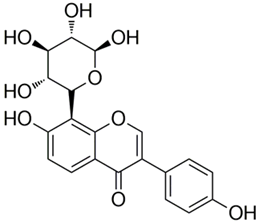
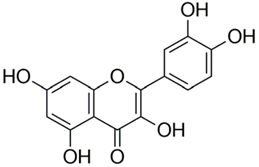
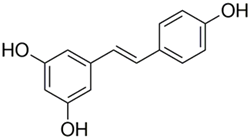
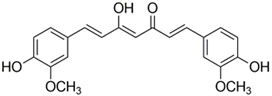
Abstract
Secondary metabolites, polyphenols, are widespread in the entire kingdom of plants. They contain one or more hydroxyl groups that have a variety of biological functions in the natural environment. These uses include polyphenols in food, beauty products, dietary supplements, and medicinal products and have grown rapidly during the past 20 years. Antimicrobial polyphenols are described together with their sources, classes, and subclasses. Polyphenols are found in different sources, such as dark chocolate, olive oil, red wine, almonds, cashews, walnuts, berries, green tea, apples, artichokes, mushrooms, etc. Examples of benefits are antiallergic, antioxidant, anticancer agents, anti-inflammatory, antihypertensive, and antimicrobe properties. From these sources, different classes of polyphenols are helpful for the growth of internal functional systems of the human body, providing healthy fats, vitamins, and minerals, lowering the risk of cardiovascular diseases, improving brain health, and rebooting our cellular microbiome health by mitochondrial uncoupling. Among the various health benefits of polyphenols (curcumin, naringenin, quercetin, catechin, etc.) primarily different antimicrobial activities are discussed along with possible future applications. For polyphenols and antimicrobial agents to be proven safe, adverse health impacts must be substantiated by reliable scientific research as well as in vitro and in vivo clinical data. Future research may be influenced by this evaluation.
1. Introduction
Polyphenols are natural organic products having several hydroxyl groups. (-OH) is the aromatic ring largely found in fruits, vegetables, cereals, and beverages [1,2,3,4,5,6,7]. They generally fall under three main categories: phenolic acids, flavonoids, and non-flavonoids. These three are thought to have a variety of antimicrobial health advantages [8,9,10,11,12,13]. The need for healthy ingredients, nutritional supplements, and medicines has stimulated an interest in research involving the use of polyphenols as antimicrobial compounds. To deal with current difficulties that restrict the use of polyphenols, such as their quick elimination from the body, low water solubility, unstable acidic conditions, and their particle size, research is being undertaken. More than 8000 various types of polyphenols have been recognized to date [14,15,16,17]. Since the dawn of civilization, infectious microbes have posed a threat as a significant source of illness and mortality. The year 1928 marked the discovery of the first antibiotic penicillin, and then the 1930s saw development of sulfa medicines. These, and the use of different plant extracts, were the only effective treatments for infections. These treatments, however, had varying degrees of success in medical science [18,19,20]. Whereas antibiotics have served as an important part of treating infectious diseases brought on by fungal and bacterial infections for the last few decades, harmful and antibiotic-resistant bacteria have been seen more frequently in recent years. Almost 100,000 different plant species have been examined for the possibility of being used as medicines for the treatment of various diseases. According to 2007 WHO estimates, 25% of medicines on the market come from plants used in traditional medicine [21,22,23]. In addition to their lengthy history of therapeutic use, plant-based substances show favorable reception among patients and tolerances, making them appear to be a reliable source of antibacterial agents [24,25,26].
This review, a comprehensive overview of flavonoid structure, fundamental characteristics, and distribution, discusses the range of their antibacterial action as a potential alternative to traditional medications. We have examined newly discovered flavonoid compounds that exhibit strong antibacterial properties, and we have provided examples of flavonoids that exhibit reciprocal and combined consequences and also can be used in medicinal and food chemistry [27].
Various microbial agents are destroyed by bioactive polyphenols through their entry into cellular systems by rupturing the cell membrane by hydrophobic interactions as well as inhibiting enzyme activities, DNA gyrase, and RNA synthesis. As a result, foreign bodies are not capable of living in the human body and are not able to interrupt cellular functionality. Diets high in plant polyphenols for a substantial amount of time may safeguard against the onset of cancer, heart disease, diabetes, osteoporosis, and neurological illnesses, according to epidemiological research and related analyses [28,29,30,31,32]. Here, we discuss the antimicrobial effects of naturally occurring polyphenols, examining their potential impact on human health. Polyphenols, as secondary metabolites with a variety of important functions in plant physiology, offer promising health benefits for humans. These benefits include acting as antioxidant compounds, antiallergics, anti-inflammatories, anticancer agents, antihypertensives, and antimicrobial agents [33,34,35,36]. The antibacterial (Gram-positive and Gram-negative), antiviral, and antifungal properties of the most potent polyphenols are discussed in this research, with an emphasis mostly on the way they work as well as the structure–activity connections that have been studied. Bioactive polyphenols are obtained in plant-based foods. They are reviewed in this article, including in combination with their effective biological activities The antibacterial properties of polyphenols derived from various natural sources have been documented by extensive research. For instance, it has been demonstrated that polyphenols derived from green tea have antibacterial action against several bacterial species, such as Escherichia coli, Staphylococcus aureus, and Streptococcus mutans [37,38,39]. Similarly, it has been demonstrated that polyphenols derived from grape seeds have antibacterial activity against several bacterial species, including Salmonella enteritidis, Listeria monocytogenes, and Bacillus cereus [40,41,42,43]. It has also been discovered that polyphenols have antifungal properties. For example, it has been demonstrated that polyphenols derived from ginger have antifungal action against Candida albicans, a fungus that can infect people. Moreover, it has been demonstrated that polyphenols derived from turmeric exhibit antifungal action against Aspergillus flavus, a fungus that can contaminate food and lead to health issues [44,45,46,47]. There is variation in the distribution of phenolics in plants at tissues, cellular, and subatomic levels. Plant cell vacuoles contain soluble phenolics, whereas insoluble phenolics are located in the walls of the cells. Quercetin is one of the polyphenols that can be found in all plant products, including fruit, vegetables, cereals, tea, wine, and infusions. On the other hand, flavanones and isoflavones are unique to certain foods [48,49,50]. Foods typically contain complex polyphenol combinations. Plants with outer layers have concentrations of phenolics greater than those with interior layers. Rainfall, sunlight, soil type, and other edaphic and natural variables all have a significant impact on the polyphenolic constituents of food. Considerable ripeness influences the ratios and quantities of different types of polyphenols. Phenolic acid content has often been shown to decrease as anthocyanin contents rise during the ripening process. Numerous polyphenols, particularly phenolic acids, play a direct role in how plants react to various stresses. They have antibacterial qualities that help injured areas heal, and their amount may rise following an outbreak of infection [51,52,53].
Overall, polyphenols isolated from natural sources are attractive candidates in medicinal industries as well as in the food industry for the creation of novel antimicrobial medicines to fight infectious diseases due to the ability of their effective hydrophobicity to destroy the foreign microbial cell membrane as well as cell functionality [54,55,56,57].
2. Basic Information: Classifications, Molecular Structure, and Natural Sources
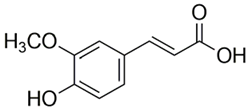
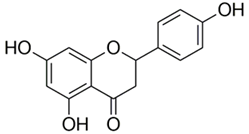
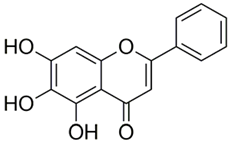
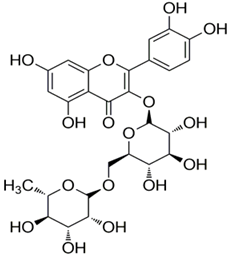
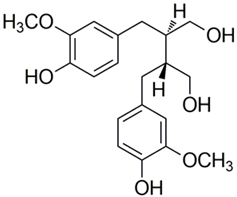
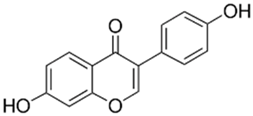
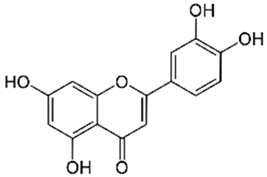
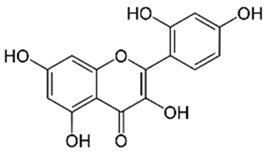
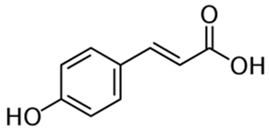
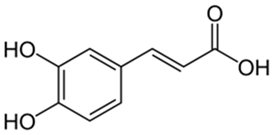
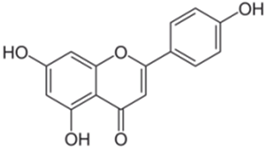
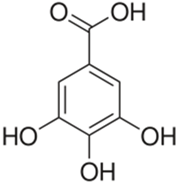
Polyphenolic acids are classified into two categories, benzoic acid and cinnamic acid derivatives. Several subclasses of non-flavonoids and flavonoids have general molecular structures: A, B, and C (Figure 1). A few examples of polyphenols are structurally represented here along with their classification [58,59].
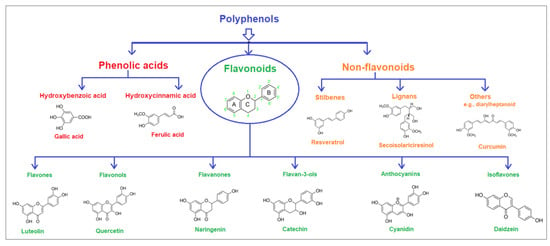
Figure 1.
Classification of the polyphenols with examples based on structural elements that bind the rings one to another.
2.1. Phenolic Acids
Foods include large amounts of phenolic acids. They are broken down into two classes: hydroxybenzoic acid and hydroxycinnamic acid derivatives. Except for some red fruits, black radish, and onions, which can have quantities of 10 mg/kg, the hydroxybenzoic acid level of food plants is typically modest. p-Coumaric, caffeic, ferulic, and sinapic acids make up the majority of the hydroxycinnamic acids, which are more prevalent than hydroxybenzoic acids [60,61,62,63]. Three primary marine macroalgae groups can also produce several antimicrobial polyphenols (mainly phenolic acids and flavonoids): brown algae (Phaeophyta), red algae (Rhodophyta), and green algae (Chlorophyta) [64].
2.2. Flavonoids
Naturally phenolic substances that are classified as flavonoids have a C6-C3-C6 carbon structure (phenyl benzopyran). The 2-phenyl-benzo-c-pyrane center of a flavonoid is composed of two benzene rings A and B connected by a heterocyclic pyran or pyrone ring C. Flavonoids can be divided into several subclasses according to their degree of unsaturation and oxidation, including flavones, isoflavones, flavonols, flavanols, flavanones, and flavanonols. Recently, a group of polyphenols known as flavonoids has received the most research attention. Two aromatic rings joined by three C-atoms to form an oxygenated heterocycle make up the general structure of this category (shown in Figure 1) [65]. Flavonoids are found in more than 4000 different forms, many of which are in charge of giving flowers, fruits, and leaves their eye-catching hues. Flavonoids can be classified into six subclasses (described in the Table 1) depending on the type of heterocycle involved as shown in Figure 1 [65,66].
Six Subclasses of Flavonoids
There are various classes of polyphenols based on their different poly hydroxyl group positions in the C6-C3-C6 carbon structure. In this section, six different classes of flavonoids are discussed, including their various antimicrobial activities. Due to the presence of polyphenolic compounds in their molecular structure, flavonoids, as a broad class of phytonutrients (chemicals found in plants), are classified as polyphenols. That indicates the structures of flavonoids contain several phenol units. “Polyphenol” derives from the words “poly” (many) and “phenol”, a chemical compound consisting of a six-carbon ring joined to a hydroxyl group (-OH). Among their many applications in biology, bioactive polyphenols are well known for their antioxidant, anticancer, and antimicrobial qualities.
The six groups of flavonoids are the following:
Flavonols (e.g., quercetin, kaempferol, etc.)—Found in onions, leeks, and broccoli.
Flavones (e.g., luteolin, apigenin, etc.)—Found in parsley, celery, and chamomile.
Flavanones (e.g., hesperidin, naringenin, etc.)—Found in citrus fruits.
Flavan-3-ols (e.g., catechin, epicatechin, etc.)—Found in green tea, chocolate, and grapes.
Anthocyanins (e.g., cyanidin, delphinidin, etc.)—Found in red, purple, blue colors in many fruits and vegetables.
Isoflavones (e.g., genistein, daidzein, etc.)—Found in soy products and other legumes.
Although all of these classes have a similar phenolic structure, they are divided into distinct six classes due to variations in the number of hydroxyl groups (-OH) attached and the positioning of carbon atoms, accordingly categorizing them into different classes of flavonoids. Their antimicrobial properties and effectiveness as antioxidants, which are essential for shielding human beings from oxidative damage and associated illnesses, are bolstered by their polyphenolic content. Henceforth, flavonoids having different numbers of –OH groups, as well as different positions in the phenyl benzopyran molecular structures of the corresponding flavonoids, provide different new classes and subclasses of polyphenols and are described with examples in tabular form (Table 1).
2.3. Non-Flavonoids
Among the non-flavonoids, two major classes (stilbenes and lignans) are considered in this section. Other sections are also important, since naturally occurring polyphenols (curcumin) have good antimicrobial characteristics [67]. Although non-flavonoids have lower antioxidant activity than flavonoids, as previously documented, several studies are nevertheless worthwhile to mention. Certain phenolic acids, such as ferulic, caffeic, and gallic acids, demonstrate antibacterial action against both Gram-negative (E. coli and Pseudomonas aeruginosa) and Gram-positive (S. aureus and L. monocytogenes) bacteria. It was discovered that these substances are more effective than common antibiotics like gentamicin and streptomycin against the bacteria. On the other hand, chlorogenic acid was ineffective against Gram-positive bacteria [67,68,69].
Stilbenes: Two Ph-moieties are joined by a two “C” methylene bridges in stilbenes. These are rather rare in human nutrition. Most stilbenes in vegetables serve as phytoalexins, which are compounds that are only generated in response to infection or injury. Resveratrol (3,4′,5-trihydroxystilbene), a naturally occurring stilbene that is mostly abundant in grapes, is one of the most well-studied polyphenol stilbenes. Red wine, a grape-based drink, has a sizable portion of resveratrol [70,71].
Lignans: The 2,3-dibenzyl-butane moiety of lignans, which are diphenolic substances, is created when two cinnamic acid residues are dimerized. Secoisolariciresinol is one of the lignans which acts as a phytoestrogen. The most abundant nutritional resource is linseed, which has significant amounts of matairesinol and secoisolariciresinol. Antimicrobial activity against Gram-positive (S. aureus, S. epidermidis, etc.) and Gram-negative (Pseudomonas aeruginosa, Escherichia coli, etc.) bacteria at low temperatures is also demonstrated by the presence of methoxy and phenolic hydroxyl groups in the lignans [72,73].
