Pharmacogenomics of 3,4-Methylenedioxymethamphetamine (MDMA): A Narrative Review of the Literature
Abstract
1. Introduction
2. Genetic Factors Influencing the Metabolism of MDMA
3. Genetic Factors Influencing Pharmacological MDMA Targets
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bernschneider-Reif, S.; Oxler, F.; Freudenmann, R.W. The origin of MDMA (‘ecstasy’)—Separating the facts from the myth. Pharmazie 2006, 61, 966–972. [Google Scholar] [PubMed]
- De La Torre, R.; Farré, M.; Roset, P.N.; Pizarro, N.; Abanades, S.; Segura, M.; Segura, J.; Cami, J. Human pharmacology of MDMA: Pharmacokinetics, metabolism, and disposition. Ther. Drug Monit. 2004, 26, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Kalant, H. The pharmacology and toxicology of ‘ecstasy’ (MDMA) and related drugs. Can. Med. Assoc. J. 2001, 165, 917–928. [Google Scholar]
- Rietjens, S.J.; Hondebrink, L.; Westerink, R.H.; Meulenbelt, J. Pharmacokinetics and pharmacodynamics of 3,4-methylenedioxymethamphetamine (MDMA): Interindividual differences due to polymorphisms and drug–drug interactions. Crit. Rev. Toxicol. 2012, 42, 854–876. [Google Scholar] [CrossRef] [PubMed]
- Schenk, S.; Highgate, Q. Methylenedioxymethamphetamine (MDMA): Serotonergic and dopaminergic mechanisms related to its use and misuse. J. Neurochem. 2021, 157, 1714–1724. [Google Scholar] [CrossRef]
- Dumont, G.J.H.; Sweep, F.C.G.J.; Van der Steen, R.; Hermsen, R.; Donders, A.R.T.; Touw, D.J.; Van Gerven, J.M.A.; Buitelaar, J.K.; Verkes, R.J. Increased oxycotin concentrations and prosocial feelings in human after ecstasy (3,4-methylenedioxymethamphetamine) administration. Soc. Neurosci. 2009, 4, 359–366. [Google Scholar] [CrossRef]
- Hysek, C.M.; Simmler, L.D.; Nicola, V.G.; Vischer, N.; Donzelli, M.; Krahenbuhl, S.; Grouzmann, E.; Huwyler, J.; Hoener, M.C.; Liechti, M.E. Duloxetine inhibits effects of MDMA (“ecstasy”) in vitro and in humans in a randomized placebo-controlled laboratory study. PLoS ONE 2012, 7, e36476. [Google Scholar] [CrossRef]
- Kuwayama, K.; Inoue, H.; Kanamori, T.; Tsujikawa, K.; Miyaguchi, H.; Iwata, Y.; Miyauchi, S.; Kamo, N.; Kishi, T. Uptake of 3,4-methylenedioxymethamphetamine and its related compounds by a proton-coupled transport system in Caco-2 cells. Biochim. Biophys. Acta. 2008, 1778, 42–50. [Google Scholar] [CrossRef]
- Bertelsen, K.M.; Greenblatt, D.J.; Von Moltke, L.L. Apparent active transport of MDMA is not mediated by P-glycoprotein: A comparison with MDCK and Caco-2 monolayers. Biopharm. Drug Dispos. 2006, 27, 219–227. [Google Scholar] [CrossRef] [PubMed]
- De La Torre, R.; Farré, M.; Ortuño, J.; Mas, M.; Brenneisen, R.; Roset, P.N.; Segura, J.; Cami, J. Non-linear pharmacokinetics of MDMA (‘ecstasy’) in humans. Br. J. Clin. Pharmacol. 2000, 49, 104–109. [Google Scholar] [CrossRef]
