Hot-Melt Extrusion-Based Dexamethasone–PLGA Implants: Physicochemical, Physicomechanical, and Surface Morphological Properties and In Vitro Release Corrected for Drug Degradation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials for Implant Manufacture
2.2. Material for LC-MS/MS Analysis
3. Methods—Implant Manufacture
3.1. Implant Formulations
3.2. Implant Manufacture by Hot-Melt Extrusion (HME)
3.3. Methods—Physicochemical Characterization
3.3.1. Implant Dexamethasone Content Determined by HPLC
3.3.2. Thermal Analysis—Using Differential Scanning Calorimetry (DSC)
3.4. Methods—Physicomechanical Characterization
3.4.1. Melt Viscosity
3.4.2. Influence of Humidity
Three-Point Bend (TPB) Tests Using a Texture Analyzer
Moisture Adsorption of Implants under High Relative Humidity
3.5. Methods—Surface Morphological Properties
3.5.1. Atomic Force Microscopy (AFM)
3.5.2. Polarized Light Microscopy (PLM) on Implant Films
3.5.3. Scanning Electron Microscopy (SEM)
3.5.4. Powder X-ray Diffraction (XRD) Analysis
3.6. Methods—In Vitro Release Testing
3.6.1. Large-Volume (100 mL) In Vitro Release Studies
3.6.2. HPLC-ESI+-MS/MS Detection and Quantification of Dexamethasone Release
3.6.3. Degradation Rate Constant and Concentration Correction in Phosphate-Buffered Saline Solution (PBS pH 7.4)
4. Results and Discussion
4.1. Dexamethasone Implant Manufacturing
4.2. Physiochemical Characterizations
4.2.1. Implant Dexamethasone Content Determined by HPLC
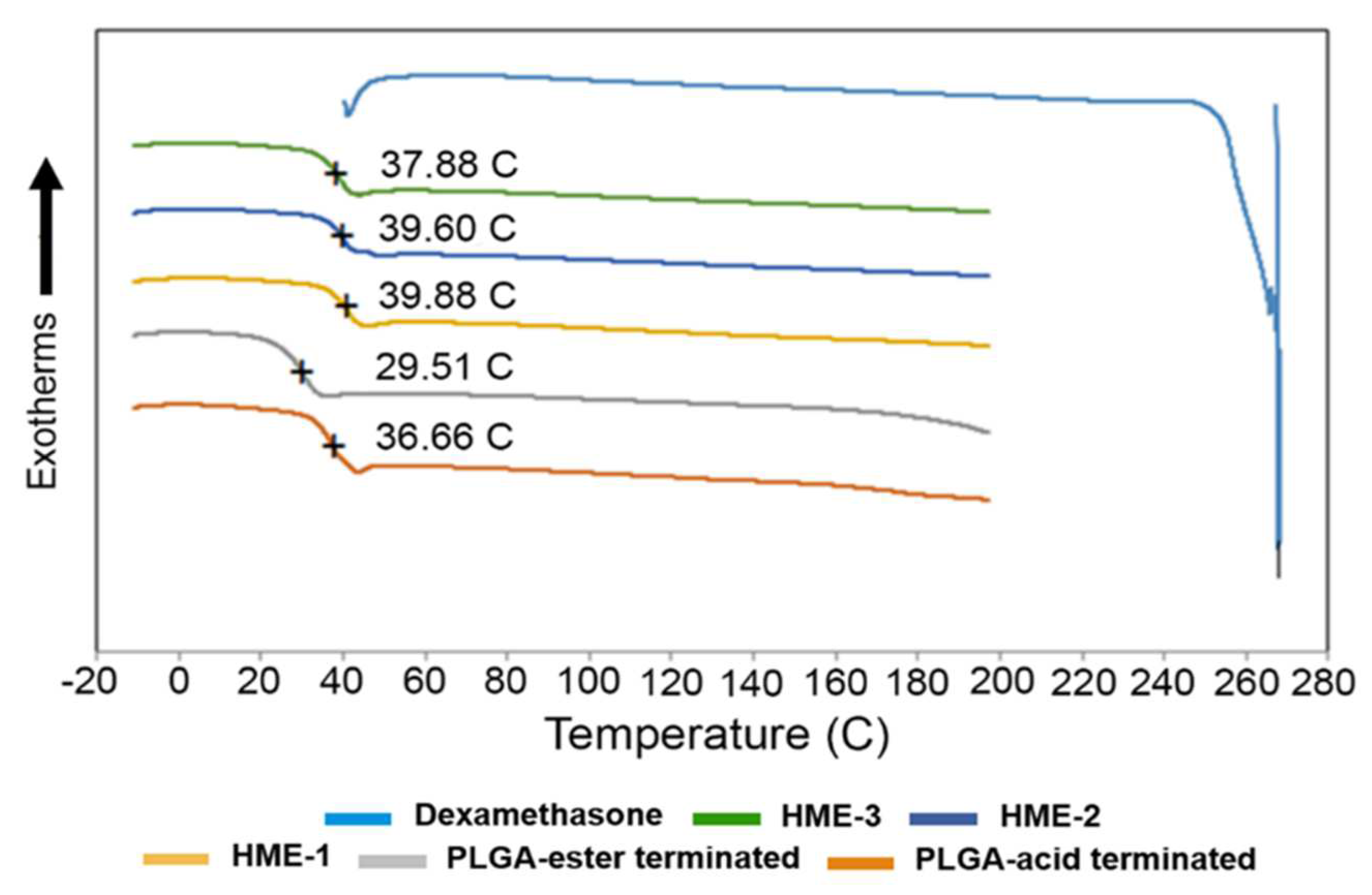
4.2.2. Thermal Analysis—Glass Transition Temperature (Tg) for Implants and Formulation Components Using Differential Scanning Calorimetry (DSC)
4.3. Physicomechanical Characterizations
4.3.1. Melt Viscosity of Implant Compositions
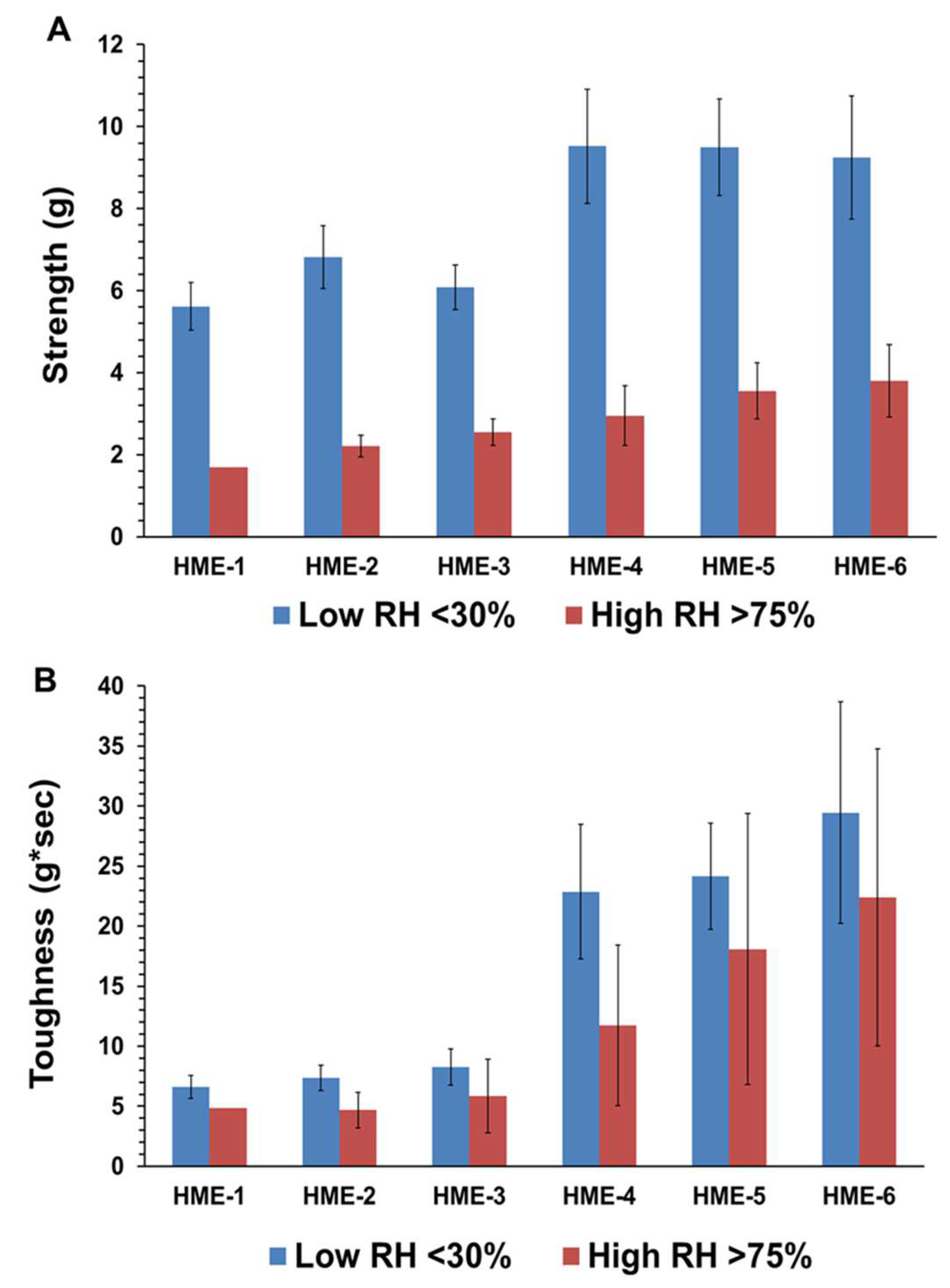
4.3.2. Three-Point Bend (TPB) Results
Influence of Humidity on Moisture Adsorption and TPB Mechanical Properties
4.4. Surface Morphological Properties
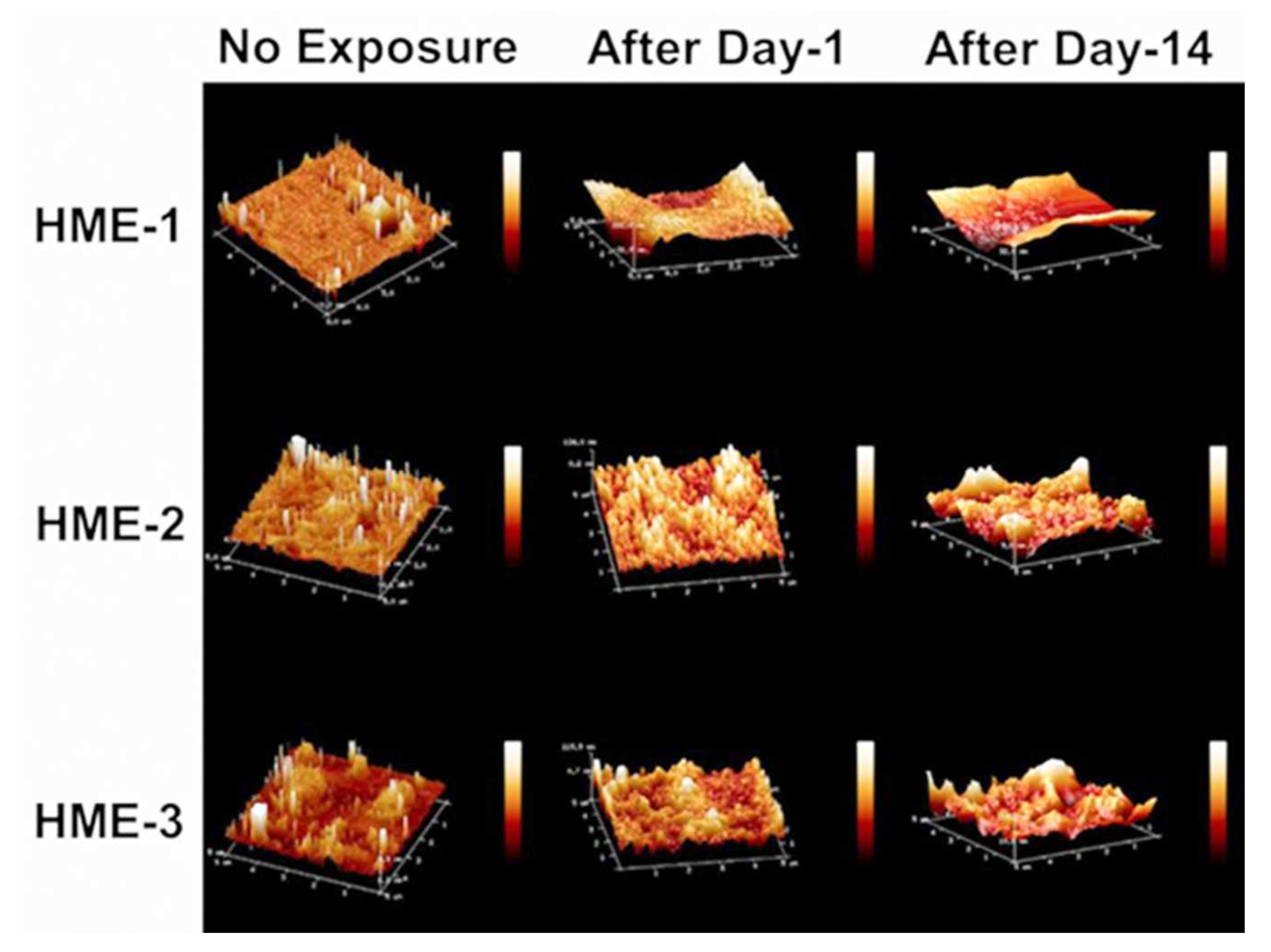
4.4.1. Surface Roughness of Dexamethasone-Loaded Implants by AFM
4.4.2. Crystalline Structure of Implants with/without Dexamethasone Based on PLM and XRD
4.4.3. Scanning Electron Microscopy (SEM) Imaging of HME Implants
4.5. In Vitro Dexamethasone Release Studies
