Long-Term Stability of Glycopyrrolate Oral Solution Galenic Compound at Different Storage Conditions
Abstract
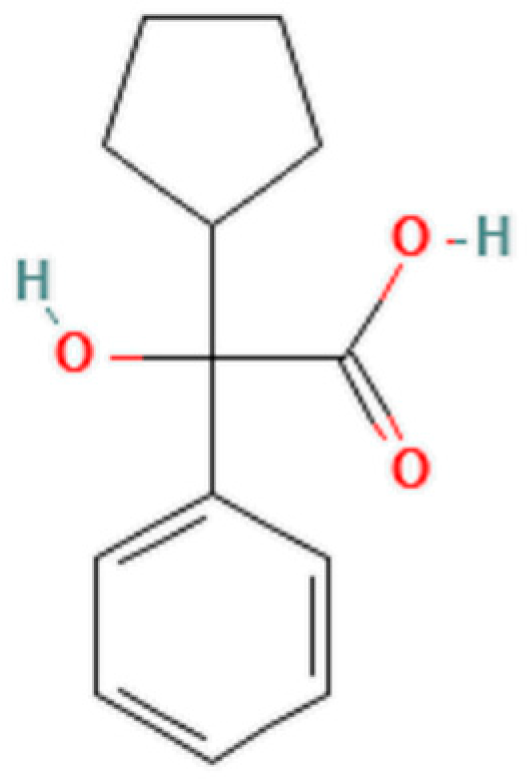
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Galenic Compound
2.3. Working Solutions
2.4. Method Development
2.5. Method Validation
2.6. Stability Study by HPLC-UV