- Steuer, A.E.; Schmidhauser, C.; Tingelhoff, E.H.; Schmid, Y.; Rickli, A.; Kraemer, T.; Liechti, M.E. Impact of cytochrome P450 2D6 function on the chiral blood plasma pharmacokinetics of 3,4-methylenedioxymethamphetamine (MDMA) and its phase I and II metabolites in humans. PLoS ONE 2016, 11, e0150955. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.; Elayan, I.; Hanson, G.R.; Foltz, R.L.; Gibb, J.W.; Lim, H.K. Effects of 3,4-dihydroxymethamphetamine and 2,4,5-trihydroxymethamphetamine, two metabolites of 3,4-methylenedioxyamphetamine, on central serotoninergic and dopaminergic systems. J. Pharmacol. Exp. Ther. 1992, 216, 447–453. [Google Scholar]
- Mueller, M.; Goodwin, A.K.; Ator, N.A.; McCann, U.D.; Ricaurte, G.A. Metabolism and disposition of 3,4-methylenedioxymethamphetamine (“Ecstasy”) in baboons after oral administration: Comparision with humans reveals marked differences. J. Pharmacol. Exp. Ther. 2011, 338, 310–317. [Google Scholar] [CrossRef]
- Pizarro, N.; De La Torre, R.; Joglar, J.; Okumura, N.; Perfetti, X.; Lau, S.S.; Monks, T.J. Serotonergic neurotoxic thioether metabolites of 3,4-methylenedioxymethamphetamine (MDMA, “Ecstasy”): Synthesis, isolation, and characterization of diastereoisomers. Chem. Res. Toxicol. 2008, 21, 2272–2279. [Google Scholar] [CrossRef]
- Baez, S.; Segura-Aguilar, J.; Widersten, M.; Johansson, A.S.; Mannervik, B. Glutathione transferases catalyse the detoxication of oxidized metabolites (o-quinones) of catecholamines and may serve as an antioxidant system preventing degenerative cellular processes. Biochem. J. 1997, 324, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Shulgin, A.T.; Nichols, D.E. Characterization of three new psychotomimetics. In The Psychopharmacology of Hallucinogens; Stillman, R.C., Willette, R.E., Eds.; PErgamon Press: New York, NY, USA, 1978; pp. 74–83. [Google Scholar]
- Nichols, D.E. Entactogens: How the name for a novel class of psychoactive agents originated. Front. Psychiatry 2022, 13, 863088. [Google Scholar] [CrossRef]
- Yazar-Klosinski, B.; Mithoefer, M. Potential psychiatric uses for MDMA. Clin. Pharmacol. Ther. 2017, 101, 194–196. [Google Scholar] [CrossRef]
- Sessa, B.; Higbed, L.; Nutt, D. A review of 3,4-methylenedioxymethamphetamine (MDMA)-assisted psychotherapy. Front. Psychiatry 2019, 10, 138. [Google Scholar] [CrossRef]
- Mitchell, J.M.; Ot’alora, G.M.; Van Der Kolk, B.; Shannon, S.; Bogenschutz, M.; Gelfand, Y.; Paleos, C.; Nicholas, C.R.; Quevedos, S.; Balliett, B.; et al. MDMA-assisted therapy for moderate to severe PTSD: A randomized, placebo-controlled phase 3 trial. Nat. Med. 2023, 29, 2473–2480. [Google Scholar] [CrossRef]
- Sessa, B. Why MDMA therapy for alcohol use disorder? And why now? Neuropharmacology 2018, 142, 83–88. [Google Scholar] [CrossRef]
- Roxburgh, A.; Sam, B.; Kriikku, P.; Mounteney, J.; Castanera, A.; Dias, M.; Giraudon, I. Trends in MDMA-related mortality across four countries. Addiction 2021, 116, 3094–3103. [Google Scholar] [CrossRef] [PubMed]
- Roxburgh, A.; Lappin, J. MDMA-related deaths in Australia 2000 to 2018. Int. J. Drug Policy 2020, 76, 102630. [Google Scholar] [CrossRef]
- Vizeli, P.; Liechti, M.E. No influence of dopamine system gene variations on acute effects of MDMA. Front. Psychiatry 2019, 10, 755. [Google Scholar] [CrossRef] [PubMed]
