Nanodrug Delivery Systems for Myasthenia Gravis: Advances and Perspectives
Abstract
1. Introduction
2. Pathogenesis of MG
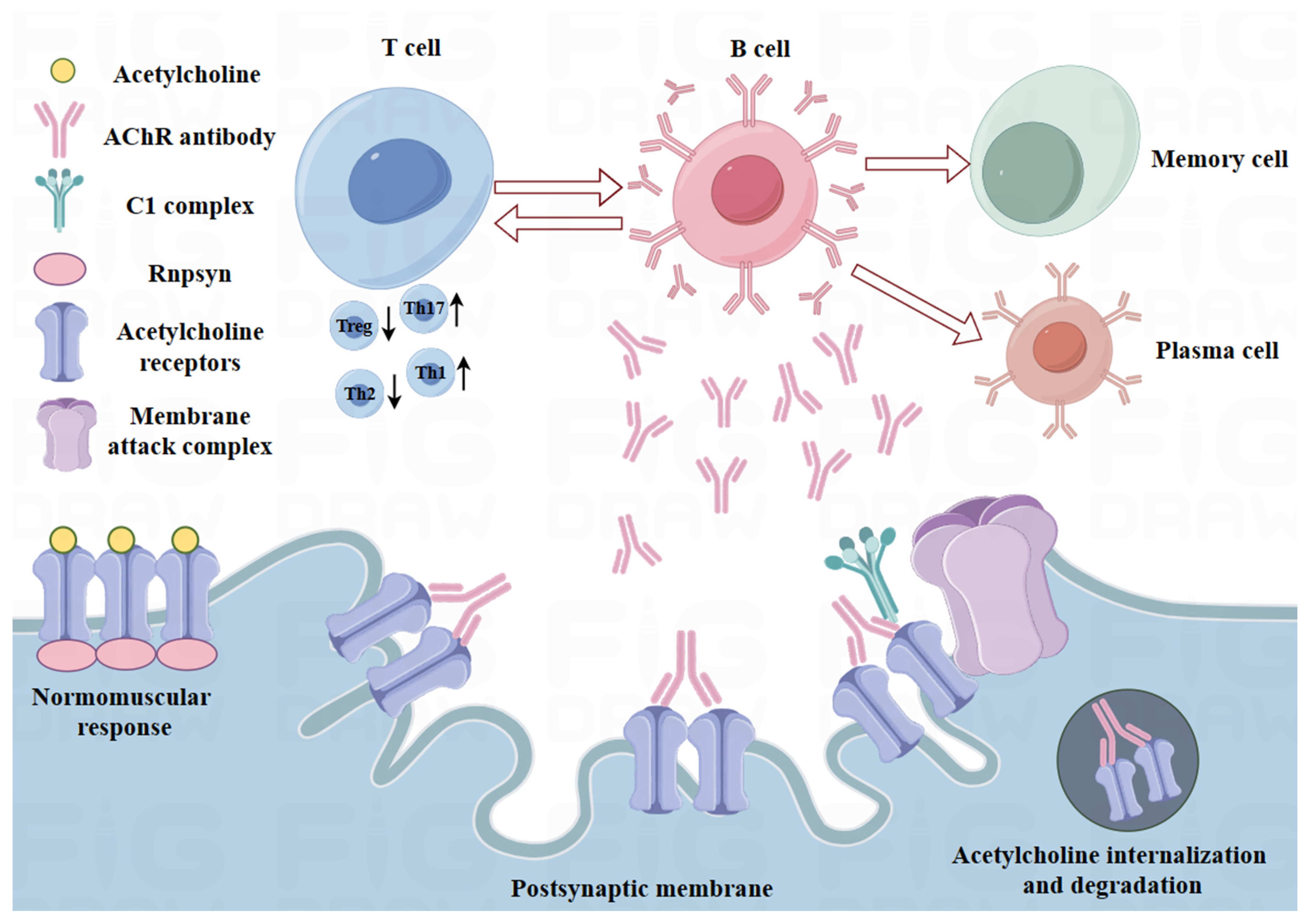
2.1. Immunoregulatory Defects in MG
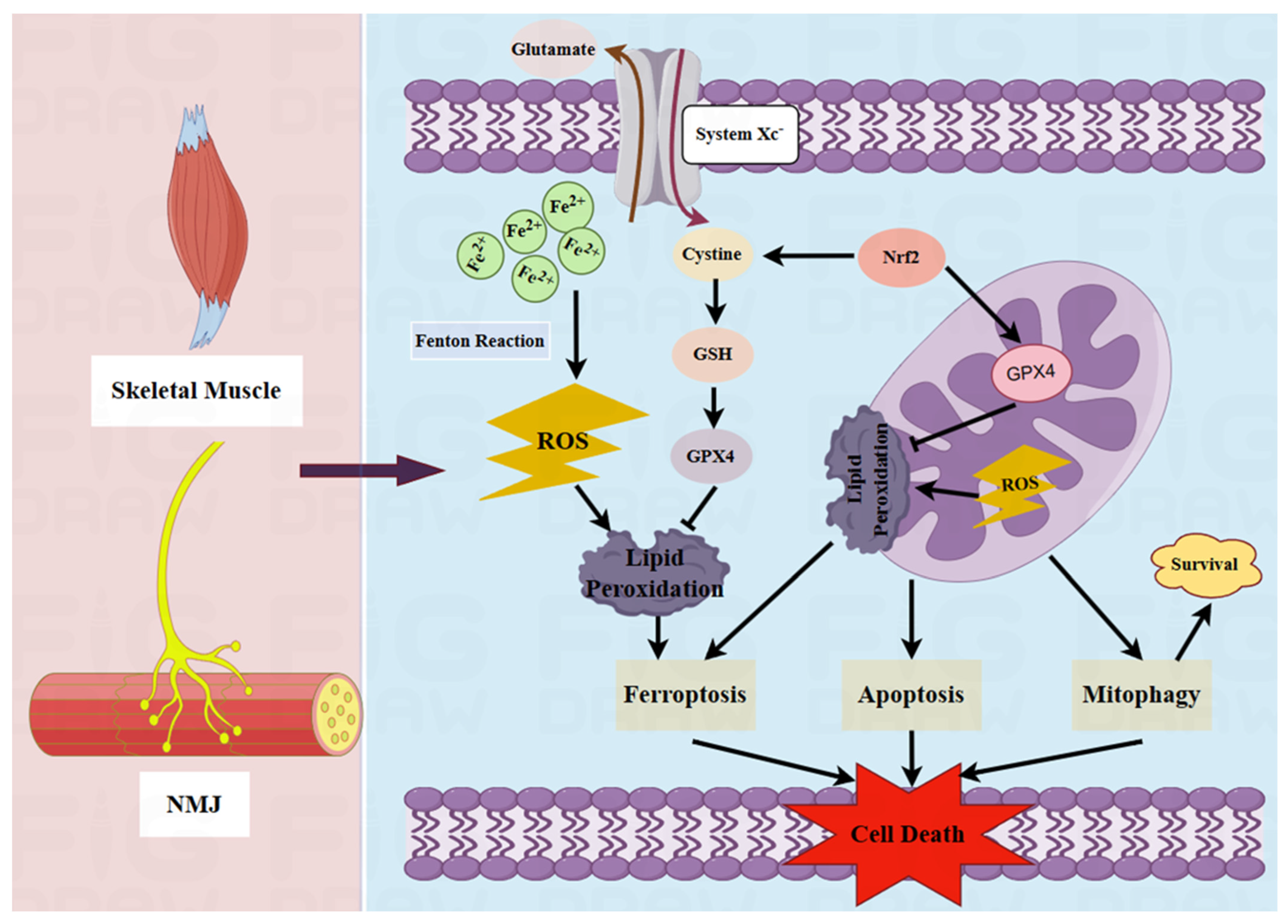
2.2. Pathological Role of Mitochondria in MG
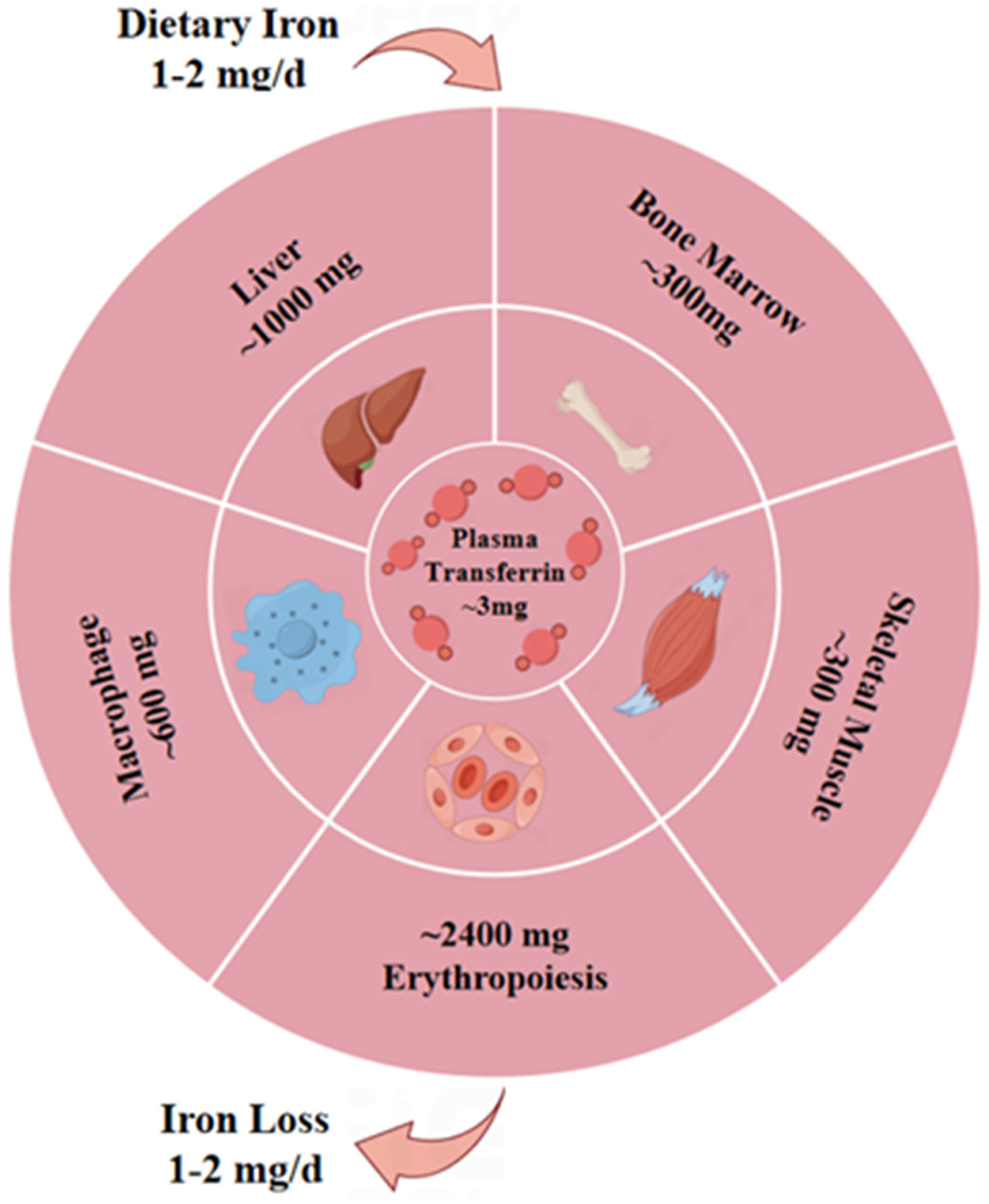
2.3. Potential Pathogenic Effects of Ferroptosis on MG
3. Therapeutic Potential of Nano-Biomedicines in MG
3.1. Potential Role of Nano-Biomedicines in Immunomodulation
3.2. Potential Role of Nano-Biomedicines in Targeted Mitochondrial Therapy
3.3. Potential Effects of Nano-Biomedicines on Ferroptosis
| Target | Delivery System | Active Drug/Agent | Treatment Outcomes | References |
|---|---|---|---|---|
| Immune system | Extracellular vesicles | Caspase-1 inhibitor | Targeted macrophages to inhibit the Th17 response and GC response and thereby improve EAMG | [115] |
| AuNPs | IL-4 or IL-10 | Shifted the immune response in chronically inflamed dystrophic muscle | [146] | |
| PLA and nano-HAP | Doxycycline | Decreased salivary MMP-8 and plasma IL-1 and TNF-α concentrations | [147] | |
| Nano-liposomes | MPS | Decreased serum TGF-β levels and reduced macrophage infiltration in the diaphragm | [119] | |
| PLGA composites | Polydeoxyribonucleotide | Regulated the M1-to-M2 polarization of macrophages and caused immune modulation | [148] | |
| LNPs and polyplex nanomicelles | mRNA | Supported rapid mRNA expression and a potent immune response | [149] | |
| Liposomes | Alendronate | Regulated the M1-to-M2 polarization of macrophages and T-cell functionality | [150] | |
| Flexible liposome hydrogel | DEX | Reduced joint swelling by increasing macrophage uptake | [151] | |
| GO nanosheets | GO | Reversed the dynamic changes to CKs and reduced the activity of Ca2+ | [152] | |
| Erythrocyte membrane-camouflaged NPs | CD22-shRNA, Aβ aptamers | Ameliorated a pro-inflammatory immune environment and could be used to visualize Aβ plaques | [153] | |
| AuNPs | IL-4 | Directed M2 macrophage polarization and promoted regeneration | [154] | |
| Mitochondria | PLGA NPs | Sonosensitizer IR780 and ferroptosis activator RSL-3 | Inhibited the activity of GPX4 and induced ROS generation | [155] |
| Lipid-polymer hybrid nano-system | Calycosin and tanshinone | Increased drug accumulation in cardiac tissue and enabled better infarct size reduction | [156] | |
| Lipid nanocarriers | siRNA-loaded magnesium phosphate core | Reversed mitochondrial dysfunction and alleviated AD neuropathology | [157] | |
| Ceria NPs | Atorvastatin | Eliminated excessive ROS and protected mitochondrial structure | [158] | |
| Polydopamine-coated NPs | PDA and α-TOS | Enabled nanomedicine accumulation in mitochondria to destroy tumor cells | [159] | |
| Molecularly imprinted polymer NPs | Molecularly imprinted polymer | Blocked the catalytic activity of DHFR to inhibit DNA synthesis | [160] | |
| Porous silicon NPs | Bovine serum albumin | Disrupted the mitochondrial respiratory chain | [161] | |
| PLGA-b-PEG NPs | CoQ10 | Effectively increased the tricarboxylic acid cycle rate | [128] | |
| Lipidosomes | Quercetin | Decreased ROS generation, increased ATP levels, and enhanced lactate dehydrogenase activity | [130] | |
| Biomimetic nanocrystals | Curcumin | Reversed mitochondrial dysfunction, TH+ neuron injury, and abnormal α-syn aggregation | [162] | |
| ZIF-8-coated Prussian blue nanocomposite | Quercetin | Restored mitochondrial function, restored energy metabolism, and reduced ROS | [163] | |
| BPNSs | Matrine | Improved neurotransmitter delivery, removed excess ROS, and decreased neuroinflammation | [164] | |
| BPNSs-based hydrogel | Methylene blue | Improved mitochondrial function, and suppressed tau neuropathology | [165] | |
| Platelet membranes-ICG-SS31-PLGA | Indocyanine green and elamipretide | Reduced mitochondrial oxidative stress, inflammation, and apoptosis | [166] | |
| Ferroptosis | Polydopamine NPs | Polydopamine | Depleted ROS, chelated iron, and inhibited the ubiquitination of GPX4 | [144] |
| Ceria-based NPs | Cerium oxide | Alleviated oxidative stress and lipid peroxidation and increased GPX4 activity | [143] | |
| DSPE-PEG 2000 NPs | Iron oxide | Regulated the Beclin1/ATG5-dependent autophagy pathway | [167] | |
| Melanin NPs | Melanin | Inhibited ROS-related ferroptosis to reduce myocardial injury | [168] | |
| Poly-PLGA co-polymers | Alpha lipoic acid | Reduced ROS-induced damage and restored heart function. | [169] | |
| Metal-phenolic nanocomplexes | Quercetin | Attenuated the free radical burst induced by iron overload and restored iron metabolism homeostasis | [170] | |
| PDN@AGL | AGL | Decreased lipid peroxidation, reduced ROS levels, and attenuated ferroptosis | [171] | |
| MPEG-PTMC NPs | Curcumin | Enhanced the delivery of Cur to inhibit ferroptosis | [141] | |
| Polymer NPs | Resveratrol | Inhibited ROS generation and excessive accumulation to attenuate ferroptosis | [172] | |
| PAA@Mn3O4 NPs | Mn3O4 | Resisted lipid peroxidation and detoxified ROS to suppress ferroptosis | [173] |
4. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Payet, C.A.; You, A.; Fayet, O.M.; Dragin, N.; Berrih-Aknin, S.; Le Panse, R. Myasthenia Gravis: An Acquired Interferonopathy? Cells 2022, 11, 1218. [Google Scholar] [CrossRef] [PubMed]
- Albazli, K.; Kaminski, H.J.; Howard, J.F., Jr. Complement Inhibitor Therapy for Myasthenia Gravis. Front. Immunol. 2020, 11, 917. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S.R.; Shah, S.B.; Lovering, R.M. The Neuromuscular Junction: Roles in Aging and Neuromuscular Disease. Int. J. Mol. Sci. 2021, 22, 8058. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.S.; Cardwell, C.R.; McCarron, P.O.; McConville, J. A systematic review of population based epidemiological studies in Myasthenia Gravis. BMC Neurol. 2010, 10, 46. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Tian, D.C.; Zhang, C.; Li, Z.; Zhai, Y.; Xiu, Y.; Gu, H.; Li, H.; Wang, Y.; Shi, F.D. Incidence, mortality, and economic burden of myasthenia gravis in China: A nationwide population-based study. Lancet Reg. Health West. Pac. 2020, 5, 100063. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Su, Y.; Chang, T. Knowledge mapping of global trends for myasthenia gravis development: A bibliometrics analysis. Front. Immunol. 2023, 14, 1132201. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Vicente, E.; Álvarez-Velasco, R.; Segovia, S.; Paradas, C.; Casasnovas, C.; Guerrero-Sola, A.; Pardo, J.; Ramos-Fransi, A.; Sevilla, T.; López de Munain, A.; et al. Clinical and therapeutic features of myasthenia gravis in adults based on age at onset. Neurology 2020, 94, e1171–e1180. [Google Scholar] [CrossRef] [PubMed]