2.7. Identification of Degradation Products by Liquid Chromatography Coupled with Mass Spectrometry (LC-MS) Analysis
2.8. Statistical Analysis
3. Results
3.1. Method Validation
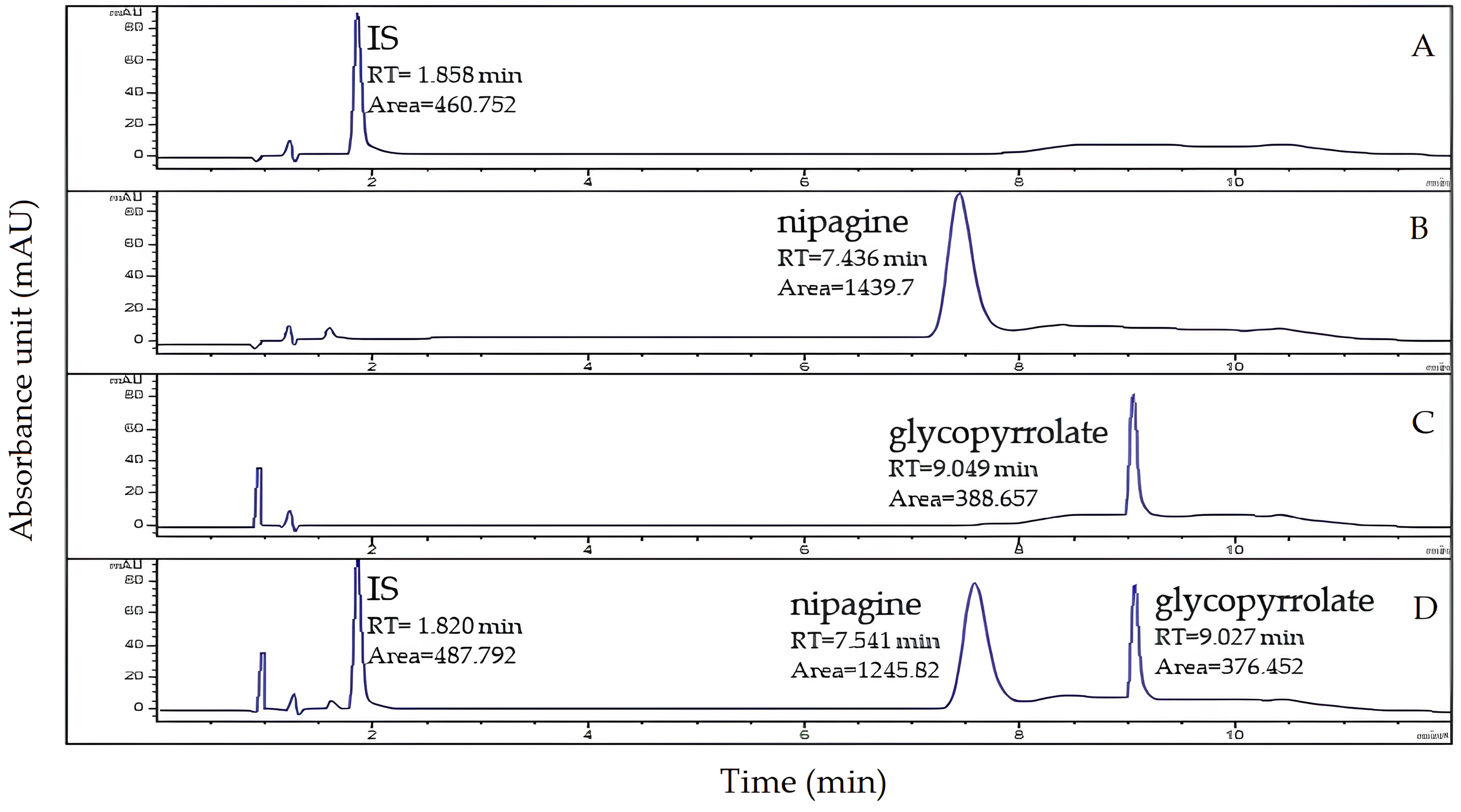
3.1.1. Specificity
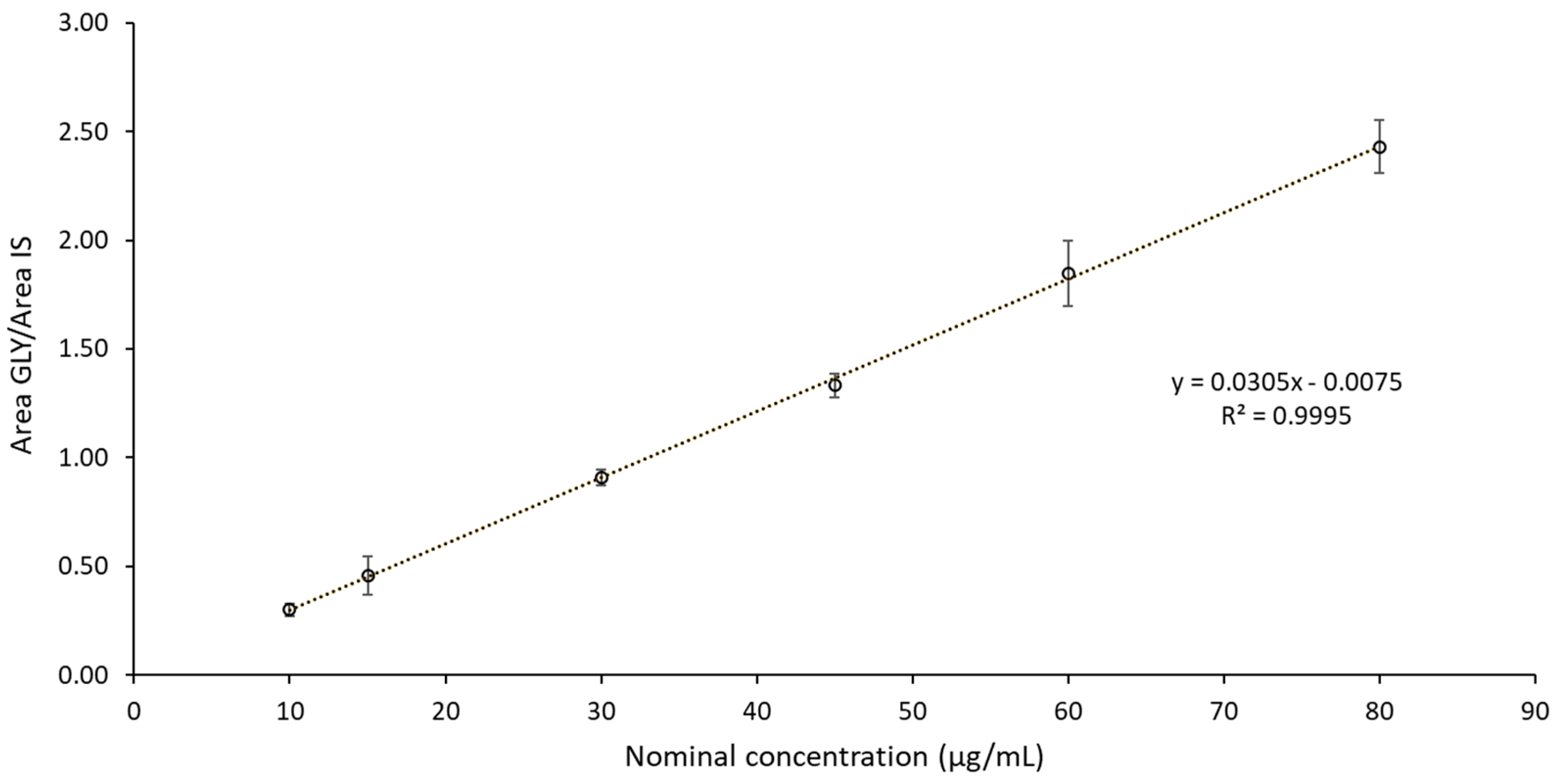
3.1.2. Linearity