- Vizeli, P.; Meyer Zu Schwabedissen, H.E.; Liechti, M.E. Role of serotonin transporter and receptor gene variations in the acute effects of MDMA in healthy subjects. ACS Chem. Neurosci. 2019, 10, 3120–3131. [Google Scholar] [CrossRef]
- Tucker, G.T.; Lennard, M.S.; Ellis, S.W.; Woods, H.F.; Cho, A.K.; Lin, L.Y.; Hiratsuka, A.; Schmitz, D.A.; Chu, T.Y.Y. The demethylenation of methylenedioxyamphetamine (“ectasy”) by debrisoquine hydroxylase (CYP2D6). Biochem. Pharmacol. 1994, 7, 1151–1156. [Google Scholar] [CrossRef]
- Leeder, J.S.; Gaedigk, A. CYP2D6 and pharmacogenomics: Where does future research need to focus? Part 2: Clinical aspects. Pharmacogenomics 2014, 15, 1055–1058. [Google Scholar] [CrossRef]
- Taylor, C.; Crosby, I.; Yip, V.; Maguire, P.; Pirmohamed, M.; Turner, R.M. A review of the important role of CYP2D6 in pharmacogenomics. Genes 2020, 11, 1295. [Google Scholar] [CrossRef] [PubMed]
- Lassen, D.; Damkier, P.; Brøsen, K. The pharmacogenetics of tramadol. Clin. Pharmacokinet. 2015, 54, 825–836. [Google Scholar] [CrossRef]
- Schwab, M.; Griese, U.; Hermle, L.; Gouzoulis, E.; Zanger, U.; Mikus, G. Is there an impact of CYP2D6 genotype on the toxicity of’ecstasy and related designer drugs? Naunyn Schmiedebergs Arch Pharmacol 1998, 4, 163. [Google Scholar]
- O’Donohoe, A.; O’Flynn, K.; Shields, K.; Hawi, Z.; Gill, M. MDMA toxicity: No evidence for a major influence of metabolic genotype at CYP2D6. Addict Biol. 1998, 3, 309–314. [Google Scholar] [CrossRef]
- Gilhooly, T.C.; Daly, A.K. CYP2D6 deficiency, a factor in ecstasy related deaths? Br. J. Clin. Pharmacol. 2002, 54, 69–70. [Google Scholar]
- Haufroid, V.; Hantson, P. CYP2D6 genetic polymorphisms and their relevance for poisoning due to amfetamines, opioid analgesics and antidepressants. Clin. Toxicol. 2015, 53, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Jamei, M.; Heydari, A.; Yeo, K.R.; De La Torre, R.; Farré, M.; Tucker, G.T.; Rostami-Hodjegan, A. Implications of mechanism-based inhibition of CYP2D6 for the pharmacokinetics and toxicity of MDMA. J. Psychopharmacol. 2006, 20, 842–849. [Google Scholar] [CrossRef] [PubMed]
- Hysek, C.M.; Fink, A.E.; Simmler, L.D.; Donzelli, M.; Grouzmann, E.; Liechti, M.E. α1-adrenergic receptors contribute to the acute effects of 3,4-methylenedioxymethamphetamine in humans. J. Clin. Psychopharmacol. 2013, 5, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Hysek, C.M.; Simmler, L.D.; Schillinger, N.; Meyer, N.; Schmid, Y.; Donzelli, M.; Grouzmann, E.; Lietchti, M.E. Pharmacokinetic and pharmacodynamic effects of methylphenidate and MDMA administered alone or in combination. Int. J. Neuropsychopharmacol. 2014, 17, 371–381. [Google Scholar] [CrossRef]
- O’Mathúna, B.; Farré, M.; Rostami-Hodjegan, A.; Yang, J.; Cuyàs, E.; Torrens, M.; Pardo, R.; Abanades, S.; Maluf, S.; Tucker, G.T.; et al. The consequences of 3,4-methylenedioxymethamphetamine induced CYP2D6 inhibition in humans. J. Clin. Psychopharmacol. 2008, 28, 523–529. [Google Scholar] [CrossRef]
