Lentisk (Pistacia lentiscus) Oil Nanoemulsions Loaded with Levofloxacin: Phytochemical Profiles and Antibiofilm Activity against Staphylococcus spp.
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Mass Spectrometry Analysis
2.3. Ternary Phase Diagram Construction and Nanoemulsion Preparation
2.4. Dynamic Light Scattering and ζ-Potential Measurements
2.5. Oil Droplet Characterization by DPH Fluorescence Anisotropy
2.6. Morphological Analysis
2.7. Stability Studies
2.8. Release Studies
2.9. In Vitro Artificial Skin Permeation Experiments
2.10. Bacterial Strains
2.11. Determination of Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
2.12. Bacterial Biofilm Production
2.13. Biofilm Inhibition and Eradication Assay
2.14. Statistical Analysis
3. Results and Discussion
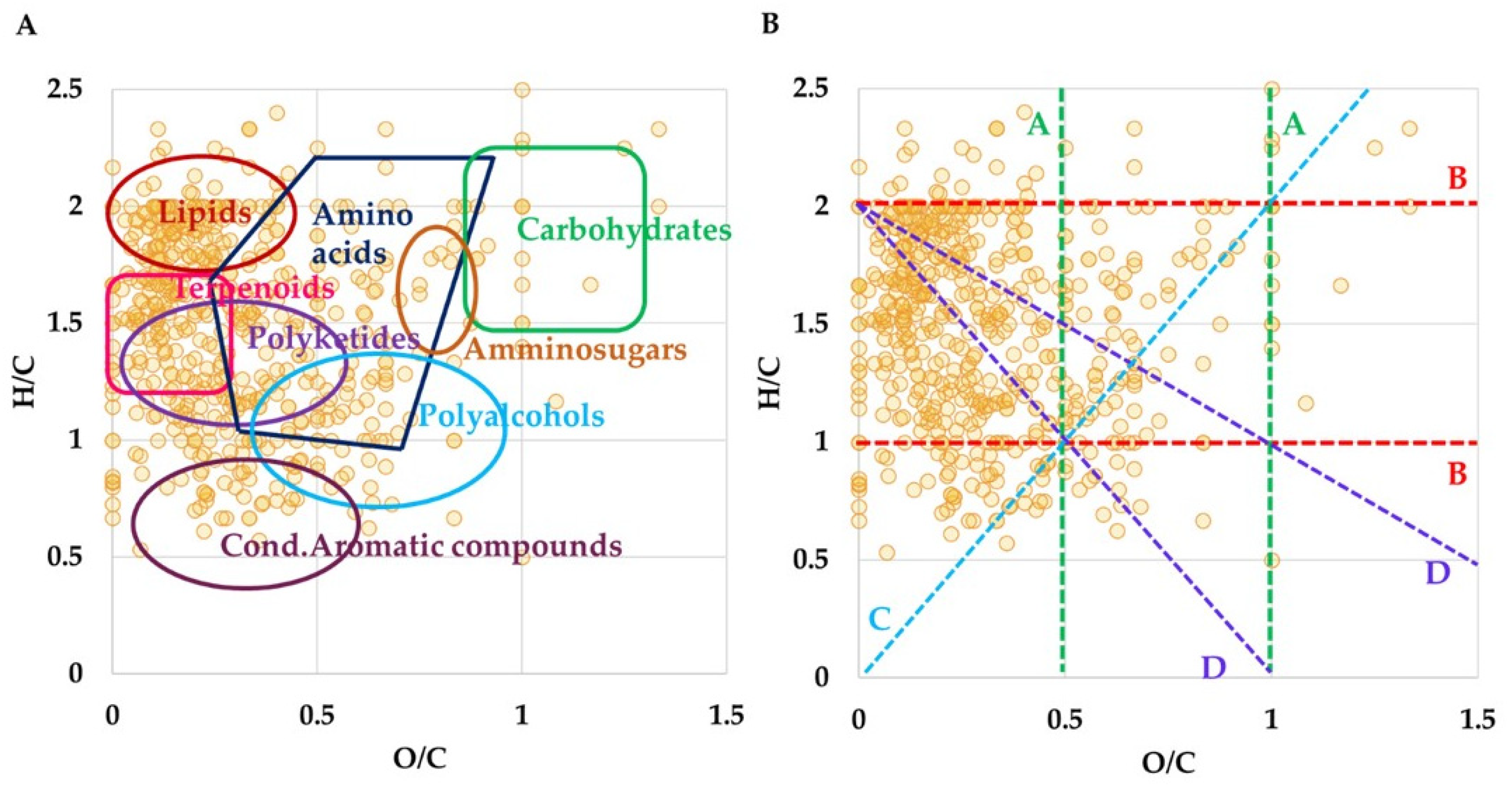
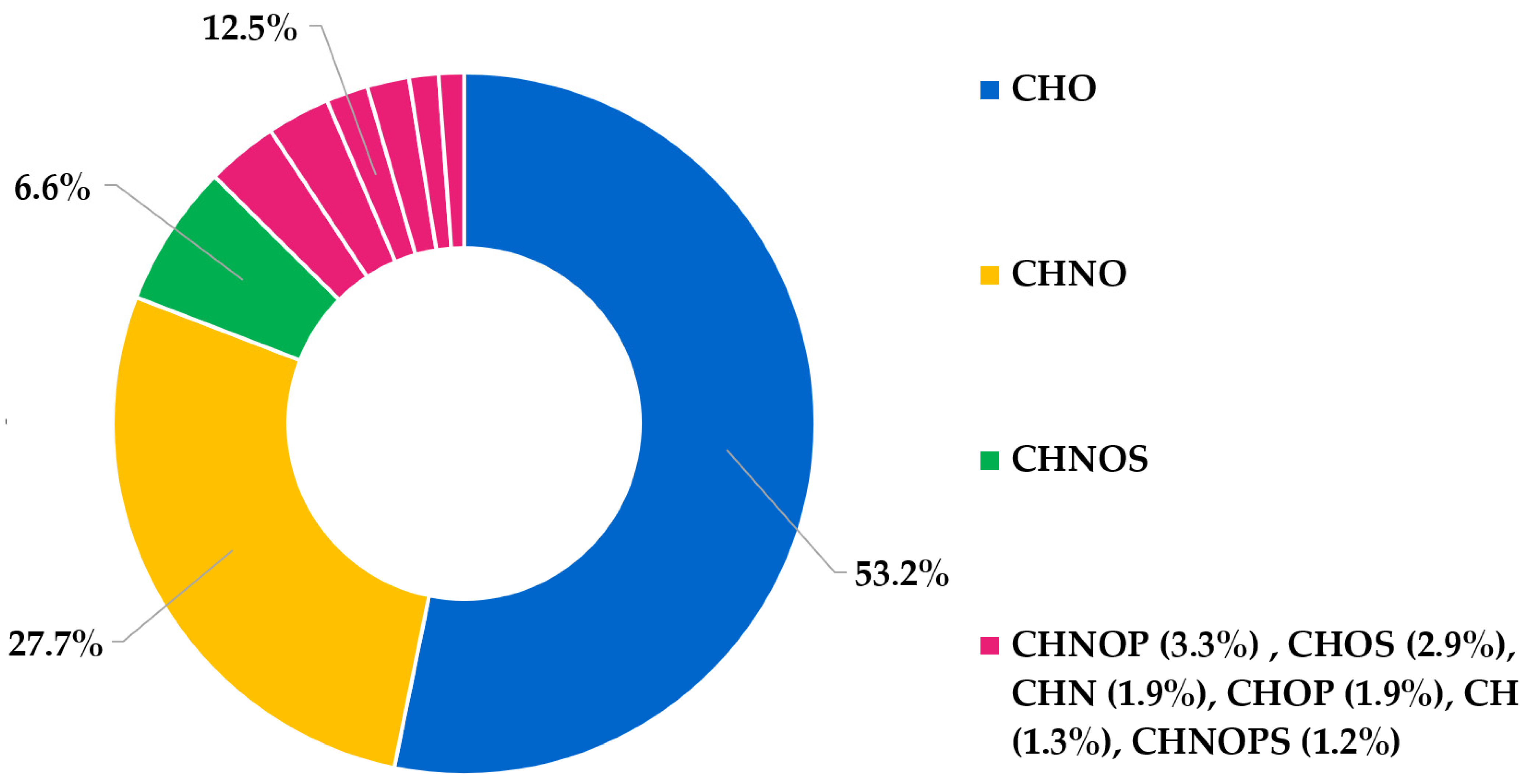
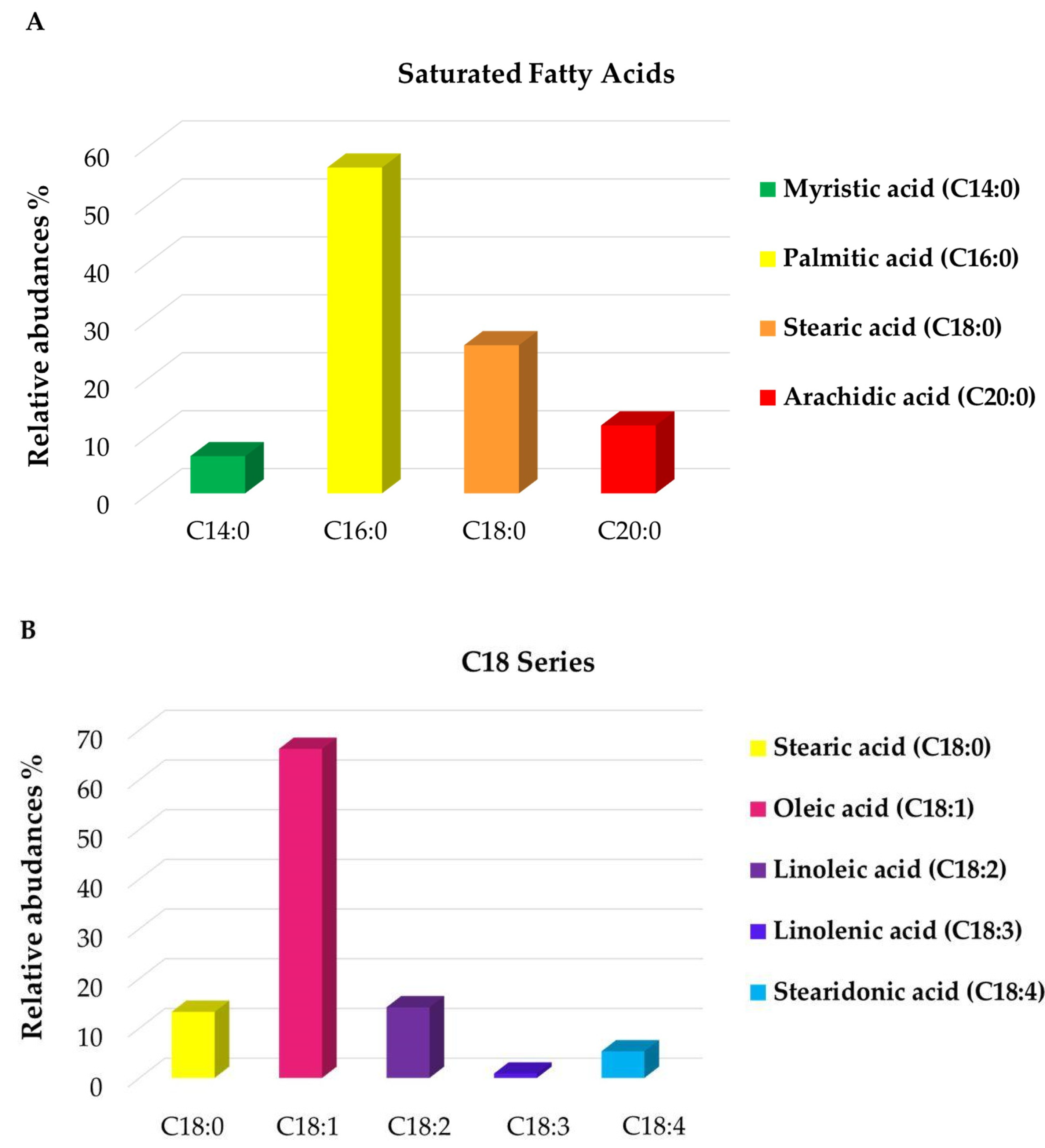
3.1. High-Resolution Mass Spectrometry Analysis
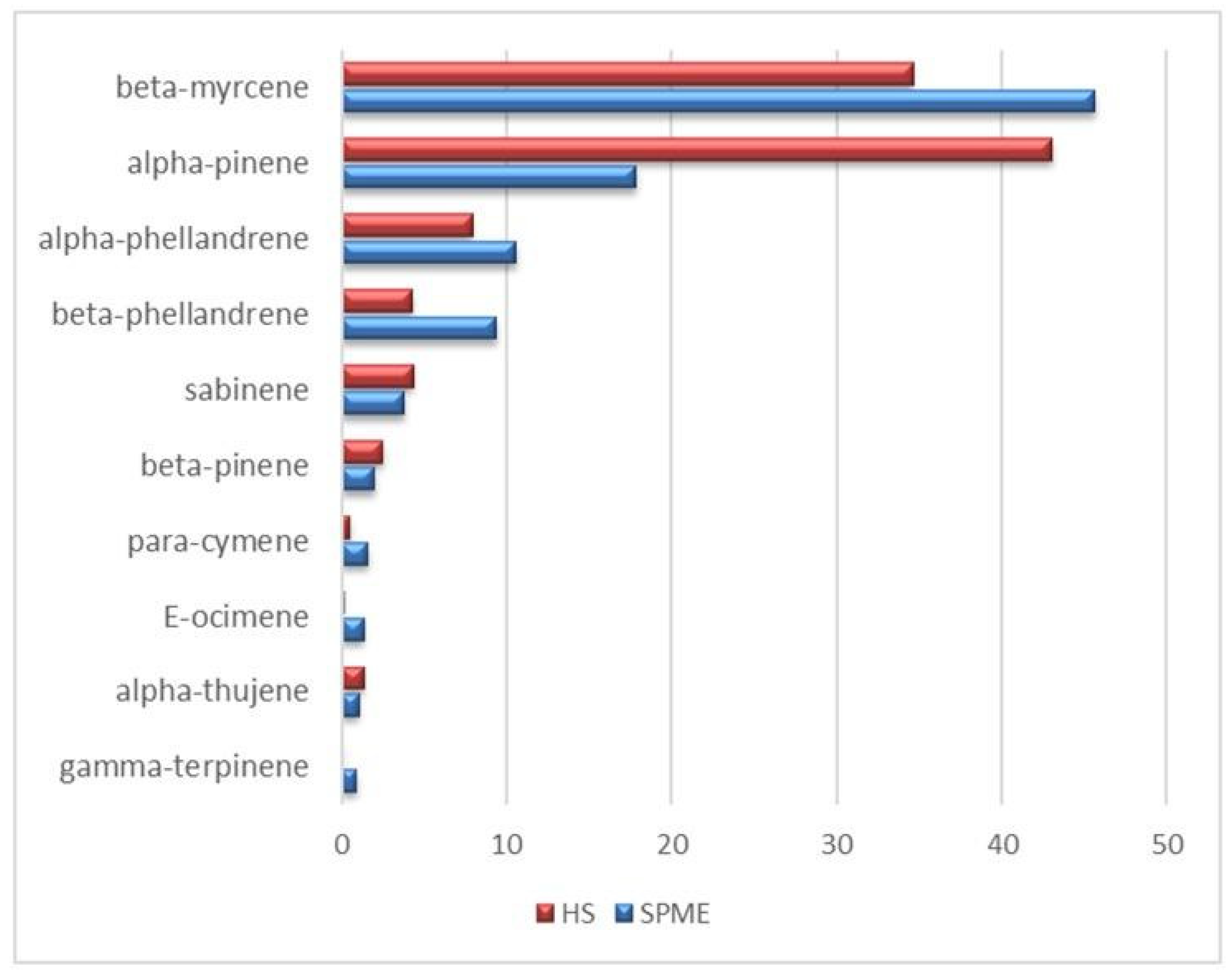
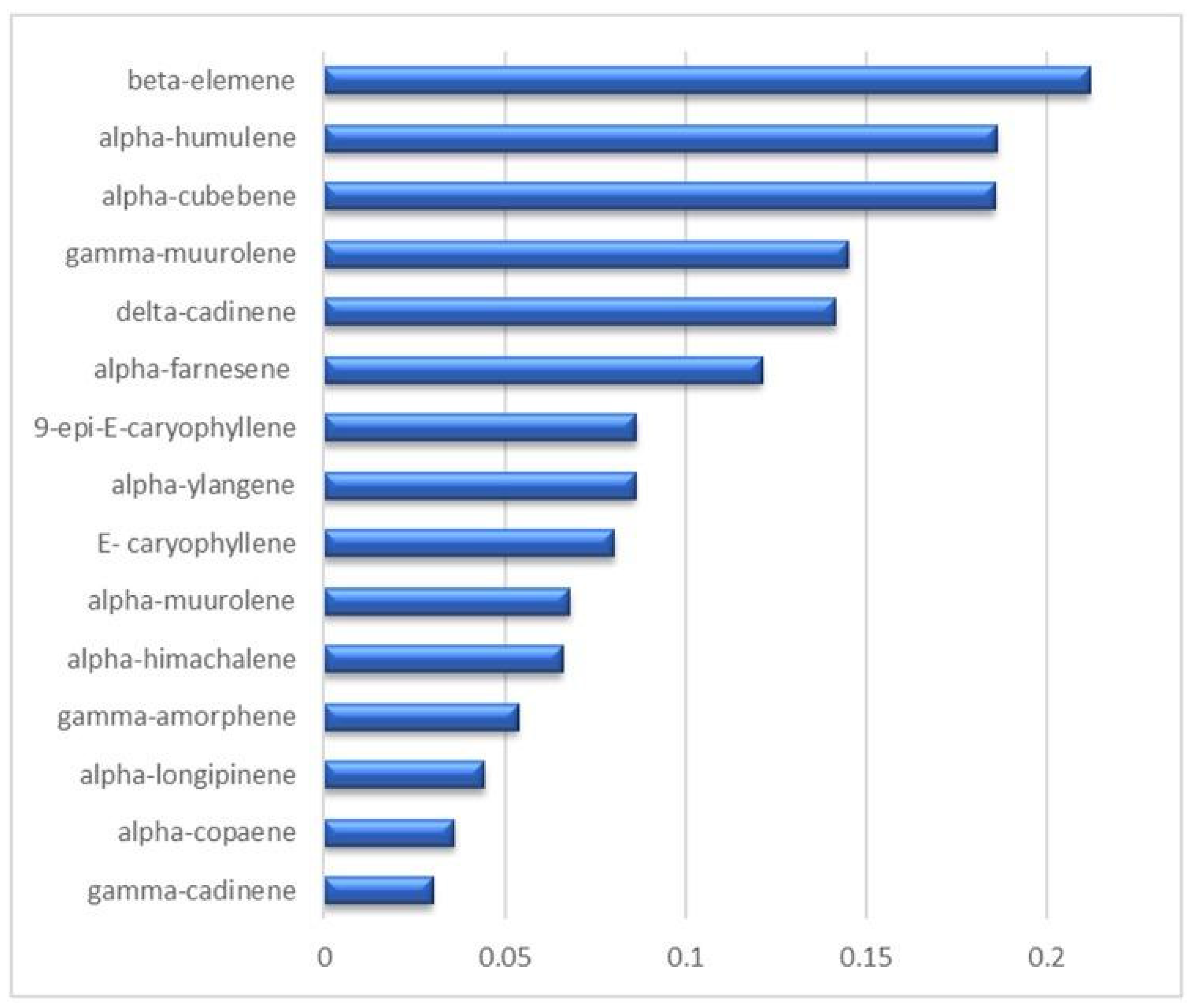
3.2. Static HS and HS-SPME-GC-MS Analysis of the VOC Profile
3.3. NEs Design and Physicochemical Characterization
3.4. Physicochemical Stability
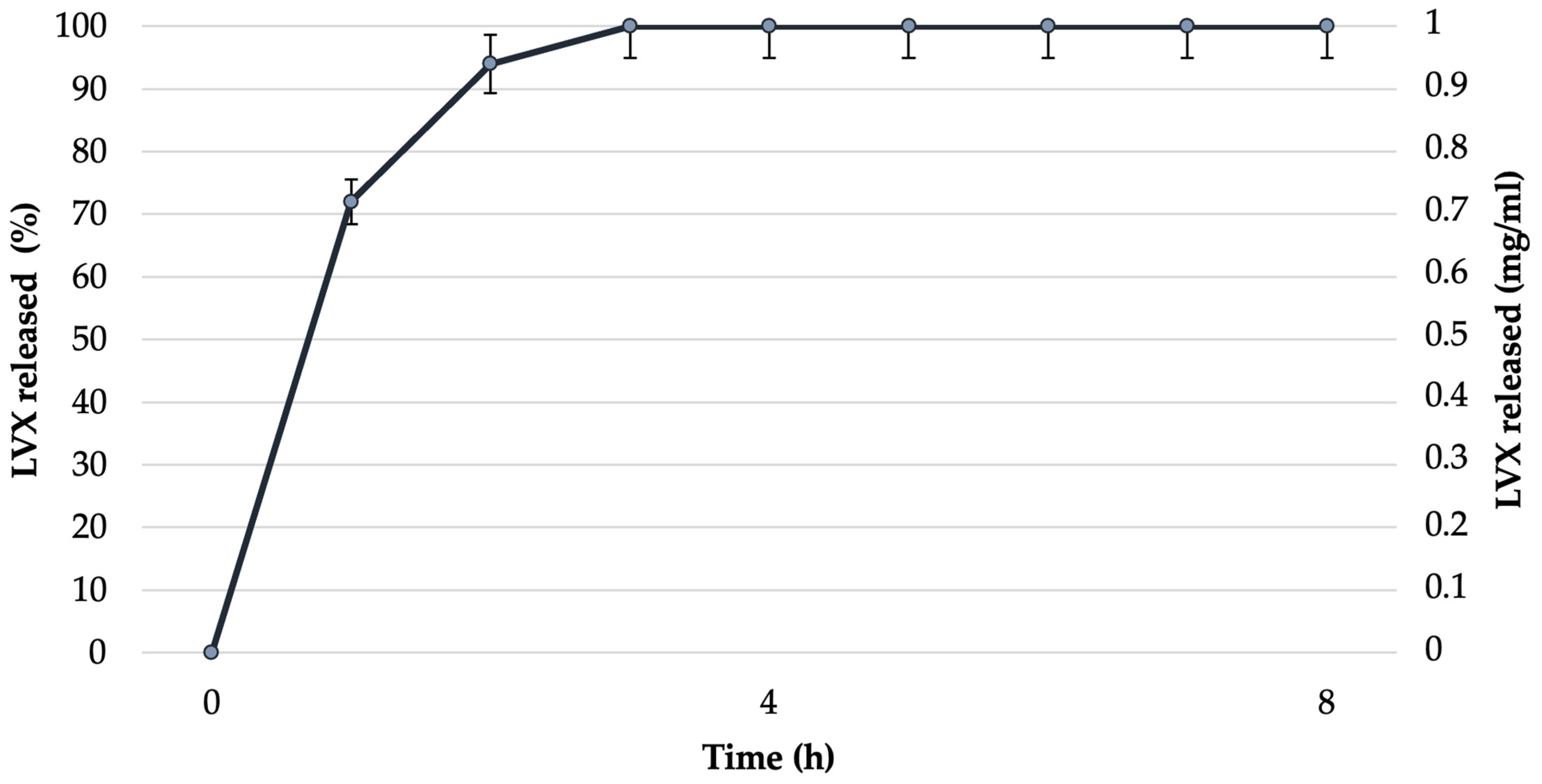
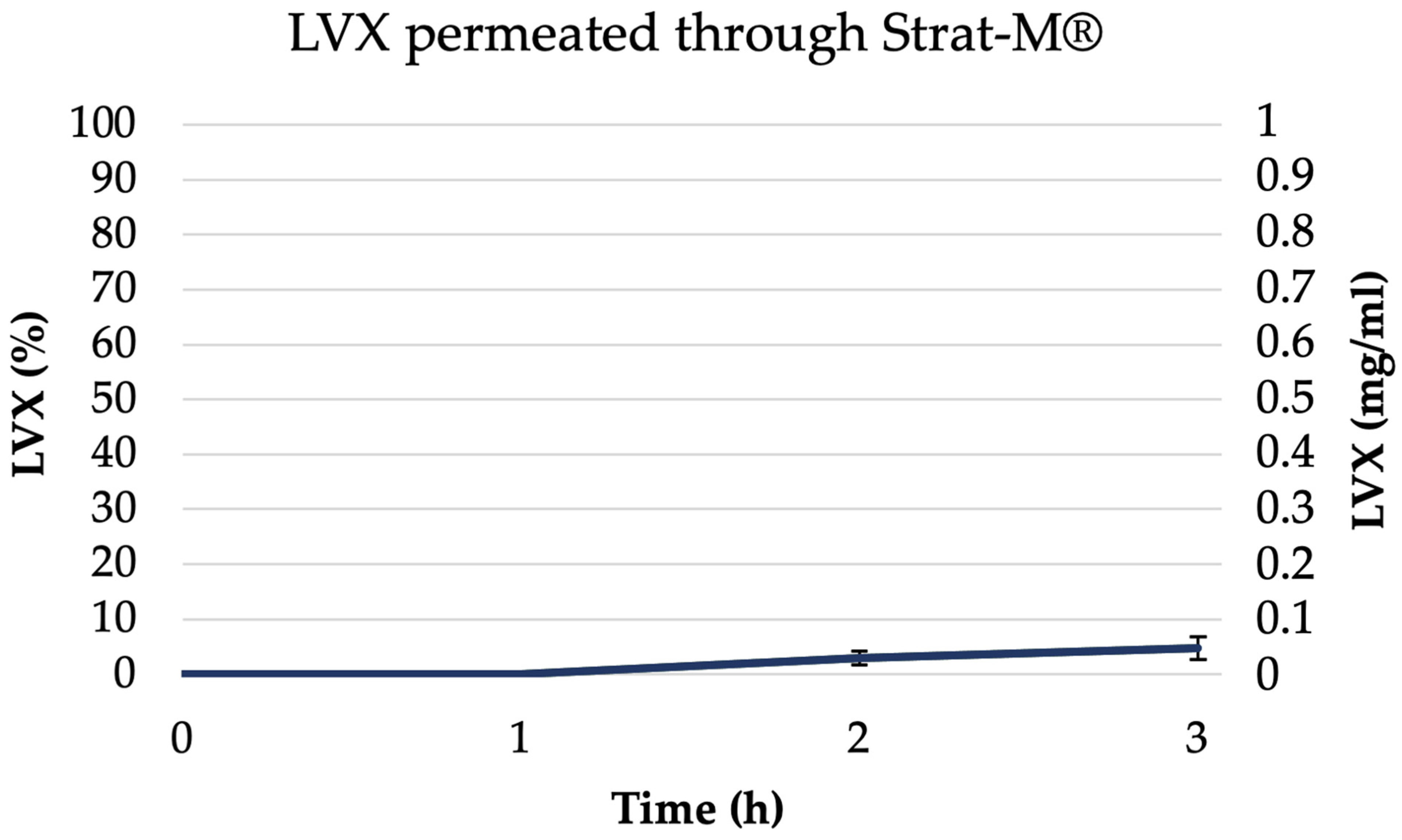
3.5. In Vitro Release Studies and Permeability Studies
3.6. Antibacterial Activity
3.7. Biofilm Inhibition and Eradication Ability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Szalay, S.; Wertz, P.W. Protective Barriers Provided by the Epidermis. Int. J. Mol. Sci. 2023, 24, 3145. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.S.; Park, H.S.; Cho, S.; Yoon, H.S. Antibiotic Susceptibility and Treatment Response in Bacterial Skin Infection. Ann. Dermatol. 2018, 30, 186–191. [Google Scholar] [CrossRef]
- Sollid, J.U.E.; Furberg, A.S.; Hanssen, A.M.; Johannessen, M. Staphylococcus aureus: Determinants of Human Carriage. Infect. Genet. Evol. 2014, 21, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, Y.; Ito, T.; Tamai, M.; Nakagawa, S.; Nakamura, Y. The Role of Staphylococcus aureus Quorum Sensing in Cutaneous and Systemic Infections. Inflamm. Regener. 2024, 44, 9. [Google Scholar] [CrossRef] [PubMed]
- Akbar, M.U.; Haque, A.; Liaquat, S.; Schierack, P.; Ali, A. Biofilm Formation by Staphylococcus epidermidis and Its Inhibition Using Carvacrol, 2-Aminobenzemidazole, and 3-Indole Acetonitrile. ACS Omega 2022, 8, 682–687. [Google Scholar] [CrossRef]
- Wu, X.; Wang, H.; Xiong, J.; Yang, G.-X.; Hu, J.-F.; Zhu, Q.; Chen, Z. Staphylococcus aureus Biofilm: Formulation, Regulatory, and Emerging Natural Products-Derived Therapeutics. Biofilm 2024, 7, 100175. [Google Scholar] [CrossRef] [PubMed]
- Aboelnaga, N.; Elsayed, S.W.; Abdelsalam, N.A.; Salem, S.; Saif, N.A.; Elsayed, M.; Ayman, S.; Nasr, M.; Elhadidy, M. Deciphering the Dynamics of Methicillin-Resistant Staphylococcus aureus biofilm Formation: From Molecular Signaling to Nanotherapeutic Advances. Cell Commun. Signal. 2024, 22, 188. [Google Scholar] [CrossRef] [PubMed]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, Research, and Development of New Antibiotics: The WHO Priority List of Antibiotic-Resistant Bacteria and Tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Rajapandi, S.; Nangan, S.; Natesan, T.; Kumar, A.; Dharman, G.; Pandeeswaran, M.; Verma, D.; Ubaidullah, M.; Pandit, B.; Dhaliwal, N.; et al. Ziziphus Mauritiana-Derived Nitrogen-Doped Biogenic Carbon Dots: Eco-Friendly Catalysts for Dye Degradation and Antibacterial Applications. Chemosphere 2023, 338, 139584. [Google Scholar] [CrossRef]