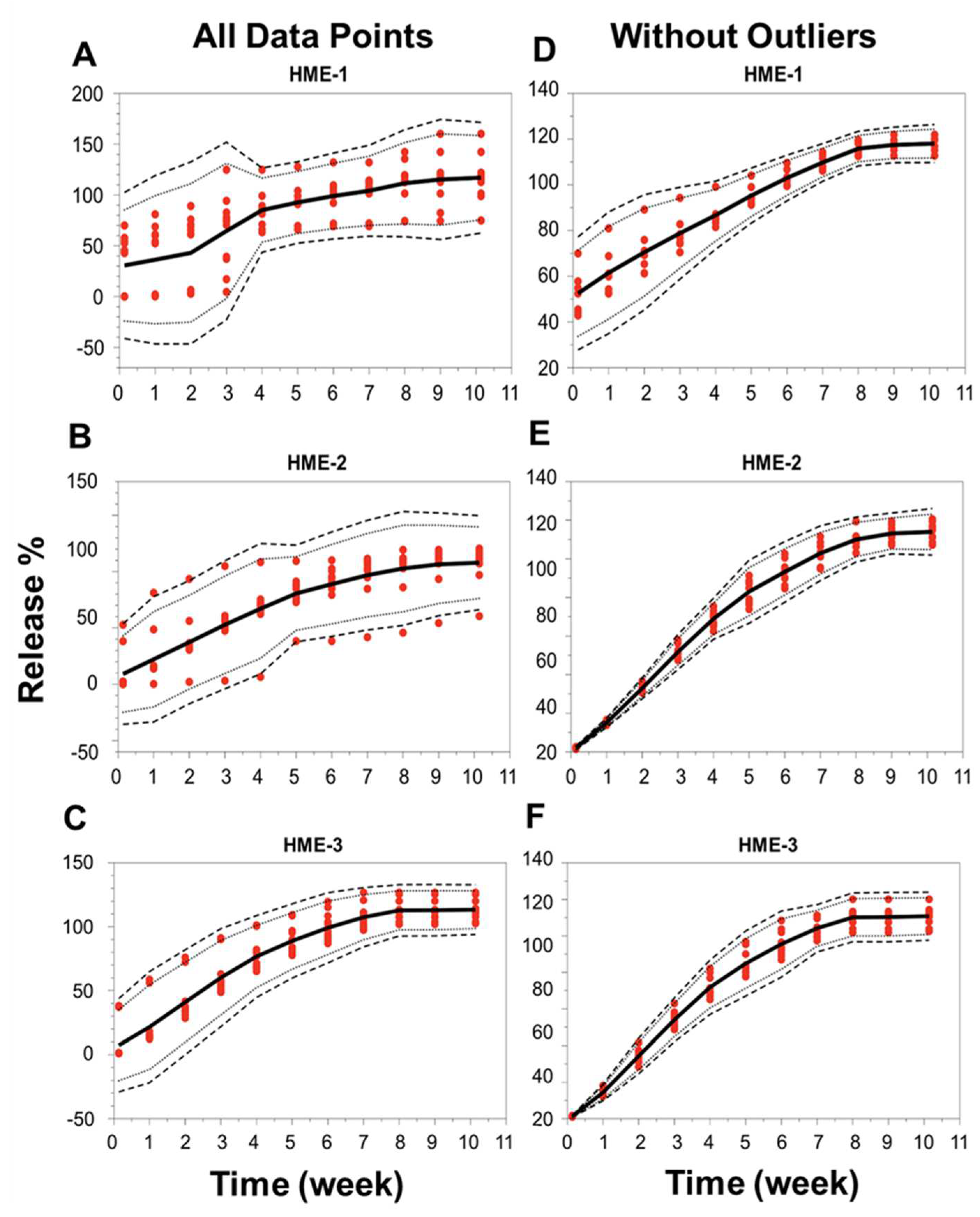
4.5.1. In Vitro Dexamethasone Release Testing
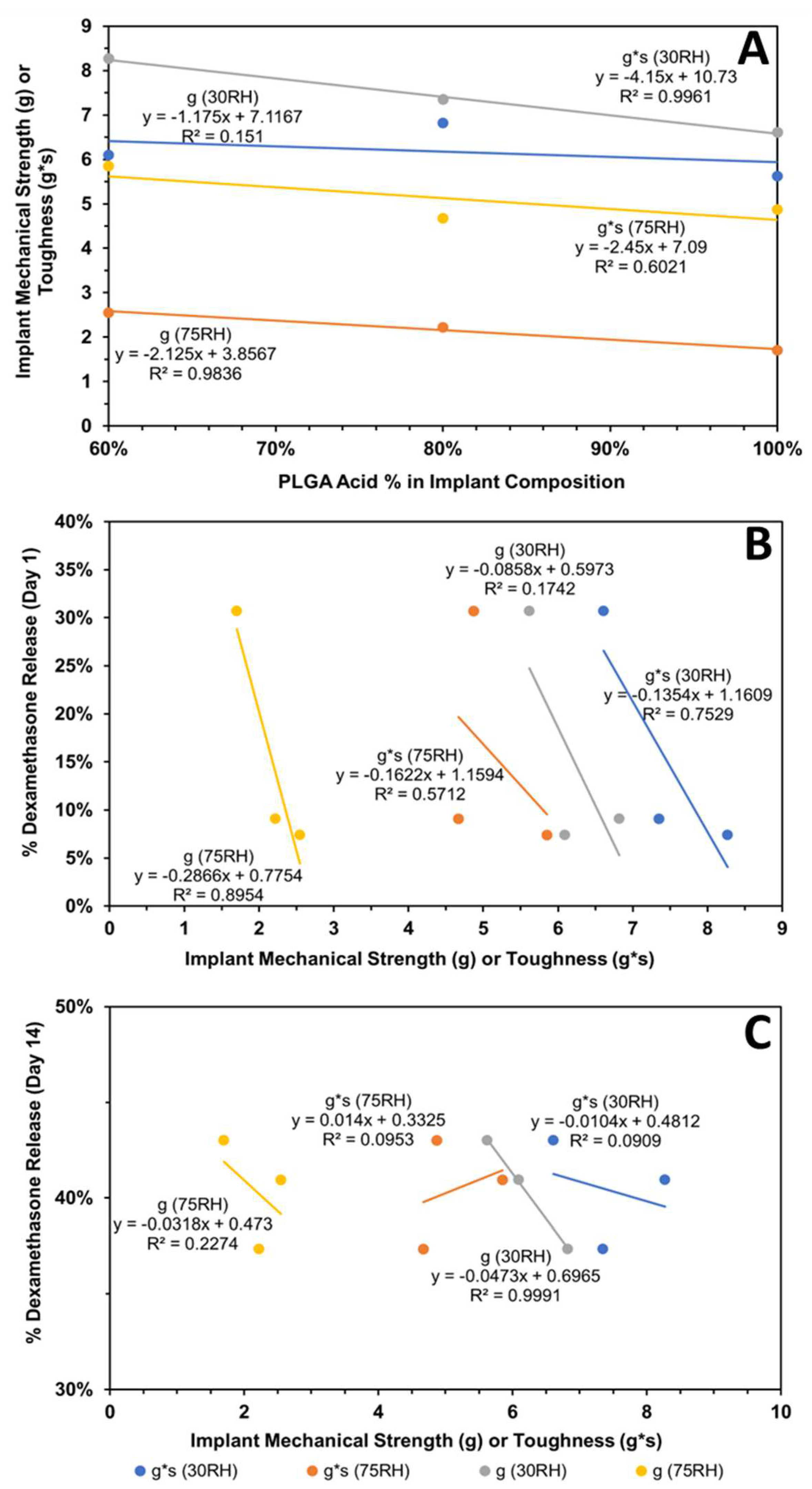
4.5.2. Correlation of Implant Physicomechanical Properties with Dexamethasone Release
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hui, H.W.; Robinson, J.R. Effect of particle dissolution rate on ocular drug bioavailability. J. Pharm. Sci. 1986, 75, 280–287. [Google Scholar] [CrossRef] [PubMed]
- FDA, 21 CFR §314.94 (a)(9)(iv). Available online: https://www.ecfr.gov/current/title-21/chapter-I/subchapter-D/part-314 (accessed on 21 June 2024).
- FDA, 21 CFR 320.22 (B)(1). Available online: https://www.ecfr.gov/current/title-21/chapter-I/subchapter-D/part-320 (accessed on 21 June 2024).
- RothenWeinhold, A.; Besseghir, K.; Gurny, R. Analysis of the influence of polymer characteristics and core loading on the in vivo release of a somatostatin analogue. Eur. J. Pharm. Sci. 1997, 5, 303–313. [Google Scholar] [CrossRef]
- Houchin, M.L.; Topp, E.M. Chemical degradation of peptides and proteins in PLGA: A review of reactions and mechanisms. J. Pharm. Sci. 2008, 97, 2395–2404. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.; Bundgaard, H. Studies on the stability of corticosteroids. 5. The degradation pattern of hydrocortisone in aqueous-solution. Int. J. Pharm. 1980, 6, 307–319. [Google Scholar] [CrossRef]
- Kelley, R.A.; Ghaffari, A.; Wang, Y.; Choi, S.; Taylor, J.R.; Hartman, R.R.; Kompella, U.B. Manufacturing of Dexamethasone-Poly(d,l-Lactide-co-Glycolide) Implants Using Hot-Melt Extrusion: Within- and Between-Batch Product Performance Comparisons. J. Ocul. Pharmacol. Ther. 2020, 36, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Bakri, S.J.; Boyer, D.S.; Albini, T.A.; Holekamp, N.M.; Ferrone, P.J.; Bhagat, R.; Novakovic, Z. Comparison of the tissue penetration and glide force of 22-gauge thin-wall needles for intravitreal implant administration. Ophthalmic Surg Lasers Imaging Retin. 2014, 45, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Jenekhe, S.A.; Roberts, M.F. Effects of intermolecular forces on the glass-transition of polymers. Macromolecules 1993, 26, 4981–4983. [Google Scholar] [CrossRef]
