.; Huang, D.; Guo, Y.; Yao, X.; Wang, C.; Zhang, W.-Q.; Zhang, X. Tumor-specific pH-responsive peptide-modified pH-sensitive liposomes containing doxorubicin for enhancing glioma targeting and anti-tumor activity. J. Control. Release 2016, 222, 56–66. [Google Scholar] [CrossRef]
- Pandey, P.; Arya, D.K.; Deepak, P.; Ali, D.; Alarifi, S.; Srivastava, S.; Lavasanifar, A.; Rajinikanth, P.S. αvβ3 integrin and folate-targeted pH-sensitive liposomes with dual ligand modification for metastatic breast cancer treatment. Bioengineering 2024, 11, 800. [Google Scholar] [CrossRef]
- Vila-Caballer, M.; Codolo, G.; Munari, F.; Malfanti, A.; Fassan, M.; Rugge, M.; Balasso, A.; de Bernard, M.; Salmaso, S. A pH-sensitive stearoyl-PEG-poly (methacryloyl sulfadimethoxine)-decorated liposome system for protein delivery: An application for bladder cancer treatment. J. Control. Release 2016, 238, 31–42. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, J.; Xu, J.; Ruan, N. Remodeling tumor microenvironment using pH-sensitive biomimetic co-delivery of TRAIL/R848 liposomes against colorectal cancer. Oncol. Res. 2024, 32, 1765. [Google Scholar] [CrossRef]
- Nunes, S.S.; Fernandes, R.S.; Cavalcante, C.H.; da Costa César, I.; Leite, E.A.; Lopes, S.C.A.; Ferretti, A.; Rubello, D.; Townsend, D.M.; de Oliveira, M.C. Influence of PEG coating on the biodistribution and tumor accumulation of pH-sensitive liposomes. Drug Deliv. Transl. Res. 2019, 9, 123–130. [Google Scholar] [CrossRef]
- Park, Y.I.; Kwon, S.-H.; Lee, G.; Motoyama, K.; Kim, M.W.; Lin, M.; Niidome, T.; Choi, J.H.; Lee, R. pH-sensitive multi-drug liposomes targeting folate receptor β for efficient treatment of non-small cell lung cancer. J. Control. Release 2021, 330, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Park, H.; Oh, K.T.; Lee, E.S. PH-Responsive hyaluronated liposomes for docetaxel delivery. Int. J. Pharm. 2018, 547, 377–384. [Google Scholar] [CrossRef]
- Swami, R.; Kumar, Y.; Chaudhari, D.; Katiyar, S.S.; Kuche, K.; Katare, P.B.; Banerjee, S.K.; Jain, S. pH sensitive liposomes assisted specific and improved breast cancer therapy using co-delivery of SIRT1 shRNA and Docetaxel. Mater. Sci. Eng. C 2021, 120, 111664. [Google Scholar] [CrossRef]
- Rustad, E.A.L.; von Hofsten, S.; Kumar, R.; Lænsman, E.A.; Berge, G.; Škalko-Basnet, N. The pH-responsive liposomes—The effect of PEGylation on release kinetics and cellular uptake in glioblastoma cells. Pharmaceutics 2022, 14, 1125. [Google Scholar] [CrossRef]
- Shen, Q.; Shen, Y.; Jin, F.; Du, Y.; Ying, X. Paclitaxel/hydroxypropyl-β-cyclodextrin complex-loaded liposomes for overcoming multidrug resistance in cancer chemotherapy. J. Liposome Res. 2020, 30, 12–20. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Silva, J.; Fernandes, R.S.; de Alcântara Lemos, J.; Cassali, G.D.; de Paula Sabino, A.; Townsend, D.M.; Oliveira, M.C.; de Barros, A.L.B. Evaluation of acute toxicity and in vitro antitumor activity of a novel doxorubicin-loaded folate-coated pH-sensitive liposome. Biomed. Pharmacother. 2023, 165, 115280. [Google Scholar] [CrossRef]
- Sonju, J.J.; Dahal, A.; Singh, S.S.; Gu, X.; Johnson, W.D.; Muthumula, C.M.R.; Meyer, S.A.; Jois, S.D. A pH-sensitive liposome formulation of a peptidomimetic-Dox conjugate for targeting HER2+ Cancer. Int. J. Pharm. 2022, 612, 121364. [Google Scholar] [CrossRef] [PubMed]
- Alrbyawi, H.; Poudel, I.; Annaji, M.; Boddu, S.H.S.; Arnold, R.D.; Tiwari, A.K.; Babu, R.J. pH-sensitive liposomes for enhanced cellular uptake and cytotoxicity of daunorubicin in melanoma (B16-BL6) cell lines. Pharmaceutics 2022, 14, 1128. [Google Scholar] [CrossRef]
- Duarte, J.A.; Gomes, E.R.; De Barros, A.L.B.; Leite, E.A. Co-encapsulation of simvastatin and doxorubicin into pH-sensitive liposomes enhances antitumoral activity in breast cancer cell lines. Pharmaceutics 2023, 15, 369. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Deore, S.V.; Ghadi, R.; Chaudhari, D.; Kuche, K.; Katiyar, S.S. Tumor microenvironment responsive VEGF-antibody functionalized pH sensitive liposomes of docetaxel for augmented breast cancer therapy. Mater. Sci. Eng. C 2021, 121, 111832. [Google Scholar] [CrossRef]
- Ding, X.; Yin, C.; Zhang, W.; Sun, Y.; Zhang, Z.; Yang, E.; Sun, D.; Wang, W. Designing aptamer-gold nanoparticle-loaded pH-sensitive liposomes encapsulate morin for treating cancer. Nanoscale Res. Lett. 2020, 15, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Lafi, Z.; Alshaer, W.; Ma’mon, M.H.; Zihlif, M.; Alqudah, D.A.; Nsairat, H.; Azzam, H.; Aburjai, T.; Bustanji, Y.; Awidi, A. Aptamer-functionalized pH-sensitive liposomes for a selective delivery of echinomycin into cancer cells. RSC Adv. 2021, 11, 29164–29177. [Google Scholar] [CrossRef] [PubMed]
- Pinho, J.O.; da Silva, I.V.; Amaral, J.D.; Rodrigues, C.M.P.; Casini, A.; Soveral, G.; Gaspar, M.M. Therapeutic potential of a copper complex loaded in pH-sensitive long circulating liposomes for colon cancer management. Int. J. Pharm. 2021, 599, 120463. [Google Scholar] [CrossRef] [PubMed]
- Nunes, S.S.; Miranda, S.E.M.; de Oliveira Silva, J.; Fernandes, R.S.; de Alcântara Lemos, J.; de Aguiar Ferreira, C.; Townsend, D.M.; Cassali, G.D.; Oliveira, M.C.; de Barros, A.L.B. pH-responsive and folate-coated liposomes encapsulating irinotecan as an alternative to improve efficacy of colorectal cancer treatment. Biomed. Pharmacother. 2021, 144, 112317. [Google Scholar] [CrossRef]
