Development of a Web Application for Simulating Plasma Drug Concentrations in Patients with Zolpidem Intoxication
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Data
2.2. Population PK Model
2.3. Model Evaluation
2.4. RxODE Conversion and Simulation
2.5. Development of A Web-Application
3. Results
3.1. Pharmacokinetic Analysis
3.2. Population PK Model
3.3. RxODE Conversion and Simulation
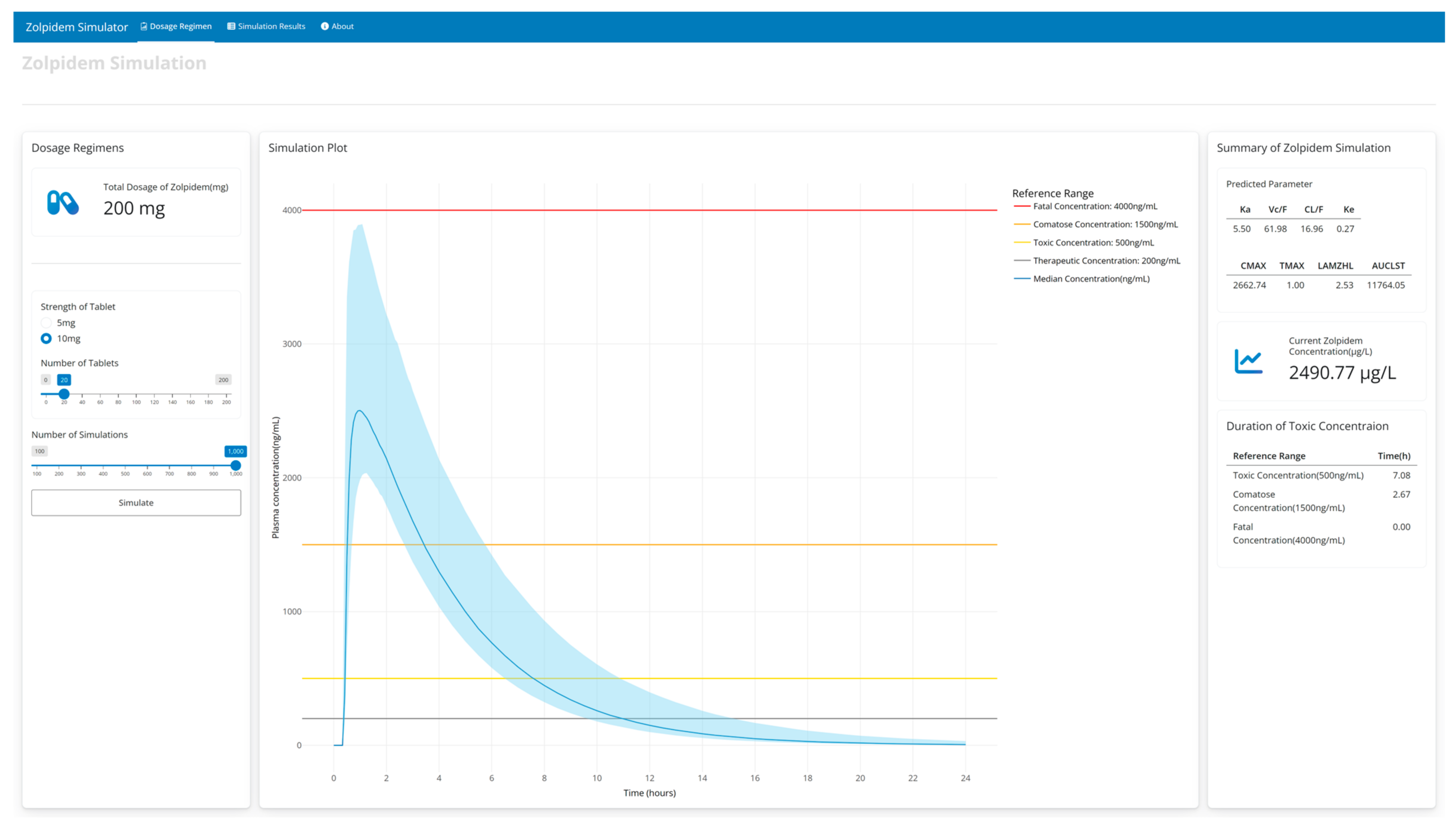
3.4. Zolpidemsim: Web Application
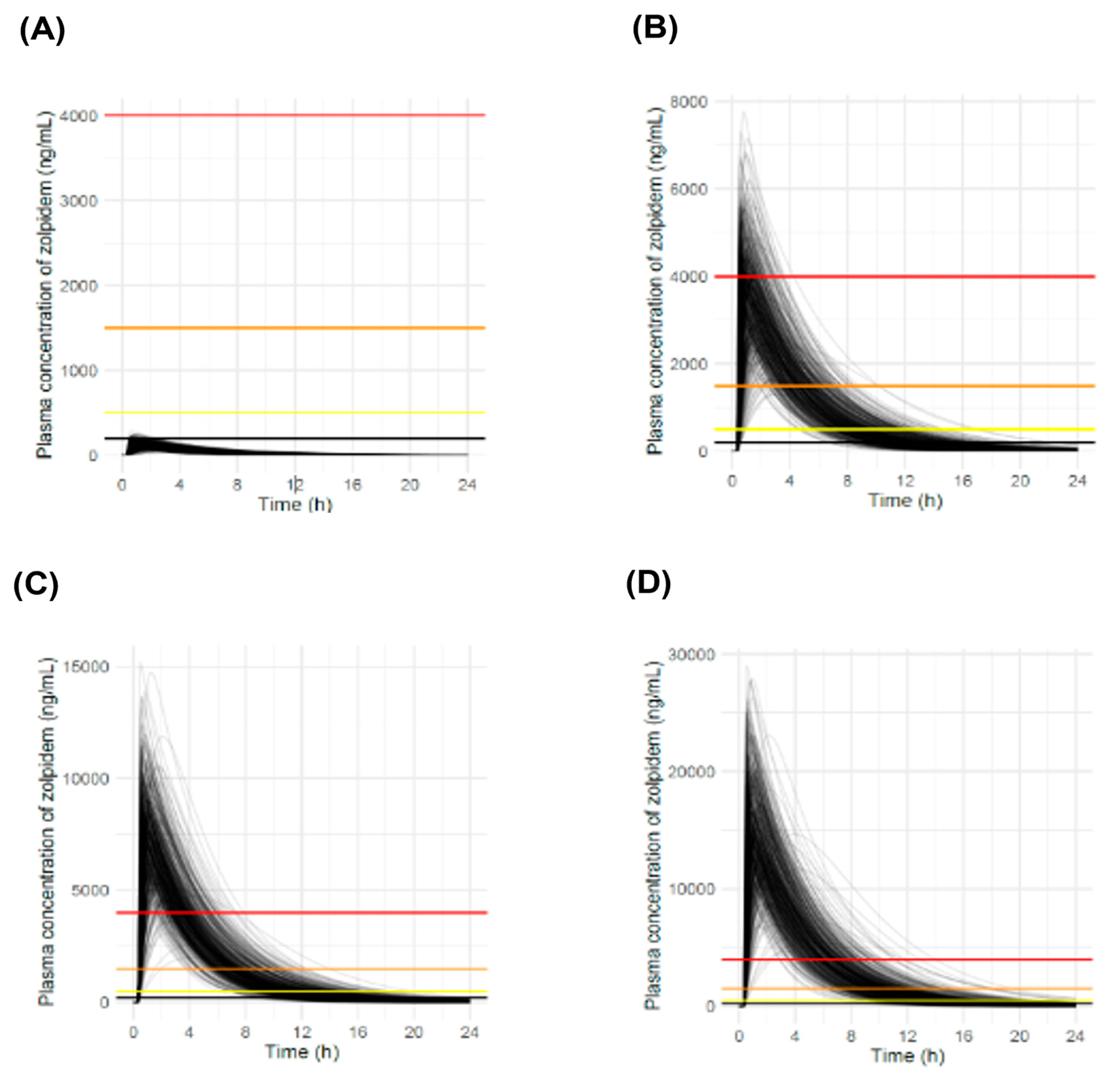
3.5. Simulation Results at Various Doses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stahl, S.M.; Grady, M.M.; Muntner, N. Stahl’s Essential Psychopharmacology, Neuroscientific Basis and Practical Application, 5th ed.; Cambridge University Press: Cambridge, UK, 2021; pp. 418–424. [Google Scholar] [CrossRef]
- Holm, K.J.; Goa, K.L. Zolpidem: An update of its pharmacology, therapeutic efficacy and tolerability in the treatment of insomnia. Drugs 2000, 59, 865–889. [Google Scholar] [CrossRef] [PubMed]
- National Institutes of Health. National Institutes of Health State of the Science Conference statement on Manifestations and Management of Chronic Insomnia in Adults, 13–15 June 2005. Sleep 2005, 28, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Moore, T.J.; Mattison, D.R. Assessment of patterns of potentially unsafe use of zolpidem. JAMA Intern. Med. 2018, 178, 1275–1277. [Google Scholar] [CrossRef] [PubMed]
- Morlock, R.J.; Tan, M.; Mitchell, D.Y. Patient characteristics and patterns of drug use for sleep complaints in the United States: Analysis of National Ambulatory Medical Survey data, 1997–2002. Clin. Ther. 2006, 28, 1044–1053. [Google Scholar] [PubMed]