- Slark, A.T. The effect of intermolecular forces on the glass transition of solute-polymer blends. 2. Extension to different solutes. Polymer 1997, 38, 4477–4483. [Google Scholar] [CrossRef]
- Slark, A.T. The effect of intermolecular forces on the glass transition of solute-polymer blends. Polymer 1997, 38, 2407–2414. [Google Scholar] [CrossRef]
- Engelburg, I.; Kohn, J. Physicomechanical properties of degradable polymers used in medical applications: A comparative study. Biomaterials 1991, 12, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Grijpma, D.W.; Pennings, A.J. (Co) polymers of L-lactide, 2. Mechanical properties. Macromol. Chem. Phys. 1994, 195, 1649–1663. [Google Scholar] [CrossRef]
- Sweeney, J.; Ward, I.M. Mechanical Properties of Solid Polymers; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Chitchumnong, P.; Brooks, S.C.; Stafford, G.D. Comparison of three- and four-point flexural strength testing of denture-base polymers. Dent. Mater. 1989, 5, 2–5. [Google Scholar] [CrossRef]
- Callister, W.D., Jr.; Rethwisch, D.G. Material Science and Engineering: An Introduction, 10th ed.; Wiley: Hoboken, NJ, USA, 2018; p. 992. [Google Scholar]
- Landel, R.F.; Nielsen, L.E. Mechanical Properties of Polymers and Composites; CRC Press: Boca Raton, FL, USA, 1993. [Google Scholar]
- Su, C.; Shi, J.; Hu, Y.; Hu, S.; Ma, J. Method and Apparatus of Using Peak Force Tapping Mode to Measure Physical Properties of a Sample; Bruker Nano, Inc.: Billerica, MA, USA, 2016. [Google Scholar]
- Li, Q.; Fuks, G.; Moulin, E.; Maaloum, M.; Rawiso, M.; Kulic, I.; Foy, J.T.; Giuseppone, N. Macroscopic contraction of a gel induced by the integrated motion of light-driven molecular motors. Nat. Nanotechnol. 2015, 10, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liu, L.-Q.; Xi, N.; Wang, Y.-C. Nanoscale monitoring of drug actions on cell membrane using atomic force microscopy. Acta Pharmacol. Sin. 2015, 36, 769–782. [Google Scholar] [CrossRef]
- Parikh, T.; Gupta, S.S.; Meena, A.K.; Vitez, I.; Mahajan, N.; Serajuddin, A.T. Application of film-casting technique to investigate drug-polymer miscibility in solid dispersion and hot-melt extrudate. J. Pharm. Sci. 2015, 104, 2142–2152. [Google Scholar] [CrossRef] [PubMed]