- Huang, R.; Gyanani, V.; Zhao, S.; Lu, Y.; Guo, X. Imidazole-based pH-sensitive convertible liposomes for anticancer drug delivery. Pharmaceuticals 2022, 15, 306. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Wang, T.; Liu, Y.; Zhang, N. Co-delivery of sorafenib and VEGF-siRNA via pH-sensitive liposomes for the synergistic treatment of hepatocellular carcinoma. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1374–1383. [Google Scholar] [CrossRef] [PubMed]
- De Leo, V.; Milano, F.; Mancini, E.; Comparelli, R.; Giotta, L.; Nacci, A.; Longobardi, F.; Garbetta, A.; Agostiano, A.; Catucci, L. Encapsulation of curcumin-loaded liposomes for colonic drug delivery in a pH-responsive polymer cluster using a pH-driven and organic solvent-free process. Molecules 2018, 23, 739. [Google Scholar] [CrossRef] [PubMed]
- Aneja, P.; Negi, P.; Aneja, S.; Garad, S.R.; Kumar, S. Formulation optimization of pH-sensitive liposomes based drug delivery of Carboplatin and anti-proliferative evaluation against A549 (human lung carcinoma) cell lines. Main. Group. Chemistry 2023, 22, 403–421. [Google Scholar] [CrossRef]
- de Oliveira Silva, J.; Fernandes, R.S.; Oda, C.M.R.; Ferreira, T.H.; Botelho, A.F.M.; Melo, M.M.; de Miranda, M.C.; Gomes, D.A.; Cassali, G.D.; Townsend, D.M. Folate-coated, long-circulating and pH-sensitive liposomes enhance doxorubicin antitumor effect in a breast cancer animal model. Biomed. Pharmacother. 2019, 118, 109323. [Google Scholar]
- Tie, Y.; Zheng, H.; He, Z.; Yang, J.; Shao, B.; Liu, L.; Luo, M.; Yuan, X.; Liu, Y.; Zhang, X. Targeting folate receptor β positive tumor-associated macrophages in lung cancer with a folate-modified liposomal complex. Signal Transduct. Target. Ther. 2020, 5, 6. [Google Scholar] [CrossRef]
- Samaniego, R.; Domínguez-Soto, Á.; Ratnam, M.; Matsuyama, T.; Sánchez-Mateos, P.; Corbí, Á.L.; Puig-Kröger, A. Folate receptor β (FRβ) expression in tissue-resident and tumor-associated macrophages associates with and depends on the expression of PU. 1. Cells 2020, 9, 1445. [Google Scholar] [CrossRef] [PubMed]
- Nsairat, H.; Khater, D.; Sayed, U.; Odeh, F.; Al Bawab, A.; Alshaer, W. Liposomes: Structure, composition, types, and clinical applications. Heliyon 2022, 8, e09394. [Google Scholar] [CrossRef] [PubMed]
- Yanagihara, S.; Kitayama, Y.; Yuba, E.; Harada, A. Preparing size-controlled liposomes modified with polysaccharide derivatives for pH-responsive drug delivery applications. Life 2023, 13, 2158. [Google Scholar] [CrossRef]
- Nunes, S.S.; de Oliveira Silva, J.; Fernandes, R.S.; Miranda, S.E.M.; Leite, E.A.; de Farias, M.A.; Portugal, R.V.; Cassali, G.D.; Townsend, D.M.; Oliveira, M.C. PEGylated versus non-PEGylated pH-sensitive liposomes: New insights from a comparative antitumor activity study. Pharmaceutics 2022, 14, 272. [Google Scholar] [CrossRef]
- Gadisa, D.A.; Assefa, M.; Wang, S.-H.; Yimer, G. Toxicity profile of Doxorubicin-Cyclophosphamide and Doxorubicin-Cyclophosphamide followed by Paclitaxel regimen and its associated factors among women with breast cancer in Ethiopia: A prospective cohort study. J. Oncol. Pharm. Pract. 2020, 26, 1912–1920. [Google Scholar] [CrossRef] [PubMed]
- Buranrat, B.; Senggunprai, L.; Prawan, A.; Kukongviriyapan, V. Effects of Simvastatin in Combination with Anticancer Drugs on Proliferation and Migration in Cholangiocarcinoma Cells. Indian. J. Pharm. Sci. 2022, 84, 72. [Google Scholar] [CrossRef]
- Duarte, J.A.; de Barros, A.L.B.; Leite, E.A. The potential use of simvastatin for cancer treatment: A review. Biomed. Pharmacother. 2021, 141, 111858. [Google Scholar] [CrossRef] [PubMed]
- Rojas, L.A.; Sethna, Z.; Soares, K.C.; Olcese, C.; Pang, N.; Patterson, E.; Lihm, J.; Ceglia, N.; Guasp, P.; Chu, A. Personalized RNA neoantigen vaccines stimulate T cells in pancreatic cancer. Nature 2023, 618, 144–150. [Google Scholar] [CrossRef]
- Thi, T.T.H.; Suys, E.J.A.; Lee, J.S.; Nguyen, D.H.; Park, K.D.; Truong, N.P. Lipid-based nanoparticles in the clinic and clinical trials: From cancer nanomedicine to COVID-19 vaccines. Vaccines 2021, 9, 359. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Tang, L.; Tian, Y.; Ji, X.; Hu, Q.; Zhou, B.; Ding, Z.; Xu, H.; Yang, L. DP7-C-modified liposomes enhance immune responses and the antitumor effect of a neoantigen-based mRNA vaccine. J. Control. Release 2020, 328, 210–221. [Google Scholar] [CrossRef] [PubMed]
- Mai, Y.; Guo, J.; Zhao, Y.; Ma, S.; Hou, Y.; Yang, J. Intranasal delivery of cationic liposome-protamine complex mRNA vaccine elicits effective anti-tumor immunity. Cell Immunol. 2020, 354, 104143. [Google Scholar] [CrossRef]
- Kapalatiya, H.; Madav, Y.; Tambe, V.S.; Wairkar, S. Enzyme-responsive smart nanocarriers for targeted chemotherapy: An overview. Drug Deliv. Transl. Res. 2022, 12, 1293–1305. [Google Scholar] [CrossRef]
- Antoniou, A.I.; Giofrè, S.; Seneci, P.; Passarella, D.; Pellegrino, S. Stimulus-responsive liposomes for biomedical applications. Drug Discov. Today 2021, 26, 1794–1824. [Google Scholar] [CrossRef]
- Nikolova, M.P.; Kumar, E.M.; Chavali, M.S. Updates on responsive drug delivery based on liposome vehicles for cancer treatment. Pharmaceutics 2022, 14, 2195. [Google Scholar] [CrossRef] [PubMed]
