Mechanistic Investigation into Crystallization of Hydrated Co-Amorphous Systems of Flurbiprofen and Lidocaine
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Sample Preparation
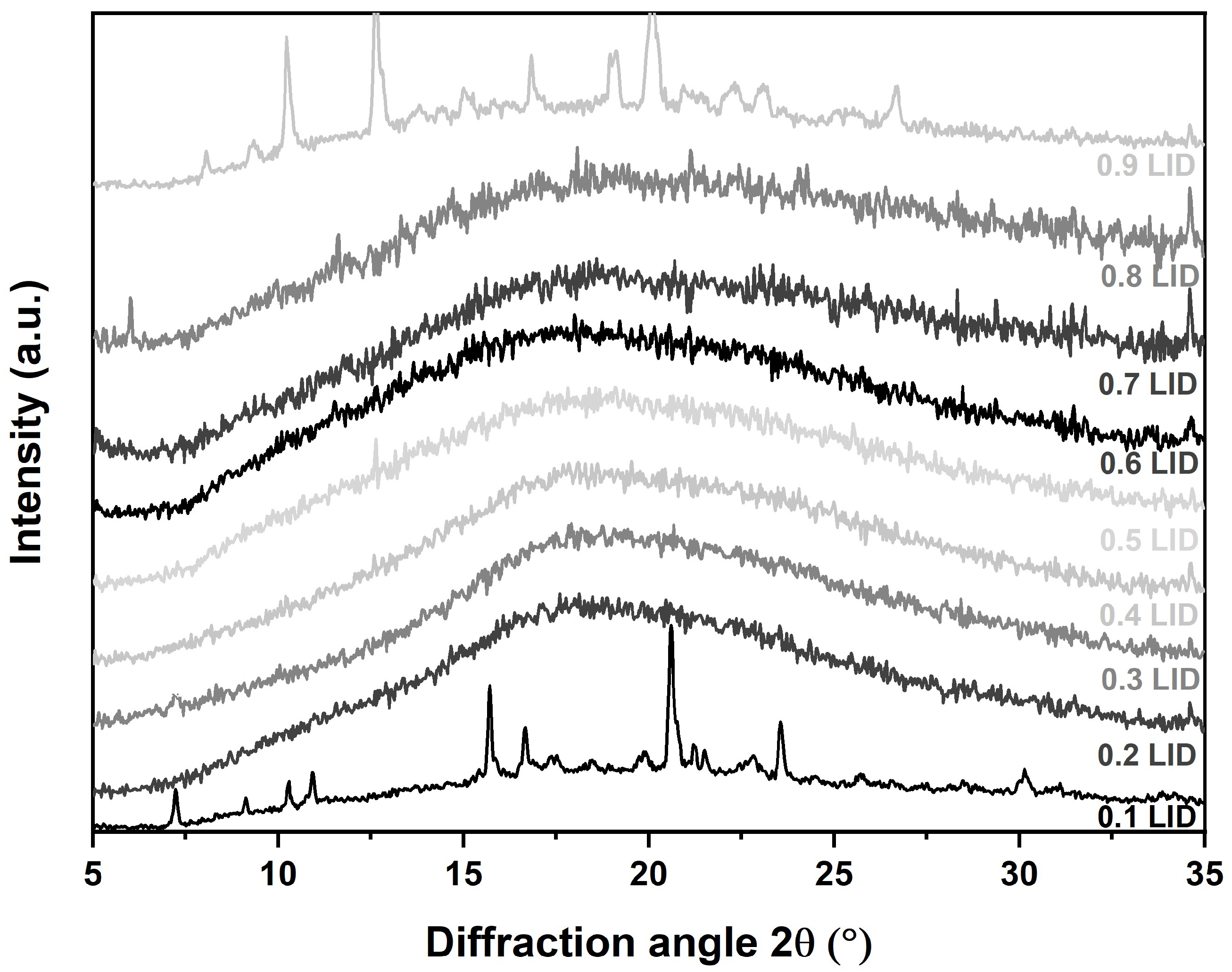
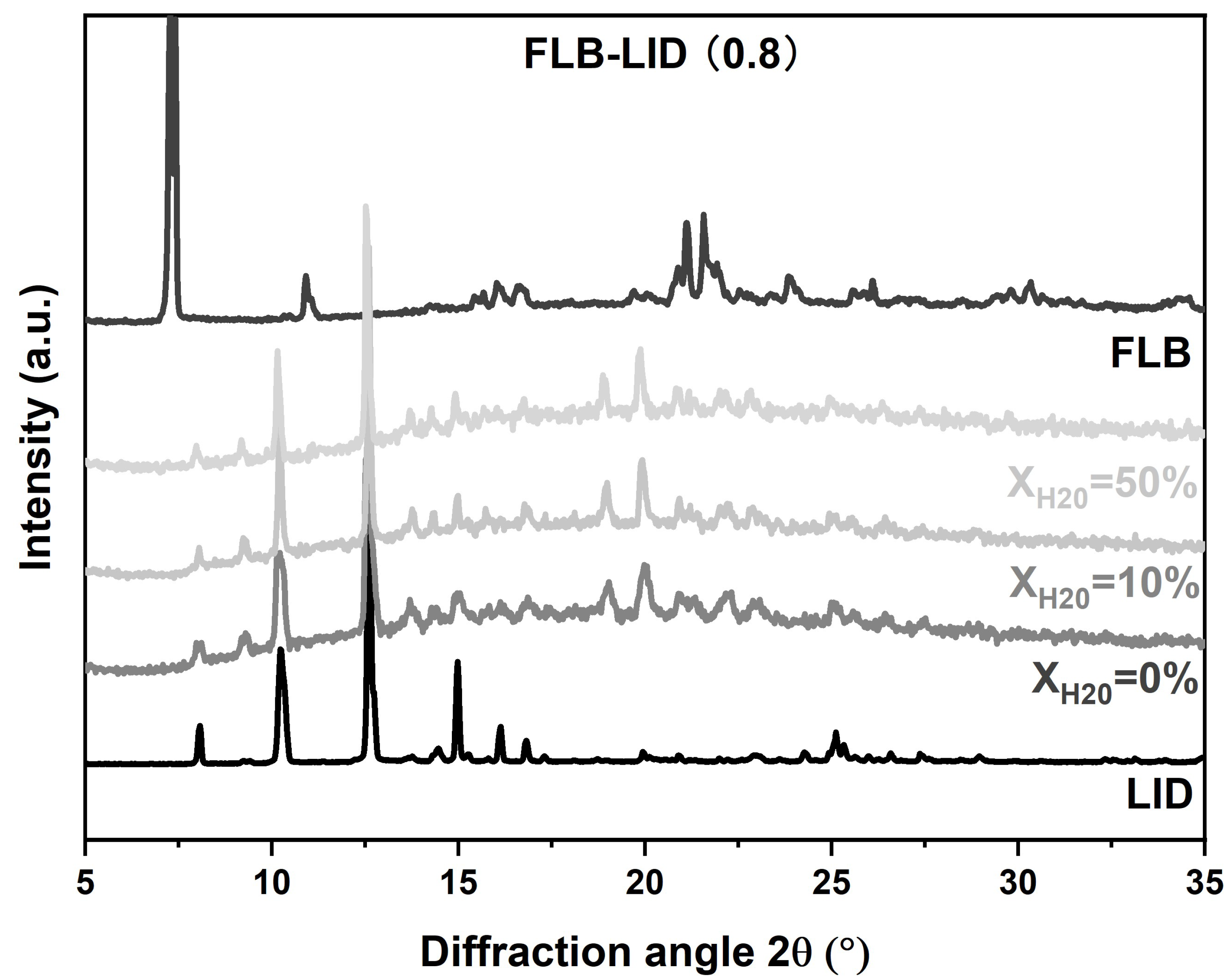
2.2.2. X-ray Powder Diffraction (XRPD)
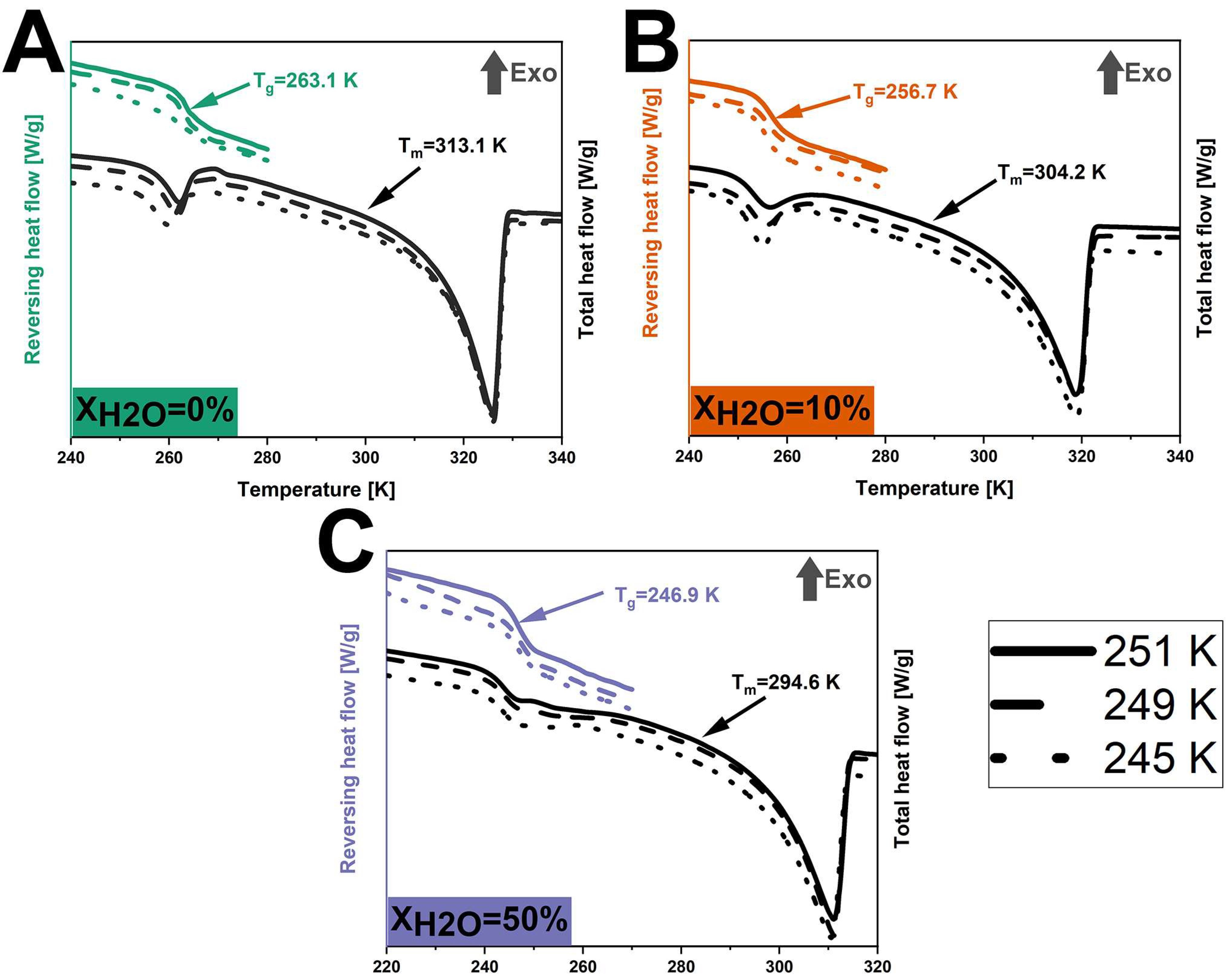
2.2.3. Differential Scanning Calorimetry (DSC)
2.2.4. Isothermal Crystallization
2.2.5. Broadband Dielectric Spectroscopy (BDS)
2.2.6. Molecular Mobility
3. Results and Discussions
3.1. Tgs of Anhydrous and Hydrated Co-Amorphous FLB-LID Systems
3.2. Isothermal Crystallization
3.2.1. Kinetics of Crystallization
3.2.2. Form of Water
3.3. Molecular Mobility
3.4. Configurational Thermodynamics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hancock, B.C.; Zografi, G. Characteristics and significance of the amorphous state in pharmaceutical systems. J. Pharm. Sci. 1997, 86, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kasten, G.; Löbmann, K.; Grohganz, H.; Rades, T. Co-former selection for co-amorphous drug-amino acid formulations. Int. J. Pharm. 2019, 557, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Dengale, S.J.; Grohganz, H.; Rades, T.; Löbmann, K. Recent advances in co-amorphous drug formulations. Adv. Drug Deliv. Rev. 2016, 100, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Löbmann, K.; Laitinen, R.; Grohganz, H.; Gordon, K.C.; Strachan, C.; Rades, T. Coamorphous drug systems: Enhanced physical stability and dissolution rate of indomethacin and naproxen. Mol. Pharm. 2011, 8, 1919–1928. [Google Scholar] [CrossRef]
- Zhou, D.; Zhang, G.G.; Law, D.; Grant, D.J.; Schmitt, E.A. Physical stability of amorphous pharmaceuticals: Importance of configurational thermodynamic quantities and molecular mobility. J. Pharm. Sci. 2002, 91, 1863–1872. [Google Scholar] [CrossRef]