- Bertoni, S.; Passerini, N.; Albertini, B. Liquid Lipids Act as Polymorphic Modifiers of Tristearin-Based Formulations Produced by Melting Technologies. Pharmaceutics 2021, 13, 1089. [Google Scholar] [CrossRef] [PubMed]
- Desai, P.M.; Hogan, R.C.; Brancazio, D.; Puri, V.; Jensen, K.D.; Chun, J.-H.; Myerson, A.S.; Trout, B.L. Integrated hot-melt extrusion–injection molding continuous tablet manufacturing platform: Effects of critical process parameters and formulation attributes on product robustness and dimensional stability. Int. J. Pharm. 2017, 531, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Cullity, B.D.; Stock, S.R. Elements of X-ray Diffraction; Prentice Hall: Upper Saddle River, NJ, USA, 2001; pp. 101–102. [Google Scholar]
- Jendrzejewska, I.; Goryczka, T.; Pietrasik, E.; Klimontko, J.; Jampilek, J. X-ray and Thermal Analysis of Selected Drugs Containing Acetaminophen. Molecules 2020, 25, 5909. [Google Scholar] [CrossRef] [PubMed]
- Verdaguer, N.; Garriga, D.; Fita, I. X-ray crystallography of viruses. Subcell Biochem. 2013, 68, 117–144. [Google Scholar] [PubMed]
- Aoto, P.C.; Ta, D.T.; Cupp-Vickery, J.R.; Vickery, L.E. X-ray diffraction analysis of a crystal of HscA from Escherichia coli. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2005, 61 Pt 7, 715–717. [Google Scholar] [CrossRef] [PubMed]
- Sawaya, M.R. Characterizing a crystal from an initial native dataset. Methods Mol. Biol. 2007, 364, 95–120. [Google Scholar] [PubMed]
- Kato, K.; Ito, K.; Hoshino, T. Anisotropic Amorphous X-ray Diffraction Attributed to the Orientation of Cyclodextrin. J. Phys. Chem. Lett. 2020, 11, 6201–6205. [Google Scholar] [CrossRef] [PubMed]
- Rongpipi, S.; Del Mundo, J.T.; Gomez, E.D.; Gomez, E.W. Resonant X-ray scattering of biological assemblies. MRS Commun. 2021, 11, 1–17. [Google Scholar] [CrossRef]
- Poulsen, H.F. An introduction to three-dimensional X-ray diffraction microscopy. J. Appl. Crystallogr. 2012, 45, 1084–1097. [Google Scholar] [CrossRef]
- Matter, B.; Ghaffari, A.; Bourne, D.; Wang, Y.; Choi, S.; Kompella, U.B. Dexamethasone Degradation in Aqueous Medium and Implications for Correction of In Vitro Release from Sustained Release Delivery Systems. Aaps. Pharmscitech. 2019, 20, 320. [Google Scholar] [CrossRef] [PubMed]