- Lazaridis, K.; Tzartos, S.J. Myasthenia Gravis: Autoantibody Specificities and Their Role in MG Management. Front. Neurol. 2020, 11, 596981. [Google Scholar] [CrossRef] [PubMed]
- Ruiter, A.M.; Verschuuren, J.; Tannemaat, M.R. Prevalence and associated factors of fatigue in autoimmune myasthenia gravis. Neuromuscul. Disord. 2021, 31, 612–621. [Google Scholar] [CrossRef]
- Huijbers, M.G.; Marx, A.; Plomp, J.J.; Le Panse, R.; Phillips, W.D. Advances in the understanding of disease mechanisms of autoimmune neuromuscular junction disorders. Lancet Neurol. 2022, 21, 163–175. [Google Scholar] [CrossRef]
- Dresser, L.; Wlodarski, R.; Rezania, K.; Soliven, B. Myasthenia Gravis: Epidemiology, Pathophysiology and Clinical Manifestations. J. Clin. Med. 2021, 10, 2235. [Google Scholar] [CrossRef]
- Lehnerer, S.; Jacobi, J.; Schilling, R.; Grittner, U.; Marbin, D.; Gerischer, L.; Stascheit, F.; Krause, M.; Hoffmann, S.; Meisel, A. Burden of disease in myasthenia gravis: Taking the patient’s perspective. J. Neurol. 2022, 269, 3050–3063. [Google Scholar] [CrossRef] [PubMed]
- Son, J.M.; Lee, C. Aging: All roads lead to mitochondria. Semin. Cell Dev. Biol. 2021, 116, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Makinde, E.; Ma, L.; Mellick, G.D.; Feng, Y. Mitochondrial Modulators: The Defender. Biomolecules 2023, 13, 226. [Google Scholar] [CrossRef] [PubMed]
- Picard, M.; Shirihai, O.S. Mitochondrial signal transduction. Cell Metab. 2022, 34, 1620–1653. [Google Scholar] [CrossRef] [PubMed]
- Valenti, D.; Atlante, A. Mitochondrial Bioenergetics in Different Pathophysiological Conditions 2.0. Int. J. Mol. Sci. 2022, 23, 5552. [Google Scholar] [CrossRef] [PubMed]
- Green, A.; Hossain, T.; Eckmann, D.M. Mitochondrial dynamics involves molecular and mechanical events in motility, fusion and fission. Front. Cell Dev. Biol. 2022, 10, 1010232. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Liang, K.; Zhu, H.; Zhao, C.; Hu, H.; Yin, S. Ferroptosis and Its Role in Chronic Diseases. Cells 2022, 11, 2040. [Google Scholar] [CrossRef]
- Rochette, L.; Dogon, G.; Rigal, E.; Zeller, M.; Cottin, Y.; Vergely, C. Lipid Peroxidation and Iron Metabolism: Two Corner Stones in the Homeostasis Control of Ferroptosis. Int. J. Mol. Sci. 2022, 24, 449. [Google Scholar] [CrossRef]
- Li, J.; Jia, B.; Cheng, Y.; Song, Y.; Li, Q.; Luo, C. Targeting Molecular Mediators of Ferroptosis and Oxidative Stress for Neurological Disorders. Oxid. Med. Cell Longev. 2022, 2022, 3999083. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, R.; Gupta, R.; Sahu, M.; Srivastava, D.; Das, A.; Ambasta, R.K.; Kumar, P. Free radical biology in neurological manifestations: Mechanisms to therapeutics interventions. Environ. Sci. Pollut. Res. Int. 2022, 29, 62160–62207. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Liu, Y.; Chen, X.; Zhong, H.; Wang, Y. Ferroptosis in life: To be or not to be. Biomed. Pharmacother. 2023, 159, 114241. [Google Scholar] [CrossRef] [PubMed]
- Villalón-García, I.; Povea-Cabello, S.; Álvarez-Córdoba, M.; Talaverón-Rey, M.; Suárez-Rivero, J.M.; Suárez-Carrillo, A.; Munuera-Cabeza, M.; Reche-López, D.; Cilleros-Holgado, P.; Piñero-Pérez, R.; et al. Vicious cycle of lipid peroxidation and iron accumulation in neurodegeneration. Neural Regen. Res. 2023, 18, 1196–1202. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Li, Y. The interaction between ferroptosis and lipid metabolism in cancer. Signal Transduct. Target. Ther. 2020, 5, 108. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xin, L.; Xiang, M.; Shang, C.; Wang, Y.; Wang, Y.; Cui, X.; Lu, Y. The molecular mechanisms of ferroptosis and its role in cardiovascular disease. Biomed. Pharmacother. 2022, 145, 112423. [Google Scholar] [CrossRef]
- Lai, B.; Wu, C.H.; Wu, C.Y.; Luo, S.F.; Lai, J.H. Ferroptosis and Autoimmune Diseases. Front. Immunol. 2022, 13, 916664. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Yan, Y.; Niu, F.; Wang, Y.; Chen, X.; Su, G.; Liu, Y.; Zhao, X.; Qian, L.; Liu, P.; et al. Ferroptosis: A cell death connecting oxidative stress, inflammation and cardiovascular diseases. Cell Death Discov. 2021, 7, 193. [Google Scholar] [CrossRef]
- Chen, X.; Li, J.; Kang, R.; Klionsky, D.J.; Tang, D. Ferroptosis: Machinery and regulation. Autophagy 2021, 17, 2054–2081. [Google Scholar] [CrossRef]
- Ren, J.X.; Li, C.; Yan, X.L.; Qu, Y.; Yang, Y.; Guo, Z.N. Crosstalk between Oxidative Stress and Ferroptosis/Oxytosis in Ischemic Stroke: Possible Targets and Molecular Mechanisms. Oxid. Med. Cell. Longev. 2021, 2021, 6643382. [Google Scholar] [CrossRef]
- Joseph, T.M.; Kar Mahapatra, D.; Esmaeili, A.; Piszczyk, Ł.; Hasanin, M.S.; Kattali, M.; Haponiuk, J.; Thomas, S. Nanoparticles: Taking a Unique Position in Medicine. Nanomaterials 2023, 13, 574. [Google Scholar] [CrossRef] [PubMed]
- Dewi, M.K.; Chaerunisaa, A.Y.; Muhaimin, M.; Joni, I.M. Improved Activity of Herbal Medicines through Nanotechnology. Nanomaterials 2022, 12, 4073. [Google Scholar] [CrossRef] [PubMed]
- Patel, C.; Pande, S.; Sagathia, V.; Ranch, K.; Beladiya, J.; Boddu, S.H.S.; Jacob, S.; Al-Tabakha, M.M.; Hassan, N.; Shahwan, M. Nanocarriers for the Delivery of Neuroprotective Agents in the Treatment of Ocular Neurodegenerative Diseases. Pharmaceutics 2023, 15, 837. [Google Scholar] [CrossRef] [PubMed]
- Makhathini, S.S.; Mdanda, S.; Kondiah, P.J.; Kharodia, M.E.; Rumbold, K.; Alagidede, I.; Pathak, Y.; Bulbulia, Z.; Rants’o, T.A.; Kondiah, P.P.D. Biomedicine Innovations and Its Nanohydrogel Classifications. Pharmaceutics 2022, 14, 2839. [Google Scholar] [CrossRef] [PubMed]
- Vodyashkin, A.A.; Kezimana, P.; Vetcher, A.A.; Stanishevskiy, Y.M. Biopolymeric Nanoparticles-Multifunctional Materials of the Future. Polymers 2022, 14, 2287. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Poon, K.; Masonsong, G.S.P.; Ramaswamy, Y.; Singh, G. Sustainable Nanomaterials for Biomedical Applications. Pharmaceutics 2023, 15, 922. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Yu, Z.; Xu, T.; Wang, L.; Meng, N.; Jin, H.; Xu, B. Novel Nano-Drug Delivery System for Brain Tumor Treatment. Cells 2022, 11, 3761. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.A.; Kim, J.H.; Ryu, K.; Kaushik, N. Current Nanomedicine for Targeted Vascular Disease Treatment: Trends and Perspectives. Int. J. Mol. Sci. 2022, 23, 12397. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.H.; Khattak, S.; Rauf, M.A.; Ansari, M.A.; Alomary, M.N.; Razak, S.; Yang, C.Y.; Wu, D.D.; Ji, X.Y. Role of Nanomedicine-Based Therapeutics in the Treatment of CNS Disorders. Molecules 2023, 28, 1283. [Google Scholar] [CrossRef]
