Porous Silica Nanomaterials as Carriers of Biologically Active Natural Polyphenols: Effect of Structure and Surface Modification
Abstract
1. Introduction
2. Porous Silica Nanomaterials
3. Biomedical Application of Porous Nanomaterials
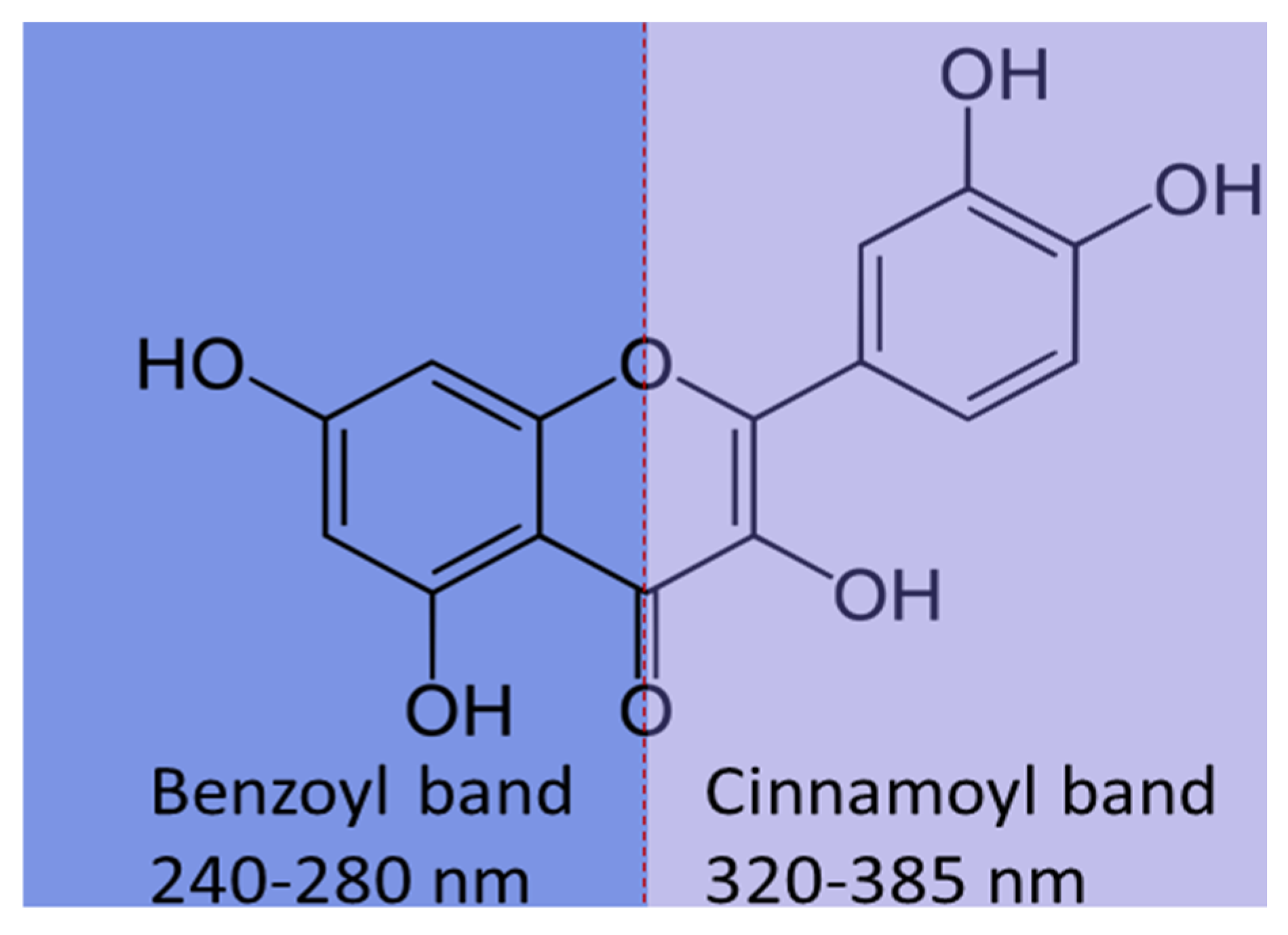
4. Polyphenols and Their Biopharmaceutical Properties
5. Porous Silica Materials with Different Structures as Carriers in Delivery Systems of Polyphenols
6. Functionalized Porous Silica Materials as Carriers in Delivery Systems of Polyphenols
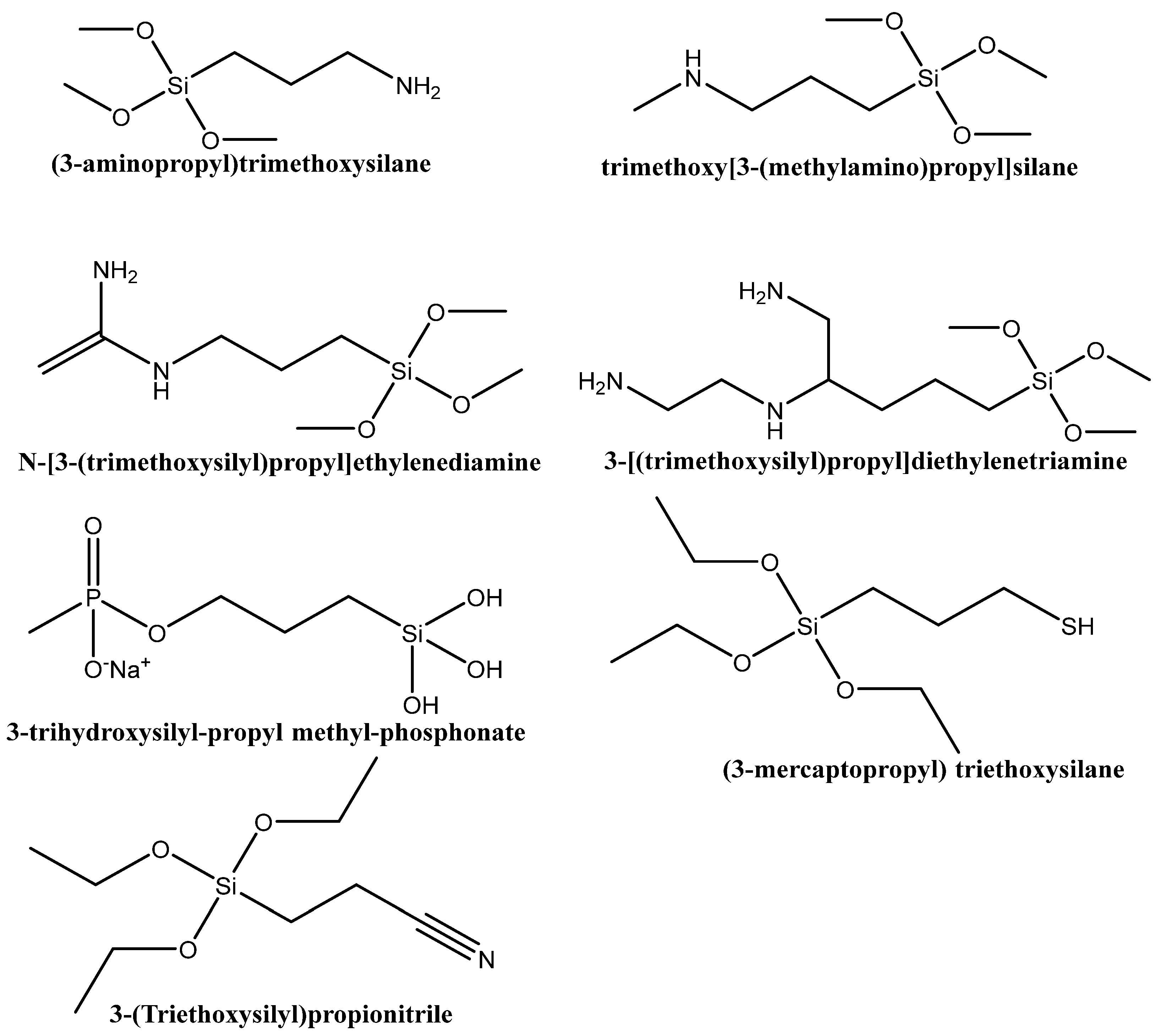
6.1. Functionalization with Organic Groups
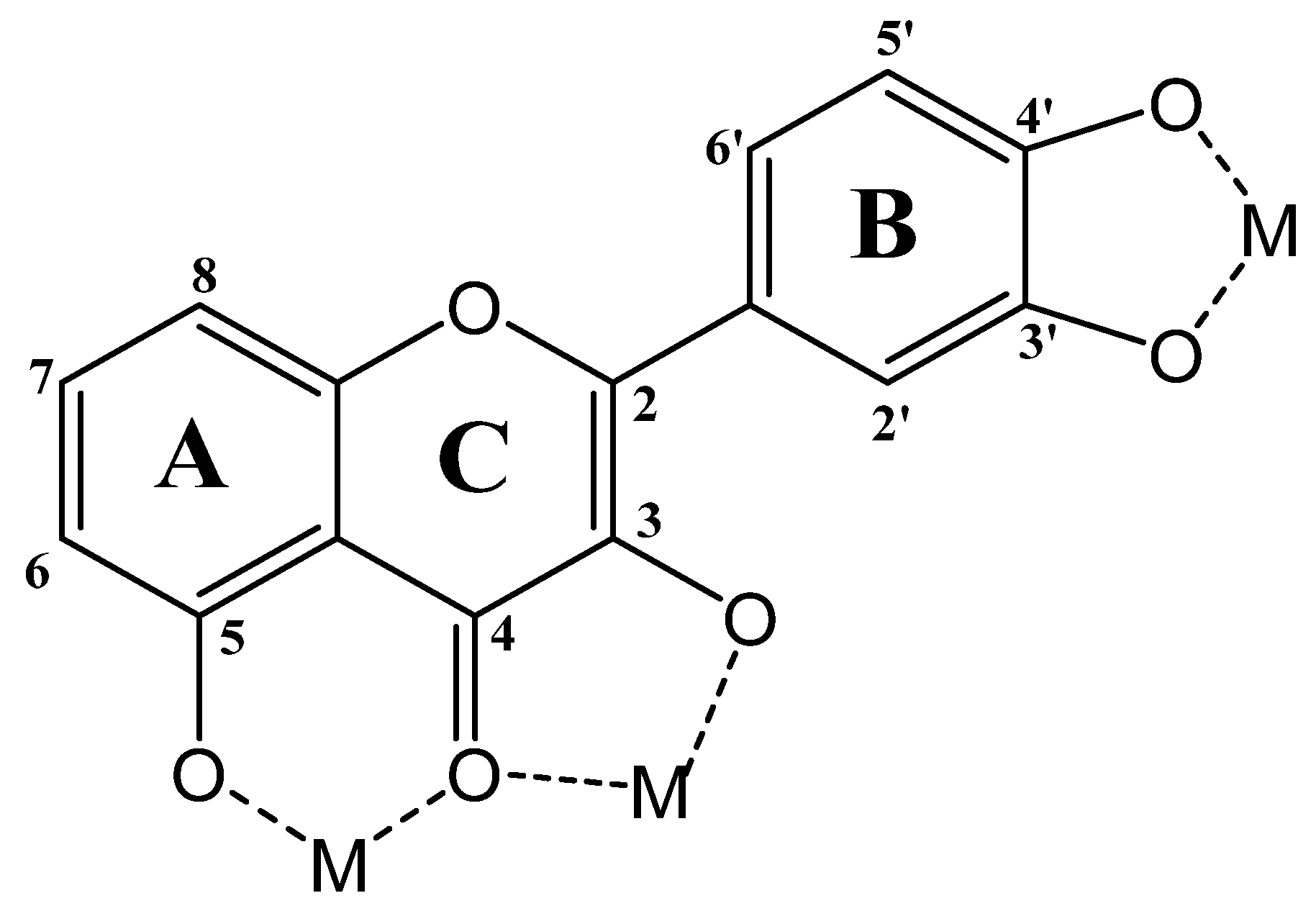
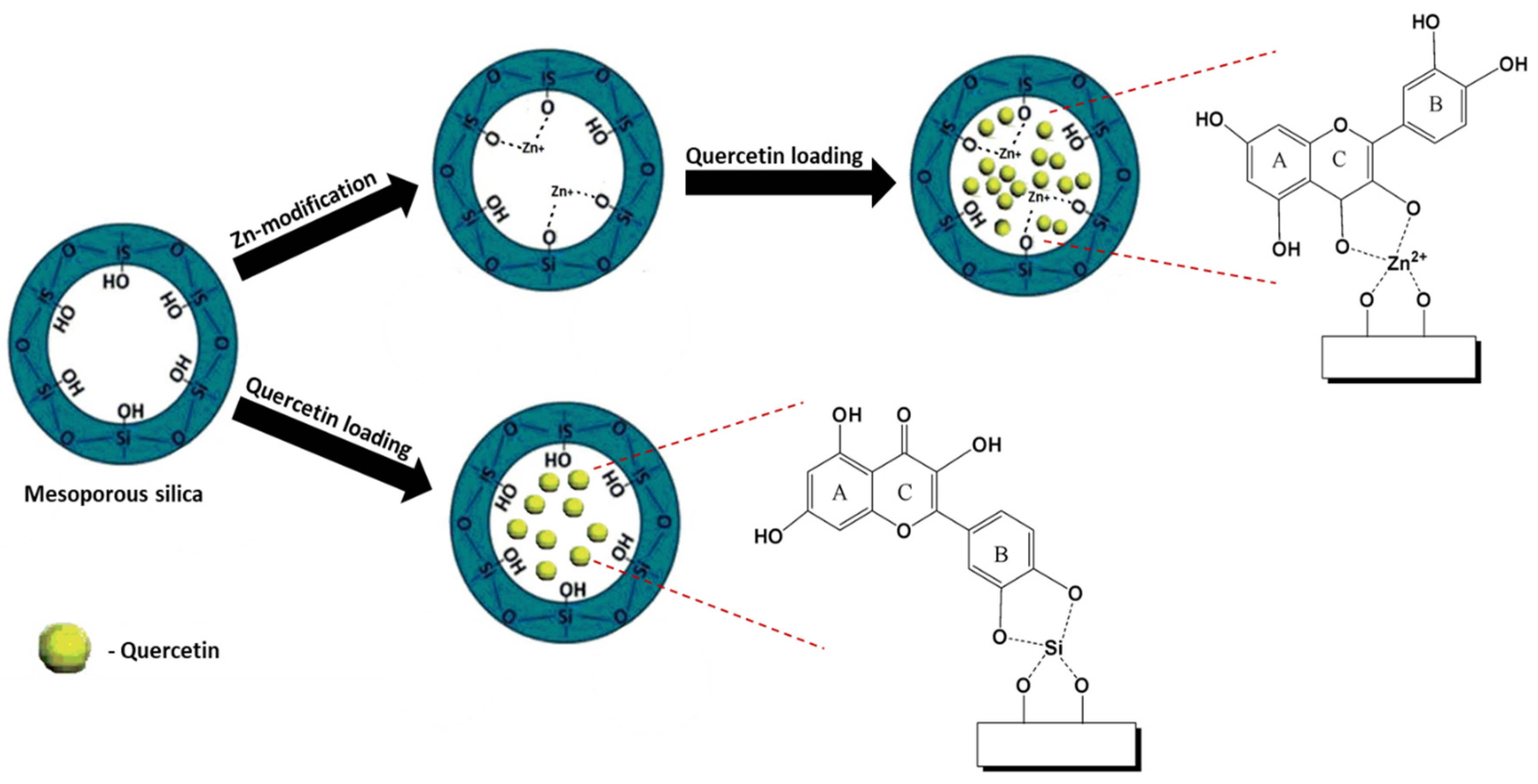
6.2. Functionalization with Metal Species
7. Conclusions and Final Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nano.DE-Report 2013; Federal Ministry of Eduation and Research: Bonn, Germany, 2013.
- Mishra, B.; Patel, B.B.; Tiwari, S. Colloidal Nanocarriers: A Review on Formulation Technology, Types and Applications toward Targeted Drug Delivery. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 9–24. [Google Scholar] [CrossRef] [PubMed]
- How, C.W.; Rasedee, A.; Manickam, S.; Rosli, R. Tamoxifen-Loaded Nanostructured Lipid Carrier as a Drug Delivery System: Characterization, Stability Assessment and Cytotoxicity. Colloids Surf. B Biointerfaces 2013, 112, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Zhang, Y.S.; Pang, B.; Hyun, D.C.; Yang, M.; Xia, Y. Engineered Nanoparticles for Drug Delivery in Cancer Therapy. Angew. Chem. Int. Ed. 2014, 53, 12320–12364. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Kompella, U. Nanoparticle Technology for Drug Delivery, 1st ed.; Taylor & Francis: Boca Raton, FL, USA, 2006; ISBN 9780429133657. [Google Scholar]