- Thompson, K.S.; Lewis, R.J.; Ritter, R.M. Analysis of zolpidem in postmortem fluids and tissues using ultra-performance liquid chromatography-mass spectrometry. J. Anal. Toxicol. 2014, 38, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Choi, J.W.; Lee, J.; Shin, A.; Oh, S.M.; Jung, S.J.; Lee, Y.J. Trends in prescriptions for sedative-hypnotics among Korean adults: A nationwide prescription database study for 2011–2015. Soc. Psychiatry Psychiatr. Epidemiol. 2019, 54, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Salvà, P.; Costa, J. Clinical Pharmacokinetics and Pharmacodynamics of Zolpidem. Clin. Pharmacokinet. 1995, 29, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Monti, J.M.; Spence, D.W.; Buttoo, K.; Randi-Perumal, S.R. Zolpidem’s use for insomnia. Asian J. Psychiatry 2017, 25, 79–90. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. FDA Drug Safety Communication: FDA Approves New Label Changes and Dosing for Zolpidem Products and Recommendation to Avoid Driving the Day after Using Ambien CR. US FDA. 2013. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-approves-new-label-changes-and-dosing-zolpidem-products-and (accessed on 21 January 2024).
- Scharner, V.; Hasieber, L.; Sönnichsen, A.; Mann, E. Efficacy and safety of Z-substances in the management of insomnia in older adults: A systematic review for the development of recommendations to reduce potentially inappropriate prescribing. BMC Geriatr. 2022, 22, 87. [Google Scholar] [CrossRef] [PubMed]
- Sanofi-aventis. U.S. LLC. AMBIEN® (Zolpidem Tartrate) Tablets, for Oral Use. Label. 2022. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/019908s40s044s047lbl.pdf (accessed on 9 April 2024).
- Uges, D.R. TIAFT Reference Blood Level List of Therapeutic and Toxic Substances. Available online: https://www.fnusa.cz/wp-content/uploads/therap_tox_levels.pdf (accessed on 13 January 2024).