- Ko, C.N.; Zang, S.; Zhou, Y.; Zhong, Z.; Yang, C. Nanocarriers for effective delivery: Modulation of innate immunity for the management of infections and the associated complications. J. Nanobiotechnol. 2022, 20, 380. [Google Scholar] [CrossRef]
- Waheed, S.; Li, Z.; Zhang, F.; Chiarini, A.; Armato, U.; Wu, J. Engineering nano-drug biointerface to overcome biological barriers toward precision drug delivery. J. Nanobiotechnol. 2022, 20, 395. [Google Scholar] [CrossRef]
- Kumari, S.; Goyal, A.; Sönmez Gürer, E.; Algın Yapar, E.; Garg, M.; Sood, M.; Sindhu, R.K. Bioactive Loaded Novel Nano-Formulations for Targeted Drug Delivery and Their Therapeutic Potential. Pharmaceutics 2022, 14, 1091. [Google Scholar] [CrossRef] [PubMed]
- Alsaab, H.O.; Alharbi, F.D.; Alhibs, A.S.; Alanazi, N.B.; Alshehri, B.Y.; Saleh, M.A.; Alshehri, F.S.; Algarni, M.A.; Almugaiteeb, T.; Uddin, M.N.; et al. PLGA-Based Nanomedicine: History of Advancement and Development in Clinical Applications of Multiple Diseases. Pharmaceutics 2022, 14, 2728. [Google Scholar] [CrossRef] [PubMed]
- Pozharov, V.P.; Minko, T. Nanotechnology-Based RNA Vaccines: Fundamentals, Advantages and Challenges. Pharmaceutics 2023, 15, 194. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, Z.; Lei, H.; Zhang, D. Recent progress in nanotechnology-based drug carriers for resveratrol delivery. Drug Deliv. 2023, 30, 2174206. [Google Scholar] [CrossRef] [PubMed]
- Fichtner, M.L.; Jiang, R.; Bourke, A.; Nowak, R.J.; O’Connor, K.C. Autoimmune Pathology in Myasthenia Gravis Disease Subtypes Is Governed by Divergent Mechanisms of Immunopathology. Front. Immunol. 2020, 11, 776. [Google Scholar] [CrossRef] [PubMed]
- Berrih-Aknin, S.; Le Panse, R. Myasthenia gravis: A comprehensive review of immune dysregulation and etiological mechanisms. J. Autoimmun. 2014, 52, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Le Panse, R.; Cizeron-Clairac, G.; Cuvelier, M.; Truffault, F.; Bismuth, J.; Nancy, P.; De Rosbo, N.K.; Berrih-Aknin, S. Regulatory and pathogenic mechanisms in human autoimmune myasthenia gravis. Ann. N. Y. Acad. Sci. 2008, 1132, 135–142. [Google Scholar] [CrossRef]
- Castañeda, J.; Hidalgo, Y.; Sauma, D.; Rosemblatt, M.; Bono, M.R.; Núñez, S. The Multifaceted Roles of B Cells in the Thymus: From Immune Tolerance to Autoimmunity. Front. Immunol. 2021, 12, 766698. [Google Scholar] [CrossRef]
- Berrih-Aknin, S.; Morel, E.; Raimond, F.; Safar, D.; Gaud, C.; Binet, J.P.; Levasseur, P.; Bach, J.F. The role of the thymus in myasthenia gravis: Immunohistological and immunological studies in 115 cases. Ann. N. Y. Acad. Sci. 1987, 505, 50–70. [Google Scholar] [CrossRef]
- DuPage, M.; Bluestone, J.A. Harnessing the plasticity of CD4+ T cells to treat immune-mediated disease. Nat. Rev. Immunol. 2016, 16, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Gertel-Lapter, S.; Mizrachi, K.; Berrih-Aknin, S.; Fuchs, S.; Souroujon, M.C. Impairment of regulatory T cells in myasthenia gravis: Studies in an experimental model. Autoimmun. Rev. 2013, 12, 894–903. [Google Scholar] [CrossRef] [PubMed]
- Akamine, H.; Uzawa, A.; Kojima, Y.; Ozawa, Y.; Yasuda, M.; Onishi, Y.; Kuwabara, S. Role of soluble forms of follicular helper T-cell membrane molecules in the pathogenesis of myasthenia gravis. J. Neuroimmunol. 2023, 375, 578014. [Google Scholar] [CrossRef] [PubMed]
- Danikowski, K.M.; Jayaraman, S.; Prabhakar, B.S. Regulatory T cells in multiple sclerosis and myasthenia gravis. J. Neuroinflammation 2017, 14, 117. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, X.S.; Wang, Y.G.; Lu, C.; Li, J.; Zhang, P. Imbalance of Th17 and Tregs in thymoma may be a pathological mechanism of myasthenia gravis. Mol. Immunol. 2021, 133, 67–76. [Google Scholar] [CrossRef]
- Villegas, J.A.; Van Wassenhove, J.; Le Panse, R.; Berrih-Aknin, S.; Dragin, N. An imbalance between regulatory T cells and T helper 17 cells in acetylcholine receptor-positive myasthenia gravis patients. Ann. N. Y. Acad. Sci. 2018, 1413, 154–162. [Google Scholar] [CrossRef]
- Shibui, A.; Shimura, E.; Nambu, A.; Yamaguchi, S.; Leonard, W.J.; Okumura, K.; Sugano, S.; Sudo, K.; Nakae, S. Th17 cell-derived IL-17 is dispensable for B cell antibody production. Cytokine 2012, 59, 108–114. [Google Scholar] [CrossRef]
- Uzawa, A.; Kuwabara, S.; Suzuki, S.; Imai, T.; Murai, H.; Ozawa, Y.; Yasuda, M.; Nagane, Y.; Utsugisawa, K. Roles of cytokines and T cells in the pathogenesis of myasthenia gravis. Clin. Exp. Immunol. 2021, 203, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Y.; Gu, W.; He, L.; Sun, B. Th1/Th2 cell’s function in immune system. Adv. Exp. Med. Biol. 2014, 841, 45–65. [Google Scholar] [CrossRef]
- Alahgholi-Hajibehzad, M.; Kasapoglu, P.; Jafari, R.; Rezaei, N. The role of T regulatory cells in immunopathogenesis of myasthenia gravis: Implications for therapeutics. Expert Rev. Clin. Immunol. 2015, 11, 859–870. [Google Scholar] [CrossRef]
- Sakaguchi, S.; Ono, M.; Setoguchi, R.; Yagi, H.; Hori, S.; Fehervari, Z.; Shimizu, J.; Takahashi, T.; Nomura, T. Foxp3+CD25+CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol. Rev. 2006, 212, 8–27. [Google Scholar] [CrossRef]
- Dominguez-Villar, M.; Hafler, D.A. Regulatory T cells in autoimmune disease. Nat. Immunol. 2018, 19, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Thiruppathi, M.; Rowin, J.; Ganesh, B.; Sheng, J.R.; Prabhakar, B.S.; Meriggioli, M.N. Impaired regulatory function in circulating CD4+CD25highCD127low/− T cells in patients with myasthenia gravis. Clin. Immunol. 2012, 145, 209–223. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Ma, Q.; Yu, L.; Huang, H.; Liu, X.; Chen, P.; Ran, H.; Liu, W. JAK2 inhibitor ameliorates the progression of experimental autoimmune myasthenia gravis and balances Th17/Treg cells via regulating the JAK2/STAT3-AKT/mTOR signaling pathway. Int. Immunopharmacol. 2023, 115, 109693. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Tang, X. Gut Microbiota as Regulators of Th17/Treg Balance in Patients With Myasthenia Gravis. Front. Immunol. 2021, 12, 803101. [Google Scholar] [CrossRef] [PubMed]