- Janjua, T.I.; Cao, Y.; Kleitz, F.; Linden, M.; Yu, C.; Popat, A. Silica Nanoparticles: A Review of Their Safety and Current Strategies to Overcome Biological Barriers. Adv. Drug Deliv. Rev. 2023, 203, 115115. [Google Scholar] [CrossRef] [PubMed]
- Panyam, J.; Labhasetwar, V. Biodegradable Nanoparticles for Drug and Gene Delivery to Cells and Tissue. Adv. Drug Deliv. Rev. 2003, 55, 329–347. [Google Scholar] [CrossRef] [PubMed]
- Moghimi, S.M.; Hunter, A.C.; Murray, J.C. Long-Circulating and Target-Specific Nanoparticles: Theory to Practice. Pharmacol. Rev. 2001, 53, 283–318. [Google Scholar] [PubMed]
- Sing, K.S.W. Reporting Physisorption Data for Gas/Solid Systems with Special Reference to the Determination of Surface Area and Porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Davis, M.E.; Saldarriaga, C.; Montes, C.; Garces, J.; Crowdert, C. A Molecular Sieve with Eighteen-Membered Rings. Nature 1988, 331, 698–699. [Google Scholar] [CrossRef]
- Schoeman, B.J.; Sterte, J.; Otterstedt, J.-E. Synthesis and Size Tailoring of Colloidal Zeolite Particles. J. Chem. Soc. Chem. Commun. 1993, 12, 994–995. [Google Scholar] [CrossRef]
- Schmidt, I.; Boisen, A.; Gustavsson, E.; Ståhl, K.; Pehrson, S.; Dahl, S.; Carlsson, A.; Jacobsen, C.J.H. Carbon Nanotube Templated Growth of Mesoporous Zeolite Single Crystals. Chem. Mater. 2001, 13, 4416–4418. [Google Scholar] [CrossRef]
- Le Page, M.; Beau, R.; Duchene, J. Porous Silica Particles Containing a Crystallized Phase and Method. U.S. Patent No. 3,493,341, 3 February 1970. [Google Scholar]
- Kresge, C.T.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.C.; Beck, J.S. Ordered Mesoporous Molecular Sieves Synthesized by a Liquid-Crystal Template Mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Available online: https://www.cfsanappsexternal.fda.gov/scripts/fdcc/index.cfm?set=GRASNotices (accessed on 3 June 2024).
