Long-Term Stability of Glycopyrrolate Oral Solution Galenic Compound at Different Storage Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Galenic Compound
2.3. Working Solutions
2.4. Method Development
2.5. Method Validation
2.6. Stability Study by HPLC-UV
2.7. Identification of Degradation Products by Liquid Chromatography Coupled with Mass Spectrometry (LC-MS) Analysis
2.8. Statistical Analysis
3. Results
3.1. Method Validation
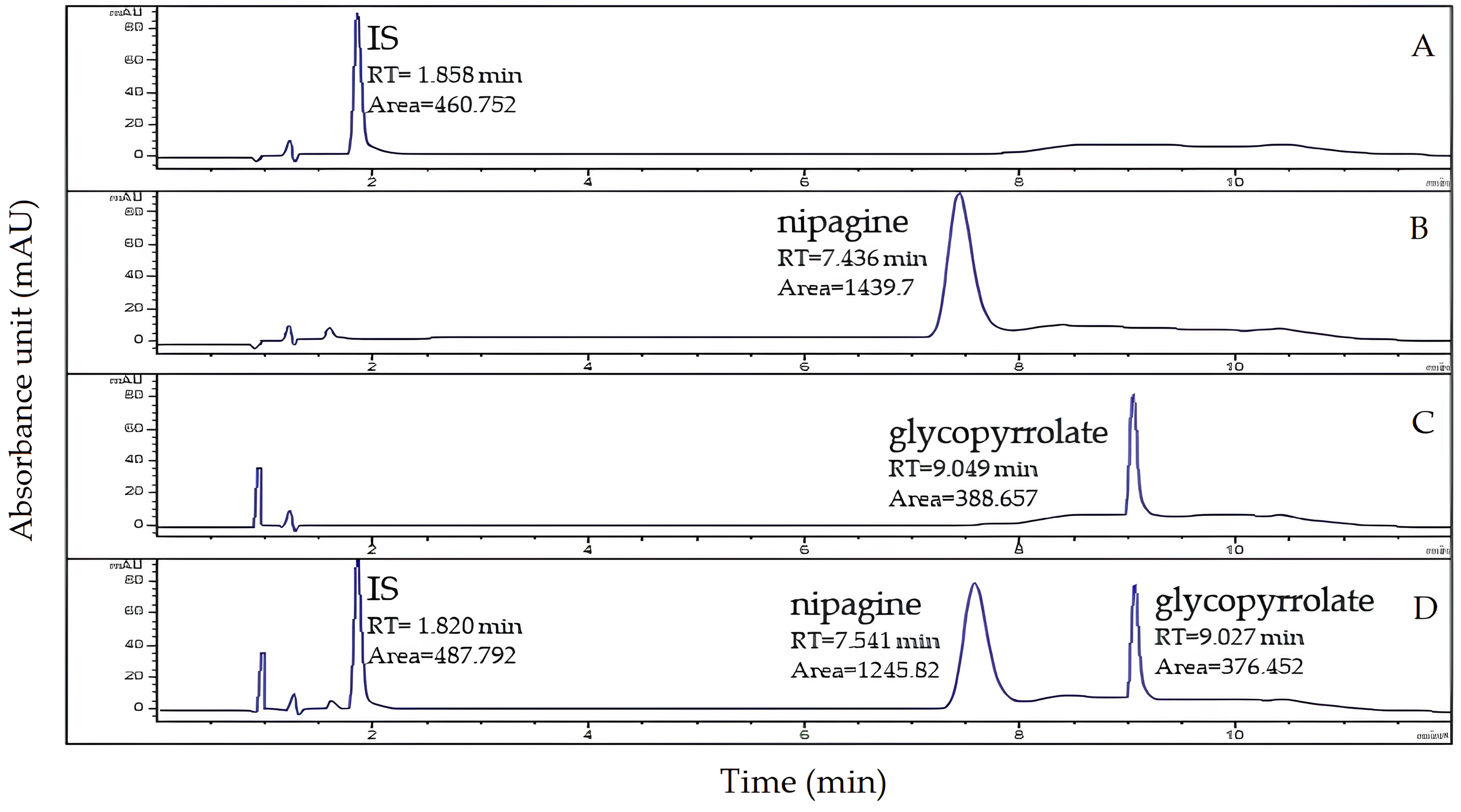
3.1.1. Specificity
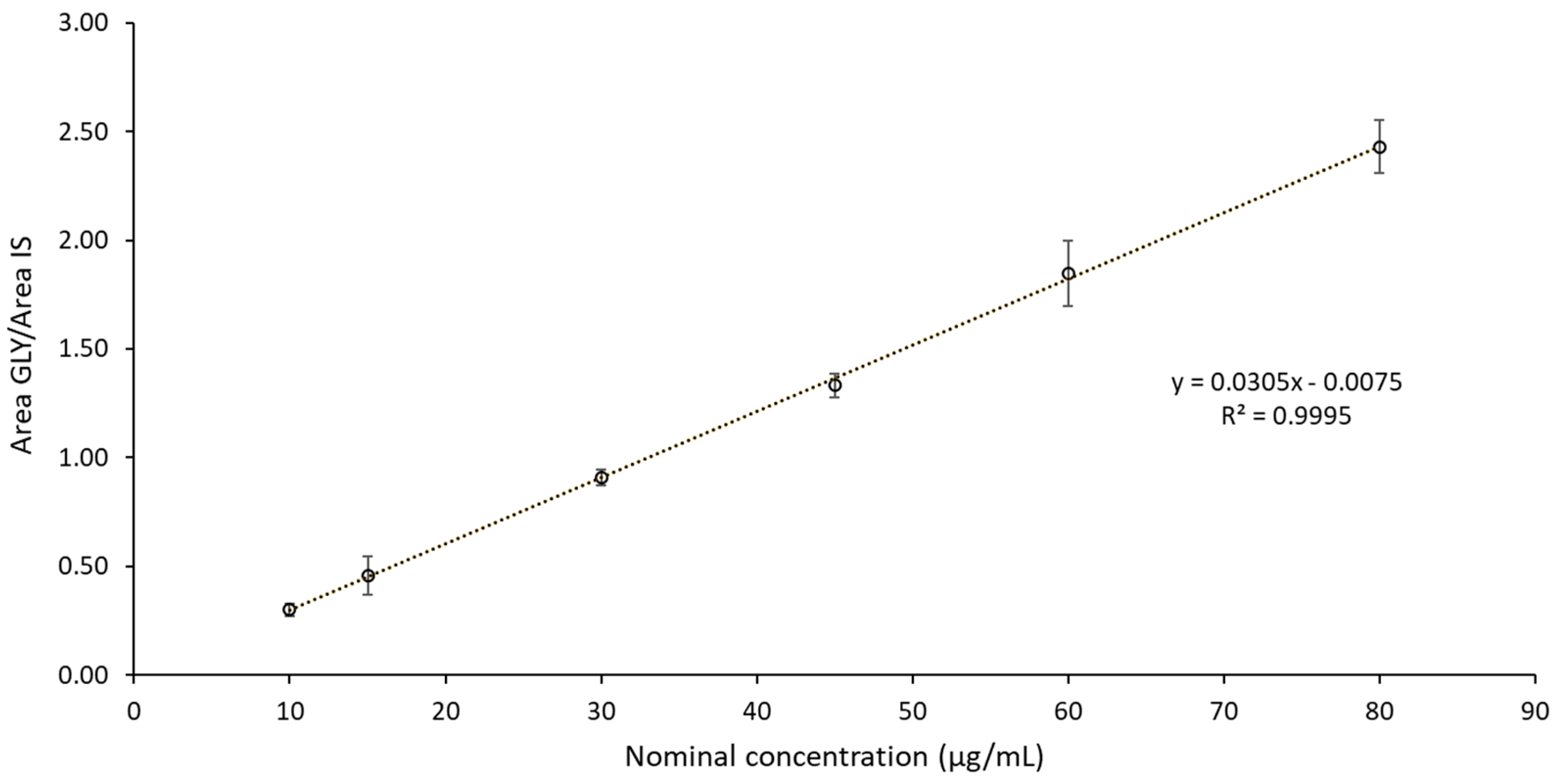
3.1.2. Linearity
3.1.3. Sensitivity
3.1.4. Accuracy and Precision