- De La Torre, R.; Farré, M.; Ó Mathúna, B.; Roset, P.N.; Pizarro, N.; Segura, M.; Torrens, M.; Ortuno, J.; Pujadas, M.; Cami, J. MDMA (ecstasy) pharmacokinetics in a CYP2D6 poor metaboliser and in nine CYP2D6 extensive metabolisers. Eur. J. Clin. Pharmacol. 2005, 61, 551–554. [Google Scholar] [CrossRef] [PubMed]
- Pardo-Lozano, R.; Farré, M.; Yubero-Lahoz, S.; O’Mathúna, B.; Torrens, M.; Mustata, C.; Pérez-Mana, C.; Langohr, K.; Cuyas, E.; Li Carbo, M.; et al. Clinical pharmacology of 3,4-methylenedioxymethamphetamine (MDMA, “Ecstasy”): The influence of gender and genetics (CYP2D6, COMT, 5-HTT). PLoS ONE 2012, 7, e47599. [Google Scholar] [CrossRef]
- De La Torre, R.; Yubero-Lahoz, S.; Pardo-Lozano, R.; Farré, M. MDMA, methamphetamine, and CYP2D6 pharmacogenetics: What is clinically relevant? Front. Genet. 2012, 3, 235. [Google Scholar] [CrossRef]
- Schmid, Y.; Vizeli, P.; Hysek, C.M.; Prestin, K.; Meyer Zu Schwabedissen, H.E.; Liechti, M.E. CYP2D6 function moderates the pharmacokinetics and pharmacodynamics of 3,4-methylene-dioxymethamphetamine in a controlled study in healthy individuals. Pharmacogenet. Genom. 2016, 26, 397–401. [Google Scholar] [CrossRef]
- Aitchison, K.J.; Tsapakis, E.M.; Huezo-Diaz, P.; Kerwin, R.W.; Forsling, M.L.; Wolff, K. Ecstasy (MDMA)-induced hyponatraemia is associated with genetic variants in CYP2D6 and COMT. J. Psychopharmacol. 2012, 26, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Cuyàs, E.; Verdejo-García, A.; Fagundo, A.B.; Khymenets, O.; Rodríguez, J.; Cuenca, A.; De Sola Llopis, S.; Langhor, K.; Pena-Casanova, J.; Torrens, M.; et al. The influence of genetic and environmental factors among MDMA users in cognitive performance. PLoS ONE 2011, 6, e27206. [Google Scholar] [CrossRef]
- Wolff, K.; Tsapakis, E.M.; Pariante, C.M.; Kerwin, R.W.; Forsling, M.L.; Aitchison, K.J. Pharmacogenetic studies of change in cortisol on ecstasy (MDMA) consumption. J. Psychopharmacol. 2012, 26, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Papaseit, E.; Torrens, M.; Perez-Mana, C.; Muga, R.; Farré, M. Key individual determinants in MDMA pharmacodynamics. Expert. Opin. Drug Metab. Toxicol. 2018, 14, 183–195. [Google Scholar] [CrossRef]
- Vizeli, P.; Schmid, Y.; Prestin, K.; Meyer Zu Schwabedissen, H.E.; Liechti, M.E. Pharmacogenetics of ecstasy: CYP1A2, CYP2C19, and CYP2B6 polymorphisms moderate pharmacokinetics of MDMA in healthy subjects. Eur. Neuropsychopharmacol. 2017, 27, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Antolino-Lobo, I.; Meulenbelt, J.; Nijmeijer, S.M.; Scherpenisse, P.; Van Den Berg, M.; Van Duursen, M.B.M. Differential roles of phase I and phase II enzymes in 3,4-methylendioxymethamphetamine-induced cytotoxicity. Drug Metab. Dispos. 2010, 38, 1105–1112. [Google Scholar] [CrossRef]
- Perfetti, X.; O’Mathúna, B.; Pizarro, N.; Cuyàs, E.; Khymenets, O.; Almeida, B.; Pellegrini, M.; Pichini, S.; Lau, S.S.; Monks, T.J.; et al. Neurotoxic thioether adducts of 3,4-methylenedioxymethamphetamine identified in human urine after ecstasy ingestion. Drug Metab. Dispos. 2009, 37, 1448–1455. [Google Scholar] [CrossRef] [PubMed]
