Cancer Vaccines and Beyond: The Transformative Role of Nanotechnology in Immunotherapy
Abstract
1. Introduction
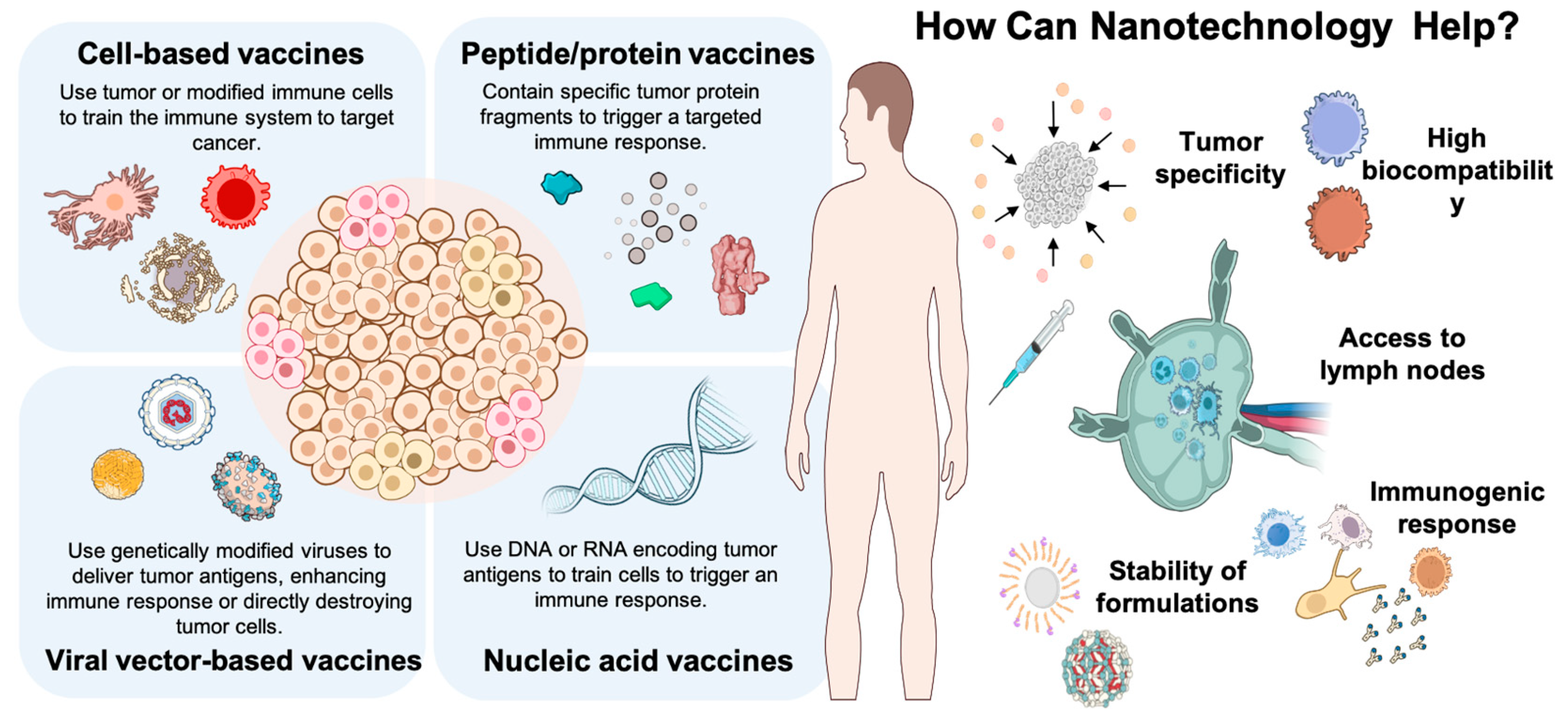
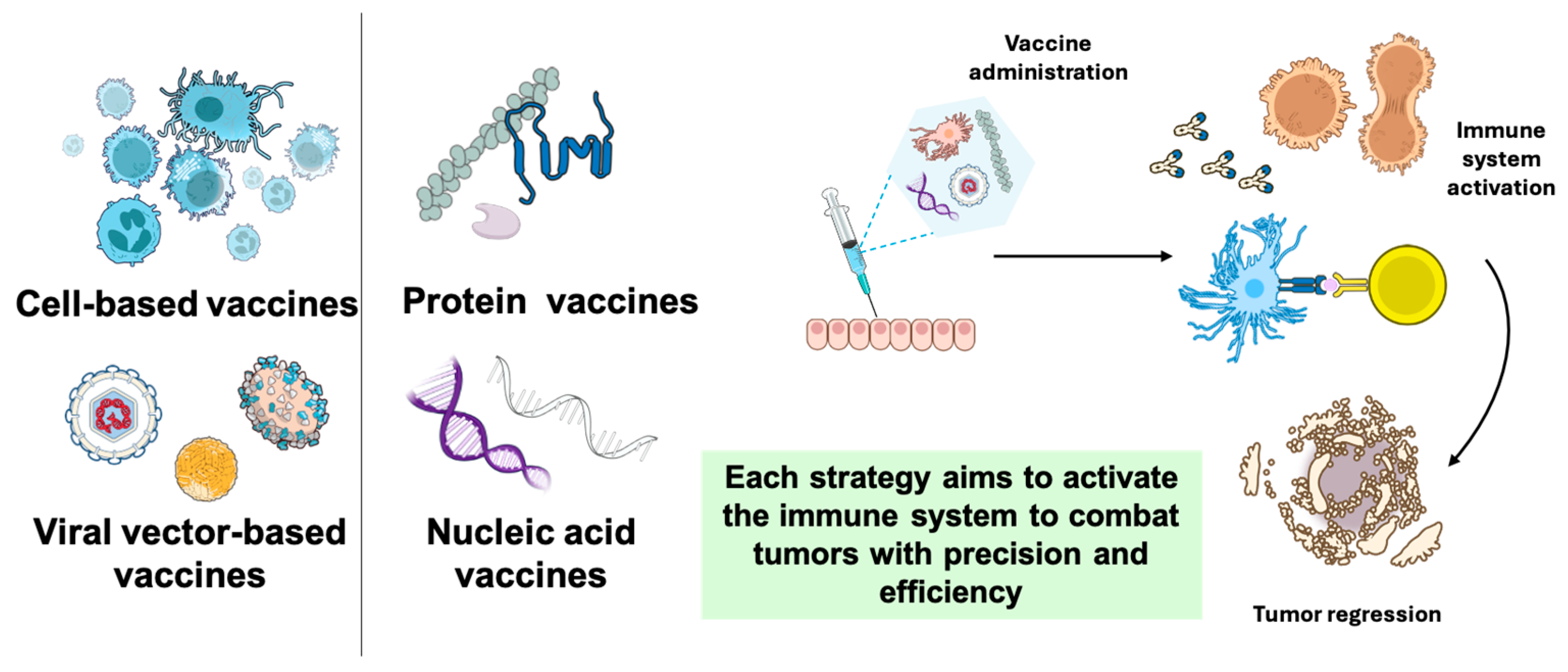
2. Cell-Based Vaccines
2.1. GVAX, Provenge, and Canvaxin
2.2. Improving Immunogenicity
2.3. Limitations of Monotherapy Vaccines
2.4. Glioblastoma Multiforme
- -
- The presence of the blood–brain barrier, which limits the access of many immune system cells to brain tissue, restricting their ability to exert an effective response against tumors.
- -
- The lack of antigens, specific to the glioma, identified.
- -
- The ability of the glioma to create an immunosuppressive tumor microenvironment.
3. Peptide and Protein Vaccines
3.1. Classification and Clinical Trials of Peptide Vaccines
3.2. Improving the Efficacy of Peptide Vaccines
3.3. Combination with Other Therapies
4. Nucleic Acid Vaccines
4.1. DNA Vaccines
4.2. Messenger RNA (mRNA) Vaccines
4.3. Clinical Outcomes in DNA and RNA Cancer Vaccines
5. Viral Vector-Based Vaccines
5.1. New Approaches in Vaccines Based on Viral Vectors: Heterologous Prime-Boost Vaccination
5.2. Types of Viral Vectors
5.2.1. Adenovirus Vectors
5.2.2. Poxvirus Vectors
5.3. Clinical Advances in Viral Vector Cancer Vaccines
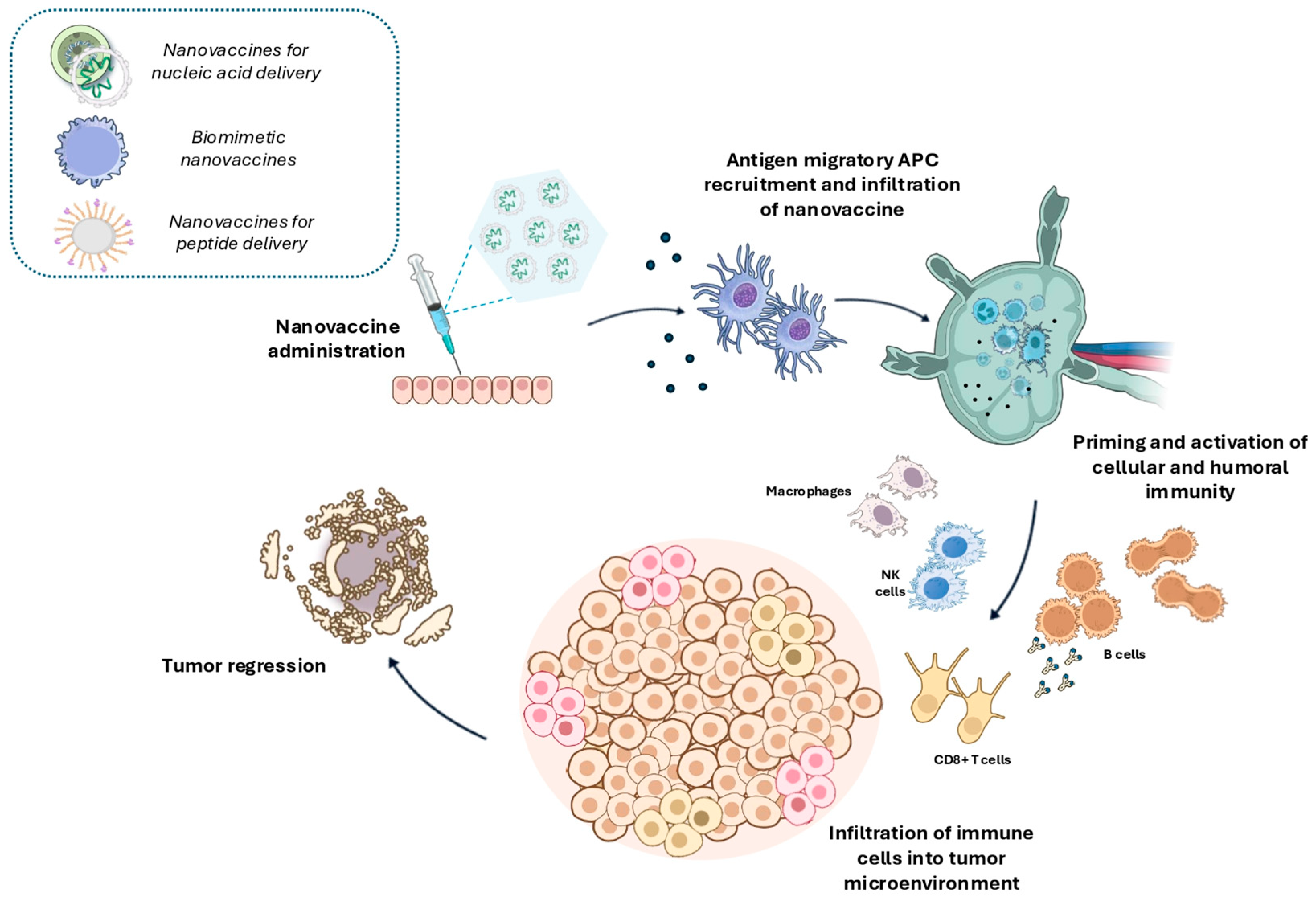
6. Nanovaccines
6.1. Efficiency of Nanovaccines in Activating the Immune System
6.2. Nanovaccine-Based Systems
6.2.1. Nanovaccines for Nucleic Acid Delivery
6.2.2. Biomimetic Nanovaccines
6.2.3. Nanovaccines for Peptide Delivery
6.3. Clinical Trials in Nanovaccines for Cancer
7. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Sellars, M.C.; Wu, C.J.; Fritsch, E.F. Cancer vaccines: Building a bridge over troubled waters. Cell 2022, 185, 2770–2788. [Google Scholar] [CrossRef] [PubMed]
- Garg, P.; Malhotra, J.; Kulkarni, P.; Horne, D.; Salgia, R.; Singhal, S.S. Emerging Therapeutic Strategies to Overcome Drug Resistance in Cancer Cells. Cancers 2024, 16, 2478. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Hong, Z.; Zhang, C.; Wang, L.; Han, Z.; Ma, D. Immune checkpoint therapy for solid tumours: Clinical dilemmas and future trends. Signal Transduct. Target. Ther. 2023, 8, 320. [Google Scholar] [CrossRef]
- Yang, L.; Ning, Q.; Tang, S.-S. Recent Advances and Next Breakthrough in Immunotherapy for Cancer Treatment. J. Immunol. Res. 2022, 2022, 8052212. [Google Scholar] [CrossRef] [PubMed]
- Binnewies, M.; Roberts, E.W.; Kersten, K.; Chan, V.; Fearon, D.F.; Merad, M.; Coussens, L.M.; Gabrilovich, D.I.; Ostrand-Rosenberg, S.; Hedrick, C.C.; et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 2018, 24, 541–550. [Google Scholar] [CrossRef]
- Dunn, G.P.; Old, L.J.; Schreiber, R.D. The immunobiology of cancer immunosurveillance and immunoediting. Immunity 2004, 21, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, R.D.; Old, L.J.; Smyth, M.J. Cancer immunoediting: Integrating immunity’s roles in cancer suppression and promotion. Science 2011, 331, 1565–1570. [Google Scholar] [CrossRef] [PubMed]
- Togashi, Y.; Shitara, K.; Nishikawa, H. Regulatory T cells in cancer immunosuppression—Implications for anticancer therapy. Nat. Rev. Clin. Oncol. 2019, 16, 356–371. [Google Scholar] [CrossRef]
- Sun, C.; Xu, S. Advances in personalized neoantigen vaccines for cancer immunotherapy. Biosci. Trends 2020, 14, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Zhang, R.; Yang, A.G.; Zheng, G. Diversity of immune checkpoints in cancer immunotherapy. Front. Immunol. 2023, 14, 1121285. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Shen, D.; Shang, M.; Yu, H.; Zuo, X.; Chen, L.; Huang, Z.; Li, L.; Wang, L. Immunotherapy: A new target for cancer cure (Review). Oncol. Rep. 2023, 49, 100. [Google Scholar] [CrossRef]
- Blaya-Cánovas, J.L.; Griñán-Lisón, C.; Blancas, I.; Marchal, J.A.; Ramírez-Tortosa, C.; López-Tejada, A.; Benabdellah, K.; Cortijo-Gutiérrez, M.; Cano-Cortés, M.V.; Graván, P.; et al. Autologous patient-derived exhausted nano T-cells exploit tumor immune evasion to engage an effective cancer therapy. Mol. Cancer 2024, 23, 83. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, Y.; Sasada, T. Cancer Vaccines: Toward the Next Breakthrough in Cancer Immunotherapy. J. Immunol. Res. 2020, 2020, 5825401. [Google Scholar] [CrossRef] [PubMed]
- Seclì, L.; Leoni, G.; Ruzza, V.; Siani, L.; Cotugno, G.; Scarselli, E.; D’Alise, A.M. Personalized Cancer Vaccines Go Viral: Viral Vectors in the Era of Personalized Immunotherapy of Cancer. Int. J. Mol. Sci. 2023, 24, 16591. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.; Zhang, M.; Yang, J.; Zhu, Z.; Cao, W.; Dong, C. Therapeutic cancer vaccines: Advancements, challenges, and prospects. Signal Transduct. Target. Ther. 2023, 8, 450. [Google Scholar] [CrossRef]
- Malacopol, A.T.; Holst, P.J. Cancer Vaccines: Recent Insights and Future Directions. Int. J. Mol. Sci. 2024, 25, 11256. [Google Scholar] [CrossRef] [PubMed]
- Richard, G.; Ruggiero, N.; Steinberg, G.D.; Martin, W.D.; De Groot, A.S. Neoadjuvant personalized cancer vaccines: The final frontier? Expert. Rev. Vaccines 2024, 23, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Kshirsagar, P.G.; Gautam, S.K.; Gulati, M.; Wafa, E.I.; Christiansen, J.C.; White, B.M.; Mallapragada, S.K.; Wannemuehler, M.J.; Kumar, S.; et al. Nanocarriers for pancreatic cancer imaging, treatments, and immunotherapies. Theranostics 2022, 12, 1030–1060. [Google Scholar] [CrossRef] [PubMed]
- Nooraei, S.; Bahrulolum, H.; Hoseini, Z.S.; Katalani, C.; Hajizade, A.; Easton, A.J.; Ahmadian, G. Virus-like particles: Preparation, immunogenicity and their roles as nanovaccines and drug nanocarriers. J. Nanobiotechnol. 2021, 19, 59. [Google Scholar] [CrossRef]
- Fang, X.; Lan, H.; Jin, K.; Gong, D.; Qian, J. Nanovaccines for Cancer Prevention and Immunotherapy: An Update Review. Cancers 2022, 14, 3842. [Google Scholar] [CrossRef] [PubMed]
- Mainini, F.; Eccles, M.R. Lipid and Polymer-Based Nanoparticle siRNA Delivery Systems for Cancer Therapy. Molecules 2020, 25, 2692. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jiang, M.; Du, G.; Zhong, X.; He, C.; Qin, M.; Hou, Y.; Liu, R.; Sun, X. An antigen self-assembled and dendritic cell-targeted nanovaccine for enhanced immunity against cancer. Acta Pharm. Sin. B 2023, 13, 3518–3534. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Ali, N. Nanovaccine: An emerging strategy. Expert. Rev. Vaccines 2021, 20, 1273–1290. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Li, Y.; Zhang, Y.; Zhang, J.; Wu, D. Current status and future directions of nanovaccine for cancer: A bibliometric analysis during 2004–2023. Front. Immunol. 2024, 15, 1423212. [Google Scholar] [CrossRef] [PubMed]
- Koyande, N.P.; Srivastava, R.; Padmakumar, A.; Rengan, A.K. Advances in Nanotechnology for Cancer Immunoprevention and Immunotherapy: A Review. Vaccines 2022, 10, 1727. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Fu, M.; Wang, M.; Wan, D.; Wei, Y.; Wei, X. Cancer vaccines as promising immuno-therapeutics: Platforms and current progress. J. Hematol. Oncol. 2022, 15, 28. [Google Scholar] [CrossRef]
- Hoover, H.C., Jr.; Surdyke, M.G.; Dangel, R.B.; Peters, L.C.; Hanna, M.G., Jr. Prospectively randomized trial of adjuvant active-specific immunotherapy for human colorectal cancer. Cancer 1985, 55, 1236–1243. [Google Scholar] [CrossRef]
- Gardner, T.; Elzey, B.; Hahn, N.M. Sipuleucel-T (Provenge) autologous vaccine approved for treatment of men with asymptomatic or minimally symptomatic castrate-resistant metastatic prostate cancer. Hum. Vaccines Immunother. 2012, 8, 534–539. [Google Scholar] [CrossRef]
- Rai, A.; Deshpande, S.G.; Vaidya, A.; Shinde, R.K. Advancements in Immunotherapy for Breast Cancer: Mechanisms, Efficacy, and Future Directions. Cureus 2024, 16, e68351. [Google Scholar] [CrossRef]
- Ghemrawi, R.; Abuamer, L.; Kremesh, S.; Hussien, G.; Ahmed, R.; Mousa, W.; Khoder, G.; Khair, M. Revolutionizing Cancer Treatment: Recent Advances in Immunotherapy. Biomedicines 2024, 12, 2158. [Google Scholar] [CrossRef]
- Panuccio, G.; Correale, P.; d’Apolito, M.; Mutti, L.; Giannicola, R.; Pirtoli, L.; Giordano, A.; Labate, D.; Macheda, S.; Carabetta, N.; et al. Immuno-related cardio-vascular adverse events associated with immuno-oncological treatments: An under-estimated threat for cancer patients. Basic. Res. Cardiol. 2024, 120, 153–169. [Google Scholar] [CrossRef]
- Rüttinger, D.; Van Den Engel, N.K.; Winter, H.; Schlemmer, M.; Pohla, H.; Grützner, S.; Wagner, B.; Schendel, D.J.; Fox, B.A.; Jauch, K.W.; et al. Adjuvant therapeutic vaccination in patients with non-small cell lung cancer made lymphopenic and reconstituted with autologous PBMC: First clinical experience and evidence of an immune response. J. Transl. Med. 2007, 5, 43. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Dong, H.; Ren, S.; Chen, J.; Zhang, Y.; Dai, M.; Wu, Y.; Zhang, X. Exploration of novel clusters and prognostic value of immune-related signatures and identify HAMP as hub gene in colorectal cancer. Oncol. Lett. 2023, 26, 360. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstern, C.R.; Ngu, R.K.; Shalapour, S.; Karin, M. Immunotherapy, Inflammation and Colorectal Cancer. Cells 2020, 9, 618. [Google Scholar] [CrossRef]
- Morton, D.L. Immune response to postsurgical adjuvant active immunotherapy with Canvaxin polyvalent cancer vaccine: Correlations with clinical course of patients with metastatic melanoma. Dev. Biol. 2004, 116, 209–217; discussion 229–236. [Google Scholar]