- Dheer, D.; Nicolas, J.; Shankar, R. Cathepsin-sensitive nanoscale drug delivery systems for cancer therapy and other diseases. Adv. Drug Deliv. Rev. 2019, 151, 130–151. [Google Scholar] [CrossRef]
- Korkmaz, B.; Lamort, A.-S.; Domain, R.; Beauvillain, C.; Gieldon, A.; Yildirim, A.Ö.; Stathopoulos, G.T.; Rhimi, M.; Jenne, D.E.; Kettritz, R. Cathepsin C inhibition as a potential treatment strategy in cancer. Biochem. Pharmacol. 2021, 194, 114803. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Song, S.J.; Lee, J.; Ha, T.H.; Choi, J.S. Cathepsin B-responsive liposomes for controlled anticancer drug delivery in Hep G2 cells. Pharmaceutics 2020, 12, 876. [Google Scholar] [CrossRef] [PubMed]
- Letsiou, E.; Htwe, Y.M.; Dudek, S.M. Secretory phospholipase A2 enzymes in acute lung injury. Cell Biochem. Biophys. 2021, 79, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Feng, C.; Xie, J.; Zhang, X.; Dai, H.; Yan, L. Recent progress of nanomedicine in secreted phospholipase A2 as a potential therapeutic target. J. Mater. Chem. B 2022, 10, 7349–7360. [Google Scholar] [CrossRef] [PubMed]
- Shchegravina, E.S.; Tretiakova, D.S.; Alekseeva, A.S.; Galimzyanov, T.R.; Utkin, Y.N.; Ermakov, Y.A.; Svirshchevskaya, E.V.; Negrebetsky, V.V.; Karpechenko, N.Y.; Chernikov, V.P. Phospholipidic colchicinoids as promising prodrugs incorporated into enzyme-responsive liposomes: Chemical, biophysical, and enzymological aspects. Bioconjug Chem. 2019, 30, 1098–1113. [Google Scholar] [CrossRef] [PubMed]
- Proshkina, G.; Shramova, E.; Ryabova, A.; Katrivas, L.; Giannini, C.; Malpicci, D.; Levi-Kalisman, Y.; Deyev, S.; Kotlyar, A. Novel Small Multilamellar Liposomes Containing Large Quantities of Peptide Nucleic Acid Selectively Kill Breast Cancer Cells. Cancers 2022, 14, 4806. [Google Scholar] [CrossRef] [PubMed]
- Alidousti, R.; Shakhsi-Niaee, M. Recent applicable delivery approaches of peptide nucleic acids to the target cells. arXiv 2019, arXiv:1910.08000. [Google Scholar]
- Ghavami, M.; Shiraishi, T.; Nielsen, P.E. Enzyme-triggered release of the antisense octaarginine-pna conjugate from phospholipase A2 sensitive liposomes. ACS Appl. Bio Mater. 2020, 3, 1018–1025. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wu, B.; Li, Q.; Chen, S.; Jin, X.; Liu, Y.; Zhou, Z.; Shen, Y.; Huang, P. Active transportation of liposome enhances tumor accumulation, penetration, and therapeutic efficacy. Small 2020, 16, 2004172. [Google Scholar] [CrossRef] [PubMed]
- Duan, N.; Li, J.; Song, S.; Wang, F.; Yang, Y.; Nie, D.; Wang, C.; Sheng, Y.; Tao, Y.; Gao, J. Enzyme-activated prodrug-based smart liposomes specifically enhance tumor hemoperfusion with efficient drug delivery to pancreatic cancer cells and stellate cells. Adv. Funct. Mater. 2021, 31, 2100605. [Google Scholar] [CrossRef]
- Zhang, Z.-T.; Huang-Fu, M.-Y.; Xu, W.-H.; Han, M. Stimulus-responsive nanoscale delivery systems triggered by the enzymes in the tumor microenvironment. Eur. J. Pharm. Biopharm. 2019, 137, 122–130. [Google Scholar] [CrossRef]
- Shahriari, M.; Zahiri, M.; Abnous, K.; Taghdisi, S.M.; Ramezani, M.; Alibolandi, M. Enzyme responsive drug delivery systems in cancer treatment. J. Control. Release 2019, 308, 172–189. [Google Scholar] [CrossRef]
- Juul, C.A.; Engel, T.B.; Fliedner, F.P.; Ringgaard, L.; Eliasen, R.; Melander, F.; Bak, M.; Kjær, A.; Henriksen, J.R.; Elema, D.R. HER2-targeted, enzyme-activated liposomes show superior in vivo efficacy in an ovarian cancer model. J. Control. Release 2024, 371, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Mirhadi, E.; Mashreghi, M.; Askarizadeh, A.; Mehrabian, A.; Alavizadeh, S.H.; Arabi, L.; Badiee, A.; Jaafari, M.R. Redox-sensitive doxorubicin liposome: A formulation approach for targeted tumor therapy. Sci. Rep. 2022, 12, 11310. [Google Scholar] [CrossRef]
- Abed, H.F.; Abuwatfa, W.H.; Husseini, G.A. Redox-responsive drug delivery systems: A chemical perspective. Nanomaterials 2022, 12, 3183. [Google Scholar] [CrossRef] [PubMed]
- Kalinina, E.V.; Gavriliuk, L.A. Glutathione synthesis in cancer cells. Biochemistry 2020, 85, 895–907. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Shen, Y.; Zhao, H.; Lu, X.; Wang, Z.; Zhao, Y. Redox-manipulating nanocarriers for anticancer drug delivery: A systematic review. J. Nanobiotechnol. 2024, 22, 587. [Google Scholar] [CrossRef]
- Wang, T.; He, W.; Du, Y.; Wang, J.; Li, X. Redox-sensitive irinotecan liposomes with active ultra-high loading and enhanced intracellular drug release. Colloids Surf. B Biointerfaces 2021, 206, 111967. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Zhang, J.; Li, H. Selenium, aging and aging-related diseases. Aging Clin. Exp. Res. 2019, 31, 1035–1047. [Google Scholar] [CrossRef]
- Qian, Y.; An, X.; Huang, X.; Pan, X.; Zhu, J.; Zhu, X. Recyclable self-healing polyurethane cross-linked by alkyl diselenide with enhanced mechanical properties. Polymers 2019, 11, 773. [Google Scholar] [CrossRef] [PubMed]
- Farmanfarma, K.H.K.; Mohammadian, M.; Shahabinia, Z.; Hassanipour, S.; Salehiniya, H. Brain cancer in the world: An epidemiological review. World Cancer Res. J. 2019, 6, 1–5. [Google Scholar]
- Butenschoen, V.M.; Kelm, A.; Meyer, B.; Krieg, S.M. Quality-adjusted life years in glioma patients: A systematic review on currently available data and the lack of evidence-based utilities. J. Neurooncol 2019, 144, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Sun, G.; Sun, X.; Li, F.; Zhao, L.; Zhong, R.; Peng, Y. The potential of lonidamine in combination with chemotherapy and physical therapy in cancer treatment. Cancers 2020, 12, 3332. [Google Scholar] [CrossRef]