- Beck, J.S.; Chu, C.T.W.; Johnson, I.D.; Kresge, C.T.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.W.W. Synthesis of mesoporous crystalline material. U.S. Patent No. US5108725A, 28 April 1992. [Google Scholar]
- Monnier, A.; Schüth, F.; Huo, Q.; Kumar, D.; Margolese, D.; Maxwell, R.S.; Stucky, G.D.; Krishnamurty, M.; Petroff, P.; Firouzi, A.; et al. Cooperative Formation of Inorganic-Organic Interfaces in the Synthesis of Silicate Mesostructures. Science 1993, 261, 1299–1303. [Google Scholar] [CrossRef] [PubMed]
- Biriaei, R.; Nohair, B.; Kaliaguine, S. A Facile Route to Synthesize Mesoporous ZSM-5 with Hexagonal Arrays Using P123 Triblock Copolymer. Microporous Mesoporous Mater. 2020, 298, 110067. [Google Scholar] [CrossRef]
- Firouzi, A.; Kumar, D.; Bull, L.M.; Besier, T.; Sieger, P.; Huo, Q.; Walker, S.A.; Zasadzinski, J.A.; Glinka, C.; Nicol, J.; et al. Cooperative Organization of Inorganic-Surfactant and Biomimetic Assemblies. Science 1995, 267, 1138–1143. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Cui, K.; Jin, S. Temperature-Driven Structural Evolution during Preparation of MCM−41 Mesoporous Silica. Materials 2024, 17, 1711. [Google Scholar] [CrossRef]
- Zhao, D.; Feng, J.; Huo, Q.; Melosh, N.; Fredrickson, G.H.; Chmelka, B.F.; Stucky, G.D. Triblock Copolymer Syntheses of Mesoporous Silica with Periodic 50 to 300 Angstrom Pores. Science 1998, 279, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Rahman, I.A.; Padavettan, V. Synthesis of Silica Nanoparticles by Sol-Gel: Size-Dependent Properties, Surface Modification, and Applications in Silica-Polymer Nanocomposites—A Review. J. Nanomater. 2012, 2012, 132424. [Google Scholar] [CrossRef]
- Ghaedi, H.; Zhao, M. Review on Template Removal Techniques for Synthesis of Mesoporous Silica Materials. Energy Fuels 2022, 36, 2424–2446. [Google Scholar] [CrossRef]
- Caltagirone, C.; Bettoschi, A.; Garau, A.; Montis, R. Silica-Based Nanoparticles: A Versatile Tool for the Development of Efficient Imaging Agents. Chem. Soc. Rev. 2015, 44, 4645–4671. [Google Scholar] [CrossRef]
- Kesse, S.; Boakye-Yiadom, K.O.; Ochete, B.O.; Opoku-Damoah, Y.; Akhtar, F.; Filli, M.S.; Asim Farooq, M.; Aquib, M.; Maviah Mily, B.J.; Murtaza, G.; et al. Mesoporous Silica Nanomaterials: Versatile Nanocarriers for Cancer Theranostics and Drug and Gene Delivery. Pharmaceutics 2019, 11, 77. [Google Scholar] [CrossRef]
- Mebert, A.M.; Baglole, C.J.; Desimone, M.F.; Maysinger, D. Nanoengineered Silica: Properties, Applications and Toxicity. Food Chem. Toxicol. 2017, 109, 753–770. [Google Scholar] [CrossRef]
- Vallet-Regi, M.; Rámila, A.; Del Real, R.P.; Pérez-Pariente, J. A New Property of MCM-41: Drug Delivery System. Chem. Mater. 2001, 13, 308–311. [Google Scholar] [CrossRef]
- Horcajada, P.; Rámila, A.; Férey, G.; Vallet-Regí, M. Influence of Superficial Organic Modification of MCM-41 Matrices on Drug Delivery Rate. Solid State Sci. 2006, 8, 1243–1249. [Google Scholar] [CrossRef]
- Slowing, I.I.; Vivero-Escoto, J.L.; Wu, C.-W.; Lin, V.S.-Y. Mesoporous Silica Nanoparticles as Controlled Release Drug Delivery and Gene Transfection Carriers. Adv. Drug Deliv. Rev. 2008, 60, 1278–1288. [Google Scholar] [CrossRef]
- Wang, S. Ordered Mesoporous Materials for Drug Delivery. Microporous Mesoporous Mater. 2009, 117, 1–9. [Google Scholar] [CrossRef]
- Available online: https://www.Technavio.Com/Report/Botanical-and-Plant-Derived-Drugs-Market-Industry-Analysis (accessed on 7 June 2024).
- Li, A.-N.; Li, S.; Zhang, Y.-J.; Xu, X.-R.; Chen, Y.-M.; Li, H.-B. Resources and Biological Activities of Natural Polyphenols. Nutrients 2014, 6, 6020–6047. [Google Scholar] [CrossRef]
- Li, W.; Chen, H.; Xu, B.; Wang, Y.; Zhang, C.; Cao, Y.; Xing, X. Research Progress on Classification, Sources and Functions of Dietary Polyphenols for Prevention and Treatment of Chronic Diseases. J. Futur. Foods 2023, 3, 289–305. [Google Scholar] [CrossRef]
- Ahmad, A.; Ahmad, V.; Zamzami, M.A.; Chaudhary, H.; Baothman, O.A.; Hosawi, S.; Kashif, M.; Akhtar, M.S.; Khan, M.J. Introduction and Classification of Natural Polyphenols BT. In Polyphenols-Based Nanotherapeutics for Cancer Management; Tabrez, S., Imran Khan, M., Eds.; Springer: Singapore, 2021; pp. 1–16. ISBN 978-981-16-4935-6. [Google Scholar]
