Advances in the Optimization of Fe Nanoparticles: Unlocking Antifungal Properties for Biomedical Applications
Abstract
1. Introduction
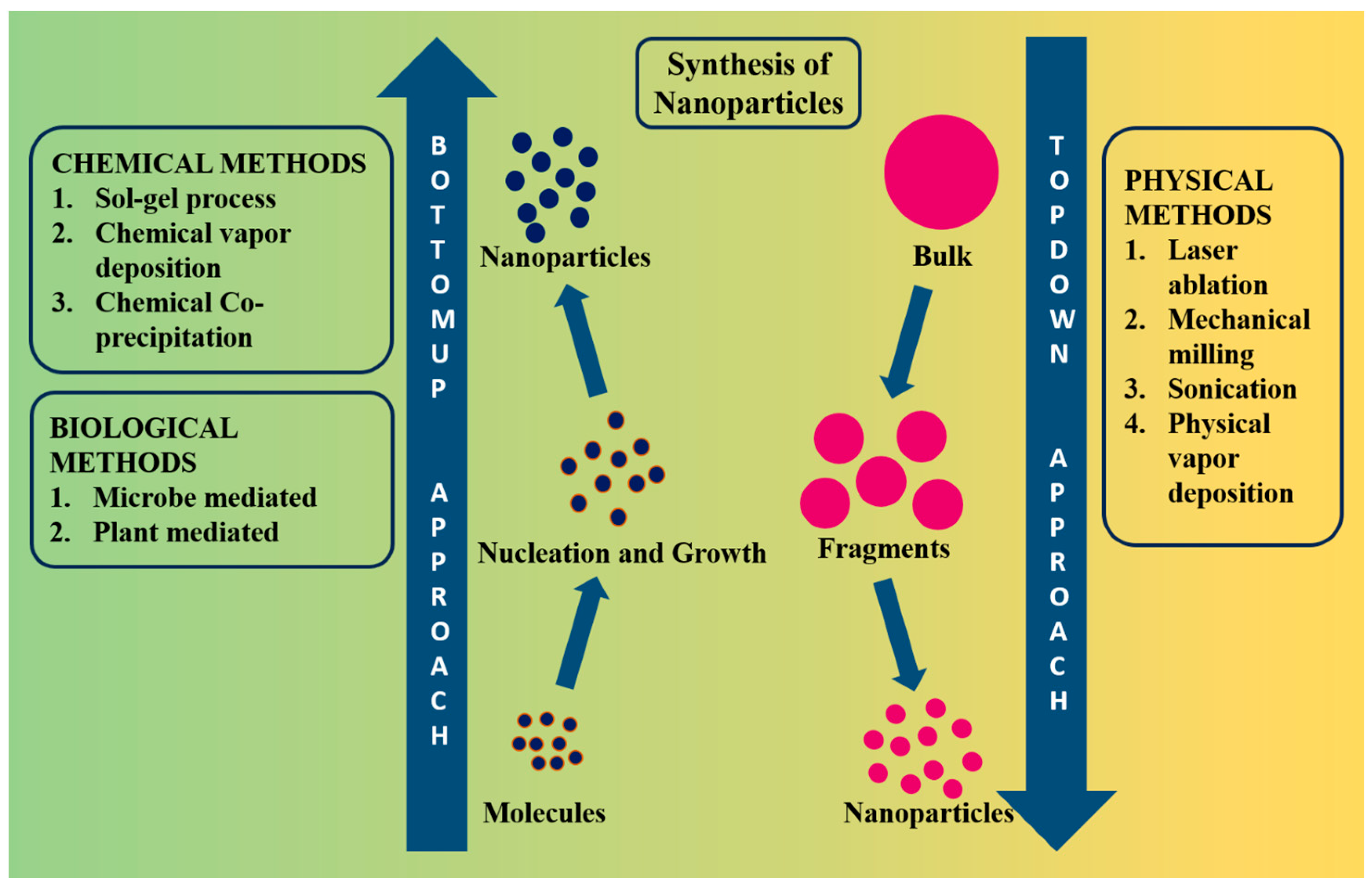
2. Various Synthesis Methods for Fe NPs
2.1. Chemical Methods
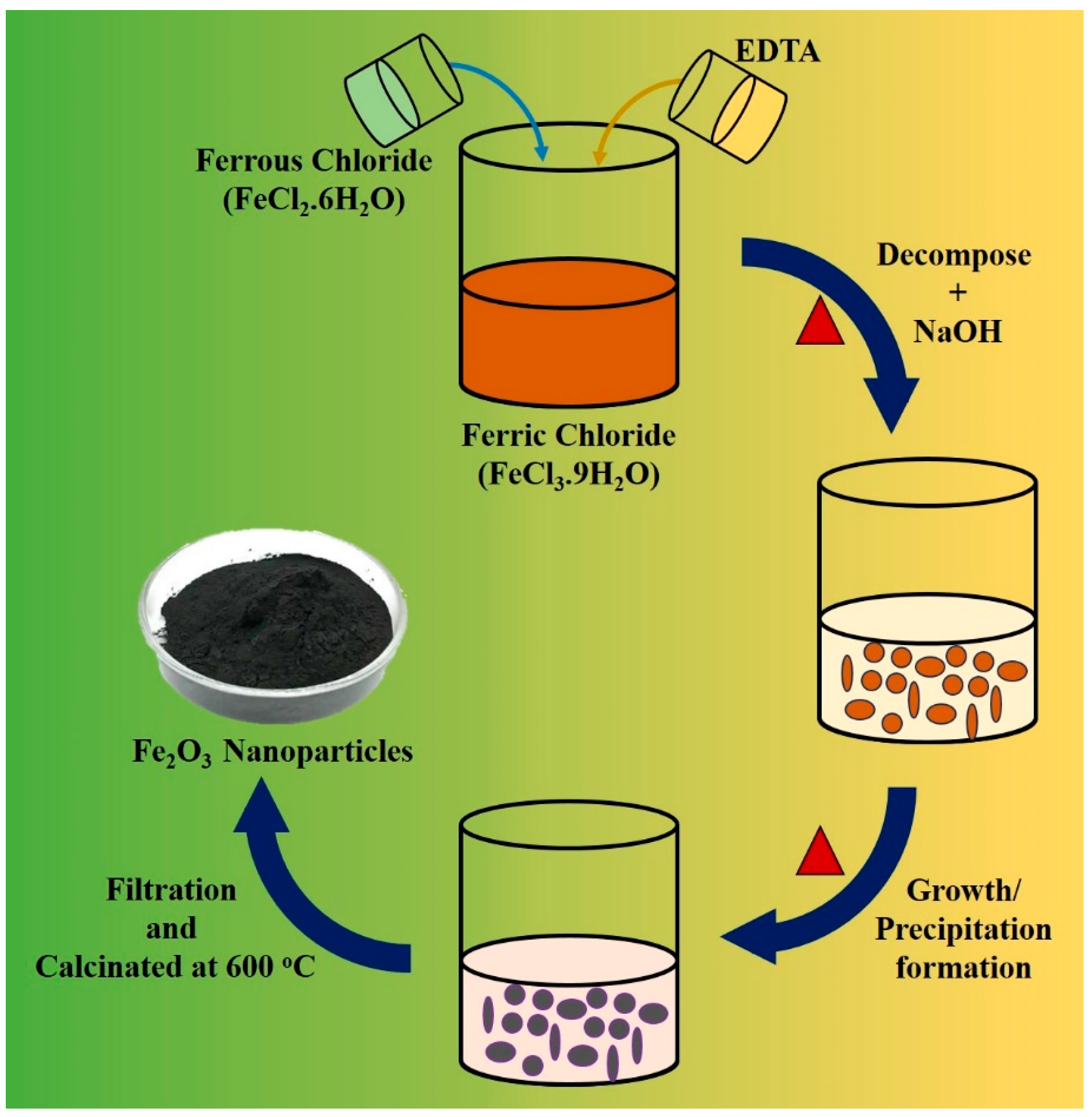
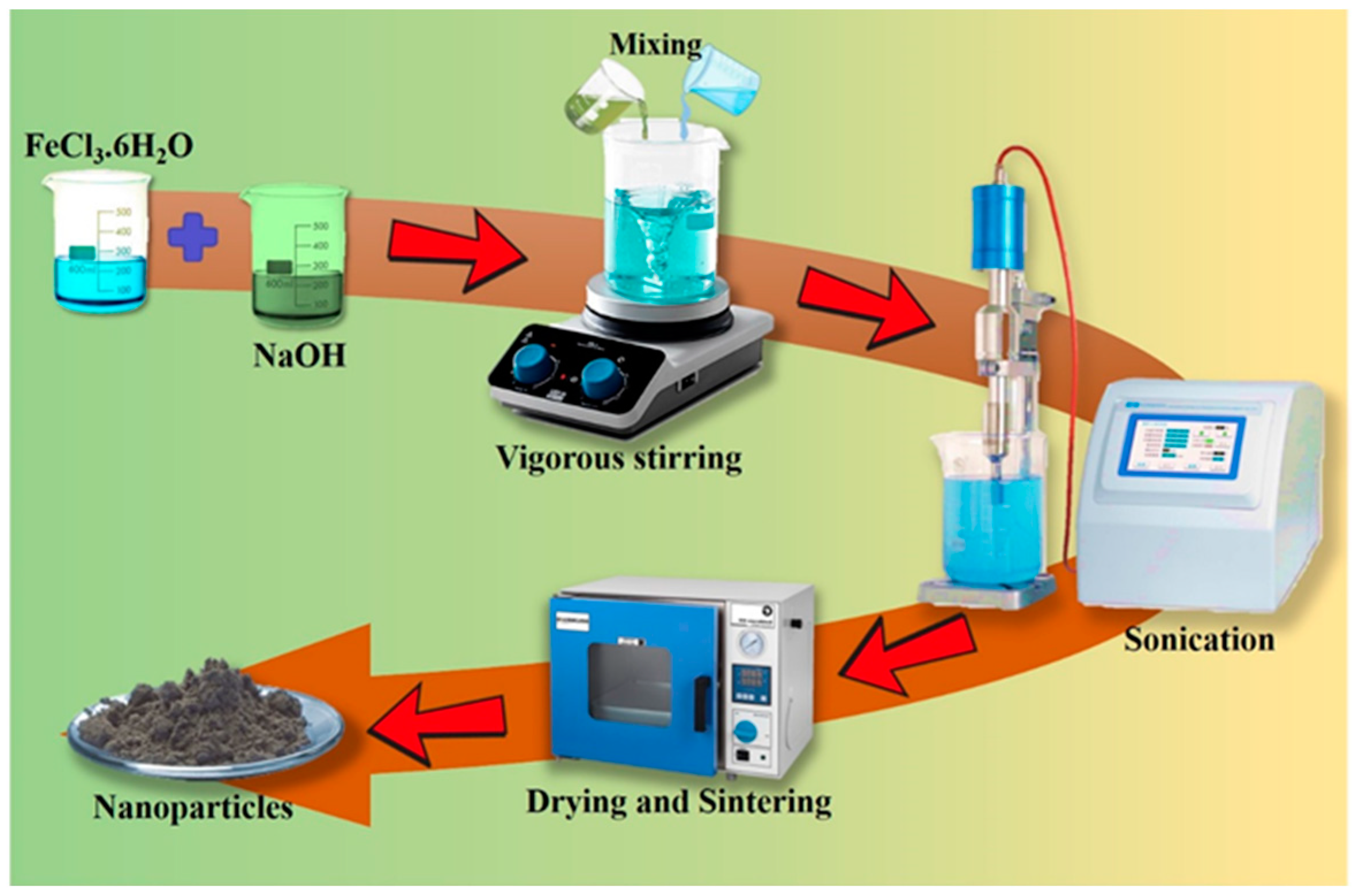
2.1.1. Co-Precipitation Methodology
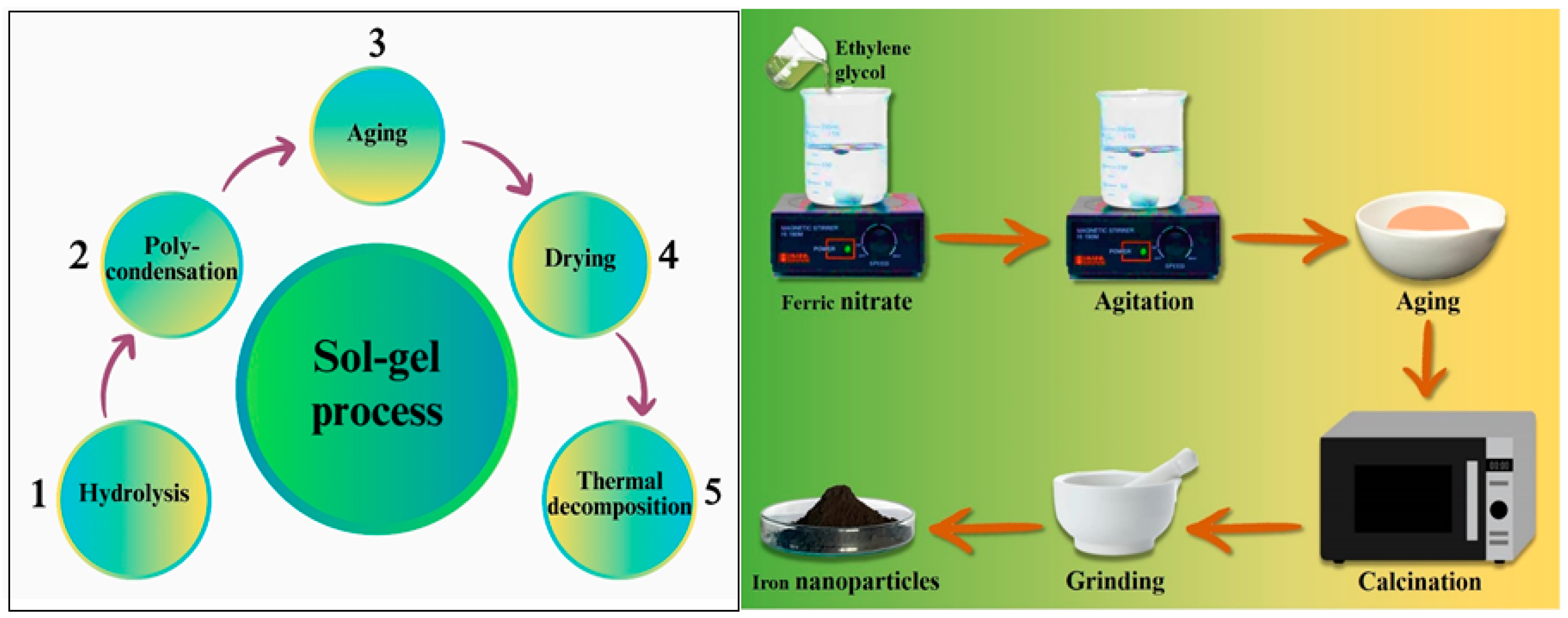
2.1.2. Sol–Gel Process
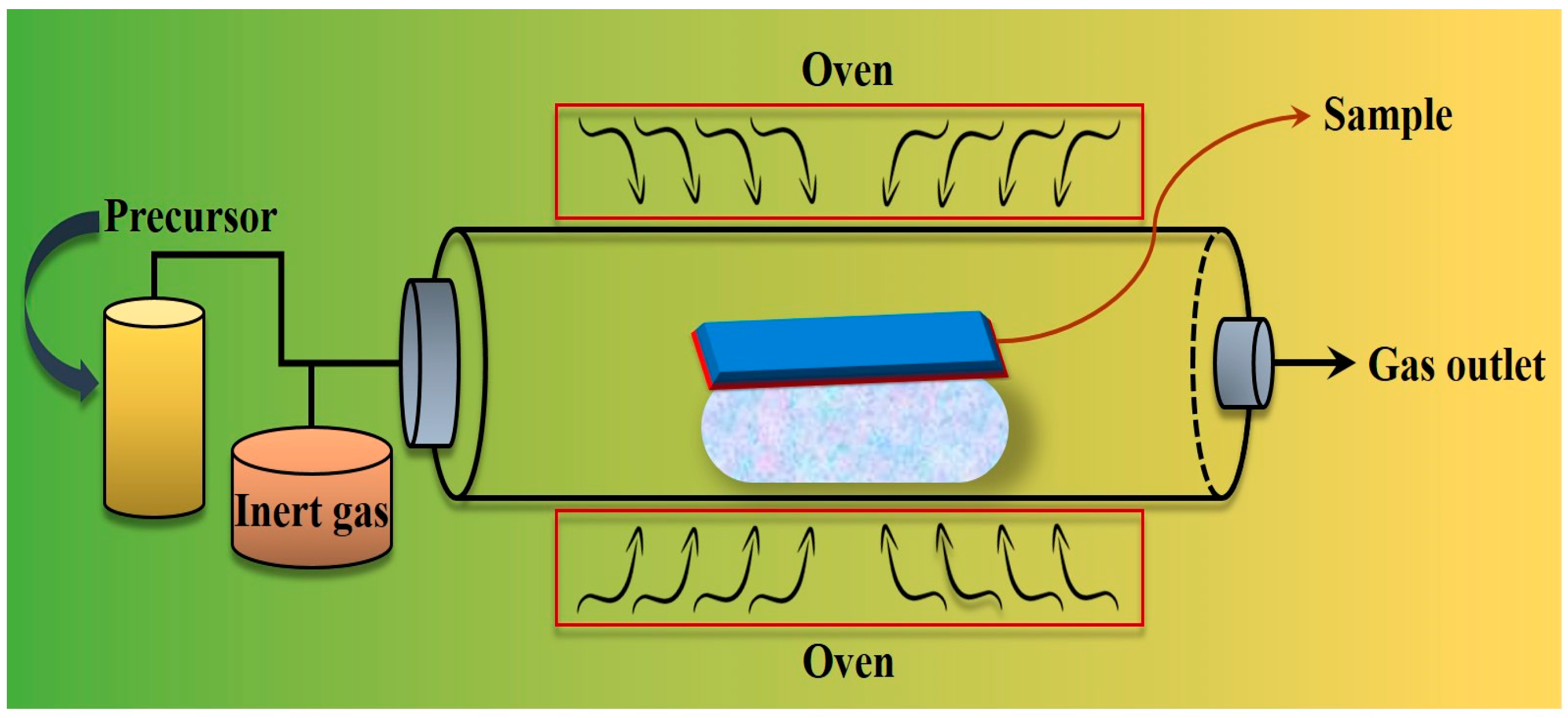
2.1.3. Chemical Vapor Deposition (CVD)
2.2. Physical Methods
2.2.1. Laser Ablation
2.2.2. Mechanical Milling
2.2.3. Sonication Approach
2.3. Biological Methods (Green Synthetic Method)
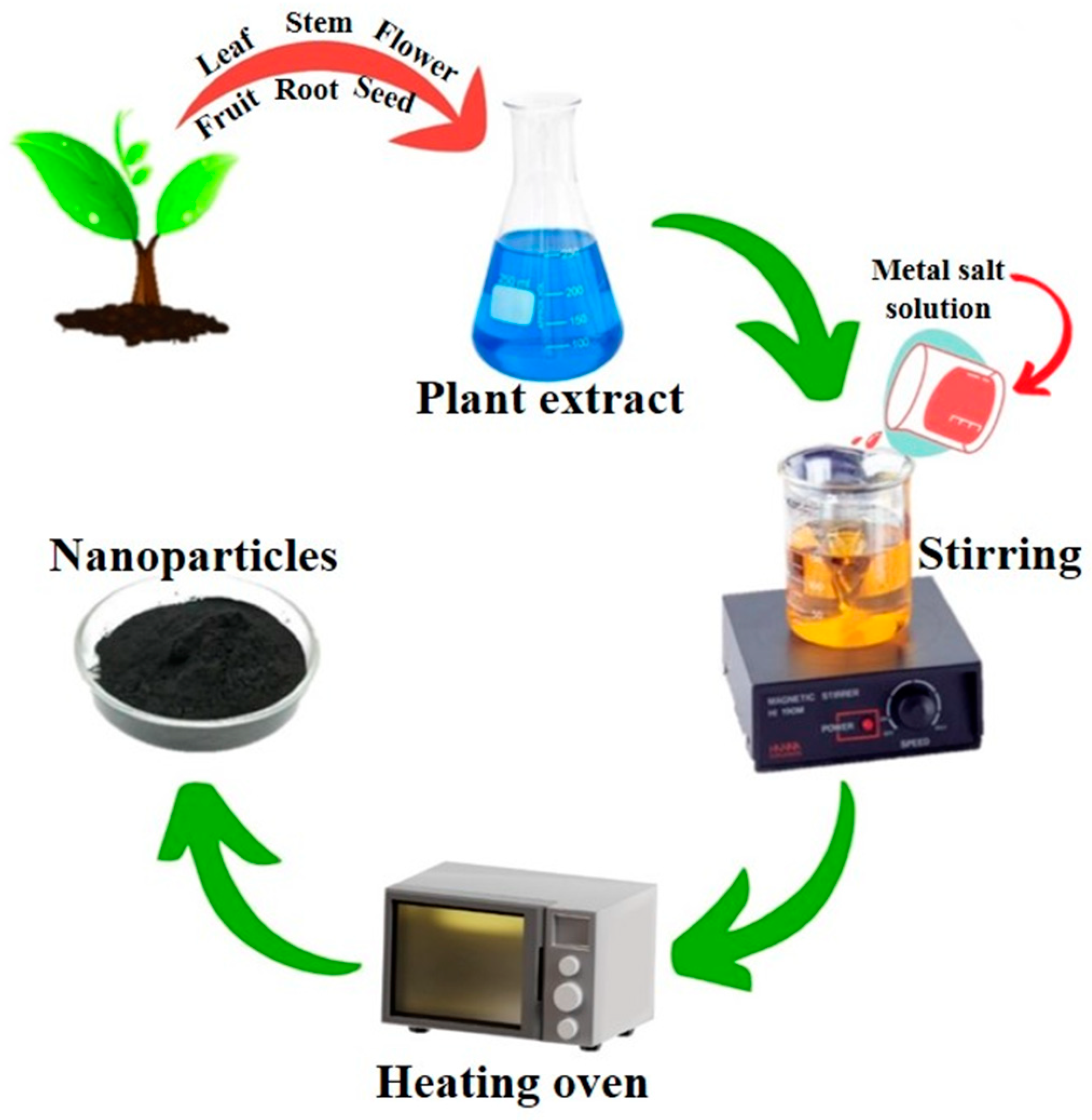
2.3.1. Plant-Mediated Synthesis of NPs
2.3.2. Microbe-Mediated Synthesis of NPs
3. Synthetic and Structural Insights of Fe NPs
4. Factors Influencing the Size, Shape, and Surface Properties of Fe NPs
5. Characterization Techniques for Analyzing the Physiochemical Properties of Fe NPs
5.1. X-ray-Based Techniques (XRD)
5.2. UV–Visible Spectroscopy
5.3. Dynamic Light Scattering (DLS)
5.4. Fourier-Transform Infrared Spectroscopy (FT-IR)
5.5. Scanning Electron Microscopy (SEM)—Energy Dispersive X-ray Spectroscopy (EDX)
6. Antifungal Mechanism of Fe NPs
7. Optimization Strategies for Enhancing Antifungal Properties
8. Antifungal, Biocompatibility and Toxicity Considerations
8.1. Antifungal Activity of Fe NPs
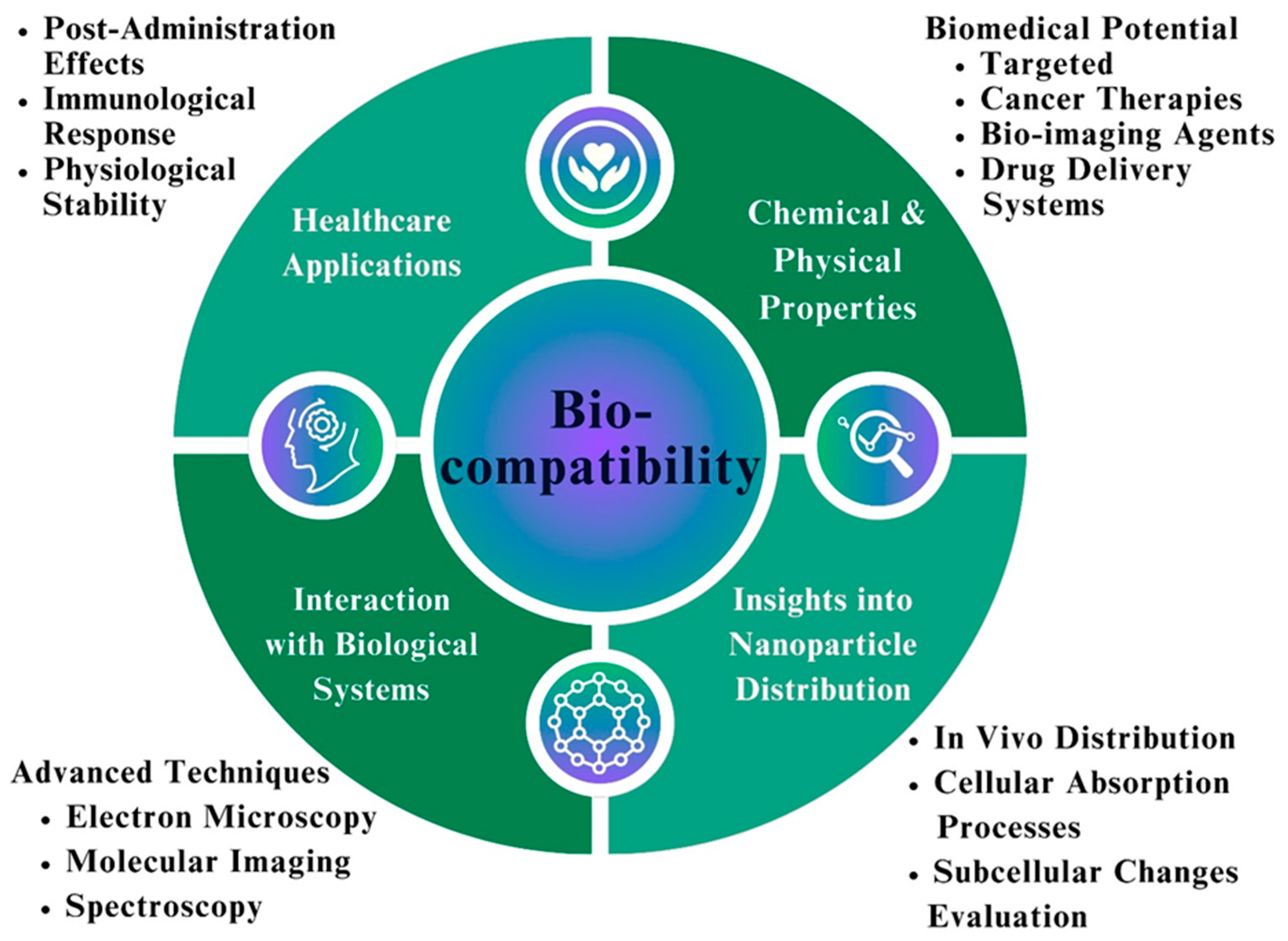
8.2. Assessment of the Biocompatibility of Fe NPs
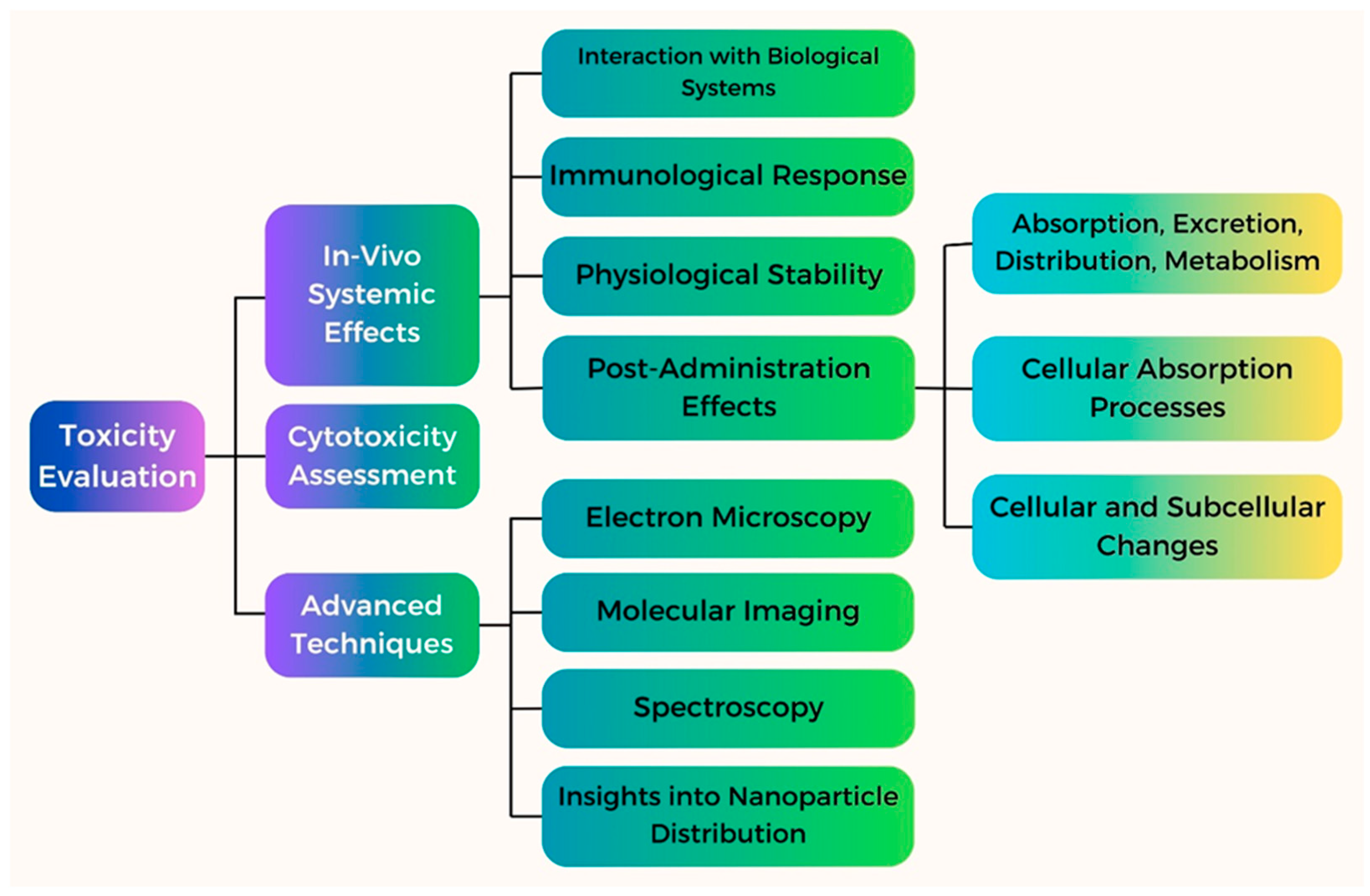
8.3. Assessment of Toxicity of Fe NPs and Strategies to Mitigate Risks
8.4. In Vitro and In Vivo Studies on the Biocompatibility and Toxicity of Fe NPs
9. Applications of Optimized Fe NPs in Biomedicine
9.1. Antifungal Coatings for Medical Devices and implants
9.2. Fe NPs as Targeted Drug Delivery Systems for Antifungal Agents
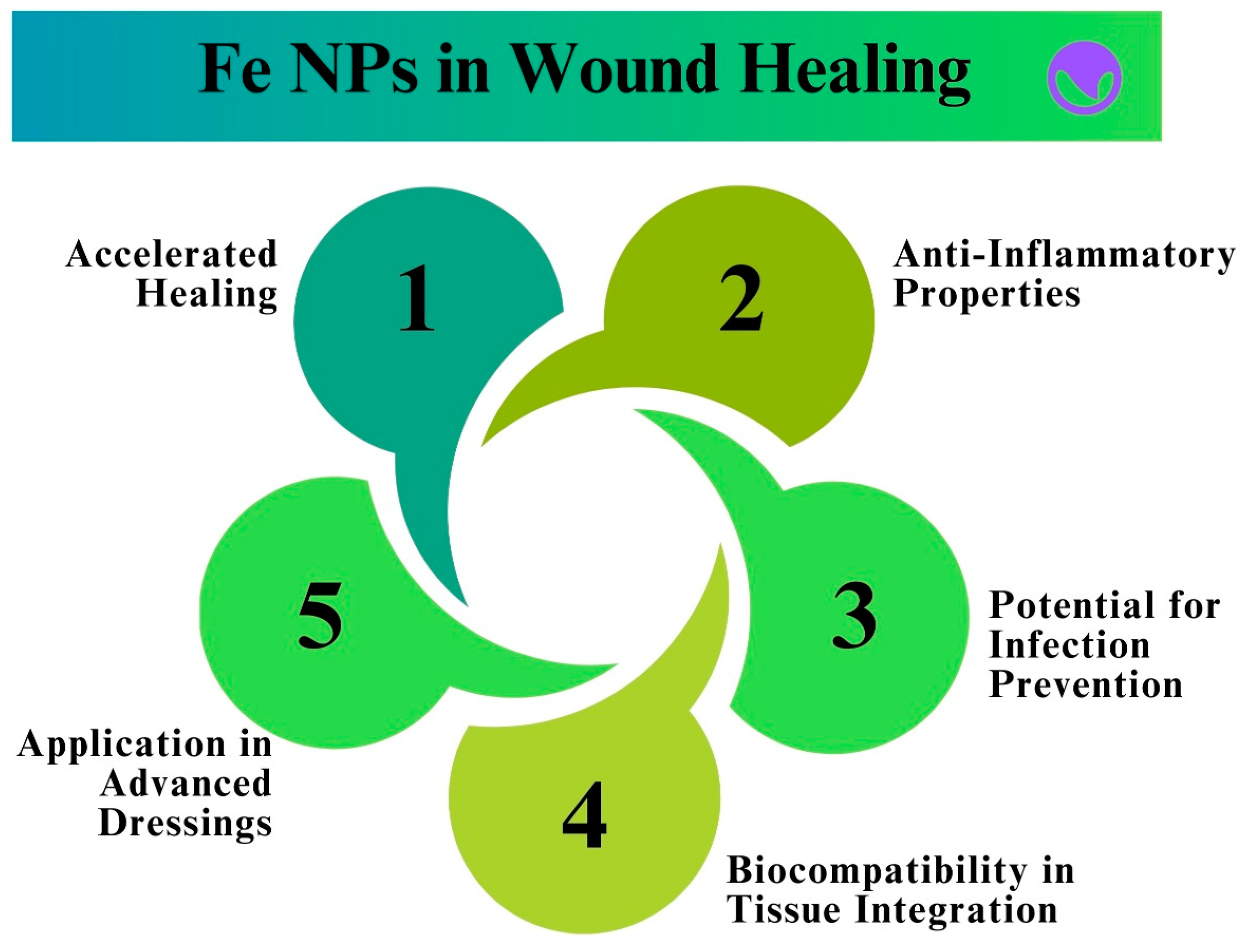
9.3. Fe NPs in Wound Healing and Tissue Regeneration
9.4. Fe NPs in Cancer Therapy and Diagnosis
9.5. Fe NPs in Magnetic Resonance Imaging (MRI)
9.6. Applications of Fe NPs in Hyperthermia
9.7. Applications of Fe NPs in the Food Industry
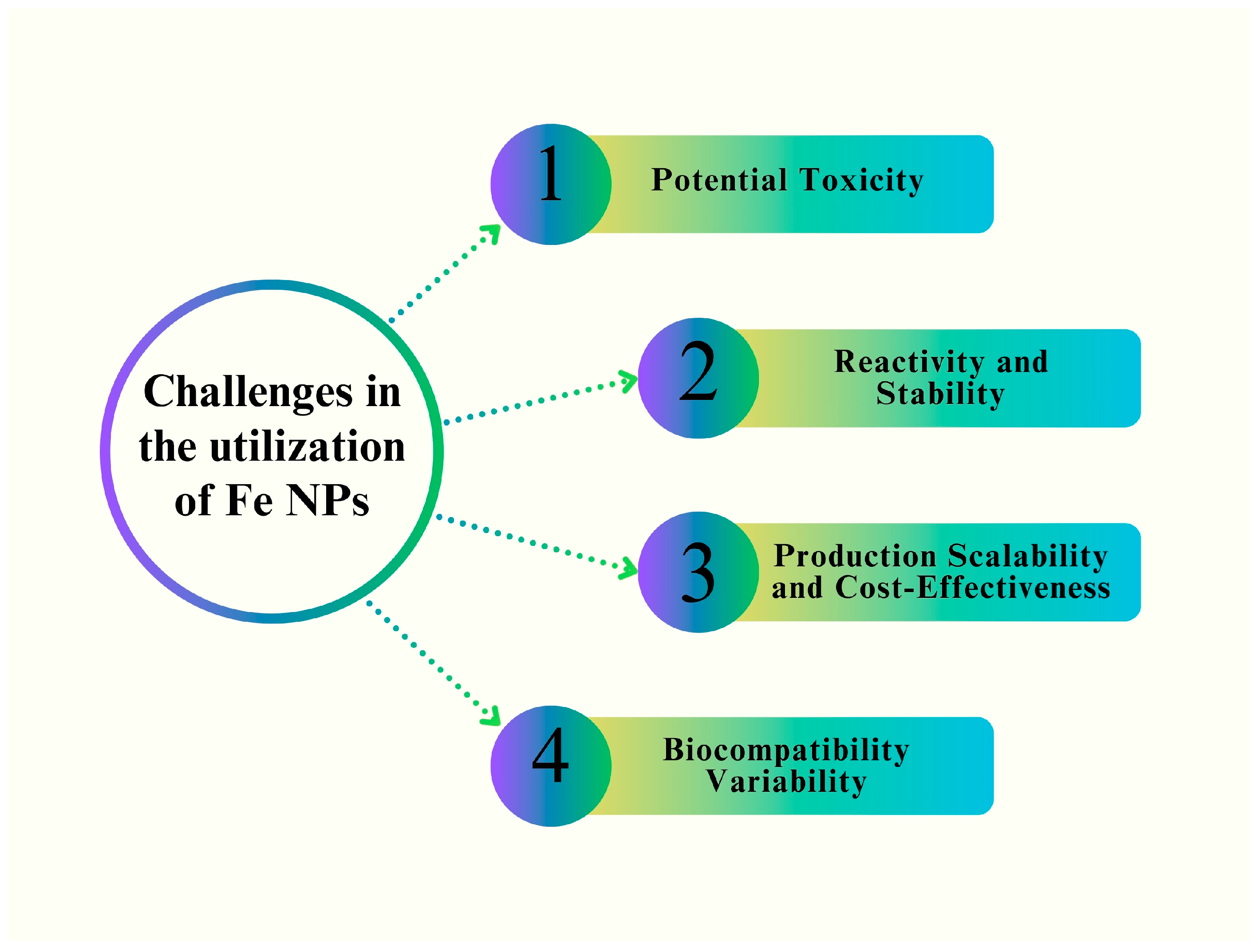
10. Future Perspectives and Challenges
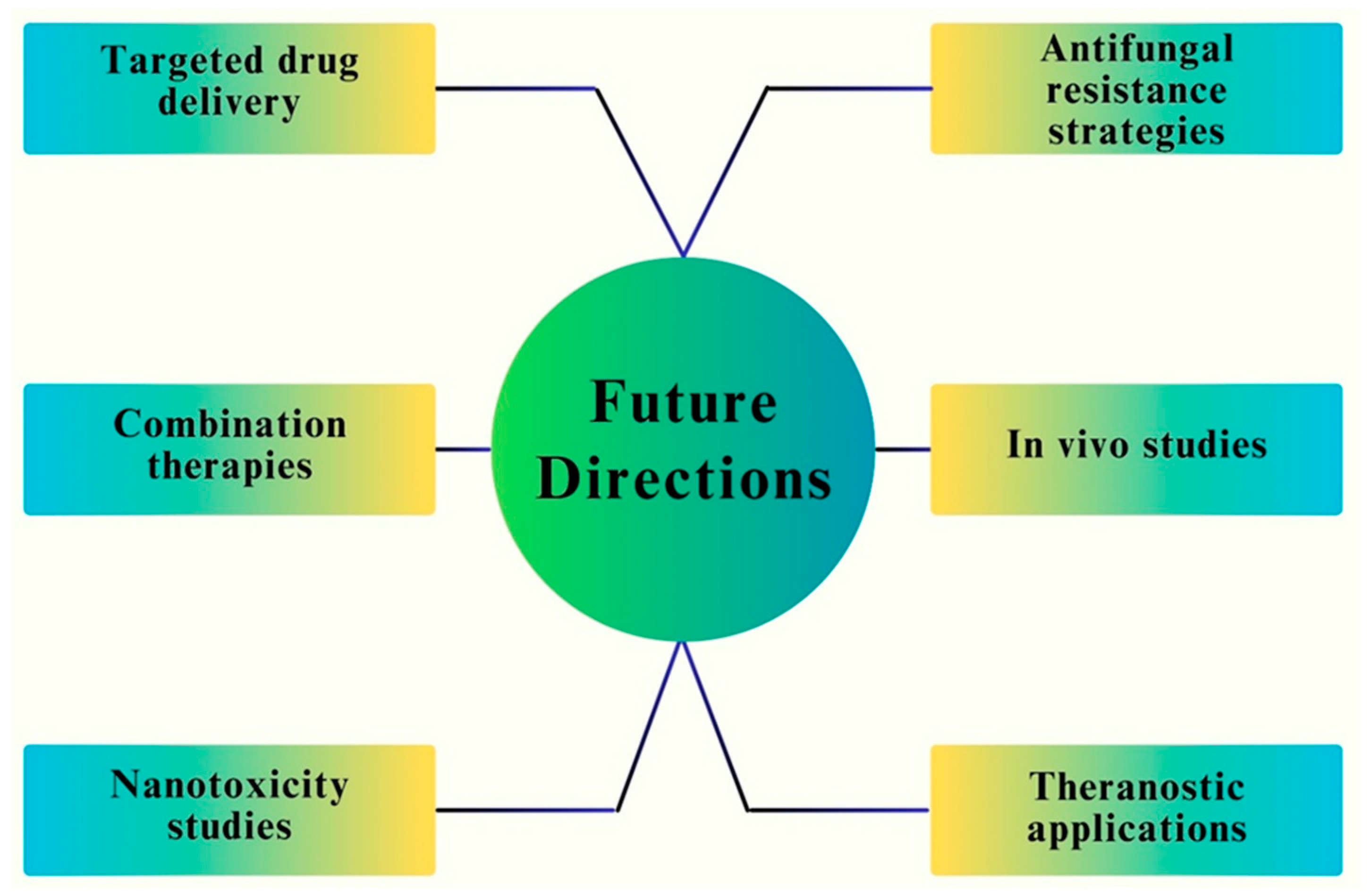
11. Emerging Trends and Future Directions in Optimizing Fe NPs for Antifungal Applications
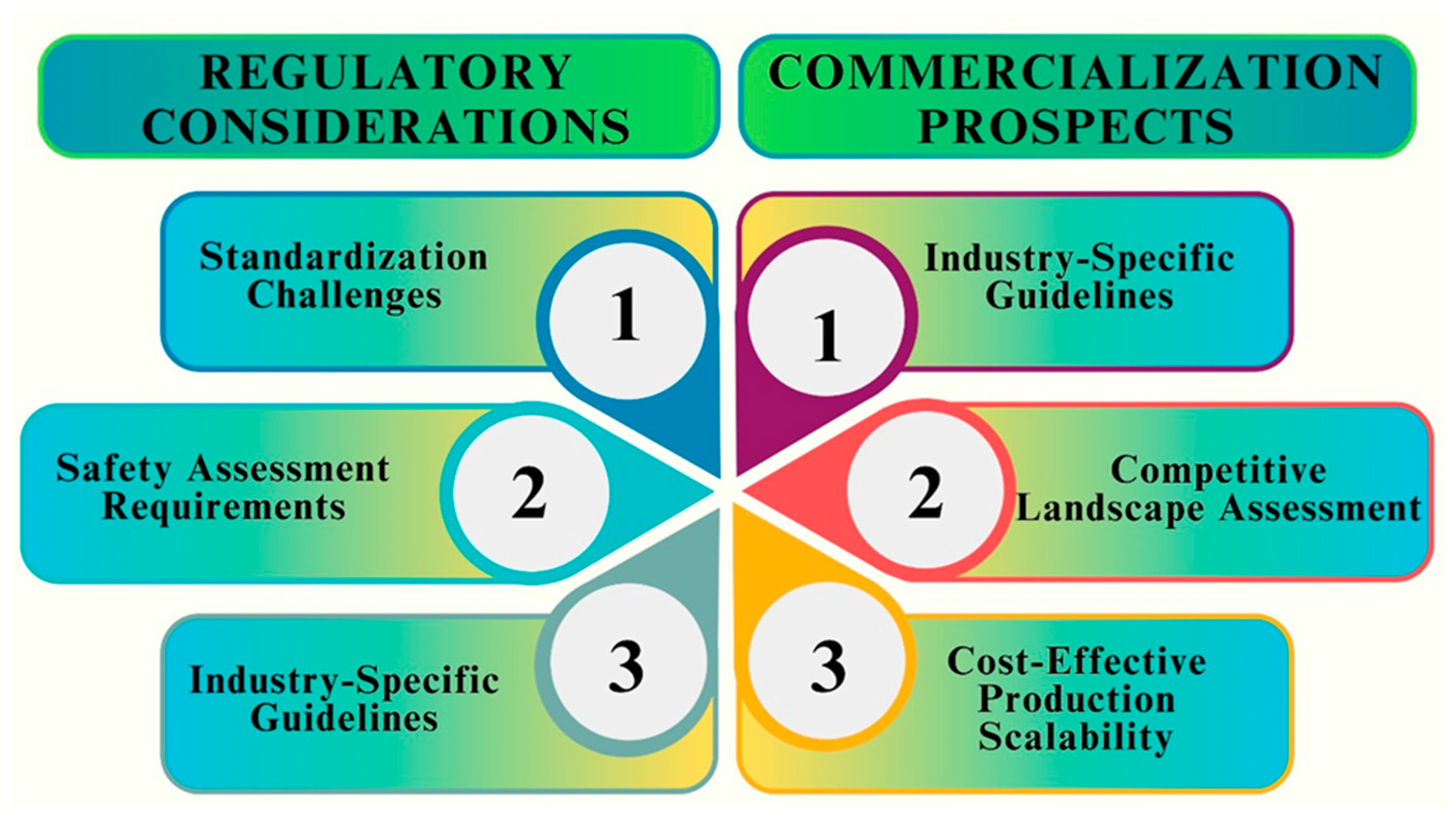
11.1. Overcoming Challenges and Barriers regarding the Translation of Fe NPs into Clinical Settings
11.2. Regulatory and Commercialization Prospects for Fe NP-Based Antifungal Products
12. Implications and Potential Impact of Optimized Fe NPs in Biomedical Applications
13. Closing Remarks and Suggestions for Future Research
14. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yang, X.; Yu, Y.; Lai, Q.; Yang, X.; Luo, P.; Zhang, B.; Zhang, X.; Wei, Y. Recent development and advances on fabrication and biomedical applications of Ga-based liquid metal micro/nanoparticles. Compos. Part B Eng. 2022, 248, 110384. [Google Scholar] [CrossRef]
- Nahari, M.H.; Al Ali, A.; Asiri, A.; Mahnashi, M.H.; Shaikh, I.A.; Shettar, A.K.; Hoskeri, J. Green Synthesis and Characterization of Iron Nanoparticles Synthesized from Aqueous Leaf Extract of Vitex leucoxylon and Its Biomedical Applications. Nanomaterials 2022, 12, 2404. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.I.; Dias, A.M.; Zille, A. Synergistic effects between metal nanoparticles and commercial antimicrobial agents: A review. ACS Appl. Nano Mater. 2022, 5, 3030–3064. [Google Scholar] [CrossRef] [PubMed]
- Pattanayak, D.; Pal, D.; Thakur, C.; Kumar, S.; Devnani, G. Bio-synthesis of iron nanoparticles for environmental remediation: Status till date. Mater. Today Proc. 2021, 44, 3150–3155. [Google Scholar] [CrossRef]
- Zhao, J.; Yu, Y.; Wang, Y.; Cheng, S. Green formulation and characterization of Fe nanoparticles containing Calendula extract and investigation of the antioxidant, cytotoxic and anti-human cholangiocarcinoma properties. Arch. Med. Sci. 2022. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, F.; Men, K.; Huang, R.; Zhou, B.; Zhang, R.; Zou, R.; Yang, L. Modified Fe3O4 magnetic nanoparticle delivery of CpG inhibits tumor growth and spontaneous pulmonary metastases to enhance immunotherapy. Nanoscale Res. Lett. 2018, 13, 240. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, J. One-pot Synthesis of Multifunctional PAM@FeNPs Composite Microspheres. IOP Conf. Ser. Mater. Sci. Eng. 2020, 768, 022004. [Google Scholar] [CrossRef]
- Batool, F.; Iqbal, M.S.; Khan, S.-U.-D.; Khan, J.; Ahmed, B.; Qadir, M.I. Biologically synthesized iron nanoparticles (FeNPs) from Phoenix dactylifera have anti-bacterial activities. Sci. Rep. 2021, 11, 22132. [Google Scholar] [CrossRef] [PubMed]
- Janbon, G.; Quintin, J.; Lanternier, F.; d’Enfert, C. Studying fungal pathogens of humans and fungal infections: Fungal diversity and diversity of approaches. Microbes Infect. 2019, 21, 237–245. [Google Scholar] [CrossRef]
- Sanyaolu, A.; Okorie, C.; Marinkovic, A.; Abbasi, A.F.; Prakash, S.; Mangat, J.; Hosein, Z.; Haider, N.; Chan, J. Candida auris: An overview of the emerging drug-resistant fungal infection. Infect. Chemother. 2022, 54, 236. [Google Scholar] [CrossRef]
- Geddes-McAlister, J.; Shapiro, R.S. New pathogens, new tricks: Emerging, drug-resistant fungal pathogens and future prospects for antifungal therapeutics. Ann. N. Y. Acad. Sci. 2019, 1435, 57–78. [Google Scholar] [CrossRef] [PubMed]
- Fatima, F.; Siddiqui, S.; Khan, W.A. Nanoparticles as novel emerging therapeutic antibacterial agents in the antibiotics resistant era. Biol. Trace Elem. Res. 2021, 199, 2552–2564. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.L.; Lim, Y.C.; Ng, L.Y.; Lim, Y.P. Plant-mediated synthesis of iron nanoparticles for environmental application: Mini review. Mater. Today Proc. 2023, 87, 64–69. [Google Scholar] [CrossRef]
- Singh, K.; Chopra, D.S.; Singh, D.; Singh, N. Optimization and ecofriendly synthesis of iron oxide nanoparticles as potential antioxidant. Arab. J. Chem. 2020, 13, 9034–9046. [Google Scholar] [CrossRef]
- Shafiei, M.; Peyton, L.; Hashemzadeh, M.; Foroumadi, A. History of the development of antifungal azoles: A review on structures, SAR, and mechanism of action. Bioorganic Chem. 2020, 104, 104240. [Google Scholar] [CrossRef] [PubMed]
- Sawant, B.; Khan, T. Recent advances in delivery of antifungal agents for therapeutic management of candidiasis. Biomed. Pharmacother. 2017, 96, 1478–1490. [Google Scholar] [CrossRef]
- Alghuthaymi, M.A.; Hassan, A.A.; Kalia, A.; Sayed El Ahl, R.M.; El Hamaky, A.A.; Oleksak, P.; Kuca, K.; Abd-Elsalam, K.A. Antifungal nano-therapy in veterinary medicine: Current status and future prospects. J. Fungi 2021, 7, 494. [Google Scholar] [CrossRef] [PubMed]
- Shastri, R.; Todkar, V.; Malabade, S.S.; Habbu, P.; Patil, B.; Kulkarni, V. Synthesis and Characterization of Metal Based Nanoparticles from Bark Extracts of Terminalia paniculata (Roxb) for Invitro Antimicrobial and Anticancer Activity. J. Pharm. Negat. Results 2023, 13, 7004–7024. [Google Scholar]
- Lončar, M.; Gašo-Sokač, D.; Molnar, M. Coumarin derivatives as antifungal agents—A review. Czech J. Food Sci. 2023, 41, 79–91. [Google Scholar] [CrossRef]
- Riera, F.O.; Caeiro, J.P.; Angiolini, S.C.; Vigezzi, C.; Rodriguez, E.; Icely, P.A.; Sotomayor, C.E. Invasive candidiasis: Update and current challenges in the management of this mycosis in south america. Antibiotics 2022, 11, 877. [Google Scholar] [CrossRef]
- de Almeida Campos, L.; Fin, M.T.; Santos, K.S.; de Lima Gualque, M.W.; Freire Cabral, A.K.L.; Khalil, N.M.; Fusco-Almeida, A.M.; Mainardes, R.M.; Mendes-Giannini, M.J.S. Nanotechnology-Based Approaches for Voriconazole Delivery Applied to Invasive Fungal Infections. Pharmaceutics 2023, 15, 266. [Google Scholar] [CrossRef] [PubMed]
- Akbayrak, S.; Çakmak, G.; Öztürk, T.; Özkar, S. Rhodium (0), Ruthenium (0) and Palladium (0) nanoparticles supported on carbon-coated iron: Magnetically isolable and reusable catalysts for hydrolytic dehydrogenation of ammonia borane. Int. J. Hydrogen Energy 2021, 46, 13548–13560. [Google Scholar] [CrossRef]
- Eskandari, M.J.; Hasanzadeh, I. Size-controlled synthesis of Fe3O4 magnetic nanoparticles via an alternating magnetic field and ultrasonic-assisted chemical co-precipitation. Mater. Sci. Eng. B 2021, 266, 115050. [Google Scholar] [CrossRef]
- Takai, Z.I.; Mustafa, M.K.; Asman, S.; Sekak, K.A. Preparation and characterization of magnetite (Fe3O4) nanoparticles by sol-gel method. Int. J. Nanoelectron. Mater 2019, 12, 37–46. [Google Scholar]
- Dai, L.; Zeng, Z.; Yang, Q.; Yang, S.; Wang, Y.; Liu, Y.; Ruan, R.; He, C.; Yu, Z.; Jiang, L. Synthesis of iron nanoparticles-based hydrochar catalyst for ex-situ catalytic microwave-assisted pyrolysis of lignocellulosic biomass to renewable phenols. Fuel 2020, 279, 118532. [Google Scholar]