- Spangler, M.; Mularz, E. A validated, stability-indicating method for the assay of dexamethasone in drug substance and drug product analyses, and the assay of preservatives in drug product. Chromatographia 2001, 54, 329–334. [Google Scholar] [CrossRef]
- Ummiti, K.; Vakkala, S.; Panuganti, V.; Annarapu, M.R. Isolation, Identification, and Characterization of 17-Oxo Dexamethasone, an Oxidative Degradation Impurity of Dexamethasone Using Flash Chromatography and NMR/HRMS/IR. J. Liq. Chromatogr. Relat. Technol. 2014, 37, 2403–2419. [Google Scholar] [CrossRef]
- Chen, Q.; Zielinski, D.; Chen, J.; Koski, A.; Werst, D.; Nowak, S. A validated, stability-indicating HPLC method for the determination of dexamethasone related substances on dexamethasone-coated drug-eluting stents. J. Pharm. Biomed. Anal. 2008, 48, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Bundgaard, H.; Hansen, J. Studies on the stability of corticosteroids VI. Kinetics of the rearrangement of betamethasone-17-valerate to the 21-valerate ester in aqueous solution. Int. J. Pharm. 1981, 7, 197–203. [Google Scholar] [CrossRef]
- Walker, J.; Albert, J.; Liang, D.; Sun, J.; Schutzman, R.; Kumar, R.; White, C.; Beck-Broichsitter, M.; Schwendeman, S.P. In vitro degradation and erosion behavior of commercial PLGAs used for controlled drug delivery. Drug Deliv. Transl. Res. 2023, 13, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Bao, Q.; Wang, R.; Kwok, O.; Maurus, K.; Wang, Y.; Qin, B.; Burgess, D.J. In situ forming risperidone implants: Effect of PLGA attributes on product performance. J. Control. Release 2023, 361, 777–791. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Bao, Q.; Wang, R.; Kwok, O.; Maurus, K.; Wang, Y.; Qin, B.; Burgess, D.J. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef] [PubMed]
- Alexis, F. Factors affecting the degradation and drug-release mechanism of poly(lactic acid) and poly (lactic acid)-co-(glycolic acid). Polym. Int. 2005, 54, 36–46. [Google Scholar] [CrossRef]
- Tsuji, H.; Mizuno, A.; Ikada, Y. Properties and morphology of poly(L-lactide).: III.: Effects of initial crystallinity on long-term in vitro hydrolysis of high molecular weight poly(L-lactide) film in phosphate-buffered solution. J. Appl. Polym. Sci. 2000, 77, 1452–1464. [Google Scholar] [CrossRef]