- Karak, P. Biological Activities of Flavonoids: An Overview. Int. J. Pharm. Sci. Res. 2019, 10, 1567–1574. [Google Scholar] [CrossRef]
- Chen, S.; Wang, X.; Cheng, Y.; Gao, H.; Chen, X. A Review of Classification, Biosynthesis, Biological Activities and Potential Applications of Flavonoids. Molecules 2023, 28, 4982. [Google Scholar] [CrossRef] [PubMed]
- Hao, B.; Yang, Z.; Liu, H.; Liu, Y.; Wang, S. Advances in Flavonoid Research: Sources, Biological Activities, and Developmental Prospectives. Curr. Issues Mol. Biol. 2024, 46, 2884–2925. [Google Scholar] [CrossRef]
- Abbas, M.; Saeed, F.; Anjum, F.M.; Afzaal, M.; Tufail, T.; Bashir, M.S.; Ishtiaq, A.; Hussain, S.; Suleria, H.A.R. Natural Polyphenols: An Overview. Int. J. Food Prop. 2017, 20, 1689–1699. [Google Scholar] [CrossRef]
- Khan, H.; Ullah, H.; Martorell, M.; Valdes, S.E.; Belwal, T.; Tejada, S.; Sureda, A.; Kamal, M.A. Flavonoids Nanoparticles in Cancer: Treatment, Prevention and Clinical Prospects. Semin. Cancer Biol. 2021, 69, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Popova, M.; Szegedi, A.; Mavrodinova, V.; Novak Tušar, N.; Mihály, J.; Klébert, S.; Benbassat, N.; Yoncheva, K. Preparation of Resveratrol-Loaded Nanoporous Silica Materials with Different Structures. J. Solid State Chem. 2014, 219, 37–42. [Google Scholar] [CrossRef]
- Petrisor, G.; Motelica, L.; Ficai, D.; Trusca, R.D.; Surdu, V.-A.; Voicu, G.; Oprea, O.C.; Ficai, A.; Andronescu, E. New Mesoporous Silica Materials Loaded with Polyphenols: Caffeic Acid, Ferulic Acid and p-Coumaric Acid as Dietary Supplements for Oral Administration. Materials 2022, 15, 7982. [Google Scholar] [CrossRef] [PubMed]
- Morante-Zarcero, S.; Endrino, A.; Casado, N.; Pérez-Quintanilla, D.; Sierra, I. Evaluation of Mesostructured Silica Materials with Different Structures and Morphologies as Carriers for Quercetin and Naringin Encapsulation. J. Porous Mater. 2022, 29, 33–48. [Google Scholar] [CrossRef]
- Ravinayagam, V.; Rabindran Jermy, B. Studying the Loading Effect of Acidic Type Antioxidant on Amorphous Silica Nanoparticle Carriers. J. Nanoparticle Res. 2017, 19, 190. [Google Scholar] [CrossRef]
- Rashidi, L.; Vasheghani-Farahani, E.; Rostami, K.; Ganji, F.; Fallahpour, M. Mesoporous Silica Nanoparticles with Different Pore Sizes for Delivery of PH-Sensitive Gallic Acid. Asia-Pac. J. Chem. Eng. 2014, 9, 845–853. [Google Scholar] [CrossRef]
- Petrisor, G.; Ficai, D.; Motelica, L.; Trusca, R.D.; Bîrcă, A.C.; Vasile, B.S.; Voicu, G.; Oprea, O.C.; Semenescu, A.; Ficai, A.; et al. Mesoporous Silica Materials Loaded with Gallic Acid with Antimicrobial Potential. Nanomaterials 2022, 12, 1648. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, Z.; Subramaniam, S.; Khan, G.M.; Abeer, M.M.; Qu, Z.; Janjua, T.; Kumeria, T.; Batra, J.; Popat, A. Encapsulation and Controlled Release of Resveratrol Within Functionalized Mesoporous Silica Nanoparticles for Prostate Cancer Therapy. Front. Bioeng. Biotechnol. 2019, 7, 225. [Google Scholar] [CrossRef]
- Deligiannakis, Y.; Sotiriou, G.A.; Pratsinis, S.E. Antioxidant and Antiradical SiO2 Nanoparticles Covalently Functionalized with Gallic Acid. ACS Appl. Mater. Interfaces 2012, 4, 6609–6617. [Google Scholar] [CrossRef]
- Szewczyk, A.; Brzezińska-Rojek, J.; Ośko, J.; Majda, D.; Prokopowicz, M.; Grembecka, M. Antioxidant-Loaded Mesoporous Silica—An Evaluation of the Physicochemical Properties. Antioxidants 2022, 11, 1417. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, S.; Liu, J.; Xu, Z. Tannic Acid Adsorption on Amino-Functionalized Magnetic Mesoporous Silica. Chem. Eng. J. 2010, 165, 10–16. [Google Scholar] [CrossRef]
- Liu, H.; Yu, H.; Jin, P.; Jiang, M.; Zhu, G.; Duan, Y.; Yang, Z.; Qiu, H. Preparation of Mesoporous Silica Materials Functionalized with Various Amino-Ligands and Investigation of Adsorption Performances on Aromatic Acids. Chem. Eng. J. 2020, 379, 122405. [Google Scholar] [CrossRef]
- Szegedi, Á.; Shestakova, P.; Trendafilova, I.; Mihayi, J.; Tsacheva, I.; Mitova, V.; Kyulavska, M.; Koseva, N.; Momekova, D.; Konstantinov, S.; et al. Modified Mesoporous Silica Nanoparticles Coated by Polymer Complex as Novel Curcumin Delivery Carriers. J. Drug Deliv. Sci. Technol. 2019, 49, 700–712. [Google Scholar] [CrossRef]