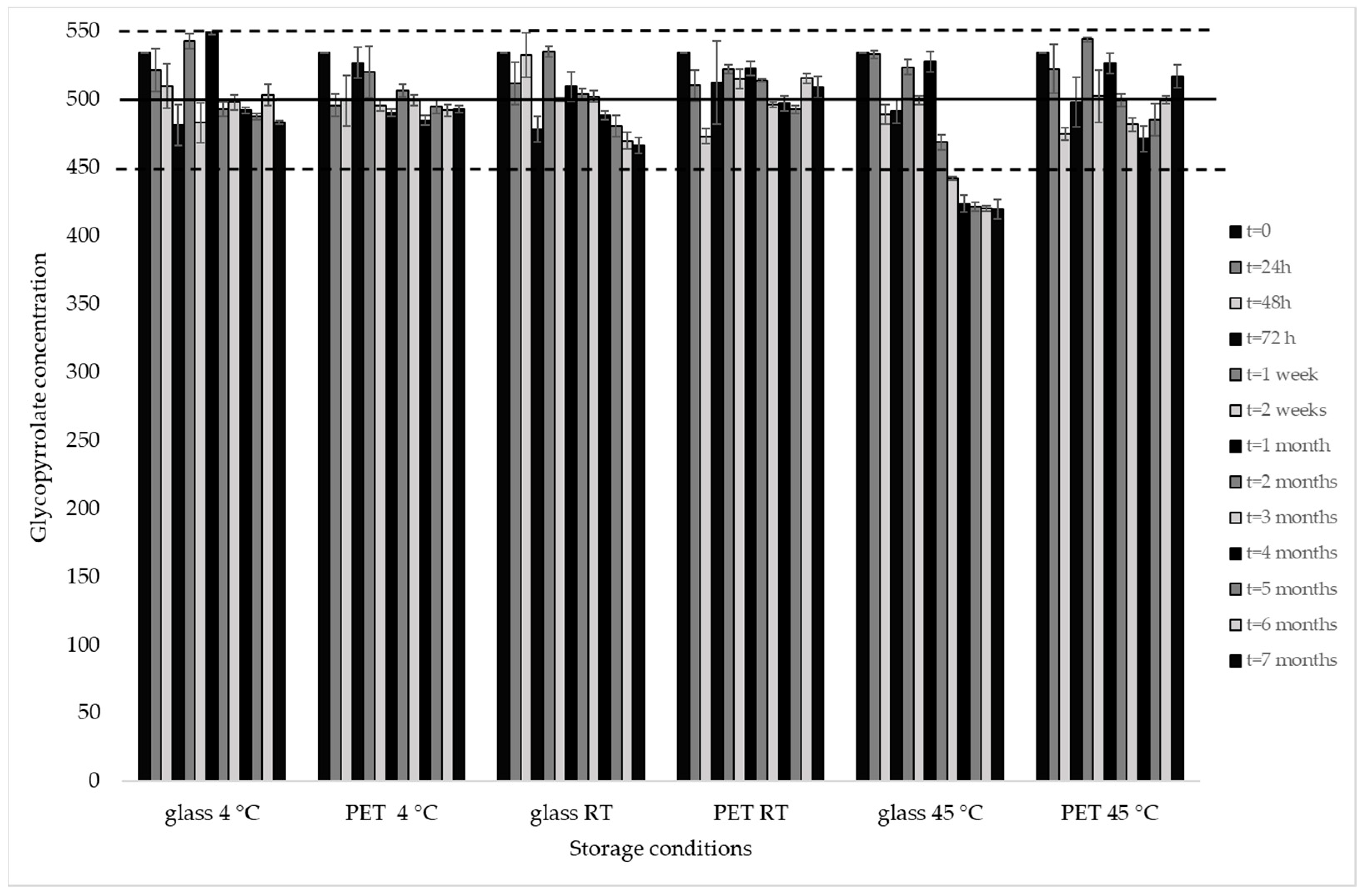
3.2. Stability Assessment
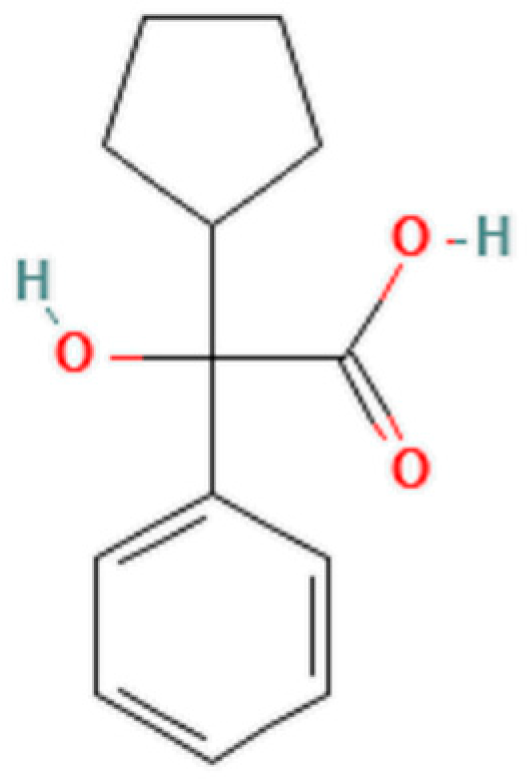
3.3. LC-MS Analysis of Degraded Compound
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- DrugBank Online. Available online: https://go.drugbank.com/drugs/DB00986 (accessed on 10 May 2024).