- Tyurikova, I.A.; Alexandrov, S.E.; Tyurikov, K.S.; Kirilenko, D.A.; Speshilova, A.B.; Shakhmin, A.L. Fast and controllable synthesis of core–shell Fe3O4–C nanoparticles by aerosol CVD. ACS Omega 2020, 5, 8146–8150. [Google Scholar] [CrossRef] [PubMed]
- El-Khawaga, A.M.; Zidan, A.; Abd El-Mageed, A.I. Preparation methods of different nanomaterials for various potential applications: A Review. J. Mol. Struct. 2023, 1281, 135148. [Google Scholar] [CrossRef]
- Qasim, S.; Zafar, A.; Saif, M.S.; Ali, Z.; Nazar, M.; Waqas, M.; Haq, A.U.; Tariq, T.; Hassan, S.G.; Iqbal, F. Green synthesis of iron oxide nanorods using Withania coagulans extract improved photocatalytic degradation and antimicrobial activity. J. Photochem. Photobiol. B Biol. 2020, 204, 111784. [Google Scholar] [CrossRef]
- Vasantharaj, S.; Sathiyavimal, S.; Senthilkumar, P.; LewisOscar, F.; Pugazhendhi, A. Biosynthesis of iron oxide nanoparticles using leaf extract of Ruellia tuberosa: Antimicrobial properties and their applications in photocatalytic degradation. J. Photochem. Photobiol. B Biol. 2019, 192, 74–82. [Google Scholar] [CrossRef]
- Perwez, M.; Fatima, H.; Arshad, M.; Meena, V.; Ahmad, B. Magnetic iron oxide nanosorbents effective in dye removal. Int. J. Environ. Sci. Technol. 2023, 20, 5697–5714. [Google Scholar] [CrossRef]
- Al-Alawy, A.F.; Al-Abodi, E.E.; Kadhim, R.M. Synthesis and characterization of magnetic iron oxide nanoparticles by co-precipitation method at different conditions. J. Eng. 2018, 24, 60–72. [Google Scholar] [CrossRef]
- Al-Madhagi, H.; Yazbik, V.; Abdelwahed, W.; Alchab, L.J.B. Magnetite Nanoparticle Co-precipitation Synthesis, Characterization, and Applications: Mini Review. BioNanoScience 2023, 13, 853–859. [Google Scholar] [CrossRef]
- Malik, M.A.; AlHarbi, L.; Nabi, A.; Alzahrani, K.A.; Narasimharao, K.; Kamli, M.R. Facile Synthesis of Magnetic Nigella sativa Seeds: Advances on Nano-Formulation Approaches for Delivering Antioxidants and Their Antifungal Activity against Candida albicans. Pharmaceutics 2023, 15, 642. [Google Scholar] [CrossRef] [PubMed]
- Parashar, M.; Shukla, V.K.; Singh, R. Metal oxides nanoparticles via sol–gel method: A review on synthesis, characterization and applications. J. Mater. Sci. Mater. Electron. 2020, 31, 3729–3749. [Google Scholar] [CrossRef]
- Batool, T.; Shah, Z.H.; Ashraf, H.; Ali, D.; Shamaila, S.; Anjum, T.; Naseem, S.; Riaz, S. Solar energy driven photo catalytic action and antimicrobial activities of Iron oxide nanoparticles. J. Sol-Gel Sci. Technol. 2023, 108, 655–671. [Google Scholar] [CrossRef]
- Tadic, M.; Panjan, M.; Tadic, B.V.; Lazovic, J.; Damnjanovic, V.; Kopani, M.; Kopanja, L. Magnetic properties of hematite (−FeO) nanoparticles synthesized by sol-gel synthesis method: The influence of particle size and particle size distribution. J. Electr. Eng. 2019, 70, 71–76. [Google Scholar]
- Marjeghal, M.A.; Sedghi, A.; Baghshahi, S. The effect of the citric acid to metal nitrates molar ratio on the structural and magnetic properties of strontium hexaferrite nanoparticles synthesized by the sol-gel combustion method. J. Alloy. Compd. 2023, 968, 171765. [Google Scholar] [CrossRef]
- Saeed, M.; Alshammari, Y.; Majeed, S.A.; Al-Nasrallah, E. Chemical vapour deposition of graphene—Synthesis, characterisation, and applications: A review. Molecules 2020, 25, 3856. [Google Scholar] [CrossRef] [PubMed]
- Samrot, A.V.; Sahithya, C.S.; Selvarani, J.; Purayil, S.K.; Ponnaiah, P. A review on synthesis, characterization and potential biological applications of superparamagnetic iron oxide nanoparticles. Curr. Res. Green Sustain. Chem. 2021, 4, 100042. [Google Scholar] [CrossRef]
- Bansal, R.; Verduzco, R.; Wong, M.S.; Westerhoff, P.; Garcia-Segura, S. Development of nano boron-doped diamond electrodes for environmental applications. J. Electroanal. Chem. 2022, 907, 116028. [Google Scholar] [CrossRef]
- Atchudan, R.; Jebakumar Immanuel Edison, T.N.; Perumal, S.; RanjithKumar, D.; Lee, Y.R. Direct growth of iron oxide nanoparticles filled multi-walled carbon nanotube via chemical vapour deposition method as high-performance supercapacitors. Int. J. Hydrogen Energy 2019, 44, 2349–2360. [Google Scholar] [CrossRef]
- Ağaoğulları, D.; Madsen, S.J.; Ögüt, B.; Koh, A.L.; Sinclair, R. Synthesis and characterization of graphite-encapsulated iron nanoparticles from ball milling-assisted low-pressure chemical vapor deposition. Carbon 2017, 124, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Saraswati, T.E.; Prasiwi, O.D.I.; Masykur, A.; Anwar, M. Synthesis of Magnetic Composite of Iron Compounds/Carbon Nanotubes in Chemical Vapor Deposition. J. Sains Materi Indones. 2019, 20, 111–119. [Google Scholar] [CrossRef]
- Davodi, F.; Mühlhausen, E.; Settipani, D.; Rautama, E.-L.; Honkanen, A.-P.; Huotari, S.; Marzun, G.; Taskinen, P.; Kallio, T. Comprehensive study to design advanced metal-carbide@garaphene and metal-carbide@iron oxide nanoparticles with tunable structure by the laser ablation in liquid. J. Colloid Interface Sci. 2019, 556, 180–192. [Google Scholar] [CrossRef]
- Rivera-Chaverra, M.J.; Restrepo-Parra, E.; Acosta-Medina, C.D.; Mello, A.; Ospina, R. Synthesis of oxide iron nanoparticles using laser ablation for possible hyperthermia applications. Nanomaterials 2020, 10, 2099. [Google Scholar] [CrossRef] [PubMed]
- Waag, F.; Fares, W.I.; Li, Y.; Andronescu, C.; Gökce, B.; Barcikowski, S. Identification of the main mixing process in the synthesis of alloy nanoparticles by laser ablation of compacted micropowder mixtures. J. Mater. Sci. 2022, 57, 3041–3056. [Google Scholar] [CrossRef]
- Kupracz, P.; Coy, E.; Grochowska, K.; Karczewski, J.; Rysz, J.; Siuzdak, K. The pulsed laser ablation synthesis of colloidal iron oxide nanoparticles for the enhancement of TiO2 nanotubes photo-activity. Appl. Surf. Sci. 2020, 530, 147097. [Google Scholar] [CrossRef]
- Curcio, M.; Rau, J.V.; Santagata, A.; Teghil, R.; Laureti, S.; De Bonis, A. Laser synthesis of iron nanoparticle for Fe doped hydroxyapatite coatings. Mater. Chem. Phys. 2019, 225, 365–370. [Google Scholar] [CrossRef]
- Zhang, Z.; Wen, G. Synthesis and characterization of carbon-encapsulated magnetite, martensite and iron nanoparticles by high-energy ball milling method. Mater. Charact. 2020, 167, 110502. [Google Scholar] [CrossRef]
- Reverberi, A.P.; Vocciante, M.; Salerno, M.; Ferretti, M.; Fabiano, B. Green synthesis of silver nanoparticles by low-energy wet bead milling of metal spheres. Materials 2019, 13, 63. [Google Scholar] [CrossRef]
- Ribas, D.; Pešková, K.; Jubany, I.; Parma, P.; Černik, M.; Benito, J.; Marti, V. High reactive nano zero-valent iron produced via wet milling through abrasion by alumina. Chem. Eng. J. 2019, 366, 235–245. [Google Scholar] [CrossRef]
- Darvina, Y.; Yulfriska, N.; Rifai, H.; Dwiridal, L.; Ramli, R. Synthesis of magnetite nanoparticles from iron sand by ball-milling. J. Phys. Conf. Ser. 2019, 1185, 012017. [Google Scholar] [CrossRef]
- Seyedi, M.; Haratian, S.; Khaki, J.V. Mechanochemical synthesis of Fe2O3 nanoparticles. Procedia Mater. Sci. 2015, 11, 309–313. [Google Scholar] [CrossRef]
- Rasouli, K.; Alamdari, A.; Sabbaghi, S. Ultrasonic-assisted synthesis of α-Fe2O3@TiO2 photocatalyst: Optimization of effective factors in the fabrication of photocatalyst and removal of non-biodegradable cefixime via response surface methodology-central composite design. Sep. Purif. Technol. 2023, 307, 122799. [Google Scholar] [CrossRef]
- Hachemaoui, M.; Mokhtar, A.; Ismail, I.; Mohamedi, M.W.; Iqbal, J.; Taha, I.; Bennabi, F.; Zaoui, F.; Bengueddach, A.; Hamacha, R. M (M: Cu, Co, Cr or Fe) nanoparticles-loaded metal-organic framework MIL-101 (Cr) material by sonication process: Catalytic activity and antibacterial properties. Microporous Mesoporous Mater. 2021, 323, 111244. [Google Scholar] [CrossRef]
- Jamkhande, P.G.; Ghule, N.W.; Bamer, A.H.; Kalaskar, M.G. Metal nanoparticles synthesis: An overview on methods of preparation, advantages and disadvantages, and applications. J. Drug Deliv. Sci. Technol. 2019, 53, 101174. [Google Scholar] [CrossRef]
- Afolalu, S.; Ikumapayi, O.; Ogedengbe, T.; Kayode, J.; Ogundipe, A.; Jen, T. A review on emerging trends in the scientific application of nano-coatings and nanoparticle synthesis. Mater. Today Proc. 2023, in press. [Google Scholar] [CrossRef]
- Braim, F.S.; Razak, N.N.A.N.A.; Aziz, A.A.; Dheyab, M.A.; Ismael, L.Q. Optimization of ultrasonic-assisted approach for synthesizing a highly stable biocompatible bismuth-coated iron oxide nanoparticles using a face-centered central composite design. Ultrason. Sonochemistry 2023, 95, 106371. [Google Scholar] [CrossRef]