- Tani, K.; Azuma, M.; Nakazaki, Y.; Oyaizu, N.; Hase, H.; Ohata, J.; Takahashi, K.; Oiwamonna, M.; Hanazawa, K.; Wakumoto, Y.; et al. Phase I Study of Autologous Tumor Vaccines Transduced with the GM-CSF Gene in Four Patients with Stage IV Renal Cell Cancer in Japan: Clinical and Immunological Findings. Mol. Ther. 2004, 10, 799–816. [Google Scholar] [CrossRef]
- Gu, Y.-Z.; Fan, C.-W.; Lu, R.; Shao, B.; Sang, Y.-X.; Huang, Q.-R.; Li, X.; Meng, W.-T.; Mo, X.-M.; Wei, Y.-Q. Forced co-expression of IL-21 and IL-7 in whole-cell cancer vaccines promotes antitumor immunity. Sci. Rep. 2016, 6, 32351. [Google Scholar] [CrossRef]
- Zhao, Y.; Wei, K.; Chi, H.; Xia, Z.; Li, X. IL-7: A promising adjuvant ensuring effective T cell responses and memory in combination with cancer vaccines? Front. Immunol. 2022, 13, 1022808. [Google Scholar] [CrossRef]
- Jaffee, E.M.; Hruban, R.H.; Biedrzycki, B.; Laheru, D.; Schepers, K.; Sauter, P.R.; Goemann, M.; Coleman, J.; Grochow, L.; Donehower, R.C.; et al. Novel Allogeneic Granulocyte-Macrophage Colony-Stimulating Factor–Secreting Tumor Vaccine for Pancreatic Cancer: A Phase I Trial of Safety and Immune Activation. J. Clin. Oncol. 2001, 19, 145–156. [Google Scholar] [CrossRef]
- Hato, L.; Vizcay, A.; Eguren, I.; Pérez-Gracia, J.L.; Rodríguez, J.; Gállego Pérez-Larraya, J.; Sarobe, P.; Inogés, S.; Díaz De Cerio, A.L.; Santisteban, M. Dendritic Cells in Cancer Immunology and Immunotherapy. Cancers 2024, 16, 981. [Google Scholar] [CrossRef]
- Sun, C.; Ma, X.; Zhou, C.; Zhang, Z.; Guo, J. Irreversible Electroporation Combined With Dendritic Cell-based Vaccines for the Treatment of Osteosarcoma. Anticancer. Res. 2023, 43, 3389–3400. [Google Scholar] [CrossRef]
- Vincent, B.G.; File, D.M.; McKinnon, K.P.; Moore, D.T.; Frelinger, J.A.; Collins, E.J.; Ibrahim, J.G.; Bixby, L.; Reisdorf, S.; Laurie, S.J.; et al. Efficacy of a Dual-Epitope Dendritic Cell Vaccine as Part of Combined Immunotherapy for HER2-Expressing Breast Tumors. J. Immunol. 2023, 211, 219–228. [Google Scholar] [CrossRef]
- Yin, T.; Shi, P.; Gou, S.; Shen, Q.; Wang, C. Dendritic Cells Loaded with Pancreatic Cancer Stem Cells (CSCs) Lysates Induce Antitumor Immune Killing Effect In Vitro. PLoS ONE 2014, 9, e114581. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Rao, W.; Chen, Y.; Xie, J. In vitro induction of anti-lung cancer immune response by the A549 lung cancer stem cell lysate-sensitized dendritic cell vaccine. Oncol. Lett. 2024, 28, 550. [Google Scholar] [CrossRef]
- Hu, Y.; Lu, L.; Xia, Y.; Chen, X.; Chang, A.E.; Hollingsworth, R.E.; Hurt, E.; Owen, J.; Moyer, J.S.; Prince, M.E.P.; et al. Therapeutic Efficacy of Cancer Stem Cell Vaccines in the Adjuvant Setting. Cancer Res. 2016, 76, 4661–4672. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, F.; Razmi, M.; Tajik, F.; Zöller, M.; Dehghan Manshadi, M.; Mahdavinezhad, F.; Tiyuri, A.; Ghods, R.; Madjd, Z. Efficacy of Whole Cancer Stem Cell-Based Vaccines: A Systematic Review of Preclinical and Clinical Studies. Stem Cells 2023, 41, 207–232. [Google Scholar] [CrossRef]
- Baek, B.-S.; Park, H.; Choi, J.-W.; Lee, E.-Y.; Youn, J.-I.; Seong, S.-Y. Dendritic cells pulsed with penetratin-OLFM4 inhibit the growth and metastasis of melanoma in mice. Biomed. Pharmacother. 2024, 177, 117083. [Google Scholar] [CrossRef] [PubMed]
- Alqarni, A.; Jasim, S.A.; Altalbawy, F.M.A.; Kaur, H.; Kaur, I.; Rodriguez-Benites, C.; Deorari, M.; Alwaily, E.R.; Al-Ani, A.M.; Redhee, A.H. Challenges and opportunities for cancer stem cell-targeted immunotherapies include immune checkpoint inhibitor, cancer stem cell-dendritic cell vaccine, chimeric antigen receptor immune cells, and modified exosomes. J. Biochem. Mol. Toxicol. 2024, 38, e23719. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.S.; Parkinson, R.; Jaffee, E.M.; Sugar, E.; Zheng, L.; Onners, B.; Weiss, M.J.; Wolfgang, C.L.; Cameron, J.L.; Pawlik, T.M.; et al. Phase I Study of Adjuvant Allogeneic GM-CSF-Transduced Pancreatic Tumor Cell Vaccine, Low Dose Cyclophosphamide, and SBRT followed by FFX in High-Risk Resected Pancreatic Ductal Adenocarcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2024. [Google Scholar] [CrossRef]
- Jacobsen, E.; Plant, A.; Redd, R.; Armand, P.; McDonough, M.; Ihuoma, U.; Fisher, D.C.; Lacasce, A.; Ritz, J.; Dranoff, G.; et al. A phase I trial of vaccination with lethally irradiated lymphoma cells admixed with granulocyte-macrophage colony-stimulating factor secreting K562 cells for the treatment of follicular lymphoma. Leuk. Lymphoma 2024, 65, 1864–1874. [Google Scholar] [CrossRef] [PubMed]
- Nemunaitis, J. GVAX (GMCSF gene modified tumor vaccine) in advanced stage non small cell lung cancer. J. Control. Release 2003, 91, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Nemunaitis, J.; Jahan, T.; Ross, H.; Sterman, D.; Richards, D.; Fox, B.; Jablons, D.; Aimi, J.; Lin, A.; Hege, K. Phase 1/2 trial of autologous tumor mixed with an allogeneic GVAX® vaccine in advanced-stage non-small-cell lung cancer. Cancer Gene Ther. 2006, 13, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Lutz, E.; Yeo, C.J.; Lillemoe, K.D.; Biedrzycki, B.; Kobrin, B.; Herman, J.; Sugar, E.; Piantadosi, S.; Cameron, J.L.; Solt, S.; et al. A lethally irradiated allogeneic granulocyte-macrophage colony stimulating factor-secreting tumor vaccine for pancreatic adenocarcinoma. A Phase II trial of safety, efficacy, and immune activation. Ann. Surg. 2011, 253, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Cheever, M.A.; Higano, C.S. PROVENGE (Sipuleucel-T) in Prostate Cancer: The First FDA-Approved Therapeutic Cancer Vaccine. Clin. Cancer Res. 2011, 17, 3520–3526. [Google Scholar] [CrossRef] [PubMed]
- Small, E.J.; Fratesi, P.; Reese, D.M.; Strang, G.; Laus, R.; Peshwa, M.V.; Valone, F.H. Immunotherapy of Hormone-Refractory Prostate Cancer With Antigen-Loaded Dendritic Cells. J. Clin. Oncol. 2000, 18, 3894–3903. [Google Scholar] [CrossRef] [PubMed]
- Najafi, S.; Mortezaee, K. Advances in dendritic cell vaccination therapy of cancer. Biomed. Pharmacother. 2023, 164, 114954. [Google Scholar] [CrossRef]
- Kong, Z.; Wang, Y.; Ma, W. Vaccination in the immunotherapy of glioblastoma. Hum. Vaccines Immunother. 2018, 14, 255–268. [Google Scholar] [CrossRef]
- Anassi, E.; Ndefo, U. Sipuleucel-T (Provenge) injection the first immunotherapy agent (Vaccine) for hormone-refractory prostate cancer. Pharm. Ther. 2011, 36, 197–202. [Google Scholar]
- Sims, R.B. Development of sipuleucel-T: Autologous cellular immunotherapy for the treatment of metastatic castrate resistant prostate cancer. Vaccine 2012, 30, 4394–4397. [Google Scholar] [CrossRef] [PubMed]
- Morton, D.L.; Hsueh, E.C.; Essner, R.; Foshag, L.J.; O’Day, S.J.; Bilchik, A.; Gupta, R.K.; Hoon, D.S.B.; Ravindranath, M.; Nizze, J.A.; et al. Prolonged Survival of Patients Receiving Active Immunotherapy With Canvaxin Therapeutic Polyvalent Vaccine After Complete Resection of Melanoma Metastatic to Regional Lymph Nodes. Ann. Surg. 2002, 236, 438–449. [Google Scholar] [CrossRef] [PubMed]
- Simons, J.W.; Carducci, M.A.; Mikhak, B.; Lim, M.; Biedrzycki, B.; Borellini, F.; Clift, S.M.; Hege, K.M.; Ando, D.G.; Piantadosi, S.; et al. Phase I/II Trial of an Allogeneic Cellular Immunotherapy in Hormone-Naïve Prostate Cancer. Clin. Cancer Res. 2006, 12, 3394–3401. [Google Scholar] [CrossRef]
- Seledtsov, V.; Goncharov, A.; Seledtsova, G. Clinically feasible approaches to potentiating cancer cell-based immunotherapies. Hum. Vaccines Immunother. 2015, 11, 851–869. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.L.-L.; Kandalaft, L.E.; Coukos, G. Adjuvants for Enhancing the Immunogenicity of Whole Tumor Cell Vaccines. Int. Rev. Immunol. 2011, 30, 150–182. [Google Scholar] [CrossRef] [PubMed]
- Raja, J.; Ludwig, J.M.; Gettinger, S.N.; Schalper, K.A.; Kim, H.S. Oncolytic virus immunotherapy: Future prospects for oncology. J. Immunother. Cancer 2018, 6, 140. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Nam, G.-H.; Kim, G.B.; Kim, Y.K.; Kim, I.-S. Intrinsic cancer vaccination. Adv. Drug Deliv. Rev. 2019, 151–152, 2–22. [Google Scholar] [CrossRef]
- Miyamoto, S.; Inoue, H.; Nakamura, T.; Yamada, M.; Sakamoto, C.; Urata, Y.; Okazaki, T.; Marumoto, T.; Takahashi, A.; Takayama, K.; et al. Coxsackievirus B3 Is an Oncolytic Virus with Immunostimulatory Properties That Is Active against Lung Adenocarcinoma. Cancer Res. 2012, 72, 2609–2621. [Google Scholar] [CrossRef] [PubMed]
- Hemminki, O.; Dos Santos, J.M.; Hemminki, A. Oncolytic viruses for cancer immunotherapy. J. Hematol. Oncol. 2020, 13, 84. [Google Scholar] [CrossRef]
- Yamano, T.; Kubo, S.; Fukumoto, M.; Yano, A.; Mawatari-Furukawa, Y.; Okamura, H.; Tomita, N. Whole cell vaccination using immunogenic cell death by an oncolytic adenovirus is effective against a colorectal cancer model. Mol. Ther.-Oncolytics 2016, 3, 16031. [Google Scholar] [CrossRef]
- Wang-Bishop, L.; Wehbe, M.; Shae, D.; James, J.; Hacker, B.C.; Garland, K.; Chistov, P.P.; Rafat, M.; Balko, J.M.; Wilson, J.T. Potent STING activation stimulates immunogenic cell death to enhance antitumor immunity in neuroblastoma. J. Immunother. Cancer 2020, 8, e000282. [Google Scholar] [CrossRef]
- Srinivasan, P.; Wu, X.; Basu, M.; Rossi, C.; Sandler, A.D. PD-L1 checkpoint inhibition and anti-CTLA-4 whole tumor cell vaccination counter adaptive immune resistance: A mouse neuroblastoma model that mimics human disease. PLoS Med. 2018, 15, e1002497. [Google Scholar] [CrossRef]
- Hailemichael, Y.; Woods, A.; Fu, T.; He, Q.; Nielsen, M.C.; Hasan, F.; Roszik, J.; Xiao, Z.; Vianden, C.; Khong, H.; et al. Cancer vaccine formulation dictates synergy with CTLA-4 and PD-L1 checkpoint blockade therapy. J. Clin. Investig. 2018, 128, 1338–1354. [Google Scholar] [CrossRef]