- Organization for Economic Cooperation and Development. OECD Factbook 2015–2016: Economic, Environmental and Social Statistics; OECD Publishing: Paris, France. 2016. Available online: https://www.oecd-ilibrary.org/economics/oecd-factbook-2015-2016_factbook-2015-en (accessed on 21 January 2024).
- Lee, E.S.; Kim, S.J.; Cho, G.C.; Lee, M.J.; So, B.H.; Kim, K.S.; Song, J.; Lee, S.W. The 2022 annual report on toxicology surveillance and severe poisoning cases at emergency departments in Korea. J. Korean Soc. Clin. Toxicol. 2023, 21, 1–16. [Google Scholar] [CrossRef]
- Pai, K.H.; Lee, S.W.; Kim, S.J.; Han, K.S.; Song, J.; Lee, S.; Park, J.H.; Song, J. Patterns of self-harm/suicide attempters who visited emergency department over the past 10 years and changes in poisoning as a major method (2011–2020). J. Korean Soc. Clin. Toxicol. 2023, 21, 69–80. [Google Scholar] [CrossRef]
- Park, M.; Park, J.; Lee, S.; In, S. Analysis of death due to poisoning in the national capital region (2014–2016). J. Korean Soc. Clin. Toxicol. 2017, 15, 101–106. [Google Scholar] [CrossRef]
- Powell, J.R.; Gobburu, J.V.S. Pharmacometrics at FDA: Evolution and impact on decisions. Clin. Pharmacol. Ther. 2007, 82, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Bauer, R.J. NONMEM Tutorial Part I: Description of Commands and Options, With Simple Examples of Population Analysis. CPT Pharmacomet. Syst. Pharmacol. 2019, 8, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Korea Clinical Trials Information Center. Available online: https://cris.nih.go.kr/cris/search/listDetail.do?search_yn=Y&searchWord=KCT0003934 (accessed on 12 January 2024).
- Rohatgi, A. WebPlotDititizer, Version 4.7 [Computer Software]. 2021. Available online: https://apps.automeris.io/wpd (accessed on 12 January 2024).