- Fagundo, A.B.; Cuyàs, E.; Verdejo-Garcia, A.; Khymenets, O.; Langohr, K.; Martín-Santos, R.; Farré, M.; De la Torre, R. The influence of 5-HTT and COMT genotypes on verbal fluency in ecstasy users. J. Psychopharmacol. 2010, 24, 1381–1393. [Google Scholar] [CrossRef]
- Bag, H.G.G. Association between COMT gene rs165599 SNP and schizophrenia: A meta-analysis of case-control studies. Mol. Genet. Genom. Med. 2018, 6, 845–854. [Google Scholar]
- Lamb, Y.N.; Thompson, J.M.D.; Murphy, R.; Wall, C.; Kirk, I.J.; Morgan, A.R.; Ferguson, L.R.; Mitchell, E.A.; Waldie, K.E.; ABC Study group. Perceived stress during pregnancy and the catechol-O-methyltransferase (COMT) rs165599 polymorphism impacts on childhood IQ. Cognition 2014, 132, 461–470. [Google Scholar] [CrossRef]
- Bamalan, O.A.; Moore, M.J.; Al Khalili, Y. Physiology, Serotonin. StatPearls Publishing. Available online: https://www.ncbi.nlm.nih.gov/books/NBK545168/ (accessed on 30 July 2023).
- Ikegame, T.; Hidaka, Y.; Nakachi, Y.; Murata, Y.; Watanabe, R.; Sugawara, H.; Asai, T.; Kiyota, E.; Ikeda, M.; Sasaki, T.; et al. Identification and functional characterization of the extremely long allele of the serotonin transporter-linked polymorphic region. Transl. Psychiatry 2021, 11, 119. [Google Scholar] [CrossRef] [PubMed]
- Pungercic, G.; Videtic, A.; Pestotnik, A.; Pajnic, I.Z.; Zupanc, T.; Balazic, J.; Tomori, M.; Komel, R. Serotonin transporter gene promoter (5-HTTLPR) and intron 2 (VNTR) polymorphisms: A study on Slovenian population of suicide victims. Psychiatr. Genet. 2006, 16, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Roiser, J.P.; Cook, L.J.; Cooper, J.D.; Rubinsztein, D.C.; Sahakian, B.J. Association of a functional polymorphism in the serotonin transporter gene with abnormal emotional processing in ecstasy users. Am. J. Psychiatry. 2005, 162, 609–612. [Google Scholar] [CrossRef]
- Martín-Santos, R.; Torrens, M.; Poudevida, S.; Langohr, K.; Cuyás, E.; Pacifici, R.; Farré, M.; Pichini, S.; De la Torre, R. 5-HTTLPR polymorphism, mood disorders and MDMA use in a 3-year follow-up study. Addict. Biol. 2010, 15, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Kuypers, K.P.C.; De la Torre, R.; Farre, M.; Xicota, L.; De Sousa Ferandes Perna, E.B.; Theunissen, E.L.; Ramakers, J.G. Depressive mood ratings are reduced by MDMA in female polydrug ecstasy users homozygous for the l-allele of serotonin transporter. Sci. Rep. 2018, 8, 1061. [Google Scholar] [CrossRef] [PubMed]
- Reneman, L.; Schilt, T.; De Win, M.M.; Booij, J.; Schmand, B.; Den Brink, W.V.; Bakker, O. Memory function and serotonin transporter promoter gene polymorphism in ecstasy (MDMA) users. J. Psychopharmacol. 2006, 20, 389–399. [Google Scholar] [CrossRef]
- Ates, O.; Karakus, N.; Sezer, S.; Bozkurt, N. Genetic association of 5-HT1A and 5-HT1B gene polymorphisms with migraine in a Turkish population. J. Neurol. Sci. 2013, 326, 64–67. [Google Scholar] [CrossRef] [PubMed]