3.1.3. Sensitivity
3.1.4. Accuracy and Precision
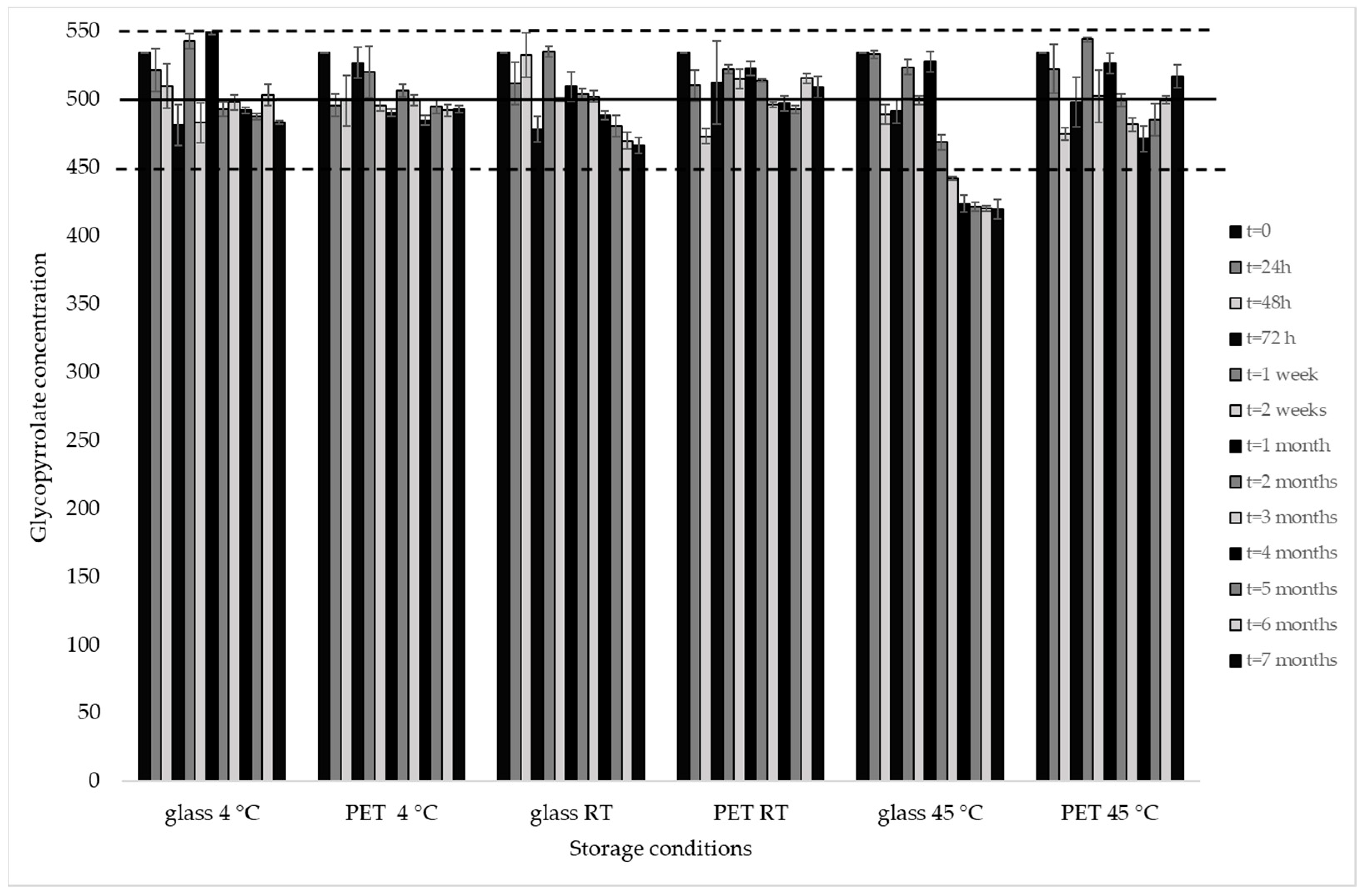
3.2. Stability Assessment
3.3. LC-MS Analysis of Degraded Compound
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- DrugBank Online. Available online: https://go.drugbank.com/drugs/DB00986 (accessed on 10 May 2024).