- Cenacchi, G.; Papa, V.; Fanin, M.; Pegoraro, E.; Angelini, C. Comparison of muscle ultrastructure in myasthenia gravis with anti-MuSK and anti-AChR antibodies. J. Neurol. 2011, 258, 746–752. [Google Scholar] [CrossRef] [PubMed]
- Schubert, M.; Pelz, A.; Trautmann, G.; Block, K.; Furlan, S.; Gutsmann, M.; Kohler, S.; Volpe, P.; Blottner, D.; Meisel, A.; et al. Opposite Regulation of Homer Signal at the NMJ Postsynaptic Micro Domain between Slow- and Fast-Twitch Muscles in an Experimentally Induced Autoimmune Myasthenia Gravis (EAMG) Mouse Model. Int. J. Mol. Sci. 2022, 23, 15052. [Google Scholar] [CrossRef] [PubMed]
- Lisowski, P.; Kannan, P.; Mlody, B.; Prigione, A. Mitochondria and the dynamic control of stem cell homeostasis. EMBO Rep. 2018, 19, e45432. [Google Scholar] [CrossRef] [PubMed]
- Reggiani, C.; Marcucci, L. A controversial issue: Can mitochondria modulate cytosolic calcium and contraction of skeletal muscle fibers? J. Gen. Physiol. 2022, 154, e202213167. [Google Scholar] [CrossRef]
- Bolaños, P.; Calderón, J.C. Excitation-contraction coupling in mammalian skeletal muscle: Blending old and last-decade research. Front. Physiol. 2022, 13, 989796. [Google Scholar] [CrossRef]
- Ke, L.; Li, Q.; Song, J.; Jiao, W.; Ji, A.; Chen, T.; Pan, H.; Song, Y. The mitochondrial biogenesis signaling pathway is a potential therapeutic target for myasthenia gravis via energy metabolism (Review). Exp. Ther. Med. 2021, 22, 702. [Google Scholar] [CrossRef]
- Slavin, M.B.; Memme, J.M.; Oliveira, A.N.; Moradi, N.; Hood, D.A. Regulatory networks coordinating mitochondrial quality control in skeletal muscle. Am. J. Physiol. Cell Physiol. 2022, 322, C913–C926. [Google Scholar] [CrossRef]
- Oudbier, S.J.; Goh, J.; Looijaard, S.; Reijnierse, E.M.; Meskers, C.G.M.; Maier, A.B. Pathophysiological Mechanisms Explaining the Association Between Low Skeletal Muscle Mass and Cognitive Function. J. Gerontol. A Biol. Sci. Med. Sci. 2022, 77, 1959–1968. [Google Scholar] [CrossRef]
- Alizadeh Pahlavani, H.; Laher, I.; Knechtle, B.; Zouhal, H. Exercise and mitochondrial mechanisms in patients with sarcopenia. Front. Physiol. 2022, 13, 1040381. [Google Scholar] [CrossRef]
- Saoji, M.; Petersen, C.E.; Sen, A.; Tripoli, B.A.; Smyth, J.T.; Cox, R.T. Reduction of Drosophila Mitochondrial RNase P in Skeletal and Heart Muscle Causes Muscle Degeneration, Cardiomyopathy, and Heart Arrhythmia. Front. Cell Dev. Biol. 2022, 10, 788516. [Google Scholar] [CrossRef]
- Xu, H.; Ranjit, R.; Richardson, A.; Van Remmen, H. Muscle mitochondrial catalase expression prevents neuromuscular junction disruption, atrophy, and weakness in a mouse model of accelerated sarcopenia. J. Cachexia Sarcopenia Muscle 2021, 12, 1582–1596. [Google Scholar] [CrossRef]
- Jiao, W.; Hu, F.; Li, J.; Song, J.; Liang, J.; Li, L.; Song, Y.; Chen, Z.; Li, Q.; Ke, L. Qiangji Jianli Decoction promotes mitochondrial biogenesis in skeletal muscle of myasthenia gravis rats via AMPK/PGC-1α signaling pathway. Biomed. Pharmacother. 2020, 129, 110482. [Google Scholar] [CrossRef]
- Song, J.; Lei, X.; Jiao, W.; Song, Y.; Chen, W.; Li, J.; Chen, Z. Effect of Qiangji Jianli decoction on mitochondrial respiratory chain activity and expression of mitochondrial fusion and fission proteins in myasthenia gravis rats. Sci. Rep. 2018, 8, 8623. [Google Scholar] [CrossRef]
- Li, L.; Cai, D.; Zhong, H.; Liu, F.; Jiang, Q.; Liang, J.; Li, P.; Song, Y.; Ji, A.; Jiao, W.; et al. Mitochondrial dynamics and biogenesis indicators may serve as potential biomarkers for diagnosis of myasthenia gravis. Exp. Ther. Med. 2022, 23, 307. [Google Scholar] [CrossRef]
- Li, L.; Huang, T.; Yang, J.; Yang, P.; Lan, H.; Liang, J.; Cai, D.; Zhong, H.; Jiao, W.; Song, Y. PINK1/Parkin pathway-mediated mitophagy by AS-IV to explore the molecular mechanism of muscle cell damage. Biomed. Pharmacother. 2023, 161, 114533. [Google Scholar] [CrossRef]
- Pereyra, A.S.; Lin, C.T.; Sanchez, D.M.; Laskin, J.; Spangenburg, E.E.; Neufer, P.D.; Fisher-Wellman, K.; Ellis, J.M. Skeletal muscle undergoes fiber type metabolic switch without myosin heavy chain switch in response to defective fatty acid oxidation. Mol. Metab. 2022, 59, 101456. [Google Scholar] [CrossRef]
- Salles, J.; Chanet, A.; Guillet, C.; Vaes, A.M.; Brouwer-Brolsma, E.M.; Rocher, C.; Giraudet, C.; Patrac, V.; Meugnier, E.; Montaurier, C.; et al. Vitamin D status modulates mitochondrial oxidative capacities in skeletal muscle: Role in sarcopenia. Commun. Biol. 2022, 5, 1288. [Google Scholar] [CrossRef]
- Chatzinikita, E.; Maridaki, M.; Palikaras, K.; Koutsilieris, M.; Philippou, A. The Role of Mitophagy in Skeletal Muscle Damage and Regeneration. Cells 2023, 12, 716. [Google Scholar] [CrossRef]
- Gambarotto, L.; Metti, S.; Chrisam, M.; Cerqua, C.; Sabatelli, P.; Armani, A.; Zanon, C.; Spizzotin, M.; Castagnaro, S.; Strappazzon, F.; et al. Ambra1 deficiency impairs mitophagy in skeletal muscle. J. Cachexia Sarcopenia Muscle 2022, 13, 2211–2224. [Google Scholar] [CrossRef]
- Huot, J.R.; Pin, F.; Chatterjee, R.; Bonetto, A. PGC1α overexpression preserves muscle mass and function in cisplatin-induced cachexia. J. Cachexia Sarcopenia Muscle 2022, 13, 2480–2491. [Google Scholar] [CrossRef]
- Europa, T.A.; Nel, M.; Lebeko, M.R.; Heckmann, J.M. Mitochondrial bioenergetics in ocular fibroblasts of two myasthenia gravis cases. IBRO Neurosci. Rep. 2022, 12, 297–302. [Google Scholar] [CrossRef]
- López-Bellón, S.; Rodríguez-López, S.; González-Reyes, J.A.; Burón, M.I.; de Cabo, R.; Villalba, J.M. CYB5R3 overexpression preserves skeletal muscle mitochondria and autophagic signaling in aged transgenic mice. GeroScience 2022, 44, 2223–2241. [Google Scholar] [CrossRef]
- Wang, D.; Jiang, D.M.; Yu, R.R.; Zhang, L.L.; Liu, Y.Z.; Chen, J.X.; Chen, H.C.; Liu, Y.P. The Effect of Aerobic Exercise on the Oxidative Capacity of Skeletal Muscle Mitochondria in Mice with Impaired Glucose Tolerance. J. Diabetes Res. 2022, 2022, 3780156. [Google Scholar] [CrossRef]
- Qualls, A.E.; Southern, W.M.; Call, J.A. Mitochondria-cytokine crosstalk following skeletal muscle injury and disuse: A mini-review. Am. J. Physiol. Cell Physiol. 2021, 320, C681–C688. [Google Scholar] [CrossRef]
- Wesolowski, L.T.; Semanchik, P.L.; White-Springer, S.H. Beyond antioxidants: Selenium and skeletal muscle mitochondria. Front. Vet. Sci. 2022, 9, 1011159. [Google Scholar] [CrossRef] [PubMed]