- Li, H.; Xu, W.; Li, F.; Zeng, R.; Zhang, X.; Wang, X.; Zhao, S.; Weng, J.; Li, Z.; Sun, L. Amplification of anticancer efficacy by co-delivery of doxorubicin and lonidamine with extracellular vesicles. Drug Deliv. 2022, 29, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Nimgampalle, M.; Chakravarthy, H.; Devanathan, V. Glucose metabolism in the brain: An update. In Recent Developments in Applied Microbiology and Biochemistry; Elsevier: Amsterdam, The Netherlands, 2021; pp. 77–88. [Google Scholar]
- Guo, X.; Yang, N.; Ji, W.; Zhang, H.; Dong, X.; Zhou, Z.; Li, L.; Shen, H.; Yao, S.Q.; Huang, W. Mito-bomb: Targeting mitochondria for cancer therapy. Adv. Mater. 2021, 33, 2007778. [Google Scholar] [CrossRef]
- Li, J.; Zhao, J.; Tan, T.; Liu, M.; Zeng, Z.; Zeng, Y.; Zhang, L.; Fu, C.; Chen, D.; Xie, T. Nanoparticle drug delivery system for glioma and its efficacy improvement strategies: A comprehensive review. Int. J. Nanomed. 2020, 15, 2563–2582. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Lu, J.; Li, R.; Zhao, Y.; Hai, L.; Guo, L.; Wu, Y. Glucose and triphenylphosphonium co-modified redox-sensitive liposomes to synergistically treat glioma with doxorubicin and lonidamine. ACS Appl. Mater. Interfaces 2021, 13, 26682–26693. [Google Scholar] [CrossRef]
- Zhang, J.-L.; Gong, L.; Yan, Q.; Zhou, N.-N.; Lee, V.H.-F.; Guan, X. Advances in surface markers of liver cancer stem cell. Hepatoma Res. 2019, 5, 27. [Google Scholar] [CrossRef]
- Jia, X.; Guo, M.; Han, Q.; Tian, Y.; Yuan, Y.; Wang, Z.; Qian, Y.; Wang, W. Synergetic tumor probes for facilitating therapeutic delivery by combined-functionalized peptide ligands. Anal. Chem. 2020, 92, 5650–5655. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, M.; Li, W.; Fan, L.; Zhou, Y.; Hu, Z. A Novel CD133-and EpCAM-targeted liposome with redox-responsive properties capable of synergistically eliminating liver cancer stem cells. Front. Chem. 2020, 8, 649. [Google Scholar] [CrossRef]
- Wu, H.; Gao, Y.; Ma, J.; Hu, M.; Xia, J.; Bao, S.; Liu, Y.; Feng, K. Cytarabine delivered by CD44 and bone targeting redox-sensitive liposomes for treatment of acute myelogenous leukemia. Regen. Biomater. 2022, 9, rbac058. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Jia, D.; Ma, X.; Liang, M.; Xue, P.; Kang, Y.; Xu, Z. Cylindrical polymer brushes-anisotropic unimolecular micelle drug delivery system for enhancing the effectiveness of chemotherapy. Bioact. Mater. 2021, 6, 2894–2904. [Google Scholar] [CrossRef] [PubMed]
- Hirschhorn, T.; Stockwell, B.R. The development of the concept of ferroptosis. Free Radic. Biol. Med. 2019, 133, 130–143. [Google Scholar] [CrossRef]
- Fu, L.; Wan, Y.; Qi, C.; He, J.; Li, C.; Yang, C.; Xu, H.; Lin, J.; Huang, P. Nanocatalytic theranostics with glutathione depletion and enhanced reactive oxygen species generation for efficient cancer therapy. Adv. Mater. 2021, 33, 2006892. [Google Scholar] [CrossRef]
- He, Y.-J.; Liu, X.-Y.; Xing, L.; Wan, X.; Chang, X.; Jiang, H.-L. Fenton reaction-independent ferroptosis therapy via glutathione and iron redox couple sequentially triggered lipid peroxide generator. Biomaterials 2020, 241, 119911. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zuo, S.; Li, L.; Kuang, X.; Li, J.; Sun, B.; Wang, S.; He, Z.; Sun, J. Iron-doxorubicin prodrug loaded liposome nanogenerator programs multimodal ferroptosis for efficient cancer therapy. Asian J. Pharm. Sci. 2021, 16, 784–793. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Takada, K. Reactive oxygen species in cancer: Current findings and future directions. Cancer Sci. 2021, 112, 3945–3952. [Google Scholar] [CrossRef]
- Aggarwal, V.; Tuli, H.S.; Varol, A.; Thakral, F.; Yerer, M.B.; Sak, K.; Varol, M.; Jain, A.; Khan, M.A.; Sethi, G. Role of reactive oxygen species in cancer progression: Molecular mechanisms and recent advancements. Biomolecules 2019, 9, 735. [Google Scholar] [CrossRef]
- Saikolappan, S.; Kumar, B.; Shishodia, G.; Koul, S.; Koul, H.K. Reactive oxygen species and cancer: A complex interaction. Cancer Lett. 2019, 452, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Nordström, R.; Zhu, L.; Härmark, J.; Levi-Kalisman, Y.; Koren, E.; Barenholz, Y.; Levinton, G.; Shamrakov, D. Quantitative cryo-TEM reveals new structural details of doxil-like PEGylated liposomal doxorubicin formulation. Pharmaceutics 2021, 13, 123. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, Z.; Ding, Y.; Wang, L.; Cheng, Y.; Meng, L.; Wu, J.; Yuan, A.; Hu, Y.; Zhu, Y. Bifunctional liposomes reduce the chemotherapy resistance of doxorubicin induced by reactive oxygen species. Biomater. Sci. 2019, 7, 4782–4789. [Google Scholar] [CrossRef]
- Du, Y.; He, W.; Xia, Q.; Zhou, W.; Yao, C.; Li, X. Thioether phosphatidylcholine liposomes: A novel ROS-responsive platform for drug delivery. ACS Appl. Mater. Interfaces 2019, 11, 37411–37420. [Google Scholar] [CrossRef] [PubMed]