- Mirakhur, R.K.; Dundee, J.W. Glycopyrrolate: Pharmacology and clinical use. Anaesthesia 1983, 38, 1195–1204. [Google Scholar] [CrossRef] [PubMed]
- Chabicovsky, M.; Winkler, S.; Soeberdt, M.; Kilic, A.; Masur, C.; Abels, C. Pharmacology, toxicology and clinical safety of glycopyrrolate. Toxicol. Appl. Pharmacol. 2019, 370, 154–169. [Google Scholar] [CrossRef] [PubMed]
- Bradford, C.V.; Parman, A.M.; Johnson, P.N.; Miller, J.L. Pharmacologic Management of Sialorrhea in Neonatal and Pediatric Patients. J. Pediatr. Pharmacol. Ther. 2024, 29, 6–21. [Google Scholar] [CrossRef]
- Parr, J.R.; Weldon, E.; Pennington, L.; Steen, N.; Williams, J.; Fairhurst, C.; O’hare, A.; Lodh, R.; Colver, A. The drooling reduction intervention trial (DRI): A single blind trial comparing the efficacy of glycopyrronium and hyoscine on drooling in children with neurodisability. Trials 2014, 15, 60. [Google Scholar] [CrossRef]
- Zanon, D.; Tumminelli, C.; Galimberti, A.M.C.; Torelli, L.; Maestro, A.; Barbi, E.; Maximova, N. Compounded glycopyrrolate is a compelling choice for drooling children: Five years of facility experience. Ital. J. Pediatr. 2021, 47, 222. [Google Scholar] [CrossRef]
- Gupta, V.D. Stability of oral liquid dosage forms of glycopyrrolate prepared with the use of powder. Int. J. Pharm. Compd. 2003, 7, 386–388. [Google Scholar]
- Flerlage, J.; Engorn, B. The Harriet Lane Handbook: A Manual for Pediatric House Officers; Mosby: Agder County, Norway, 2015. [Google Scholar]
- EMA, ICH Harmonised Tripartite Guideline. Validation of Analytical Procedures Q2(R2); International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use: Geneva, Switzerland, 2022; Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-guideline-q2r2-validation-analytical-procedures-step-2b_en.pdf (accessed on 19 July 2024).
- European Directorate for the Quality of Medicines and HealthCare. European Pharmacopoeia, 10th ed.; Council of Europe: Geneva, Switzerland, 2024. [Google Scholar]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.-M.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed minimum reporting standards for chemical analysis. Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef]
- Teale, P.; Lowes, S. Electrospray LC/MS Analysis of Glycopyrrolate in Equine Sport. Micromass Application Note 213. Available online: https://gimitec.com/file/an213.pdf (accessed on 19 June 2024).