- Gupta, P.; Chawla, G.; Bansal, A.K. Physical stability and solubility advantage from amorphous celecoxib: The role of thermodynamic quantities and molecular mobility. Mol. Pharm. 2004, 1, 406–413. [Google Scholar] [CrossRef]
- Mehta, M.; Ragoonanan, V.; McKenna, G.B.; Suryanarayanan, R. Correlation between Molecular Mobility and Physical Stability in Pharmaceutical Glasses. Mol. Pharm. 2016, 13, 1267–1277. [Google Scholar] [CrossRef]
- Kissi, E.O.; Grohganz, H.; Löbmann, K.; Ruggiero, M.T.; Zeitler, J.A.; Rades, T. Glass-transition temperature of the β-Relaxation as the major predictive parameter for recrystallization of neat amorphous drugs. J. Phys. Chem. B 2018, 122, 2803–2808. [Google Scholar] [CrossRef]
- Krishna Kumar, N.S.; Suryanarayanan, R. Crystallization propensity of amorphous pharmaceuticals: Kinetics and thermodynamics. Mol. Pharm. 2022, 19, 472–483. [Google Scholar] [CrossRef]
- Heng, W.; Song, Y.; Luo, M.; Hu, E.; Wei, Y.; Gao, Y.; Pang, Z.; Zhang, J.; Qian, S. Mechanistic insights into the crystallization of coamorphous drug systems. J. Control Release 2023, 354, 489–502. [Google Scholar] [CrossRef]
- Graeser, K.A.; Patterson, J.E.; Zeitler, J.A.; Rades, T. The role of configurational entropy in amorphous systems. Pharmaceutics 2010, 2, 224–244. [Google Scholar] [CrossRef] [PubMed]
- Chuang, L.; Panyoyai, N.; Katopo, L.; Shanks, R.; Kasapis, S. Calcium chloride effects on the glass transition of condensed systems of potato starch. Food Chem. 2016, 199, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Mascia, L.; Kouparitsas, Y.; Nocita, D.; Bao, X. Antiplasticization of polymer materials: Structural aspects and effects on mechanical and diffusion-controlled properties. Polymers 2020, 12, 769. [Google Scholar] [CrossRef] [PubMed]