- Burt, S. Essential Oils: Their Antibacterial Properties and Potential Applications in Foods—A Review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Chi, Y.; Wang, Y.; Ji, M.; Li, Y.; Zhu, H.; Yan, Y.; Fu, D.; Zou, L.; Ren, B. Natural Products from Traditional Medicine as Promising Agents Targeting at Different Stages of Oral Biofilm Development. Front. Microbiol. 2022, 13, 955459. [Google Scholar] [CrossRef] [PubMed]
- Sehaki, C.; Jullian, N.; Ayati, F.; Fernane, F.; Gontier, E. A Review of Pistacia lentiscus Polyphenols: Chemical Diversity and Pharmacological Activities. Plants 2023, 12, 279. [Google Scholar] [CrossRef]
- Ben Khedir, S.; Moalla, D.; Jardak, N.; Mzid, M.; Sahnoun, Z.; Rebai, T. Pistacia Lentiscus Fruit Oil Reduces Oxidative Stress in Human Skin Explants Caused by Hydrogen Peroxide. Biotech. Histochem. 2016, 91, 480–491. [Google Scholar] [CrossRef]
- Poljšak, N.; Kreft, S.; Kočevar Glavač, N. Vegetable Butters and Oils in Skin Wound Healing: Scientific Evidence for New Opportunities in Dermatology. Phytother. Res. 2020, 34, 254–269. [Google Scholar] [CrossRef]
- Orrù, G.; Demontis, C.; Mameli, A.; Tuveri, E.; Coni, P.; Pichiri, G.; Coghe, F.; Rosa, A.; Rossi, P.; D’hallewin, G. The Selective Interaction of Pistacia lentiscus Oil vs. Human Streptococci, an Old Functional Food Revisited with New Tools. Front. Microbiol. 2017, 8, 2067. [Google Scholar] [CrossRef]
- Post, M.; Okruszko, A.; Szaflik, J.P. Levofloxacin in Everyday Ophthalmic Practice—Review. Klin. Ocz./Acta Ophthalmol. Pol. 2021, 123, 122–128. [Google Scholar] [CrossRef]
- Majalekar, P.P.; Shirote, P.J. Fluoroquinolones: Blessings Or Curses. Curr. Drug Targets 2020, 21, 1354–1370. [Google Scholar] [CrossRef]
- Madsen, M.; Messenger, K.; Papich, M.G. Pharmacokinetics of Levofloxacin Following Oral Administration of a Generic Levofloxacin Tablet and Intravenous Administration to Dogs. Am. J. Vet. Res. 2019, 80, 957–962. [Google Scholar] [CrossRef] [PubMed]
- Kistler, J.M.; Vroome, C.M.; Ramsey, F.V.; Ilyas, A.M. Increasing Multidrug Antibiotic Resistance in MRSA Infections of the Hand: A 10-Year Analysis of Risk Factors. Hand 2020, 15, 877–881. [Google Scholar] [CrossRef]
- Aboelenin, A.M.; El-Mowafy, M.; Saleh, N.M.; Shaaban, M.I.; Barwa, R. Ciprofloxacin- and Levofloxacin-Loaded Nanoparticles Efficiently Suppressed Fluoroquinolone Resistance and biofilm Formation in Acinetobacter baumannii. Sci. Rep. 2024, 14, 3125. [Google Scholar] [CrossRef]
- Zaharieva, M.M.; Kaleva, M.; Kroumov, A.; Slavkova, M.; Benbassat, N.; Yoncheva, K.; Najdenski, H. Advantageous Combinations of Nanoencapsulated Oregano Oil with Selected Antibiotics for Skin Treatment. Pharmaceutics 2022, 14, 2773. [Google Scholar] [CrossRef] [PubMed]
- Bastari, K.; Arshath, M.; Ng, Z.H.; Chia, J.H.; Yow, Z.X.; Sana, B.; Tan, M.F.; Lim, S.; Loo, S.C. A controlled release of antibiotics from calcium phosphate-coated poly(lactic-co-glycolic acid) particles and their in vitro efficacy against Staphylococcus aureus biofilm. J. Mater. Sci. Mater. Med. 2014, 25, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Czyrski, A. The Spectrophotometric Determination of Lipophilicity and Dissociation Constants of Ciprofloxacin and Levofloxacin. Spectrochim. Acta Part. A Mol. Biomol. Spectrosc. 2022, 265, 120343. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, F.; Hanieh, P.N.; Maurizi, L.; Longhi, C.; Uccelletti, D.; Schifano, E.; Del Favero, E.; Cantù, L.; Ricci, C.; Ammendolia, M.G.; et al. Neem Oil or Almond Oil Nanoemulsions for Vitamin E Delivery: From Structural Evaluation to In Vivo Assessment of Antioxidant and Anti-Inflammatory Activity. IJN 2022, 17, 6447–6465. [Google Scholar] [CrossRef] [PubMed]
- Mosallam, F.M.; Abbas, H.A.; Shaker, G.H.; Gomaa, S.E. Alleviating the Virulence of Pseudomonas aeruginosa and Staphylococcus aureus by Ascorbic Acid Nanoemulsion. Res. Microbiol. 2023, 174, 104084. [Google Scholar] [CrossRef] [PubMed]