- Ruocco, A.C.; Rodrigo, A.H.; Carcone, D.; McMain, S.; Jacobs, G.; Kennedy, J.L. Tryptophan hydroxylase 1 gene polymorphisms alter prefrontal cortex activation during response inhibition. Neuropsychology 2016, 30, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Pan, Z.; Jiao, Z.; Li, F.; Zhao, G.; Wei, Q.; Pan, F.; Evangelou, E. TPH2 gene polymorphisms and major depression—A meta-analysis. PLoS ONE 2012, 7, e36721. [Google Scholar] [CrossRef][Green Version]
- Genis-Mendoza, A.D.; Ruiz-Ramos, D.; López-Narvaez, M.L.; Tovilla-Zárate, C.A.; García, A.R.; Meda, G.C.; Martinez-Magana, J.J.; Gonzales-Castro, T.B.; Juarez-Rojop, I.E.; Nicolini, H. Genetic association analysis of 5-HTR2A gene variants in eating disorders in a Mexican population. Brain Behav. 2019, 9, e01286. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.C.; MacKillop, J.; Weafer, J.; Hernandez, K.M.; Gao, J.; Palmer, A.A.; De Wit, H. Genetic analysis of impulsive personality traits: Examination of a priori candidates and genome-wide variation. Psychiatry Res. 2018, 259, 398–404. [Google Scholar] [CrossRef]
- Kaur, G.; Singh Chavan, B.; Gupta, D.; Sinhmar, V.; Prasad, R.; Tripathi, A.; Garg, P.D.; Gupta, R.; Khurana, H.; Gautam, S.; et al. An association study of dopaminergic (DRD2) and serotoninergic (5-HT2) gene polymorphism and schizophrenia in a North Indian population. Asian J. Psychiatry 2019, 39, 178–184. [Google Scholar] [CrossRef]
- Ni, J.; Lu, W.; Wu, Z.; Chen, J.; Yi, Z.; Zhang, C. T102C polymorphism of serotonin 2 A type receptor gene confers susceptibility to (early onset) schizophrenia in Han Chinese: An association study and meta-analysis. Asia Pac. Psychiatry 2013, 5, 24–30. [Google Scholar] [CrossRef]
- Marsden, C.A. Dopamine: The rewarding years. Br. J. Pharmacol. 2006, 147, S136–S144. [Google Scholar] [CrossRef] [PubMed]
- Missale, C.; Nash, S.R.; Robinson, S.W.; Jaber, M.; Caron, M.G. Dopamine receptors: From structure to function. Physiol. Rev. 1998, 78, 189–225. [Google Scholar] [CrossRef] [PubMed]
- Noble, E.P. The DRD2 gene in psychiatric and neurological disorders and its phenotypes. Pharmacogenomics 2000, 1, 309–333. [Google Scholar] [CrossRef]
- He, M.; Yan, H.; Duan, Z.X.; Qu, W.; Gong, H.Y.; Fan, Z.L.; Kang, J.Y.; Li, B.C.; Wang, J.M. Genetic distribution and association analysis of DRD2 gene polymorphisms with major depressive disorder in the Chinese Han population. Int. J. Clin. Exp. Pathol. 2013, 6, 1142–1149. [Google Scholar]
- Della Torre, O.H.; Paes, L.A.; Henriques, T.B.; De Mello, M.P.; Celeri, E.H.R.V.; Dalgalarrondo, P.; Guerra-Junior, G.; Dos Santos-Junior, A. Dopamine D2 receptor gene polymorphisms and externalizing behaviors in children and adolescents. BMC Med. Genet. 2018, 19, 65. [Google Scholar] [CrossRef] [PubMed]