- Mirakhur, R.K.; Dundee, J.W. Glycopyrrolate: Pharmacology and clinical use. Anaesthesia 1983, 38, 1195–1204. [Google Scholar] [CrossRef] [PubMed]
- Chabicovsky, M.; Winkler, S.; Soeberdt, M.; Kilic, A.; Masur, C.; Abels, C. Pharmacology, toxicology and clinical safety of glycopyrrolate. Toxicol. Appl. Pharmacol. 2019, 370, 154–169. [Google Scholar] [CrossRef] [PubMed]
- Bradford, C.V.; Parman, A.M.; Johnson, P.N.; Miller, J.L. Pharmacologic Management of Sialorrhea in Neonatal and Pediatric Patients. J. Pediatr. Pharmacol. Ther. 2024, 29, 6–21. [Google Scholar] [CrossRef]
- Parr, J.R.; Weldon, E.; Pennington, L.; Steen, N.; Williams, J.; Fairhurst, C.; O’hare, A.; Lodh, R.; Colver, A. The drooling reduction intervention trial (DRI): A single blind trial comparing the efficacy of glycopyrronium and hyoscine on drooling in children with neurodisability. Trials 2014, 15, 60. [Google Scholar] [CrossRef]
- Zanon, D.; Tumminelli, C.; Galimberti, A.M.C.; Torelli, L.; Maestro, A.; Barbi, E.; Maximova, N. Compounded glycopyrrolate is a compelling choice for drooling children: Five years of facility experience. Ital. J. Pediatr. 2021, 47, 222. [Google Scholar] [CrossRef]
- Gupta, V.D. Stability of oral liquid dosage forms of glycopyrrolate prepared with the use of powder. Int. J. Pharm. Compd. 2003, 7, 386–388. [Google Scholar]
- Flerlage, J.; Engorn, B. The Harriet Lane Handbook: A Manual for Pediatric House Officers; Mosby: Agder County, Norway, 2015. [Google Scholar]
- EMA, ICH Harmonised Tripartite Guideline. Validation of Analytical Procedures Q2(R2); International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use: Geneva, Switzerland, 2022; Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-guideline-q2r2-validation-analytical-procedures-step-2b_en.pdf (accessed on 19 July 2024).
- European Directorate for the Quality of Medicines and HealthCare. European Pharmacopoeia, 10th ed.; Council of Europe: Geneva, Switzerland, 2024. [Google Scholar]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.-M.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed minimum reporting standards for chemical analysis. Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef]
- Teale, P.; Lowes, S. Electrospray LC/MS Analysis of Glycopyrrolate in Equine Sport. Micromass Application Note 213. Available online: https://gimitec.com/file/an213.pdf (accessed on 19 June 2024).