- Sklenarova, R.; Allaw, M.; Perra, M.; Castangia, I.; Frankova, J.; Luis Pedraz, J.; Letizia Manca, M.; Manconi, M. Co-Delivering of Oleuropein and Lentisk Oil in Phospholipid Vesicles as an Effective Approach to Modulate Oxidative Stress, Cytokine Secretion and Promote Skin Regeneration. Eur. J. Pharm. Biopharm. 2023, 185, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Pachi, V.K.; Mikropoulou, E.V.; Gkiouvetidis, P.; Siafakas, K.; Argyropoulou, A.; Angelis, A.; Mitakou, S.; Halabalaki, M. Traditional Uses, Phytochemistry and Pharmacology of Chios Mastic Gum (Pistacia lentiscus Var. Chia, Anacardiaceae): A Review. J. Ethnopharmacol. 2020, 254, 112485. [Google Scholar] [CrossRef] [PubMed]
- Allaw, M.; Manconi, M.; Caboni, P.; Bacchetta, G.; Escribano-Ferrer, E.; Peris, J.E.; Nacher, A.; Diez-Sales, O.; Manca, M.L. Formulation of Liposomes Loading Lentisk Oil to Ameliorate Topical Delivery, Attenuate Oxidative Stress Damage and Improve Cell Migration in Scratch Assay. Biomed. Pharmacother. 2021, 144, 112351. [Google Scholar] [CrossRef] [PubMed]
- Murugesan, V.; Govindarasu, M.; Manoharadas, S.; Pandiaraj, S.; Thiruvengadam, M.; Govindasamy, R.; Vaiyapuri, M. Combinatorial Anticancer Effects of Multi Metal Ion and Drug Substitute with Hydroxyapatite Coatings on Surgical Grade 316LSS Stainless Steel Alloys towards Biomedical Applications. J. Mater. Res. Technol. 2023, 27, 7244–7258. [Google Scholar] [CrossRef]
- Maccelli, A.; Vitanza, L.; Imbriano, A.; Fraschetti, C.; Filippi, A.; Goldoni, P.; Maurizi, L.; Ammendolia, M.G.; Crestoni, M.E.; Fornarini, S.; et al. Satureja montana L. Essential Oils: Chemical Profiles/Phytochemical Screening, Antimicrobial Activity and O/W NanoEmulsion Formulations. Pharmaceutics 2019, 12, 7. [Google Scholar] [CrossRef]
- Djebari, S.; Wrona, M.; Boudria, A.; Madani, K.; Nerin, C. Pistacia lentiscus L. Vegetable Oil: Physicochemical Quality, Composition and Antibacterial Capacity. Flavour Fragr. J. 2023, 38, 426–441. [Google Scholar] [CrossRef]
- Spano, M.; Di Matteo, G.; Fernandez Retamozo, C.A.; Lasalvia, A.; Ruggeri, M.; Sandri, G.; Cordeiro, C.; Sousa Silva, M.; Totaro Fila, C.; Garzoli, S.; et al. A Multimethodological Approach for the Chemical Characterization of Edible Insects: The Case Study of Acheta Domesticus. Foods 2023, 12, 2331. [Google Scholar] [CrossRef]
- Fraschetti, C.; Goci, E.; Nicolescu, A.; Cairone, F.; Carradori, S.; Filippi, A.; Palmieri, V.; Mocan, A.; Cesa, S. Pomegranate Fruit Cracking during Maturation: From Waste to Valuable Fruits. Foods 2023, 12, 1908. [Google Scholar] [CrossRef] [PubMed]
- Recinella, L.; Chiavaroli, A.; Masciulli, F.; Fraschetti, C.; Filippi, A.; Cesa, S.; Cairone, F.; Gorica, E.; De Leo, M.; Braca, A.; et al. Protective Effects Induced by a Hydroalcoholic Allium sativum Extract in Isolated Mouse Heart. Nutrients 2021, 13, 2332. [Google Scholar] [CrossRef] [PubMed]
- Lioupi, A.; Sampsonidis, I.; Virgiliou, C.; Papoti, V.T.; Zinoviadou, K.G.; Spyros, A.; Theodoridis, G. Optimisation of the HS-SPME/GC-MS Approach by Design of Experiments Combined with Chemometrics for the Classification of Cretan Virgin Olive Oils. Metabolites 2022, 12, 114. [Google Scholar] [CrossRef] [PubMed]
- Loyte, A.; Suryawanshi, J.; Bhiogade, G.; Devarajan, Y.; T, R.; T, G. Novel Approach for Efficient Operation and Reduced Harmful Emissions on a Dual-Fuel Research Engine Propelled with Hydrogen-Enriched Natural Gas and Diesel. Energy Sources Part A Recovery Util. Environ. Eff. 2023, 45, 8218–8238. [Google Scholar] [CrossRef]