- Raymakers, L.; Demmers, T.J.; Meijer, G.J.; Molenaar, I.Q.; Van Santvoort, H.C.; Intven, M.P.W.; Leusen, J.H.W.; Olofsen, P.A.; Daamen, L.A. The Effect of Radiation Treatment of Solid Tumors on Neutrophil Infiltration and Function: A Systematic Review. Int. J. Radiat. Oncol. Biol. Phys. 2024, 120, 845–861. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.; Matulonis, U. Immunotherapy and radiation combinatorial trials in gynecologic cancer: A potential synergy? Gynecol. Oncol. 2019, 154, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Walle, T.; Martinez Monge, R.; Cerwenka, A.; Ajona, D.; Melero, I.; Lecanda, F. Radiation effects on antitumor immune responses: Current perspectives and challenges. Ther. Adv. Med. Oncol. 2018, 10, 175883401774257. [Google Scholar] [CrossRef] [PubMed]
- Joyce, J.A.; Fearon, D.T. T cell exclusion, immune privilege, and the tumor microenvironment. Science 2015, 348, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Li, S.; Li, M.; Zhou, H.; Yang, X. Upregulation of CD3ζ and L-selectin in antigen-specific cytotoxic T lymphocytes by eliminating myeloid-derived suppressor cells with doxorubicin to improve killing efficacy of neuroblastoma cells in vitro. J. Clin. Lab. Anal. 2022, 36, e24158. [Google Scholar] [CrossRef] [PubMed]
- Borges, F.; Laureano, R.S.; Vanmeerbeek, I.; Sprooten, J.; Demeulenaere, O.; Govaerts, J.; Kinget, L.; Saraswat, S.; Beuselinck, B.; De Vleeschouwer, S.; et al. Trial watch: Anticancer vaccination with dendritic cells. Oncoimmunology 2024, 13, 2412876. [Google Scholar] [CrossRef]
- Luo, Y.; Shreeder, B.; Jenkins, J.W.; Shi, H.; Lamichhane, P.; Zhou, K.; Bahr, D.A.; Kurian, S.; Jones, K.A.; Daum, J.I.; et al. Th17-inducing dendritic cell vaccines stimulate effective CD4 T cell-dependent antitumor immunity in ovarian cancer that overcomes resistance to immune checkpoint blockade. J. Immunother. Cancer 2023, 11, e007661. [Google Scholar] [CrossRef]
- Sprooten, J.; Garg, A.D. Next-generation DC vaccines with an immunogenic trajectory against cancer: Therapeutic opportunities vs. resistance mechanisms. Genes. Immun. 2024, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Roberts, E.W.; Broz, M.L.; Binnewies, M.; Headley, M.B.; Nelson, A.E.; Wolf, D.M.; Kaisho, T.; Bogunovic, D.; Bhardwaj, N.; Krummel, M.F. Critical Role for CD103+/CD141+ Dendritic Cells Bearing CCR7 for Tumor Antigen Trafficking and Priming of T Cell Immunity in Melanoma. Cancer Cell 2016, 30, 324–336. [Google Scholar] [CrossRef]
- Zhou, Y.; Slone, N.; Chrisikos, T.T.; Kyrysyuk, O.; Babcock, R.L.; Medik, Y.B.; Li, H.S.; Kleinerman, E.S.; Watowich, S.S. Vaccine efficacy against primary and metastatic cancer with in vitro-generated CD103+ conventional dendritic cells. J. Immunother. Cancer 2020, 8, e000474. [Google Scholar] [CrossRef]
- Schiller, J.T.; Lowy, D.R.; Frazer, I.H.; Finn, O.J.; Vilar, E.; Lyerly, H.K.; Gnjatic, S.; Zaidi, N.; Ott, P.A.; Balachandran, V.P.; et al. Cancer vaccines. Cancer Cell 2022, 40, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.W.; Quail, D.F. Immunotherapy for Glioblastoma: Current Progress and Challenges. Front. Immunol. 2021, 12, 676301. [Google Scholar] [CrossRef]
- Lynes, J.; Sanchez, V.; Dominah, G.; Nwankwo, A.; Nduom, E. Current Options and Future Directions in Immune Therapy for Glioblastoma. Front. Oncol. 2018, 8, 578. [Google Scholar] [CrossRef]
- Liau, L.M.; Black, K.L.; Martin, N.A.; Sykes, S.N.; Bronstein, J.M.; Jouben-Steele, L.; Mischel, P.S.; Belldegrun, A.; Cloughesy, T.F. Treatment of a patient by vaccination with autologous dendritic cells pulsed with allogeneic major histocompatibility complex class I-matched tumor peptides. Case Report. Neurosurg. Focus. 2000, 9, e8. [Google Scholar] [CrossRef]
- Yu, J.S.; Wheeler, C.J.; Zeltzer, P.M.; Ying, H.; Finger, D.N.; Lee, P.K.; Yong, W.H.; Incardona, F.; Thompson, R.C.; Riedinger, M.S.; et al. Vaccination of malignant glioma patients with peptide-pulsed dendritic cells elicits systemic cytotoxicity and intracranial T-cell infiltration. Cancer Res. 2001, 61, 842–847. [Google Scholar]
- Wen, P.Y.; Reardon, D.A.; Armstrong, T.S.; Phuphanich, S.; Aiken, R.D.; Landolfi, J.C.; Curry, W.T.; Zhu, J.J.; Glantz, M.; Peereboom, D.M.; et al. A Randomized Double-Blind Placebo-Controlled Phase II Trial of Dendritic Cell Vaccine ICT-107 in Newly Diagnosed Patients with Glioblastoma. Clin. Cancer Res. 2019, 25, 5799–5807. [Google Scholar] [CrossRef] [PubMed]
- Pour, M.E.; Moghadam, S.G.; Shirkhani, P.; Sahebkar, A.; Mosaffa, F. Therapeutic cell-based vaccines for glioblastoma multiforme. Med. Oncol. 2023, 40, 354. [Google Scholar] [CrossRef]
- da Silva, L.H.R.; Catharino, L.C.C.; da Silva, V.J.; Evangelista, G.C.M.; Barbuto, J.A.M. The War Is on: The Immune System against Glioblastoma-How Can NK Cells Drive This Battle? Biomedicines 2022, 10, 400. [Google Scholar] [CrossRef]
- Rapp, M.; Grauer, O.M.; Kamp, M.; Sevens, N.; Zotz, N.; Sabel, M.; Sorg, R.V. A randomized controlled phase II trial of vaccination with lysate-loaded, mature dendritic cells integrated into standard radiochemotherapy of newly diagnosed glioblastoma (GlioVax): Study protocol for a randomized controlled trial. Trials 2018, 19, 293. [Google Scholar] [CrossRef]
- Inogés, S.; Tejada, S.; de Cerio, A.L.; Gállego Pérez-Larraya, J.; Espinós, J.; Idoate, M.A.; Domínguez, P.D.; de Eulate, R.G.; Aristu, J.; Bendandi, M.; et al. A phase II trial of autologous dendritic cell vaccination and radiochemotherapy following fluorescence-guided surgery in newly diagnosed glioblastoma patients. J. Transl. Med. 2017, 15, 104. [Google Scholar] [CrossRef] [PubMed]
- Ardon, H.; Van Gool, S.W.; Verschuere, T.; Maes, W.; Fieuws, S.; Sciot, R.; Wilms, G.; Demaerel, P.; Goffin, J.; Van Calenbergh, F.; et al. Integration of autologous dendritic cell-based immunotherapy in the standard of care treatment for patients with newly diagnosed glioblastoma: Results of the HGG-2006 phase I/II trial. Cancer Immunol. Immunother. 2012, 61, 2033–2044. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.; Huang, J.; Xi, H.; Zhou, X. Efficacy and safety of dendritic cell vaccines for patients with glioblastoma: A meta-analysis of randomized controlled trials. Int. Immunopharmacol. 2020, 83, 106336. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.B.; Luo, C.; O’Connell, D.J.; Lefkovith, A.; Brown, E.M.; Yassour, M.; Varma, M.; Abelin, J.G.; Conway, K.L.; Jasso, G.J.; et al. Antigen discovery and specification of immunodominance hierarchies for MHCII-restricted epitopes. Nat. Med. 2018, 24, 1762–1772. [Google Scholar] [CrossRef] [PubMed]
- Schneidman-Duhovny, D.; Khuri, N.; Dong, G.Q.; Winter, M.B.; Shifrut, E.; Friedman, N.; Craik, C.S.; Pratt, K.P.; Paz, P.; Aswad, F.; et al. Predicting CD4 T-cell epitopes based on antigen cleavage, MHCII presentation, and TCR recognition. PLoS ONE 2018, 13, e0206654. [Google Scholar] [CrossRef]
- Bojarska, J.; Wolf, W.M. Short Peptides as Powerful Arsenal for Smart Fighting Cancer. Cancers 2024, 16, 3254. [Google Scholar] [CrossRef]
- Roudko, V.; Greenbaum, B.; Bhardwaj, N. Computational Prediction and Validation of Tumor-Associated Neoantigens. Front. Immunol. 2020, 11, 27. [Google Scholar] [CrossRef]
- Peng, M.; Mo, Y.; Wang, Y.; Wu, P.; Zhang, Y.; Xiong, F.; Guo, C.; Wu, X.; Li, Y.; Li, X.; et al. Neoantigen vaccine: An emerging tumor immunotherapy. Mol. Cancer 2019, 18, 128. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Yu, L.; Xu, X.; Yu, S.; Yu, Z. Neoantigen vaccine and neoantigen-specific cell adoptive transfer therapy in solid tumors: Challenges and future directions. Cancer Innov. 2022, 1, 168–182. [Google Scholar] [CrossRef]
- Melero, I.; Gaudernack, G.; Gerritsen, W.; Huber, C.; Parmiani, G.; Scholl, S.; Thatcher, N.; Wagstaff, J.; Zielinski, C.; Faulkner, I.; et al. Therapeutic vaccines for cancer: An overview of clinical trials. Nat. Rev. Clin. Oncol. 2014, 11, 509–524. [Google Scholar] [CrossRef] [PubMed]
- Nelde, A.; Rammensee, H.G.; Walz, J.S. The Peptide Vaccine of the Future. Mol. Cell Proteom. 2021, 20, 100022. [Google Scholar] [CrossRef] [PubMed]
- Hamley, I.W. Peptides for Vaccine Development. ACS Appl. Bio Mater. 2022, 5, 905–944. [Google Scholar] [CrossRef] [PubMed]
- Kumai, T.; Lee, S.; Cho, H.I.; Sultan, H.; Kobayashi, H.; Harabuchi, Y.; Celis, E. Optimization of Peptide Vaccines to Induce Robust Antitumor CD4 T-cell Responses. Cancer Immunol. Res. 2017, 5, 72–83. [Google Scholar] [CrossRef]
- Liu, D.; Liu, L.; Li, X.; Wang, S.; Wu, G.; Che, X. Advancements and Challenges in Peptide-Based Cancer Vaccination: A Multidisciplinary Perspective. Vaccines 2024, 12, 950. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Tang, H.; Li, L.; Wang, X.; Yu, Z.; Li, J. Peptide-based therapeutic cancer vaccine: Current trends in clinical application. Cell Prolif. 2021, 54, e13025. [Google Scholar] [CrossRef] [PubMed]
- Jou, J.; Harrington, K.J.; Zocca, M.B.; Ehrnrooth, E.; Cohen, E.E.W. The Changing Landscape of Therapeutic Cancer Vaccines-Novel Platforms and Neoantigen Identification. Clin. Cancer Res. 2021, 27, 689–703. [Google Scholar] [CrossRef] [PubMed]
- Phan, T.; Fan, D.; Melstrom, L.G. Developing Vaccines in Pancreatic Adenocarcinoma: Trials and Tribulations. Curr. Oncol. 2024, 31, 4855–4884. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, C.; Li, Y.; Li, Z.; Zhu, Y.; Yang, L.; Hu, H.; Sun, Q.; Liu, M.; Cao, S. Anti-cancer immune effect of human colorectal cancer neoantigen peptide based on MHC class I molecular affinity screening. Front. Immunol. 2024, 15, 1473145. [Google Scholar] [CrossRef]