- Newman, A.; Zografi, G. An Examination of Water Vapor Sorption by Multicomponent Crystalline and Amorphous Solids and Its Effects on Their Solid-State Properties. J. Pharm. Sci. 2019, 108, 1061–1080. [Google Scholar] [CrossRef]
- Hancock, B.C.; Zografi, G. The relationship between the glass transition temperature and the water content of amorphous pharmaceutical solids. Pharm. Res. 1994, 11, 471–477. [Google Scholar] [CrossRef]
- Heljo, V.P.; Nordberg, A.; Tenho, M.; Virtanen, T.; Jouppila, K.; Salonen, J.; Maunu, S.L.; Juppo, A.M. The effect of water plasticization on the molecular mobility and crystallization tendency of amorphous disaccharides. Pharm. Res. 2012, 29, 2684–2697. [Google Scholar] [CrossRef]
- Ruiz, G.N.; Romanini, M.A.-O.X.; Hauptmann, A.; Loerting, T.; Shalaev, E.; Tamarit, J.A.-O.; Pardo, L.A.-O.; Macovez, R.A.-O. Genuine antiplasticizing effect of water on a glass-former drug. Sci. Rep. 2017, 7, 7470. [Google Scholar] [CrossRef]
- Xu, X.; Grohganz, H.; Rades, T. Influence of water on amorphous lidocaine. Mol. Pharm. 2022, 19, 3199–3205. [Google Scholar] [CrossRef]
- Chen, Y.; Tang, T.; Ayranci, C. Moisture-induced anti-plasticization of polylactic acid: Experiments and modeling. J. Appl. Polym. Sci. 2022, 139, 52369. [Google Scholar] [CrossRef]
- Wang, J.L.; Cheng, F.; Zhu, P.X. Structure and properties of urea-plasticized starch films with different urea contents. Carbohydr. Polym. 2014, 101, 1109–1115. [Google Scholar] [CrossRef]
- Lerbret, A.; Affouard, F. Molecular Packing, Hydrogen bonding, and fast dynamics in lysozyme/trehalose/glycerol and trehalose/glycerol glasses at low hydration. J. Phys. Chem. B 2017, 121, 9437–9451. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Rades, T.; Grohganz, H. Molecular interactions of hydrated co-amorphous systems of prilocaine and lidocaine. Int. J. Pharm. 2024, 651, 123807. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Grohganz, H.; Rades, T. Anti-plasticizing effect of water on prilocaine and lidocaine—The role of the hydrogen bonding pattern. Phys. Chem. Chem. Phys. 2024, 26, 14149–14159. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Rades, T.; Grohganz, H. Thermal investigation on hydrated co-amorphous systems of nicotinamide and prilocaine. Eur. J. Pharm. Biopharm. 2023, 186, 1–6. [Google Scholar] [CrossRef]
- Flurbiprofen. SCIFINDER. American Chemical Society. n.d. (CAS RN: 5104-49-4). Available online: https://scifinder-n.cas.org/searchDetail/substance/66ab53ab990d45148c8b114f/substanceDetails (accessed on 1 August 2024).
- Lidocaine. SCIFINDER. American Chemical Society. n.d. (CAS RN: 137-58-6). Available online: https://scifinder-n.cas.org/searchDetail/substance/66ab5441990d45148c8b1f9d/substanceDetails (accessed on 1 August 2024).
- Childs, S.L.; Stahly, G.P.; Park, A. The salt-cocrystal continuum: The influence of crystal structure on ionization state. Mol. Pharm. 2007, 4, 323–338. [Google Scholar] [CrossRef]
- Moreira, D.N.; Fresno, N.; Pérez-Fernández, R.; Frizzo, C.P.; Goya, P.; Marco, C.; Martins, M.A.P.; Elguero, J. Brønsted acid–base pairs of drugs as dual ionic liquids: NMR ionicity studies. Tetrahedron 2015, 71, 676–685. [Google Scholar] [CrossRef]
- Wang, H.; Gurau, G.; Shamshina, J.; Cojocaru, O.A.; Janikowski, J.; MacFarlane, D.R.; Davis, J.H.; Rogers, R.D. Simultaneous membrane transport of two active pharmaceutical ingredients by charge assisted hydrogen bond complex formation. Chem. Sci. 2014, 5, 3449–3456. [Google Scholar] [CrossRef]