- Rinaldi, F.; Maurizi, L.; Conte, A.L.; Marazzato, M.; Maccelli, A.; Crestoni, M.E.; Hanieh, P.N.; Forte, J.; Conte, M.P.; Zagaglia, C.; et al. Nanoemulsions of Satureja montana Essential Oil: Antimicrobial and Antibiofilm Activity against Avian Escherichia coli Strains. Pharmaceutics 2021, 13, 134. [Google Scholar] [CrossRef] [PubMed]
- Singh, Y.; Meher, J.G.; Raval, K.; Khan, F.A.; Chaurasia, M.; Jain, N.K.; Chourasia, M.K. Nanoemulsion: Concepts, Development and Applications in Drug Delivery. J. Control. Release 2017, 252, 28–49. [Google Scholar] [CrossRef] [PubMed]
- Provencher, S.W. CONTIN: A General Purpose Constrained Regularization Program for Inverting Noisy Linear Algebraic and Integral Equations. Comput. Phys. Commun. 1982, 27, 229–242. [Google Scholar] [CrossRef]
- De Vos, C.; Deriemaeker, L.; Finsy, R. Quantitative Assessment of the Conditioning of the Inversion of Quasi-Elastic and Static Light Scattering Data for Particle Size Distributions. Langmuir 1996, 12, 2630–2636. [Google Scholar] [CrossRef]
- Rinaldi, F.; Hanieh, P.N.; Imbriano, A.; Passeri, D.; Del Favero, E.; Rossi, M.; Marianecci, C.; De Panfilis, S.; Carafa, M. Different Instrumental Approaches to Understand the Chitosan Coated Niosomes/Mucin Interaction. J. Drug Deliv. Sci. Technol. 2020, 55, 101339. [Google Scholar] [CrossRef]
- Rinaldi, F.; Hanieh, P.N.; Sennato, S.; Santis, F.D.; Forte, J.; Fraziano, M.; Casciardi, S.; Marianecci, C.; Bordi, F.; Carafa, M. Rifampicin–Liposomes for Mycobacterium abscessus Infection Treatment: Intracellular Uptake and Antibacterial Activity Evaluation. Pharmaceutics 2021, 13, 1070. [Google Scholar] [CrossRef] [PubMed]
- Alhasso, B.; Ghori, M.U.; Conway, B.R. Development of Nanoemulsions for Topical Application of Mupirocin. Pharmaceutics 2023, 15, 378. [Google Scholar] [CrossRef]
- Stepanović, S.; Vuković, D.; Hola, V.; Bonaventura, G.D.; Djukić, S.; Ćirković, I.; Ruzicka, F. Quantification of Biofilm in Microtiter Plates: Overview of Testing Conditions and Practical Recommendations for Assessment of Biofilm Production by Staphylococci. APMIS 2007, 115, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Lagha, M.; Bothma, J.P.; Levine, M. Mechanisms of Transcriptional Precision in Animal Development. Trends Genet. 2012, 28, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Souilah, N.; Amina, B.; Hamdi, B.; Miara, M.D.; Daoud, N.; Mustafa, A.M.; Yilmaz, M.A.; Öztürk, M.; Caprioli, G.; Maggi, F. Ethnobotanical Investigation of Pistacia lentiscus L. Grown in El Kala (Algeria), and Phytochemical Study and Antioxidant Activity of Its Essential Oil and Extracts. Nat. Prod. Res. 2023, 37, 1583–1588. [Google Scholar] [CrossRef] [PubMed]
- Ingallina, C.; Maccelli, A.; Spano, M.; Matteo, G.D.; Sotto, A.D.; Giusti, A.M.; Vinci, G.; Giacomo, S.D.; Rapa, M.; Ciano, S.; et al. Chemico-Biological Characterization of Torpedino Di Fondi® Tomato Fruits: A Comparison with San Marzano Cultivar at Two Ripeness Stages. Antioxidants 2020, 9, 1027. [Google Scholar] [CrossRef] [PubMed]
- Kitahara, T.; Koyama, N.; Matsuda, J.; Aoyama, Y.; Hirakata, Y.; Kamihira, S.; Kohno, S.; Nakashima, M.; Sasaki, H. Antimicrobial activity of saturated fatty acids and fatty amines against methicillin-resistant Staphylococcus aureus. Biol. Pharm. Bull. 2004, 27, 1321–1326. [Google Scholar] [CrossRef]
- Zheng, C.J.; Yoo, J.-S.; Lee, T.-G.; Cho, H.-Y.; Kim, Y.-H.; Kim, W.-G. Fatty Acid Synthesis Is a Target for Antibacterial Activity of Unsaturated Fatty Acids. FEBS Lett. 2005, 579, 5157–5162. [Google Scholar] [CrossRef]
- Watanabe, T.; Yamamoto, Y.; Miura, M.; Konno, H.; Yano, S.; Nonomura, Y. Systematic Analysis of Selective Bactericidal Activity of Fatty Acids against Staphylococcus aureus with Minimum Inhibitory Concentration and Minimum Bactericidal Concentration. J. Oleo Sci. 2019, 68, 291–296. [Google Scholar] [CrossRef]