- Bershad, A.K.; Weafer, J.J.; Kirkpatrick, M.G.; Wardle, M.C.; Miller, M.A.; De Wit, H. Oxytocin receptor gene variation predicts subjective response to MDMA. Soc. Neurosci. 2016, 11, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Vizeli, P.; Liechti, M.E. Oxytocin receptor gene variations and socio-emotional effects of MDMA: A pooled analysis of controlled studies in healthy subjects. PLoS ONE 2018, 13, e0199384. [Google Scholar] [CrossRef]
- Newton, T.F. A perhaps unexpected role of noreipenephrine in actions of MDMA. Clin. Pharmacol. Ther. 2011, 90, 215–216. [Google Scholar] [CrossRef] [PubMed]
- Vizeli, P.; Meyer Zu Schwabedissen, H.E.; Liechti, M.E. No major role of norepinephrine transporter gene variations in the cardiostimulant effects of MDMA. Eur. J. Pharmacol. 2018, 74, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-K.; Hwang, J.-A.; Lee, H.-J.; Yoon, H.-K.; Ko, Y.-H.; Lee, B.-H.; Jung, H.-Y.; Hahn, S.-W.; Na, K.-S. Association between norepinephrine transporter gene (SLC6A2) polymorphisms and suicide in patients with major depressive disorder. J. Affect. Disord. 2014, 158, 127–132. [Google Scholar] [CrossRef]
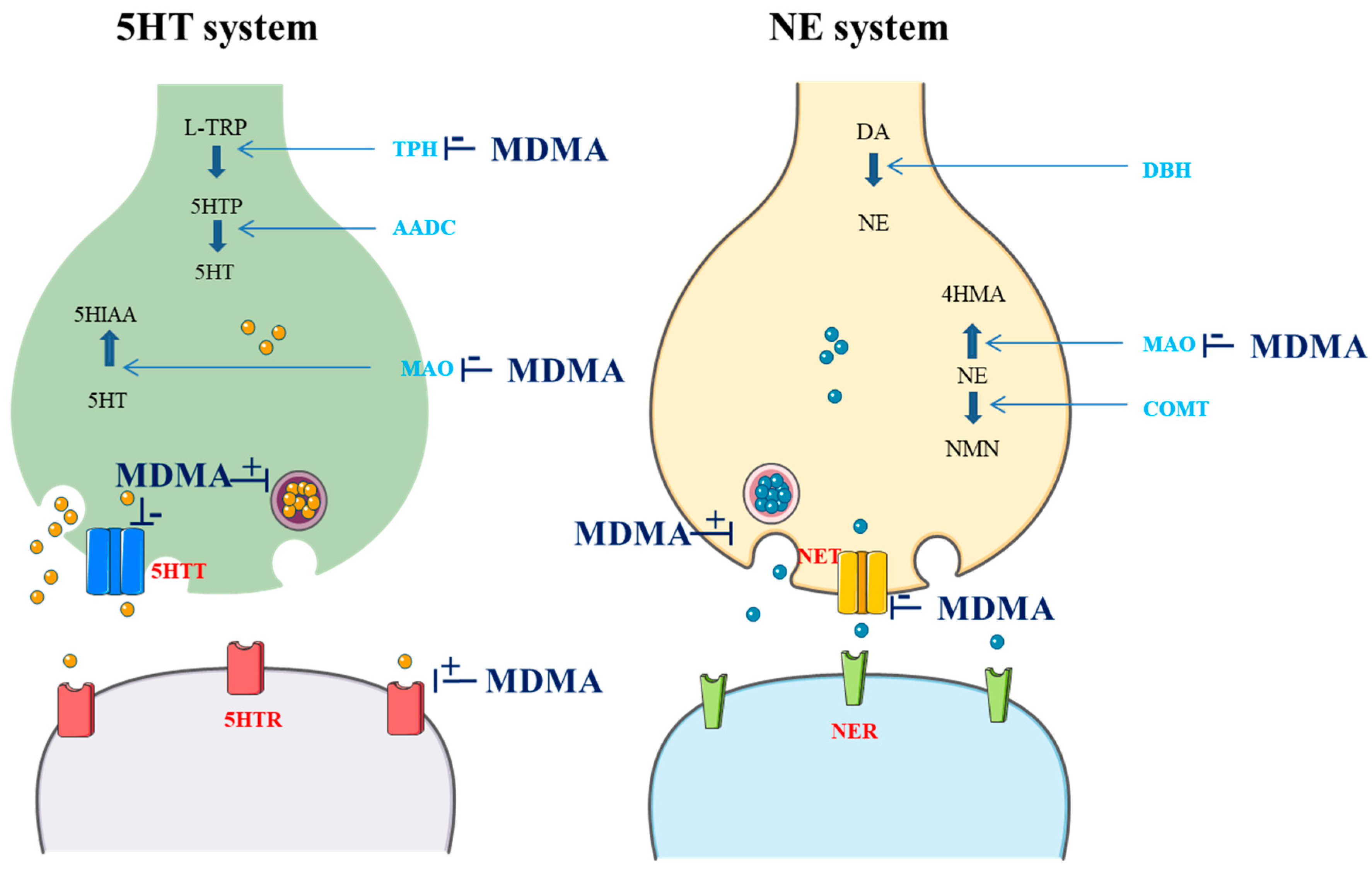
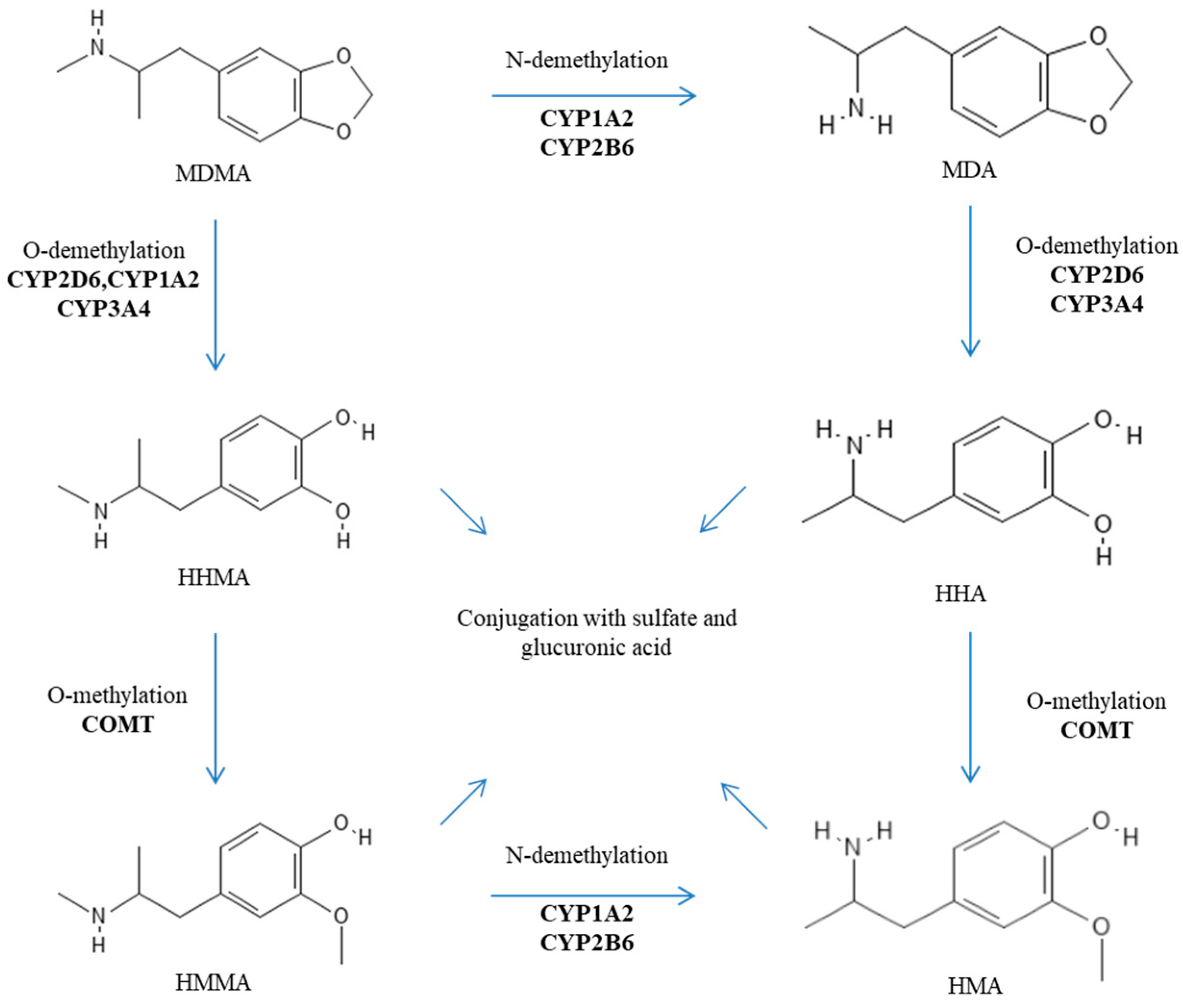
| Gene | Polymorphisms (rsId) | Minor Allele Frequency (dbSNP) | Enzyme Activity | Studies | Sample Size | Results/Outcomes | References |
|---|---|---|---|---|---|---|---|
| CYP2B6 | rs3745274 | T = 0.255351/35,719 (ALFA) | Low activity | Vizeli et al. (2017) | 142 | Altered metabolism of MDMA | [46] |
| CYP2C19 | rs4244285 | A = 0.149748/38,985 (ALFA) | Altered metabolism of MDMA Enhanced cardiovascular response | ||||
| rs28399504 | G = 0.00319/863 (ALFA) | ||||||
| CYP1A2 | rs762551 | C = 0.318648/21,670 (ALFA) | High activity | Altered metabolism of MDMA | |||
| CYP2D6 | Not reported | N/A | Low activity | Wolff et al. (2012) | 48 | Increased production of cortisol | [44] |
| Aitchison et al. (2012) | 48 | Increased risk of hyponatremia | [42] | ||||
| De la Torre (2005) | 10 | Altered metabolism of MDMA | [40] | ||||
| Schmid et al. (2016) | 139 | Increased blood pressure Altered metabolism of MDMA | [41] | ||||
| High activity | Cuyas et al. (2011) | 60 | Altered metabolism of MDMA Altered cognitive effects | [43] | |||
| COMT | rs4680 | A = 0.489085/138,095 (ALFA) | Low activity (met/*) | Cuyas et al. (2011) | 60 | Altered metabolism of MDMA Altered cognitive effects | [43] |
| Wolff et al. (2012) | 48 | Increased production of cortisol | [44] | ||||
| Aitchison et al. (2012) | 48 | Increased risk of hyponatremia | [42] | ||||
| Pardo-Lozano et al. (2012) | 27 | Increased cardiovascular effects | [39] | ||||
| rs165599 | G = 0.335031/76,442 (ALFA) | Low activity? | Fagundo et al. (2010) | 30 | Impaired language performances | [49] |
| Gene | Genotypes | Studies | Sample Sizes | Results/Outcomes | References |
|---|---|---|---|---|---|
| 5HTTLPR | s/s | Roiser et al. (2005) | 66 | Emotional disturbances | [55] |
| Martin-Santos et al. (2009) | 37 | Mood disorders (comorbid primary mood disorder) | [56] | ||
| Fagundo et al. (2010) | 30 | Impaired cognitive performance (verbal fluency) | [49] | ||
| Cuyas et al. (2011) | 60 | Impaired cognitive performance (visuospatial attention and memory) | [43] | ||