- Hong, X.; Isern, J.; Campanario, S.; Perdiguero, E.; Ramírez-Pardo, I.; Segalés, J.; Hernansanz-Agustín, P.; Curtabbi, A.; Deryagin, O.; Pollán, A.; et al. Mitochondrial dynamics maintain muscle stem cell regenerative competence throughout adult life by regulating metabolism and mitophagy. Cell Stem Cell 2022, 29, 1298–1314.e1210. [Google Scholar] [CrossRef]
- Yan, Y.; Li, M.; Lin, J.; Ji, Y.; Wang, K.; Yan, D.; Shen, Y.; Wang, W.; Huang, Z.; Jiang, H.; et al. Adenosine monophosphate activated protein kinase contributes to skeletal muscle health through the control of mitochondrial function. Front. Pharmacol. 2022, 13, 947387. [Google Scholar] [CrossRef] [PubMed]
- Alway, S.E.; Paez, H.G.; Pitzer, C.R.; Ferrandi, P.J.; Khan, M.M.; Mohamed, J.S.; Carson, J.A.; Deschenes, M.R. Mitochondria transplant therapy improves regeneration and restoration of injured skeletal muscle. J. Cachexia Sarcopenia Muscle 2023, 14, 493–507. [Google Scholar] [CrossRef]
- Zhang, R.F.; Zeng, M.; Lv, N.; Wang, L.M.; Yang, Q.Y.; Gan, J.L.; Li, H.H.; Yu, B.; Jiang, X.J.; Yang, L. Ferroptosis in neurodegenerative diseases: Inhibitors as promising candidate mitigators. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 46–65. [Google Scholar] [CrossRef]
- Tong, X.; Tang, R.; Xiao, M.; Xu, J.; Wang, W.; Zhang, B.; Liu, J.; Yu, X.; Shi, S. Targeting cell death pathways for cancer therapy: Recent developments in necroptosis, pyroptosis, ferroptosis, and cuproptosis research. J. Hematol. Oncol. 2022, 15, 174. [Google Scholar] [CrossRef]
- Fang, X.; Ardehali, H.; Min, J.; Wang, F. The molecular and metabolic landscape of iron and ferroptosis in cardiovascular disease. Nat. Rev. Cardiol. 2023, 20, 7–23. [Google Scholar] [CrossRef]
- Galaris, D.; Pantopoulos, K. Oxidative stress and iron homeostasis: Mechanistic and health aspects. Crit. Rev. Clin. Lab. Sci. 2008, 45, 1–23. [Google Scholar] [CrossRef]
- Huang, Y.; Wu, B.; Shen, D.; Chen, J.; Yu, Z.; Chen, C. Ferroptosis in a sarcopenia model of senescence accelerated mouse prone 8 (SAMP8). Int. J. Biol. Sci. 2021, 17, 151–162. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Li, R.; Peng, Y.; Wang, Z.; Huang, J.; Meng, H.; Min, J.; Wang, F.; Ma, Q. ACSL4 contributes to ferroptosis-mediated rhabdomyolysis in exertional heat stroke. J. Cachexia Sarcopenia Muscle 2022, 13, 1717–1730. [Google Scholar] [CrossRef]
- Wang, D.; Liang, W.; Huo, D.; Wang, H.; Wang, Y.; Cong, C.; Zhang, C.; Yan, S.; Gao, M.; Su, X.; et al. SPY1 inhibits neuronal ferroptosis in amyotrophic lateral sclerosis by reducing lipid peroxidation through regulation of GCH1 and TFR1. Cell Death Differ. 2023, 30, 369–382. [Google Scholar] [CrossRef]
- La Rosa, P.; Petrillo, S.; Turchi, R.; Berardinelli, F.; Schirinzi, T.; Vasco, G.; Lettieri-Barbato, D.; Fiorenza, M.T.; Bertini, E.S.; Aquilano, K.; et al. The Nrf2 induction prevents ferroptosis in Friedreich’s Ataxia. Redox Biol. 2021, 38, 101791. [Google Scholar] [CrossRef]
- Huang, P. The relationship between serum iron levels and AChR-Ab and IL-6 in patients with myasthenia gravis. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 98–102. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, J.; Song, C.; Sun, J.; Liu, W. Association Between Serum Iron Status and Muscle Mass in Adults: Results From NHANES 2015-2018. Front. Nutr. 2022, 9, 941093. [Google Scholar] [CrossRef]
- Li, K.; Hou, L.; Tan, Y.; Huang, Y.; Shi, J.; Han, J.; Yan, J.; Guan, Y.; Cui, L. Iron metabolism in non-anemic myasthenia gravis patients: A cohort study. J. Neuroimmunol. 2023, 375, 578015. [Google Scholar] [CrossRef]
- Scaramellini, N.; Fischer, D.; Agarvas, A.R.; Motta, I.; Muckenthaler, M.U.; Mertens, C. Interpreting Iron Homeostasis in Congenital and Acquired Disorders. Pharmaceuticals 2023, 16, 329. [Google Scholar] [CrossRef]
- Gao, J.; Zhou, Q.; Wu, D.; Chen, L. Mitochondrial iron metabolism and its role in diseases. Clin. Chim. Acta 2021, 513, 6–12. [Google Scholar] [CrossRef]
- Duan, G.; Li, J.; Duan, Y.; Zheng, C.; Guo, Q.; Li, F.; Zheng, J.; Yu, J.; Zhang, P.; Wan, M.; et al. Mitochondrial Iron Metabolism: The Crucial Actors in Diseases. Molecules 2022, 28, 29. [Google Scholar] [CrossRef]
- Rouault, T.A. Mitochondrial iron overload: Causes and consequences. Curr. Opin. Genet. Dev. 2016, 38, 31–37. [Google Scholar] [CrossRef]
- Sung, H.K.; Murugathasan, M.; Abdul-Sater, A.A.; Sweeney, G. Autophagy deficiency exacerbates iron overload induced reactive oxygen species production and apoptotic cell death in skeletal muscle cells. Cell Death Dis. 2023, 14, 252. [Google Scholar] [CrossRef]
- Zhang, Q.; Qu, H.; Chen, Y.; Luo, X.; Chen, C.; Xiao, B.; Ding, X.; Zhao, P.; Lu, Y.; Chen, A.F.; et al. Atorvastatin Induces Mitochondria-Dependent Ferroptosis via the Modulation of Nrf2-xCT/GPx4 Axis. Front. Cell Dev. Biol. 2022, 10, 806081. [Google Scholar] [CrossRef]
- Ding, H.; Chen, S.; Pan, X.; Dai, X.; Pan, G.; Li, Z.; Mai, X.; Tian, Y.; Zhang, S.; Liu, B.; et al. Transferrin receptor 1 ablation in satellite cells impedes skeletal muscle regeneration through activation of ferroptosis. J. Cachexia Sarcopenia Muscle 2021, 12, 746–768. [Google Scholar] [CrossRef] [PubMed]
- Alves, F.M.; Kysenius, K.; Caldow, M.K.; Hardee, J.P.; Crouch, P.J.; Ayton, S.; Bush, A.I.; Lynch, G.S.; Koopman, R. Iron accumulation in skeletal muscles of old mice is associated with impaired regeneration after ischaemia-reperfusion damage. J. Cachexia Sarcopenia Muscle 2021, 12, 476–492. [Google Scholar] [CrossRef] [PubMed]
- Menon, D.; Barnett, C.; Bril, V. Novel Treatments in Myasthenia Gravis. Front. Neurol. 2020, 11, 538. [Google Scholar] [CrossRef]
- Al-Lawati, H.; Aliabadi, H.M.; Makhmalzadeh, B.S.; Lavasanifar, A. Nanomedicine for immunosuppressive therapy: Achievements in pre-clinical and clinical research. Expert Opin. Drug Deliv. 2018, 15, 397–418. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Du, T.; Yang, C.L.; Li, T.; Li, X.L.; Liu, W.; Zhang, P.; Dong, J.; Si, W.Y.; Duan, R.S.; et al. Extracellular vesicles encapsulated with caspase-1 inhibitor ameliorate experimental autoimmune myasthenia gravis through targeting macrophages. J. Control. Release 2023, 364, 458–472. [Google Scholar] [CrossRef] [PubMed]
- Azharuddin, M.; Zhu, G.H.; Sengupta, A.; Hinkula, J.; Slater, N.K.H.; Patra, H.K. Nano toolbox in immune modulation and nanovaccines. Trends Biotechnol. 2022, 40, 1195–1212. [Google Scholar] [CrossRef] [PubMed]