- Baccouri, B.; Rajhi, I. Potential antioxidant activity of terpenes. In Terpenes and Terpenoids—Recent Advances; IntechOpen: London, UK, 2021; pp. 53–62. [Google Scholar]
- Coman, V.; Vodnar, D.C. Hydroxycinnamic Acids and Human Health: Recent Advances. J. Sci. Food Agric. 2020, 100, 483–499. [Google Scholar] [CrossRef] [PubMed]
- Lou, Z.; Wang, H.; Zhu, S.; Ma, C.; Wang, Z. Antibacterial Activity and Mechanism of Action of Chlorogenic Acid. J. Food Sci. 2011, 76, 398–403. [Google Scholar] [CrossRef]
- Guimarães, A.C.; Meireles, L.M.; Lemos, M.F.; Guimarães, M.C.C.; Endringer, D.C.; Fronza, M.; Scherer, R. Antibacterial Activity of Terpenes and Terpenoids Present in Essential Oils. Molecules 2019, 24, 2471. [Google Scholar] [CrossRef] [PubMed]
- Walczak, M.; Michalska-Sionkowska, M.; Olkiewicz, D.; Tarnawska, P.; Warżyńska, O. Potential of Carvacrol and Thymol in Reducing Biofilm Formation on Technical Surfaces. Molecules 2021, 6, 2723. [Google Scholar] [CrossRef] [PubMed]
- Sarria, S.; Wong, B.; García Martín, H.; Keasling, J.D.; Peralta-Yahya, P. Microbial synthesis of pinene. ACS Synth. Biol. 2014, 8, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Cushnie, T.P.; Lamb, A.J. Recent advances in understanding the antibacterial properties of flavonoids. Int. J. Antimicrob. Agents 2011, 38, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Kamal-Eldin, A.; Appelqvist, L.A. The chemistry and antioxidant properties of tocopherols and tocotrienols. Lipids 1996, 31, 671–701. [Google Scholar] [CrossRef] [PubMed]
- Vuković, S.; Popović-Djordjević, J.B.; Kostić, A.Ž.; Pantelić, N.D.; Srećković, N.; Akram, M.; Laila, U.; Stanković, J.S.K. Allium Species in the Balkan Region—Major Metabolites, Antioxidant and Antimicrobial Properties. Horticulturae 2023, 9, 408. [Google Scholar] [CrossRef]
- Rigling, M.; Fraatz, M.A.; Trögel, S.; Sun, J.; Zorn, H.; Zhang, Y. Aroma Investigation of Chios Mastic Gum (Pistacia Lentiscus Variety Chia) Using Headspace Gas Chromatography Combined with Olfactory Detection and Chiral Analysis. J. Agric. Food Chem. 2019, 67, 13420. [Google Scholar] [CrossRef]
- Tsala, D.E.; Amadou, D.; Habtemariam, S. Natural Wound Healing and Bioactive Natural Products. Phytopharmacology 2013, 4, 532–560. [Google Scholar]
- Li, H.Y.; Yang, W.Q.; Zhou, X.Z.; Shao, F.; Shen, T.; Guan, H.Y.; Zheng, J.; Zhang, L.M. Antibacterial and antifungal sesquiterpenoids: Chemistry, resource, and activity. Biomolecules 2022, 12, 1271. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, C. Effect of Stirring Speed on Particle Dispersion in Silica Synthesis. Nano-Struct. Nano-Objects 2023, 35, 100994. [Google Scholar] [CrossRef]
- Rashid, S.A.; Bashir, S.; Ullah, H.; Shah, K.U.; Khan, D.H.; Shah, P.A.; Danish, M.Z.; Khan, M.H.; Mahmood, S.; Sohaib, M.; et al. Development, Characterization and Optimization of Methotrexate-Olive Oil Nano-Emulsion for Topical Application. Pak. J. Pharm. Sci. 2021, 43, 205–215. [Google Scholar]
- Moraru, C.; Mincea, M.; Menghiu, G.; Ostafe, V. Understanding the Factors Influencing Chitosan-Based Nanoparticles-Protein Corona Interaction and Drug Delivery Applications. Molecules 2020, 25, 4758. [Google Scholar] [CrossRef]
- Ferreira, M.; Pinto, S.N.; Aires-da-Silva, F.; Bettencourt, A.; Aguiar, S.I.; Gaspar, M.M. Liposomes as a Nanoplatform to Improve the Delivery of Antibiotics into Staphylococcus aureus biofilms. Pharmaceutics 2021, 13, 321. [Google Scholar] [CrossRef] [PubMed]