- Saldanha, L.; Costa, B.; Vale, N. In silico personalized study for zolpidem based on sex difference. Future Pharmacol. 2022, 2, 99–116. [Google Scholar] [CrossRef]
- Kim, H.; Han, S.; Cho, Y.S.; Yoon, S.K.; Bae, K.S. Development of R packages: ‘NonCompart’ and ‘ncar’ for noncompartmental analysis (NCA). Transl. Clin. Pharmacol. 2018, 26, 10–15. [Google Scholar] [CrossRef] [PubMed]
- NonCompart: Noncompartmental Analysis for Pharmacokinetic Data. R package Version 0.7.0. Available online: https://cran.r-project.org/web/packages/NonCompart/ (accessed on 2 April 2024).
- NONMEM. ICON plc. Available online: https://iconplc.com/solutions/technologies/nonmem (accessed on 21 January 2024).
- Yoon, S.; Jeong, S.; Jung, E.; Kim, K.S.; Jeon, I.; Lee, Y.; Cho, J.Y.; Oh, W.Y.; Chung, J.Y. Effect of CYP3A4 metabolism on sex differences in the pharmacokinetics and pharmacodynamics of zolpidem. Sci. Rep. 2021, 11, 19150. [Google Scholar] [CrossRef] [PubMed]
- The R Project for Statistical Computing. R. Available online: http://www.r-project.org (accessed on 22 November 2023).
- nonmem2rx: ‘nonmem2rx’ Converts ‘NONMEM’ Models to ‘rxode2’. R Package Version 0.1.3. Available online: https://cran.r-project.org/web/packages/nonmem2rx/ (accessed on 12 December 2023).
- Rxode2: Facilities for Simulating from ODE-Based Models. R Package Version 2.1.0. Available online: https://cran.r-project.org/package=rxode2/ (accessed on 30 January 2024).
- Wang, W.; Hallow, K.M. A Tutorial on RxODE: Simulating Differential Equation Pharmacometric Models in R. CPT Pharmacomet. Syst. Pharmacol. 2016, 5, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Shiny: Web Application Framework for, R. R Package Version 1.8.1.1. Available online: https://cran.r-project.org/web/packages/shiny/ (accessed on 2 April 2024).
- bslib: Custom ‘Bootstrap’ ‘Sass’ Themes for ‘Shiny’ and ‘Rmarkdown’. R Package Version 0.7.0. Available online: https://cran.r-project.org/web/packages/bslib/ (accessed on 9 April 2024).
- Lee, Y.; Oh, W.Y.; Kim, K.S.; Jung, E.; Jeong, S.M.; Lee, J.G.; Yi, J.Y.; Chung, J.; Ryu, S.A.; Park, Z.W. Development of Pharmacometrics-Based Modeling for Optimal Dosage (Korean). Research Report of Ministry of Food and Drug Safety (MFDS), Republic of Korea. 2018. Available online: https://rnd.mfds.go.kr/download/rnd/1125833?mode=download (accessed on 22 January 2024).
- Drover, D.; Lemmens, H.; Naidu, S.; Cevallos, W.; Darwish, M.; Stanski, D. Pharmacokinetics, pharmacodynamics, and relative pharmacokinetic/pharmacodynamic profiles of zaleplon and zolpidem. Clin. Ther. 2000, 22, 1443–1461. [Google Scholar] [CrossRef] [PubMed]
- de Haas, S.L.; Schoemaker, R.C.; van Gerven, J.M.A.; Hoever, P.; Cohen, A.F.; Dingemanse, J. Pharmacokinetics, pharmacodynamics and the pharmacokinetic/pharmacodynamic relationship of zolpidem in healthy subjects. J. Psychopharmacol. 2010, 24, 1619–1629. [Google Scholar] [CrossRef] [PubMed]
- mrgsolve: Simulate from ODE-Based Models. R Package Version 1.4.1. Available online: https://cran.r-project.org/web/packages/mrgsolve/ (accessed on 9 April 2024).
- PKPDsim: Tools for Performing Pharmacokinetic-Pharmacodynamic Simulations. R Package Version 1.3.0. Available online: https://cran.r-project.org/web/packages/PKPDsim/ (accessed on 9 April 2024).
- deSolve: Solvers for Initial Value Problems of Differential Equations (‘ODE’, ‘DAE’, ‘DDE’) R Package Version 1.40. Available online: https://cran.r-project.org/web/packages/deSolve/ (accessed on 9 April 2024).