- Peng, G.; Fadeel, B. Understanding the bidirectional interactions between two-dimensional materials, microorganisms, and the immune system. Adv. Drug Deliv. Rev. 2022, 188, 114422. [Google Scholar] [CrossRef]
- Gomes, G.S.; Frank, L.A.; Contri, R.V.; Longhi, M.S.; Pohlmann, A.R.; Guterres, S.S. Nanotechnology-based alternatives for the topical delivery of immunosuppressive agents in psoriasis. Int. J. Pharm. 2023, 631, 122535. [Google Scholar] [CrossRef] [PubMed]
- Turjeman, K.; Yanay, N.; Elbaz, M.; Bavli, Y.; Gross, M.; Rabie, M.; Barenholz, Y.; Nevo, Y. Liposomal steroid nano-drug is superior to steroids as-is in mdx mouse model of Duchenne muscular dystrophy. Nanomedicine 2019, 16, 34–44. [Google Scholar] [CrossRef]
- Ahmed, Z.; Qaisar, R. Nanomedicine for Treating Muscle Dystrophies: Opportunities, Challenges, and Future Perspectives. Int. J. Mol. Sci. 2022, 23, 12039. [Google Scholar] [CrossRef]
- Chen, B.Q.; Zhao, Y.; Zhang, Y.; Pan, Y.J.; Xia, H.Y.; Kankala, R.K.; Wang, S.B.; Liu, G.; Chen, A.Z. Immune-regulating camouflaged nanoplatforms: A promising strategy to improve cancer nano-immunotherapy. Bioact. Mater. 2023, 21, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Steffens, R.C.; Wagner, E. Directing the Way-Receptor and Chemical Targeting Strategies for Nucleic Acid Delivery. Pharm. Res. 2023, 40, 47–76. [Google Scholar] [CrossRef] [PubMed]
- Bellanti, F.; Lo Buglio, A.; Vendemiale, G. Muscle Delivery of Mitochondria-Targeted Drugs for the Treatment of Sarcopenia: Rationale and Perspectives. Pharmaceutics 2022, 14, 2588. [Google Scholar] [CrossRef] [PubMed]
- Pin, F.; Huot, J.R.; Bonetto, A. The Mitochondria-Targeting Agent MitoQ Improves Muscle Atrophy, Weakness and Oxidative Metabolism in C26 Tumor-Bearing Mice. Front. Cell Dev. Biol. 2022, 10, 861622. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Perumal, E.; Bi, X.; Wang, Y.; Ding, W. Potential mechanisms of uremic muscle wasting and the protective role of the mitochondria-targeted antioxidant Mito-TEMPO. Int. Urol. Nephrol. 2020, 52, 1551–1561. [Google Scholar] [CrossRef]
- van de Weijer, T.; Phielix, E.; Bilet, L.; Williams, E.G.; Ropelle, E.R.; Bierwagen, A.; Livingstone, R.; Nowotny, P.; Sparks, L.M.; Paglialunga, S.; et al. Evidence for a direct effect of the NAD+ precursor acipimox on muscle mitochondrial function in humans. Diabetes 2015, 64, 1193–1201. [Google Scholar] [CrossRef]
- Pirinen, E.; Cantó, C.; Jo, Y.S.; Morato, L.; Zhang, H.; Menzies, K.J.; Williams, E.G.; Mouchiroud, L.; Moullan, N.; Hagberg, C.; et al. Pharmacological Inhibition of poly(ADP-ribose) polymerases improves fitness and mitochondrial function in skeletal muscle. Cell Metab. 2014, 19, 1034–1041. [Google Scholar] [CrossRef] [PubMed]
- Sena Ozbay, H.; Yabanoglu-Ciftci, S.; Baysal, I.; Gultekinoglu, M.; Can Eylem, C.; Ulubayram, K.; Nemutlu, E.; Topaloglu, R.; Ozaltin, F. Mitochondria-targeted CoQ10 loaded PLGA-b-PEG-TPP nanoparticles: Their effects on mitochondrial functions of COQ8B-/- HK-2 cells. Eur. J. Pharm. Biopharm. 2022, 173, 22–33. [Google Scholar] [CrossRef]
- Faria, R.; Boisguérin, P.; Sousa, Â.; Costa, D. Delivery Systems for Mitochondrial Gene Therapy: A Review. Pharmaceutics 2023, 15, 572. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Ling, L.; Lu, J.; Jiang, F.; Sun, J.; Zhang, Z.; Huang, Y.; Liu, X.; Zhu, Y.; Fu, X.; et al. Reactive oxygen species-responsive mitochondria-targeted liposomal quercetin attenuates retinal ischemia-reperfusion injury via regulating SIRT1/FOXO3A and p38 MAPK signaling pathways. Bioeng. Transl. Med. 2023, 8, e10460. [Google Scholar] [CrossRef]
- Liu, X.; Gao, J.; Yan, Y.; Georgiou, E.A.; Lou, J.; Feng, M.; Zhang, X.; Gao, F.; Liu, J.; Kostakis, I.K.; et al. Mitochondria-Targeted Triphenylphosphonium-Hydroxytyrosol Prevents Lipotoxicity-Induced Endothelial Injury by Enhancing Mitochondrial Function and Redox Balance via Promoting FoxO1 and Nrf2 Nuclear Translocation and Suppressing Inflammation via Inhibiting p38/NF-кB Pathway. Antioxidants 2023, 12, 175. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yao, Z.; Zhang, X.; Li, J.; Huang, C.; Ouyang, Y.; Qian, Y.; Fan, C. Energy-Supporting Enzyme-Mimic Nanoscaffold Facilitates Tendon Regeneration Based on a Mitochondrial Protection and Microenvironment Remodeling Strategy. Adv. Sci. 2022, 9, e2202542. [Google Scholar] [CrossRef] [PubMed]
- Guglielmi, V.; Carton, F.; Vattemi, G.; Arpicco, S.; Stella, B.; Berlier, G.; Marengo, A.; Boschi, F.; Malatesta, M. Uptake and intracellular distribution of different types of nanoparticles in primary human myoblasts and myotubes. Int. J. Pharm. 2019, 560, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Van Steenbergen, V.; Lavoie-Cardinal, F.; Kazwiny, Y.; Decet, M.; Martens, T.; Verstreken, P.; Boesmans, W.; De Koninck, P.; Vanden Berghe, P. Nano-positioning and tubulin conformation contribute to axonal transport regulation of mitochondria along microtubules. Proc. Natl. Acad. Sci. USA 2022, 119, e2203499119. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.; Qin, X.; Yang, Z.; Song, S.; Liu, X.; Wu, C.; Qian, J.; Huang, X.; Zhang, Y.; He, W. Engineered a dual-targeting HA-TPP/A nanoparticle for combination therapy against KRAS-TP53 co-mutation in gastrointestinal cancers. Bioact. Mater. 2024, 32, 277–291. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zheng, F.; Liu, K.; Liu, S.; Xiao, T.; Zhu, Y.; Xu, L. Mitochondria-Targeting Polymer Micelles in Stepwise Response Releasing Gemcitabine and Destroying the Mitochondria and Nucleus for Combined Antitumor Chemotherapy. Int. J. Mol. Sci. 2022, 23, 12624. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Lee, G.H.; Yoo, K.H.; Khang, D. Overcoming multidrug-resistant lung cancer by mitochondrial-associated ATP inhibition using nanodrugs. J. Nanobiotechnol. 2023, 21, 12. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.C.; Chang, H.S.; Yeh, C.Y.; Chang, H.J.; Cheng, W.L.; Lin, T.T.; Liu, C.S.; Chen, S.T. Regulation of mitochondrial fusion and mitophagy by intra-tumoral delivery of membrane-fused mitochondria or Midiv-1 enhances sensitivity to doxorubicin in triple-negative breast cancer. Biomed. Pharmacother. 2022, 153, 113484. [Google Scholar] [CrossRef] [PubMed]
- Jaganjac, M.; Borovic Sunjic, S.; Zarkovic, N. Utilizing Iron for Targeted Lipid Peroxidation as Anticancer Option of Integrative Biomedicine: A Short Review of Nanosystems Containing Iron. Antioxidants 2020, 9, 191. [Google Scholar] [CrossRef]