- Santus, P.; Radovanovic, D.; Cristiano, A.; Valenti, V.; Rizzi, M. Role of nebulized glycopyrrolate in the treatment of chronic obstructive pulmonary disease. Drug Des. Dev. Ther. 2017, 11, 3257–3271. [Google Scholar] [CrossRef] [PubMed]
- Chawla, R.K.; Rao, G.S.N.K.; Kulandaivelu, U.; Panda, S.P.; Alavala, R.R. A Selective and Sensitive Method Development and Validation of 1,1-Dimethyl-3-Hydroxy-Pyrrolidinium Bromide Impurity in Glycopyrrolate Oral Solution by Liquid Chromatography–Tandem Mass Spectroscopy. J. Chromatogr. Sci. 2021, 59, 566–575. [Google Scholar] [CrossRef] [PubMed]

| Standard | Nominal Concentration (µg/mL) | First Analytical Session | Second Analytical Session | Third Analytical Session | Inter-Day ACC (%) | |||
|---|---|---|---|---|---|---|---|---|
| Calculated Concentration (µg/mL) | Intra-Day ACC (%) | Calculated Concentration (µg/mL) | Intra-Day ACC (%) | Calculated Concentration (µg/mL) | Intra-Day ACC (%) | |||
| CAL1 | 10 | 8.88 | 88.84 | 10.11 | 101.05 | 11.15 | 111.46 | 100.45 |
| CAL2 | 15 | 16.47 | 109.83 | 14.54 | 96.95 | 14.75 | 98.36 | 101.71 |
| CAL3 | 30 | 28.95 | 96.50 | 30.63 | 102.12 | 30.31 | 101.03 | 99.88 |
| CAL4 | 45 | 44.46 | 98.80 | 45.81 | 101.79 | 41.94 | 93.20 | 97.93 |
| CAL5 | 60 | 62.27 | 103.78 | 58.63 | 97.71 | 61.48 | 102.46 | 101.32 |
| CAL6 | 80 | 78.63 | 98.29 | 80.69 | 100.86 | 80.46 | 100.58 | 99.91 |
| Standard | Nominal Concentration (µg/mL) | First Analytical Session | Second Analytical Session | Third Analytical Session | Inter-Day ACC (%) | Inter-Day CV (%) | |||
|---|---|---|---|---|---|---|---|---|---|
| Intra-Day ACC (%) | Intra-Day CV (%) | Intra-Day ACC (%) | Intra-Day CV (%) | Intra-Day ACC (%) | Intra-Day CV (%) | ||||
| LQC | 12.5 | 98.59 | 3.96 | 101.25 | 1.41 | 98.89 | 4.62 | 99.58 | 3.33 |
| MQC | 22.5 | 98.94 | 7.22 | 96.17 | 3.13 | 94.59 | 4.27 | 96.57 | 4.87 |
| HQC | 35 | 95.79 | 1.13 | 92.09 | 2.72 | 92.81 | 4.94 | 93.56 | 2.93 |
| Glass 4 °C | PET 4 °C | Glass RT | PET RT | Glass 45 °C | PET 45 °C | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time | Mean ± SE (μg/mL) | ACC (%) | Mean ± SE (μg/mL) | ACC (%) | Mean ± SE (μg/mL) | ACC (%) | Mean ± SE (μg/mL) | ACC (%) | Mean ± SE (μg/mL) | ACC (%) | Mean ± SE (μg/mL) | ACC (%) |
| 0 | 534.44 ± 0.22 | 106.89 | 534.44 ± 0.22 | 106.89 | 534.44 ± 0.22 | 106.89 | 534.44 ± 0.22 | 106.89 | 534.44 ± 0.22 | 106.89 | 534.44 ± 0.22 | 106.89 |
| 24 h | 521.55 ± 15.44 | 104.31 | 495.90 ± 8.34 | 99.18 | 511.62 ± 15.57 | 102.32 | 510.21 ± 11.23 | 102.04 | 533.15 ± 2.93 | 106.63 | 522.45 ± 17.79 | 104.49 |
| 48 h | 510.14 ± 16.21 | 102.03 | 499.36 ± 18.46 | 99.87 | 532.79 ± 16.32 | 106.56 | 472.93 ± 5.47 | 94.59 | 489.08 ± 7.34 | 97.82 | 474.57 ± 4.49 | 94.91 |
| 72 h | 481.27 ± 14.85 | 96.25 | 527.04 ± 11.13 | 105.41 | 478.11 ± 9.41 | 95.62 | 512.36 ± 30.61 | 102.47 | 491.57 ± 8.70 | 98.31 | 498.20 ± 18.19 | 99.64 |
| 1 w | 542.82 ± 5.47 | 108.56 | 520.26 ± 18.92 | 104.05 | 535.46 ± 3.81 | 107.09 | 522.31 ± 3.12 | 104.46 | 523.77 ± 5.70 | 104.75 | 544.25 ± 1.59 | 108.85 |
| 2 w | 482.96 ± 14.52 | 96.59 | 495.74 ± 4.13 | 99.15 | 499.95 ± 1.44 | 99.99 | 515.07 ± 7.09 | 103.01 | 499.49 ± 3.30 | 99.90 | 502.41 ± 19.46 | 100.48 |