- Schliecker, G.; Schmidt, C.; Fuchs, S.; Wombacher, R.; Kissel, T. Hydrolytic degradation of poly(lactide-co-glycolide) films: Effect of oligomers on degradation rate and crystallinity. Int. J. Pharm. 2003, 266, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Rapier, C.E.; Shea, K.J.; Lee, A.P. Investigating PLGA microparticle swelling behavior reveals an interplay of expansive intermolecular forces. Sci. Rep. 2021, 11, 12. [Google Scholar] [CrossRef]
- O’Neil, M.J.E. The Merck Index—An Encyclopedia of Chemicals, Drugs, and Biologicals; Whitehouse Station: Hunterdon County, NJ, USA, 2001; p. 518. [Google Scholar]
- Bruker Corp. The NanoScope Analysis 1.50 User Manual; Bruker Corp.: Billerica, MA, USA, 2013. [Google Scholar]
- McCool, J.I. Relating Profile Instrument Measurements to the Functional Performance of Rough Surfaces. J. Tribol. 1987, 109, 264–270. [Google Scholar] [CrossRef]
- Cometa, S.; Bonifacio, M.A.; Ferreira, A.M.; Gentile, P.; De Giglio, E. Surface Characterization of Electro-Assisted Titanium Implants: A Multi-Technique Approach. Materials 2020, 13, 705. [Google Scholar] [CrossRef]
- Troy, D.B.; Beringer, P. Remington: The Science and Practice of Pharmacy; Lippincott Williams & Wilkins: Philadelphia, PN, USA, 2005; pp. 657–658. [Google Scholar]
- Gupta, S.S.; Solanki, N.; Serajuddin, A.T. Investigation of Thermal and Viscoelastic Properties of Polymers Relevant to Hot Melt Extrusion, IV: Affinisol™ HPMC HME Polymers. AAPS PharmSciTech. 2016, 17, 148–157. [Google Scholar] [CrossRef]
- Bates, S.; Zografi, G.; Engers, D.; Morris, K.; Crowley, K.; Newman, A. Analysis of Amorphous and Nanocrystalline Solids from Their X-ray Diffraction Patterns. Pharm. Res. 2006, 23, 2333–2349. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Li, X.; He, R.; Cheng, J.; Ni, Z.; Zhao, G. Preparation and evaluation of poly(D, L-lactic acid)/poly(L-lactide-co-ε-caprolactone) blends for tunable sirolimus release. Colloids Surf. A Physicochem. Eng. Asp. 2020, 590, 124518. [Google Scholar] [CrossRef]
- McFaddin, D.C.; Russell, K.E.; Wu, G.; Heyding, R.D. Characterization of polyethylenes by X-ray-diffraction and C-13-NMR temperature studies and the nature of the amorphous halo. J. Polym. Sci. Part B Polym. Phys. 1993, 31, 175–183. [Google Scholar] [CrossRef]