- Hampson, I.N.; Oliver, A.W. Update on Effects of the Prophylactic HPV Vaccines on HPV Type Prevalence and Cervical Pathology. Viruses 2024, 16, 1245. [Google Scholar] [CrossRef]
- Mbulawa, Z.Z.A.; Kondlo, S.; Toni, S.; Faye, L.M.; Businge, C.B. Prevalence, Characteristics, and Distribution of Human Papillomavirus According to Age and HIV Status in Women of Eastern Cape Province, South Africa. Viruses 2024, 16, 1751. [Google Scholar] [CrossRef] [PubMed]
- Zahedipour, F.; Jamialahmadi, K.; Zamani, P.; Reza Jaafari, M. Improving the efficacy of peptide vaccines in cancer immunotherapy. Int. Immunopharmacol. 2023, 123, 110721. [Google Scholar] [CrossRef]
- Wang, X.; Niu, Y.; Bian, F. The progress of tumor vaccines clinical trials in non-small cell lung cancer. Clin. Transl. Oncol. 2024, 1–13. [Google Scholar] [CrossRef]
- Fu, Q.; Luo, Y.; Li, J.; Li, H.; Liu, X.; Chen, Z.; Ni, G.; Wang, T. Caerin 1.1 and 1.9 peptides halt B16 melanoma metastatic tumours via expanding cDC1 and reprogramming tumour macrophages. J. Transl. Med. 2024, 22, 973. [Google Scholar] [CrossRef]
- Mittendorf, E.A.; Clifton, G.T.; Holmes, J.P.; Schneble, E.; Van Echo, D.; Ponniah, S.; Peoples, G.E. Final report of the phase I/II clinical trial of the E75 (nelipepimut-S) vaccine with booster inoculations to prevent disease recurrence in high-risk breast cancer patients. Ann. Oncol. 2014, 25, 1735–1742. [Google Scholar] [CrossRef] [PubMed]
- Onodi, F.; Maherzi-Mechalikh, C.; Mougel, A.; Ben Hamouda, N.; Taboas, C.; Gueugnon, F.; Tran, T.; Nozach, H.; Marcon, E.; Gey, A.; et al. High Therapeutic Efficacy of a New Survivin LSP-Cancer Vaccine Containing CD4+ and CD8+ T-Cell Epitopes. Front. Oncol. 2018, 8, 517. [Google Scholar] [CrossRef]
- Stephens, A.J.; Burgess-Brown, N.A.; Jiang, S. Beyond Just Peptide Antigens: The Complex World of Peptide-Based Cancer Vaccines. Front. Immunol. 2021, 12, 696791. [Google Scholar] [CrossRef] [PubMed]
- Stergiou, N.; Gaidzik, N.; Heimes, A.S.; Dietzen, S.; Besenius, P.; Jäkel, J.; Brenner, W.; Schmidt, M.; Kunz, H.; Schmitt, E. Reduced Breast Tumor Growth after Immunization with a Tumor-Restricted MUC1 Glycopeptide Conjugated to Tetanus Toxoid. Cancer Immunol. Res. 2019, 7, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Stergiou, N.; Glaffig, M.; Jonuleit, H.; Schmitt, E.; Kunz, H. Immunization with a Synthetic Human MUC1 Glycopeptide Vaccine against Tumor-Associated MUC1 Breaks Tolerance in Human MUC1 Transgenic Mice. ChemMedChem 2017, 12, 1424–1428. [Google Scholar] [CrossRef] [PubMed]
- Naz, R.K.; Dabir, P. Peptide vaccines against cancer, infectious diseases, and conception. Front. Biosci. 2007, 12, 1833–1844. [Google Scholar] [CrossRef] [PubMed]
- Boumelha, J.; Molina-Arcas, M.; Downward, J. Facts and Hopes on RAS Inhibitors and Cancer Immunotherapy. Clin. Cancer Res. 2023, 29, 5012–5020. [Google Scholar] [CrossRef]
- Mugarza, E.; van Maldegem, F.; Boumelha, J.; Moore, C.; Rana, S.; Llorian Sopena, M.; East, P.; Ambler, R.; Anastasiou, P.; Romero-Clavijo, P.; et al. Therapeutic KRAS(G12C) inhibition drives effective interferon-mediated antitumor immunity in immunogenic lung cancers. Sci. Adv. 2022, 8, eabm8780. [Google Scholar] [CrossRef] [PubMed]
- Molina-Arcas, M.; Downward, J. Exploiting the therapeutic implications of KRAS inhibition on tumor immunity. Cancer Cell 2024, 42, 338–357. [Google Scholar] [CrossRef]
- Sakamoto, S.; Noguchi, M.; Yamada, A.; Itoh, K.; Sasada, T. Prospect and progress of personalized peptide vaccinations for advanced cancers. Expert. Opin. Biol. Ther. 2016, 16, 689–698. [Google Scholar] [CrossRef]
- Desrichard, A.; Snyder, A.; Chan, T.A. Cancer Neoantigens and Applications for Immunotherapy. Clin. Cancer Res. 2016, 22, 807–812. [Google Scholar] [CrossRef]
- Liu, Z.; Lv, J.; Dang, Q.; Liu, L.; Weng, S.; Wang, L.; Zhou, Z.; Kong, Y.; Li, H.; Han, Y.; et al. Engineering neoantigen vaccines to improve cancer personalized immunotherapy. Int. J. Biol. Sci. 2022, 18, 5607–5623. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, S.; Han, N.; Jiang, J.; Xu, Y.; Ma, D.; Lu, L.; Guo, X.; Qiu, M.; Huang, Q.; et al. A Neoantigen-Based Peptide Vaccine for Patients With Advanced Pancreatic Cancer Refractory to Standard Treatment. Front. Immunol. 2021, 12, 691605. [Google Scholar] [CrossRef] [PubMed]
- Criscitiello, C.; Viale, G.; Curigliano, G. Peptide vaccines in early breast cancer. Breast 2019, 44, 128–134. [Google Scholar] [CrossRef]
- Tu, H.F.; Wong, M.; Tseng, S.H.; Ingavat, N.; Olczak, P.; Notarte, K.I.; Hung, C.F.; Roden, R.B.S. Virus-like particle vaccine displaying an external, membrane adjacent MUC16 epitope elicits ovarian cancer-reactive antibodies. J. Ovarian Res. 2024, 17, 19. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, L.; Li, P.; Liu, S.; Yu, S.; Chen, Z.; Zhu, M.; Xie, S.; Ling, D.; Li, F. An Immunomodulatory Zinc-Alum/Ovalbumin Nanovaccine Boosts Cancer Metalloimmunotherapy Through Erythrocyte-Assisted Cascade Immune Activation. Adv. Sci. 2024, 11, e2307389. [Google Scholar] [CrossRef]
- Phung, I.; Rodrigues, K.A.; Marina-Zárate, E.; Maiorino, L.; Pahar, B.; Lee, W.H.; Melo, M.; Kaur, A.; Allers, C.; Fahlberg, M.; et al. A combined adjuvant approach primes robust germinal center responses and humoral immunity in non-human primates. Nat. Commun. 2023, 14, 7107. [Google Scholar] [CrossRef] [PubMed]
- Makino, T.; Miyata, H.; Yasuda, T.; Kitagawa, Y.; Muro, K.; Park, J.H.; Hikichi, T.; Hasegawa, T.; Igarashi, K.; Iguchi, M.; et al. A phase 3, randomized, double-blind, multicenter, placebo-controlled study of S-588410, a five-peptide cancer vaccine as an adjuvant therapy after curative resection in patients with esophageal squamous cell carcinoma. Esophagus 2024, 21, 447–455. [Google Scholar] [CrossRef]
- Melssen, M.M.; Fisher, C.T.; Slingluff, C.L.; Melief, C.J.M. Peptide emulsions in incomplete Freund’s adjuvant create effective nurseries promoting egress of systemic CD4+ and CD8+ T cells for immunotherapy of cancer. J. Immunother. Cancer 2022, 10, e004709. [Google Scholar] [CrossRef]
- Roehnisch, T.; Martos-Contreras, M.C.; Manoochehri, M.; Nogueira, M.; Bremm, F.; Dörrie, J.; Christoph, J.; Kunz, M.; Schönharting, W. Individualized neoantigen peptide immunization of a metastatic pancreatic cancer patient: A case report of combined tumor and liquid biopsy. Front. Immunol. 2024, 15, 1414737. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; O’Cearbhaill, R.E.; Block, M.S.; Hamilton, E.; Konner, J.A.; Knutson, K.L.; Potts, J.; Garrett, G.; Kenney, R.T.; Wenham, R.M. Vaccination with folate receptor-alpha peptides in patients with ovarian cancer following response to platinum-based therapy: A randomized, multicenter clinical trial. Gynecol. Oncol. 2024, 189, 90–97. [Google Scholar] [CrossRef]
- Liao, J.-Y.; Zhang, S. Safety and Efficacy of Personalized Cancer Vaccines in Combination With Immune Checkpoint Inhibitors in Cancer Treatment. Front. Oncol. 2021, 11, 663264. [Google Scholar] [CrossRef] [PubMed]
- Soltani, M.; Savvateeva, L.V.; Ganjalikhani-Hakemi, M.; Zamyatnin, A.A. Clinical Combinatorial Treatments Based on Cancer Vaccines: Combination with Checkpoint Inhibitors and Beyond. Curr. Drug Targets 2022, 23, 1072–1084. [Google Scholar] [CrossRef] [PubMed]
- Tagliabue, E.; Campiglio, M.; Pupa, S.M.; Ménard, S.; Balsari, A. Activity and resistance of trastuzumab according to different clinical settings. Cancer Treat. Rev. 2012, 38, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Gall, V.A.; Philips, A.V.; Qiao, N.; Clise-Dwyer, K.; Perakis, A.A.; Zhang, M.; Clifton, G.T.; Sukhumalchandra, P.; Ma, Q.; Reddy, S.M.; et al. Trastuzumab Increases HER2 Uptake and Cross-Presentation by Dendritic Cells. Cancer Res. 2017, 77, 5374–5383. [Google Scholar] [CrossRef]
- Hickerson, A.; Clifton, G.T.; Hale, D.F.; Peace, K.M.; Holmes, J.P.; Vreeland, T.J.; Litton, J.K.; Murthy, R.K.; Lukas, J.J.; Mittendorf, E.A.; et al. Final analysis of nelipepimut-S plus GM-CSF with trastuzumab versus trastuzumab alone to prevent recurrences in high-risk, HER2 low-expressing breast cancer: A prospective, randomized, blinded, multicenter phase IIb trial. J. Clin. Oncol. 2019, 37, 1. [Google Scholar] [CrossRef]
- Huijts, C.M.; Santegoets, S.J.; van den Eertwegh, A.J.; Pijpers, L.S.; Haanen, J.B.; de Gruijl, T.D.; Verheul, H.M.; van der Vliet, H.J. Phase I-II study of everolimus and low-dose oral cyclophosphamide in patients with metastatic renal cell cancer. BMC Cancer 2011, 11, 505. [Google Scholar] [CrossRef]
- Scurr, M.; Pembroke, T.; Bloom, A.; Roberts, D.; Thomson, A.; Smart, K.; Bridgeman, H.; Adams, R.; Brewster, A.; Jones, R.; et al. Effect of Modified Vaccinia Ankara-5T4 and Low-Dose Cyclophosphamide on Antitumor Immunity in Metastatic Colorectal Cancer: A Randomized Clinical Trial. JAMA Oncol. 2017, 3, e172579. [Google Scholar] [CrossRef]
- Shirahama, T.; Muroya, D.; Matsueda, S.; Yamada, A.; Shichijo, S.; Naito, M.; Yamashita, T.; Sakamoto, S.; Okuda, K.; Itoh, K.; et al. A randomized phase II trial of personalized peptide vaccine with low dose cyclophosphamide in biliary tract cancer. Cancer Sci. 2017, 108, 838–845. [Google Scholar] [CrossRef]
- Puigmal, N.; Ramos, V.; Artzi, N.; Borrós, S. Poly(β-amino ester)s-Based Delivery Systems for Targeted Transdermal Vaccination. Pharmaceutics 2023, 15, 1262. [Google Scholar] [CrossRef] [PubMed]