- Marei, H.F.; Arafa, M.F.; Essa, E.A.; El Maghraby, G.M. Lidocaine as eutectic forming drug for enhanced transdermal delivery of nonsteroidal anti-inflammatory drugs. J. Drug Deliv. Sci. Technol. 2021, 61, 102338. [Google Scholar] [CrossRef]
- Fiandaca, M.; Dalwadi, G.; Wigent, R.; Gupta, P. Ionic liquid formation with deep eutectic forces at an atypical ratio (2:1) of naproxen to lidocaine in the solid-state, thermal characterization and FTIR investigation. Int. J. Pharm. 2020, 575, 118946. [Google Scholar] [CrossRef]
- Blaabjerg, L.I.; Lindenberg, E.; Löbmann, K.; Grohganz, H.; Rades, T. Glass Forming Ability of Amorphous Drugs Investigated by Continuous Cooling and Isothermal Transformation. Mol. Pharm. 2016, 13, 3318–3325. [Google Scholar] [CrossRef]
- Toda, A.; Arita, T.; Tomita, C.; Hikosaka, M. Temperature-modulated DSC applied to the transformation kinetics of polymer crystallization. Polym. J. 1999, 31, 790–794. [Google Scholar] [CrossRef]
- Pak, J.; Wunderlich, B. Melting and crystallization of polyethylene of different molar mass by calorimetry. Macromolecules 2001, 34, 4492–4503. [Google Scholar] [CrossRef]
- Righetti, M.C.; Prevosto, D.; Tombari, E. Time and temperature evolution of the rigid amorphous fraction and differently constrained amorphous fractions in PLLA. Macromol. Chem. Phys. 2016, 217, 2013–2026. [Google Scholar] [CrossRef]
- Righetti, M.C. Crystallization of polymers investigated by temperature-modulated DSC. Materials 2017, 10, 442. [Google Scholar] [CrossRef]
- Otun, S.O.; Meehan, E.; Qi, S.; Craig, D.Q. The use of quasi-isothermal modulated temperature differential scanning calorimetry for the characterization of slow crystallization processes in lipid-based solid self-emulsifying systems. Pharm. Res. 2015, 32, 1316–1324. [Google Scholar] [CrossRef]