- Santus, P.; Radovanovic, D.; Cristiano, A.; Valenti, V.; Rizzi, M. Role of nebulized glycopyrrolate in the treatment of chronic obstructive pulmonary disease. Drug Des. Dev. Ther. 2017, 11, 3257–3271. [Google Scholar] [CrossRef] [PubMed]
- Chawla, R.K.; Rao, G.S.N.K.; Kulandaivelu, U.; Panda, S.P.; Alavala, R.R. A Selective and Sensitive Method Development and Validation of 1,1-Dimethyl-3-Hydroxy-Pyrrolidinium Bromide Impurity in Glycopyrrolate Oral Solution by Liquid Chromatography–Tandem Mass Spectroscopy. J. Chromatogr. Sci. 2021, 59, 566–575. [Google Scholar] [CrossRef] [PubMed]

| Standard | Nominal Concentration (µg/mL) | First Analytical Session | Second Analytical Session | Third Analytical Session | Inter-Day ACC (%) | |||
|---|---|---|---|---|---|---|---|---|
| Calculated Concentration (µg/mL) | Intra-Day ACC (%) | Calculated Concentration (µg/mL) | Intra-Day ACC (%) | Calculated Concentration (µg/mL) | Intra-Day ACC (%) | |||
| CAL1 | 10 | 8.88 | 88.84 | 10.11 | 101.05 | 11.15 | 111.46 | 100.45 |
| CAL2 | 15 | 16.47 | 109.83 | 14.54 | 96.95 | 14.75 | 98.36 | 101.71 |
| CAL3 | 30 | 28.95 | 96.50 | 30.63 | 102.12 | 30.31 | 101.03 | 99.88 |
| CAL4 | 45 | 44.46 | 98.80 | 45.81 | 101.79 | 41.94 | 93.20 | 97.93 |
| CAL5 | 60 | 62.27 | 103.78 | 58.63 | 97.71 | 61.48 | 102.46 | 101.32 |
| CAL6 | 80 | 78.63 | 98.29 | 80.69 | 100.86 | 80.46 | 100.58 | 99.91 |
| Standard | Nominal Concentration (µg/mL) | First Analytical Session | Second Analytical Session | Third Analytical Session | Inter-Day ACC (%) | Inter-Day CV (%) | |||
|---|---|---|---|---|---|---|---|---|---|
| Intra-Day ACC (%) | Intra-Day CV (%) | Intra-Day ACC (%) | Intra-Day CV (%) | Intra-Day ACC (%) | Intra-Day CV (%) | ||||
| LQC | 12.5 | 98.59 | 3.96 | 101.25 | 1.41 | 98.89 | 4.62 | 99.58 | 3.33 |
| MQC | 22.5 | 98.94 | 7.22 | 96.17 | 3.13 | 94.59 | 4.27 | 96.57 | 4.87 |
| HQC | 35 | 95.79 | 1.13 | 92.09 | 2.72 | 92.81 | 4.94 | 93.56 | 2.93 |
| Glass 4 °C | PET 4 °C | Glass RT | PET RT | Glass 45 °C | PET 45 °C | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time | Mean ± SE (μg/mL) | ACC (%) | Mean ± SE (μg/mL) | ACC (%) | Mean ± SE (μg/mL) | ACC (%) | Mean ± SE (μg/mL) | ACC (%) | Mean ± SE (μg/mL) | ACC (%) | Mean ± SE (μg/mL) | ACC (%) |
| 0 | 534.44 ± 0.22 | 106.89 | 534.44 ± 0.22 | 106.89 | 534.44 ± 0.22 | 106.89 | 534.44 ± 0.22 | 106.89 | 534.44 ± 0.22 | 106.89 | 534.44 ± 0.22 | 106.89 |
| 24 h | 521.55 ± 15.44 | 104.31 | 495.90 ± 8.34 | 99.18 | 511.62 ± 15.57 | 102.32 | 510.21 ± 11.23 | 102.04 | 533.15 ± 2.93 | 106.63 | 522.45 ± 17.79 | 104.49 |
| 48 h | 510.14 ± 16.21 | 102.03 | 499.36 ± 18.46 | 99.87 | 532.79 ± 16.32 | 106.56 | 472.93 ± 5.47 | 94.59 | 489.08 ± 7.34 | 97.82 | 474.57 ± 4.49 | 94.91 |
| 72 h | 481.27 ± 14.85 | 96.25 | 527.04 ± 11.13 | 105.41 | 478.11 ± 9.41 | 95.62 | 512.36 ± 30.61 | 102.47 | 491.57 ± 8.70 | 98.31 | 498.20 ± 18.19 | 99.64 |
| 1 w | 542.82 ± 5.47 | 108.56 | 520.26 ± 18.92 | 104.05 | 535.46 ± 3.81 | 107.09 | 522.31 ± 3.12 | 104.46 | 523.77 ± 5.70 | 104.75 | 544.25 ± 1.59 | 108.85 |
| 2 w | 482.96 ± 14.52 | 96.59 | 495.74 ± 4.13 | 99.15 | 499.95 ± 1.44 | 99.99 | 515.07 ± 7.09 | 103.01 | 499.49 ± 3.30 | 99.90 | 502.41 ± 19.46 | 100.48 |