- Putri, N.R.E.; Firdausi, S.I.; Najmina, M.; Amelia, S.; Timotius, D.; Kusumastuti, Y.; Petrus, H.T.B.M. Effect of sonication time and particle size for synthesis of magnetic nanoparticle from local iron sand. J. Eng. Sci. Technol. 2020, 15, 894–904. [Google Scholar]
- Deshmukh, A.R.; Gupta, A.; Kim, B.S. Ultrasound assisted green synthesis of silver and iron oxide nanoparticles using fenugreek seed extract and their enhanced antibacterial and antioxidant activities. BioMed Res. Int. 2019, 2019, 1714358. [Google Scholar] [CrossRef]
- Ijaz, I.; Bukhari, A.; Gilani, E.; Nazir, A.; Zain, H.; Saeed, R. Green synthesis of silver nanoparticles using different plants parts and biological organisms, characterization and antibacterial activity. Environ. Nanotechnol. Monit. Manag. 2022, 18, 100704. [Google Scholar] [CrossRef]
- El-Seedi, H.R.; El-Shabasy, R.M.; Khalifa, S.A.; Saeed, A.; Shah, A.; Shah, R.; Iftikhar, F.J.; Abdel-Daim, M.M.; Omri, A.; Hajrahand, N.H. Metal nanoparticles fabricated by green chemistry using natural extracts: Biosynthesis, mechanisms, and applications. RSC Adv. 2019, 9, 24539–24559. [Google Scholar] [CrossRef]
- Pandit, C.; Roy, A.; Ghotekar, S.; Khusro, A.; Islam, M.N.; Emran, T.B.; Lam, S.E.; Khandaker, M.U.; Bradley, D.A. Biological agents for synthesis of nanoparticles and their applications. J. King Saud Univ.-Sci. 2022, 34, 101869. [Google Scholar] [CrossRef]
- Ebrahiminezhad, A.; Zare-Hoseinabadi, A.; Sarmah, A.K.; Taghizadeh, S.; Ghasemi, Y.; Berenjian, A. Plant-mediated synthesis and applications of iron nanoparticles. Mol. Biotechnol. 2018, 60, 154–168. [Google Scholar] [CrossRef]
- Karimi, P.; Javanshir, S.; Sayadi, M.H.; Arabyarmohammadi, H. Arsenic removal from mining effluents using plant-mediated, green-synthesized iron nanoparticles. Processes 2019, 7, 759. [Google Scholar] [CrossRef]
- Yusefi, M.; Shameli, K.; Ali, R.R.; Pang, S.-W.; Teow, S.-Y. Evaluating Anticancer Activity of Plant-Mediated Synthesized Iron Oxide Nanoparticles Using Punica granatum Fruit Peel Extract. J. Mol. Struct. 2020, 1204, 127539. [Google Scholar] [CrossRef]
- Nadeem, A.; Sumbal; Ali, J.S.; Latif, M.; Rizvi, Z.F.; Naz, S.; Mannan, A.; Zia, M. Green synthesis and characterization of Fe, Cu and Mg oxide nanoparticles using Clematis orientalis leaf extract: Salt concentration modulates physiological and biological properties. Mater. Chem. Phys. 2021, 271, 124900. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Mofijur, M.; Rafa, N.; Chowdhury, A.T.; Chowdhury, S.; Nahrin, M.; Islam, A.S.; Ong, H.C. Green approaches in synthesising nanomaterials for environmental nanobioremediation: Technological advancements, applications, benefits and challenges. Environ. Res. 2022, 204, 111967. [Google Scholar] [CrossRef] [PubMed]
- Koul, B.; Poonia, A.K.; Yadav, D.; Jin, J.-O. Microbe-mediated biosynthesis of nanoparticles: Applications and future prospects. Biomolecules 2021, 11, 886. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, M.; Khan, R.; Shah, N.; Bangash, I.R.; Abbasi, B.H.; Hano, C.; Liu, C.; Ullah, S.; Hashmi, S.S.; Nadhman, A.; et al. A review of microbial mediated iron nanoparticles (IONPs) and its biomedical applications. Nanomaterials 2021, 12, 130. [Google Scholar] [CrossRef]
- Khalil, A.T.; Ovais, M.; Iqbal, J.; Ali, A.; Ayaz, M.; Abbas, M.; Ahmad, I.; Devkota, H.P. Microbes-mediated synthesis strategies of metal nanoparticles and their potential role in cancer therapeutics. Semin. Cancer Biol. 2022, 86, 693–705. [Google Scholar] [CrossRef]
- Jadhav, P.; Khalid, Z.B.; Zularisam, A.W.; Krishnan, S.; Nasrullah, M. The role of iron-based nanoparticles (Fe-NPs) on methanogenesis in anaerobic digestion (AD) performance. Environ. Res. 2022, 204, 112043. [Google Scholar] [CrossRef] [PubMed]
- Amiri, S.; Vatanpour, V.; Mansourpanah, Y.; Khataee, A. Recent trends in application of nanoscale zero-valent metals and metal single atoms in membrane processes. J. Environ. Chem. Eng. 2022, 10, 107457. [Google Scholar] [CrossRef]
- Antony, V.S.; Sahithya, C.S.; Durga Sruthi, P.; Selvarani, J.; Raji, P.; Prakash, P.; Ponnaiah, P.; Petchi, I.; Pattammadath, S.; Keeyari, S. Itraconazole coated super paramagnetic iron oxide nanoparticles for antimicrobial studies. Biointerface Res. Appl. Chem. 2020, 10, 6218–6225. [Google Scholar]
- Parveen, S.; Wani, A.H.; Shah, M.A.; Devi, H.S.; Bhat, M.Y.; Koka, J.A. Preparation, characterization and antifungal activity of iron oxide nanoparticles. Microb. Pathog. 2018, 115, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Nehra, P.; Chauhan, R.; Garg, N.; Verma, K. Antibacterial and antifungal activity of chitosan coated iron oxide nanoparticles. Br. J. Biomed. Sci. 2018, 75, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Mousa, S.A.; El-Sayed, E.-S.R.; Mohamed, S.S.; Abo El-Seoud, M.A.; Elmehlawy, A.A.; Abdou, D.A. Novel mycosynthesis of Co3O4, CuO, Fe3O4, NiO, and ZnO nanoparticles by the endophytic Aspergillus terreus and evaluation of their antioxidant and antimicrobial activities. Appl. Microbiol. Biotechnol. 2021, 105, 741–753. [Google Scholar] [CrossRef]
- Jaison, J.P.; Balasubramanian, B.; Gangwar, J.; James, N.; Pappuswamy, M.; Anand, A.V.; Al-Dhabi, N.A.; Valan Arasu, M.; Liu, W.-C.; Sebastian, J.K. Green Synthesis of Bioinspired Nanoparticles Mediated from Plant Extracts of Asteraceae Family for Potential Biological Applications. Antibiotics 2023, 12, 543. [Google Scholar] [CrossRef] [PubMed]
- El-Refai, A.A.; Ghoniem, G.A.; El-Khateeb, A.Y.; Hassaan, M.M. Eco-friendly synthesis of metal nanoparticles using ginger and garlic extracts as biocompatible novel antioxidant and antimicrobial agents. J. Nanostructure Chem. 2018, 8, 71–81. [Google Scholar] [CrossRef]
- Perveen, S.; Nadeem, R.; ur Rehman, S.; Afzal, N.; Anjum, S.; Noreen, S.; Saeed, R.; Amami, M.; Al-Mijalli, S.H.; Iqbal, M. Green synthesis of iron (Fe) nanoparticles using Plumeria obtusa extract as a reducing and stabilizing agent: Antimicrobial, antioxidant and biocompatibility studies. Arab. J. Chem. 2022, 15, 103764. [Google Scholar] [CrossRef]
- Win, T.T.; Khan, S.; Bo, B.; Zada, S.; Fu, P. Green synthesis and characterization of Fe3O4 nanoparticles using Chlorella-K01 extract for potential enhancement of plant growth stimulating and antifungal activity. Sci. Rep. 2021, 11, 21996. [Google Scholar] [CrossRef] [PubMed]
- Devi, H.S.; Boda, M.A.; Shah, M.A.; Parveen, S.; Wani, A.H. Green synthesis of iron oxide nanoparticles using Platanus orientalis leaf extract for antifungal activity. Green Process. Synth. 2019, 8, 38–45. [Google Scholar] [CrossRef]
- Kulkarni, S.; Jadhav, M.; Raikar, P.; Barretto, D.A.; Vootla, S.K.; Raikar, U. Green synthesized multifunctional Ag@Fe2O3 nanocomposites for effective antibacterial, antifungal and anticancer properties. New J. Chem. 2017, 41, 9513–9520. [Google Scholar] [CrossRef]
- Abdel-Khalek, E.; Rayan, D.; Askar, A.A.; Maksoud, M.A.; El-Bahnasawy, H. Synthesis and characterization of SrFeO3-δ nanoparticles as antimicrobial agent. J. Sol-Gel Sci. Technol. 2021, 97, 27–38. [Google Scholar] [CrossRef]
- Padilla-Cruz, A.; Garza-Cervantes, J.; Vasto-Anzaldo, X.G.; García-Rivas, G.; León-Buitimea, A.; Morones-Ramírez, J. Synthesis and design of Ag–Fe bimetallic nanoparticles as antimicrobial synergistic combination therapies against clinically relevant pathogens. Sci. Rep. 2021, 11, 5351. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.; Uwa, P. Eco-friendly synthesis of iron nanoparticles using Uvaria chamae: Characterization and biological activity. Inorg. Nano-Met. Chem. 2019, 49, 431–442. [Google Scholar] [CrossRef]
- Asghar, M.A.; Zahir, E.; Shahid, S.M.; Khan, M.N.; Asghar, M.A.; Iqbal, J.; Walker, G. Iron, copper and silver nanoparticles: Green synthesis using green and black tea leaves extracts and evaluation of antibacterial, antifungal and aflatoxin B1 adsorption activity. LWT 2018, 90, 98–107. [Google Scholar] [CrossRef]
- Ali, Z.; Andreassen, J.-P.; Bandyopadhyay, S. Fine-Tuning of Particle Size and Morphology of Silica Coated Iron Oxide Nanoparticles. Ind. Eng. Chem. Res. 2023, 62, 4831–4839. [Google Scholar] [CrossRef]
- Prabha, A.S.; Dorothy, R.; Jancirani, S.; Rajendran, S.; Singh, G.; Kumaran, S.S. Recent advances in the study of toxicity of polymer-based nanomaterials. In Nanotoxicity; Elsevier: Amsterdam, The Netherlands, 2020; pp. 143–165. [Google Scholar]
- Niroumand, U.; Firouzabadi, N.; Goshtasbi, G.; Hassani, B.; Ghasemiyeh, P.; Mohammadi-Samani, S. The effect of size, morphology and surface properties of mesoporous silica nanoparticles on pharmacokinetic aspects and potential toxicity concerns. Front. Mater. 2023, 10, 1189463. [Google Scholar] [CrossRef]
- Fouad, D.E.; Zhang, C.; El-Didamony, H.; Yingnan, L.; Mekuria, T.D.; Shah, A.H. Improved size, morphology and crystallinity of hematite (α-Fe2O3) nanoparticles synthesized via the precipitation route using ferric sulfate precursor. Results Phys. 2019, 12, 1253–1261. [Google Scholar] [CrossRef]