- Boratto, F.A.; Lages, E.B.; Loures, C.M.C.; Sabino, A.P.; Malachias, A.; Townsend, D.M.; Branco De Barros, A.L.; Miranda Ferreira, L.A.; Amaral Leite, E. Alpha-Tocopheryl Succinate and Doxorubicin-Loaded Liposomes Improve Drug Uptake and Tumor Accumulation in a Murine Breast Tumor Model. Biomed. Pharmacother. 2023, 165, 115034. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Kang, B.; Yang, E.; Kim, K.; Kwak, M.K.; Chang, P.-S.; Jung, H.-S. Precise Control of Liposome Size Using Characteristic Time Depends on Solvent Type and Membrane Properties. Sci. Rep. 2023, 13, 4728. [Google Scholar] [CrossRef]
- Shah, S.; Dhawan, V.; Holm, R.; Nagarsenker, M.S.; Perrie, Y. Liposomes: Advancements and innovation in the manufacturing process. Adv. Drug Deliv. Rev. 2020, 154–155, 102–122. [Google Scholar] [CrossRef]
- Gayathiri, E.; Prakash, P.; Karmegam, N.; Varjani, S.; Awasthi, M.K.; Ravindran, B. Biosurfactants: Potential and Eco-Friendly Material for Sustainable Agriculture and Environmental Safety—A Review. Agronomy 2022, 12, 662. [Google Scholar] [CrossRef]
- Montes de Oca-Ávalos, J.M.; Candal, R.J.; Herrera, M.L. Nanoemulsions: Stability and physical properties. Curr. Opin. Food Sci. 2017, 16, 1–6. [Google Scholar] [CrossRef]
- Sennato, S.; Bordi, F.; Cametti, C.; Marianecci, C.; Carafa, M.; Cametti, M. Hybrid Niosome Complexation in the Presence of Oppositely Charged Polyions. J. Phys. Chem. B 2008, 112, 3720–3727. [Google Scholar] [CrossRef] [PubMed]
- Crommelin, D.J.A. Influence of Lipid Composition and Ionic Strength on the Physical Stability of Liposomes. J. Pharm. Sci. 1984, 73, 1559–1563. [Google Scholar] [CrossRef]
- Alshawwa, S.Z.; Kassem, A.A.; Farid, R.M.; Mostafa, S.K.; Labib, G.S. Nanocarrier Drug Delivery Systems: Characterization, Limitations, Future Perspectives and Implementation of Artificial Intelligence. Pharmaceutics 2022, 14, 883. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.K.; Thareja, S. In vitro and in vivo characterization of pharmaceutical nanocarriers used for drug delivery. Artif. Cells Nanomed. Biotechnol. 2019, 47, 524–539. [Google Scholar] [CrossRef]
- D’Souza, S. A Review of In Vitro Drug Release Test Methods for Nano-Sized Dosage Forms. Adv. Pharm. 2014, 2014, 304757. [Google Scholar] [CrossRef]
- Venkatesan, P.; Govindasamy, R.; Periyasami, G.; Rahaman, M.; Pandiaraj, S.; Thiruvengadam, M.; Thirumalaivasan, N.; Wu, S.-P. Eco-Friendly, Bright Luminescent Carbon Dots and Their Potential Applications for Detecting Hypochlorous Acid in Water and Live Cell Imaging. J. Mater. Res. Technol. 2023, 24, 6522–6532. [Google Scholar] [CrossRef]
- Pulsoni, I.; Lubda, M.; Aiello, M.; Fedi, A.; Marzagalli, M.; von Hagen, J.; Scaglione, S. Comparison Between Franz Diffusion Cell and a novel Micro-physiological System for In Vitro Penetration Assay Using Different Skin Models. SLAS Technol. 2022, 27, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Blokhina, S.; Sharapova, A.V.; Ol’khovich, M.; Volkova, T.V.; Perlovich, G.L. Solubility, lipophilicity and membrane permeability of some fluoroquinolone antimicrobials. Eur. J. Pharm. Sci. 2016, 93, 29–37. [Google Scholar] [CrossRef]
- Casillas-Vargas, G.; Ocasio-Malavé, C.; Medina, S.; Morales-Guzmán, C.; Del Valle, R.G.; Carballeira, N.M.; Sanabria-Ríos, D.J. Antibacterial fatty acids: An update of possible mechanisms of action and implications in the development of the next-generation of antibacterial agents. Prog. Lipid Res. 2021, 82, 101093. [Google Scholar] [CrossRef]
- Milia, E.; Bullitta, S.M.; Mastandrea, G.; Szotáková, B.; Schoubben, A.; Langhansová, L.; Quartu, M.; Bortone, A.; Eick, S. Leaves and Fruits Preparations of Pistacia lentiscus L.: A Review on the Ethnopharmacological Uses and Implications in Inflammation and Infection. Antibiotics 2021, 10, 425. [Google Scholar] [CrossRef]