- Sang, M.; Luo, R.; Bai, Y.; Dou, J.; Zhang, Z.; Liu, F.; Feng, F.; Xu, J.; Liu, W. Mitochondrial membrane anchored photosensitive nano-device for lipid hydroperoxides burst and inducing ferroptosis to surmount therapy-resistant cancer. Theranostics 2019, 9, 6209–6223. [Google Scholar] [CrossRef]
- Yang, C.; Han, M.; Li, R.; Zhou, L.; Zhang, Y.; Duan, L.; Su, S.; Li, M.; Wang, Q.; Chen, T.; et al. Curcumin Nanoparticles Inhibiting Ferroptosis for the Enhanced Treatment of Intracerebral Hemorrhage. Int. J. Nanomed. 2021, 16, 8049–8065. [Google Scholar] [CrossRef] [PubMed]
- Mavridi-Printezi, A.; Menichetti, A.; Mordini, D.; Amorati, R.; Montalti, M. Recent Applications of Melanin-like Nanoparticles as Antioxidant Agents. Antioxidants 2023, 12, 863. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, S.; Peng, J.; Cheng, S.; Zhang, Q.; Zhang, N.; Zhou, Z.; Zhang, Y.; Zhao, Y.; Liu, T. Biomimetic Nanozymes Suppressed Ferroptosis to Ameliorate Doxorubicin-Induced Cardiotoxicity via Synergetic Effect of Antioxidant Stress and GPX4 Restoration. Nutrients 2023, 15, 1090. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Chen, Y.; Guo, J.; Li, J.; Zhang, P.; Yang, H.; Rong, K.; Zhou, T.; Fu, J.; Zhao, J. Polydopamine Nanoparticles Targeting Ferroptosis Mitigate Intervertebral Disc Degeneration Via Reactive Oxygen Species Depletion, Iron Ions Chelation, and GPX4 Ubiquitination Suppression. Adv. Sci. 2023, 10, e2207216. [Google Scholar] [CrossRef] [PubMed]
- She, H.; Tan, L.; Du, Y.; Zhou, Y.; Guo, N.; Zhang, J.; Du, Y.; Wang, Y.; Wu, Z.; Ma, C.; et al. VDAC2 malonylation participates in sepsis-induced myocardial dysfunction via mitochondrial-related ferroptosis. Int. J. Biol. Sci. 2023, 19, 3143–3158. [Google Scholar] [CrossRef] [PubMed]
- Raimondo, T.M.; Mooney, D.J. Anti-inflammatory nanoparticles significantly improve muscle function in a murine model of advanced muscular dystrophy. Sci. Adv. 2021, 7, eabh3693. [Google Scholar] [CrossRef] [PubMed]
- Andrei, V.; Andrei, S.; Gal, A.F.; Rus, V.; Gherman, L.M.; Boșca, B.A.; Niculae, M.; Barabas, R.; Cadar, O.; Dinte, E.; et al. Immunomodulatory Effect of Novel Electrospun Nanofibers Loaded with Doxycycline as an Adjuvant Treatment in Periodontitis. Pharmaceutics 2023, 15, 707. [Google Scholar] [CrossRef]
- Lee, H.Y.; Kim, D.S.; Hwang, G.Y.; Lee, J.K.; Lee, H.L.; Jung, J.W.; Hwang, S.Y.; Baek, S.W.; Yoon, S.L.; Ha, Y.; et al. Multi-modulation of immune-inflammatory response using bioactive molecule-integrated PLGA composite for spinal fusion. Mater. Today Bio. 2023, 19, 100611. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Yada, E.; Terai, Y.; Takahashi, T.; Nakanishi, H.; Tanaka, H.; Akita, H.; Itaka, K. Comprehensive Evaluation of Lipid Nanoparticles and Polyplex Nanomicelles for Muscle-Targeted mRNA Delivery. Pharmaceutics 2023, 15, 2291. [Google Scholar] [CrossRef]
- Islam, M.R.; Patel, J.; Back, P.I.; Shmeeda, H.; Kallem, R.R.; Shudde, C.; Markiewski, M.; Putnam, W.C.; Gabizon, A.A.; La-Beck, N.M. Pegylated Liposomal Alendronate Biodistribution, Immune Modulation, and Tumor Growth Inhibition in a Murine Melanoma Model. Biomolecules 2023, 13, 1309. [Google Scholar] [CrossRef]
- Zhao, Y.P.; Han, J.F.; Zhang, F.Y.; Liao, T.T.; Na, R.; Yuan, X.F.; He, G.B.; Ye, W. Flexible nano-liposomes-based transdermal hydrogel for targeted delivery of dexamethasone for rheumatoid arthritis therapy. Drug Deliv. 2022, 29, 2269–2282. [Google Scholar] [CrossRef] [PubMed]
- Di Mauro, G.; Amoriello, R.; Lozano, N.; Carnasciali, A.; Guasti, D.; Becucci, M.; Cellot, G.; Kostarelos, K.; Ballerini, C.; Ballerini, L. Graphene Oxide Nanosheets Reduce Astrocyte Reactivity to Inflammation and Ameliorate Experimental Autoimmune Encephalomyelitis. ACS Nano 2023, 17, 1965–1978. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Huang, Y.; Kou, Q.; Lu, L.; Jiang, H.; Li, X.; Gui, R.; Huang, R.; Huang, X.; Ma, J.; et al. Study on the Role of an Erythrocyte Membrane-Coated Nanotheranostic System in Targeted Immune Regulation of Alzheimer’s Disease. Adv. Sci. 2023, 10, e2301361. [Google Scholar] [CrossRef] [PubMed]
- Raimondo, T.M.; Mooney, D.J. Functional muscle recovery with nanoparticle-directed M2 macrophage polarization in mice. Proc. Natl. Acad. Sci. USA 2018, 115, 10648–10653. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, Z.; Liu, Y.; Cao, X.; Li, F.; Ran, H.; Cao, Y.; Wu, C. ‘Mito-Bomb’: A novel mitochondria-targeting nanosystem for ferroptosis-boosted sonodynamic antitumor therapy. Drug Deliv. 2022, 29, 3111–3122. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Guo, J.; Wang, Y.; Xing, X.; Zhang, X.; Zhang, G.; Dong, Z. Acute myocardial infarction therapy using calycosin and tanshinone co-loaded mitochondria targeted lipid-polymer hybrid nano-system: Preparation, characterization, and anti myocardial infarction activity assessment. Biomed. Pharmacother. 2022, 155, 113650. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Song, Q.; Yu, R.; Wang, A.; Jiang, G.; Huang, Y.; Chen, J.; Xu, J.; Wang, D.; Chen, H.; et al. Nano-Brake Halts Mitochondrial Dysfunction Cascade to Alleviate Neuropathology and Rescue Alzheimer’s Cognitive Deficits. Adv. Sci. 2023, 10, e2204596. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Jin, F.; Liu, D.; Shu, G.; Wang, X.; Qi, J.; Sun, M.; Yang, P.; Jiang, S.; Ying, X.; et al. ROS-responsive nano-drug delivery system combining mitochondria-targeting ceria nanoparticles with atorvastatin for acute kidney injury. Theranostics 2020, 10, 2342–2357. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Wang, B.; Liu, Y.; Wan, Y.; Liu, Q.; Xu, H.; Liang, R.; Shi, Y.; Tu, P.; Wu, H.; et al. Mitochondria-targeting polydopamine-coated nanodrugs for effective photothermal- and chemo-synergistic therapies against lung cancer. Regen. Biomater. 2022, 9, rbac051. [Google Scholar] [CrossRef]
- Qin, Y.T.; Ma, Y.J.; Feng, Y.S.; He, X.W.; Li, W.Y.; Zhang, Y.K. Targeted Mitochondrial Fluorescence Imaging-Guided Tumor Antimetabolic Therapy with the Imprinted Polymer Nanomedicine Capable of Specifically Recognizing Dihydrofolate Reductase. ACS Appl. Mater. Interfaces 2021, 13, 40332–40341. [Google Scholar] [CrossRef]