- Qin, F.; Xia, F.; Chen, H.; Cui, B.; Feng, Y.; Zhang, P.; Chen, J.; Luo, M. A Guide to Nucleic Acid Vaccines in the Prevention and Treatment of Infectious Diseases and Cancers: From Basic Principles to Current Applications. Front. Cell Dev. Biol. 2021, 9, 633776. [Google Scholar] [CrossRef] [PubMed]
- Jahanafrooz, Z.; Baradaran, B.; Mosafer, J.; Hashemzaei, M.; Rezaei, T.; Mokhtarzadeh, A.; Hamblin, M.R. Comparison of DNA and mRNA vaccines against cancer. Drug Discov. Today 2020, 25, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Lopes, A.; Vandermeulen, G.; Préat, V. Cancer DNA vaccines: Current preclinical and clinical developments and future perspectives. J. Exp. Clin. Cancer Res. 2019, 38, 146. [Google Scholar] [CrossRef]
- Liu, M.A. A Comparison of Plasmid DNA and mRNA as Vaccine Technologies. Vaccines 2019, 7, 37. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, B.; Morrow, M.P.; Hutnick, N.A.; Shin, T.H.; Lucke, C.E.; Weiner, D.B. Clinical applications of DNA vaccines: Current progress. Clin. Infect. Dis. 2011, 53, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Lim, J.M.; Yu, B.; Song, S.; Neeli, P.; Sobhani, N.; K, P.; Bonam, S.R.; Kurapati, R.; Zheng, J.; et al. The next-generation DNA vaccine platforms and delivery systems: Advances, challenges and prospects. Front. Immunol. 2024, 15, 1332939. [Google Scholar] [CrossRef] [PubMed]
- Pandya, A.; Shah, Y.; Kothari, N.; Postwala, H.; Shah, A.; Parekh, P.; Chorawala, M.R. The future of cancer immunotherapy: DNA vaccines leading the way. Med. Oncol. 2023, 40, 200. [Google Scholar] [CrossRef]
- Tiptiri-Kourpeti, A.; Spyridopoulou, K.; Pappa, A.; Chlichlia, K. DNA vaccines to attack cancer: Strategies for improving immunogenicity and efficacy. Pharmacol. Ther. 2016, 165, 32–49. [Google Scholar] [CrossRef]
- Dang, Y.; Guan, J. Nanoparticle-based drug delivery systems for cancer therapy. Smart Mater. Med. 2020, 1, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Shi, Y.; Zhang, J.; Liu, Q. Nanoparticle-based Drug Delivery Systems for Targeted Epigenetics Cancer Therapy. Curr. Drug Targets 2020, 21, 1084–1098. [Google Scholar] [CrossRef]
- Xin, Y.; Yin, M.; Zhao, L.; Meng, F.; Luo, L. Recent progress on nanoparticle-based drug delivery systems for cancer therapy. Cancer Biol. Med. 2017, 14, 228–241. [Google Scholar] [CrossRef] [PubMed]
- Lorentzen, C.L.; Haanen, J.B.; Met, Ö.; Svane, I.M. Clinical advances and ongoing trials on mRNA vaccines for cancer treatment. Lancet Oncol. 2022, 23, e450–e458. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Tian, Y.; Song, J.; An, G.; Yang, P. mRNA Vaccines: The Dawn of a New Era of Cancer Immunotherapy. Front. Immunol. 2022, 13, 887125. [Google Scholar] [CrossRef]
- Sayour, E.J.; Boczkowski, D.; Mitchell, D.A.; Nair, S.K. Cancer mRNA vaccines: Clinical advances and future opportunities. Nat. Rev. Clin. Oncol. 2024, 21, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.; Wang, X. RNA modification in mRNA cancer vaccines. Clin. Exp. Med. 2023, 23, 1917–1931. [Google Scholar] [CrossRef]
- Chehelgerdi, M.; Chehelgerdi, M. The use of RNA-based treatments in the field of cancer immunotherapy. Mol. Cancer 2023, 22, 106. [Google Scholar] [CrossRef]
- Omidi, Y.; Pourseif, M.M.; Ansari, R.A.; Barar, J. Design and development of mRNA and self-amplifying mRNA vaccine nanoformulations. Nanomedicine 2024, 19, 2699–2725. [Google Scholar] [CrossRef] [PubMed]
- Zong, Y.; Lin, Y.; Wei, T.; Cheng, Q. Lipid Nanoparticle (LNP) Enables mRNA Delivery for Cancer Therapy. Adv. Mater. 2023, 35, e2303261. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xia, X. RNA cancer vaccines: Developing mRNA nanovaccine with self-adjuvant property for cancer immunotherapy. Hum. Vaccin. Immunother. 2021, 17, 2995–2998. [Google Scholar] [CrossRef]
- Zhou, L.; Yi, W.; Zhang, Z.; Shan, X.; Zhao, Z.; Sun, X.; Wang, J.; Wang, H.; Jiang, H.; Zheng, M.; et al. STING agonist-boosted mRNA immunization via intelligent design of nanovaccines for enhancing cancer immunotherapy. Natl. Sci. Rev. 2023, 10, nwad214. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, K.; Zhao, Z.; Shan, X.; Wang, Y.; Feng, Z.; Li, B.; Luo, C.; Chen, X.; Sun, J. Self-Adjuvanting Polyguanidine Nanovaccines for Cancer Immunotherapy. ACS Nano 2024, 18, 7136–7147. [Google Scholar] [CrossRef]
- Vishweshwaraiah, Y.L.; Dokholyan, N.V. mRNA vaccines for cancer immunotherapy. Front. Immunol. 2022, 13, 1029069. [Google Scholar] [CrossRef] [PubMed]
- Disis, M.L.N.; Guthrie, K.A.; Liu, Y.; Coveler, A.L.; Higgins, D.M.; Childs, J.S.; Dang, Y.; Salazar, L.G. Safety and Outcomes of a Plasmid DNA Vaccine Encoding the ERBB2 Intracellular Domain in Patients With Advanced-Stage ERBB2-Positive Breast Cancer: A Phase 1 Nonrandomized Clinical Trial. JAMA Oncol. 2023, 9, 71–78. [Google Scholar] [CrossRef]
- Awad, M.M.; Govindan, R.; Balogh, K.N.; Spigel, D.R.; Garon, E.B.; Bushway, M.E.; Poran, A.; Sheen, J.H.; Kohler, V.; Esaulova, E.; et al. Personalized neoantigen vaccine NEO-PV-01 with chemotherapy and anti-PD-1 as first-line treatment for non-squamous non-small cell lung cancer. Cancer Cell 2022, 40, 1010–1026.e1011. [Google Scholar] [CrossRef] [PubMed]
- Keskin, D.B.; Anandappa, A.J.; Sun, J.; Tirosh, I.; Mathewson, N.D.; Li, S.; Oliveira, G.; Giobbie-Hurder, A.; Felt, K.; Gjini, E.; et al. Neoantigen vaccine generates intratumoral T cell responses in phase Ib glioblastoma trial. Nature 2019, 565, 234–239. [Google Scholar] [CrossRef]
- Pant, S.; Wainberg, Z.A.; Weekes, C.D.; Furqan, M.; Kasi, P.M.; Devoe, C.E.; Leal, A.D.; Chung, V.; Basturk, O.; VanWyk, H.; et al. Lymph-node-targeted, mKRAS-specific amphiphile vaccine in pancreatic and colorectal cancer: The phase 1 AMPLIFY-201 trial. Nat. Med. 2024, 30, 531–542. [Google Scholar] [CrossRef]
- Rojas, L.A.; Sethna, Z.; Soares, K.C.; Olcese, C.; Pang, N.; Patterson, E.; Lihm, J.; Ceglia, N.; Guasp, P.; Chu, A.; et al. Personalized RNA neoantigen vaccines stimulate T cells in pancreatic cancer. Nature 2023, 618, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Muth, S.T.; Saung, M.T.; Blair, A.B.; Henderson, M.G.; Thomas, D.L., 2nd; Zheng, L. CD137 agonist-based combination immunotherapy enhances activated, effector memory T cells and prolongs survival in pancreatic adenocarcinoma. Cancer Lett. 2021, 499, 99–108. [Google Scholar] [CrossRef]
- Cafri, G.; Gartner, J.J.; Zaks, T.; Hopson, K.; Levin, N.; Paria, B.C.; Parkhurst, M.R.; Yossef, R.; Lowery, F.J.; Jafferji, M.S.; et al. mRNA vaccine-induced neoantigen-specific T cell immunity in patients with gastrointestinal cancer. J. Clin. Investig. 2020, 130, 5976–5988. [Google Scholar] [CrossRef]
- Sharma, P.; Hu-Lieskovan, S.; Wargo, J.A.; Ribas, A. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell 2017, 168, 707–723. [Google Scholar] [CrossRef] [PubMed]
- Postow, M.A.; Callahan, M.K.; Wolchok, J.D. Immune Checkpoint Blockade in Cancer Therapy. J. Clin. Oncol. 2015, 33, 1974–1982. [Google Scholar] [CrossRef] [PubMed]
- Emens, L.A.; Ascierto, P.A.; Darcy, P.K.; Demaria, S.; Eggermont, A.M.M.; Redmond, W.L.; Seliger, B.; Marincola, F.M. Cancer immunotherapy: Opportunities and challenges in the rapidly evolving clinical landscape. Eur. J. Cancer 2017, 81, 116–129. [Google Scholar] [CrossRef] [PubMed]
- Fusciello, M.; Ylösmäki, E.; Cerullo, V. Viral Nanoparticles: Cancer Vaccines and Immune Modulators. Adv. Exp. Med. Biol. 2021, 1295, 317–325. [Google Scholar] [CrossRef]
- Fukuhara, H.; Ino, Y.; Todo, T. Oncolytic virus therapy: A new era of cancer treatment at dawn. Cancer Sci. 2016, 107, 1373–1379. [Google Scholar] [CrossRef]
- Sasso, E.; D’Alise, A.M.; Zambrano, N.; Scarselli, E.; Folgori, A.; Nicosia, A. New viral vectors for infectious diseases and cancer. Semin. Immunol. 2020, 50, 101430. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.Y.; Sun, T.K.; Chen, M.S.; Munir, M.; Liu, H.J. Oncolytic viruses-modulated immunogenic cell death, apoptosis and autophagy linking to virotherapy and cancer immune response. Front. Cell Infect. Microbiol. 2023, 13, 1142172. [Google Scholar] [CrossRef]
- Mathlouthi, S.; Kuryk, L.; Prygiel, M.; Lupo, M.G.; Zasada, A.A.; Pesce, C.; Ferri, N.; Rinner, B.; Salmaso, S.; Garofalo, M. Extracellular vesicles powered cancer immunotherapy: Targeted delivery of adenovirus-based cancer vaccine in humanized melanoma model. J. Control Release 2024, 376, 777–793. [Google Scholar] [CrossRef]
- Cote, G.M.; Conley, A.P.; Attia, S.; Van Tine, B.A.; Seetharam, M.; Chen, Y.L.; Gafoor, Z.; Heery, C.; Pico-Navarro, C.; Adams, T. A phase 2 study of a brachyury-targeting vaccine in combination with radiation therapy for the treatment of advanced chordoma. Cancer 2024, 130, 3845–3854. [Google Scholar] [CrossRef] [PubMed]
- Cyrelle Ornella, M.S.; Kim, J.J.; Cho, E.; Cho, M.; Hwang, T.H. Dose Considerations for Vaccinia Oncolytic Virus Based on Retrospective Reanalysis of Early and Late Clinical Trials. Vaccines 2024, 12, 1010. [Google Scholar] [CrossRef]
- Del Médico Zajac, M.P.; Molinari, P.; Gravisaco, M.J.; Maizon, D.O.; Morón, G.; Gherardi, M.M.; Calamante, G. MVAΔ008 viral vector encoding the model protein OVA induces improved immune response against the heterologous antigen and equal levels of protection in a mice tumor model than the conventional MVA. Mol. Immunol. 2021, 139, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, L.H. Cancer vaccines. Bmj 2015, 350, h988. [Google Scholar] [CrossRef] [PubMed]
- Larocca, C.; Schlom, J. Viral vector-based therapeutic cancer vaccines. Cancer J. 2011, 17, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Lu, S. Heterologous prime-boost vaccination. Curr. Opin. Immunol. 2009, 21, 346–351. [Google Scholar] [CrossRef]