- Voorwerk, L.; Slagter, M.; Horlings, H.M.; Sikorska, K.; van de Vijver, K.K.; de Maaker, M.; Nederlof, I.; Kluin, R.J.C.; Warren, S.; Ong, S. Immune induction strategies in metastatic triple-negative breast cancer to enhance the sensitivity to PD-1 blockade: The TONIC trial. Nat. Med. 2019, 25, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Hu, L. Immunomodulators targeting the PD-1/PD-L1 protein-protein interaction: From antibodies to small molecules. Med. Res. Rev. 2019, 39, 265–301. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhu, Z.; Lv, H.; Li, F.; Sun, S.; Li, J.; Lee, C. Immune checkpoint blockade mediated by a small-molecule nanoinhibitor targeting the PD-1/PD-L1 pathway synergizes with photodynamic therapy to elicit antitumor immunity and antimetastatic effects on breast cancer. Small 2019, 15, 1903881. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, J.; Li, D.; Zhao, L.; Sun, B.; Wang, J.; Wang, Z.; Zhou, S.; Wang, M.; Yang, Y. Paclitaxel derivative-based liposomal nanoplatform for potentiated chemo-immunotherapy. J. Control. Release 2022, 341, 812–827. [Google Scholar] [CrossRef]
- Gaona-Luviano, P.; Medina-Gaona, L.A.; Magaña-Pérez, K. Epidemiology of ovarian cancer. Chin. Clin. Oncol. 2020, 9, 47. [Google Scholar] [CrossRef]
- Tang, L.; Wang, Y.-J.; Wang, Y.-Y.; Li, S.-T.; Kong, L.; Li, X.-T.; Ma, L.-L.; Liu, X.-X. Construction of ROS-Responsive Hyaluronic Acid Modified Paclitaxel and Diosgenin Liposomes and Study on Synergistic Enhancement of Anti-Ovarian Cancer Efficacy. Int. J. Nanomed. 2024, 19, 5193–5211. [Google Scholar] [CrossRef]
- Hu, H.; Yang, W.; Liang, Z.; Zhou, Z.; Song, Q.; Liu, W.; Deng, X.; Zhu, J.; Xing, X.; Zhong, B. Amplification of oxidative stress with lycorine and gold-based nanocomposites for synergistic cascade cancer therapy. J Nanobiotechnol. 2021, 19, 1–18. [Google Scholar] [CrossRef]
- Kawabata, H. Transferrin and transferrin receptors update. Free Radic. Biol. Med. 2019, 133, 46–54. [Google Scholar] [CrossRef]
- Quindoza III, G.M.; Nakagawa, Y.; Anraku, Y.; Ikoma, T. Adsorption of l-buthionine sulfoximine on Bi (III) and Eu (III) co-substituted hydroxyapatite nanocrystals for enhancing radiosensitization effects. Colloids Surf. B Biointerfaces 2023, 228, 113403. [Google Scholar] [CrossRef]
- Yu, X.; Lu, M.; Luo, Y.; Hu, Y.; Zhang, Y.; Xu, Z.; Gong, S.; Wu, Y.; Ma, X.-N.; Yu, B.-Y. A cancer-specific activatable theranostic nanodrug for enhanced therapeutic efficacy via amplification of oxidative stress. Theranostics 2020, 10, 371. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Bouyahya, A.; Hachlafi, N.E.L.; El Menyiy, N.; Akram, M.; Sultana, S.; Zengin, G.; Ponomareva, L.; Shariati, M.A.; Ojo, O.A. Anticancer properties of medicinal plants and their bioactive compounds against breast cancer: A review on recent investigations. Environ. Sci. Pollut. Res. 2022, 29, 24411–24444. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.K.; Biswal, B.K. Piperlongumine, a potent anticancer phytotherapeutic: Perspectives on contemporary status and future possibilities as an anticancer agent. Pharmacol. Res. 2020, 156, 104772. [Google Scholar] [CrossRef] [PubMed]
- Parveen, S.; Kumar, S.; Pal, S.; Yadav, N.P.; Rajawat, J.; Banerjee, M. Enhanced of Piperlongumine for cancer treatment using nano-liposomes mediated delivery. Int. J. Pharm. 2023, 643, 123212. [Google Scholar] [CrossRef] [PubMed]
- Kumari, R.; Sunil, D.; Ningthoujam, R.S. Hypoxia-responsive nanoparticle based drug delivery systems in cancer therapy: An up-to-date review. J. Control. Release 2020, 319, 135–156. [Google Scholar] [CrossRef]
- Zhang, B.; Huang, X.; Wang, H.; Gou, S. Promoting antitumor efficacy by suppressing hypoxia via nano self-assembly of two irinotecan-based dual drug conjugates having a HIF-1α inhibitor. J. Mater. Chem. B 2019, 7, 5352–5362. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhu, Y.; Wu, C.; Shi, J. Nanoplatform-based cascade engineering for cancer therapy. Chem. Soc. Rev. 2020, 49, 9057–9094. [Google Scholar] [CrossRef]
- Li, Y.; Jeon, J.; Park, J.H. Hypoxia-responsive nanoparticles for tumor-targeted drug delivery. Cancer Lett. 2020, 490, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Shah, V.M.; Dorrell, C.; Al-Fatease, A.; Allen-Petersen, B.L.; Woo, Y.; Bortnyak, Y.; Gheewala, R.; Sheppard, B.C.; Sears, R.C.; Alani, A.W.G. Microfluidics formulated liposomes of hypoxia activated prodrug for treatment of pancreatic cancer. Pharmaceutics 2022, 14, 713. [Google Scholar] [CrossRef]
- Rottenberg, S.; Disler, C.; Perego, P. The rediscovery of platinum-based cancer therapy. Nat. Rev. Cancer 2021, 21, 37–50. [Google Scholar] [CrossRef]
- Chen, J.; Wang, X.; Yuan, Y.; Chen, H.; Zhang, L.; Xiao, H.; Chen, J.; Zhao, Y.; Chang, J.; Guo, W. Exploiting the acquired vulnerability of cisplatin-resistant tumors with a hypoxia-amplifying DNA repair–inhibiting (HYDRI) nanomedicine. Sci. Adv. 2021, 7, eabc5267. [Google Scholar] [CrossRef]
- Wang, Y.; Hou, Y.; Wang, Y.; Zheng, L.; Wang, Q. A novel cold-adapted nitroreductase from Psychrobactersp. ANT206: Heterologous expression, characterization and nitrobenzene reduction capacity. Enzyme Microb. Technol. 2019, 131, 109434. [Google Scholar] [CrossRef]
- Li, Y.; Lu, A.; Long, M.; Cui, L.; Chen, Z.; Zhu, L. Nitroimidazole derivative incorporated liposomes for hypoxia-triggered drug delivery and enhanced therapeutic efficacy in patient-derived tumor xenografts. Acta Biomater. 2019, 83, 334–348. [Google Scholar] [CrossRef]
- Long, M.; Lu, A.; Lu, M.; Weng, L.; Chen, Q.; Zhu, L.; Chen, Z. Azo-inserted responsive hybrid liposomes for hypoxia-specific drug delivery. Acta Biomater. 2020, 115, 343–357. [Google Scholar] [CrossRef]