3. Activities as Antimicrobial Agents of Several Polyphenols
Salicylic acid suppresses the growth of various fungi and bacteria, including Propionibacterium acnes, linked to acne development. It disrupts bacterial membranes and hinders their metabolism [74]. Gallic acid/ellagic acid/4-hydroxybenzoic acid kills the microbial agents by interfering with cell walls, and metabolic processes disrupt enzyme activity, effectively combating bacteria, fungi, etc. p-coumaric acid is the main antibacterial component in alkaline hydrolysates compared to caffeic and ferulic acid while hydroxycinnamic acids also exhibit strong antibacterial effects. Particularly, p-coumaric acid, with IC50 ranging from 200–400 µg/mL, showed a stronger effect against various enterobacteria strains [75]. Apigenin demonstrates notable antibacterial and antibiotic-synergistic action toward oral infections. Significant growth inhibition of bacteria and fungi has been achieved by luteolin, baicalein, and tangetin as observed in earlier studies. Luteolin is a more effective antimicrobial agent through nucleic acid and protein synthesis inhibition, bacterial cell membrane impairment, cell morphological modification, and biofilm inhibition [76,77,78]. Algae like Jania rubens red seaweed [79] inhibited the growth of several bacteria, fungi, and viruses and, in addition, microbial cell growth is prevented by quercetin, rutin, morin, and myricetin. Flavonols like galangin and kaempferol, which is frequently present in propolis samples, have inhibitory effects against the aspergilli Aspergillus tamarii, Aspergillus flavus, Cladosporium sphaerospermum, Penicillium digitatum, and Penicillium italicum [20,80,81]. Naringenin and naringin treatments decrease lipid peroxidation and elevate antioxidant levels in rats. The pharmacological effects of hesperidin and eriodictyol include antibacterial and antifungal properties were observed and the antifungal and antibacterial activity of eriodictyol was high [82,83]. Catechin and arbutin restrict the growth of Salmonella typhimurium. ATCC 13311 and phloretin and phlioridzin inhibit fungi and bacterial species through decreased lymphatic node metastases and kill the microorganisms [3,84]. The antimicrobial properties of pelargonidin, delphinidin, cyanidin chloride, cyanidin 3-glucoside, malvidin, petunidin, and phenolic extracts from various berries were tested against Lactobacillus species and other intestinal bacteria [85]. The growth of Listeria monocytogenes, Staphylococcus aureus, Bacillus subtilis, Enterococcus faecalis, and yeast strains (Debaryomyceshansenii, Trichosporoncutaneum, etc.) was inhibited by anthocyanins [86,87,88]. C. albicans and T. rubrum are affected by daidzein and genistein. Glycitein shows effective antifungal as well as antibacterial properties [89,90]. In experiments using antimicrobial agents, strains susceptible to resveratrol (50 µg/mL) and curcumin (20 µg/mL) show sub-MIC levels lowering evaluated virulence factors by at least 50% (IC50). In the majority of isolations (57–94%), hemagglutination and hemolysin activities were 50% reduced. Protease and biofilm IC50 values were observed in 6.5–17.8% of isolates utilizing a methicillin-resistant strain of Staphylococcus aureus [91].

Table 1.
Description of sources and different antimicrobial activity of corresponding polyphenols with IC50 values.
Table 1.
Description of sources and different antimicrobial activity of corresponding polyphenols with IC50 values.
| Class/Subclass of Polyphenols | Sources | Polyphenols | IC50 Values | Inhibition Target | Ref. |
|---|---|---|---|---|---|
| Hydrobenzoic acid | Red huckleberries, coriander, black radish, garden onions, etc. | Salicylic acid (SA) | 5 mM | Fusarium oxysporum | [92] |
| Gallic acid (GA) | 1600 μg/mL | Staphylococcus aureus | [93] | ||
| Ellagic acid (EA) | 1 mM | Helicobacter pylori | [94] | ||
| 4-Hydroxybenzoic acid | 926 μg/mL | Staphylococcus aureus | [95] | ||
| Hydroxycinnamic acids | Fruits, vegetables, and drinks (tea, wine, and coffee). | p-Coumaric acid | 652 ± 3.3 μM | B. subtilis | [96] |
| Caffeic acid | 334 μM | Y. enterocolitica | [97] | ||
| Ferulic acid | 700 ± 3.4μM | B subtilis | [96] | ||
| Flavones | Leaves, flowers, and fruits including celery, parsley, red peppers, chamomile, mint, and Ginkgo biloba. | Luteolin | 50 μM | Helicobacter pylori | [98] |
| Apigenin | 9.59 ± 0.54 mM | S. aureus | [99] | ||
| Baicalein | 25.86 μg/mL | S. aureus | [100] | ||
| Flavonols | Onions, kale, lettuce, tomatoes, apples, grapes, berries, tea, and red wine. In addition, other sources are algae like Jania rubens red seaweed. | Quercetin | 65± 5 μM | S. aureus NCTC 5655 strain | [101] |
| Morin | 50 μM | Vibrio cholerae | [102] | ||
| Myricetin | 46.2 μM | Escherichia coli | [103] | ||
| Rutin | 357.8 ± 15.5 µg/mL | F. oxysporum f. sp. vasinfectum | [104] | ||
| Kaempferol | 50 ± 2 μM 22 ± 2 μM | P. aeruginosa S. aureus PriA | [105] | ||
| Galangin | 65 ± 5 µM | Staphylococcus aureus | [106] | ||
| Flavanones | Naringenin in grapefruit, tomatoes, mint, and citrus fruits and eriodictyol in lemons. | Naringenin | 6.8 ± 0.4 μM | Viral replication | [107] |
| Naringin | 62.5 μg/mL | S. aureus | [108] | ||
| Hesperidin | 125 μg/mL | Staphylococcus aureus | [83] | ||
| Eriodictyol | 2.229 ± 0.014 μg/mL | Staphylococcus aureus | [109] | ||
| Flavan-3-ols | Important bioactives in tea, pome fruits, berries, cocoa-derived products, nuts, and other foods. | Catechin | 5.65 µg/mL | M. luteus, B. subtilis, and S. aureus | [110] |
| Arbutin | 200 μg/mL | Human melanoma cell line (Fema-x) | [111] | ||
| Phloretin | 671.76 ± 9.03 µg/mL | E. coli | [112] | ||
| Phlioridzin | 5.1 ± 1.2 µg/mL | Trichophyton violaceum | [113] | ||
| Proanthocyanidin | 312 µg/mL | Staphylococcus aureus ATCC 25923 | [114] | ||
| Anthocyanins | Red and purple berries, grapes, apples, plums, cabbage, and natural colorants. | Cyanidin | 21.91 µg/mL | Staphylococcus aureus | [115] |
| Malvidin | 4 mg/mL | B. cereus ATCC 11778 | [116] | ||
| Delphindin | 12.5 mg/mL | Vibrio parahaemolyticus | [116] | ||
| Petunidin | 30.78 ± 1.17 μM | Saccharomyces cerevisiae | [117] | ||
| Pelagonidin | 3.02 µg/mL | Staphylococcus aureus ATCC 29213 | [118] | ||
| Isoflavones | Soy and its products, as well as legume seeds (lentils, beans, and peas). | Daidzein | 15.1 μM | E. coli | [119] |
| Glycitein | 7.49–10.46 μM | E. limosum | [120] | ||
| Genistein | 281 ± 29 μM | SW480 cell | [121] | ||
| Non-flavonoids | Grapes, apples, blueberries, plums, peanuts, lentils, wheat, algae, some vegetables (carrots, asparagus, garlic), and curcumin in turmeric. | Resveratrol | 16.23 µg/mL | Leishmania major | [122] |
| Curcumin | 13.67 μM | Anti-ZIKV (strain PAN2016) | [123] |
4. Microbial (Bacteria, Fungi, Viruses) Species Sensitive to Polyphenols
One major group of bacterial species is multidrug resistant, posing a special risk to people who work in nursing homes and hospitals. Several microbes (e.g., Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, Acinetobacter sp., Proteus sp., Micrococcus sp., Klebsiella pneumonia, Staphylococcus epidermidis, Bacillus subtilis) are very much responsible for problems in our lives. These types of microbes are resisted by polyphenolic compounds (phenolic acids, flavonoids, and non-flavonoids) like catechin, phenolic acid, cinnamic acid, ferulic acid, sinapic acid, p-coumaric acid, epigallocatechin gallate, daidzein, resveratrol, curcumin, etc.
Microbes like C. famata, C. utilis, C. albicans, and S. cerevisiae are types of fungi that also have been restricted using broth dilution and agar disk diffusion methods [124]. Baicalein was shown by Chen et al. to inhibit replication with cell adhesion in S. aureus [125]. Since EGCG is capable of binding to porins, this is likely how it influences the ability to damage the exterior cell membrane of Gram-negative bacteria through porin pores. Several flavonoids successfully killed microbes like Escherichia coli by forming complexes with extracellular and dissolved proteins [126]. Some viruses destroy our lives easily. Among these, the most common viral pathogens are the influenza virus, rhinovirus, adenovirus, coronavirus, and respiratory syncytial virus (RSV), which are very much responsible for the above. In the presence of polyphenols like resveratrol, luteolin, ellagic acid, cyanidin, etc., harmful viruses like hepatitis B, influenza, etc., can be destroyed and save our lives. Using these more important polyphenolic compounds can protect from microorganisms like bacteria, fungi, viruses, etc. [127,128,129,130].
In addition, several profound antimicrobial properties of polyphenols in wound healing are discussed in this section. A wound or injury to the skin can be caused by several factors, such as heat damage, trauma, or long-term ulcerations brought on by diseases like diabetes mellitus. Healing is hampered by chronic wounds, which make treatment more difficult, lowering patient satisfaction and increasing expenses. Such wounds are quite prone to becoming infected. Novel antimicrobial polyphenolic systems are being investigated in light of the growing antibiotic resistance in conventional therapy. Although stability concerns may restrict the effectiveness of polyphenols as alternatives to antibiotics for the treatment of chronic wounds, they still show potential [51]. To accomplish this, developments in efficiency by delivering bioactive polyphenols as naturally occurring antibacterial agents aim to stabilize their use for complex wounds while improving wound healing. S. aureus and methicillin-resistant S. aureus are the primary microorganisms identified in the early stages of chronic wounds, while E. coli and other pathogens are found later as the disease progresses. Polyphenolic substances that promote wound healing include kaempferol, chlorogenic acid, resveratrol, and ferulic acid. Tannic acid is a useful substance for treating wounds because of several advantageous qualities. Nurettin Sahiner et al., developed an antioxidant antimicrobial hydrogel against various pathogens for chronic wounds. A recent study confirms the wound-healing effects of tannic acid in both lab and animal tests, demonstrating its ability to stimulate hair follicles and accelerate skin regrowth. This suggests tannic acid could be valuable in natural wound care materials. The results demonstrate that whereas pectin- and nanoparticulate-based systems improve resveratrol distribution by resolving its solubility and bioavailability issues, microencapsulation of polyphenols boosts cutaneous wound healing with curcumin. Resveratrol in capsules exhibits more antimicrobial action compared to its natural version [52,53,131].
5. Possible Antimicrobial Mechanisms (Inhibition Pathways)
Polyphenols have a variety of antimicrobial properties to counter bacteria, fungi, and viruses. They have the ability to cause quorum sensing to interfere with and break cell membranes, inhibit enzymes, produce reactive oxygen species (ROS), chelate metal ions, alter the host immunological response, and prevent viral entry and reproduction [132]. Through these processes, polyphenols are able to kill the microbial species. While enzyme inhibition can cause metabolic pathways to be disrupted, disruption of cell membranes has the potential to cause cell death. Microbiological elements may sustain oxidative damage from reactive oxygen species, which can result in cell death. Additionally, polyphenols can disrupt quorum-sensing commands, which can impact gene expression and the development of biofilms. Additionally, by modifying the host immune response, they can strengthen defenses against microbiological infections. The particular mechanisms at play may differ based on the objective microorganism, the molecular structure of secondary metabolites, and the surrounding circumstances. Part of the antibacterial capacity of polyphenols comes from their ability to break down microbe cell membranes [26,133]. They cause cellular loss and death through their interactions via lipid bilayers, membrane permeability, and disruption of protein chains in the membrane that releases reactive oxygen species, damaging the integrity and functionality of membranes and ultimately causing cell lysis. This process may cause the microorganisms to lose their internal contents and disrupt their biological functions. Studies indicated that polyphenols, which are effective antifungal, antiviral, and antibacterial agents, have emerged as a potent subject in anti-infective investigations [134,135,136]. This review’s goal is to investigate the antibacterial properties of polyphenolic compounds utilizing in vitro assays. Their applications and possible antimicrobial mechanisms are shown in Figure 2 [137,138,139].
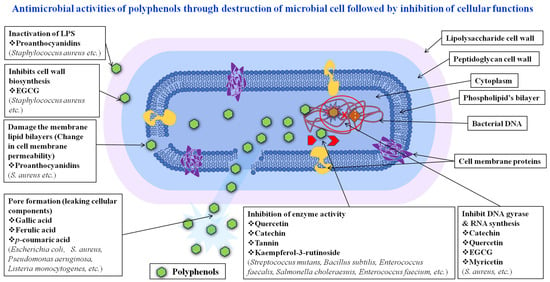
Figure 2.
Schematic diagram of antimicrobial effect of natural polyphenols through inhibition of intracellular functions.
Of the most biologically active polyphenols, few of their antibacterial, antiviral, antifungal, etc., properties have been described. Here are a few instances of polyphenols and their listed antimicrobial properties, also reviewed in tabular form [140,141]:
Catechins: These are the types of flavonoids present in cocoa, green tea, and other foods. Catechins have the highest antimicrobial activity against a variety of bacteria, including Helicobacter pylori, which is linked to gastritis and peptic ulcer illness, and Streptococcus mutans, which causes dental caries. EGC, EGCG, etc. and catechins act as strong antimicrobial agents through DNA gyrase (binding at ATP sites), inhibition of intracellular functions, as well as destruction of biofilm formation in the microbial cells. A slight alteration within the toxin’s configuration causes a reduction in the toxin’s required membrane communications. That, in turn, causes catechin substances to inhibit leukotoxin action.
We believe that these substances can be utilize internally to cure periodontal diseases and boost patients through improvement therapy protocols. Catechins from tea, such as EGCG, prevent planktonic growth of the bacterium P. gingivalis [142,143,144].