- Li, J.; Fan, J.; Gao, Y.; Huang, S.; Huang, D.; Li, J.; Wang, X.; Santos, H.A.; Shen, P.; Xia, B. Porous Silicon Nanocarriers Boost the Immunomodulation of Mitochondria-Targeted Bovine Serum Albumins on Macrophage Polarization. ACS Nano 2023, 17, 1036–1053. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, J.; Liu, Y.; Liu, W.; Yu, G.; Huang, Y.; Yang, Y.; Chen, X.; Chen, T. Brain-Targeted Biomimetic Nanodecoys with Neuroprotective Effects for Precise Therapy of Parkinson’s Disease. ACS Cent. Sci. 2022, 8, 1336–1349. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hong, H.; Xue, J.; Luo, J.; Liu, Q.; Chen, X.; Pan, Y.; Zhou, J.; Liu, Z.; Chen, T. Near-Infrared Radiation-Assisted Drug Delivery Nanoplatform to Realize Blood-Brain Barrier Crossing and Protection for Parkinsonian Therapy. ACS Appl. Mater. Interfaces 2021, 13, 37746–37760. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Li, Z.; Liu, Y.; Ma, R.; Chen, X.; Liu, W.; Song, Y.; Zhang, Y.; Yu, G.; Wu, Z.; et al. “Swiss Army Knife” black phosphorus-based nanodelivery platform for synergistic antiparkinsonian therapy via remodeling the brain microenvironment. J. Control. Release 2023, 353, 752–766. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tan, Y.; Cheng, G.; Ni, Y.; Xie, A.; Zhu, X.; Yin, C.; Zhang, Y.; Chen, T. Customized Intranasal Hydrogel Delivering Methylene Blue Ameliorates Cognitive Dysfunction against Alzheimer’s Disease. Adv. Mater. 2024, e2307081. [Google Scholar] [CrossRef]
- Yao, S.; Wu, D.; Hu, X.; Chen, Y.; Fan, W.; Mou, X.; Cai, Y.; Yang, X. Platelet membrane-coated bio-nanoparticles of indocyanine green/elamipretide for NIR diagnosis and antioxidant therapy in acute kidney injury. Acta Biomater. 2023, 173, 482–497. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Chen, H.; Ren, Z.; Zhang, P.; Chen, J.; Jiang, S. Ultrasmall iron oxide nanoparticles induced ferroptosis via Beclin1/ATG5-dependent autophagy pathway. Nano Converg. 2021, 8, 10. [Google Scholar] [CrossRef]
- Liu, C.; Zou, Q.; Tang, H.; Liu, J.; Zhang, S.; Fan, C.; Zhang, J.; Liu, R.; Liu, Y.; Liu, R.; et al. Melanin nanoparticles alleviate sepsis-induced myocardial injury by suppressing ferroptosis and inflammation. Bioact. Mater. 2023, 24, 313–321. [Google Scholar] [CrossRef]
- Xie, D.M.; Zhong, Q.; Xu, X.; Li, Y.; Chen, S.; Li, M.; Peng, C. Alpha lipoic acid-loaded electrospun fibrous patch films protect heart in acute myocardial infarction mice by inhibiting oxidative stress. Int. J. Pharm. 2023, 632, 122581. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, D.; Yang, F.; Ye, C.; Chen, Z.; Chen, Y.; Yu, X.; Xie, J.; Dou, Y.; Chang, J. In Situ Self-Assembled Phytopolyphenol-Coordinated Intelligent Nanotherapeutics for Multipronged Management of Ferroptosis-Driven Alzheimer’s Disease. ACS Nano 2024, 18, 7890–7906. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, Z.; Wu, G.; Yin, L.; Xu, L.; Wang, N.; Peng, J. Apigenin-7-glucoside-loaded nanoparticle alleviates intestinal ischemia-reperfusion by ATF3/SLC7A11-mediated ferroptosis. J. Control. Release 2024, 366, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Mo, Y.; Duan, L.; Yang, Y.; Liu, W.; Zhang, Y.; Zhou, L.; Su, S.; Lo, P.C.; Cai, J.; Gao, L.; et al. Nanoparticles improved resveratrol brain delivery and its therapeutic efficacy against intracerebral hemorrhage. Nanoscale 2021, 13, 3827–3840. [Google Scholar] [CrossRef] [PubMed]
- Shan, X.; Li, J.; Liu, J.; Feng, B.; Zhang, T.; Liu, Q.; Ma, H.; Wu, H.; Wu, H. Targeting ferroptosis by poly(acrylic) acid coated Mn3O4 nanoparticles alleviates acute liver injury. Nat. Commun. 2023, 14, 7598. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.S.; Qin, C.; Dong, M.H.; Heming, M.; Zhou, L.Q.; Wang, W.; Cai, S.B.; You, Y.F.; Shang, K.; Xiao, J.; et al. B cell lineage reconstitution underlies CAR-T cell therapeutic efficacy in patients with refractory myasthenia gravis. EMBO Mol. Med. 2024, 16, 966–987. [Google Scholar] [CrossRef]
- Dalakas, M.C. Progress in the therapy of myasthenia gravis: Getting closer to effective targeted immunotherapies. Curr. Opin. Neurol. 2020, 33, 545–552. [Google Scholar] [CrossRef]
| Treatment Strategy | NCT Number | Drug | Actual Enrollment | Age | Phase | Status |
|---|---|---|---|---|---|---|
| Antagonize neonatal Fc receptor | NCT05681715 | Rozanolixizumab | 62 | ≥18 | Phase 3 | On going |
| NCT04951622 | Nipocalimab | 198 | ≥18 | Phase 3 | Recruiting | |
| NCT05265273 | Nipocalimab | 12 | 2~17 | Phase 2 Phase 3 | Recruiting | |
| NCT05403541 | Batoclimab | 240 | ≥18 | Phase 3 | Recruiting | |
| NCT04980495 | Efgartigimod | 69 | ≥18 | Phase 3 | On going | |
| NCT05374590 | Efgartigimod | 12 | 2~18 | Phase 2 Phase 3 | Recruiting | |
| NCT04833894 | Efgartigimod | 12 | 2~18 | Phase 2 Phase 3 | Recruiting | |
| NCT04818671 | Efgartigimod | 183 | ≥18 | Phase 3 | On going | |
| Inhibit complement | NCT06055959 | Zilucoplan | 8 | 12~17 | Phase 2 Phase 3 | Recruiting |
| NCT04225871 | Zilucoplan | 200 | ≥18 | Phase 3 | On going | |
| NCT05514873 | Zilucoplan | 26 | 18~85 | Phase 3 | On going | |
| NCT05644561 | Ravulizumab | 12 | Not limited | Phase 3 | Recruiting | |
| NCT05070858 | Pozelimab and Cemdisiran | 235 | ≥18 | Phase 3 | Recruiting | |
| NCT06282159 | DNTH103 | 60 | 18~75 | Phase 2 | Recruiting | |
| Target IL-6R | NCT05067348 | Tocilizumab | 64 | 18~80 | Phase 2 | Recruiting |
| NCT05716035 | Tocilizumab | 64 | 18~80 | Phase 2 Phase 3 | Recruiting | |
| NCT04963270 | Satralizumab | 185 | ≥12 | Phase 3 | Recruiting | |
| CAR-T cells | NCT05828225 | CD19 CAR-T cells | 9 | ≥18 | Phase 1 | Recruiting |
| NCT04146051 | Descartes-08 | 30 | ≥18 | Phase 2 | Recruiting | |
| Target B cells | NCT04524273 | Inebilizumab | 238 | ≥18 | Phase 3 | On going |
| NCT05737160 | Telitacicept | 100 | 18~80 | Phase 3 | Recruiting |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Yan, Z.; Song, Y.; Chen, T. Nanodrug Delivery Systems for Myasthenia Gravis: Advances and Perspectives. Pharmaceutics 2024, 16, 651. https://doi.org/10.3390/pharmaceutics16050651
Huang J, Yan Z, Song Y, Chen T. Nanodrug Delivery Systems for Myasthenia Gravis: Advances and Perspectives. Pharmaceutics. 2024; 16(5):651. https://doi.org/10.3390/pharmaceutics16050651
Chicago/Turabian StyleHuang, Jiayan, Zhao Yan, Yafang Song, and Tongkai Chen. 2024. "Nanodrug Delivery Systems for Myasthenia Gravis: Advances and Perspectives" Pharmaceutics 16, no. 5: 651. https://doi.org/10.3390/pharmaceutics16050651
APA StyleHuang, J., Yan, Z., Song, Y., & Chen, T. (2024). Nanodrug Delivery Systems for Myasthenia Gravis: Advances and Perspectives. Pharmaceutics, 16(5), 651. https://doi.org/10.3390/pharmaceutics16050651