- Petrișor, G.; Motelica, L.; Ficai, D.; Ilie, C.-I.; Trușcǎ, R.D.; Surdu, V.-A.; Oprea, O.-C.; Ficai, A.; Vasilievici, G.; Semenescu, A.; et al. Increasing Bioavailability of Trans-Ferulic Acid by Encapsulation in Functionalized Mesoporous Silica. Pharmaceutics 2023, 15, 660. [Google Scholar] [CrossRef]
- Brezoiu, A.-M.; Bajenaru, L.; Berger, D.; Mitran, R.-A.; Deaconu, M.; Lincu, D.; Stoica Guzun, A.; Matei, C.; Moisescu, M.G.; Negreanu-Pirjol, T. Effect of Nanoconfinement of Polyphenolic Extract from Grape Pomace into Functionalized Mesoporous Silica on Its Biocompatibility and Radical Scavenging Activity. Antioxidants 2020, 9, 696. [Google Scholar] [CrossRef]
- Popova, M.; Trendafilova, I.; Szegedi, Á.; Mihály, J.; Németh, P.; Marinova, S.G.; Aleksandrov, H.A.; Vayssilov, G.N. Experimental and Theoretical Study of Quercetin Complexes Formed on Pure Silica and Zn-Modified Mesoporous MCM-41 and SBA-16 Materials. Microporous Mesoporous Mater. 2016, 228, 256–265. [Google Scholar] [CrossRef]
- Trendafilova, I.; Szegedi, A.; Mihály, J.; Momekov, G.; Lihareva, N.; Popova, M. Preparation of Efficient Quercetin Delivery System on Zn-Modified Mesoporous SBA-15 Silica Carrier. Mater. Sci. Eng. C 2017, 73, 285–292. [Google Scholar] [CrossRef]
- Trendafilova, I.; Momekova, D.; Szegedi, A.; Momekov, G.; Zgureva, D.; Boycheva, S.; Popova, M. Silver and Quercetin Loaded Nanostructured Silica Materials as Potential Dermal Formulations. Bulg. Chem. Commun. 2017, 49, 51–58. [Google Scholar]
- Trendafilova, I.; Mihály, J.; Momekova, D.; Chimshirova, R.; Lazarova, H.; Momekov, G.; Popova, M. Antioxidant Activity and Modified Release Profiles of Morin and Hesperetin Flavonoids Loaded in Mg- or Ag-Modified SBA-16 Carriers. Mater. Today Commun. 2020, 24, 101198. [Google Scholar] [CrossRef]
- Trendafilova, I.; Lazarova, H.; Chimshirova, R.; Trusheva, B.; Koseva, N.; Popova, M. Novel Kaempferol Delivery Systems Based on Mg-Containing MCM-41 Mesoporous Silicas. J. Solid State Chem. 2021, 301, 122323. [Google Scholar] [CrossRef]
- Chen, J.; Qiu, L.; Li, Q.; Ai, J.; Liu, H.; Chen, Q. Rapid Hemostasis Accompanied by Antibacterial Action of Calcium Crosslinking Tannic Acid-Coated Mesoporous Silica/Silver Janus Nanoparticles. Mater. Sci. Eng. C 2021, 123, 111958. [Google Scholar] [CrossRef] [PubMed]
- Rananaware, P.; Brahmkhatria, V.P.; Dasgupta, D.; Patel, A. Functionalized Mesoporous Silica for Drug Delivery of Poorly Soluble Polyphenols: Synthesis, Characterization, and Antimicrobial Action. J. Solid State Chem. 2023, 326, 124214. [Google Scholar] [CrossRef]
- Wu, I.-T.; Chu, Y.-H.; Huang, Y.-R.; Chen, C.-C.; Ding, S.-J. Antibacterial Ability and Osteogenic Activity of Polyphenol-Tailored Calcium Silicate Bone Cement. J. Mater. Chem. B 2022, 10, 4640–4649. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.-H.; Chen, C.-Y.; Chang, C.-Y.; Chen, Y.-W.; Lin, C.-P. The Synergistic Effects of Quercetin-Containing 3D-Printed Mesoporous Calcium Silicate/Calcium Sulfate/Poly-ε-Caprolactone Scaffolds for the Promotion of Osteogenesis in Mesenchymal Stem Cells. J. Formos. Med. Assoc. 2021, 120, 1627–1634. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Zhang, J.; Qian, J.; Li, Q.; Li, H.; Yan, Y.; Wei, S.; Wei, J.; Su, J. The Effects of Surface Bioactivity and Sustained-Release of Genistein from a Mesoporous Magnesium-Calcium-Silicate/PK Composite Stimulating Cell Responses in Vitro, and Promoting Osteogenesis and Enhancing Osseointegration In Vivo. Biomater. Sci. 2018, 6, 842–853. [Google Scholar] [CrossRef] [PubMed]
- Brezoiu, A.-M.; Matei, C.; Deaconu, M.; Stanciuc, A.-M.; Trifan, A.; Gaspar-Pintiliescu, A.; Berger, D. Polyphenols Extract from Grape Pomace. Characterization and Valorisation through Encapsulation into Mesoporous Silica-Type Matrices. Food Chem. Toxicol. 2019, 133, 110787. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Xing, J.; Lang, Y.; Liu, H. Synthesis of Amino-Silane Modified Magnetic Silica Adsorbents and Application for Adsorption of Flavonoids from Glycyrrhiza Uralensis Fisch. Sci. China Ser. B Chem. 2008, 51, 145–151. [Google Scholar] [CrossRef]
- Feng, H.; Li, M.; Xing, Z.; Ouyang, X.; Ling, J. Efficient Delivery of Fucoxanthin Using Metal–Polyphenol Network-Coated Magnetic Mesoporous Silica. J. Drug Deliv. Sci. Technol. 2022, 77, 103842. [Google Scholar] [CrossRef]