- Skinner, M.A.; Wedlock, D.N.; De Lisle, G.W.; Cooke, M.L.M.; Tascon, R.E.; Ferraz, J.C.; Lowrie, D.B.; Vordermeier, H.M.; Hewinson, R.G.; Buddle, B.M. The Order of Prime-Boost Vaccination of Neonatal Calves with Mycobacterium bovis BCG and a DNA Vaccine Encoding Mycobacterial Proteins Hsp65, Hsp70, and Apa Is Not Critical for Enhancing Protection against Bovine Tuberculosi. Infect. Immun. 2005, 73, 4441–4444. [Google Scholar] [CrossRef]
- Meseda, C.A.; Elkins, K.L.; Merchlinsky, M.J.; Weir, J.P. Prime-boost immunization with DNA and modified vaccinia virus ankara vectors expressing herpes simplex virus-2 glycoprotein D elicits greater specific antibody and cytokine responses than DNA vaccine alone. J. Infect. Dis. 2002, 186, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Yang, S.H.; Lee, C.G.; Youn, J.W.; Chang, J.; Sung, Y.C. Efficient induction of T helper 1 CD4+ T-cell responses to hepatitis C virus core and E2 by a DNA prime-adenovirus boost. Vaccine 2003, 21, 4555–4564. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Hernandez, M.D.L.L.; Gray, A.; Hubby, B.; Kast, W.M. In vivo Effects of Vaccination with Six-Transmembrane Epithelial Antigen of the Prostate: A Candidate Antigen for Treating Prostate Cancer. Cancer Res. 2007, 67, 1344–1351. [Google Scholar] [CrossRef]
- Kaufman, H.L.; Cohen, S.; Cheung, K.; Deraffele, G.; Mitcham, J.; Moroziewicz, D.; Schlom, J.; Hesdorffer, C. Local Delivery of Vaccinia Virus Expressing Multiple Costimulatory Molecules for the Treatment of Established Tumors. Hum. Gene Ther. 2006, 17, 239–244. [Google Scholar] [CrossRef]
- Bezeljak, U. Cancer gene therapy goes viral: Viral vector platforms come of age. Radiol. Oncol. 2022, 56, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Capone, S.; Naddeo, M.; D’Alise, A.M.; Abbate, A.; Grazioli, F.; Del Gaudio, A.; Del Sorbo, M.; Esposito, M.L.; Ammendola, V.; Perretta, G.; et al. Fusion of HCV Nonstructural Antigen to MHC Class II–associated Invariant Chain Enhances T-cell Responses Induced by Vectored Vaccines in Nonhuman Primates. Mol. Ther. 2014, 22, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Bots, S.T.F.; Kemp, V.; Cramer, S.J.; van den Wollenberg, D.J.M.; Hornsveld, M.; Lamfers, M.L.M.; van der Pluijm, G.; Hoeben, R.C. Nonhuman Primate Adenoviruses of the Human Adenovirus B Species Are Potent and Broadly Acting Oncolytic Vector Candidates. Hum. Gene Ther. 2022, 33, 275–289. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.S.; Lu, B.; Guo, Z.; Giehl, E.; Feist, M.; Dai, E.; Liu, W.; Storkus, W.J.; He, Y.; Liu, Z.; et al. Vaccinia virus-mediated cancer immunotherapy: Cancer vaccines and oncolytics. J. Immunother. Cancer 2019, 7, 6. [Google Scholar] [CrossRef]
- Zuo, S.; Wei, M.; He, B.; Chen, A.; Wang, S.; Kong, L.; Zhang, Y.; Meng, G.; Xu, T.; Wu, J.; et al. Enhanced antitumor efficacy of a novel oncolytic vaccinia virus encoding a fully monoclonal antibody against T-cell immunoglobulin and ITIM domain (TIGIT). EBioMedicine 2021, 64, 103240. [Google Scholar] [CrossRef]
- Yang, X.; Huang, B.; Deng, L.; Hu, Z. Progress in gene therapy using oncolytic vaccinia virus as vectors. J. Cancer Res. Clin. Oncol. 2018, 144, 2433–2440. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.J.; Schneider, J. Plasmid DNA and viral vector-based vaccines for the treatment of cancer. Vaccine 2007, 25 (Suppl. S2), B24–B34. [Google Scholar] [CrossRef] [PubMed]
- Velu, T.; Ramlau, R.; Quoix, E.; Pawlicki, M.; Pless, M.; Lena, H.; Levy, E.; Krzakowski, M.; Limacher, J.-M.; Bizouarne, N. A phase II study evaluating the clinical efficacy of TG4010 (MVA-MUC1-IL2) in association with chemotherapy in patients with non small cell lung cancer. J. Clin. Oncol. 2005, 23, 7132. [Google Scholar] [CrossRef]
- Le Chevalier, T.; Brisgand, D.; Douillard, J.Y.; Pujol, J.L.; Alberola, V.; Monnier, A.; Riviere, A.; Lianes, P.; Chomy, P.; Cigolari, S.; et al. Randomized study of vinorelbine and cisplatin versus vindesine and cisplatin versus vinorelbine alone in advanced non-small-cell lung cancer: Results of a european multicenter trial including 612 patients. J. Clin. Oncol. 1994, 12, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Scagliotti, G.V.; De Marinis, F.; Rinaldi, M.; Crinò, L.; Gridelli, C.; Ricci, S.; Matano, E.; Boni, C.; Marangolo, M.; Failla, G.; et al. Phase III randomized trial comparing three platinum-based doublets in advanced non-small-cell lung cancer. J. Clin. Oncol. 2002, 20, 4285–4291. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, P.; Kaufman, R.; Goff, B.; Mathioudakis, G.; Baudin, M.; Balloul, J.; Bourgault-Villadas, I. Immunogenicity of a non-replicative Vaccinia virus expressing the E6 and E7 early genes of HPV16 in patients with cervical neoplasia. J. Investig. Dermatol. 2004, 123, A108. [Google Scholar]
- Harrop, R.; Hawkins, R.; Anthoney, A.; Steven, N.; Habib, N.; Griffiths, R.; Melcher, A.; Wassan, H.; Naylor, S. Open label phase II studies of modified vaccinia ankara expressing the tumor antigen 5T4 given in conjunction with IFL and FOLFOX chemotherapy regimens: Final analysis of safety and immunogenicity of MVA 5T4 given before, during and after chemotherapy. J. Clin. Oncol. 2006, 24, 2527. [Google Scholar] [CrossRef]
- Spaner, D.E.; Astsaturov, I.; Vogel, T.; Petrella, T.; Elias, I.; Burdett-Radoux, S.; Verma, S.; Iscoe, N.; Hamilton, P.; Berinstein, N.L. Enhanced viral and tumor immunity with intranodal injection of canary pox viruses expressing the melanoma antigen, gp100. Cancer 2006, 106, 890–899. [Google Scholar] [CrossRef] [PubMed]
- Hörig, H.; Lee, D.S.; Conkright, W.; Divito, J.; Hasson, H.; LaMare, M.; Rivera, A.; Park, D.; Tine, J.; Guito, K.; et al. Phase I clinical trial of a recombinant canarypoxvirus (ALVAC) vaccine expressing human carcinoembryonic antigen and the B7.1 co-stimulatory molecule. Cancer Immunol. Immunother. 2000, 49, 504–514. [Google Scholar] [CrossRef]
- Rosenberg, S.A.; Zhai, Y.; Yang, J.C.; Schwartzentruber, D.J.; Hwu, P.; Marincola, F.M.; Topalian, S.L.; Restifo, N.P.; Seipp, C.A.; Einhorn, J.H.; et al. Immunizing Patients With Metastatic Melanoma Using Recombinant Adenoviruses Encoding MART-1 or gp100 Melanoma Antigens. J. Natl. Cancer Inst. 1998, 90, 1870–1872. [Google Scholar] [CrossRef]
- Nemunaitis, J.; Meyers, T.; Senzer, N.; Cunningham, C.; West, H.; Vallieres, E.; Anthony, S.; Vukelja, S.; Berman, B.; Tully, H.; et al. Phase I Trial of Sequential Administration of Recombinant DNA and Adenovirus Expressing L523S Protein in Early Stage Non-Small-Cell Lung Cancer. Mol. Ther. 2006, 13, 1185–1191. [Google Scholar] [CrossRef]
- Chen, F.; Wang, Y.; Gao, J.; Saeed, M.; Li, T.; Wang, W.; Yu, H. Nanobiomaterial-based vaccination immunotherapy of cancer. Biomaterials 2021, 270, 120709. [Google Scholar] [CrossRef] [PubMed]
- Hashemi Goradel, N.; Nemati, M.; Bakhshandeh, A.; Arashkia, A.; Negahdari, B. Nanovaccines for cancer immunotherapy: Focusing on complex formation between adjuvant and antigen. Int. Immunopharmacol. 2023, 117, 109887. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Zhang, H.; Zhou, Y.; Umeshappa, C.S.; Gao, H. Nanovaccine-Based Strategies to Overcome Challenges in the Whole Vaccination Cascade for Tumor Immunotherapy. Small 2021, 17, 2006000. [Google Scholar] [CrossRef]
- Kim, J.-E.; Cho, M.-H. Nanomedicine in Cancer Treatment; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014; pp. 161–188. [Google Scholar]
- Zhang, Y.; Ma, S.; Liu, X.; Xu, Y.; Zhao, J.; Si, X.; Li, H.; Huang, Z.; Wang, Z.; Tang, Z.; et al. Supramolecular Assembled Programmable Nanomedicine As In Situ Cancer Vaccine for Cancer Immunotherapy. Adv. Mater. 2021, 33, 2007293. [Google Scholar] [CrossRef] [PubMed]
- Bezbaruah, R.; Chavda, V.P.; Nongrang, L.; Alom, S.; Deka, K.; Kalita, T.; Ali, F.; Bhattacharjee, B.; Vora, L. Nanoparticle-Based Delivery Systems for Vaccines. Vaccines 2022, 10, 1946. [Google Scholar] [CrossRef] [PubMed]
- Desai, N. Challenges in development of nanoparticle-based therapeutics. Aaps J. 2012, 14, 282–295. [Google Scholar] [CrossRef]
- Patel, D.M.; Patel, N.N.; Patel, J.K. Nanomedicine Scale-Up Technologies: Feasibilities and Challenges; Springer International Publishing: Berlin/Heidelberg, Germany, 2021; pp. 511–539. [Google Scholar]
- Zhou, L.; Zou, M.; Xu, Y.; Lin, P.; Lei, C.; Xia, X. Nano Drug Delivery System for Tumor Immunotherapy: Next-Generation Therapeutics. Front. Oncol. 2022, 12, 864301. [Google Scholar] [CrossRef] [PubMed]
- Yadav, H.K.S.; Dibi, M.; Mohammad, A.; Srouji, A.E. Nanovaccines formulation and applications-a review. J. Drug Deliv. Sci. Technol. 2018, 44, 380–387. [Google Scholar] [CrossRef]
- Dash, P.; Piras, A.M.; Dash, M. Cell membrane coated nanocarriers—An efficient biomimetic platform for targeted therapy. J. Control. Release 2020, 327, 546–570. [Google Scholar] [CrossRef]
- Guo, K.; Xiao, N.; Liu, Y.; Wang, Z.; Tóth, J.; Gyenis, J.; Thakur, V.K.; Oyane, A.; Shubhra, Q.T.H. Engineering polymer nanoparticles using cell membrane coating technology and their application in cancer treatments: Opportunities and challenges. Nano Mater. Sci. 2022, 4, 295–321. [Google Scholar] [CrossRef]
- Liu, H.; Miao, Z.; Zha, Z. Cell membrane-coated nanoparticles for immunotherapy. Chin. Chem. Lett. 2022, 33, 1673–1680. [Google Scholar] [CrossRef]
- Zhang, L.-X.; Sun, X.-M.; Jia, Y.-B.; Liu, X.-G.; Dong, M.; Xu, Z.P.; Liu, R.-T. Nanovaccine’s rapid induction of anti-tumor immunity significantly improves malignant cancer immunotherapy. Nano Today 2020, 35, 100923. [Google Scholar] [CrossRef]