| 1 m | 549.93 ± 2.33 | 109.99 | 490.14 ± 2.58 | 98.03 | 509.59 ± 10.81 | 101.92 | 523.13 ± 5.21 | 104.63 | 527.81 ± 7.58 | 105.56 | 526.58 ± 7.62 | 105.32 |
| 2 m | 493.20 ± 5.10 | 98.64 | 506.61 ± 4.60 | 101.32 | 503.96 ± 4.13 | 100.79 | 513.96 ± 1.15 | 102.79 | 468.86 ± 5.55 | 93.77 | 499.53 ± 4.62 | 99.91 |
| 3 m | 498.00 ± 5.36 | 99.60 | 499.40 ± 3.94 | 99.88 | 502.24 ± 4.28 | 100.45 | 496.21 ± 2.11 | 99.24 | 442.56 ± 1.29 | 88.51 | 481.68 ± 4.79 | 96.34 |
| 4 m | 492.21 ± 2.35 | 98.44 | 484.68 ± 3.71 | 96.94 | 488.47 ± 2.98 | 97.69 | 497.41 ± 5.58 | 99.48 | 423.64 ± 6.28 | 84.73 | 471.23 ± 9.59 | 94.25 |
| 5 m | 487.48 ± 2.25 | 97.50 | 494.97 ± 5.46 | 98.99 | 480.44 ± 7.87 | 96.09 | 492.89 ± 2.86 | 98.58 | 421.57 ± 3.39 | 84.31 | 485.18 ± 11.77 | 97.04 |
| 6 m | 503.53 ± 7.84 | 100.71 | 492.02 ± 4.12 | 98.40 | 469.81 ± 6.35 | 93.96 | 515.66 ± 3.51 | 103.13 | 420.27 ± 1.97 | 84.05 | 499.67 ± 2.98 | 99.93 |
| 7 m | 483.09 ± 1.24 | 96.62 | 493.05 ± 2.66 | 98.61 | 466.2 ± 5.74 | 93.26 | 509.0 ± 7.79 | 101.82 | 419.4 ± 7.31 | 83.90 | 516.9 ± 8.70 | 103.39 |
| Compound | Molecular Formula | Retention Time (min) | Identified Ion Mass (m/z) |
|---|---|---|---|
| Glycopyrrolate | C19H28NO3 | 4.738 | [M+H]+ 318.2062 |
| Metabolite M9 α-cyclopentylmandelic acid | C13H16O3 | 10.10 | [M+H-H2O]+ 203.1066 |
| Compound | Median Area ± SD (Glass 45 °C = Sample) | Median Area ± SD (Glass 4 °C = Control) | Ratio Sample/Control | p-Value |
|---|---|---|---|---|
| Glycopyrrolate | 6.86 × 107 ± 2.75 × 106 | 8.93 × 107 ± 2.23 × 106 | 0.768 | 7.0 × 10−6 |
| Metabolite M9 | 1.50 × 106 ± 1.08 × 105 | 1.78 × 106 ± 4.81 × 104 | 1.187 | 1.1 × 10−2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bellich, B.; Franzin, M.; Curci, D.; Cirino, M.; Maestro, A.; Bennati, G.; Stocco, G.; Adami, G.; Maximova, N.; Grasso, D.L.; et al. Long-Term Stability of Glycopyrrolate Oral Solution Galenic Compound at Different Storage Conditions. Pharmaceutics 2024, 16, 1018. https://doi.org/10.3390/pharmaceutics16081018
Bellich B, Franzin M, Curci D, Cirino M, Maestro A, Bennati G, Stocco G, Adami G, Maximova N, Grasso DL, et al. Long-Term Stability of Glycopyrrolate Oral Solution Galenic Compound at Different Storage Conditions. Pharmaceutics. 2024; 16(8):1018. https://doi.org/10.3390/pharmaceutics16081018
Chicago/Turabian StyleBellich, Barbara, Martina Franzin, Debora Curci, Mario Cirino, Alessandra Maestro, Giada Bennati, Gabriele Stocco, Gianpiero Adami, Natalia Maximova, Domenico Leonardo Grasso, and et al. 2024. "Long-Term Stability of Glycopyrrolate Oral Solution Galenic Compound at Different Storage Conditions" Pharmaceutics 16, no. 8: 1018. https://doi.org/10.3390/pharmaceutics16081018
APA StyleBellich, B., Franzin, M., Curci, D., Cirino, M., Maestro, A., Bennati, G., Stocco, G., Adami, G., Maximova, N., Grasso, D. L., Barbi, E., & Zanon, D. (2024). Long-Term Stability of Glycopyrrolate Oral Solution Galenic Compound at Different Storage Conditions. Pharmaceutics, 16(8), 1018. https://doi.org/10.3390/pharmaceutics16081018