- Jiang, W.; Wang, Q.; Cui, D.; Han, L.; Chen, L.; Xu, J.; Niu, N. Metal-Polyphenol Network Coated Magnetic Hydroxyapatite for PH-Activated MR Imaging and Drug Delivery. Colloids Surf. B Biointerfaces 2023, 222, 113076. [Google Scholar] [CrossRef]
- Taguchi, A.; Schüth, F. Ordered Mesoporous Materials in Catalysis. Microporous Mesoporous Mater. 2005, 77, 1–45. [Google Scholar] [CrossRef]
- Buyl, F. Organo-Functional Silanes. In Inorganic Polymers; Jaeger De, R., Gleria, M., Eds.; Nova Science: Hauppauge, NY, USA, 2009. [Google Scholar]
- Qin, J.; Guo, N.; Yang, J.; Chen, Y. Recent Advances of Metal–Polyphenol Coordination Polymers for Biomedical Applications. Biosensors 2023, 13, 776. [Google Scholar] [CrossRef]
- Santos, E.L.; Maia, B.H.L.N.S.; Ferriani, A.P.; Teixeira, S.D. Flavonoids: Classification, Biosynthesis and Chemical Ecology. In Flavonoids; Justino, G.C., Ed.; IntechOpen: Rijeka, Croatia, 2017. [Google Scholar]
- Selvaraj, S.; Krishnaswamy, S.; Devashya, V.; Sethuraman, S.; Krishnan, U.M. Flavonoid-Metal Ion Complexes: A Novel Class of Therapeutic Agents. Med. Res. Rev. 2014, 34, 677–702. [Google Scholar] [CrossRef]
- Khater, M.; Ravishankar, D.; Greco, F.; Osborn, H.M. Metal Complexes of Flavonoids: Their Synthesis, Characterization and Enhanced Antioxidant and Anticancer Activities. Future Med. Chem. 2019, 11, 2845–2867. [Google Scholar] [CrossRef]
- Qian, L.-L.; Lu, Y.; Xu, Y.; Yang, Z.-Y.; Yang, J.; Zhou, Y.-M.; Han, R.-M.; Zhang, J.-P.; Skibsted, L.H. Alkaline Earth Metal Ion Coordination Increases the Radical Scavenging Efficiency of Kaempferol. RSC Adv. 2020, 10, 30035–30047. [Google Scholar] [CrossRef]
- de Souza, R.F.V.; De Giovani, W.F. Antioxidant Properties of Complexes of Flavonoids with Metal Ions. Redox Rep. 2004, 9, 97–104. [Google Scholar] [CrossRef]
- Kejík, Z.; Kaplánek, R.; Masařík, M.; Babula, P.; Matkowski, A.; Filipenský, P.; Veselá, K.; Gburek, J.; Sýkora, D.; Martásek, P.; et al. Iron Complexes of Flavonoids-Antioxidant Capacity and Beyond. Int. J. Mol. Sci. 2021, 22, 646. [Google Scholar] [CrossRef]
- Halevas, E.; Mavroidi, B.; Pelecanou, M.; Hatzidimitriou, A.G. Structurally Characterized Zinc Complexes of Flavonoids Chrysin and Quercetin with Antioxidant Potential. Inorganica Chim. Acta 2021, 523, 120407. [Google Scholar] [CrossRef]
- Cazarolli, L.H.; Zanatta, L.; Jorge, A.P.; de Sousa, E.; Horst, H.; Woehl, V.M.; Pizzolatti, M.G.; Szpoganicz, B.; Silva, F.R.M.B. Follow-up Studies on Glycosylated Flavonoids and Their Complexes with Vanadium: Their Anti-Hyperglycemic Potential Role in Diabetes. Chem. Biol. Interact. 2006, 163, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Naso, L.G.; Martínez, V.R.; Ferrer, E.G.; Williams, P.A.M. Antimetastatic Effects of VOflavonoid Complexes on A549 Cell Line. J. Trace Elements Med. Biol. 2021, 64, 126690. [Google Scholar] [CrossRef] [PubMed]
- Małecka, M.; Skoczyńska, A.; Goodman, D.M.; Hartinger, C.G.; Budzisz, E. Biological Properties of Ruthenium(II)/(III) Complexes with Flavonoids as Ligands. Coord. Chem. Rev. 2021, 436, 213849. [Google Scholar] [CrossRef]
- Kostyuk, V.A.; Potapovich, A.I.; Kostyuk, T.V.; Cherian, M.G. Metal Complexes of Dietary Flavonoids: Evaluation of Radical Scavenger Properties and Protective Activity against Oxidative Stress in Vivo. Cell. Mol. Biol. 2007, 53, 62–69. [Google Scholar]
- Jurd, L.; Geissman, T.A. Absorption Spectra of Metal Complexes of Flavonoid Compounds. J. Org. Chem. 1956, 21, 1395–1401. [Google Scholar] [CrossRef]
- Costa, F.; Sousa Gomes, P.; Fernandes, M.H. Osteogenic and Angiogenic Response to Calcium Silicate-Based Endodontic Sealers. J. Endod. 2016, 42, 113–119. [Google Scholar] [CrossRef]
- Wu, C.; Chang, J.; Fan, W. Bioactive Mesoporous Calcium–Silicate Nanoparticles with Excellent Mineralization Ability, Osteostimulation, Drug-Delivery and Antibacterial Properties for Filling Apex Roots of Teeth. J. Mater. Chem. 2012, 22, 16801–16809. [Google Scholar] [CrossRef]
- Fan, W.; Wu, D.; Tay, F.R.; Ma, T.; Wu, Y.; Fan, B. Effects of Adsorbed and Templated Nanosilver in Mesoporous Calcium-Silicate Nanoparticles on Inhibition of Bacteria Colonization of Dentin. Int. J. Nanomed. 2014, 9, 5217–5230. [Google Scholar] [CrossRef]