- Benković, M.; Valinger, D.; Jurina, T.; Gajdoš Kljusurić, J.; Jurinjak Tušek, A. Biocatalysis as a Green Approach for Synthesis of Iron Nanoparticles—Batch and Microflow Process Comparison. Catalysts 2023, 13, 112. [Google Scholar] [CrossRef]
- Muzafar, W.; Kanwal, T.; Rehman, K.; Perveen, S.; Jabri, T.; Qamar, F.; Faizi, S.; Shah, M.R. Green synthesis of iron oxide nanoparticles using Melia azedarach flowers extract and evaluation of their antimicrobial and antioxidant activities. J. Mol. Struct. 2022, 1269, 133824. [Google Scholar] [CrossRef]
- Mourdikoudis, S.; Pallares, R.M.; Thanh, N.T. Characterization techniques for nanoparticles: Comparison and complementarity upon studying nanoparticle properties. Nanoscale 2018, 10, 12871–12934. [Google Scholar] [CrossRef] [PubMed]
- Sadhasivam, S.; Vinayagam, V.; Balasubramaniyan, M. Recent advancement in biogenic synthesis of iron nanoparticles. J. Mol. Struct. 2020, 1217, 128372. [Google Scholar] [CrossRef]
- Hammad, E.N.; Salem, S.S.; Mohamed, A.A.; El-Dougdoug, W. Environmental impacts of ecofriendly iron oxide nanoparticles on dyes removal and antibacterial activity. Appl. Biochem. Biotechnol. 2022, 194, 6053–6067. [Google Scholar] [CrossRef] [PubMed]
- Kayani, Z.N.; Arshad, S.; Riaz, S.; Naseem, S. Synthesis of iron oxide nanoparticles by sol–gel technique and their characterization. IEEE Trans. Magn. 2014, 50, 1–4. [Google Scholar] [CrossRef]
- Ashraf, I.; Singh, N.B.; Agarwal, A. Green synthesis of iron oxide nanoparticles using Amla seed for methylene blue dye removal from water. Mater. Today Proc. 2023, 72, 311–316. [Google Scholar] [CrossRef]
- Kamal, A.; Saba, M.; Kamal, A.; Batool, M.; Asif, M.; Al-Mohaimeed, A.M.; Al Farraj, D.A.; Habib, D.; Ahmad, S. Bioinspired Green Synthesis of Bimetallic Iron and Zinc Oxide Nanoparticles Using Mushroom Extract and Use against Aspergillus niger; The Most Devastating Fungi of the Green World. Catalysts 2023, 13, 400. [Google Scholar] [CrossRef]
- Joudeh, N.; Linke, D. Nanoparticle classification, physicochemical properties, characterization, and applications: A comprehensive review for biologists. J. Nanobiotechnology 2022, 20, 262. [Google Scholar] [CrossRef]
- Devatha, C.; Thalla, A.K.; Katte, S.Y. Green synthesis of iron nanoparticles using different leaf extracts for treatment of domestic waste water. J. Clean. Prod. 2016, 139, 1425–1435. [Google Scholar] [CrossRef]
- Rocha, F.S.; Gomes, A.J.; Lunardi, C.N.; Kaliaguine, S.; Patience, G.S. Experimental methods in chemical engineering: Ultraviolet visible spectroscopy—UV-Vis. Can. J. Chem. Eng. 2018, 96, 2512–2517. [Google Scholar] [CrossRef]
- Afrouz, M.; Ahmadi-Nouraldinvand, F.; Elias, S.G.; Alebrahim, M.T.; Tseng, T.M.; Zahedian, H. Green synthesis of spermine coated iron nanoparticles and its effect on biochemical properties of Rosmarinus officinalis. Sci. Rep. 2023, 13, 775. [Google Scholar] [CrossRef]
- Iqbal, J.; Abbasi, B.A.; Ahmad, R.; Shahbaz, A.; Zahra, S.A.; Kanwal, S.; Munir, A.; Rabbani, A.; Mahmood, T. Biogenic synthesis of green and cost effective iron nanoparticles and evaluation of their potential biomedical properties. J. Mol. Struct. 2020, 1199, 126979. [Google Scholar] [CrossRef]
- Lohrasbi, S.; Kouhbanani, M.A.J.; Beheshtkhoo, N.; Ghasemi, Y.; Amani, A.M.; Taghizadeh, S. Green Synthesis of Iron Nanoparticles Using Plantago major Leaf Extract and Their Application as a Catalyst for the Decolorization of Azo Dye. BioNanoScience 2019, 9, 317–322. [Google Scholar] [CrossRef]
- Jia, Z.; Li, J.; Gao, L.; Yang, D.; Kanaev, A. Dynamic Light Scattering: A Powerful Tool for In Situ Nanoparticle Sizing. Colloids Interfaces 2023, 7, 15. [Google Scholar] [CrossRef]
- Rizvi, M.; Bhatia, T.; Gupta, R. Green & sustainable synthetic route of obtaining iron oxide nanoparticles using Hylocereus undantus (pitaya or dragon fruit). Mater. Today Proc. 2022, 50, 1100–1106. [Google Scholar] [CrossRef]
- Roy, A.; Singh, V.; Sharma, S.; Ali, D.; Azad, A.K.; Kumar, G.; Emran, T.B. Antibacterial and dye degradation activity of green synthesized iron nanoparticles. J. Nanomater. 2022, 2022, 3636481. [Google Scholar] [CrossRef]
- Aksu Demirezen, D.; Yıldız, Y.Ş.; Yılmaz, Ş.; Demirezen Yılmaz, D. Green synthesis and characterization of iron oxide nanoparticles using Ficus carica (common fig) dried fruit extract. J. Biosci. Bioeng. 2019, 127, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Harmansah, C.; Karatay Kutman, M.; Biber Muftuler, F.Z. Preparation of iron oxide nanoparticles by banana peels extract and its usage in NDT. Measurement 2022, 204, 112081. [Google Scholar] [CrossRef]
- Al-Karagoly, H.; Rhyaf, A.; Naji, H.; Albukhaty, S.; AlMalki, F.A.; Alyamani, A.A.; Albaqami, J.; Aloufi, S. Green synthesis, characterization, cytotoxicity, and antimicrobial activity of iron oxide nanoparticles using Nigella sativa seed extract. Green Process. Synth. 2022, 11, 254–265. [Google Scholar] [CrossRef]
- Drozdz, A.; Matusiak, K.; Setkowicz, Z.; Ciarach, M.; Janeczko, K.; Sandt, C.; Borondics, F.; Horak, D.; Babic, M.; Chwiej, J. FTIR microspectroscopy revealed biochemical changes in liver and kidneys as a result of exposure to low dose of iron oxide nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 236, 118355. [Google Scholar] [CrossRef]
- Zambri, N.D.S.; Taib, N.I.; Abdul Latif, F.; Mohamed, Z. Utilization of neem leaf extract on biosynthesis of iron oxide nanoparticles. Molecules 2019, 24, 3803. [Google Scholar] [CrossRef]
- Stoia, M.; Istratie, R.; Păcurariu, C. Investigation of magnetite nanoparticles stability in air by thermal analysis and FTIR spectroscopy. J. Therm. Anal. Calorim. 2016, 125, 1185–1198. [Google Scholar] [CrossRef]
- Alangari, A.; Alqahtani, M.S.; Mateen, A.; Kalam, M.A.; Alshememry, A.; Ali, R.; Kazi, M.; AlGhamdi, K.M.; Syed, R. Iron Oxide Nanoparticles: Preparation, Characterization, and Assessment of Antimicrobial and Anticancer Activity. Adsorpt. Sci. Technol. 2022, 2022, 1562051. [Google Scholar] [CrossRef]
- Zakariya, N.A.; Majeed, S.; Jusof, W.H.W. Investigation of antioxidant and antibacterial activity of iron oxide nanoparticles (IONPS) synthesized from the aqueous extract of Penicillium spp. Sens. Int. 2022, 3, 100164. [Google Scholar] [CrossRef]
- Mohammed, A.; Abdullah, A. Scanning electron microscopy (SEM): A review. In Proceedings of the 2018 International Conference on Hydraulics and Pneumatics—HERVEX, Băile Govora, Romania, 7–9 November 2018; pp. 7–9. [Google Scholar]
- Kiwumulo, H.F.; Muwonge, H.; Ibingira, C.; Lubwama, M.; Kirabira, J.B.; Ssekitoleko, R.T. Green synthesis and characterization of iron-oxide nanoparticles using Moringa oleifera: A potential protocol for use in low and middle income countries. BMC Res. Notes 2022, 15, 149. [Google Scholar] [CrossRef]
- Haris, M.; Fatima, N.; Iqbal, J.; Chalgham, W.; Mumtaz, A.S.; El-Sheikh, M.A.; Tavafoghi, M. Oscillatoria limnetica Mediated Green Synthesis of Iron Oxide (Fe2O3) Nanoparticles and Their Diverse In Vitro Bioactivities. Molecules 2023, 28, 2091. [Google Scholar] [CrossRef]
- Cruz-Luna, A.R.; Cruz-Martínez, H.; Vásquez-López, A.; Medina, D.I. Metal Nanoparticles as Novel Antifungal Agents for Sustainable Agriculture: Current Advances and Future Directions. J. Fungi 2021, 7, 1033. [Google Scholar] [CrossRef]
- Slavin, Y.N.; Bach, H. Mechanisms of Antifungal Properties of Metal Nanoparticles. Nanomaterials 2022, 12, 4470. [Google Scholar] [CrossRef] [PubMed]
- Madkhali, O.A. A comprehensive review on potential applications of metallic nanoparticles as antifungal therapies to combat human fungal diseases. Saudi Pharm. J. 2023, 31, 101733. [Google Scholar] [CrossRef] [PubMed]
- Wahab, S.; Salman, A.; Khan, Z.; Khan, S.; Krishnaraj, C.; Yun, S.-I. Metallic Nanoparticles: A Promising Arsenal against Antimicrobial Resistance—Unraveling Mechanisms and Enhancing Medication Efficacy. Int. J. Mol. Sci. 2023, 24, 14897. [Google Scholar] [CrossRef]
- Periakaruppan, R.; Kumar, T.S.; Vanathi, P.; Al-Awsi, G.R.L.; Al-Dayan, N.; Dhanasekaran, S. Phyto-synthesis and characterization of parthenium-mediated iron oxide nanoparticles and an evaluation of their antifungal and antioxidant activities and effect on seed germination. JOM 2023, 75, 5235–5242. [Google Scholar] [CrossRef]
- Anjum, S.A.; Ashraf, U.; Tanveer, M.; Khan, I.; Hussain, S.; Shahzad, B.; Zohaib, A.; Abbas, F.; Saleem, M.F.; Ali, I. Drought induced changes in growth, osmolyte accumulation and antioxidant metabolism of three maize hybrids. Front. Plant Sci. 2017, 8, 69. [Google Scholar] [CrossRef] [PubMed]
- Abdal Dayem, A.; Hossain, M.K.; Lee, S.B.; Kim, K.; Saha, S.K.; Yang, G.-M.; Choi, H.Y.; Cho, S.-G. The role of reactive oxygen species (ROS) in the biological activities of metallic nanoparticles. Int. J. Mol. Sci. 2017, 18, 120. [Google Scholar] [CrossRef]