Quercetin: Quercetins, having antimicrobial activities, were investigated using the broth dilution technique against: S. aureus and Pseudomonas aeruginosa ((quercetin = 20 µ/mL); E. coli (400 mcg/mL); Proteus vulgaris (300 µ/mL); Shigella flexneri and Lactobacillus casei var. Shirota (500 µ/mL)) [101,145,146]. Quercetin shows antifungal activity in literature studies on C. albicans. The C. albicans strain grew aerobically overnight in 4 mL of Sabouraud broth at 37 °C. After 48 h at 37 °C, viable Candida albicans cells were counted, and CFU values were recorded on Sabouraud agar plates, producing around 107 CFU/mL after overnight incubation [147]. The MIC values of quercetin derivative solutions, after 48 h at 37 °C (the minimal amount of flavonoid products that prevent C. albicans from growing visibly), were determined to range from 0.06 to 2.50 mg/mL [148].
Resveratrol: This is a stilbene found (with a high content) in red wine and grapes. It has been demonstrated that resveratrol exerts antimicrobial effects on several microorganisms, including Staphylococcus aureus and Helicobacter pylori. Therefore, in parallel to the biochemical processes stated previously, the main focus of research is to investigate the potential as an inhibitor of the growth of specific harmful microbes like Gram-positive and Gram-negative bacteria, fungi, etc. It could be used in the future to prevent and treat various diseases caused by particular pathogens. Staphylococcus aureus, Bacillus cereus, Escherichia coli ATCC 25922, etc. have been used to test the antimicrobial activities of resveratrol in different settings like agar dilution, microdilution, time–kill curve methods, etc. [149,150,151]. Resveratrol’s antiviral properties are linked to restrictions of viral transmission, the production of proteins, gene transcription, and the generation of nucleic acids, whereas the antioxidant benefit is primarily generated via inhibition of essential genetic routes, such as the nuclear factor-kappa β (NF-κβ) pathway. Resveratrol is beneficial in treating several viral (Epstein–Barr virus (EBV), herpes simplex virus-1 and herpes simplex virus-2 (HSV-1 and HSV-2), as well as respiratory syncytial viral (RPSV), etc.) infections. An anti-inflammatory intermediary by blocking NF-κβ activity, procyclooxygenase-2 activity, and prostaglandin synthesis, in addition to cellular growth at the G1 and G1/S stages, can be observed using resveratrol.
Ellagic acid: The simultaneous use of ellagic acid with other antioxidants, which are well known for their numerous potential antimicrobial benefits, has also been shown to have effective medicinal properties. Antibacterial, antiallergic, antimicrobial, antiviral, and antiparasitic properties were additionally demonstrated for this material [152,153]. Furthermore, ellagic acid has demonstrated immunity toward naturally occurring toxins as well as the toxicity of metals and metalloids. This is a polyphenol found in nuts, berries, and pomegranates. Streptococcus mutans and Helicobacter pylori are just two of the microorganisms in which ellagic acid has been demonstrated to have an antibacterial effect [154,155,156]. Human rhinoviruses, or HRVs, such as HRV-2, HRV-3, and HRV-4, are the reason for widespread flu and account for millions of dollars’ worth of healthcare expenditures each year. Naturally, ellagic acid (EA) was found to have IC50 values that indicated that it is around 2 times more toxic than ribavirin against HRV-2 (38 μg/mL), HRV-3 (31 μg/mL), and HRV-4 (29 μg/mL). Moreover, 50 μg/mL EA significantly suppresses HRV-4’s RNA synthesis in HeLa cells, according to real-time PCR techniques, indicating that EA blocks viral transformations by focusing on cell molecules instead of virus particles, as well as protecting the cell from the virus.
Curcumin: Curcumin has antimicrobial properties against a variety of bacteria, viruses, fungi, etc. Turmeric contains a polyphenol called curcumin. Pseudomonas aeruginosa, Staphylococcus aureus, Escherichia coli, etc. are strongly inhibited through the destruction of the microorganism’s cell in the presence of curcumin [157,158,159]. Additionally, curcumin has demonstrated strong antibacterial properties towards 65 clinical specimens of Helicobacter pylori, with MIC values of 5 and 50 μg/mL, and inhibitory effects toward methicillin-resistant S. aureus (MRSA) strains with a minimal inhibitory concentration (MIC) of 125–250 μg/mL. Curcumin’s various bioconjugates, such as di-O-tryptophan-phenylalanine curcumin, di-O-decanoyl curcumin, di-O-palmitoyl curcumin, etc., have demonstrated strong antiviral activity towards a range of viruses, including flock house virus (FHV), parainfluenza virus-3 (PIV-3), feline-infectious peritonitis virus (FIPV), etc. Di-O-decanoyl and di-O tryptophan–phenylalanine curcumins, with half maximal effective concentration (EC50) values of 0.011 μM and 0.029 μM, respectively, have demonstrated notable beneficial activity towards FIPV/FHV. On the other hand, in MT-4 cells, bioconjugates showed no appreciable antiviral activity towards HIV-1 (IIIB) and HIV-2 (ROD) varieties [160]. Even so, the strong antibacterial, antioxidant, antifungal, anti-inflammatory, and antiviral properties of curcumin have been proven. However, antimicrobial studies are required for further detailed investigation of more clinical isolates [161,162,163].
Naringenin: Through disruption of DNA replication, cell membranes, and enzyme functions, naringenin demonstrates antibacterial properties against Salmonella typhimurium, Escherichia coli, Pseudomonas aeruginosa, and Staphylococcus aureus. Moreover, it prevents the growth of bacterial biofilms, which may enhance the effectiveness of treatment [164]. Naringenin possesses antifungal properties towards a range of fungi, especially Aspergillus fumigatus, Candida albicans, Cryptococcus neoformans, and dermatophytes like Trichophyton rubrum. These molds are linked to a variety of human infections, such as dermatophytosis (fungal skin infections), systemic fungal infections, and oral thrush. Naringenin fights influenza A and B strains, inhibits HSV-1 and HSV-2, which cause genital and oral herpes, and has antiviral activity against HCV, which causes chronic hepatitis. It also prevents dengue virus replication [165].
Luteolin: Luteolin exhibits antibacterial activity against a range of bacteria, including Helicobacter pylori, Pseudomonas aeruginosa, Escherichia coli, and Staphylococcus aureus. Its efficacy is attributed to its mechanisms of action, which include rupturing bacterial cell membranes and blocking bacterial enzymes [166,167]. Numerous fungal pathogens, such as Botrytis cinerea and Penicillium expansum, have been shown to be susceptible to luteolin’s antifungal activity [168]. Luteolin’s antiviral properties and low toxicity were analyzed using LDH and MTT assays. Cell survival trends improved in the presence of PRV, indicating luteolin’s beneficial effects at concentrations below 10 μM. By inducing HO-1 expression and downregulating STAT1/3-dependent NF-κB activation, luteolin suppresses viral inflammation [169,170,171].
Cyanidin: It has been used in antibiotics, inhibits S. aureus and K. pneumoniae microbes about 87% observed by in-vitro method. Elevated levels of cyanidin improve K. pneumoniae’s susceptibility to pertinent antibiotics. Generally, synergistic effects are evident in the values of fractional inhibitory concentration (FIC). Antibiotic action is enhanced synergistically by sub-MIC cyanidin [172]. Cyanidin has exhibited antifungal properties against a range of fungal species, including Botrytis cinerea, Candida albicans, and Colletotrichum gloeosporioides. As concentrations are raised, cyanidin-3-glucoside more effectively controls the fungus [173]. Fruit-derived flavonoid cyanidin inhibits the NF-κB and IL-17A pathways to fight disease. In RSV-infected mice, it reduces cytokine levels, inhibits multiple viruses, and minimizes lung damage. It restricts RSV, HSV-1, influenza virus, canine coronavirus, and SARS-CoV-2 multiplication, indicating a wide range of antiviral activity [127].
Daidzein: Daidzein is used to treat menopausal symptoms, thrombosis, hypertension, vertigo, and deafness. When it comes to Staphylococcus aureus, Escherichia coli, Acinetobacter baumannii, Klebsiella pneumoniae, and Pseudomonas aeruginosa, daizenin has a clear antibacterial effect [174,175]. Elicitation causes daidzein prenylation, indicating that interactions between A. oryzae and soybeans produce beneficial phytoalexins. Daidzein has also shown significant antifungal activity against Pyrrhoderma noxium, C. lindemuthianum, and P. citricarpa [176]. Preclinical research on daidzein has revealed possible antiviral activity against a range of viruses. This covers viruses like hepatitis C virus (HCV), human immunodeficiency virus (HIV), influenza, etc. Daidzein’s efficacy against these and other viruses varies with its concentration [177,178].
Bioactive polyphenols, which are abundant in nature, are summarized in tabular form to describe their chemical molecular structures, molecular weight (Mol. Wt.), and aqueous solubility (mg/mL), including extracted amounts from different natural sources (Table 2).

Table 2.
Aqueous solubility and extracted amounts from natural sources of a few polyphenols are described in the following table.
Different polyphenolic compounds were considered for their antimicrobial activity for further use in different applications, such as for medicinal purposes or in food industries. Antibacterial, antifungal, and antiviral studies are mainly considered for checking primarily for further use in formulation processes. Among them, most polyphenols show strong antimicrobial activities. Antibacterial studies have been observed in the literature, including biological applications.
6. Determination Assay
6.1. Determination of the Antibacterial Activity
Polyphenols cause destabilization or disruption in the cell membrane by combining with the cytoplasmic microbial cell membrane’s lipid bilayer. This breakdown occurs because polyphenolic substances can irreversibly change the structures of cell membranes and interfere with their hydrophobic properties [209]. They can also start spot ruptures that compromise the impermeability of a structure. This produces pores that can be penetrated by cellular material. The physical structure of a cell membrane is distorted when polyphenols are present, which causes the membrane to expand, become structurally unstable, and enhance the material’s inactive penetration [210,211]. The loosening of a microbial cell’s membrane robs it of ATP, ions, and nucleic acids, all of which are necessary for the cell to survive. Certain polyphenols, like flavone-3-ol (tannin), can interfere with the replication of genes required for the synthesis of p-fimbriae proteins. Microbial agents, like bacteria (Escherichia coli), are unable to stick to the surface of the epithelium due to a modification in molecular structure. Uropathogenic E. coli cannot spread to the surface of the mucilaginous epithelium and subsequently cause a barrier, and they are unable to form an adhesive connection with the receptors found on the host’s cell membranes [212,213]. Several bacterial strains (such as S. aureus CNRZ3 [214], L. monocytogenes ATCC19115 [215], P. aeruginosa ATCC27853 [216], S. enteritidis E0220, B. subtilis ATCC6633, and E. coli ATCC25922 [214]), are used separately in broth microdilution method to investigate the antibacterial activity of the corresponding polyphenols by cell growth monitoring [217,218]. Mainly, the DMSO solvent was used within a suitable concentration range for antibacterial studies, since solubility of polyphenols are much less or insoluble in aqueous medium. Generally, the buffer could not be used for making the polyphenolic solutions. Antibacterial property shows the percentage of OD at a fixed wavelength range of reduction after 24 h at 37 °C, which is computed using the following formula [214,219].
where OD = optical density (nm), bacteria load difference (BLD) = percent reduction of OD420−580 after 24 h of incubation.
The efficiency of antibacterial drugs on microorganisms is commonly assessed through the use of minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) assays. Associated methods include agar dilution method, microdilution method, E-test (epsilometer test), and broth dilution method. Here, antibacterial activity determination procedures have been stepwise extrapolated through the general disk diffusion process as follows.
First, prepare the nutrient agar plates, which should be prepared using conventional procedures through transferring the liquefied agar onto sterilized Petri plates and letting them set. Bacterial inoculums have to be prepared by choosing which bacterial strains to test. This will depend on the goals, ensuring the turbidity of the bacterial culture is adjusted to meet the 0.5 McFarland standard (about 1.5 × 108 CFU/mL) by suspending it in a sterile solution of saline or broth. (i) After that, if the antibacterial agent is solid, dilute it in a suitable solvent (such as ethanol or DMSO or water) to create a sample solution with a specified concentration. To obtain the appropriate concentrations for testing, dilute the stock solution. (ii) Impregnation of disks: Place sterile filter paper disks onto a sterile surface using sterile forceps. (iii) Then, on each disk, apply a known volume of the antibacterial agent solution. To make sure the disks are completely impregnated, let them air dry. (iv) Evenly smear bacterial suspension across the agar plates’ surface using a sterile cotton swab. To make sure the inoculum is properly absorbed, let the inoculated plates dry for a few minutes. (v) On the surface of the infected agar plates, place the impregnated disks containing the antibacterial agent using sterile forceps. (vi) Incubation: After inverting the plates, incubate them for about 18–24 h at the proper temperature (37 °C) for most bacterial strains [220]. (vii) Zones of inhibition measurement: Using a caliper or ruler, measure the diameter of the clear zones (zones of inhibition) surrounding the disks after incubation. (viii) Note the zone widths for every antimicrobial agent’s strength that was evaluated and analyze the data by calculating the average diameter of the zones of inhibition for each antibacterial agent concentration. (ix) To find the minimum inhibitory concentration (MIC) and evaluate the antibacterial agent’s potency, plot a dose–response curve. Then, determine the tested agent’s antibacterial activity and compare the zone widths and MIC values to published data or standard reference values. Finally, to verify the results, use suitable −ve and +ve controls in the test, such as solvent-only disks and standard antibiotics. Using the technique of disk diffusion, this process offers a fundamental framework for assessing a substance’s antibacterial properties [220,221,222].
6.2. Determination of the Antifungal Activity
Identifying an element’s antifungal properties entails evaluating its capacity to prevent the reproduction or survival of fungi. Materials with antifungal action include drugs, natural products, and chemicals. Usually, this is accomplished by using a variety of tests and experimental techniques. The following are some typical procedures and techniques for assessing antifungal activity [223,224,225,226].
First, select the particular fungi toward which the antifungal properties of the selected microorganisms are investigated. They might include experimental strains of various species of fungi, standard strains, or medical isolates. Then comes testing material preparation. If testing a chemical compound or natural extract, prepare the ingredient in various doses. It may be dissolved in the proper solvents to produce a variety of strengths. In this manner and in the future, systems have to be considered for antifungal activities using different methods. Examples are: (i) Agar dilution method: Create several agar plates with progressively higher test material concentrations. Inject the plates with fungus spores. Place the plates in an incubator at the right temperatures for fungi to thrive. The areas surrounding the wells or streaks that hold the drug are the inhibition areas. The stronger the antifungal action, the bigger the inhibition area. (ii) Broth dilution method (minimum inhibitory concentration, MIC): Prepare a series of tubes containing the test material in a growth medium at escalating concentrations. Add fungi spores to each tube. Set the tubes’ temperature to the ideal range for fungus growth. The MIC is the level of the chemical at which visible fungal growth is inhibited. (iii) Disk diffusion method: The filtering disks should be impregnated using the test material. Put the disks on a plate of agar that has been freshly inoculated with fungi. Determine the area of inhibition surrounding the disks after incubating the plate. (iv) Time–kill assay: Use a precise concentration of the experiment’s chemical to the fungus culture. Specimens taken at various times should be plated onto agar to determine the number of growing fungal colonies. This technique reveals how rapidly the chemical eliminates the fungus. (v) Biofilm inhibition assay: Certain molds can create biofilms that become more resistant to antifungal medications. Compounds are evaluated for their impact on biofilm viability and development using particular tests. Analysis of gene expression and quantitative PCR are two methods that can be used to measure how the test drug influences particular fungal genes or pathways.