- Chen, S.; Greasley, S.L.; Ong, Z.Y.; Naruphontjirakul, P.; Page, S.J.; Hanna, J.V.; Redpath, A.N.; Tsigkou, O.; Rankin, S.; Ryan, M.P.; et al. Biodegradable Zinc-Containing Mesoporous Silica Nanoparticles for Cancer Therapy. Mater. Today Adv. 2020, 6, 100066. [Google Scholar] [CrossRef]
- Yu, L.; Chen, Y.; Lin, H.; Gao, S.; Chen, H.; Shi, J. Magnesium-Engineered Silica Framework for PH-Accelerated Biodegradation and DNAzyme-Triggered Chemotherapy. Small 2018, 14, e1800708. [Google Scholar] [CrossRef]
- Pohaku Mitchell, K.K.; Liberman, A.; Kummel, A.C.; Trogler, W.C. Iron(III)-Doped, Silica Nanoshells: A Biodegradable Form of Silica. J. Am. Chem. Soc. 2012, 134, 13997–14003. [Google Scholar] [CrossRef]
- Adhikari, C.; Mishra, A.; Nayak, D.; Chakraborty, A. Metal Organic Frameworks Modified Mesoporous Silica Nanoparticles (MSN): A Nano-Composite System to Inhibit Uncontrolled Chemotherapeutic Drug Delivery from Bare-MSN. J. Drug Deliv. Sci. Technol. 2018, 47, 1–11. [Google Scholar] [CrossRef]
- Yang, B.; Zhou, S.; Zeng, J.; Zhang, L.; Zhang, R.; Liang, K.; Xie, L.; Shao, B.; Song, S.; Huang, G.; et al. Super-Assembled Core-Shell Mesoporous Silica-Metal-Phenolic Network Nanoparticles for Combinatorial Photothermal Therapy and Chemotherapy. Nano Res. 2020, 13, 1013–1019. [Google Scholar] [CrossRef]
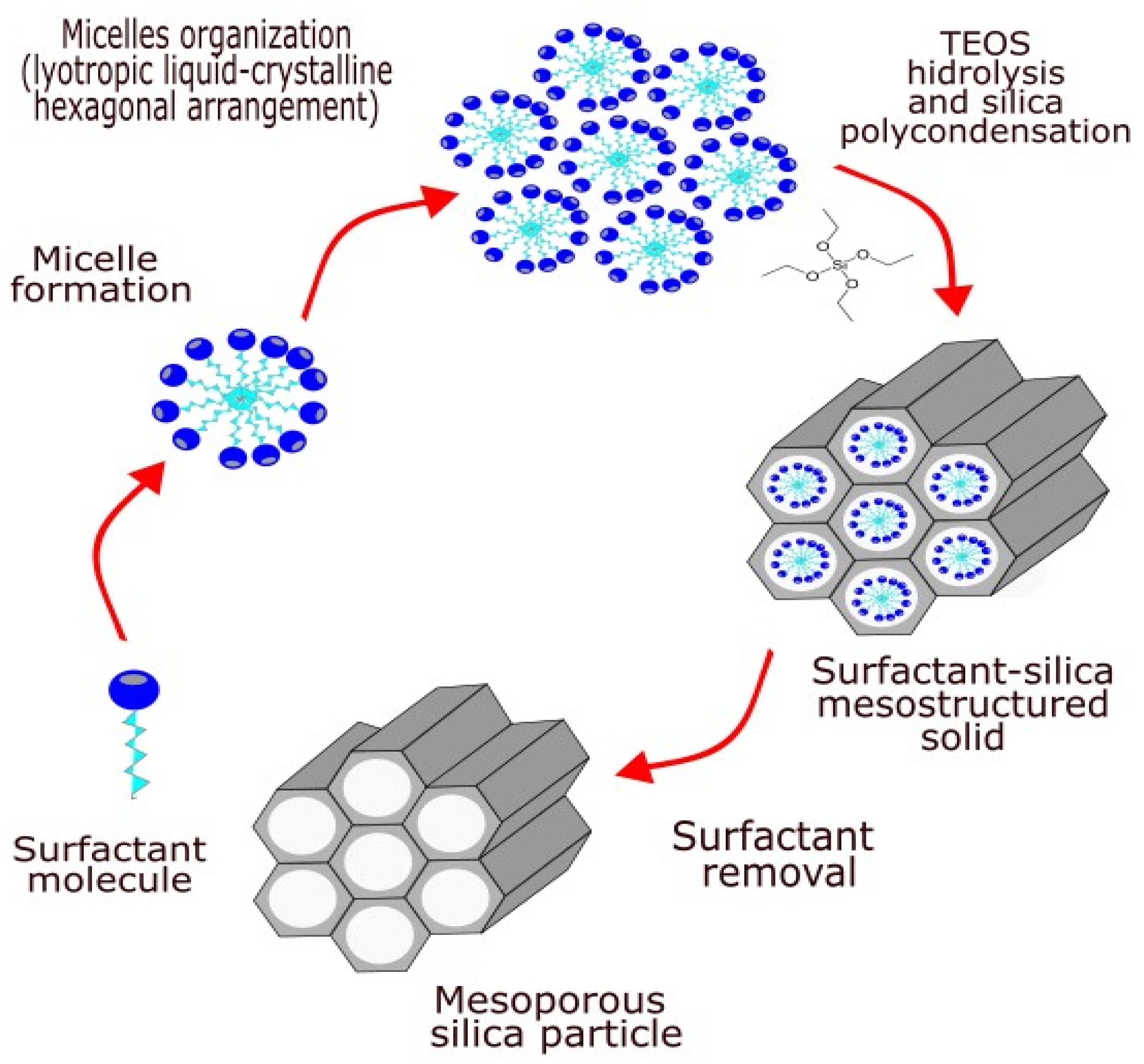

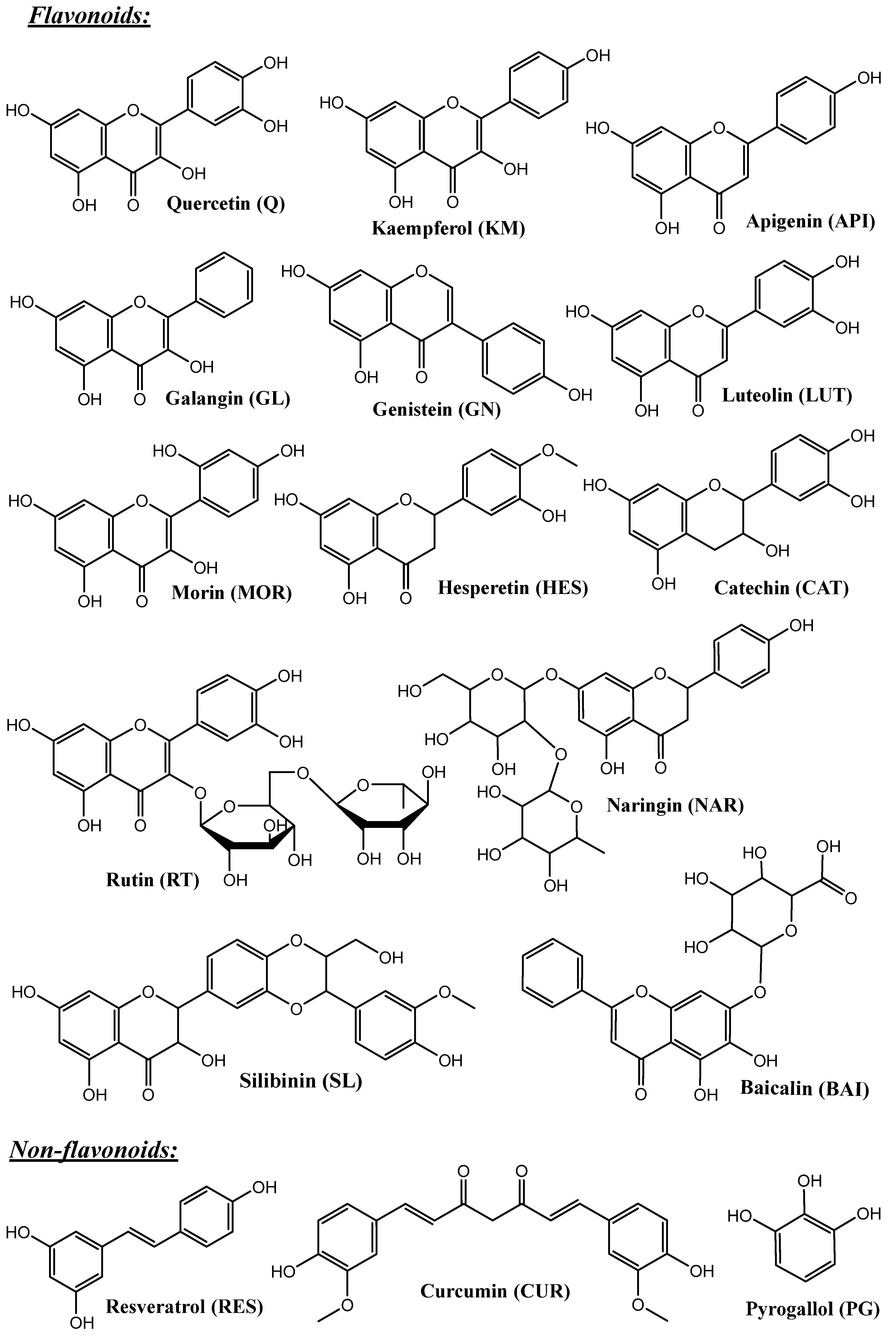
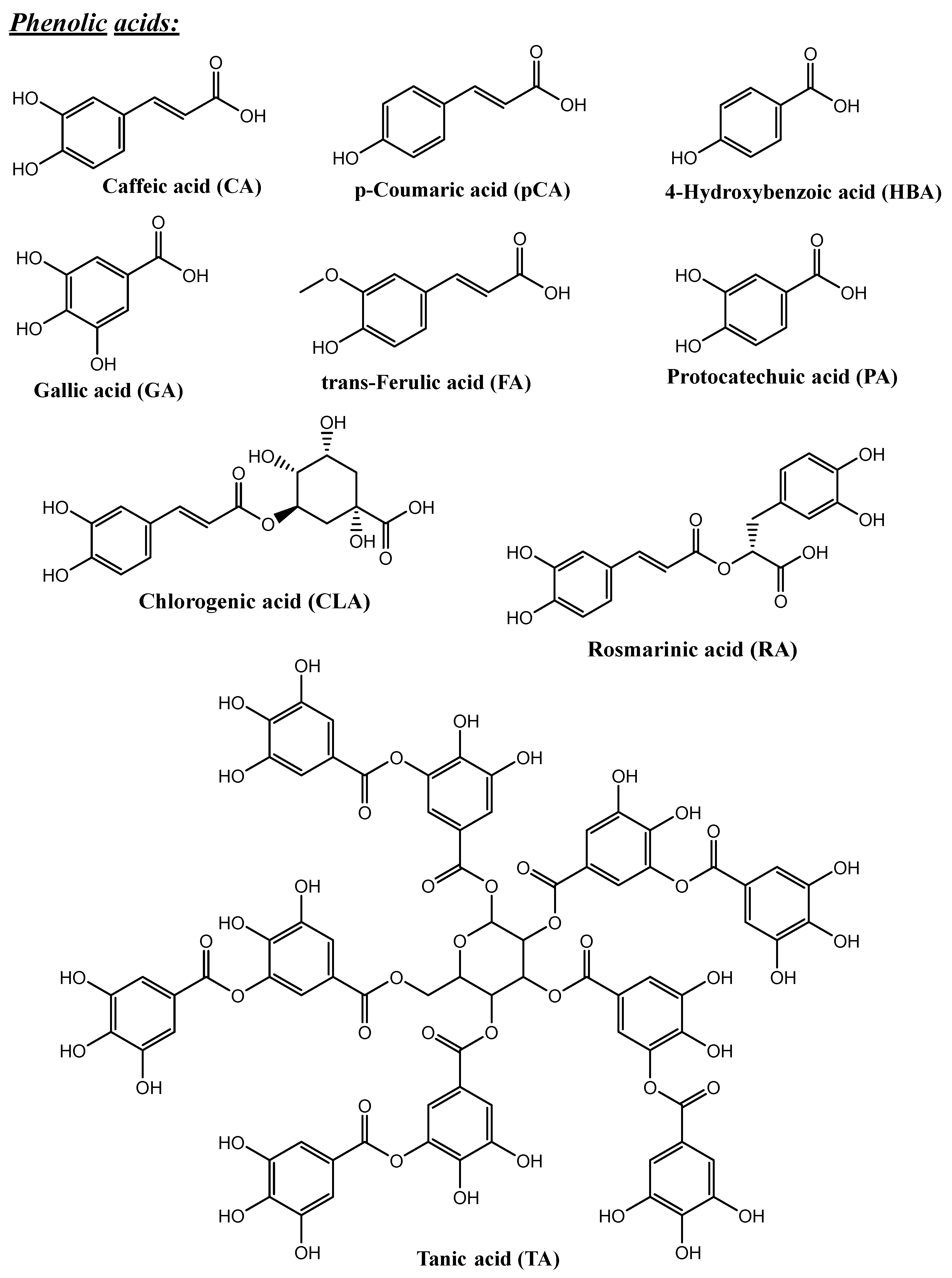
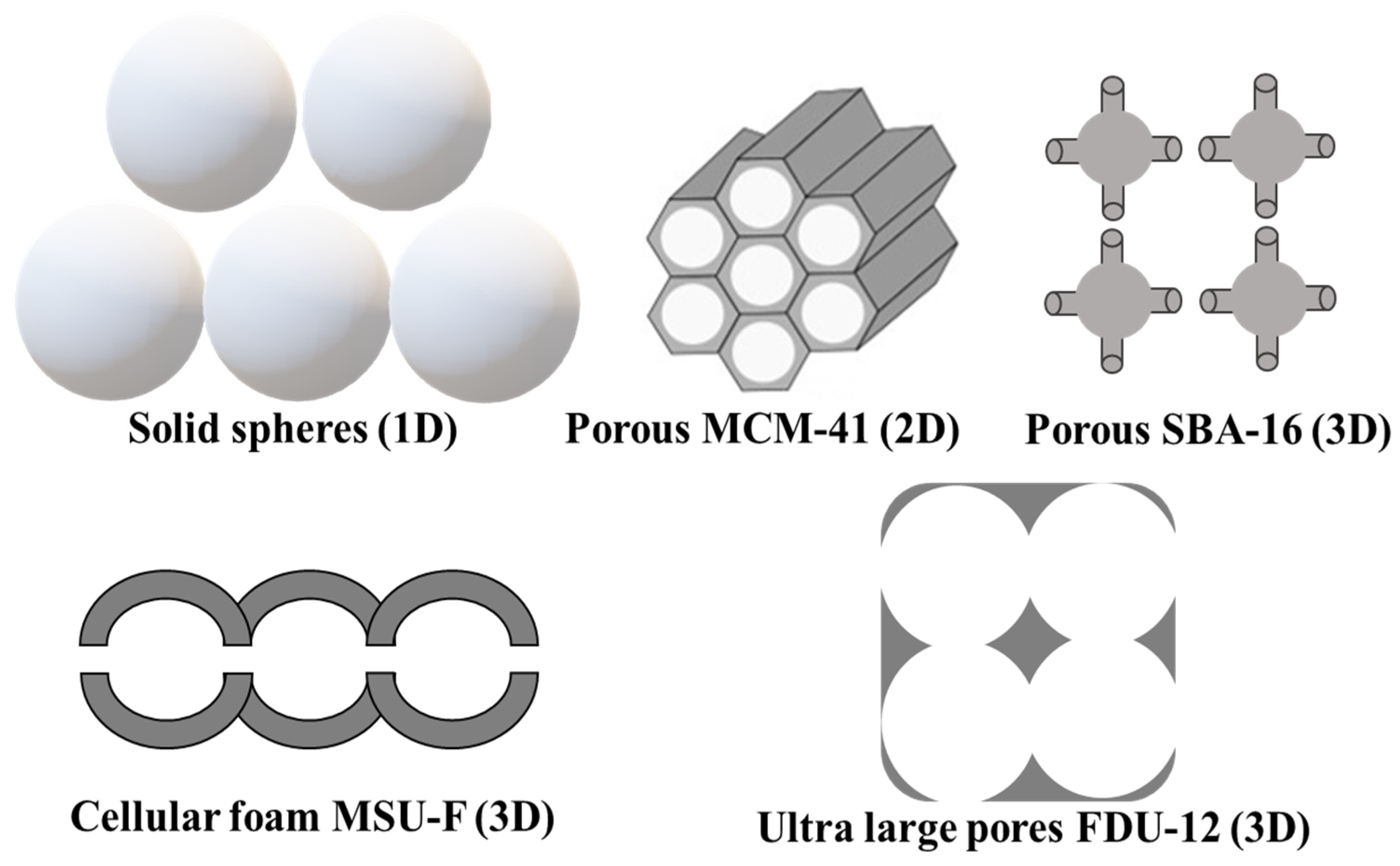


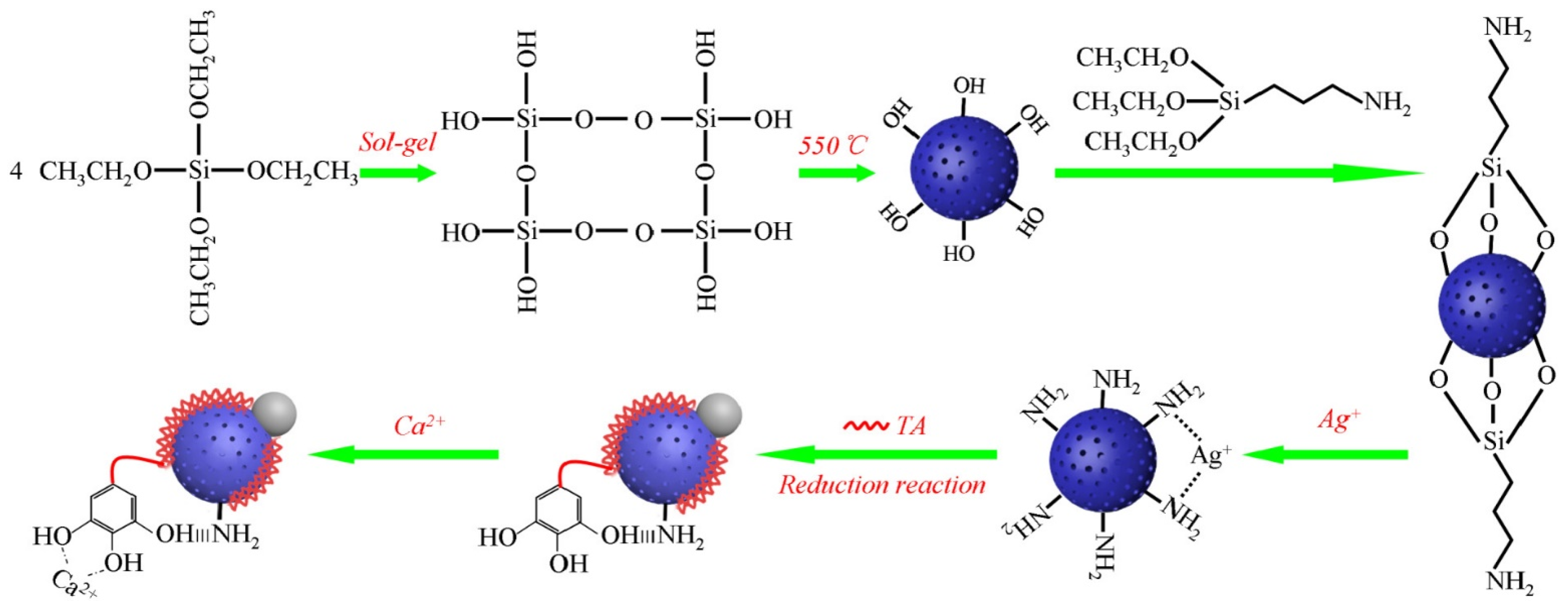
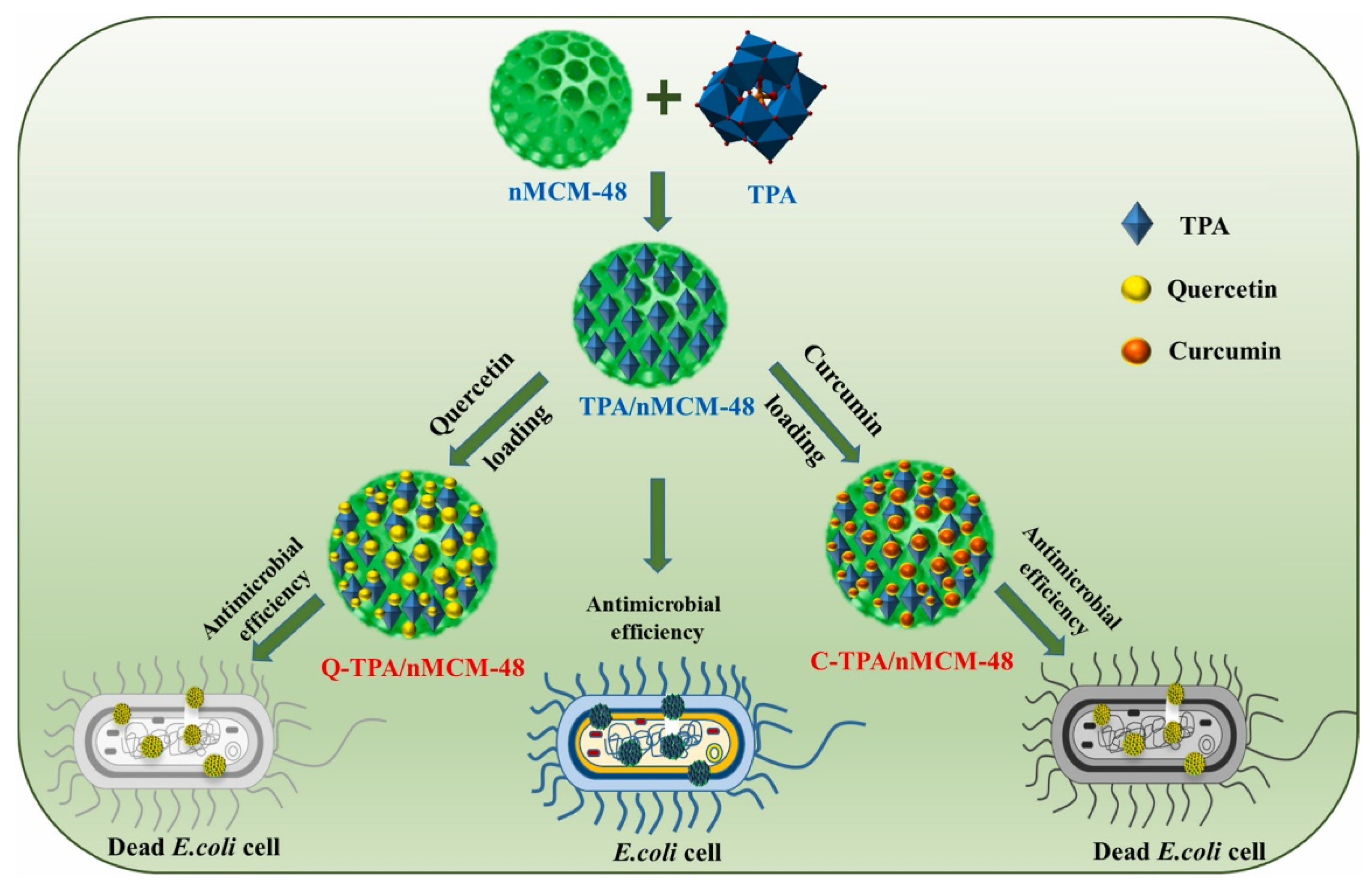
| Type of Silica | Pore Structure | Average Pore Size, nm | Surface Modification | Loaded Polyphenol (Amount) | Ref. |
|---|---|---|---|---|---|
| MCM-41 | 2D cylindrical mesopores, hexagonal arrangement | 2.7 | - | RES (32 wt%) | [40] |
| KIL-2 | Wormhole-like | 15.2 | - | RES (37 wt%) | [40] |
| Zeolite BEA | 3D | - | - | RES (40 wt%) | [40] |
| MCM-41 | 2D cylindrical mesopores, hexagonal arrangement | - | - | CA (45 wt%), p-CA (39 wt%), FA (33 wt%) | [41] |
| MCM-48 | 3D interlaced pore system divided by a continuous pore wall | -- | - | CA (35 wt%), p-CA (34 wt%), FA (32 wt%) | [41] |
| HMS | Wormhole-like | 2.5 | - | Q (40% EE **), NAR (41% EE) | [42] |
| MSU-2 | Wormhole-like | 3.1 | - | Q (40% EE), NAR (43% EE) | [42] |
| SBA-15 | 2D hexagonal | 5.6 | - | Q (32% EE), NAR (47% EE) | [42] |
| SBA-16 | 3D cage-like, body-centered-cubic array | 3.3 | - | G | [43] |
| KIT-6 | 3D interpenetrating cylindrical, bicontinuous cubic | 5.7 | - | GA | [43] |
| MCM-41 | 2D cylindrical mesopores, hexagonal arrangement | 3.1 | - | GA | [43] |
| ULPFDU-12 | 3D spherical cage-like, face-centered cubic | 4.7 | - | GA | [43] |
| Q10 SiO2 *** | 1D | 18.6 | - | GA | [43] |
| MSU-F | cellular foam | 16.4 | - | GA | [43] |
| MSN * | Wormhole-like | 1.8 | NH2 | RES (10 wt%) | [46] |
| MSN * | Wormhole-like | 2.3 | PO3 | RES (11 wt%) | [46] |
| Aerosil A90 *** | Solid particles | - | NH2 | GA (100 μM/g) | [47] |
| Aerosil A150 *** | Solid particles | - | NH2 | GA (152 μM/g) | [47] |
| Aerosil A300 *** | Solid particles | - | NH2 | GA (259 μM/g) | [47] |
| Aerosil A380 *** | Solid particles | - | NH2 | GA (366 μM/g) | [47] |
| MCM-41 | 2D cylindrical mesopores, hexagonal arrangement | 2.7 | - | GA (19%) | [48] |
| SBA-15 | 2D hexagonal | 6.3 | - | GA (13% EE) | [48] |
| SBA-15 | 2D hexagonal | 6.3 | NH2 | GA (26% EE) | [48] |
| SBA-15 | 2D hexagonal | 6.3 | SH | GA (13% EE) | [48] |
| SBA-15 | 2D hexagonal | 6.3 | NH2 | CGA (55% EE) | [48] |
| SBA-15 | 2D hexagonal | 6.3 | NH2 | PA (8% EE) | [48] |
| SBA-15 | 2D hexagonal | 6.3 | NH2 | 4-HBA (3% EE) | [48] |
| MCM-41 | 2D cylindrical mesopores, hexagonal arrangement | 2.4 | TA | [49] | |
| MCM-41 | 2D cylindrical mesopores, hexagonal arrangement | 2.4 | NH2 | TA | [49] |
| MSN | - | 8.4 | NH2 | GA (36 μmol), CLA (48 μmol), CA (40 μmol), pCA (46 μmol), RA (53 μmol) | [50] |
| MSN | - | 8.8 | NHCH3 | GA (30 μmol), CLA (46 μmol), CA (31 μmol), pCA (46 μmol), RA (58 μmol) | [50] |
| MSN | - | 8.8 | NHC2H4NH2 | GA (48 μmol), CLA (60 μmol), CA (48 μmol), pCA (60 μmol), RA (76 μmol) | [50] |
| MSN | - | 8.6 | NHC2H4NHC2H4NH2 | GA (60 μmol), CLA (70 μmol), CA (68 μmol), pCA (68 μmol), RA (83 μmol) | [50] |