- Brownlee, W.J.; Seib, F.P. Impact of the hypoxic phenotype on the uptake and efflux of nanoparticles by human breast cancer cells. Sci. Rep. 2018, 8, 12318. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, L.; Zhu, W.; Guo, R.; Sun, H.; Chen, X.; Deng, N. Barriers and strategies of cationic liposomes for cancer gene therapy. Mol. Ther. Methods Clin. Dev. 2020, 18, 751–764. [Google Scholar] [CrossRef] [PubMed]
- Zarazvand, F.; Karimi, M.; Moosavian, S.A.; Arabi, L.; Badiee, A.; Jaafari, M.R.; Mashreghi, M. Efficacy Comparison of TAT peptide-functionalized PEGylated liposomal doxorubicin in C26 and B16F0 tumor mice models. Int. J. Pept. Res. Ther. 2021, 27, 2099–2109. [Google Scholar] [CrossRef]
- Mashreghi, M.; Maleki, M.F.; Askarizadeh, A.; Farshchi, H.; Farhoudi, L.; Nasrollahzadeh, M.S.; Bazaz, M.R.; Hadizadeh, F.; Jaafari, M.R. A novel and easy to prepare azo-based bioreductive linker and its application in hypoxia-sensitive cationic liposomal doxorubicin: Synthesis, characterization, in vitro and in vivo studies in mice bearing C26 tumor. Chem. Phys. Lipids 2022, 247, 105226. [Google Scholar] [CrossRef]
- Zhu, S.; Zhang, T.; Zheng, L.; Liu, H.; Song, W.; Liu, D.; Li, Z.; Pan, C. Combination strategies to maximize the benefits of cancer immunotherapy. J. Hematol. Oncol. 2021, 14, 156. [Google Scholar] [CrossRef] [PubMed]
- Christofi, T.; Baritaki, S.; Falzone, L.; Libra, M.; Zaravinos, A. Current perspectives in cancer immunotherapy. Cancers 2019, 11, 1472. [Google Scholar] [CrossRef]
- Kruger, S.; Ilmer, M.; Kobold, S.; Cadilha, B.L.; Endres, S.; Ormanns, S.; Schuebbe, G.; Renz, B.W.; D’Haese, J.G.; Schloesser, H. Advances in cancer immunotherapy 2019–latest trends. J. Exp. Clin. Cancer Res. 2019, 38, 1–11. [Google Scholar]
- Xue, Y.; Gao, S.; Gou, J.; Yin, T.; He, H.; Wang, Y.; Zhang, Y.; Tang, X.; Wu, R. Platinum-based chemotherapy in combination with PD-1/PD-L1 inhibitors: Preclinical and clinical studies and mechanism of action. Expert. Opin. Drug Deliv. 2021, 18, 187–203. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Zhang, T.; Qin, S.; Huang, Z.; Zhou, L.; Shi, J.; Nice, E.C.; Xie, N.; Huang, C.; Shen, Z. Enhancing the therapeutic efficacy of nanoparticles for cancer treatment using versatile targeted strategies. J. Hematol. Oncol. 2022, 15, 132. [Google Scholar] [CrossRef]
- Song, L.; Hao, Y.; Wang, C.; Han, Y.; Zhu, Y.; Feng, L.; Miao, L.; Liu, Z. Liposomal oxaliplatin prodrugs loaded with metformin potentiate immunotherapy for colorectal cancer. J. Control. Release 2022, 350, 922–932. [Google Scholar] [CrossRef]
- Zafar, S.; Beg, S.; Panda, S.K.; Rahman, M.; Alharbi, K.S.; Jain, G.K.; Ahmad, F.J. Novel therapeutic interventions in cancer treatment using protein and peptide based targeted smart systems. Semin. Cancer Biol. 2021, 69, 249–267. [Google Scholar] [CrossRef]
- Yao, Y.; Chen, H.; Tan, N. Cancer-cell-biomimetic nanoparticles systemically eliminate hypoxia tumors by synergistic chemotherapy and checkpoint blockade immunotherapy. Acta Pharm. Sin. B 2022, 12, 2103–2119. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Tang, Z.; Peng, S.; Zhang, J.; Wang, W.; Wang, Q.; Lin, W.; Lin, X.; Zu, X.; Luo, H. pH/redox/UV irradiation multi-stimuli responsive nanogels from star copolymer micelles and Fe3+ complexation for “on-demand” anticancer drug delivery. React. Funct. Polym. 2020, 149, 104532. [Google Scholar] [CrossRef]
- García, M.C.; Cuggino, J.C. Stimulus-responsive nanogels for drug delivery. In Stimuli Responsive Polymeric Nanocarriers for Drug Delivery Applications; Elsevier: Amsterdam, The Netherlands, 2018; Volume 1, pp. 321–341. [Google Scholar]
- Lyu, D.; Chen, S.; Guo, W. Liposome crosslinked polyacrylamide/DNA hydrogel: A smart controlled-release system for small molecular payloads. Small 2018, 14, 1704039. [Google Scholar] [CrossRef] [PubMed]
- Palmese, L.L.; Fan, M.; Scott, R.A.; Tan, H.; Kiick, K.L. Multi-stimuli-responsive, liposome-crosslinked poly (ethylene glycol) hydrogels for drug delivery. J. Biomater. Sci. Polym. Ed. 2020, 32, 635–656. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; You, C.; Ling, Y.; Wu, H.; Sun, B. pH and thermo dual stimulus-responsive liposome nanoparticles for targeted delivery of platinum-acridine hybrid agent. Life Sci. 2019, 217, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Sentoukas, T.; Demetzos, C.; Pispas, S. Chimeric liposomes incorporating functional copolymers: Preparation and pH/thermo-responsive behaviour in aqueous solutions. J. Liposome Res. 2021, 31, 279–290. [Google Scholar] [CrossRef]
- Sugimoto, T.; Yamazaki, N.; Hayashi, T.; Yuba, E.; Harada, A.; Kotaka, A.; Shinde, C.; Kumei, T.; Sumida, Y.; Fukushima, M. Preparation of dual-stimuli-responsive liposomes using methacrylate-based copolymers with pH and temperature sensitivities for precisely controlled release. Colloids Surf. B Biointerfaces 2017, 155, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Ta, T.; Convertine, A.J.; Reyes, C.R.; Stayton, P.S.; Porter, T.M. Thermosensitive liposomes modified with poly (N-isopropylacrylamide-co-propylacrylic acid) copolymers for triggered release of doxorubicin. Biomacromolecules 2010, 11, 1915–1920. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Montizaan, D.; Yang, K.; Reker-Smit, C.; Salvati, A. Comparison of the uptake mechanisms of zwitterionic and negatively charged liposomes by HeLa Cells. Nanomedicine 2020, 30, 102300. [Google Scholar] [CrossRef] [PubMed]