- Ong, K.S.; Cheow, Y.L.; Lee, S.M. The role of reactive oxygen species in the antimicrobial activity of pyochelin. J. Adv. Res. 2017, 8, 393–398. [Google Scholar] [CrossRef]
- Shekhova, E.; Kniemeyer, O.; Brakhage, A.A. Induction of mitochondrial reactive oxygen species production by itraconazole, terbinafine, and amphotericin B as a mode of action against Aspergillus fumigatus. Antimicrob. Agents Chemother. 2017, 61, e00978-17. [Google Scholar] [CrossRef]
- Geetha, R.G.; Krishnankutty Nair Chandrika, S.; Saraswathy, G.G.; Nair Sivakumari, A.; Sakuntala, M. ROS Dependent Antifungal and Anticancer Modulations of Piper colubrinum Osmotin. Molecules 2021, 26, 2239. [Google Scholar] [CrossRef]
- Gold, K.; Slay, B.; Knackstedt, M.; Gaharwar, A.K. Antimicrobial activity of metal and metal-oxide based nanoparticles. Adv. Ther. 2018, 1, 1700033. [Google Scholar] [CrossRef]
- Stanford, F.A.; Voigt, K. Iron assimilation during emerging infections caused by opportunistic fungi with emphasis on Mucorales and the development of antifungal resistance. Genes 2020, 11, 1296. [Google Scholar] [CrossRef] [PubMed]
- Amasha, R.H.H.; Jastaniah, S.D.S.; Al-qaim, Z.H.; Hussam, F.; Shakir, A.; Kazemnejadi, M. Antibiotic-modified ionic liquids-assisted preparation of biomedical silver NPs with antibacterial, anti-colon cancer, antioxidant, cytotoxicity, and antifungal activity. Inorg. Chem. Commun. 2023, 149, 110375. [Google Scholar] [CrossRef]
- Shahbaz, M.; Akram, A.; Raja, N.I.; Mukhtar, T.; Mehak, A.; Fatima, N.; Ajmal, M.; Ali, K.; Mustafa, N.; Abasi, F. Antifungal activity of green synthesized selenium nanoparticles and their effect on physiological, biochemical, and antioxidant defense system of mango under mango malformation disease. PLoS ONE 2023, 18, e0274679. [Google Scholar] [CrossRef]
- Poznanski, P.; Hameed, A.; Orczyk, W. Chitosan and Chitosan Nanoparticles: Parameters Enhancing Antifungal Activity. Molecules 2023, 28, 2996. [Google Scholar] [CrossRef]
- Jebril, S.; Jenana, R.K.B.; Dridi, C. Green synthesis of silver nanoparticles using Melia azedarach leaf extract and their antifungal activities: In vitro and in vivo. Mater. Chem. Phys. 2020, 248, 122898. [Google Scholar] [CrossRef]
- Ong, Z.Y.; Wiradharma, N.; Yang, Y.Y. Strategies employed in the design and optimization of synthetic antimicrobial peptide amphiphiles with enhanced therapeutic potentials. Adv. Drug Deliv. Rev. 2014, 78, 28–45. [Google Scholar] [CrossRef]
- Lei, C.; Sun, Y.; Tsang, D.C.; Lin, D. Environmental transformations and ecological effects of iron-based nanoparticles. Environ. Pollut. 2018, 232, 10–30. [Google Scholar] [CrossRef]
- Obaid, H.S.; Halbus, A.F. Boosting iron oxide nanoparticles activity for dyes removal and antifungal applications by modifying its surface with polyelectrolytes. Chem. Phys. Impact 2023, 6, 100244. [Google Scholar] [CrossRef]
- Makvandi, P.; Wang, C.y.; Zare, E.N.; Borzacchiello, A.; Niu, L.n.; Tay, F.R. Metal-based nanomaterials in biomedical applications: Antimicrobial activity and cytotoxicity aspects. Adv. Funct. Mater. 2020, 30, 1910021. [Google Scholar] [CrossRef]
- Duan, S.; Wu, R.; Xiong, Y.-H.; Ren, H.-M.; Lei, C.; Zhao, Y.-Q.; Zhang, X.-Y.; Xu, F.-J. Multifunctional antimicrobial materials: From rational design to biomedical applications. Prog. Mater. Sci. 2022, 125, 100887. [Google Scholar] [CrossRef]
- Manzoor, A.; Yousuf, B.; Pandith, J.A.; Ahmad, S. Plant-derived active substances incorporated as antioxidant, antibacterial or antifungal components in coatings/films for food packaging applications. Food Biosci. 2023, 53, 102717. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, Z.; Chen, F. Surface modification of carbon nanotubes with an enhanced antifungal activity for the control of plant fungal pathogen. Materials 2017, 10, 1375. [Google Scholar] [CrossRef] [PubMed]
- Caizer, C.; Rai, M. Magnetic Nanoparticles in Alternative Tumors Therapy: Biocompatibility, Toxicity, and Safety Compared with Classical Methods. In Magnetic Nanoparticles in Human Health and Medicine: Current Medical Applications and Alternative Therapy of Cancer; Wiley: Hoboken, NJ, USA, 2021; pp. 355–379. [Google Scholar]
- Thangudu, S.; Huang, E.-Y.; Su, C.-H. Safe magnetic resonance imaging on biocompatible nanoformulations. Biomater. Sci. 2022, 10, 5032–5053. [Google Scholar] [CrossRef]
- Prucek, R.; Tuček, J.; Kilianová, M.; Panáček, A.; Kvítek, L.; Filip, J.; Kolář, M.; Tománková, K.; Zbořil, R. The targeted antibacterial and antifungal properties of magnetic nanocomposite of iron oxide and silver nanoparticles. Biomaterials 2011, 32, 4704–4713. [Google Scholar] [CrossRef]
- Niemirowicz, K.; Durnaś, B.; Tokajuk, G.; Głuszek, K.; Wilczewska, A.Z.; Misztalewska, I.; Mystkowska, J.; Michalak, G.; Sodo, A.; Wątek, M.; et al. Magnetic nanoparticles as a drug delivery system that enhance fungicidal activity of polyene antibiotics. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 2395–2404. [Google Scholar] [CrossRef]
- Seddighi, N.S.; Salari, S.; Izadi, A.R. Evaluation of antifungal effect of iron-oxide nanoparticles against different Candida species. IET Nanobiotechnol. 2017, 11, 883–888. [Google Scholar] [CrossRef]
- Salari, S.; Sadat Seddighi, N.; Ghasemi Nejad Almani, P. Evaluation of biofilm formation ability in different Candida strains and anti-biofilm effects of Fe3O4-NPs compared with Fluconazole: An in vitro study. J. Mycol. Med. 2018, 28, 23–28. [Google Scholar] [CrossRef]
- de Lima, T.M.T.; Arias, L.S.; Afanaci, L.F.; Ferraresse, R.F.B.; de S Neto, F.N.; de Lima, B.H.R.; Straioto, F.G.; de Camargo, E.R.; Pessan, J.P.; Monteiro, D.R. Assembly and antifungal effect of a new fluconazole-carrier nanosystem. Future Microbiol. 2020, 15, 273–285. [Google Scholar] [CrossRef]
- Zare-Khafri, M.; Alizadeh, F.; Nouripour-Sisakht, S.; Khodavandi, A.; Gerami, M. Inhibitory effect of magnetic iron-oxide nanoparticles on the pattern of expression of lanosterol 14α -demethylase (ERG11) in fluconazole-resistant colonising isolate of Candida albicans. IET Nanobiotechnol. 2020, 14, 375–381. [Google Scholar] [CrossRef]
- Sriramulu, M.; Balaji; Sumathi, S. Photo Catalytic, Antimicrobial and Antifungal Activity of Biogenic Iron Oxide Nanoparticles Synthesised Using Aegle marmelos Extracts. J. Inorg. Organomet. Polym. Mater. 2021, 31, 1738–1744. [Google Scholar] [CrossRef]
- Yassin, M.T.; Al-Otibi, F.O.; Al-Askar, A.A.; Alharbi, R.I. Green Synthesis, Characterization, and Antifungal Efficiency of Biogenic Iron Oxide Nanoparticles. Appl. Sci. 2023, 13, 9942. [Google Scholar] [CrossRef]
- Azadi, S.; Azizipour, E.; Amani, A.M.; Vaez, A.; Zareshahrabadi, Z.; Abbaspour, A.; Firuzyar, T.; Dortaj, H.; Kamyab, H.; Chelliapan, S.; et al. Antifungal activity of Fe3O4@SiO2/Schiff-base/Cu(II) magnetic nanoparticles against pathogenic Candida species. Sci. Rep. 2024, 14, 5855. [Google Scholar] [CrossRef]
- Wu, L.; Wang, C.; Li, Y. Iron oxide nanoparticle targeting mechanism and its application in tumor magnetic resonance imaging and therapy. Nanomed. Nanotechnol. Biol. Med. 2022, 17, 1567–1583. [Google Scholar] [CrossRef] [PubMed]
- Wuttke, S.; Zimpel, A.; Bein, T.; Braig, S.; Stoiber, K.; Vollmar, A.; Müller, D.; Haastert-Talini, K.; Schaeske, J.; Stiesch, M. Validating metal-organic framework nanoparticles for their nanosafety in diverse biomedical applications. Adv. Healthc. Mater. 2017, 6, 1600818. [Google Scholar] [CrossRef] [PubMed]
- Arias, L.S.; Pessan, J.P.; Vieira, A.P.M.; Lima, T.M.T.d.; Delbem, A.C.B.; Monteiro, D.R. Iron oxide nanoparticles for biomedical applications: A perspective on synthesis, drugs, antimicrobial activity, and toxicity. Antibiotics 2018, 7, 46. [Google Scholar] [CrossRef] [PubMed]
- Matschegewski, C.; Kowalski, A.; Müller, K.; Teller, H.; Grabow, N.; Großmann, S.; Schmitz, K.-P.; Siewert, S. Biocompatibility of magnetic iron oxide nanoparticles for biomedical applications. Curr. Dir. Biomed. Eng. 2019, 5, 573–576. [Google Scholar] [CrossRef]
- Singh, N.; Qutub, S.; Khashab, N.M. Biocompatibility and biodegradability of metal organic frameworks for biomedical applications. J. Mater. Chem. B 2021, 9, 5925–5934. [Google Scholar] [CrossRef] [PubMed]
- McNamara, K.; Tofail, S.A. Nanoparticles in biomedical applications. Adv. Phys. X 2017, 2, 54–88. [Google Scholar] [CrossRef]
- Xie, W.; Guo, Z.; Gao, F.; Gao, Q.; Wang, D.; Liaw, B.-s.; Cai, Q.; Sun, X.; Wang, X.; Zhao, L. Shape-, size-and structure-controlled synthesis and biocompatibility of iron oxide nanoparticles for magnetic theranostics. Theranostics 2018, 8, 3284. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.S.; Shedbalkar, U.U.; Truskewycz, A.; Chopade, B.A.; Ball, A.S. Nanoparticles for environmental clean-up: A review of potential risks and emerging solutions. Environ. Technol. Innov. 2016, 5, 10–21. [Google Scholar] [CrossRef]