- Qian, Y.; Jin, H.; Qiao, S.; Dai, Y.; Huang, C.; Lu, L.; Luo, Q.; Zhang, Z. Targeting dendritic cells in lymph node with an antigen peptide-based nanovaccine for cancer immunotherapy. Biomaterials 2016, 98, 171–183. [Google Scholar] [CrossRef]
- Zhu, G.; Zhang, F.; Ni, Q.; Niu, G.; Chen, X. Efficient Nanovaccine Delivery in Cancer Immunotherapy. ACS Nano 2017, 11, 2387–2392. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.T.; Van Der Vlies, A.J.; Simeoni, E.; Angeli, V.; Randolph, G.J.; O’Neil, C.P.; Lee, L.K.; Swartz, M.A.; Hubbell, J.A. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat. Biotechnol. 2007, 25, 1159–1164. [Google Scholar] [CrossRef] [PubMed]
- Gutjahr, A.; Phelip, C.; Coolen, A.-L.; Monge, C.; Boisgard, A.-S.; Paul, S.; Verrier, B. Biodegradable polymeric nanoparticles-based vaccine adjuvants for lymph nodes targeting. Vaccines 2016, 4, 34. [Google Scholar] [CrossRef]
- Cai, T.; Liu, H.; Zhang, S.; Hu, J.; Zhang, L. Delivery of nanovaccine towards lymphoid organs: Recent strategies in enhancing cancer immunotherapy. J. Nanobiotechnol. 2021, 19, 389. [Google Scholar] [CrossRef]
- Hailemichael, Y.; Dai, Z.; Jaffarzad, N.; Ye, Y.; Medina, M.A.; Huang, X.-F.; Dorta-Estremera, S.M.; Greeley, N.R.; Nitti, G.; Peng, W. Persistent antigen at vaccination sites induces tumor-specific CD8+ T cell sequestration, dysfunction and deletion. Nat. Med. 2013, 19, 465–472. [Google Scholar] [CrossRef]
- Grenier, P.; Chénard, V.; Bertrand, N. The mechanisms of anti-PEG immune response are different in the spleen and the lymph nodes. J. Control. Release 2023, 353, 611–620. [Google Scholar] [CrossRef]
- Yang, B.; Jeang, J.; Yang, A.; Wu, T.C.; Hung, C.-F. DNA vaccine for cancer immunotherapy. Hum. Vaccines Immunother. 2014, 10, 3153–3164. [Google Scholar] [CrossRef]
- Fan, Y.-N.; Li, M.; Luo, Y.-L.; Chen, Q.; Wang, L.; Zhang, H.-B.; Shen, S.; Gu, Z.; Wang, J. Cationic lipid-assisted nanoparticles for delivery of mRNA cancer vaccine. Biomater. Sci. 2018, 6, 3009–3018. [Google Scholar] [CrossRef]
- Liu, M.A. DNA vaccines: An historical perspective and view to the future. Immunol. Rev. 2011, 239, 62–84. [Google Scholar] [CrossRef]
- Peng, X.; Fang, J.; Lou, C.; Yang, L.; Shan, S.; Wang, Z.; Chen, Y.; Li, H.; Li, X. Engineered nanoparticles for precise targeted drug delivery and enhanced therapeutic efficacy in cancer immunotherapy. Acta Pharm. Sin. B 2024, 14, 3432–3456. [Google Scholar] [CrossRef] [PubMed]
- Jneid, B.; Bochnakian, A.; Hoffmann, C.; Delisle, F.; Djacoto, E.; Sirven, P.; Denizeau, J.; Sedlik, C.; Gerber-Ferder, Y.; Fiore, F.; et al. Selective STING stimulation in dendritic cells primes antitumor T cell responses. Sci. Immunol. 2023, 8, eabn6612. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef]
- Rudin, C.M.; Marshall, J.L.; Huang, C.H.; Kindler, H.L.; Zhang, C.; Kumar, D.; Gokhale, P.C.; Steinberg, J.; Wanaski, S.; Kasid, U.N.; et al. Delivery of a Liposomalc-raf-1Antisense Oligonucleotide by Weekly Bolus Dosing in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2004, 10, 7244–7251. [Google Scholar] [CrossRef] [PubMed]
- Sinani, G.; Durgun, M.E.; Cevher, E.; Özsoy, Y. Polymeric-Micelle-Based Delivery Systems for Nucleic Acids. Pharmaceutics 2023, 15, 2021. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Jiang, Q.; Zhao, X.; Zhao, R.; Wang, Y.; Wang, Y.; Liu, J.; Shang, Y.; Zhao, S.; Wu, T.; et al. A DNA nanodevice-based vaccine for cancer immunotherapy. Nat. Mater. 2021, 20, 421–430. [Google Scholar] [CrossRef]
- Zhou, J.; Kroll, A.V.; Holay, M.; Fang, R.H.; Zhang, L. Biomimetic Nanotechnology toward Personalized Vaccines. Adv. Mater. 2020, 32, 1901255. [Google Scholar] [CrossRef]
- Li, X.; Cai, X.; Zhang, Z.; Ding, Y.; Ma, R.; Huang, F.; Liu, Y.; Liu, J.; Shi, L. Mimetic Heat Shock Protein Mediated Immune Process to Enhance Cancer Immunotherapy. Nano Lett. 2020, 20, 4454–4463. [Google Scholar] [CrossRef]
- Luan, X.; Sansanaphongpricha, K.; Myers, I.; Chen, H.; Yuan, H.; Sun, D. Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacol. Sin. 2017, 38, 754–763. [Google Scholar] [CrossRef]
- Filipović, L.; Kojadinović, M.; Popović, M. Exosomes and exosome-mimetics as targeted drug carriers: Where we stand and what the future holds? J. Drug Deliv. Sci. Technol. 2022, 68, 103057. [Google Scholar] [CrossRef]
- Laotee, S.; Arunmanee, W. Genetically surface-modified Escherichia coli outer membrane vesicles targeting MUC1 antigen in cancer cells. Biotechnol. Rep. 2024, 44, e00854. [Google Scholar] [CrossRef]
- Ho, M.Y.; Liu, S.; Xing, B. Bacteria extracellular vesicle as nanopharmaceuticals for versatile biomedical potential. Nano Converg. 2024, 11, 28. [Google Scholar] [CrossRef]
- Norouzi, P.; Amini, M.; Mottaghitalab, F.; Mirzazadeh Tekie, F.S.; Dinarvand, R.; Mirzaie, Z.H.; Atyabi, F. Design and fabrication of dual-targeted delivery system based on gemcitabine-conjugated human serum albumin nanoparticles. Chem. Biol. Drug Des. 2020, 96, 745–757. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Teng, Z.; Zhang, Y.; Yuwen, L.; Zhang, Q.; Su, X.; Dang, M.; Tian, Y.; Tao, J.; Bao, L.; et al. Flexible MoS2-Embedded Human Serum Albumin Hollow Nanocapsules with Long Circulation Times and High Targeting Ability for Efficient Tumor Ablation. Adv. Funct. Mater. 2018, 28, 1804081. [Google Scholar] [CrossRef]
- Hu, S.; Ma, J.; Su, C.; Chen, Y.; Shu, Y.; Qi, Z.; Zhang, B.; Shi, G.; Zhang, Y.; Zhang, Y.; et al. Engineered exosome-like nanovesicles suppress tumor growth by reprogramming tumor microenvironment and promoting tumor ferroptosis. Acta Biomater. 2021, 135, 567–581. [Google Scholar] [CrossRef] [PubMed]
- Kroll, A.V.; Fang, R.H.; Jiang, Y.; Zhou, J.; Wei, X.; Yu, C.L.; Gao, J.; Luk, B.T.; Dehaini, D.; Gao, W.; et al. Nanoparticulate Delivery of Cancer Cell Membrane Elicits Multiantigenic Antitumor Immunity. Adv. Mater. 2017, 29, 1703969. [Google Scholar] [CrossRef] [PubMed]
- Mu, Y.; Zhang, Z.; Zhou, H.; Jin, M.; Ma, L.; Liu, B.; Ma, C.; Hu, X.; Zhang, Y.; Wang, D.-A. A biomimetic targeted nanosystem delivering synergistic inhibitors for glioblastoma immune microenvironment reprogramming and treatment. Mater. Today Bio 2024, 28, 101222. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-L.; Zou, M.-Z.; Liu, T.; Zeng, J.-Y.; Li, X.; Yu, W.-Y.; Li, C.-X.; Ye, J.-J.; Song, W.; Feng, J.; et al. Cytomembrane nanovaccines show therapeutic effects by mimicking tumor cells and antigen presenting cells. Nat. Commun. 2019, 10, 3199. [Google Scholar] [CrossRef]
- Olden, B.R.; Perez, C.R.; Wilson, A.L.; Cardle, I.I.; Lin, Y.S.; Kaehr, B.; Gustafson, J.A.; Jensen, M.C.; Pun, S.H. Cell-Templated Silica Microparticles with Supported Lipid Bilayers as Artificial Antigen-Presenting Cells for T Cell Activation. Adv. Healthc. Mater. 2019, 8, 1801188. [Google Scholar] [CrossRef]
- Wang, Z.; Miao, F.; Gu, L.; Zhang, R.; Ma, Y.; Li, Y.; Zheng, J.; Lin, Z.; Gao, Y.; Huang, L.; et al. Stimulator of Interferon Genes-Activated Biomimetic Dendritic Cell Nanovaccine as a Chemotherapeutic Booster to Enhance Systemic Fibrosarcoma Treatment. ACS Nano 2024, 18, 24219–24235. [Google Scholar] [CrossRef]
- Calvo Tardón, M.; Allard, M.; Dutoit, V.; Dietrich, P.-Y.; Walker, P.R. Peptides as cancer vaccines. Curr. Opin. Pharmacol. 2019, 47, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Ott, P.A.; Hu, Z.; Keskin, D.B.; Shukla, S.A.; Sun, J.; Bozym, D.J.; Zhang, W.; Luoma, A.; Giobbie-Hurder, A.; Peter, L.; et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature 2017, 547, 217–221. [Google Scholar] [CrossRef]
- Vázquez-González, M.; Willner, I. Stimuli-responsive biomolecule-based hydrogels and their applications. Angew. Chem. Int. Ed. 2020, 59, 15342–15377. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yu, X.; Jiang, Y.; He, S.; Zhang, Y.; Luo, Y.; Pu, K. Second near-infrared photothermal semiconducting polymer nanoadjuvant for enhanced cancer immunotherapy. Adv. Mater. 2021, 33, 2003458. [Google Scholar] [CrossRef]
- Zeng, Z.; Zhang, C.; Li, J.; Cui, D.; Jiang, Y.; Pu, K. Activatable polymer nanoenzymes for photodynamic immunometabolic cancer therapy. Adv. Mater. 2021, 33, 2007247. [Google Scholar] [CrossRef] [PubMed]
- Lafuente-Gómez, N.; de Lázaro, I.; Dhanjani, M.; García-Soriano, D.; Sobral, M.C.; Salas, G.; Mooney, D.J.; Somoza, Á. Multifunctional magnetic nanoparticles elicit anti-tumor immunity in a mouse melanoma model. Mater. Today Bio 2023, 23, 100817. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Xie, D.; Hu, D.; Zhang, R.; Wang, Y.; Tang, L.; Zhou, B.; Zhao, B.; Yang, L. In Situ Cocktail Nanovaccine for Cancer Immunotherapy. Adv. Sci. 2023, 10, e2207697. [Google Scholar] [CrossRef]
- He, Q.; Jiang, X.; Zhou, X.; Weng, J. Targeting cancers through TCR-peptide/MHC interactions. J. Hematol. Oncol. 2019, 12, 139. [Google Scholar] [CrossRef] [PubMed]
- Cercek, A.; Roxburgh, C.S.D.; Strombom, P.; Smith, J.J.; Temple, L.K.F.; Nash, G.M.; Guillem, J.G.; Paty, P.B.; Yaeger, R.; Stadler, Z.K.; et al. Adoption of Total Neoadjuvant Therapy for Locally Advanced Rectal Cancer. JAMA Oncol. 2018, 4, e180071. [Google Scholar] [CrossRef] [PubMed]
- Thivat, E.; Casile, M.; Moreau, J.; Molnar, I.; Dufort, S.; Seddik, K.; Le Duc, G.; De Beaumont, O.; Loeffler, M.; Durando, X.; et al. Phase I/II study testing the combination of AGuIX nanoparticles with radiochemotherapy and concomitant temozolomide in patients with newly diagnosed glioblastoma (NANO-GBM trial protocol). BMC Cancer 2023, 23, 344. [Google Scholar] [CrossRef]