- García, M.C.; Naitlho, N.; Calderón-Montaño, J.M.; Drago, E.; Rueda, M.; Longhi, M.; Rabasco, A.M.; López-Lázaro, M.; Prieto-Dapena, F.; González-Rodríguez, M.L. Cholesterol levels affect the performance of AuNPs-decorated thermo-sensitive liposomes as nanocarriers for controlled doxorubicin delivery. Pharmaceutics 2021, 13, 973. [Google Scholar] [CrossRef] [PubMed]
- García, M.C.; Calderón-Montaño, J.M.; Rueda, M.; Longhi, M.; Rabasco, A.M.; López-Lázaro, M.; Prieto-Dapena, F.; González-Rodríguez, M.L. pH-temperature dual-sensitive nucleolipid-containing stealth liposomes anchored with PEGylated AuNPs for triggering delivery of doxorubicin. Int. J. Pharm. 2022, 619, 121691. [Google Scholar] [CrossRef] [PubMed]
- Nezhadali, A.; Shapouri, M.R.; Amoli-Diva, M. Anti-cancer combination therapy by co-delivery of hydrophilic and hydrophobic using dual temperature and pH-responsive liposomes. Micro Nano Lett. 2020, 15, 1065–1070. [Google Scholar] [CrossRef]
- Zhao, Y.; Cai, F.; Shen, X.; Su, H. A high stable ph-temperature dual-sensitive liposome for tuning anticancer drug release. Synth. Syst. Biotechnol. 2020, 5, 103–110. [Google Scholar] [CrossRef]
- Ge, J.; Zhou, K.; Li, Y.; Li, H.; Chen, F.; Li, L.; Xu, W. Human serum albumin and sialic acid co-modified liposome nanoreactors with tumor-specific activable cascade reactions for cooperative cancer therapy. J. Drug Deliv. Sci. Technol. 2024, 92, 105343. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, X.-G.; Yang, P.-P.; Qiao, Z.-Y.; Wang, H. Tumor microenvironmental pH and enzyme dual responsive polymer-liposomes for synergistic treatment of cancer immuno-chemotherapy. Biomacromolecules 2019, 20, 882–892. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, Y. Controlled Release from cleavable polymerized liposomes upon redox and pH stimulation. Bioconjug Chem. 2011, 22, 523–528. [Google Scholar] [CrossRef]
- Mavuso, S.; Choonara, Y.E.; Marimuthu, T.; Kumar, P.; du Toit, L.C.; Kondiah, P.P.D.; Pillay, V. A dual pH/redox responsive copper-ligand nanoliposome bioactive complex for the treatment of chronic inflammation. Int. J. Pharm. 2016, 509, 348–359. [Google Scholar] [CrossRef]
- Li, M.; Wang, S.; Xu, J.; Xu, S.; Liu, H. pH/Redox-controlled interaction between lipid membranes and peptide derivatives with a “Helmet”. J. Phys. Chem. B 2019, 123, 6784–6791. [Google Scholar] [CrossRef]
- Zhao, Y.; Peng, Y.; Yang, Z.; Lu, J.; Li, R.; Shi, Y.; Du, Y.; Zhao, Z.; Hai, L.; Wu, Y. pH-redox responsive cascade-targeted liposomes to intelligently deliver doxorubicin prodrugs and lonidamine for glioma. Eur. J. Med. Chem. 2022, 235, 114281. [Google Scholar] [CrossRef] [PubMed]
- Xue, P.; Li, J.; Song, J.; Yu, J.; Liu, H.; Jiang, Y.; Wang, Y. Fe3+ mediated shikonin and PPA coloaded liposomes induce robust immunogenic cell death by integrating ROS enhancement and GSH depletion. Int. J. Pharm. 2024, 649, 123657. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Feng, L.; Dong, Z.; Wang, L.; Liang, C.; Chen, J.; Ma, Q.; Chen, Q.; Wang, Y.; Liu, Z. Glucose & oxygen exhausting liposomes for combined cancer starvation and hypoxia-activated therapy. Biomaterials 2018, 162, 123–131. [Google Scholar] [PubMed]
- Yao, W.; Liu, C.; Wang, N.; Zhou, H.; Chen, H.; Qiao, W. Triple-responsive targeted hybrid liposomes with high MRI performance for tumor diagnosis and therapy. Mater. Chem. Front. 2021, 5, 6226–6243. [Google Scholar] [CrossRef]
- Torres, J.; Calderón-Montaño, J.M.; Prieto-Dapena, F.; López-Lázaro, M.; Rueda, M.; Rabasco-Álvarez, A.M.; González-Rodríguez, M.L.; García, M.C. A Quality-by-design approach for optimizing the functionalization of gold nanoparticles onto the surface of doxorubicin-encapsulated liposomes. Int. J. Pharm. 2025, 669, 125040. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, Q.; Ren, Y.; Zhong, P.; Bao, P.; Guan, S.; Qiu, X.; Qu, X. Oxygen and pH responsive theragnostic liposomes for early-stage diagnosis and photothermal therapy of solid tumours. Biomater. Sci. 2024, 12, 748–762. [Google Scholar] [CrossRef] [PubMed]
- Tasnim, K.N.; Rahman, A.; Newaj, S.M.; Mahmud, O.; Monira, S.; Khan, T.Z.; Reza, H.M.; Shin, M.; Sharker, S.M. Trackable Liposomes for In Vivo Delivery Tracing toward Personalized Medicine Care under NIR Light on Skin Tumor. ACS Appl. Bio Mater. 2024, 7, 3190–3201. [Google Scholar] [CrossRef]
- Chen, L.-J.; Yang, C.-X.; Yan, X.-P. Liposome-coated persistent luminescence nanoparticles as luminescence trackable drug carrier for Chemotherapy. Anal. Chem. 2017, 89, 6936–6939. [Google Scholar] [CrossRef]
- Payne, C.; Cressey, P.; Talianu, A.; Szychot, E.; Hargrave, D.; Thanou, M.; Pouliopoulos, A.N. Bi-modal confirmation of liposome delivery to the brain after focused ultrasound-induced blood-brain barrier opening. Heliyon 2024, 10, e39972. [Google Scholar] [CrossRef] [PubMed]
- Moloney, C.; Chaudhuri, T.R.; Spernyak, J.A.; Straubinger, R.M.; Brougham, D.F. Long-circulating magnetoliposomes as surrogates for assessing pancreatic tumour permeability and nanoparticle deposition. Acta Biomater. 2023, 158, 611–624. [Google Scholar] [CrossRef] [PubMed]
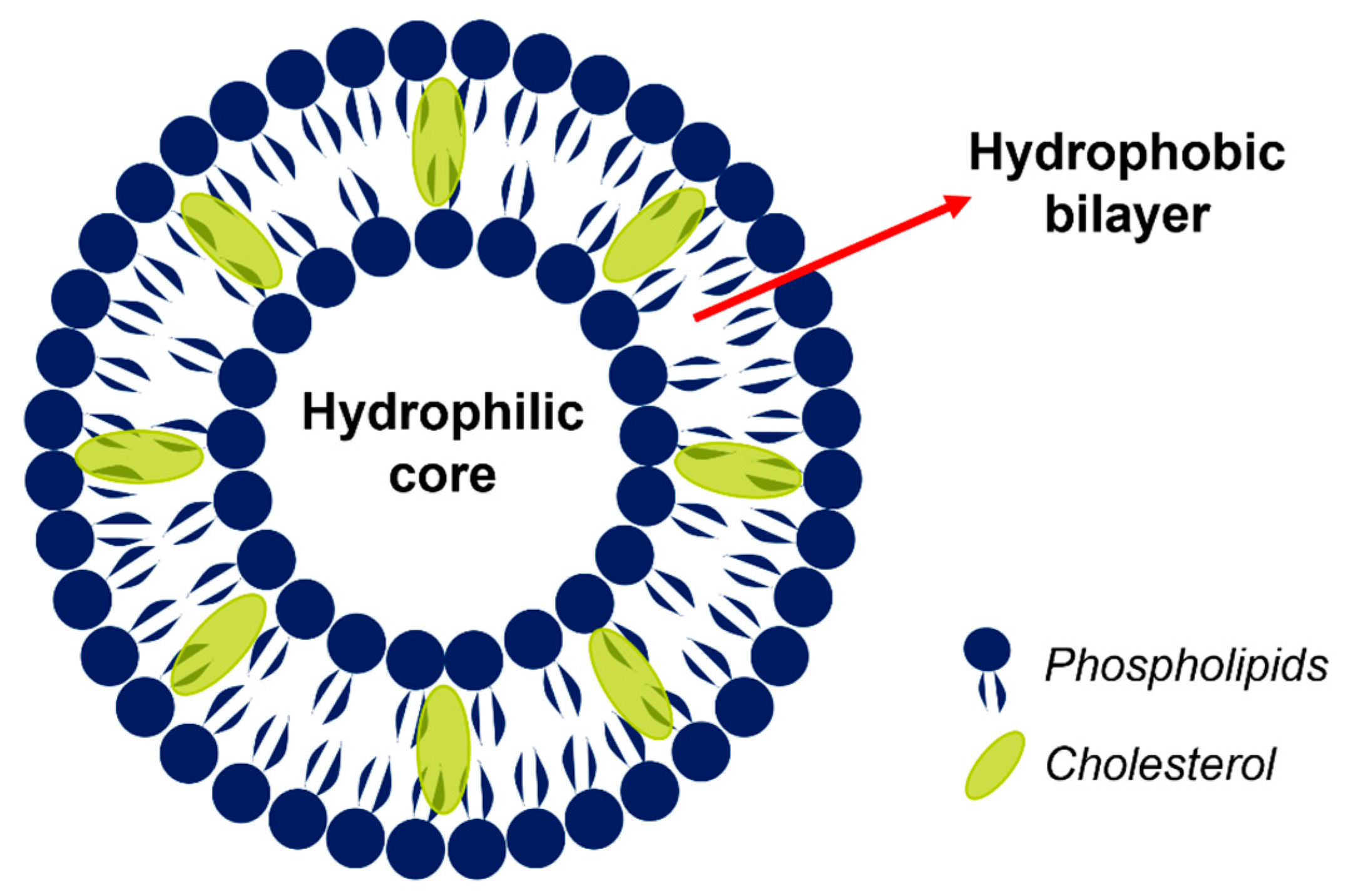

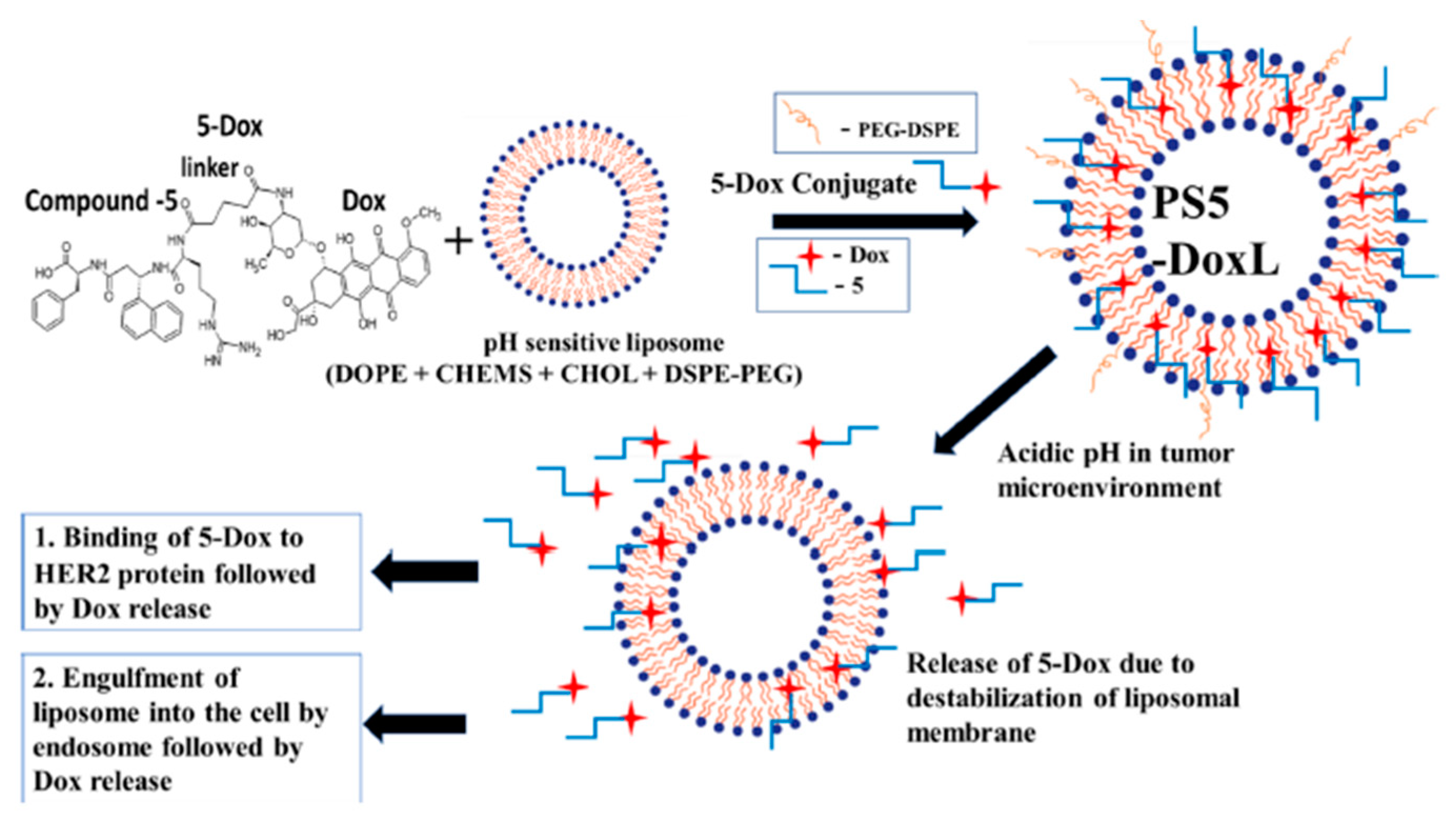
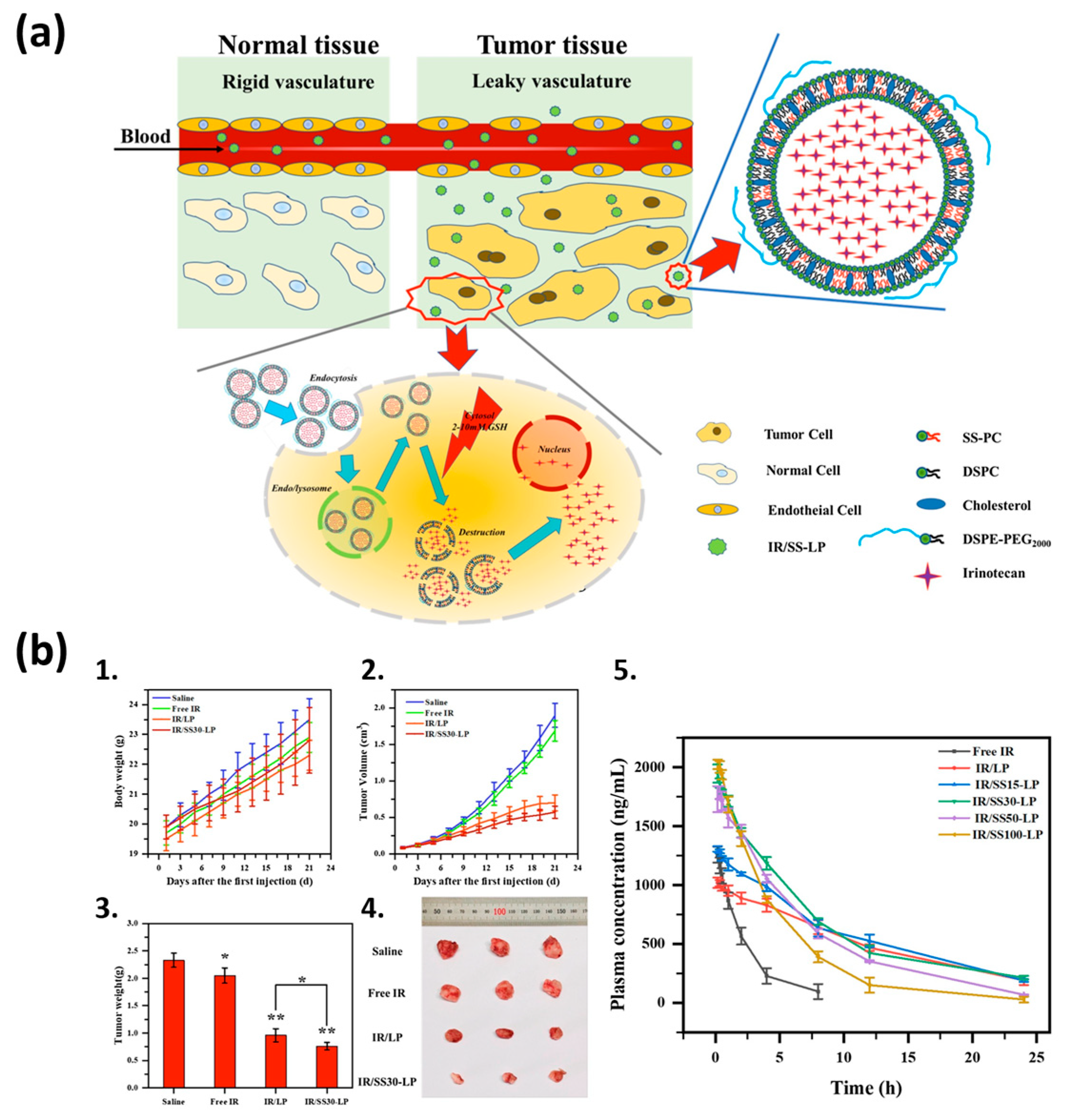