- Najahi-Missaoui, W.; Arnold, R.D.; Cummings, B.S. Safe nanoparticles: Are we there yet? Int. J. Mol. Sci. 2020, 22, 385. [Google Scholar] [CrossRef]
- Rahman, S.U.; Wang, X.; Shahzad, M.; Bashir, O.; Li, Y.; Cheng, H. A review of the influence of nanoparticles on the physiological and biochemical attributes of plants with a focus on the absorption and translocation of toxic trace elements. Environ. Pollut. 2022, 310, 119916. [Google Scholar] [CrossRef]
- Okeke, E.S.; Ezeorba, T.P.C.; Mao, G.; Chen, Y.; Feng, W.; Wu, X. Nano-enabled agrochemicals/materials: Potential human health impact, risk assessment, management strategies and future prospects. Environ. Pollut. 2022, 295, 118722. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Xiong, G.; Liu, Z. Toxicity of metal-based nanoparticles: Challenges in the nano era. Front. Bioeng. Biotechnol. 2022, 10, 1001572. [Google Scholar] [CrossRef] [PubMed]
- Bakand, S.; Hayes, A. Toxicological considerations, toxicity assessment, and risk management of inhaled nanoparticles. Int. J. Mol. Sci. 2016, 17, 929. [Google Scholar] [CrossRef] [PubMed]
- Xuan, L.; Ju, Z.; Skonieczna, M.; Zhou, P.K.; Huang, R. Nanoparticles-induced potential toxicity on human health: Applications, toxicity mechanisms, and evaluation models. MedComm 2023, 4, e327. [Google Scholar] [CrossRef]
- Pizarro, C.; Escudey, M.; Caroca, E.; Pavez, C.; Zúñiga, G.E. Evaluation of zeolite, nanomagnetite, and nanomagnetite-zeolite composite materials as arsenic (V) adsorbents in hydroponic tomato cultures. Sci. Total Environ. 2021, 751, 141623. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Keswani, C.; Abhilash, P.; Fraceto, L.F.; Singh, H.B. Integrated approach of agri-nanotechnology: Challenges and future trends. Front. Plant Sci. 2017, 8, 471. [Google Scholar] [CrossRef] [PubMed]
- Okeke, E.S.; Chukwudozie, K.I.; Nyaruaba, R.; Ita, R.E.; Oladipo, A.; Ejeromedoghene, O.; Atakpa, E.O.; Agu, C.V.; Okoye, C.O. Antibiotic resistance in aquaculture and aquatic organisms: A review of current nanotechnology applications for sustainable management. Environ. Sci. Pollut. Res. 2022, 29, 69241–69274. [Google Scholar] [CrossRef] [PubMed]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; Zhong, Y.; Zhang, J.; Wang, Z.; Wang, L.; An, Y.; Lin, M.; Gao, Z.; Zhang, D. Biocompatibility of Fe3O4@ Au composite magnetic nanoparticles in vitro and in vivo. Int. J. Nanomed. 2011, 6, 2805–2819. [Google Scholar] [CrossRef]
- Mansouri, H.; Gholibegloo, E.; Mortezazadeh, T.; Yazdi, M.H.; Ashouri, F.; Malekzadeh, R.; Najafi, A.; Foroumadi, A.; Khoobi, M. A biocompatible theranostic nanoplatform based on magnetic gadolinium-chelated polycyclodextrin: In vitro and in vivo studies. Carbohydr. Polym. 2021, 254, 117262. [Google Scholar] [CrossRef]
- Jia, Z.; Zhang, Y.; Yan, A.; Wang, M.; Han, Q.; Wang, K.; Wang, J.; Qiao, C.; Pan, Z.; Chen, C.; et al. 1,25-dihydroxyvitamin D3 signaling-induced decreases in IRX4 inhibits NANOG-mediated cancer stem-like properties and gefitinib resistance in NSCLC cells. Cell Death Dis. 2020, 11, 670. [Google Scholar] [CrossRef]
- Feng, W.; Nie, W.; Cheng, Y.; Zhou, X.; Chen, L.; Qiu, K.; Chen, Z.; Zhu, M.; He, C. In vitro and in vivo toxicity studies of copper sulfide nanoplates for potential photothermal applications. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 901–912. [Google Scholar] [CrossRef]
- Almaki, J.H.; Nasiri, R.; Idris, A.; Nasiri, M.; Majid, F.A.A.; Losic, D. Trastuzumab-decorated nanoparticles for in vitro and in vivo tumor-targeting hyperthermia of HER2+ breast cancer. J. Mater. Chem. B 2017, 5, 7369–7383. [Google Scholar] [CrossRef]
- Do, X.-H.; Nguyen, T.D.; Le, T.T.H.; To, T.T.; Bui, T.V.K.; Pham, N.H.; Lam, K.; Hoang, T.M.N.; Ha, P.T. High Biocompatibility, MRI Enhancement, and Dual Chemo-and Thermal-Therapy of Curcumin-Encapsulated Alginate/Fe3O4 Nanoparticles. Pharmaceutics 2023, 15, 1523. [Google Scholar] [CrossRef]
- Zamay, G.S.; Zamay, T.N.; Lukyanenko, K.A.; Kichkailo, A.S. Aptamers increase biocompatibility and reduce the toxicity of magnetic nanoparticles used in biomedicine. Biomedicines 2020, 8, 59. [Google Scholar] [CrossRef]
- Arami, H.; Khandhar, A.; Liggitt, D.; Krishnan, K.M. In vivo delivery, pharmacokinetics, biodistribution and toxicity of iron oxide nanoparticles. Chem. Soc. Rev. 2015, 44, 8576–8607. [Google Scholar] [CrossRef]
- Gaikwad, V.L.; Choudhari, P.B.; Bhatia, N.M.; Bhatia, M.S. Characterization of pharmaceutical nanocarriers: In vitro and in vivo studies. In Nanomaterials for Drug Delivery and Therapy; Elsevier: Amsterdam, The Netherlands, 2019; pp. 33–58. [Google Scholar]
- Coricovac, D.-E.; Moacă, E.-A.; Pinzaru, I.; Cîtu, C.; Soica, C.; Mihali, C.-V.; Păcurariu, C.; Tutelyan, V.A.; Tsatsakis, A.; Dehelean, C.-A. Biocompatible colloidal suspensions based on magnetic iron oxide nanoparticles: Synthesis, characterization and toxicological profile. Front. Pharmacol. 2017, 8, 154. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, L.; Zheng, Y.; Li, L.; Wu, C.; Yang, H.; Huang, M.; An, X. Synthesis and biocompatibility of two-dimensional biomaterials. Colloids Surf. A Physicochem. Eng. Asp. 2019, 583, 124004. [Google Scholar] [CrossRef]
- Hao, J.; Zhang, C.; Feng, C.; Wang, Q.; Liu, Z.-Y.; Li, Y.; Mu, J.; Yang, E.-C.; Wang, Y. An ultra-highly active nanozyme of Fe, N co-doped ultrathin hollow carbon framework for antibacterial application. Chin. Chem. Lett. 2023, 34, 107650. [Google Scholar] [CrossRef]
- Yang, X.; Shao, G.; Zhang, Y.; Wang, W.; Qi, Y.; Han, S.; Li, H. Applications of magnetic particle imaging in biomedicine: Advancements and prospects. Front. Physiol. 2022, 13, 898426. [Google Scholar] [CrossRef] [PubMed]
- Bossmann, S.H.; Payne, M.M.; Kalita, M.; Bristow, R.M.; Afshar, A.; Perera, A.S. Iron-based magnetic nanosystems for diagnostic imaging and drug delivery: Towards transformative biomedical applications. Pharmaceutics 2022, 14, 2093. [Google Scholar] [CrossRef] [PubMed]
- Campora, S.; Ghersi, G. Recent developments and applications of smart nanoparticles in biomedicine. Nanotechnol. Rev. 2022, 11, 2595–2631. [Google Scholar] [CrossRef]
- Liu, N.N.; Pyatakov, A.P.; Zharkov, M.N.; Pyataev, N.A.; Sukhorukov, G.B.; Alekhina, Y.A.; Perov, N.S.; Gun’ko, Y.K.; Tishin, A.M. Optimization of Zn–Mn ferrite nanoparticles for low frequency hyperthermia: Exploiting the potential of superquadratic field dependence of magnetothermal response. Appl. Phys. Lett. 2022, 120, 102403. [Google Scholar] [CrossRef]
- Borse, S.; Rafique, R.; Murthy, Z.; Park, T.J.; Kailasa, S.K. Applications of upconversion nanoparticles in analytical and biomedical sciences: A review. Anal. 2022, 147, 3155–3179. [Google Scholar] [CrossRef] [PubMed]
- Binandeh, M. Performance of unique magnetic nanoparticles in biomedicine. Eur. J. Med. Chem. Rep. 2022, 6, 100072. [Google Scholar] [CrossRef]
- Sinha, S.; Kumar, R.; Anand, J.; Gupta, R.; Gupta, A.; Pant, K.; Dohare, S.; Tiwari, P.; Kesari, K.K.; Krishnan, S. Nanotechnology-Based Solutions for Antibiofouling Applications: An Overview. ACS Appl. Nano Mater. 2023, 6, 12828–12848. [Google Scholar] [CrossRef]
- Spirescu, V.A.; Chircov, C.; Grumezescu, A.M.; Vasile, B.Ș.; Andronescu, E. Inorganic nanoparticles and composite films for antimicrobial therapies. Int. J. Mol. Sci. 2021, 22, 4595. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Chung, E.; Johnston, I.; Ren, G.; Cheong, Y.-K. Exploitation of antimicrobial nanoparticles and their applications in biomedical engineering. Appl. Sci. 2021, 11, 4520. [Google Scholar] [CrossRef]
- Dzogbewu, T.C.; du Preez, W.B. Additive manufacturing of titanium-based implants with metal-based antimicrobial agents. Metals 2021, 11, 453. [Google Scholar] [CrossRef]
- Sofi, M.A.; Sunitha, S.; Sofi, M.A.; Pasha, S.K.; Choi, D. An overview of antimicrobial and anticancer potential of silver nanoparticles. J. King Saud Univ.-Sci. 2022, 34, 101791. [Google Scholar] [CrossRef]
- Sahoo, J.; Sarkhel, S.; Mukherjee, N.; Jaiswal, A. Nanomaterial-Based Antimicrobial Coating for Biomedical Implants: New Age Solution for Biofilm-Associated Infections. ACS Omega 2022, 7, 45962–45980. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, J.; Wang, X.; Wang, J.; Chen, L.; Cao, J.; Cao, W.; Liang, S.; Luan, P.; Zheng, K. CaCO3-coated hollow mesoporous silica nanoparticles for pH-responsive fungicides release. Chin. Chem. Lett. 2024, 109697. [Google Scholar] [CrossRef]
- Sousa, F.; Ferreira, D.; Reis, S.; Costa, P. Current insights on antifungal therapy: Novel nanotechnology approaches for drug delivery systems and new drugs from natural sources. Pharmaceuticals 2020, 13, 248. [Google Scholar] [CrossRef] [PubMed]
- Nami, S.; Aghebati-Maleki, A.; Aghebati-Maleki, L. Current applications and prospects of nanoparticles for antifungal drug delivery. EXCLI J. 2021, 20, 562. [Google Scholar] [PubMed]