- Li, J.; RStayshich, M.; Meyer, T.Y. Exploiting Sequence to Control the Hydrolysis Behavior of Biodegradable PLGA Copolymers. J. Am. Chem. Soc. 2011, 133, 6910–6913. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Rothstein, S.N.; Little, S.R.; Edenborn, H.M.; Meyer, T.Y. The Effect of Monomer Order on the Hydrolysis of Biodegradable Poly(lactic-co-glycolic acid) Repeating Sequence Copolymers. J. Am. Chem. Soc. 2012, 134, 16352–16359. [Google Scholar] [CrossRef] [PubMed]
- Washington, M.A.; Swiner, D.J.; Bell, K.R.; Fedorchak, M.V.; Little, S.R.; Meyer, T.Y. The impact of monomer sequence and stereochemistry on the swelling and erosion of biodegradable poly(lactic-co-glycolic acid) matrices. Biomaterials 2017, 117, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Essa, D.; Kondiah, P.P.D.; Choonara, Y.E.; Pillay, V. The Design of Poly(lactide-co-glycolide) Nanocarriers for Medical Applications. Front. Bioeng. Biotechnol. 2020, 8, 20. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P.V. An Overview of Poly(lactic-co-glycolic) Acid (PLGA)-Based Biomaterials for Bone Tissue Engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659. [Google Scholar] [CrossRef] [PubMed]
- Tracy, M.A.; Ward, K.L.; Firouzabadian, L.; Wang, Y.; Dong, N.; Qian, R.; Zhang, Y. Factors affecting the degradation rate of poly(lactide-co-glycolide) microspheres in vivo and in vitro. Biomaterials 1999, 20, 1057–1062. [Google Scholar] [CrossRef] [PubMed]
- Costello, M.A.; Liu, J.; Chen, B.; Wang, Y.; Qin, B.; Xu, X.; Li, Q.; Lynd, N.A.; Zhang, F. Drug release mechanisms of high-drug-load, melt-extruded dexamethasone intravitreal implants. Eur. J. Pharm. Biopharm. 2023, 187, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Beig, A.; Ackermann, R.; Wang, Y.; Schutzman, R.; Schwendeman, S.P. Minimizing the initial burst of octreotide acetate from glucose star PLGA microspheres prepared by the solvent evaporation method. Int. J. Pharm. 2022, 624, 10. [Google Scholar] [CrossRef] [PubMed]
- Zolnik, B.S.; Burgess, D.J. Effect of acidic pH on PLGA microsphere degradation and release. J. Control. Release 2007, 122, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.N.; Zhao, X.C. A Simple Approach for Comparing the In Vitro Dissolution Profiles of Highly Variable Drug Products: A Proposal. AAPS J. 2018, 20, 14. [Google Scholar] [CrossRef] [PubMed]