- Radovic-Moreno, A.F.; Lu, T.K.; Puscasu, V.A.; Yoon, C.J.; Langer, R.; Farokhzad, O.C. Surface charge-switching polymeric nanoparticles for bacterial cell wall-targeted delivery of antibiotics. ACS Nano 2012, 6, 4279–4287. [Google Scholar] [CrossRef]
- Zhou, G.; Peng, H.; Wang, Y.S.; Huang, X.M.; Xie, X.B.; Shi, Q.S. Enhanced synergistic effects of xylitol and isothiazolones for inhibition of initial biofilm formation by Pseudomonas aeruginosa ATCC 9027 and Staphylococcus aureus ATCC 6538. J. Oral Sci. 2019, 61, 255–263. [Google Scholar] [CrossRef]
- Fernandez, J.; Martin-Serrano, Á.; Gómez-Casanova, N.; Falanga, A.; Galdiero, S.; Javier De La Mata, F.; Heredero-Bermejo, I.; Ortega, P. Effect of the Combination of Levofloxacin with Cationic Carbosilane Dendron and Peptide in the Prevention and Treatment of Staphylococcus aureus biofilms. Polymers 2021, 13, 2127. [Google Scholar] [CrossRef]
- King, J.A.; Ratner, B.D. Levofloxacin Impregnation and Extended Release: Concentration Model for Insulin Catheters. Biomed. Eng. Adv. 2023, 5, 100081. [Google Scholar] [CrossRef]
- Yassien, M.; Khardori, N.; Ahmedy, A.; Toama, M. Modulation of Biofilms of Pseudomonas aeruginosa by Quinolones. Antimicrob. Agents Chemother. 1995, 39, 2262–2268. [Google Scholar] [CrossRef]
- Mazzantini, D.; Massimino, M.; Calvigioni, M.; Rossi, V.; Celandroni, F.; Lupetti, A.; Batoni, G.; Ghelardi, E. Anti-Staphylococcal biofilm Effects of a Liposome-Based Formulation Containing Citrus Polyphenols. Antibiotics 2024, 13, 318. [Google Scholar] [CrossRef]
- Landis, R.F.; Li, C.-H.; Gupta, A.; Lee, Y.-W.; Yazdani, M.; Ngernyuang, N.; Altinbasak, I.; Mansoor, S.; Khichi, M.A.S.; Sanyal, A.; et al. Biodegradable Nanocomposite Antimicrobials for the Eradication of Multidrug-Resistant Bacterial Biofilms without Accumulated Resistance. J. Am. Chem. Soc. 2018, 140, 6176–6182. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Mahida, N.; Ho, G.; Pena, E.; Makabenta, J.M.V.; Aneke, S.; Jiang, M.; Bouthillette, L.M.; Holz, S.E.; Hassan, M.A.; et al. Integration of Antimicrobials and Delivery Systems: Synergistic Antibiofilm Activity with Biodegradable Nanoemulsions Incorporating Pseudopyronine Analogs. Antibiotics 2023, 12, 1240. [Google Scholar] [CrossRef] [PubMed]
- Anderl, J.N.; Franklin, M.J.; Stewart, P.S. Role of Antibiotic Penetration Limitation in Klebsiella Pneumoniae biofilm Resistance to Ampicillin and Ciprofloxacin. Antimicrob. Agents Chemother. 2000, 44, 1818–1824. [Google Scholar] [CrossRef] [PubMed]
- Idrees, M.; Sawant, S.; Karodia, N.; Rahman, A. Staphylococcus aureus biofilm: Morphology, Genetics, Pathogenesis and Treatment Strategies. Int. J. Environ. Res. Public Health 2021, 18, 7602. [Google Scholar] [CrossRef]
- Meers, P.; Neville, M.; Malinin, V.; Scotto, A.W.; Sardaryan, G.; Kurumunda, R.; Mackinson, C.; James, G.; Fisher, S.; Perkins, W.R. Biofilm Penetration, Triggered Release and in Vivo Activity of Inhaled Liposomal Amikacin in Chronic Pseudomonas aeruginosa Lung Infections. J. Antimicrob. Chemother. 2008, 61, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Raj, D.S.; Dhamodharan, D.; Thanigaivel, S.; Vickram, A.S.; Byun, H.-S. Nanoemulsion as an Effective Inhibitor of Biofilm-Forming Bacterial Associated Drug Resistance: An Insight into COVID Based Nosocomial Infections. Biotechnol. Bioprocess Eng. 2022, 27, 543–555. [Google Scholar] [CrossRef] [PubMed]
- Huh, A.J.; Kwon, Y.J. “Nanoantibiotics”: A New Paradigm for Treating Infectious Diseases Using Nanomaterials in the Antibiotics Resistant Era. J. Control. Release 2011, 156, 128–145. [Google Scholar] [CrossRef] [PubMed]
- Allkja, J.; Van Charante, F.; Aizawa, J.; Reigada, I.; Guarch-Pérez, C.; Vazquez-Rodriguez, J.A.; Cos, P.; Coenye, T.; Fallarero, A.; Zaat, S.A.J.; et al. Interlaboratory Study for the Evaluation of Three Microtiter Plate-Based biofilm Quantification Methods. Sci. Rep. 2021, 11, 13779. [Google Scholar] [CrossRef] [PubMed]