- Nagaraj, S.; Manivannan, S.; Narayan, S. Potent antifungal agents and use of nanocarriers to improve delivery to the infected site: A systematic review. J. Basic Microbiol. 2021, 61, 849–873. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos Ramos, M.A.; Da Silva, P.B.; Spósito, L.; De Toledo, L.G.; Bonifácio, B.V.; Rodero, C.F.; Dos Santos, K.C.; Chorilli, M.; Bauab, T.M. Nanotechnology-based drug delivery systems for control of microbial biofilms: A review. Int. J. Nanomed. 2018, 13, 1179–1213. [Google Scholar] [CrossRef] [PubMed]
- Nagasa, G.D.; Belete, A. Review on nanomaterials and Nano-scaled Systems for Topical and Systemic Delivery of antifungal drugs. J. Multidiscip. Healthc. 2022, 15, 1819–1840. [Google Scholar] [CrossRef] [PubMed]
- Edis, Z.; Wang, J.; Waqas, M.K.; Ijaz, M.; Ijaz, M. Nanocarriers-mediated drug delivery systems for anticancer agents: An overview and perspectives. Int. J. Nanomed. 2021, 16, 1313–1330. [Google Scholar] [CrossRef] [PubMed]
- Thakuria, A.; Kataria, B.; Gupta, D. Nanoparticle-based methodologies for targeted drug delivery—An insight. J. Nanoparticle Res. 2021, 23, 87. [Google Scholar] [CrossRef]
- Yegappan, R.; Selvaprithiviraj, V.; Amirthalingam, S.; Jayakumar, R. Carrageenan based hydrogels for drug delivery, tissue engineering and wound healing. Carbohydr. Polym. 2018, 198, 385–400. [Google Scholar] [CrossRef]
- Friedrich, R.P.; Cicha, I.; Alexiou, C. Iron oxide nanoparticles in regenerative medicine and tissue engineering. Nanomaterials 2021, 11, 2337. [Google Scholar] [CrossRef] [PubMed]
- Fathi-Achachelouei, M.; Knopf-Marques, H.; Ribeiro da Silva, C.E.; Barthès, J.; Bat, E.; Tezcaner, A.; Vrana, N.E. Use of nanoparticles in tissue engineering and regenerative medicine. Front. Bioeng. Biotechnol. 2019, 7, 113. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xia, J.; Pang, X.; Zhao, M.; Wang, B.; Yang, L.; Wan, H.; Wu, J.; Fu, S. Magnetic nanoparticle-loaded electrospun polymeric nanofibers for tissue engineering. Mater. Sci. Eng. C 2017, 73, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Nethi, S.K.; Das, S.; Patra, C.R.; Mukherjee, S. Recent advances in inorganic nanomaterials for wound-healing applications. Biomater. Sci. 2019, 7, 2652–2674. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.; Morshed, M.; Memic, A.; Hassan, S.; Webster, T.J.; Marei, H.E.-S. Nanoparticles in tissue engineering: Applications, challenges and prospects. Int. J. Nanomed. 2018, 13, 5637–5655. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Cui, J.; Shen, H.; He, C.; Wang, X.; Shen, S.G.; Lin, K. Advances of nanomaterial applications in oral and maxillofacial tissue regeneration and disease treatment. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2021, 13, e1669. [Google Scholar] [CrossRef] [PubMed]
- Basit, S.; Ashraf, Z.; Lee, K.; Latif, M. First macrocyclic 3rd-generation ALK inhibitor for treatment of ALK/ROS1 cancer: Clinical and designing strategy update of lorlatinib. Eur. J. Med. Chem. 2017, 134, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Montiel Schneider, M.G.; Martín, M.J.; Otarola, J.; Vakarelska, E.; Simeonov, V.; Lassalle, V.; Nedyalkova, M. Biomedical applications of iron oxide nanoparticles: Current insights progress and perspectives. Pharmaceutics 2022, 14, 204. [Google Scholar] [CrossRef] [PubMed]
- Tran, S.; DeGiovanni, P.-J.; Piel, B.; Rai, P. Cancer nanomedicine: A review of recent success in drug delivery. Clin. Transl. Med. 2017, 6, 44. [Google Scholar] [CrossRef]
- Rezaei, B.; Yari, P.; Sanders, S.M.; Wang, H.; Chugh, V.K.; Liang, S.; Mostufa, S.; Xu, K.; Wang, J.P.; Gómez-Pastora, J. Magnetic nanoparticles: A review on synthesis, characterization, functionalization, and biomedical applications. Small 2024, 20, 2304848. [Google Scholar] [CrossRef]
- Verma, C.; Verma, D.K.; Berdimurodov, E.; Barsoum, I.; Alfantazi, A.; Hussain, C.M. Green magnetic nanoparticles: A comprehensive review of recent progress in biomedical and environmental applications. J. Mater. Sci. 2024, 59, 325–358. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Y.; Song, L.; Cui, X.; Zhou, J.; Jin, G.; Boccaccini, A.R.; Virtanen, S. Iron oxide nanoparticle-based nanocomposites in biomedical application. Trends Biotechnol. 2023, 41, 1471–1487. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M. Magnetic resonance imaging and iron-oxide nanoparticles in the era of personalized medicine. Nanotheranostics 2023, 7, 424. [Google Scholar] [CrossRef] [PubMed]
- Vangijzegem, T.; Lecomte, V.; Ternad, I.; Van Leuven, L.; Muller, R.N.; Stanicki, D.; Laurent, S. Superparamagnetic iron oxide nanoparticles (SPION): From fundamentals to state-of-the-art innovative applications for cancer therapy. Pharmaceutics 2023, 15, 236. [Google Scholar] [CrossRef] [PubMed]
- Chavda, V.P.; Balar, P.C.; Nalla, L.V.; Bezbaruah, R.; Gogoi, N.R.; Gajula, S.N.R.; Peng, B.; Meena, A.S.; Conde, J.; Prasad, R. Conjugated Nanoparticles for Solid Tumor Theranostics: Unraveling the Interplay of Known and Unknown Factors. ACS Omega 2023, 8, 37654–37684. [Google Scholar] [CrossRef] [PubMed]
- Fleming, C.L.; Golzan, M.; Gunawan, C.; McGrath, K.C. Systematic and Bibliometric Analysis of Magnetite Nanoparticles and Their Applications in (Biomedical) Research. Glob. Chall. 2023, 7, 2200009. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Althomali, R.H.; Ansari, S.A.; Saleh, E.A.M.; Gupta, J.; Kambarov, K.D.; Alsaab, H.O.; Alwaily, E.R.; Hussien, B.M.; Mustafa, Y.F. Advances in preparation, biomedical, and pharmaceutical applications of chitosan-based gold, silver, and magnetic nanoparticles: A review. Int. J. Biol. Macromol. 2023, 251, 126390. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Zhang, Y.; Sun, L.; Wang, J.; Zhao, Z.; Huang, Z.; Mao, W.; Xue, R.; Chen, R.; Luo, J. Modulated ultrasmall γ-Fe2O3 nanocrystal assemblies for switchable magnetic resonance imaging and photothermal-ferroptotic-chemical synergistic cancer therapy. Adv. Funct. Mater. 2023, 33, 2211251. [Google Scholar] [CrossRef]
- Chen, Q.; Ma, X.; Xie, L.; Chen, W.; Xu, Z.; Song, E.; Zhu, X.; Song, Y. Iron-based nanoparticles for MR imaging-guided ferroptosis in combination with photodynamic therapy to enhance cancer treatment. Nanoscale 2021, 13, 4855–4870. [Google Scholar] [CrossRef]
- Li, Z.; Bai, R.; Yi, J.; Zhou, H.; Xian, J.; Chen, C. Designing Smart Iron Oxide Nanoparticles for MR Imaging of Tumors. Chem. Biomed. Imaging 2023, 1, 315–339. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, L.; Yu, M.; Yu, D.; Chen, Y.; Zhang, J. Engineering nanoprobes for magnetic resonance imaging of brain diseases. Chem. Eng. J. 2023, 481, 148472. [Google Scholar] [CrossRef]
- Bhattacharya, D.; Mukhopadhyay, M.; Shivam, K.; Tripathy, S.; Patra, R.; Pramanik, A. Recent developments in photodynamic therapy and its application against multidrug resistant cancers. Biomed. Mater. 2023, 18, 062005. [Google Scholar] [CrossRef]
- Savari, M.-N.; Jabali, A. Drug Conjugation Chemistry in Iron Oxide Nanoparticles (IONPs). In Theranostic Iron-Oxide Based Nanoplatforms in Oncology: Synthesis, Metabolism, and Toxicity for Simultaneous Imaging and Therapy; Springer: Berlin/Heidelberg, Germany, 2023; pp. 15–34. [Google Scholar]
- Li, X.; Yue, R.; Guan, G.; Zhang, C.; Zhou, Y.; Song, G. Recent development of pH-responsive theranostic nanoplatforms for magnetic resonance imaging-guided cancer therapy. Exploration 2023, 3, 20220002. [Google Scholar] [CrossRef]
- Segers, F.M.; Ruder, A.V.; Westra, M.M.; Lammers, T.; Dadfar, S.M.; Roemhild, K.; Lam, T.S.; Kooi, M.E.; Cleutjens, K.B.; Verheyen, F.K. Magnetic resonance imaging contrast-enhancement with superparamagnetic iron oxide nanoparticles amplifies macrophage foam cell apoptosis in human and murine atherosclerosis. Cardiovasc. Res. 2022, 118, 3346–3359. [Google Scholar] [CrossRef]
- Nelson, N.R.; Port, J.D.; Pandey, M.K. Use of superparamagnetic iron oxide nanoparticles (SPIONs) via multiple imaging modalities and modifications to reduce cytotoxicity: An educational review. J. Nanotheranostics 2020, 1, 105–135. [Google Scholar] [CrossRef]
- Dash, A.; Blasiak, B.; Tomanek, B.; Banerjee, A.; Trudel, S.; Latta, P.; van Veggel, F.C. Colloidally Stable Monodisperse Fe Nanoparticles as T2 Contrast Agents for High-Field Clinical and Preclinical Magnetic Resonance Imaging. ACS Appl. Nano Mater. 2021, 4, 1235–1242. [Google Scholar] [CrossRef]
- Liu, C.L.; Peng, Y.K.; Chou, S.W.; Tseng, W.H.; Tseng, Y.J.; Chen, H.C.; Hsiao, J.K.; Chou, P.T. One-step, room-temperature synthesis of glutathione-capped iron-oxide nanoparticles and their application in in vivo T1-weighted magnetic resonance imaging. Small 2014, 10, 3962–3969. [Google Scholar] [CrossRef]