After that, the cytotoxicity evaluation can be carried out on the test chemical for human or animal cells. When thinking about using the chemical for therapeutic purposes, this is crucial. Finally, using analytical statistics, ascertain whether the detected antifungal properties are statistically important, and run the necessary statistical analysis. The choice of method depends on factors like the nature of the test substance, the fungal species being studied, and the specific research objectives. It is also important to use appropriate controls and replicate experiments to ensure reliable and meaningful results.
Short descriptions of antifungal activity of polyphenols have been extrapolated with several examples. Different fungi survive and make our lives difficult, such as fungal infections caused by Candida, Aspergillus, Pneumocystis, and Cryptococcus, the main threatening agents. These can be killed by polyphenolic compounds through the disk diffusion method: daidzein with MIC value 516–1032 µg/mL active on C. albicans, genistein active on T. rubrum at 1000 µg/mL, naringenin active on C. graminicola and T. deformans with MIC value of about 3.125 mg/mL, quercetin active on C. albicans, C. glabrata, and C. krusei with MIC value of about 7.8–256 µg/mL, etc. [176].
6.3. Determination of Antiviral Activity
Antiviral activities were studied in the following procedure using an in vitro model. Testing substances or compounds to ascertain their efficacy in preventing the replication or spread of viruses is a common step in antiviral activity procedures. This is a basic summary of the steps taken to evaluate antiviral activity [148,165,227].
Selection of virus: (i) Determine which viruses are appropriate for the target infection or illness. (ii) Select a particular strain from the variety of viruses if known.
Culturing of cells: (i) Choose suitable cells for hosting that facilitate the selected virus’s reproduction. (ii) Keep cultivating cells in an environment conducive to the replication of viruses. (iii) Make sure the cells are uncontaminated and in good health.
Test for cytotoxicity: (i) Determine the toxicity of the test material on the host cell population. (ii) Analyses to assess the viability of cells can be carried out through methylthiazolyldiphenyl-tetrazolium bromide (MTT) assay, methoxynitrosulfophenyl-tetrazolium carboxanilide (XTT), or lactate dehydrogenase (LDH) discharge. (iii) Determine a range of concentrations that will not be harmful for upcoming antiviral tests.
Antiviral assay: (i) Introduce the chosen virus into host cells, while varying the test substance’s concentration. (ii) Add the negative controls (without the test material) and positive controls (also referred to as antiviral agents). (iii) Utilizing techniques like quantitative PCR, 50% tissue culture infectious dose (TCID50) assays, or plaque elimination assays, one can quantify the propagation or growth of a virus.
Figure of dose–response: (i) Produce a curve with a dose–response relationship to identify the amount in which the test compound displays antiviral activity. (ii) Determine additional relevant variables, such as the half-maximal inhibitory level (IC50).
Studies of time courses and action mechanisms: (i) Evaluate the antiviral activity’s kinetics as influenced by time. (ii) Find the best incubation period to observe the strongest antiviral effects. (ii) Examine the mechanism by which the antiviral effect of the test substance is achieved. (iii) Examine whether it prevents the entry, reproduction, or release of viruses.
Index of selectivity (SI) repeated and verified: (i) By contrasting the cytotoxicity and antiviral activity, determine a selectivity index. (ii) Importantly, particularity for suppressing the virus despite appreciable cytotoxic effects is indicated by a higher SI. (iii) To ensure that results can be repeated, repeat tests. (iii) To verify the wide-spectrum activity, test with various virus strains or kinds of cells.
Reporting, data analysis, and conclusion: (i) Apply statistical analysis to the data. (ii) Describe the findings using IC50 values, inhibition percentages, and other pertinent information. (iii) Make judgments regarding the tested substance’s potential for antiviral action.
7. Future Prospects
To increase the targeted and controlled release of bioactive polyphenols against microbial organisms, the solubility of polyphenols has to be increased. Modifying the delivery systems having sustained release nature of drug molecules polyphenols strongly protected us from the microorganisms [57,228]. Naturally occurring polyphenols extracted from fruits, leaves, alleges, etc. show lower solubility in aqueous medium, by increasing the solubility of the polyphenols by converting them into the salts can be more useful in different food and biomedical applications [229,230,231]. Using positively charged metals ions, creating a more soluble nature of these polyphenolic compounds through physiochemical modifications can show superior effectiveness against harmful microorganisms as well as upgraded medicinal chemistry [232,233]. It is crucial to keep in mind that the antibacterial, antioxidant, and anticancer activity of these polyphenols can vary based on elements, including the polyphenol’s quantity, solubility in different solvents, the kind of bacterium being targeted, and the tested delivery procedure [234]. A variety of distinct routes may be involved in the mechanisms by which polyphenolic systems perform their antimicrobial properties compared to pure and different modified bioactive polyphenolic substances, which are still under investigation [235,236].
8. Conclusions
The necessity to develop novel antimicrobial compounds is ongoing, as the prevalence of antibiotic resistance increases internationally. Phenols display strong antibacterial activity that can be greatly strengthened via modifications into its salt derivatives. They also have a few modular frameworks that enable a wide space of chemical modifications for different applications. S. aureus (with methicillin resistance), for example, spreads quickly among patients in healthcare institutions and is thought to be a major contributor to community-associated infections and related deaths. A major healthcare goal is the creation of successful treatment options for resistant microorganisms. Polyphenols are diversely structured substances that have been utilized for centuries to cure illnesses, especially infections. In addition to having antibacterial action, they also have antioxidant, anti-inflammatory, and anticancer properties. Based on available research on the antibacterial properties of polyphenols, they may be suggested as a replacement or supplementary treatment for pathogenic disorders. Consequently, regular and ongoing consumption of polyphenols throughout a lifetime can be described as “bioactive polyphenol schooling”, which explains how repeated and ongoing intake of foreign substances is countered by antimicrobial polyphenol molecules through the development of self-defense mechanisms in the body. Natural polyphenols represent a potentially useful resource of antimicrobial properties, either used independently or in conjunction with currently available medicines to create novel antimicrobial therapeutics and in the food industry.
Author Contributions
Writing—original draft, M.K.M.; supervision and visualization, A.J.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data can be shared upon request.
Acknowledgments
M.K. Mandal thanks Abraham J. Domb and the Hebrew University of Jerusalem for doing postdoctoral work and accessible laboratory.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Abbas, M.; Saeed, F.; Anjum, F.M.; Afzaal, M.; Tufail, T.; Bashir, M.S.; Ishtiaq, A.; Hussain, S.; Suleria, H.A.R. Natural polyphenols: An overview. Int. J. Food Prop. 2017, 20, 1689–1699. [Google Scholar] [CrossRef]
- Bié, J.; Sepodes, B.; Fernandes, P.C.; Ribeiro, M.H. Polyphenols in health and disease: Gut microbiota, bioaccessibility, and bioavailability. Compounds 2023, 3, 40–72. [Google Scholar] [CrossRef]
- Giada, M. Food phenolic compounds: Main classes, sources and their antioxidant power. Oxidative Stress Chronic Degener. Dis.-A Role Antioxid. 2013, 2013, 87–112. [Google Scholar]
- Mattila, P.; Hellström, J.; Törrönen, R. Phenolic acids in berries, fruits, and beverages. J. Agric. Food Chem. 2006, 54, 7193–7199. [Google Scholar] [CrossRef] [PubMed]
- Pietta, P.; Minoggio, M.; Bramati, L. Plant polyphenols: Structure, occurrence and bioactivity. Stud. Nat. Prod. Chem. 2003, 28, 257–312. [Google Scholar]
- Soto-Vaca, A.; Gutierrez, A.; Losso, J.N.; Xu, Z.; Finley, J.W. Evolution of phenolic compounds from color and flavor problems to health benefits. J. Agric. Food Chem. 2012, 60, 6658–6677. [Google Scholar] [CrossRef]
- Zeb, A. Concept, mechanism, and applications of phenolic antioxidants in foods. J. Food Biochem. 2020, 44, e13394. [Google Scholar] [CrossRef] [PubMed]
- Rasouli, H.; Farzaei, M.H.; Khodarahmi, R. Polyphenols and their benefits: A review. Int. J. Food Prop. 2017, 20, 1700–1741. [Google Scholar] [CrossRef]
- Cartea, M.E.; Francisco, M.; Soengas, P.; Velasco, P. Phenolic compounds in Brassica vegetables. Molecules 2010, 16, 251–280. [Google Scholar] [CrossRef]
- Durazzo, A.; Lucarini, M.; Souto, E.B.; Cicala, C.; Caiazzo, E.; Izzo, A.A.; Novellino, E.; Santini, A. Polyphenols: A concise overview on the chemistry, occurrence, and human health. Phytother. Res. 2019, 33, 2221–2243. [Google Scholar] [CrossRef]
- Katalinic, V.; Mozina, S.S.; Generalic, I.; Skroza, D.; Ljubenkov, I.; Klancnik, A. Phenolic profile, antioxidant capacity, and antimicrobial activity of leaf extracts from six Vitis vinifera L. varieties. Int. J. Food Prop. 2013, 16, 45–60. [Google Scholar] [CrossRef]
- Coppo, E.; Marchese, A. Antibacterial activity of polyphenols. Curr. Pharm. Biotechnol. 2014, 15, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Grijalva, E.P.; Picos-Salas, M.A.; Leyva-López, N.; Criollo-Mendoza, M.S.; Vazquez-Olivo, G.; Heredia, J.B. Flavonoids and phenolic acids from oregano: Occurrence, biological activity and health benefits. Plants 2017, 7, 2. [Google Scholar] [CrossRef] [PubMed]
- Rathod, N.B.; Elabed, N.; Punia, S.; Ozogul, F.; Kim, S.-K.; Rocha, J.M. Recent developments in polyphenol applications on human health: A review with current knowledge. Plants 2023, 12, 1217. [Google Scholar] [CrossRef] [PubMed]
- Ignat, I.; Volf, I.; Popa, V.I. A critical review of methods for characterisation of polyphenolic compounds in fruits and vegetables. Food Chem. 2011, 126, 1821–1835. [Google Scholar] [CrossRef] [PubMed]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouységu, L. Plant polyphenols: Chemical properties, biological activities, and synthesis. Angew. Chem. Int. Ed. 2011, 50, 586–621. [Google Scholar] [CrossRef] [PubMed]
- Tijjani, H.; Zangoma, M.H.; Mohammed, Z.S.; Obidola, S.M.; Egbuna, C.; Abdulai, S.I. Polyphenols: Classifications, biosynthesis and bioactivities. In Functional Foods and Nutraceuticals: Bioactive Components, Formulations and Innovations; Springer: Cham, Switzerland, 2020; pp. 389–414. [Google Scholar]
- Baldwin, A.; Booth, B.W. Biomedical applications of tannic acid. J. Biomater. Appl. 2022, 36, 1503–1523. [Google Scholar] [CrossRef] [PubMed]
- Nicolaou, K.e.C.; Chen, J.S.; Edmonds, D.J.; Estrada, A.A. Recent advances in the chemistry and biology of naturally occurring antibiotics. Angew. Chem. Int. Ed. 2009, 48, 660–719. [Google Scholar] [CrossRef] [PubMed]
- Górniak, I.; Bartoszewski, R.; Króliczewski, J. Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem. Rev. 2019, 18, 241–272. [Google Scholar] [CrossRef]
- El Gharras, H. Polyphenols: Food sources, properties and applications–a review. Int. J. Food Sci. Technol. 2009, 44, 2512–2518. [Google Scholar] [CrossRef]
- Ververidis, F.; Trantas, E.; Douglas, C.; Vollmer, G.; Kretzschmar, G.; Panopoulos, N. Biotechnology of flavonoids and other phenylpropanoid-derived natural products. Part I: Chemical diversity, impacts on plant biology and human health. Biotechnol. J. Healthc. Nutr. Technol. 2007, 2, 1214–1234. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Martínez, F.J.; Barrajón-Catalán, E.; Encinar, J.A.; Rodríguez-Díaz, J.C.; Micol, V. Antimicrobial capacity of plant polyphenols against gram-positive bacteria: A comprehensive review. Curr. Med. Chem. 2020, 27, 2576–2606. [Google Scholar] [CrossRef] [PubMed]