- Svoboda, R. Crystallization of glasses—When to use the Johnson-Mehl-Avrami kinetics? J. Eur. Ceram. Soc. 2021, 41, 7862–7867. [Google Scholar] [CrossRef]
- Kramarczyk, D.; Knapik-Kowalczuk, J.; Smolka, W.; Monteiro, M.F.; Tajber, L.; Paluch, M. Inhibition of celecoxib crystallization by mesoporous silica—Molecular dynamics studies leading to the discovery of the stabilization origin. Eur. J. Pharm. Sci. 2022, 171, 106132. [Google Scholar] [CrossRef]
- Knapik-Kowalczuk, J.; Rams-Baron, M.; Paluch, M. Current research trends in dielectric relaxation studies of amorphous pharmaceuticals: Physical stability, tautomerism, and the role of hydrogen bonding. TrAC Trends Anal. Chem. 2021, 134, 116097. [Google Scholar] [CrossRef]
- Androsch, R.; Wunderlich, B. Specific reversible melting of polymers. J. Polym. Sci. Part B Polym. Phys. 2003, 41, 2039–2051. [Google Scholar] [CrossRef]
- Bhugra, C.; Pikal, M.J. Role of thermodynamic, molecular, and kinetic factors in crystallization from the amorphous state. J. Pharm. Sci. 2008, 97, 1329–1349. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of Phase Change. I General Theory. J. Chem. Phys. 1939, 7, 1103–1112. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of Phase Change. II Transformation-Time Relations for Random Distribution of Nuclei. J. Chem. Phys. 1940, 8, 212–224. [Google Scholar] [CrossRef]
- Avrami, M. Granulation, Phase Change, and Microstructure Kinetics of Phase Change. III. J. Chem. Phys. 1941, 9, 177–184. [Google Scholar] [CrossRef]
- Havriliak, S.; Negami, S. A complex plane representation of dielectric and mechanical relaxation processes in some polymers. Polymer 1967, 8, 161–210. [Google Scholar] [CrossRef]
- Fulcher, G.S. Analysis of recent measurements of the viscosity of glasses. J. Am. Ceram. Soc. 1925, 8, 339–355. [Google Scholar] [CrossRef]