| Formulations | Theoretical Release Characteristic | Dexamethasone (g) | PLGA–Acid End-Capped (g) | PLGA–Ester End-Capped (g) |
|---|---|---|---|---|
| HME-1 | Fast | 2 | 8.0 | 0 |
| HME-2 | Intermediate | 2 | 6.4 | 1.6 |
| HME-3 | Slow | 2 | 4.8 | 3.2 |
| HME-4 | Fast | 0 | 10.0 | 0 |
| HME-5 | Intermediate | 0 | 8.0 | 2.0 |
| HME-6 | Slow | 0 | 6.0 | 4.0 |
| Setting Description | Setting |
|---|---|
| Test Mode | Compression |
| Test Speed | 0.1 mm/s |
| Target Mode | Distance |
| Gap Space | 1.00 cm (0.394 in) |
| Stop Plot at | Target distance |
| Distance | 5 mm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghaffari, A.; Matter, B.A.; Hartman, R.R.; Bourne, D.W.A.; Wang, Y.; Choi, S.; Kompella, U.B. Hot-Melt Extrusion-Based Dexamethasone–PLGA Implants: Physicochemical, Physicomechanical, and Surface Morphological Properties and In Vitro Release Corrected for Drug Degradation. Pharmaceutics 2024, 16, 895. https://doi.org/10.3390/pharmaceutics16070895
Ghaffari A, Matter BA, Hartman RR, Bourne DWA, Wang Y, Choi S, Kompella UB. Hot-Melt Extrusion-Based Dexamethasone–PLGA Implants: Physicochemical, Physicomechanical, and Surface Morphological Properties and In Vitro Release Corrected for Drug Degradation. Pharmaceutics. 2024; 16(7):895. https://doi.org/10.3390/pharmaceutics16070895
Chicago/Turabian StyleGhaffari, Alireza (Allen), Brock A. Matter, Rachel R. Hartman, David W. A. Bourne, Yan Wang, Stephanie Choi, and Uday B. Kompella. 2024. "Hot-Melt Extrusion-Based Dexamethasone–PLGA Implants: Physicochemical, Physicomechanical, and Surface Morphological Properties and In Vitro Release Corrected for Drug Degradation" Pharmaceutics 16, no. 7: 895. https://doi.org/10.3390/pharmaceutics16070895
APA StyleGhaffari, A., Matter, B. A., Hartman, R. R., Bourne, D. W. A., Wang, Y., Choi, S., & Kompella, U. B. (2024). Hot-Melt Extrusion-Based Dexamethasone–PLGA Implants: Physicochemical, Physicomechanical, and Surface Morphological Properties and In Vitro Release Corrected for Drug Degradation. Pharmaceutics, 16(7), 895. https://doi.org/10.3390/pharmaceutics16070895