- Papuc, C.; Goran, G.V.; Predescu, C.N.; Nicorescu, V.; Stefan, G. Plant polyphenols as antioxidant and antibacterial agents for shelf-life extension of meat and meat products: Classification, structures, sources, and action mechanisms. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1243–1268. [Google Scholar] [CrossRef] [PubMed]
- Bouarab Chibane, L.; Degraeve, P.; Ferhout, H.; Bouajila, J.; Oulahal, N. Plant antimicrobial polyphenols as potential natural food preservatives. J. Sci. Food Agric. 2019, 99, 1457–1474. [Google Scholar] [CrossRef] [PubMed]
- Daglia, M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Bomkamp, C.; Skaalure, S.C.; Fernando, G.F.; Ben-Arye, T.; Swartz, E.W.; Specht, E.A. Scaffolding biomaterials for 3D cultivated meat: Prospects and challenges. Adv. Sci. 2022, 9, 2102908. [Google Scholar] [CrossRef] [PubMed]
- Rana, A.; Samtiya, M.; Dhewa, T.; Mishra, V.; Aluko, R.E. Health benefits of polyphenols: A concise review. J. Food Biochem. 2022, 46, e14264. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Rahaman, M.S.; Islam, M.R.; Rahman, F.; Mithi, F.M.; Alqahtani, T.; Almikhlafi, M.A.; Alghamdi, S.Q.; Alruwaili, A.S.; Hossain, M.S. Role of phenolic compounds in human disease: Current knowledge and future prospects. Molecules 2021, 27, 233. [Google Scholar] [CrossRef]
- De Lima Cherubim, D.J.; Buzanello Martins, C.V.; Oliveira Fariña, L.; da Silva de Lucca, R.A. Polyphenols as natural antioxidants in cosmetics applications. J. Cosmet. Dermatol. 2020, 19, 33–37. [Google Scholar] [CrossRef]
- Guo, Y.; Sun, Q.; Wu, F.G.; Dai, Y.; Chen, X. Polyphenol-containing nanoparticles: Synthesis, properties, and therapeutic delivery. Adv. Mater. 2021, 33, 2007356. [Google Scholar] [CrossRef]
- RACCACH, M. The antimicrobial activity of phenolic antioxidants in foods: A review 1. J. Food Saf. 1984, 6, 141–170. [Google Scholar] [CrossRef]
- Sobhani, M.; Farzaei, M.H.; Kiani, S.; Khodarahmi, R. Immunomodulatory; anti-inflammatory/antioxidant effects of polyphenols: A comparative review on the parental compounds and their metabolites. Food Rev. Int. 2021, 37, 759–811. [Google Scholar] [CrossRef]
- Montenegro-Landívar, M.F.; Tapia-Quirós, P.; Vecino, X.; Reig, M.; Valderrama, C.; Granados, M.; Cortina, J.L.; Saurina, J. Polyphenols and their potential role to fight viral diseases: An overview. Sci. Total Environ. 2021, 801, 149719. [Google Scholar] [CrossRef] [PubMed]
- Zekrumah, M.; Begua, P.; Razak, A.; Wahab, J.; Moffo, N.; Ivane, A.; Oman, M.; Elrashied, H.; Zou, X.; Zhang, D. Role of dietary polyphenols in non-communicable chronic disease prevention, and interactions in food systems: An overview. Nutrition 2023, 112, 112034. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, R.; Swamy, M.K.; Jaganathan, R. Therapeutic potential of plant polyphenolics and their mechanistic action against various diseases. In Natural Bio-Active Compounds: Volume 2: Chemistry, Pharmacology and Health Care Practices; Springer: Singapore, 2019; pp. 313–351. [Google Scholar]
- Olszewska, M.A.; Gędas, A.; Simões, M. Antimicrobial polyphenol-rich extracts: Applications and limitations in the food industry. Food Res. Int. 2020, 134, 109214. [Google Scholar] [CrossRef] [PubMed]
- Efenberger-Szmechtyk, M.; Nowak, A.; Czyzowska, A. Plant extracts rich in polyphenols: Antibacterial agents and natural preservatives for meat and meat products. Crit. Rev. Food Sci. Nutr. 2021, 61, 149–178. [Google Scholar] [CrossRef]
- Rudrapal, M.; Sarkar, B.; Deb, P.K.; Bendale, A.R.; Nagar, A. Addressing Antimicrobial Resistance by Repurposing Polyphenolic Phytochemicals with Novel Antibacterial Potential. In Polyphenols: Food, Nutraceutical, and Nanotherapeutic Applications; John Wiley & Sons: Hoboken, NJ, USA, 2023; pp. 260–289. [Google Scholar]
- Cui, Y.; Oh, Y.; Lim, J.; Youn, M.; Lee, I.; Pak, H.; Park, W.; Jo, W.; Park, S. AFM study of the differential inhibitory effects of the green tea polyphenol (−)-epigallocatechin-3-gallate (EGCG) against Gram-positive and Gram-negative bacteria. Food Microbiol. 2012, 29, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Ishiyama, K.; Sheng, H.; Ikai, H.; Kanno, T.; Niwano, Y. Bactericidal activity and mechanism of photoirradiated polyphenols against Gram-positive and-negative bacteria. J. Agric. Food Chem. 2015, 63, 7707–7713. [Google Scholar] [CrossRef]
- Pagliarulo, C.; De Vito, V.; Picariello, G.; Colicchio, R.; Pastore, G.; Salvatore, P.; Volpe, M.G. Inhibitory effect of pomegranate (Punica granatum L.) polyphenol extracts on the bacterial growth and survival of clinical isolates of pathogenic Staphylococcus aureus and Escherichia coli. Food Chem. 2016, 190, 824–831. [Google Scholar] [CrossRef]
- Goswami, P.; Kalita, C.; Bhuyan, A.C. Antibacterial Activity of Black Tea Extract against S. mutans, S. aureus, L. acidophilus, Klebsiella and E. coli. J. Evol. Med. Dent. Sci. 2020, 9, 18–22. [Google Scholar] [CrossRef]
- Rojas, R.; Alvarez-Pérez, O.B.; Contreras-Esquivel, J.C.; Vicente, A.; Flores, A.; Sandoval, J.; Aguilar, C.N. Valorisation of mango peels: Extraction of pectin and antioxidant and antifungal polyphenols. Waste Biomass Valorization 2020, 11, 89–98. [Google Scholar]
- Das, P.E.; Abu-Yousef, I.A.; Majdalawieh, A.F.; Narasimhan, S.; Poltronieri, P. Green synthesis of encapsulated copper nanoparticles using a hydroalcoholic extract of Moringa oleifera leaves and assessment of their antioxidant and antimicrobial activities. Molecules 2020, 25, 555. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Suzuki, R.; Murata, I.; Nomura, H.; Isshiki, Y.; Kanamoto, I. Evaluation of antibacterial activity expression of the hinokitiol/cyclodextrin complex against bacteria. ACS Omega 2020, 5, 27180–27187. [Google Scholar] [CrossRef] [PubMed]
- Simonetti, G.; Brasili, E.; Pasqua, G. Antifungal activity of phenolic and polyphenolic compounds from different matrices of Vitis vinifera L. against human pathogens. Molecules 2020, 25, 3748. [Google Scholar] [CrossRef]
- Loo, Y.T.; Howell, K.; Chan, M.; Zhang, P.; Ng, K. Modulation of the human gut microbiota by phenolics and phenolic fiber-rich foods. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1268–1298. [Google Scholar] [CrossRef] [PubMed]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Extraction of phenolic compounds: A review. Curr. Res. Food Sci. 2021, 4, 200–214. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Han, Z.; Granato, D. Polyphenols in foods: Classification, methods of identification, and nutritional aspects in human health. Adv. Food Nutr. Res. 2021, 98, 1–33. [Google Scholar] [PubMed]
- Guimarães, I.; Baptista-Silva, S.; Pintado, M.; Oliveira, A.L. Polyphenols: A promising avenue in therapeutic solutions for wound care. Appl. Sci. 2021, 11, 1230. [Google Scholar] [CrossRef]
- Zulkefli, N.; Che Zahari, C.N.M.; Sayuti, N.H.; Kamarudin, A.A.; Saad, N.; Hamezah, H.S.; Bunawan, H.; Baharum, S.N.; Mediani, A.; Ahmed, Q.U. Flavonoids as potential wound-healing molecules: Emphasis on pathways perspective. Int. J. Mol. Sci. 2023, 24, 4607. [Google Scholar] [CrossRef]
- Yupanqui Mieles, J.; Vyas, C.; Aslan, E.; Humphreys, G.; Diver, C.; Bartolo, P. Honey: An advanced antimicrobial and wound healing biomaterial for tissue engineering applications. Pharmaceutics 2022, 14, 1663. [Google Scholar] [CrossRef]
- Rathee, P.; Sehrawat, R.; Rathee, P.; Khatkar, A.; Akkol, E.K.; Khatkar, S.; Redhu, N.; Türkcanoğlu, G.; Sobarzo-Sánchez, E. Polyphenols: Natural preservatives with promising applications in food, cosmetics and pharma industries; problems and toxicity associated with synthetic preservatives; impact of misleading advertisements; recent trends in preservation and legislation. Materials 2023, 16, 4793. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Bamunuarachchi, N.I.; Tabassum, N.; Kim, Y.-M. Caffeic acid and its derivatives: Antimicrobial drugs toward microbial pathogens. J. Agric. Food Chem. 2021, 69, 2979–3004. [Google Scholar] [CrossRef] [PubMed]
- Manso, T.; Lores, M.; de Miguel, T. Antimicrobial activity of polyphenols and natural polyphenolic extracts on clinical isolates. Antibiotics 2021, 11, 46. [Google Scholar] [CrossRef] [PubMed]
- Murthy, N.T.V.; Agrahari, V.; Chauhan, H. Polyphenols against infectious diseases: Controlled release nano-formulations. Eur. J. Pharm. Biopharm. 2021, 161, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- González-Sarrías, A.; Tomás-Barberán, F.A.; García-Villalba, R. Structural diversity of polyphenols and distribution in foods. In Dietary Polyphenols: Their Metabolism and Health Effects; John Wiley & Sons: Hoboken, NJ, USA, 2020; pp. 1–29. [Google Scholar]
- Zeb, A. A comprehensive review on different classes of polyphenolic compounds present in edible oils. Food Res. Int. 2021, 143, 110312. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, J.; Gaspar, A.; Garrido, E.M.; Garrido, J.; Borges, F. Hydroxycinnamic acid antioxidants: An electrochemical overview. BioMed Res. Int. 2013, 2013, 251754. [Google Scholar] [CrossRef] [PubMed]
- Valanciene, E.; Jonuskiene, I.; Syrpas, M.; Augustiniene, E.; Matulis, P.; Simonavicius, A.; Malys, N. Advances and prospects of phenolic acids production, biorefinery and analysis. Biomolecules 2020, 10, 874. [Google Scholar] [CrossRef] [PubMed]
- Andrés-Lacueva, C.; Medina-Remon, A.; Llorach, R.; Urpi-Sarda, M.; Khan, N.; Chiva-Blanch, G.; Zamora-Ros, R.; Rotches-Ribalta, M.; Lamuela-Raventós, R.M. Phenolic Compounds: Chemistry and Occurrence in Fruits and Vegetables. Fruit and Vegetable Phytochemicals: Chemistry, Nutritional Value, and Stability; John Wiley & Sons: Hoboken, NJ, USA, 2009; pp. 53–88. [Google Scholar]
- Besednova, N.N.; Andryukov, B.G.; Zaporozhets, T.S.; Kryzhanovsky, S.P.; Kuznetsova, T.A.; Fedyanina, L.N.; Makarenkova, I.D.; Zvyagintseva, T.N. Algae Polyphenolic Compounds and Modern Antibacterial Strategies: Current Achievements and Immediate Prospects. Biomedicines 2020, 8, 342. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- Rambaran, T.F. Nanopolyphenols: A review of their encapsulation and anti-diabetic effects. SN Appl. Sci. 2020, 2, 1335. [Google Scholar] [CrossRef]
- Vaquero, M.R.; Alberto, M.R.; De Nadra, M.M. Antibacterial effect of phenolic compounds from different wines. Food Control 2007, 18, 93–101. [Google Scholar] [CrossRef]
- Borges, A.; Saavedra, M.J.; Simões, M. The activity of ferulic and gallic acids in biofilm prevention and control of pathogenic bacteria. Biofouling 2012, 28, 755–767. [Google Scholar] [CrossRef] [PubMed]
- Welch, C.R.; Wu, Q.; Simon, J.E. Recent advances in anthocyanin analysis and characterization. Curr. Anal. Chem. 2008, 4, 75–101. [Google Scholar] [CrossRef] [PubMed]
- Dhalaria, R.; Verma, R.; Kumar, D.; Puri, S.; Tapwal, A.; Kumar, V.; Nepovimova, E.; Kuca, K. Bioactive Compounds of Edible Fruits with Their Anti-Aging Properties: A Comprehensive Review to Prolong Human Life. Antioxidants 2020, 9, 1123. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Caballero, J.M.; Coy-Barrera, E. Chapter4.10—Lignans. In Antioxidants Effects in Health; Nabavi, S.M., Silva, A.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 387–416. [Google Scholar] [CrossRef]
- Challa, S.; Ajumeera, R.; Venna, N. Phytoestrogens as a Natural Source for the Possible Colon Cancer Treatment. Anticancer. Plants Mech. Mol. Interact. 2018, 4, 259–281. [Google Scholar]
- Blaskovich, M.A.T.; Elliott, A.G.; Kavanagh, A.M.; Ramu, S.; Cooper, M.A. In vitro Antimicrobial Activity of Acne Drugs Against Skin-Associated Bacteria. Sci. Rep. 2019, 9, 14658. [Google Scholar] [CrossRef] [PubMed]
- Mitani, T.; Ota, K.; Inaba, N.; Kishida, K.; Koyama, H.A. Antimicrobial activity of the phenolic compounds of Prunus mume against Enterobacteria. Biol. Pharm. Bull. 2018, 41, 208–212. [Google Scholar] [CrossRef]
- Chagas, M.d.S.S.; Behrens, M.D.; Moragas-Tellis, C.J.; Penedo, G.X.; Silva, A.R.; Gonçalves-de-Albuquerque, C.F. Flavonols and flavones as potential anti-inflammatory, antioxidant, and antibacterial compounds. Oxid. Med. Cell. Longev. 2022, 2022, 9966750. [Google Scholar] [CrossRef]
- Adamczak, A.; Ożarowski, M.; Karpiński, T.M. Antibacterial Activity of Some Flavonoids and Organic Acids Widely Distributed in Plants. J. Clin. Med. 2020, 9, 109. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Venegas, G.; Gómez-Mora, J.A.; Meraz-Rodríguez, M.A.; Flores-Sánchez, M.A.; Ortiz-Miranda, L.F. Effect of flavonoids on antimicrobial activity of microorganisms present in dental plaque. Heliyon 2019, 5, e03013. [Google Scholar] [CrossRef] [PubMed]
- Nabil-Adam, A.; Ashour, M.L.; Tamer, T.M.; Shreadah, M.A.; Hassan, M.A. Interaction of Jania rubens Polyphenolic Extract as an Antidiabetic Agent with α-Amylase, Lipase, and Trypsin: In Vitro Evaluations and In Silico Studies. Catalysts 2023, 13, 443. [Google Scholar] [CrossRef]