- Fox, T.G., Jr.; Flory, P.J. Second-Order Transition Temperatures and Related Properties of Polystyrene. I. Influence of Molecular Weight. J. Appl. Phys. 1950, 21, 581–591. [Google Scholar] [CrossRef]
- Cheng, S.; McKenna, G.B. Isothermal crystallization and time-temperature transformation of amorphous nifedipine: A case of polymorphism formation and conversion. Mol. Pharm. 2021, 18, 2786–2802. [Google Scholar] [CrossRef]
- Ruiz, G.N.; Romanini, M.; Barrio, M.; Tamarit, J.L.; Pardo, L.C.; Macovez, R. Relaxation dynamics vs crystallization kinetics in the amorphous state: The case of stiripentol. Mol. Pharm. 2017, 14, 3636–3643. [Google Scholar] [CrossRef]
- Madbouly, S.A.; Mansour, A.A.; Abdou, N.Y. Crystallization kinetics of PHB/PVAc blends using time resolved dielectric spectroscopy. Eur. Polym. J. 2007, 43, 3933–3942. [Google Scholar] [CrossRef]
- Pirayavaraporn, C.; Rades, T.; Gordon, K.C.; Tucker, I.G. Quantification of the types of water in Eudragit RLPO polymer and the kinetics of water loss using FTIR. Int. J. Pharm. 2013, 458, 90–98. [Google Scholar] [CrossRef]
- Inoue, T. Effect of water on melting phase relations and melt composition in the system Mg2SiO4·MgSiO3·H2O up to 15 GPa. Phys. Earth Planet. Inter. 1994, 85, 237–263. [Google Scholar] [CrossRef]
- Zhou, D.; Zhang, G.G.; Law, D.; Grant, D.J.; Schmitt, E.A. Thermodynamics, molecular mobility and crystallization kinetics of amorphous griseofulvin. Mol. Pharm. 2008, 5, 927–936. [Google Scholar] [CrossRef] [PubMed]
- Lauritzen, J.I., Jr.; Hoffman, J.D. Theory of formation of polymer crystals with folded chains in dilute solution. J. Res. Natl. Bur. Stand. A Phys. Chem. 1960, 64, 73–102. [Google Scholar] [CrossRef] [PubMed]