| KIL-2 | Wormhole-like | 12.5 | NH2 | CUR (28 wt%) | [51] |
| KIT-6 | 3D interpenetrating cylindrical, bicontinuous cubic | 5.5 | NH2 | CUR (28 wt%) | [51] |
| MCM-41 | 2D cylindrical mesopores, hexagonal arrangement | - | - | FA (33 wt%) | [52] |
| MCM-41 | 2D cylindrical mesopores, hexagonal arrangement | - | NH2 | FA (32 wt%) | [52] |
| MCM-48 | 3D interlaced pore system divided by a continuous pore wall | - | - | FA (32 wt%) | [52] |
| MCM-48 | 3D interlaced pore system divided by a continuous pore wall | - | NH2 | FA (26 wt%) | [52] |
| MCM-41 | 2D cylindrical mesopores, hexagonal arrangement | 3.9 | - | grape pomace extract | [53] |
| MCM-41 | 2D cylindrical mesopores, hexagonal arrangement | 3.2 | CN | grape pomace extract | [53] |
| MCM-41 | 2D cylindrical mesopores, hexagonal arrangement | 3.2 | COOH | grape pomace extract | [53] |
| MCM-41 | 2D cylindrical mesopores, hexagonal arrangement | 3.5 | SH | grape pomace extract | [53] |
| MCM-41 | 2D cylindrical mesopores, hexagonal arrangement | 3.7 | SO3H | grape pomace extract | [53] |
| MCM-41 | 2D cylindrical mesopores, hexagonal arrangement | 2.7 | - | Q (35 wt%) | [54] |
| MCM-41 | 2D cylindrical mesopores, hexagonal arrangement | 2.7 | Zn (2/4%) | Q (42/37 wt%) | [54] |
| SBA-16 | 3D cage-like, body-centered-cubic array | 3.6 | - | Q (41 wt%) | [54] |
| SBA-16 | 3D cage-like, body-centered-cubic array | 4.6 | Zn (2/4%) | Q (43/45 wt%) | [54] |
| SBA-15 | 2D hexagonal | 6 | - | Q (42 wt%) | [55] |
| SBA-15 | 2D hexagonal | 5.8 | Zn (2/4%) | Q (44/46 wt%) | [55] |
| MCM-41 | 2D cylindrical mesopores, hexagonal arrangement | 4.2 | Ag | Q (44 wt%) | [56] |
| SBA-16 | 3D cage-like, body-centered-cubic array | 4.5 | - | MOR (29 wt%), HES (30 wt%) | [57] |
| SBA-16 | 3D cage-like, body-centered-cubic array | 4.4 | Ag | MOR (33 wt%), HES (32 wt%) | [57] |
| SBA-16 | 3D cage-like, body-centered-cubic array | 4.4 | Mg | MOR (32 wt%), HES (28 wt%) | [57] |
| MCM-41 | 2D cylindrical mesopores, hexagonal arrangement | 2.2 | - | KM (25 wt%) | [58] |
| MCM-41 | 2D cylindrical mesopores, hexagonal arrangement | 2.7 | Mg | KM (30 wt%) | [58] |
| MSN | - | 3.7 | NH2/Ag/TA/Ca2+ | - | [59] |
| MCM-48 | 3D interlaced pore system divided by a continuous pore wall | 2.25 | TPA | CUR (84 wt%), Q (86 wt%) | [60] |
| Calcium-silicate composite | - | - | - | GA, PG, TA | [61] |
| Mesoporous calcium silicate-calcium sulfate (MSCS)/polycaprolactone (PCL) | - | - | - | Q | [62] |
| Mesoporous magnesium-calcium-silicate/polyetheretherketone composite | - | - | - | GN | [63] |
| MCM-41 | 2D cylindrical mesopores, hexagonal arrangement | - | - | grape pomace extract (48 wt%) | [64] |
| MCM-41 | 2D cylindrical mesopores, hexagonal arrangement | - | ZnO | grape pomace extract (50 wt%) | [64] |
| MCM-41 | 2D cylindrical mesopores, hexagonal arrangement | - | MgO | grape pomace extract (47 wt%) | [64] |
| Magnetic silica particles | - | - | NH2 | licorice (Glycyrrhiza uralensis Fisch.) root extract | [65] |
| Magnetic silica particles | - | 3.4 | Fe3+, Al3+ | TA | [66] |
| Polyacrylic acid-coordinated Mn2+ and F− co-doped hydroxyapatite | - | 6 | Fe3 | TA | [67] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trendafilova, I.; Popova, M. Porous Silica Nanomaterials as Carriers of Biologically Active Natural Polyphenols: Effect of Structure and Surface Modification. Pharmaceutics 2024, 16, 1004. https://doi.org/10.3390/pharmaceutics16081004
Trendafilova I, Popova M. Porous Silica Nanomaterials as Carriers of Biologically Active Natural Polyphenols: Effect of Structure and Surface Modification. Pharmaceutics. 2024; 16(8):1004. https://doi.org/10.3390/pharmaceutics16081004
Chicago/Turabian StyleTrendafilova, Ivalina, and Margarita Popova. 2024. "Porous Silica Nanomaterials as Carriers of Biologically Active Natural Polyphenols: Effect of Structure and Surface Modification" Pharmaceutics 16, no. 8: 1004. https://doi.org/10.3390/pharmaceutics16081004
APA StyleTrendafilova, I., & Popova, M. (2024). Porous Silica Nanomaterials as Carriers of Biologically Active Natural Polyphenols: Effect of Structure and Surface Modification. Pharmaceutics, 16(8), 1004. https://doi.org/10.3390/pharmaceutics16081004