- Cushnie, T.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Biharee, A.; Sharma, A.; Kumar, A.; Jaitak, V. Antimicrobial flavonoids as a potential substitute for overcoming antimicrobial resistance. Fitoterapia 2020, 146, 104720. [Google Scholar] [CrossRef] [PubMed]
- Barreca, D.; Gattuso, G.; Bellocco, E.; Calderaro, A.; Trombetta, D.; Smeriglio, A.; Laganà, G.; Daglia, M.; Meneghini, S.; Nabavi, S.M. Flavanones: Citrus phytochemical with health-promoting properties. BioFactors 2017, 43, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Kırcı, D.; Demirci, F.; Demirci, B.l. Microbial Transformation of Hesperidin and Biological Evaluation. ACS Omega 2023, 8, 42610–42621. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, A.; Dar, I.H.; Bhat, S.A.; Ahmad, S. Flavonoids: Health benefits and their potential use in food systems. In Functional Food Products and Sustainable Health; Springer: Singapore, 2020; pp. 235–256. [Google Scholar]
- Cisowska, A.; Wojnicz, D.; Hendrich, A.B. Anthocyanins as antimicrobial agents of natural plant origin. Nat. Prod. Commun. 2011, 6, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Lim, S. Anthocyanin-Enriched Purple Sweet Potato for Colon Cancer Prevention; Kansas State University: Manhattan, KS, USA, 2012. [Google Scholar]
- Zia Ul Haq, M.; Riaz, M.; Saad, B.; Zia-Ul-Haq, M. The Role of Anthocyanins in Health as Antioxidant, in Bone Health and as Heart Protecting Agents. In Anthocyanins and Human Health: Biomolecular and Therapeutic Aspects; Springer: Cham, Switzerland, 2016; pp. 87–107. [Google Scholar]
- Côté, J.; Caillet, S.; Doyon, G.; Sylvain, J.-F.; Lacroix, M. Bioactive compounds in cranberries and their biological properties. Crit. Rev. Food Sci. Nutr. 2010, 50, 666–679. [Google Scholar] [CrossRef]
- Gaya, P.; Peirotén, Á.; Medina, M.; Landete, J.M. Isoflavone metabolism by a collection of lactic acid bacteria and bifidobacteria with biotechnological interest. Int. J. Food Sci. Nutr. 2016, 67, 117–124. [Google Scholar] [CrossRef]
- Alsaloom, A.N. Testing and Evaluation of Bioactive Compounds in Soybean. Iraqi J. Agric. Sci. 2023, 54, 85–92. [Google Scholar] [CrossRef]
- Alqahtani, M.; Almukainzi, M.; Alghoribi, M.F.; El-Mahdy, A.M. Antivirulence Effects of Trans-Resveratrol and Curcumin on Methicillin-Resistant Staphylococcus aureus (MRSA) from Saudi Arabia. Life 2024, 14, 491. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhu, T.; Song, Y.; Feng, L.; Kear, P.J.; Riseh, R.S.; Sitohy, M.; Datla, R.; Ren, M. Salicylic acid fights against Fusarium wilt by inhibiting target of rapamycin signaling pathway in Fusarium oxysporum. J. Adv. Res. 2022, 39, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Jiamboonsri, P.; Eurtivong, C.; Wanwong, S. Assessing the Potential of Gallic Acid and Methyl Gallate to Enhance the Efficacy of β-Lactam Antibiotics against Methicillin-Resistant Staphylococcus aureus by Targeting β-Lactamase: In Silico and In Vitro Studies. Antibiotics 2023, 12, 1622. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.G. Inhibitory actions of ellagic acid on growth and arylamine N-acetyltransferase activity in strains of Helicobacter pylori from peptic ulcer patients. Microbios 1998, 93, 115–127. [Google Scholar] [PubMed]
- Cho, J.-Y.; Moon, J.-H.; Seong, K.-Y.; Park, K.-H. Antimicrobial Activity of 4-Hydroxybenzoic Acid and trans 4-Hydroxycinnamic Acid Isolated and Identified from Rice Hull. Biosci. Biotechnol. Biochem. 1998, 62, 2273–2276. [Google Scholar] [CrossRef] [PubMed]
- Halpani, C.G.; Mishra, S. Design, synthesis, characterization of ferulic acid and p-coumaric acid amide derivatives as an antibacterial/antioxidant agent. Pharm. Sci. Adv. 2024, 2, 100023. [Google Scholar] [CrossRef]
- Kuban-Jankowska, A.; Sahu, K.K.; Gorska, M.; Tuszynski, J.A.; Wozniak, M. Chicoric acid binds to two sites and decreases the activity of the YopH bacterial virulence factor. Oncotarget 2016, 7, 2229. [Google Scholar] [CrossRef]
- Chung, J.G.; Hsia, T.C.; Kuo, H.M.; Li, Y.C.; Lee, Y.M.; Lin, S.S.; Hung, C.F. Inhibitory actions of luteolin on the growth and arylamine N-acetyltransferase activity in strains of Helicobacter pylori from ulcer patients. Toxicol. In Vitro 2001, 15, 191–198. [Google Scholar] [CrossRef]
- Aldawsari, M.F.; Ahmed, M.M.; Fatima, F.; Anwer, M.K.; Katakam, P.; Khan, A. Development and Characterization of Calcium-Alginate Beads of Apigenin: In Vitro Antitumor, Antibacterial, and Antioxidant Activities. Mar. Drugs 2021, 19, 467. [Google Scholar] [CrossRef]
- Wang, G.; Gao, Y.; Wang, H.; Niu, X.; Wang, J. Baicalin weakens Staphylococcus aureus pathogenicity by targeting sortase B. Front. Cell. Infect. Microbiol. 2018, 8, 418. [Google Scholar] [CrossRef] [PubMed]
- Veiko, A.G.; Olchowik-Grabarek, E.; Sekowski, S.; Roszkowska, A.; Lapshina, E.A.; Dobrzynska, I.; Zamaraeva, M.; Zavodnik, I.B. Antimicrobial Activity of Quercetin, Naringenin and Catechin: Flavonoids Inhibit Staphylococcus aureus-Induced Hemolysis and Modify Membranes of Bacteria and Erythrocytes. Molecules 2023, 28, 1252. [Google Scholar] [CrossRef] [PubMed]
- Nag, D.; Dastidar, D.G.; Chakrabarti, G. Natural flavonoid morin showed anti-bacterial activity against Vibrio cholera after binding with cell division protein FtsA near ATP binding site. Biochim. Et Biophys. Acta (BBA) Gen. Subj. 2021, 1865, 129931. [Google Scholar] [CrossRef] [PubMed]
- Arita-Morioka, K.-i.; Yamanaka, K.; Mizunoe, Y.; Tanaka, Y.; Ogura, T.; Sugimoto, S. Inhibitory effects of Myricetin derivatives on curli-dependent biofilm formation in Escherichia coli. Sci. Rep. 2018, 8, 8452. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Lin, Y.; Wang, C.; Niu, B.; Xu, Y.; Zhao, G.; Zhao, J. Chemical Profile, Antimicrobial and Antioxidant Activity Assessment of the Crude Extract and Its Main Flavonoids from Tartary Buckwheat Sprouts. Molecules 2022, 27, 374. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-H.; Huang, C.-C.; Chen, C.-C.; Yang, K.-J.; Huang, C.-Y. Inhibition of Staphylococcus aureus PriA helicase by flavonol kaempferol. Protein J. 2015, 34, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Cushnie, T.P.T.; Lamb, A.J. Assessment of the antibacterial activity of galangin against 4-quinolone resistant strains of Staphylococcus aureus. Phytomedicine 2006, 13, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Wen, H.; Zhou, H.; Zhang, D.; Lan, D.; Liu, S.; Li, C.; Dai, X.; Song, T.; Wang, X.; et al. Naringenin: A flavanone with anti-inflammatory and anti-infective properties. Biomed. Pharmacother. 2023, 164, 114990. [Google Scholar] [CrossRef] [PubMed]
- Jaradat, N.; Shawarb, N.; Hussein, F.; Al-Masri, M.; Warad, I.; Khasati, A.; Shehadeh, M.; Qneibi, M.; Hussein, A.M.A.; Makhamreh, S. Antibacterial and antioxidant screening of semi-synthetic naringin based hydrazone and oxime derivatives. Jundishapur J. Microbiol. 2018, 11, e65496. [Google Scholar] [CrossRef]
- Wang, L.; Li, Q.; Li, J.; Jing, S.; Jin, Y.; Yang, L.; Yu, H.; Wang, D.; Wang, T.; Wang, L. Eriodictyol as a potential candidate inhibitor of sortase A protects mice from methicillin-resistant Staphylococcus aureus-induced pneumonia. Front. Microbiol. 2021, 12, 635710. [Google Scholar] [CrossRef]
- Nuryana, I.; Ratnakomala, S.; Fahrurrozi, A.B.J.; Andriani, A.; Putra, F.J.N.; Rezamela, E.; Wulansari, R.; Prawira-Atmaja, M.I.; Lisdiyanti, P. Catechin contents, antioxidant and antibacterial activities of different types of Indonesian tea (Camellia sinensis). Ann. Bogor. 2020, 24, 107. [Google Scholar] [CrossRef]
- Kajiwara, R.; Seto, A.; Kofujita, H.; Shiba, Y.; Oishi, Y.; Shibasaki, Y. Enhanced antimicrobial activities of polymerized arbutin and its derivatives prepared by oxidative polymerization of arbutin. React. Funct. Polym. 2019, 138, 39–45. [Google Scholar] [CrossRef]
- Lee, J.-H.; Regmi, S.C.; Kim, J.-A.; Cho, M.H.; Yun, H.; Lee, C.-S.; Lee, J. Apple flavonoid phloretin inhibits Escherichia coli O157: H7 biofilm formation and ameliorates colon inflammation in rats. Infect. Immun. 2011, 79, 4819–4827. [Google Scholar] [CrossRef] [PubMed]
- Baldisserotto, A.; Malisardi, G.; Scalambra, E.; Andreotti, E.; Romagnoli, C.; Vicentini, C.B.; Manfredini, S.; Vertuani, S. Synthesis, Antioxidant and Antimicrobial Activity of a New Phloridzin Derivative for Dermo-Cosmetic Applications. Molecules 2012, 17, 13275–13289. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Hoyos, M.; Lebrón-Aguilar, R.; Quintanilla-López, J.E.; Cueva, C.; Hevia, D.; Quesada, S.; Azofeifa, G.; Moreno-Arribas, M.V.; Monagas, M.; Bartolomé, B. Proanthocyanidin Characterization and Bioactivity of Extracts from Different Parts of Uncaria tomentosa L. (Cat’s Claw). Antioxidants 2017, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Yu, H.; Wang, X.; Zhang, C.; Wang, H.; Kong, X.; Qu, Y.; Luan, Y.; Meng, Y.; Guan, J. Cyanidin chloride protects mice from methicillin-resistant Staphylococcus aureus-induced pneumonia by targeting Sortase A. Virulence 2022, 13, 1434–1445. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.C.; Nunes, A.R.; Meirinho, S.; Ayuso-Calles, M.; Roca-Couso, R.; Rivas, R.; Falcão, A.; Alves, G.; Silva, L.R.; Flores-Félix, J.D. Exploring the Antioxidant, Antidiabetic, and Antimicrobial Capacity of Phenolics from Blueberries and Sweet Cherries. Appl. Sci. 2023, 13, 6348. [Google Scholar] [CrossRef]
- Promyos, N.; Temviriyanukul, P.; Suttisansanee, U. Investigation of anthocyanidins and anthocyanins for targeting α-glucosidase in diabetes mellitus. Prev. Nutr. Food Sci. 2020, 25, 263. [Google Scholar] [CrossRef] [PubMed]
- Lamola, S.M.; Dzoyem, J.P.; Botha, F.; Van Wyk, C. Anti-bacterial, free radical scavenging activity and cytotoxicity of acetone extracts of Grewia flava. Afr. Health Sci. 2017, 17, 790–796. [Google Scholar] [CrossRef]
- Chiang, C.-M.; Wang, D.-S.; Chang, T.-S. Improving Free Radical Scavenging Activity of Soy Isoflavone Glycosides Daidzin and Genistin by 3′-Hydroxylation Using Recombinant Escherichia coli. Molecules 2016, 21, 1723. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, D.-H.; Zhang, Y.-T.; Chen, X.-M.; Li, L.-L.; Cai, S.-Q. Biotransformation on the flavonolignan constituents of Silybi Fructus by an intestinal bacterial strain Eubacterium limosum ZL-II. Fitoterapia 2014, 92, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Rendón, J.P.; Cañas, A.I.; Correa, E.; Bedoya-Betancur, V.; Osorio, M.; Castro, C.; Naranjo, T.W. Evaluation of the Effects of Genistein In Vitro as a Chemopreventive Agent for Colorectal Cancer—Strategy to Improve Its Efficiency When Administered Orally. Molecules 2022, 27, 7042. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, P.; Rahimi Esboei, B.; Pourhajibagher, M.; Fakhar, M.; Shahmoradi, Z.; Hejazi, S.H.; Hassannia, H.; Nasrollahi Omran, A.; Hasanpour, H. Anti-leishmanial effects of resveratrol and resveratrol nanoemulsion on Leishmania major. BMC Microbiol. 2022, 22, 56. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Tai, W.; Wang, N.; Li, X.; Jiang, S.; Debnath, A.K.; Du, L.; Chen, S. Identification of Novel Natural Products as Effective and Broad-Spectrum Anti-Zika Virus Inhibitors. Viruses 2019, 11, 1019. [Google Scholar] [CrossRef] [PubMed]
- Marinaş, I.C.; Chifiriuc, C.; Oprea, E.; Lazăr, V. Antimicrobial and antioxidant activities of alcoholic extracts obtained from vegetative organs of A. retroflexus. Roum. Arch. Microbiol. Immunol. 2014, 73, 35–42. [Google Scholar] [PubMed]
- Othman, L.; Sleiman, A.; Abdel-Massih, R.M. Antimicrobial activity of polyphenols and alkaloids in middle eastern plants. Front. Microbiol. 2019, 10, 911. [Google Scholar] [CrossRef] [PubMed]
- Makarewicz, M.; Drożdż, I.; Tarko, T.; Duda-Chodak, A. The Interactions between Polyphenols and Microorganisms, Especially Gut Microbiota. Antioxidants 2021, 10, 188. [Google Scholar] [CrossRef] [PubMed]
- Venu, L.N.; Austin, A. Antiviral efficacy of medicinal plants against respiratory viruses: Respiratory Syncytial Virus (RSV) and Coronavirus (CoV)/COVID 19. J Pharmacol 2020, 9, 281–290. [Google Scholar] [CrossRef]
- Park, S.W.; Kwon, M.J.; Yoo, J.Y.; Choi, H.-J.; Ahn, Y.-J. Antiviral activity and possible mode of action of ellagic acid identified in Lagerstroemia speciosa leaves toward human rhinoviruses. BMC Complement. Altern. Med. 2014, 14, 171. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Nong, Y.; Shi, Y.; Liu, M.; Yan, L.; Shang, J.; Huang, F.; Lin, Y.; Tang, H. Luteolin inhibits hepatitis B virus replication through extracellular signal-regulated kinase-mediated down-regulation of hepatocyte nuclear factor 4α expression. Mol. Pharm. 2016, 13, 568–577. [Google Scholar] [CrossRef]