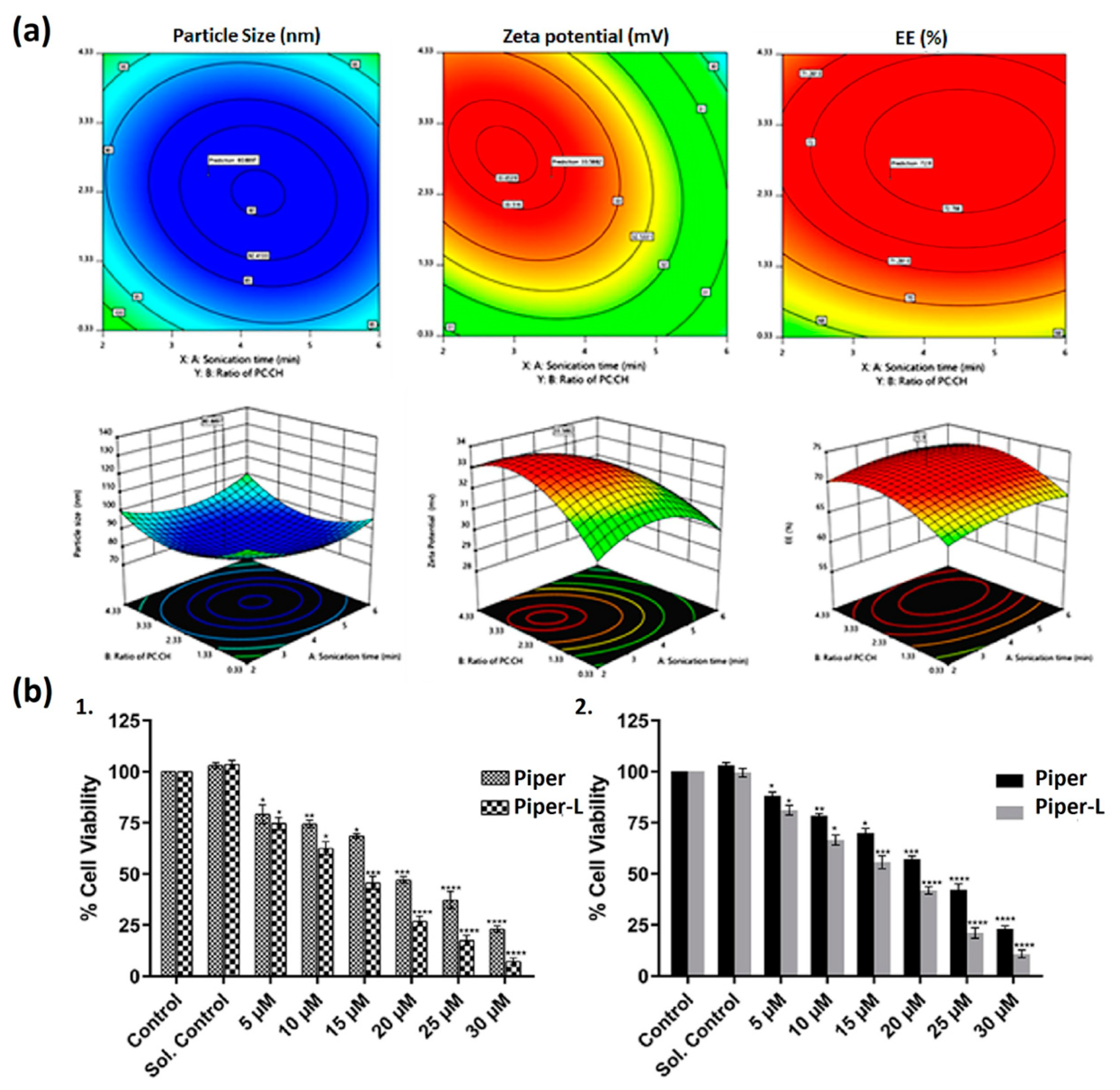

| Liposome Composition | Therapeutic Agents Loaded | Biomedical Application | Stage of Development | Ref. |
|---|---|---|---|---|
| HSPC, CHOL, and GC | Dox | Fibrosarcoma | Preclinical (murine xenograft tumor model) | [25] |
| MGlu, HA, and CHex-HA | Dox | Cervix cancer Breast cancer | Preclinical (murine colon model) | [26] |
| CHOL, DSPE, PEG2000, and SPC | Ptx | Melanoma Breast cancer | Preclinical (tumor-bearing mice) | [27] |
| Peptide KLA, DMA, DSPE, and SPC | Ptx | Lung cancer | Preclinical (nude mice) | [28] |
| EYPC and MGlu-HPG | Ovalbumin | Lymphoma | Preclinical (C57BL/6 mice) | [29] |
| DMAP, SPC, PEG, DSPE, and DCC | Dox | Melanoma | In vitro (A375 cancer cell line) | [30] |
| EYPC, DOPE, MGlu, MPLA, and DEX | Ovalbumin | Lymphoma | Preclinical (C57BL/6 mice) | [31] |
| DPPC, CHOL, and DC | Dox | Osteosarcoma | In vitro (K7M2 and NIH/3T3 cancer cell lines) | [32] |
| PC, DOPE, DSPE, PEG2000, and HA | Dox | Breast cancer | In vitro (MCF-7 cancer cell line) | [33] |
| HSPC, DOPE, DSPE, PEG2000, and CHOL | Dox | Breast cancer | Preclinical (BALB/c mice) | [34] |
| DSPE, PEG2000, and CHOL | Emtansine | Breast cancer | Preclinical (murine RAW 264.7) | [35] |
| CHEMS, DOPE, DSPE, PEG2000, and CHOL | Gemcitabine | Pancreatic cancer | Preclinical (MIA PaCa-2 cells) | [36] |
| EPC, CHEMS, DOPE, DSPE, PEG2000, and CHOL | Dox | Glioma | Preclinical (BALB/c nude mice) | [37] |
| CHEMS, DOPE, SPE, and PEG2000 | 5-Fluorouracil | Breast cancer | In vitro (MDA-MB-231 and SK-BR-3 cancer cell lines) | [38] |
| CHOL, SPC, DSPE, and PEG5000 | Bovine serum albumin | Bladder cancer | Preclinical (C57BL/6 mice) | [39] |
| DOPE, CHOL, and DC | Resiquimod | Colorectal cancer | Preclinical (BALB/c mice) | [40] |
| CHEMS, DOPE, DSPE, and PEG2000 | Radiotracer (99mTc-HYNIC-βAla-Bombesin(7–14)) | Breast cancer | Preclinical (BALB/c mice) | [41] |
| CHEMS, DPPC, DSPE, and PEG2000 | Docetaxel Doxycycline hyclate | Lung cancer | Preclinical (BALB/c mice) | [42] |
| HSPC, HA, and DEAP | Docetaxel | Colon carcinoma | Preclinical (BALB/c mice) | [43] |
| DOPE, DOTAP, and PC | Docetaxel | Breast cancer | Preclinical (Swiss albino mice) | [44] |
| DOPE, CHEMS, DSPE, and PEG750 or PEG2000 | Calcein | Glioblastoma | Preclinical (murine model of glioblastoma) | [45] |
| SPC and CHOL | Paclitaxel hydroxypropyl-β-cyclodextrin complex | Lung cancer | Preclinical (BALB/c mice) | [46] |
| DOPE, CHEMS, DSPE, CHOL, HSPC, and PEG2000 | Dox | Cervical cancer | In vitro (HeLa cancer cell line) | [24] |
| DOPE, CHEMS, DSPE, and PEG2000 | Dox | Breast cancer | Preclinical (BALB/c mice) | [47] |
| DOPE, CHEMS, CHOL, DSPE, and PEG2000 | Peptidomimetic-doxorubicin conjugate | Lung cancer Breast cancer | Preclinical (BALB/c nude mice) | [48] |
| DOPE, CHOL, DSPE, PEG2000, and CL | Daunorubicin | Melanoma | In vitro (B16-BL6 cancer cell line) | [49] |
| DOPE, CHEMS, DSPE, and PEG2000 | Simvastatin Dox | Breast cancer | In vitro (MDA-MB-231, MCF-7, and SK-BR-3 cancer cell lines) | [50] |
| DOPE, CHEMS, DSPE, PEG2000, and EDC | Docetaxel | Breast cancer | Preclinical (Dawley rats) | [51] |
| PC, CHOL, and CHEMS | Morin | Hepatocellular cancer Breast cancer Lung cancer Gastric cancer | Preclinical (BALB/c nude mice) | [52] |
| DPPC, DSPE, PEG2000, DOPE, and CHOL | Echinomycin | Breast cancer Lung cancer | In vitro (MDA-MB-231, MCF-7, and A549 cancer cell lines) | [53] |
| DOPE, CHEMS, and PEG2000 | Cu(II) complex (Cu(1,10- phenanthroline)Cl2) | Colorectal carcinoma | Preclinical (BALB/c mice) | [54] |
| DOPE, CHEMS, CHOL, DSPE, and PEG2000 | Irinotecan | Colorectal carcinoma | Preclinical (BALB/c mice) | [55] |
| DPPE and PEG2000 | Imidazole | Cervical cancer Breast cancer Lung cancer | In vitro (MDA-MB-231, MDA-MB-468, and A549 cancer cell lines) | [56] |
| DOTAP, DOPE, and CHOL | Sorafenib and VEGF-siRN | Hepatocellular carcinoma | Preclinical (Kunming mice) | [57] |
| Eudragit® S100, OA, CHOL, and SC | Curcumin | Colon cancer | In vitro (Caco-2 cancer cell line) | [58] |
| EPC and CHEMS | Carboplatin | Lung cancer | In vitro (A549 cancer cell line) | [59] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres, J.; Valenzuela Oses, J.K.; Rabasco-Álvarez, A.M.; González-Rodríguez, M.L.; García, M.C. Innovations in Cancer Therapy: Endogenous Stimuli-Responsive Liposomes as Advanced Nanocarriers. Pharmaceutics 2025, 17, 245. https://doi.org/10.3390/pharmaceutics17020245
Torres J, Valenzuela Oses JK, Rabasco-Álvarez AM, González-Rodríguez ML, García MC. Innovations in Cancer Therapy: Endogenous Stimuli-Responsive Liposomes as Advanced Nanocarriers. Pharmaceutics. 2025; 17(2):245. https://doi.org/10.3390/pharmaceutics17020245
Chicago/Turabian StyleTorres, Jazmín, Johanna Karina Valenzuela Oses, Antonio María Rabasco-Álvarez, María Luisa González-Rodríguez, and Mónica Cristina García. 2025. "Innovations in Cancer Therapy: Endogenous Stimuli-Responsive Liposomes as Advanced Nanocarriers" Pharmaceutics 17, no. 2: 245. https://doi.org/10.3390/pharmaceutics17020245
APA StyleTorres, J., Valenzuela Oses, J. K., Rabasco-Álvarez, A. M., González-Rodríguez, M. L., & García, M. C. (2025). Innovations in Cancer Therapy: Endogenous Stimuli-Responsive Liposomes as Advanced Nanocarriers. Pharmaceutics, 17(2), 245. https://doi.org/10.3390/pharmaceutics17020245