- Jorge, P.; Lourenço, A.; Pereira, M.O. Data Quality in biofilm High-Throughput Routine Analysis: Intralaboratory Protocol Adaptation and Experiment Reproducibility. J. AOAC Int. 2015, 98, 1721–1727. [Google Scholar] [CrossRef]
- Pinto, R.M.; Lopes-de-Campos, D.; Martins, M.C.L.; Van Dijck, P.; Nunes, C.; Reis, S. Impact of Nanosystems in Staphylococcus aureus biofilms Treatment. FEMS Microbiol. Rev. 2019, 43, 622–641. [Google Scholar] [CrossRef]



| Sample | LO (mg/mL) | Brij O10 (mg/mL) | LVX (mg/mL) |
|---|---|---|---|
| NEs | 0.3 | 0.1 | - |
| NEsL | 0.3 | 0.1 | 1.0 |
| Hydrodynamic Diameter ± SD (nm) | PDI ± SD | ζ-Potential ± SD (mV) | EE (%) | Anisotropy A.U. (Fluidity) | |
|---|---|---|---|---|---|
| NEs | 165.7 ± 2.5 | 0.22 ± 0.02 | −24.3 ± 0.2 | - | 0.24 |
| NEsL | 152.6 ± 2.9 | 0.21 ± 0.01 | −22.9 ± 1.2 | 100 | 0.26 |
| MIC (µg/mL) | MBC (µg/mL) | |||||
|---|---|---|---|---|---|---|
| NEs | NEsL | LVX | NEs | NEsL | LVX | |
| S. aureus ATCC 6538P | 2000 | 0.08 | 0.08 | 2000 | 0.08 | 0.08 |
| S. aureus DA | 2000 | 0.63 | 0.63 | 2000 | 0.63 | 0.63 |
| S. aureus HC | 2000 | 0.31 | 0.08 | 2000 | 0.31 | 0.08 |
| S. epidermidis ATCC 35984 | 1000 | 0.31 | 0.31 | 1000 | 0.63 | 0.31 |
| Biofilm Inhibition | Biofilm Eradication | |||||||
|---|---|---|---|---|---|---|---|---|
| Strains | NEs | NEsL | LVX | LO | NEs | NEsL | LVX | LO |
| S. aureus ATCC 6538P | 0.3 ± 1.3 * | 14.0 ± 12.8 * | 0.6 ± 1.0 | 14.7 ± 2.9 * | 24.4 ± 2.9 * | 3.2 ± 3.0 * | 16.0 ± 3.5 * | 4.5 ± 2.1 |
| S. aureus DA | 25.0 ± 2.5 | 36.25± 12.3 | 24.7 ± 22.0 | 4.75 ± 9.5 | 21.0 ± 15.6 | 71.0 ± 10.0 | 30.8 ± 3.2 | 31.0 ± 4.3 |
| S. aureus HC | 24.1 ± 5.6 | 8.0 ± 6.0 * | 23.0 ± 9.5 | 19.5 ± 6.3 | 40.0 ± 17.0 | 41.0 ± 0.3 | 33.7 ± 2.0 * | 33.0 ± 4.8 |
| S. epidermidis ATCC 35984 | 0.2 ± 1.5 | 30.0 ± 5.0 | 56.0 ± 7.0 | 29.3 ± 9.0 | 35.0 ± 2.3 | 21.0 ± 7.5 | 15.0 ± 4.2 | 15.5 ± 4.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maurizi, L.; Lasalvia, A.; Fabiano, M.G.; D’Intino, E.; Del Cioppo, F.; Fraschetti, C.; Filippi, A.; Ammendolia, M.G.; Conte, A.L.; Forte, J.; et al. Lentisk (Pistacia lentiscus) Oil Nanoemulsions Loaded with Levofloxacin: Phytochemical Profiles and Antibiofilm Activity against Staphylococcus spp. Pharmaceutics 2024, 16, 927. https://doi.org/10.3390/pharmaceutics16070927
Maurizi L, Lasalvia A, Fabiano MG, D’Intino E, Del Cioppo F, Fraschetti C, Filippi A, Ammendolia MG, Conte AL, Forte J, et al. Lentisk (Pistacia lentiscus) Oil Nanoemulsions Loaded with Levofloxacin: Phytochemical Profiles and Antibiofilm Activity against Staphylococcus spp. Pharmaceutics. 2024; 16(7):927. https://doi.org/10.3390/pharmaceutics16070927
Chicago/Turabian StyleMaurizi, Linda, Alba Lasalvia, Maria Gioia Fabiano, Eleonora D’Intino, Francesca Del Cioppo, Caterina Fraschetti, Antonello Filippi, Maria Grazia Ammendolia, Antonietta Lucia Conte, Jacopo Forte, and et al. 2024. "Lentisk (Pistacia lentiscus) Oil Nanoemulsions Loaded with Levofloxacin: Phytochemical Profiles and Antibiofilm Activity against Staphylococcus spp." Pharmaceutics 16, no. 7: 927. https://doi.org/10.3390/pharmaceutics16070927
APA StyleMaurizi, L., Lasalvia, A., Fabiano, M. G., D’Intino, E., Del Cioppo, F., Fraschetti, C., Filippi, A., Ammendolia, M. G., Conte, A. L., Forte, J., Corinti, D., Crestoni, M. E., Carafa, M., Marianecci, C., Rinaldi, F., & Longhi, C. (2024). Lentisk (Pistacia lentiscus) Oil Nanoemulsions Loaded with Levofloxacin: Phytochemical Profiles and Antibiofilm Activity against Staphylococcus spp. Pharmaceutics, 16(7), 927. https://doi.org/10.3390/pharmaceutics16070927