- Dinda, B.; Dinda, M.; Dinda, S.; Ghosh, P.S.; Das, S.K. Anti-SARS-CoV-2, antioxidant and immunomodulatory potential of dietary flavonol quercetin: Focus on molecular targets and clinical efficacy. Eur. J. Med. Chem. Rep. 2024, 10, 100125. [Google Scholar] [CrossRef]
- Sandhu, S.K.; Kumar, S.; Raut, J.; Singh, M.; Kaur, S.; Sharma, G.; Roldan, T.L.; Trehan, S.; Holloway, J.; Wahler, G. Systematic development and characterization of novel, high drug-loaded, photostable, curcumin solid lipid nanoparticle hydrogel for wound healing. Antioxidants 2021, 10, 725. [Google Scholar] [CrossRef] [PubMed]
- Mucha, P.; Skoczyńska, A.; Małecka, M.; Hikisz, P.; Budzisz, E. Overview of the Antioxidant and Anti-Inflammatory Activities of Selected Plant Compounds and Their Metal Ions Complexes. Molecules 2021, 26, 4886. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Y.; Alam, W.; Ullah, H.; Dacrema, M.; Daglia, M.; Khan, H.; Arciola, C.R. Antimicrobial potential of curcumin: Therapeutic potential and challenges to clinical applications. Antibiotics 2022, 11, 322. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M. Antibacterial, antiviral, and antifungal properties of wines and winery byproducts in relation to their flavonoid content. J. Agric. Food Chem. 2014, 62, 6025–6042. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M. Overview of antibacterial, antitoxin, antiviral, and antifungal activities of tea flavonoids and teas. Mol. Nutr. Food Res. 2007, 51, 116–134. [Google Scholar] [CrossRef] [PubMed]
- Lima, V.N.; Oliveira-Tintino, C.D.M.; Santos, E.S.; Morais, L.P.; Tintino, S.R.; Freitas, T.S.; Geraldo, Y.S.; Pereira, R.L.S.; Cruz, R.P.; Menezes, I.R.A.; et al. Antimicrobial and enhancement of the antibiotic activity by phenolic compounds: Gallic acid, caffeic acid and pyrogallol. Microb. Pathog. 2016, 99, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Tareq, A.M.; Das, R.; Emran, T.B.; Nainu, F.; Chakraborty, A.J.; Ahmad, I.; Tallei, T.E.; Idris, A.M.; Simal-Gandara, J. Polyphenols: A first evidence in the synergism and bioactivities. Food Rev. Int. 2023, 39, 4419–4441. [Google Scholar] [CrossRef]
- Abdallah, E.M.; Alhatlani, B.Y.; de Paula Menezes, R.; Martins, C.H.G. Back to Nature: Medicinal plants as promising sources for antibacterial drugs in the post-antibiotic era. Plants 2023, 12, 3077. [Google Scholar] [CrossRef]
- Hemmami, H.; Seghir, B.B.; Zeghoud, S.; Ben Amor, I.; Kouadri, I.; Rebiai, A.; Zaater, A.; Messaoudi, M.; Benchikha, N.; Sawicka, B. Desert Endemic Plants in Algeria: A Review on Traditional Uses, Phytochemistry, Polyphenolic Compounds and Pharmacological Activities. Molecules 2023, 28, 1834. [Google Scholar] [CrossRef]
- Chowdhury, M.A.H.; Ashrafudoulla, M.; Mevo, S.I.U.; Mizan, M.F.R.; Park, S.H.; Ha, S.D. Current and future interventions for improving poultry health and poultry food safety and security: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2023, 22, 1555–1596. [Google Scholar] [CrossRef] [PubMed]
- Elshafie, H.S.; Camele, I.; Mohamed, A.A. A Comprehensive review on the biological, agricultural and pharmaceutical properties of secondary metabolites based-plant origin. Int. J. Mol. Sci. 2023, 24, 3266. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.; Zhang, H.; Li, L.; Liu, Z. Effects of green tea extract epigallocatechin-3-gallate (EGCG) on oral disease-associated microbes: A review. J. Oral Microbiol. 2022, 14, 2131117. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.; Kim, N.; Shin, Y.; Kim, S.-Y.; Kim, Y.-J. Activity of catechins and their applications. Biomed. Dermatol. 2020, 4, 8. [Google Scholar] [CrossRef]
- Fan, F.-Y.; Sang, L.-X.; Jiang, M. Catechins and Their Therapeutic Benefits to Inflammatory Bowel Disease. Molecules 2017, 22, 484. [Google Scholar] [CrossRef] [PubMed]
- Jaisinghani, R. Antibacterial properties of quercetin. Microbiol. Res. 2017, 8, 6877. [Google Scholar] [CrossRef]
- Nguyen, T.L.A.; Bhattacharya, D. Antimicrobial activity of quercetin: An approach to its mechanistic principle. Molecules 2022, 27, 2494. [Google Scholar] [CrossRef] [PubMed]
- Tempesti, T.C.; Alvarez, M.G.; de Araújo, M.F.; Catunda Júnior, F.E.A.; de Carvalho, M.G.; Durantini, E.N. Antifungal activity of a novel quercetin derivative bearing a trifluoromethyl group on Candida albicans. Med. Chem. Res. 2012, 21, 2217–2222. [Google Scholar] [CrossRef]
- Badshah, S.L.; Faisal, S.; Muhammad, A.; Poulson, B.G.; Emwas, A.H.; Jaremko, M. Antiviral activities of flavonoids. Biomed. Pharmacother. 2021, 140, 111596. [Google Scholar] [CrossRef]
- Roshani, M.; Jafari, A.; Loghman, A.; Sheida, A.H.; Taghavi, T.; Tamehri Zadeh, S.S.; Hamblin, M.R.; Homayounfal, M.; Mirzaei, H. Applications of resveratrol in the treatment of gastrointestinal cancer. Biomed. Pharmacother. 2022, 153, 113274. [Google Scholar] [CrossRef]
- Mahady, G.B.; Pendland, S.L.; Chadwick, L.R. Resveratrol and Red Wine Extracts Inhibit The Growth of Caga+Strains of Helicobacter Pylori in vitro. Off. J. Am. Coll. Gastroenterol. ACG 2003, 98, 1440–1441. [Google Scholar] [CrossRef] [PubMed]
- Mattio, L.M.; Catinella, G.; Dallavalle, S.; Pinto, A. Stilbenoids: A Natural Arsenal against Bacterial Pathogens. Antibiotics 2020, 9, 336. [Google Scholar] [CrossRef] [PubMed]
- Ekrikaya, S.; Yilmaz, E.; Celik, C.; Demirbuga, S.; Ildiz, N.; Demirbas, A.; Ocsoy, I. Investigation of ellagic acid rich-berry extracts directed silver nanoparticles synthesis and their antimicrobial properties with potential mechanisms towards Enterococcus faecalis and Candida albicans. J. Biotechnol. 2021, 341, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Vattem, D.; Shetty, K. Biological functionality of ellagic acid: A review. J. Food Biochem. 2005, 29, 234–266. [Google Scholar] [CrossRef]
- Bell, C.; Hawthorne, S. Ellagic acid, pomegranate and prostate cancer—A mini review. J. Pharm. Pharmacol. 2008, 60, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Glazer, I.; Masaphy, S.; Marciano, P.; Bar-Ilan, I.; Holland, D.; Kerem, Z.; Amir, R. Partial identification of antifungal compounds from Punica granatum peel extracts. J. Agric. Food Chem. 2012, 60, 4841–4848. [Google Scholar] [CrossRef] [PubMed]
- An, J.-Y.; Wang, L.-T.; Lv, M.-J.; Wang, J.-D.; Cai, Z.-H.; Wang, Y.-Q.; Zhang, S.; Yang, Q.; Fu, Y.-J. An efficiency strategy for extraction and recovery of ellagic acid from waste chestnut shell and its biological activity evaluation. Microchem. J. 2021, 160, 105616. [Google Scholar] [CrossRef]
- Ciuca, M.D.; Racovita, R.C. Curcumin: Overview of Extraction Methods, Health Benefits, and Encapsulation and Delivery Using Microemulsions and Nanoemulsions. Int. J. Mol. Sci. 2023, 24, 8874. [Google Scholar] [CrossRef]
- Candra, A.; Prasetyo, B.E.; Darge, H.F. Honey utilization in soursop leaves (Annona muricata) kombucha: Physicochemical, cytotoxicity, and antimicrobial activity. Biocatal. Agric. Biotechnol. 2023, 52, 102815. [Google Scholar] [CrossRef]
- Lüer, S.; Troller, R.; Aebi, C. Antibacterial and antiinflammatory kinetics of curcumin as a potential antimucositis agent in cancer patients. Nutr. Cancer 2012, 64, 975–981. [Google Scholar] [CrossRef]
- Zorofchian Moghadamtousi, S.; Abdul Kadir, H.; Hassandarvish, P.; Tajik, H.; Abubakar, S.; Zandi, K. A review on antibacterial, antiviral, and antifungal activity of curcumin. BioMed Res. Int. 2014, 2014, 186864. [Google Scholar] [CrossRef] [PubMed]
- Quichaba, M.B.; Moreira, T.F.M.; de Oliveira, A.; de Carvalho, A.S.; de Menezes, J.L.; Gonçalves, O.H.; de Abreu Filho, B.A.; Leimann, F.V. Biopreservatives against foodborne bacteria: Combined effect of nisin and nanoncapsulated curcumin and co-encapsulation of nisin and curcumin. J. Food Sci. Technol. 2023, 60, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Gholipour, F.; Amini, M.; Baradaran, B.; Mokhtarzadeh, A.; Eskandani, M. Anticancer properties of curcumin-treated Lactobacillus plantarum against the HT-29 colorectal adenocarcinoma cells. Sci. Rep. 2023, 13, 2860. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Español, A.; Bravo, E.; Ribeiro-Vidal, H.; Virto, L.; Herrera, D.; Alonso, B.; Sanz, M. The Antimicrobial Activity of Curcumin and Xanthohumol on Bacterial Biofilms Developed over Dental Implant Surfaces. Int. J. Mol. Sci. 2023, 24, 2335. [Google Scholar] [CrossRef] [PubMed]
- Duda-Madej, A.; Stecko, J.; Sobieraj, J.; Szymańska, N.; Kozłowska, J. Naringenin and its derivatives—Health-promoting phytobiotic against resistant bacteria and fungi in humans. Antibiotics 2022, 11, 1628. [Google Scholar] [CrossRef] [PubMed]
- Denaro, M.; Smeriglio, A.; Barreca, D.; De Francesco, C.; Occhiuto, C.; Milano, G.; Trombetta, D. Antiviral activity of plants and their isolated bioactive compounds: An update. Phytother. Res. 2020, 34, 742–768. [Google Scholar] [CrossRef]
- Usman Amin, M.; Khurram, M.; Khan, T.A.; Faidah, H.S.; Ullah Shah, Z.; Ur Rahman, S.; Haseeb, A.; Ilyas, M.; Ullah, N.; Umar Khayam, S.M. Effects of luteolin and quercetin in combination with some conventional antibiotics against methicillin-resistant Staphylococcus aureus. Int. J. Mol. Sci. 2016, 17, 1947. [Google Scholar] [CrossRef]
- Qian, W.; Liu, M.; Fu, Y.; Zhang, J.; Liu, W.; Li, J.; Li, X.; Li, Y.; Wang, T. Antimicrobial mechanism of luteolin against Staphylococcus aureus and Listeria monocytogenes and its antibiofilm properties. Microb. Pathog. 2020, 142, 104056. [Google Scholar] [CrossRef]
- Bangar, S.P.; Kajla, P.; Chaudhary, V.; Sharma, N.; Ozogul, F. Luteolin: A flavone with myriads of bioactivities and food applications. Food Biosci. 2023, 52, 102366. [Google Scholar] [CrossRef]
- Houillé, B.; Papon, N.; Boudesocque, L.; Bourdeaud, E.; Besseau, S.; Courdavault, V.; Enguehard-Gueiffier, C.; Delanoue, G.; Guérin, L.; Bouchara, J.-P. Antifungal activity of resveratrol derivatives against Candida species. J. Nat. Prod. 2014, 77, 1658–1662. [Google Scholar] [CrossRef]
- Weber, K.; Schulz, B.; Ruhnke, M. Resveratrol and its antifungal activity against Candida species. Mycoses 2011, 54, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.-T.; Hsu, W.-C.; Lin, C.-C. Antiviral natural products and herbal medicines. J. Tradit. Complement. Med. 2014, 4, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Abedini, E.; Khodadadi, E.; Zeinalzadeh, E.; Moaddab, S.R.; Asgharzadeh, M.; Mehramouz, B.; Dao, S.; Samadi Kafil, H. A comprehensive study on the antimicrobial properties of resveratrol as an alternative therapy. Evid. Based Complement. Altern. Med. 2021, 2021, 8866311. [Google Scholar] [CrossRef] [PubMed]
- Biancatelli, R.; Berrill, M.; Catravas, J.; Marik, P. Quercetin and vitamin C: An experimental, synergistic therapy for the prevention and treatment of SARSCoV-2 related disease (COVID-19). Front. Immunol. 2020, 11, 1451. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-H.; Han, J.-H.; Kim, S.-U. Isoflavone daidzein: Chemistry and bacterial metabolism. J. Appl. Biol. Chem. 2008, 51, 253–261. [Google Scholar] [CrossRef]
- De Oliveira Santos, G.C.; Vasconcelos, C.C.; Lopes, A.J.; de Sousa Cartágenes, M.d.S.; Filho, A.K.; do Nascimento, F.R.; Ramos, R.M.; Pires, E.R.; de Andrade, M.S.; Rocha, F.M.; et al. Candida infections and therapeutic strategies: Mechanisms of action for traditional and alternative agents. Front. Microbiol. 2018, 9, 362855. [Google Scholar] [CrossRef] [PubMed]
- Al Aboody, M.S.; Mickymaray, S. Anti-fungal efficacy and mechanisms of flavonoids. Antibiotics 2020, 9, 45. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, N. Polyphenolic compounds-a promising leads for antiviral therapy. Pharmacophore 2022, 13, 36–47. [Google Scholar]
- Zakaryan, H.; Arabyan, E.; Oo, A.; Zandi, K. Flavonoids: Promising natural compounds against viral infections. Arch. Virol. 2017, 162, 2539–2551. [Google Scholar] [CrossRef]
- Gan, X.; Zhang, W.; Lan, S.; Hu, D. Novel Cyclized Derivatives of Ferulic Acid as Potential Antiviral Agents through Activation of Photosynthesis. J. Agric. Food Chem. 2023, 71, 1369–1380. [Google Scholar] [CrossRef]
- Özçelik, B.; Kartal, M.; Orhan, I. Cytotoxicity, antiviral and antimicrobial activities of alkaloids, flavonoids, and phenolic acids. Pharm. Biol. 2011, 49, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Shi, G.; Shao, J.; Wu, D.; Yan, Y.; Zhang, M.; Cui, Y.; Wang, C. In vitro antifungal activity of baicalin against Candida albicans biofilms via apoptotic induction. Microb. Pathog. 2015, 87, 21–29. [Google Scholar] [CrossRef]
- Johari, J.; Kianmehr, A.; Mustafa, M.R.; Abubakar, S.; Zandi, K. Antiviral activity of baicalein and quercetin against the Japanese encephalitis virus. Int. J. Mol. Sci. 2012, 13, 16785–16795. [Google Scholar] [CrossRef]