| 1 m | 549.93 ± 2.33 | 109.99 | 490.14 ± 2.58 | 98.03 | 509.59 ± 10.81 | 101.92 | 523.13 ± 5.21 | 104.63 | 527.81 ± 7.58 | 105.56 | 526.58 ± 7.62 | 105.32 |
| 2 m | 493.20 ± 5.10 | 98.64 | 506.61 ± 4.60 | 101.32 | 503.96 ± 4.13 | 100.79 | 513.96 ± 1.15 | 102.79 | 468.86 ± 5.55 | 93.77 | 499.53 ± 4.62 | 99.91 |
| 3 m | 498.00 ± 5.36 | 99.60 | 499.40 ± 3.94 | 99.88 | 502.24 ± 4.28 | 100.45 | 496.21 ± 2.11 | 99.24 | 442.56 ± 1.29 | 88.51 | 481.68 ± 4.79 | 96.34 |
| 4 m | 492.21 ± 2.35 | 98.44 | 484.68 ± 3.71 | 96.94 | 488.47 ± 2.98 | 97.69 | 497.41 ± 5.58 | 99.48 | 423.64 ± 6.28 | 84.73 | 471.23 ± 9.59 | 94.25 |
| 5 m | 487.48 ± 2.25 | 97.50 | 494.97 ± 5.46 | 98.99 | 480.44 ± 7.87 | 96.09 | 492.89 ± 2.86 | 98.58 | 421.57 ± 3.39 | 84.31 | 485.18 ± 11.77 | 97.04 |
| 6 m | 503.53 ± 7.84 | 100.71 | 492.02 ± 4.12 | 98.40 | 469.81 ± 6.35 | 93.96 | 515.66 ± 3.51 | 103.13 | 420.27 ± 1.97 | 84.05 | 499.67 ± 2.98 | 99.93 |
| 7 m | 483.09 ± 1.24 | 96.62 | 493.05 ± 2.66 | 98.61 | 466.2 ± 5.74 | 93.26 | 509.0 ± 7.79 | 101.82 | 419.4 ± 7.31 | 83.90 | 516.9 ± 8.70 | 103.39 |
| Compound | Molecular Formula | Retention Time (min) | Identified Ion Mass (m/z) |
|---|---|---|---|
| Glycopyrrolate | C19H28NO3 | 4.738 | [M+H]+ 318.2062 |
| Metabolite M9 α-cyclopentylmandelic acid | C13H16O3 | 10.10 | [M+H-H2O]+ 203.1066 |
| Compound | Median Area ± SD (Glass 45 °C = Sample) | Median Area ± SD (Glass 4 °C = Control) | Ratio Sample/Control | p-Value |
|---|---|---|---|---|
| Glycopyrrolate | 6.86 × 107 ± 2.75 × 106 | 8.93 × 107 ± 2.23 × 106 | 0.768 | 7.0 × 10−6 |
| Metabolite M9 | 1.50 × 106 ± 1.08 × 105 | 1.78 × 106 ± 4.81 × 104 | 1.187 | 1.1 × 10−2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bellich, B.; Franzin, M.; Curci, D.; Cirino, M.; Maestro, A.; Bennati, G.; Stocco, G.; Adami, G.; Maximova, N.; Grasso, D.L.; et al. Long-Term Stability of Glycopyrrolate Oral Solution Galenic Compound at Different Storage Conditions. Pharmaceutics 2024, 16, 1018. https://doi.org/10.3390/pharmaceutics16081018
Bellich B, Franzin M, Curci D, Cirino M, Maestro A, Bennati G, Stocco G, Adami G, Maximova N, Grasso DL, et al. Long-Term Stability of Glycopyrrolate Oral Solution Galenic Compound at Different Storage Conditions. Pharmaceutics. 2024; 16(8):1018. https://doi.org/10.3390/pharmaceutics16081018
Chicago/Turabian StyleBellich, Barbara, Martina Franzin, Debora Curci, Mario Cirino, Alessandra Maestro, Giada Bennati, Gabriele Stocco, Gianpiero Adami, Natalia Maximova, Domenico Leonardo Grasso, and et al. 2024. "Long-Term Stability of Glycopyrrolate Oral Solution Galenic Compound at Different Storage Conditions" Pharmaceutics 16, no. 8: 1018. https://doi.org/10.3390/pharmaceutics16081018
APA StyleBellich, B., Franzin, M., Curci, D., Cirino, M., Maestro, A., Bennati, G., Stocco, G., Adami, G., Maximova, N., Grasso, D. L., Barbi, E., & Zanon, D. (2024). Long-Term Stability of Glycopyrrolate Oral Solution Galenic Compound at Different Storage Conditions. Pharmaceutics, 16(8), 1018. https://doi.org/10.3390/pharmaceutics16081018