- Qian, K.K.; Bogner, R.H. Application of mesoporous silicon dioxide and silicate in oral amorphous drug delivery systems. J. Pharm. Sci. 2012, 101, 444–463. [Google Scholar] [CrossRef]
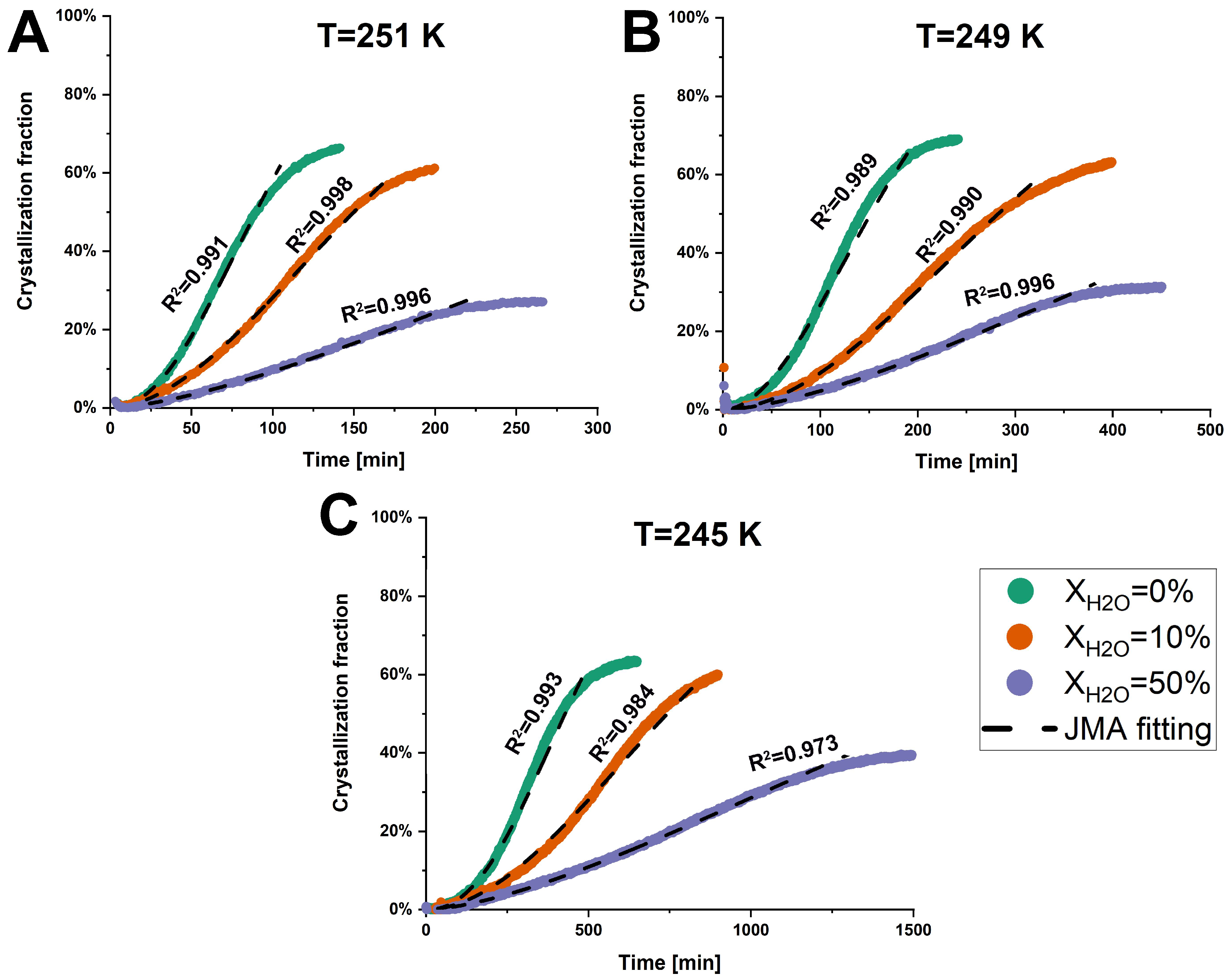


| Temperature | XH2O | Crystallization Fraction | Crystallization Complete-Time [min] | n | k [min−1] |
|---|---|---|---|---|---|
| 251 K | 0% | 66.1% | 136.7 | 2.11 | 0.0094 |
| 10% | 60.1% | 198.8 | 1.83 | 0.0055 | |
| 50% | 27.1% | 256.3 | 1.51 | 0.0021 | |
| 249 K | 0% | 69.0% | 237.7 | 1.92 | 0.0054 |
| 10% | 62.6% | 389.3 | 1.87 | 0.0029 | |
| 50% | 31.3% | 447.2 | 1.54 | 0.0014 | |
| 245 K | 0% | 62.9% | 620.7 | 2.22 | 0.0020 |
| 10% | 60.0% | 897.0 | 1.88 | 0.0011 | |
| 50% | 39.3% | 1476.2 | 1.54 | 0.0005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.; Grohganz, H.; Knapik-Kowalczuk, J.; Paluch, M.; Rades, T. Mechanistic Investigation into Crystallization of Hydrated Co-Amorphous Systems of Flurbiprofen and Lidocaine. Pharmaceutics 2025, 17, 175. https://doi.org/10.3390/pharmaceutics17020175
Xu X, Grohganz H, Knapik-Kowalczuk J, Paluch M, Rades T. Mechanistic Investigation into Crystallization of Hydrated Co-Amorphous Systems of Flurbiprofen and Lidocaine. Pharmaceutics. 2025; 17(2):175. https://doi.org/10.3390/pharmaceutics17020175
Chicago/Turabian StyleXu, Xiaoyue, Holger Grohganz, Justyna Knapik-Kowalczuk, Marian Paluch, and Thomas Rades. 2025. "Mechanistic Investigation into Crystallization of Hydrated Co-Amorphous Systems of Flurbiprofen and Lidocaine" Pharmaceutics 17, no. 2: 175. https://doi.org/10.3390/pharmaceutics17020175
APA StyleXu, X., Grohganz, H., Knapik-Kowalczuk, J., Paluch, M., & Rades, T. (2025). Mechanistic Investigation into Crystallization of Hydrated Co-Amorphous Systems of Flurbiprofen and Lidocaine. Pharmaceutics, 17(2), 175. https://doi.org/10.3390/pharmaceutics17020175