- Moghaddam, E.; Teoh, B.-T.; Sam, S.-S.; Lani, R.; Hassandarvish, P.; Chik, Z.; Yueh, A.; Abubakar, S.; Zandi, K. Baicalin, a metabolite of baicalein with antiviral activity against dengue virus. Sci. Rep. 2014, 4, 5452. [Google Scholar] [CrossRef] [PubMed]
- Gullon, B.; Lú-Chau, T.A.; Moreira, M.T.; Lema, J.M.; Eibes, G. Rutin: A review on extraction, identification and purification methods, biological activities and approaches to enhance its bioavailability. Trends Food Sci. Technol. 2017, 67, 220–235. [Google Scholar] [CrossRef]
- Orhan, D.D.; Özçelik, B.; Özgen, S.; Ergun, F. Antibacterial, antifungal, and antiviral activities of some flavonoids. Microbiol. Res. 2010, 165, 496–504. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, Q.; Yi, D.; Wu, T.; Chen, H.; Guo, S.; Li, S.; Ji, C.; Wang, L.; Zhao, D. Quantitative proteomic analysis reveals antiviral and anti-inflammatory effects of puerarin in piglets infected with porcine epidemic diarrhea virus. Front. Immunol. 2020, 11, 169. [Google Scholar] [CrossRef] [PubMed]
- Ojo, O.A.; Ojo, A.B.; Nwonuma, C.O.; Awakan, O.J.; Maimako, R.F.; Afolabi, B.L.; Taiwo, O.A. Puerarin: A Review on the Pharmacological Activity, Chemical Properties and Pharmacokinetics of Main Isoflavonoid. Nat. Prod. J. 2022, 12, 17–26. [Google Scholar] [CrossRef]
- Demir, T.; Akpınar, Ö.; Kara, H.; Güngör, H. Cherry stem phenolic compounds: Optimization of extraction conditions and in vitro evaluations of antioxidant, antimicrobial, antidiabetic, anti-inflammatory, and cytotoxic activities. J. Food Process. Preserv. 2020, 44, e14804. [Google Scholar] [CrossRef]
- Bourais, I.; Elmarrkechy, S.; Taha, D.; Mourabit, Y.; Bouyahya, A.; El Yadini, M.; Machich, O.; El Hajjaji, S.; El Boury, H.; Dakka, N. A review on medicinal uses, nutritional value, and antimicrobial, antioxidant, anti-inflammatory, antidiabetic, and anticancer potential related to bioactive compounds of J. regia. Food Rev. Int. 2023, 39, 6199–6249. [Google Scholar] [CrossRef]
- Azeem, M.; Hanif, M.; Mahmood, K.; Ameer, N.; Chughtai, F.R.S.; Abid, U. An insight into anticancer, antioxidant, antimicrobial, antidiabetic and anti-inflammatory effects of quercetin: A review. Polym. Bull. 2023, 80, 241–262. [Google Scholar] [CrossRef] [PubMed]
- Di Petrillo, A.; Orrù, G.; Fais, A.; Fantini, M.C. Quercetin and its derivates as antiviral potentials: A comprehensive review. Phytother. Res. 2022, 36, 266–278. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Sharifi-Rad, J. Resveratrol: A double-edged sword in health benefits. Biomedicines 2018, 6, 91. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-X.; Li, C.-X.; Kakar, M.U.; Khan, M.S.; Wu, P.-F.; Amir, R.M.; Dai, D.-F.; Naveed, M.; Li, Q.-Y.; Saeed, M.; et al. Resveratrol (RV): A pharmacological review and call for further research. Biomed. Pharmacother. 2021, 143, 112164. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Z.; Thu, H.E.; Amjad, M.W.; Hussain, F.; Ahmed, T.A.; Khan, S. Exploring recent developments to improve antioxidant, anti-inflammatory and antimicrobial efficacy of curcumin: A review of new trends and future perspectives. Mater. Sci. Eng. C 2017, 77, 1316–1326. [Google Scholar] [CrossRef] [PubMed]
- Bhawana; Basniwal, R.K.; Buttar, H.S.; Jain, V.; Jain, N. Curcumin nanoparticles: Preparation, characterization, and antimicrobial study. J. Agric. Food Chem. 2011, 59, 2056–2061. [Google Scholar] [CrossRef] [PubMed]
- Polat Kose, L.; Gulcin, İ. Evaluation of the antioxidant and antiradical properties of some phyto and mammalian lignans. Molecules 2021, 26, 7099. [Google Scholar] [CrossRef]
- Kyselka, J.; Rabiej, D.; Dragoun, M.; Kreps, F.; Burčová, Z.; Němečková, I.; Smolová, J.; Bjelková, M.; Szydłowska-Czerniak, A.; Schmidt, Š. Antioxidant and antimicrobial activity of linseed lignans and phenolic acids. Eur. Food Res. Technol. 2017, 243, 1633–1644. [Google Scholar] [CrossRef]
- Vázquez, L.; Flórez, A.B.; Guadamuro, L.; Mayo, B. Effect of soy isoflavones on growth of representative bacterial species from the human gut. Nutrients 2017, 9, 727. [Google Scholar] [CrossRef]
- Rajput, S.A.; Wang, X.-q.; Yan, H.-C. Morin hydrate: A comprehensive review on novel natural dietary bioactive compound with versatile biological and pharmacological potential. Biomed. Pharmacother. 2021, 138, 111511. [Google Scholar] [CrossRef]
- Ferreira, P.S.; Victorelli, F.D.; Fonseca-Santos, B.; Chorilli, M. A review of analytical methods for p-coumaric acid in plant-based products, beverages, and biological matrices. Crit. Rev. Anal. Chem. 2019, 49, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Boz, H. p-Coumaric acid in cereals: Presence, antioxidant and antimicrobial effects. Int. J. Food Sci. Technol. 2015, 50, 2323–2328. [Google Scholar] [CrossRef]
- Murtaza, G.; Karim, S.; Akram, M.R.; Khan, S.A.; Azhar, S.; Mumtaz, A.; Bin Asad, M.H.H. Caffeic acid phenethyl ester and therapeutic potentials. BioMed Res. Int. 2014, 2014, 145342. [Google Scholar] [CrossRef] [PubMed]
- Magnani, C.; Isaac, V.L.B.; Correa, M.A.; Salgado, H.R.N. Caffeic acid: A review of its potential use in medications and cosmetics. Anal. Methods 2014, 6, 3203–3210. [Google Scholar] [CrossRef]
- Ali, F.; Rahul; Naz, F.; Jyoti, S.; Siddique, Y.H. Health functionality of apigenin: A review. Int. J. Food Prop. 2017, 20, 1197–1238. [Google Scholar] [CrossRef]
- Zahrani, N.A.A.; El-Shishtawy, R.M.; Asiri, A.M. Recent developments of gallic acid derivatives and their hybrids in medicinal chemistry: A review. Eur. J. Med. Chem. 2020, 204, 112609. [Google Scholar] [CrossRef]
- Badhani, B.; Sharma, N.; Kakkar, R. Gallic acid: A versatile antioxidant with promising therapeutic and industrial applications. Rsc Adv. 2015, 5, 27540–27557. [Google Scholar] [CrossRef]
- Adhami, V.M.; Syed, D.N.; Khan, N.; Mukhtar, H. Dietary flavonoid fisetin: A novel dual inhibitor of PI3K/Akt and mTOR for prostate cancer management. Biochem. Pharmacol. 2012, 84, 1277–1281. [Google Scholar] [CrossRef]
- Imran, M.; Saeed, F.; Gilani, S.A.; Shariati, M.A.; Imran, A.; Afzaal, M.; Atif, M.; Tufail, T.; Anjum, F.M. Fisetin: An anticancer perspective. Food Sci. Nutr. 2021, 9, 3–16. [Google Scholar] [CrossRef]
- Piekarska-Radzik, L.; Klewicka, E. Mutual influence of polyphenols and Lactobacillus spp. bacteria in food: A review. Eur. Food Res. Technol. 2021, 247, 9–24. [Google Scholar] [CrossRef]
- Mai-Prochnow, A.; Clauson, M.; Hong, J.; Murphy, A.B. Gram positive and Gram negative bacteria differ in their sensitivity to cold plasma. Sci. Rep. 2016, 6, 38610. [Google Scholar] [CrossRef] [PubMed]
- Añón, A.; López, J.F.; Hernando, D.; Orriols, I.; Revilla, E.; Losada, M.M. Effect of five enological practices and of the general phenolic composition on fermentation-related aroma compounds in Mencia young red wines. Food Chem. 2014, 148, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Baranowska, M.; Bartoszek, A. Antioxidant and antimicrobial properties of bioactive phytochemicals from cranberry. Adv. Hyg. Exp. Med. 2016, 70, 1460–1468. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Cheng, N.; Zhou, J.; Wang, B.; Deng, J.; Cao, W. Antioxidant activities and phenolic compounds of date plum persimmon (Diospyros lotus L.) fruits. J. Food Sci. Technol. 2014, 51, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Vodnar, D.C. Inhibition of Listeria monocytogenes ATCC 19115 on ham steak by tea bioactive compounds incorporated into chitosan-coated plastic films. Chem. Cent. J. 2012, 6, 74. [Google Scholar] [CrossRef] [PubMed]
- Galvão, S.d.S.; Monteiro, A.d.S.; Siqueira, E.P.; Bomfim, M.R.Q.; Dias-Souza, M.V.; Ferreira, G.F.; Denadai, A.M.L.; Santos, Á.R.; Lúcia dos Santos, V.; Souza-Fagundes, E.M.d. Annona glabra flavonoids act as antimicrobials by binding to Pseudomonas aeruginosa cell walls. Front. Microbiol. 2016, 7, 2053. [Google Scholar] [CrossRef] [PubMed]
- Bouarab-Chibane, L.; Forquet, V.; Lantéri, P.; Clément, Y.; Léonard-Akkari, L.; Oulahal, N.; Degraeve, P.; Bordes, C. Antibacterial properties of polyphenols: Characterization and QSAR (Quantitative structure–activity relationship) models. Front. Microbiol. 2019, 10, 829. [Google Scholar] [CrossRef] [PubMed]
- Zammuto, V.; Rizzo, M.G.; De Pasquale, C.; Ferlazzo, G.; Caccamo, M.T.; Magazù, S.; Guglielmino, S.P.P.; Gugliandolo, C. Lichenysin-like Polypeptide Production by Bacillus licheniformis B3-15 and Its Antiadhesive and Antibiofilm Properties. Microorganisms 2023, 11, 1842. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.-N.; Wang, F.; Yuan, Y.-T.; Liu, J.; Liu, Y.-Z.; Yi, X. Antibacterial activity and mode of action of dihydromyricetin from Ampelopsis grossedentata leaves against food-borne bacteria. Molecules 2019, 24, 2831. [Google Scholar] [CrossRef]
- Pinho, E.; Magalhães, L.; Henriques, M.; Oliveira, R. Antimicrobial activity assessment of textiles: Standard methods comparison. Ann. Microbiol. 2011, 61, 493–498. [Google Scholar] [CrossRef]
- Tenover, F.C. Antimicrobial Susceptibility Testing. In Encyclopedia of Microbiology, 4th ed.; Schmidt, T.M., Ed.; Academic Press: Oxford, UK, 2019; pp. 166–175. [Google Scholar] [CrossRef]
- Klančnik, A.; Piskernik, S.; Jeršek, B.; Možina, S.S. Evaluation of diffusion and dilution methods to determine the antibacterial activity of plant extracts. J. Microbiol. Methods 2010, 81, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Valgas, C.; Souza, S.M.d.; Smânia, E.F.; Smânia Jr, A. Screening methods to determine antibacterial activity of natural products. Braz. J. Microbiol. 2007, 38, 369–380. [Google Scholar] [CrossRef]
- Dzah, C.S.; Duan, Y.; Zhang, H.; Wen, C.; Zhang, J.; Chen, G.; Ma, H. The effects of ultrasound assisted extraction on yield, antioxidant, anticancer and antimicrobial activity of polyphenol extracts: A review. Food Biosci. 2020, 35, 100547. [Google Scholar] [CrossRef]
- El Moussaoui, A.; Jawhari, F.Z.; Almehdi, A.M.; Elmsellem, H.; Benbrahim, K.F.; Bousta, D.; Bari, A. Antibacterial, antifungal and antioxidant activity of total polyphenols of Withania frutescens L. Bioorg. Chem. 2019, 93, 103337. [Google Scholar] [CrossRef] [PubMed]
- Aulakh, M.K.; Kaur, N.; Saggoo, M. Bioactive phytoconstituents of pteridophytes–a review. Indian Fern. J. 2019, 36, 37–79. [Google Scholar]
- Terry, L.A. Natural Disease Resistance in Strawberry Fruit and Geraldton Waxflower Flowers; Cranfield University: Bedford, UK, 2002. [Google Scholar]
- Chattopadhyay, D.; Chawla-Sarkar, M.; Chatterjee, T.; Dey, R.S.; Bag, P.; Chakraborti, S.; Khan, M.T.H. Recent advancements for the evaluation of anti-viral activities of natural products. New Biotechnol. 2009, 25, 347–368. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Dong, S.; Wang, X.; Xu, H.; Yang, X.; Wu, S.; Jiang, X.; Kan, M.; Xu, C. Research progress of polyphenols in nanoformulations for antibacterial application. Mater. Today Bio. 2023, 21, 100729. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, K.; King, J.W.; Howard, L.R.; Monrad, J.K. Solubility of gallic acid, catechin, and protocatechuic acid in subcritical water from (298.75 to 415.85) K. J. Chem. Eng. Data 2010, 55, 3101–3108. [Google Scholar] [CrossRef]
- Rajhard, S.; Hladnik, L.; Vicente, F.A.; Srčič, S.; Grilc, M.; Likozar, B. Solubility of luteolin and other polyphenolic compounds in water, nonpolar, polar aprotic and protic solvents by applying ftir/hplc. Processes 2021, 9, 1952. [Google Scholar] [CrossRef]
- Munin, A.; Edwards-Lévy, F. Encapsulation of natural polyphenolic compounds; a review. Pharmaceutics 2011, 3, 793–829. [Google Scholar] [CrossRef]
- Wang, H.; Wang, C.; Zou, Y.; Hu, J.; Li, Y.; Cheng, Y. Natural polyphenols in drug delivery systems: Current status and future challenges. Giant 2020, 3, 100022. [Google Scholar] [CrossRef]
- Yang, B.; Dong, Y.; Wang, F.; Zhang, Y. Nanoformulations to enhance the bioavailability and physiological functions of polyphenols. Molecules 2020, 25, 4613. [Google Scholar] [CrossRef] [PubMed]
- Ponte, T.S.d. Epigallocatechin-3-Gallate Antimycotic and Azole Resistant Modulator Potential against Resistant Fungi. Ph.D. Thesis, Instituto Politécnico de Lisboa, Lisboa, Portugal, 2021. [Google Scholar]
- Puupponen-Pimiä, R.; Nohynek, L.; Meier, C.; Kähkönen, M.; Heinonen, M.; Hopia, A.; Oksman-Caldentey, K.M. Antimicrobial properties of phenolic compounds from berries. J. Appl. Microbiol. 2001, 90, 494–507. [Google Scholar] [CrossRef]
- Alves, M.J.; Ferreira, I.C.; Froufe, H.J.; Abreu, R.; Martins, A.; Pintado, M. Antimicrobial activity of phenolic compounds identified in wild mushrooms, SAR analysis and docking studies. J. Appl. Microbiol. 2013, 115, 346–357. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).