| Parameters | Male | Female | ||
|---|---|---|---|---|
| Original (N = 15) | Digitized (N = 12) | Original (N = 15) | Digitized (N = 11) | |
| Cmax (ng/mL) | 161.21 (46.74) | 162.72 (47.04) | 187.64 (59.44) | 189.22 (59.85) |
| AUC0–12h (ng h/mL) | 537.9 (202.3) | 542.5 (197.5) | 696.9 (221.7) | 688.1 (221.3) |
| CL/F (L/h) | 19.76 (7.25) | 18.88 (6.06) | 14.72 (4.07) | 14.93 (4.22) |
| Lambda z (1/h) | 0.27 (0.09) | 0.22 (0.06) | 0.23 (0.03) | 0.24 (0.04) |
| Half-life (h) | 2.93 (1.14) | 3.58 (1.10) | 3.05 (0.35) | 3.02 (0.52) |
| Vd/F (L) | 74.8 (16.2) | 92.2 (35.7) | 63.6 (15.6) | 63.5 (16.5) |
| Tmax (h) | 0.94 (0.58–1.80) | 0.96 (0.58–1.78) | 0.83 (0.58–3.33) | 0.84 (0.57–3.36) |
| Parameters | Estimates (RSE a %) | Bootstrap | |
|---|---|---|---|
| Median | 95% CI | ||
| Fixed effects | |||
| Ka (1/h) | 5.41 (45.47) | 5.66 | 3.24–11.24 |
| Vd/F (L) | 61.70 (5.82) | 61.33 | 54.89–68.64 |
| CL/F (L/h) | 16.90 (8.40) | 16.87 | 14.29–19.50 |
| ALAG (h) | 0.394 (6.24) | 0.394 | 0.385–0.493 |
| Interindividual variability (IIV) | |||
| ω2 Ka (CV b %) | 158.91 (43.65) | 157.81 | 69.76–444.98 |
| ω2 Vd (CV%) | 22.10 (47.17) | 20.98 | 12.0–29.68 |
| ω2 CL (CV%) | 32.60 (45.54) | 31.74 | 20.96–41.65 |
| ρVd-CL | 0.853 | 0.853 | 0.796–0.869 |
| Residual variability | |||
| Proportional error | 0.284 (6.16) | 0.283 | 0.242–0.319 |
| Doses (mg) | NR a (%) | <1 h (%) | 1–2 h (%) | 2–3 h (%) | >3 h (%) | |
|---|---|---|---|---|---|---|
| Toxic concentration (500–1500 ng/mL) | 10 | 100 | 0 | 0 | 0 | 0 |
| 280 | 0 | 0 | 0 | 0 | 100 | |
| 560 | 0 | 0 | 0 | 0 | 100 | |
| 1120 | 0 | 0 | 0 | 0 | 100 | |
| Comatose concentration (1500–4000 ng/mL) | 10 | 100 | 0 | 0 | 0 | 0 |
| 280 | 0.2 | 0.4 | 0.8 | 15.0 | 83.6 | |
| 560 | 0 | 0 | 0 | 0.4 | 99.6 | |
| 1120 | 0 | 0 | 0 | 0 | 100 | |
| Fatal concentration (>4000 ng/mL) | 10 | 100 | 0 | 0 | 0 | 0 |
| 280 | 58.8 | 21.4 | 15.6 | 4.0 | 0.2 | |
| 560 | 2.6 | 1.6 | 11.2 | 31.4 | 53.2 | |
| 1120 | 0 | 0 | 0 | 1.6 | 98.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cha, H.J.; Han, S.; Pak, K.C.; Kim, H. Development of a Web Application for Simulating Plasma Drug Concentrations in Patients with Zolpidem Intoxication. Pharmaceutics 2024, 16, 689. https://doi.org/10.3390/pharmaceutics16050689
Cha HJ, Han S, Pak KC, Kim H. Development of a Web Application for Simulating Plasma Drug Concentrations in Patients with Zolpidem Intoxication. Pharmaceutics. 2024; 16(5):689. https://doi.org/10.3390/pharmaceutics16050689
Chicago/Turabian StyleCha, Hwa Jun, Sungpil Han, Kwan Cheol Pak, and Hyungsub Kim. 2024. "Development of a Web Application for Simulating Plasma Drug Concentrations in Patients with Zolpidem Intoxication" Pharmaceutics 16, no. 5: 689. https://doi.org/10.3390/pharmaceutics16050689
APA StyleCha, H. J., Han, S., Pak, K. C., & Kim, H. (2024). Development of a Web Application for Simulating Plasma Drug Concentrations in Patients with Zolpidem Intoxication. Pharmaceutics, 16(5), 689. https://doi.org/10.3390/pharmaceutics16050689