- Hurria, A.; Blanchard, M.S.; Synold, T.W.; Mortimer, J.; Chung, C.T.; Luu, T.; Katheria, V.; Rotter, A.J.; Wong, C.; Choi, A.; et al. Age-related changes in nanoparticle albumin-bound paclitaxel pharmacokinetics and pharmacodynamics: Influence of chronological versus functional age. Oncologist 2015, 20, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Srikrishna, D.; Sachsenmeier, K. We need to bring R0 < 1 to treat cancer too. Genome Med. 2021, 13, 120. [Google Scholar] [CrossRef] [PubMed]
- Sarker, D.; Plummer, R.; Meyer, T.; Sodergren, M.H.; Basu, B.; Chee, C.E.; Huang, K.W.; Palmer, D.H.; Ma, Y.T.; Evans, T.R.J.; et al. MTL-CEBPA, a Small Activating RNA Therapeutic Upregulating C/EBP-α, in Patients with Advanced Liver Cancer: A First-in-Human, Multicenter, Open-Label, Phase I Trial. Clin. Cancer Res. 2020, 26, 3936–3946. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, A.J.; Agrawal, N.; Pearson, A.T.; Gooi, Z.; Blair, E.; Portugal, L.; Cursio, J.F.; Juloori, A.; Chin, J.; Rouse, K.; et al. Phase I study of nab-paclitaxel-based induction followed by nab-paclitaxel-based concurrent chemotherapy and re-irradiation in previously treated head and neck squamous cell carcinoma. Br. J. Cancer 2022, 127, 1497–1506. [Google Scholar] [CrossRef] [PubMed]
- Zanganeh, S.; Hutter, G.; Spitler, R.; Lenkov, O.; Mahmoudi, M.; Shaw, A.; Pajarinen, J.S.; Nejadnik, H.; Goodman, S.; Moseley, M.; et al. Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat. Nanotechnol. 2016, 11, 986–994. [Google Scholar] [CrossRef]
- Trujillo-Alonso, V.; Pratt, E.C.; Zong, H.; Lara-Martinez, A.; Kaittanis, C.; Rabie, M.O.; Longo, V.; Becker, M.W.; Roboz, G.J.; Grimm, J.; et al. FDA-approved ferumoxytol displays anti-leukaemia efficacy against cells with low ferroportin levels. Nat. Nanotechnol. 2019, 14, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Xie, P.; Yang, S.T.; Huang, Y.; Zeng, C.; Xin, Q.; Zeng, G.; Yang, S.; Xia, P.; Tang, X.; Tang, K. Carbon Nanoparticles-Fe(II) Complex for Efficient Tumor Inhibition with Low Toxicity by Amplifying Oxidative Stress. ACS Appl. Mater. Interfaces 2020, 12, 29094–29102. [Google Scholar] [CrossRef]
- Rajani, K.R.; Vile, R.G. Harnessing the Power of Onco-Immunotherapy with Checkpoint Inhibitors. Viruses 2015, 7, 5889–5901. [Google Scholar] [CrossRef]
- Kohlhapp, F.J.; Kaufman, H.L. Molecular Pathways: Mechanism of Action for Talimogene Laherparepvec, a New Oncolytic Virus Immunotherapy. Clin. Cancer Res. 2016, 22, 1048–1054. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.; Reid, T.; Ruo, L.; Breitbach, C.J.; Rose, S.; Bloomston, M.; Cho, M.; Lim, H.Y.; Chung, H.C.; Kim, C.W.; et al. Randomized dose-finding clinical trial of oncolytic immunotherapeutic vaccinia JX-594 in liver cancer. Nat. Med. 2013, 19, 329–336. [Google Scholar] [CrossRef]
| NCT Number | Status | Start/Completion Date | Stage | Title | References |
|---|---|---|---|---|---|
| NCT05264974 | Suspended | - | Phase I | Novel RNA-nanoparticle Vaccine for the Treatment of Early Melanoma Recurrence Following Adjuvant Anti-PD-1 Antibody Therapy | NP |
| NCT04645147 | Active, not recruiting | 2022-03/present | Phase I | Safety and Immunogenicity of an Epstein-Barr Virus (EBV) gp350-Ferritin Nanoparticle Vaccine in Healthy Adults With or Without EBV Infection | NP |
| NCT03120832 | Completed | 2016-12/2018-12 | Phase I | Phase 1 Trial of PAN-301-1 (SNS-301) in Cancer Patients | NP |
| NCT03606967 | Recruiting | 2021-04/present | Phase II | Testing the Addition of an Individualized Vaccine to Durvalumab and Tremelimumab and Chemotherapy in Patients With Metastatic Triple Negative Breast Cancer | NP |
| NCT05456022 | Unknown status | 2022-07/- | Phase II | Therapeutic Efficacy of Quercetin Versus Its Encapsulated Nanoparticle on Tongue Squamous Cell Carcinoma Cell Line | NP |
| NCT04881032 | Active, not recruiting | 2022-03/present | Phase I Phase II | AGuIX Nanoparticles with Radiotherapy Plus Concomitant Temozolomide in the Treatment of Newly Diagnosed Glioblastoma (NANO-GBM) | [262] |
| NCT03410030 | Completed | 2017-12/2022-01 | Phase I Phase II | Trial of Ascorbic Acid (AA) + Nanoparticle Paclitaxel Protein Bound + Cisplatin + Gemcitabine (AA NABPLAGEM) (AA NABPLAGEM) | NP |
| NCT05000801 | Recruiting | 2021-07/present | Not Applicable | Clinical Study of DC-AML Cells in the Treatment of Acute Myeloid Leukemia | NP |
| NCT00609791 | Active, not recruiting | 2008-02/present | Phase II | Paclitaxel Albumin-Stabilized Nanoparticle Formulation in Treating Patients of Different Ages With Metastatic Breast Cancer | [263] |
| NCT03323398 | Terminated | 2017-08/2021-08 | Phase I Phase II | Dose Escalation and Efficacy Study of mRNA-2416 for Intratumoral Injection Alone and in Combination With Durvalumab for Participants With Advanced Malignancies | NP |
| NCT05968326 | Recruiting | 2023-10/present | Phase II | A Study of the Efficacy and Safety of Adjuvant Autogene Cevumeran Plus Atezolizumab and mFOLFIRINOX Versus mFOLFIRINOX Alone in Participants With Resected PDAC (IMCODE003) | NP |
| NCT02149225 | Completed | 2014-10/2018-06 | Phase I | GAPVAC Phase I Trial in Newly Diagnosed Glioblastoma Patients | NP |
| NCT03739931 | Active, not recruiting | 2018-11/present | Phase I | Dose Escalation Study of mRNA-2752 for Intratumoral Injection to Participants in Advanced Malignancies | [264] |
| NCT05533697 | Recruiting | 2022-08/present | Phase I Phase II | Study of mRNA-4359 Administered Alone and in Combination With Immune Checkpoint Blockade in Participants With Advanced Solid Tumors | NP |
| NCT02716012 | Active, not recruiting | 2016-03/present | Phase I | First-in-Human Safety, Tolerability and Antitumor Activity Study of MTL-CEBPA in Patients With Advanced Liver Cancer (OUTREACH) | [265] |
| NCT05631886 | Recruiting | 2023-07/present | Phase I | Combination of CAR-DC Vaccine and ICIs in Malignant Tumors | NP |
| NCT02975882 | Active, not recruiting | 2017-08/present | Phase I | Nanoparticle Albumin-Bound Rapamycin, Temozolomide, and Irinotecan Hydrochloride in Treating Pediatric Patients With Recurrent or Refractory Solid Tumors | NP |
| NCT03313778 | Recruiting | 2017-08/present | Phase I | Safety, Tolerability, and Immunogenicity of mRNA-4157 Alone and in Combination in Participants With Solid Tumors (KEYNOTE-603) | NP |
| NCT03897881 | Recruiting | 2019-07/present | Phase II | An Efficacy Study of Adjuvant Treatment With the Personalized Cancer Vaccine mRNA-4157 and Pembrolizumab in Participants With High-Risk Melanoma (KEYNOTE-942) | NP |
| NCT05062980 | Active, not recruiting | 2022-03/present | Phase I Phase II | Quaratusugene Ozeplasmid (Reqorsa) in Combination with Pembrolizumab in Previously Treated Non-Small Lung Cancer (Acclaim-2) | NP |
| NCT01847326 | Completed | 2013-03/2024-01 | Phase I | Paclitaxel Albumin-Stabilized Nanoparticle Formulation and Carboplatin Followed By Chemoradiation in Treating Patients With Recurrent Head and Neck Cancer | [266] |
| NCT06048367 | Recruiting | 2022-10/present | Phase I | Carbon Nanoparticle-Loaded Iron [CNSI-Fe(II)] in the Treatment of Advanced Solid Tumor (CNSI-Fe(II)) | [267,268,269] |
| NCT01435720 | Unknown status | 2011-09/- | Phase I Phase II | Safety and Tolerability Study of SNS01-T in Relapsed or Refractory B Cell Malignancies (Multiple Myeloma, B Cell Lymphoma, or Plasma Cell Leukemia (PCL) | NP |
| NCT01676259 | Unknown status | 2018-03/- | Phase II | A Phase 2 Study of siG12D LODER in Combination With Chemotherapy in Patients With Locally Advanced Pancreatic Cancer (PROTACT) | NP |
| NCT00436410 | Completed | 2006-12/2009-08 | Early Phase I | Tumor Necrosis Factor in Patients Undergoing Surgery for Primary Cancer or Metastatic Cancer | NP |
| NCT03020017 | Completed | 2017-05/2020-08 | Early Phase I | NU-0129 in Treating Patients With Recurrent Glioblastoma or Gliosarcoma Undergoing Surgery | NP |
| NCT01159288 | Completed | 2010-05/2015-12 | Phase II | Trial of a Vaccination With Tumor Antigen-loaded Dendritic Cell-derived Exosomes (CSET 1437) | NP |
| NCT00651703 | Completed | 2008-04/2009-12 | Phase II | Safety and Immunogenicity of CYT004-MelQbG10 Vaccine With and Without Adjuvant in Advanced Stage Melanoma Patients | NP |
| NCT03206073 | Completed | 2017-12/2022-06 | Phase I Phase II | A Phase I/II Study of Pexa-Vec Oncolytic Virus in Combination With Immune Checkpoint Inhibition in Refractory Colorectal Cancer | [270,271,272] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delgado-Almenta, V.; Blaya-Cánovas, J.L.; Calahorra, J.; López-Tejada, A.; Griñán-Lisón, C.; Granados-Principal, S. Cancer Vaccines and Beyond: The Transformative Role of Nanotechnology in Immunotherapy. Pharmaceutics 2025, 17, 216. https://doi.org/10.3390/pharmaceutics17020216
Delgado-Almenta V, Blaya-Cánovas JL, Calahorra J, López-Tejada A, Griñán-Lisón C, Granados-Principal S. Cancer Vaccines and Beyond: The Transformative Role of Nanotechnology in Immunotherapy. Pharmaceutics. 2025; 17(2):216. https://doi.org/10.3390/pharmaceutics17020216
Chicago/Turabian StyleDelgado-Almenta, Violeta, Jose L. Blaya-Cánovas, Jesús Calahorra, Araceli López-Tejada, Carmen Griñán-Lisón, and Sergio Granados-Principal. 2025. "Cancer Vaccines and Beyond: The Transformative Role of Nanotechnology in Immunotherapy" Pharmaceutics 17, no. 2: 216. https://doi.org/10.3390/pharmaceutics17020216
APA StyleDelgado-Almenta, V., Blaya-Cánovas, J. L., Calahorra, J., López-Tejada, A., Griñán-Lisón, C., & Granados-Principal, S. (2025). Cancer Vaccines and Beyond: The Transformative Role of Nanotechnology in Immunotherapy. Pharmaceutics, 17(2), 216. https://doi.org/10.3390/pharmaceutics17020216