| Pardo-Lozano et al. (2012) | 27 | Emotional disturbances | [39] | ||
| l/l | Kuypers et al. (2018) | 63 | Reduction in self-rated depressive feelings | [57] | |
| l/* | Pardo-Lozano et al. (2012) | 27 | Cardiovascular effects | [39] |
| Genes | Polymorphisms (rsId) | Minor Allele Frequency (dbSNP) | Studies | Sample Size | Results/Outcomes | References |
|---|---|---|---|---|---|---|
| TPH1 | rs1800532 | T = 0.364661/23,040 (ALFA) | Vizeli et al. (2019) | 125 | No significant impact | [25] |
| rs1799913 | T = 0.369055/34,238 (ALFA) | |||||
| TPH2 | rs7305115 | T = 0./0 (ALFA) | ||||
| 5HTR1A | rs6295 | G = 0.479109/8646 (ALFA) | ||||
| 5HTRIB | rs6296 | G = 0.264684/13,303 (ALFA) | ||||
| 5HTR2B | rs6313 | A = 0.420112/145,567 (ALFA) | ||||
| DAT1 | rs28363170 | Not reported | Vizeli et al. (2019) | 149 | [24] | |
| rs3836790 | ||||||
| rs6347 | C = 0.272059/35,631 (ALFA) | |||||
| rs11133767 | T = 0.324161/26,010 (ALFA) | |||||
| rs11564774 | G = 0.230439/4353 (ALFA) | |||||
| rs460000 | C = 0./0 (ALFA) | |||||
| rs463379 | C = 0.151071/2073 (ALFA) | |||||
| DRD2/ANKK1 | rs1800497 | A = 0.204778/41,712 (ALFA) | ||||
| DRD2 | rs6277 | A = 0.48466/33,521 (ALFA) | ||||
| rs107959 | Not reported | |||||
| DRD4 | rs1805186 | Not reported | ||||
| OXTR | rs53576 | A = 0.326459/24,474 (ALFA) | Bershad et al. (2016) | 68 | [72] | |
| Vizeli et al. (2018) | 123 | [73] | ||||
| rs1042778 | C = 0./0 (ALFA) | Greater feelings of trust | ||||
| rs2254298 | A = 0.139783/6522 (ALFA) | No significant impact | ||||
| SLC6A2 | rs168924 | G = 0.147036/22,375 (ALFA) | Vizeli et al. (2018) | 124 | [75] | |
| rs47958 | A = 0.433445/15,578 (ALFA) | |||||
| rs1861647 | A = 0.205928/2668 (ALFA) | Increased cardiovascular response | ||||
| rs2242446 | C = 0.283916/83,373 (ALFA) | |||||
| rs36029 | G = 0.381354/8267 (ALFA) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drevin, G.; Pena-Martin, M.; Bauduin, A.; Baudriller, A.; Briet, M.; Abbara, C. Pharmacogenomics of 3,4-Methylenedioxymethamphetamine (MDMA): A Narrative Review of the Literature. Pharmaceutics 2024, 16, 1091. https://doi.org/10.3390/pharmaceutics16081091
Drevin G, Pena-Martin M, Bauduin A, Baudriller A, Briet M, Abbara C. Pharmacogenomics of 3,4-Methylenedioxymethamphetamine (MDMA): A Narrative Review of the Literature. Pharmaceutics. 2024; 16(8):1091. https://doi.org/10.3390/pharmaceutics16081091
Chicago/Turabian StyleDrevin, Guillaume, Maria Pena-Martin, Aurélien Bauduin, Antoine Baudriller, Marie Briet, and Chadi Abbara. 2024. "Pharmacogenomics of 3,4-Methylenedioxymethamphetamine (MDMA): A Narrative Review of the Literature" Pharmaceutics 16, no. 8: 1091. https://doi.org/10.3390/pharmaceutics16081091
APA StyleDrevin, G., Pena-Martin, M., Bauduin, A., Baudriller, A., Briet, M., & Abbara, C. (2024). Pharmacogenomics of 3,4-Methylenedioxymethamphetamine (MDMA): A Narrative Review of the Literature. Pharmaceutics, 16(8), 1091. https://doi.org/10.3390/pharmaceutics16081091