- Szwed, M.; Marczak, A. Application of Nanoparticles for Magnetic Hyperthermia for Cancer Treatment—The Current State of Knowledge. Cancers 2024, 16, 1156. [Google Scholar] [CrossRef]
- Famiani, S. Synthesis and Characterisation of Ironbased Nanoparticles for Magnetic Hyperthermia. Ph.D. Thesis, UCL (University College London), London, UK, 2020. [Google Scholar]
- Tajabadi, M.; Rahmani, I.; Mirkazemi, S.M.; Orimi, H.G. Insights into the synthesis optimization of Fe@SiO2 Core-Shell nanostructure as a highly efficient nano-heater for magnetic hyperthermia treatment. Adv. Powder Technol. 2022, 33, 103366. [Google Scholar] [CrossRef]
- Mondal, P.; Anweshan, A.; Purkait, M.K. Green synthesis and environmental application of iron-based nanomaterials and nanocomposite: A review. Chemosphere 2020, 259, 127509. [Google Scholar] [CrossRef]
- Tian, Z.; Yu, X.; Ruan, Z.; Zhu, M.; Zhu, Y.; Hanagata, N. Magnetic mesoporous silica nanoparticles coated with thermo-responsive copolymer for potential chemo-and magnetic hyperthermia therapy. Microporous Mesoporous Mater. 2018, 256, 1–9. [Google Scholar] [CrossRef]
- Yang, G.; Gong, H.; Liu, T.; Sun, X.; Cheng, L.; Liu, Z. Two-dimensional magnetic WS2@Fe3O4 nanocomposite with mesoporous silica coating for drug delivery and imaging-guided therapy of cancer. Biomaterials 2015, 60, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Omerović, N.; Djisalov, M.; Živojević, K.; Mladenović, M.; Vunduk, J.; Milenković, I.; Knežević, N.Ž.; Gadjanski, I.; Vidić, J. Antimicrobial nanoparticles and biodegradable polymer composites for active food packaging applications. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2428–2454. [Google Scholar] [CrossRef] [PubMed]
- Atta, O.M.; Manan, S.; Shahzad, A.; Ul-Islam, M.; Ullah, M.W.; Yang, G. Biobased materials for active food packaging: A review. Food Hydrocoll. 2022, 125, 107419. [Google Scholar] [CrossRef]
- Natarajan, S.; Harini, K.; Gajula, G.P.; Sarmento, B.; Neves-Petersen, M.T.; Thiagarajan, V. Multifunctional magnetic iron oxide nanoparticles: Diverse synthetic approaches, surface modifications, cytotoxicity towards biomedical and industrial applications. BMC Mater. 2019, 1, 2. [Google Scholar] [CrossRef]
- Dey, A.; Neogi, S. Oxygen scavengers for food packaging applications: A review. Trends Food Sci. Technol. 2019, 90, 26–34. [Google Scholar] [CrossRef]
- Foltynowicz, Z. Nanoiron-Based Composite Oxygen Scavengers for Food Packaging. In Composites Materials for Food Packaging; Scrivener Publishing LLC.: Beverly, MA, USA, 2018; pp. 209–234. [Google Scholar]
- Hoseinnejad, M.; Jafari, S.M.; Katouzian, I. Inorganic and metal nanoparticles and their antimicrobial activity in food packaging applications. Crit. Rev. Microbiol. 2018, 44, 161–181. [Google Scholar] [CrossRef] [PubMed]
- Pirozzi, A.; Pataro, G.; Donsì, F.; Ferrari, G. Edible coating and pulsed light to increase the shelf life of food products. Food Eng. Rev. 2021, 13, 544–569. [Google Scholar] [CrossRef]
- Yiannikourides, A.; Latunde-Dada, G.O. A short review of iron metabolism and pathophysiology of iron disorders. Medicines 2019, 6, 85. [Google Scholar] [CrossRef]
- Perumal, A.B.; Nambiar, R.B.; Sellamuthu, P.S.; Sadiku, E.R. Application of biosynthesized nanoparticles in food, food packaging and dairy industries. In Biological Synthesis of Nanoparticles and Their Applications; CRC Press: Boca Raton, FL, USA, 2019; pp. 145–158. [Google Scholar]
- Luo, X.; Zaitoon, A.; Lim, L.T. A review on colorimetric indicators for monitoring product freshness in intelligent food packaging: Indicator dyes, preparation methods, and applications. Compr. Rev. Food Sci. Food Saf. 2022, 21, 2489–2519. [Google Scholar] [CrossRef]
- Rabajczyk, A.; Zielecka, M.; Porowski, R.; Hopke, P.K. Metal nanoparticles in the air: State of the art and future perspectives. Environ. Sci. Nano 2020, 7, 3233–3254. [Google Scholar] [CrossRef]
- Hasan, M.; Ullah, I.; Zulfiqar, H.; Naeem, K.; Iqbal, A.; Gul, H.; Ashfaq, M.; Mahmood, N. Biological entities as chemical reactors for synthesis of nanomaterials: Progress, challenges and future perspective. Mater. Today Chem. 2018, 8, 13–28. [Google Scholar] [CrossRef]
- Javed, R.; Zia, M.; Naz, S.; Aisida, S.O.; Ain, N.u.; Ao, Q. Role of capping agents in the application of nanoparticles in biomedicine and environmental remediation: Recent trends and future prospects. J. Nanobiotechnol. 2020, 18, 172. [Google Scholar] [CrossRef] [PubMed]
- Modi, S.; Prajapati, R.; Inwati, G.K.; Deepa, N.; Tirth, V.; Yadav, V.K.; Yadav, K.K.; Islam, S.; Gupta, P.; Kim, D.-H. Recent trends in fascinating applications of nanotechnology in allied health sciences. Crystals 2021, 12, 39. [Google Scholar] [CrossRef]
- Swain, S.; Kumar Sahu, P.; Beg, S.; Manohar Babu, S. Nanoparticles for cancer targeting: Current and future directions. Curr. Drug Deliv. 2016, 13, 1290–1302. [Google Scholar] [CrossRef] [PubMed]
- Baig, N. Two-dimensional nanomaterials: A critical review of recent progress, properties, applications, and future directions. Compos. Part A Appl. Sci. Manuf. 2022, 165, 107362. [Google Scholar] [CrossRef]
- Pathak, A.; Kothari, R.; Vinoba, M.; Habibi, N.; Tyagi, V. Fungal bioleaching of metals from refinery spent catalysts: A critical review of current research, challenges, and future directions. J. Environ. Manag. 2021, 280, 111789. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Goyal, A.K.; Rath, G. Recent advances in metal nanoparticles in cancer therapy. J. Drug Target. 2018, 26, 617–632. [Google Scholar] [CrossRef] [PubMed]
- Mundekkad, D.; Cho, W.C. Nanoparticles in clinical translation for cancer therapy. Int. J. Mol. Sci. 2022, 23, 1685. [Google Scholar] [CrossRef]
- Sindhwani, S.; Chan, W.C. Nanotechnology for modern medicine: Next step towards clinical translation. J. Intern. Med. 2021, 290, 486–498. [Google Scholar] [CrossRef]
- Qiao, R.; Fu, C.; Forgham, H.; Javed, I.; Huang, X.; Zhu, J.; Whittaker, A.K.; Davis, T.P. Magnetic Iron Oxide Nanoparticles for Brain Imaging and Drug Delivery. Adv. Drug Deliv. Rev. 2023, 197, 114822. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Feng, W.; Chen, Y.; Shi, J. Inorganic nanoparticles in clinical trials and translations. Nano Today 2020, 35, 100972. [Google Scholar] [CrossRef]
- Mittal, D.; Kaur, G.; Singh, P.; Yadav, K.; Ali, S.A. Nanoparticle-based sustainable agriculture and food science: Recent advances and future outlook. Front. Nanotechnol. 2020, 2, 579954. [Google Scholar] [CrossRef]
- Mohana, N.C.; Mithun, P.; Rao, H.Y.; Mahendra, C.; Satish, S. Nanoparticle applications in sustainable agriculture, poultry, and food: Trends and perspective. In Nanotoxicity; Elsevier: Amsterdam, The Netherlands, 2020; pp. 341–353. [Google Scholar]
- Pramanik, P.; Krishnan, P.; Maity, A.; Mridha, N.; Mukherjee, A.; Rai, V. Application of nanotechnology in agriculture. Environ. Nanotechnol. 2020, 4, 317–348. [Google Scholar]
- Tabassum, H.; Ahmad, I.; Ahmad, A.; Tabassum, H.; Kiyani, M.Z.; Khan, A.; Younis, M.; Asiri, A.M. Recent advancements, developments, and regulatory issues in nanomedicine. In Nanomedicine Manufacturing and Applications; Elsevier: Amsterdam, The Netherlands, 2021; pp. 39–55. [Google Scholar]
- Tekade, R.K.; Maheshwari, R.; Soni, N.; Tekade, M.; Chougule, M.B. Nanotechnology for the development of nanomedicine. In Nanotechnology-Based Approaches for Targeting and Delivery of Drugs and Genes; Elsevier: Amsterdam, The Netherlands, 2017; pp. 3–61. [Google Scholar]
- Cardoso, V.F.; Francesko, A.; Ribeiro, C.; Bañobre-López, M.; Martins, P.; Lanceros-Mendez, S. Advances in magnetic nanoparticles for biomedical applications. Adv. Healthc. Mater. 2018, 7, 1700845. [Google Scholar] [CrossRef] [PubMed]
- Sangaiya, P.; Jayaprakash, R. A review on iron oxide nanoparticles and their biomedical applications. J. Supercond. Nov. Magn. 2018, 31, 3397–3413. [Google Scholar] [CrossRef]
- Martins, P.M.; Lima, A.C.; Ribeiro, S.; Lanceros-Mendez, S.; Martins, P. Magnetic nanoparticles for biomedical applications: From the soul of the earth to the deep history of ourselves. ACS Appl. Bio Mater. 2021, 4, 5839–5870. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.; Ashraf, N.; Ashraf, T.; Zhou, R.-B.; Yin, D.-C. Biological synthesis of metallic nanoparticles (MNPs) by plants and microbes: Their cellular uptake, biocompatibility, and biomedical applications. Appl. Microbiol. Biotechnol. 2019, 103, 2913–2935. [Google Scholar] [CrossRef]
- Wang, J.; Tang, J. Fe-based Fenton-like catalysts for water treatment: Preparation, characterization and modification. Chemosphere 2021, 276, 130177. [Google Scholar] [CrossRef]
- Dinali, R.; Ebrahiminezhad, A.; Manley-Harris, M.; Ghasemi, Y.; Berenjian, A. Iron oxide nanoparticles in modern microbiology and biotechnology. Crit. Rev. Microbiol. 2017, 43, 493–507. [Google Scholar] [CrossRef]


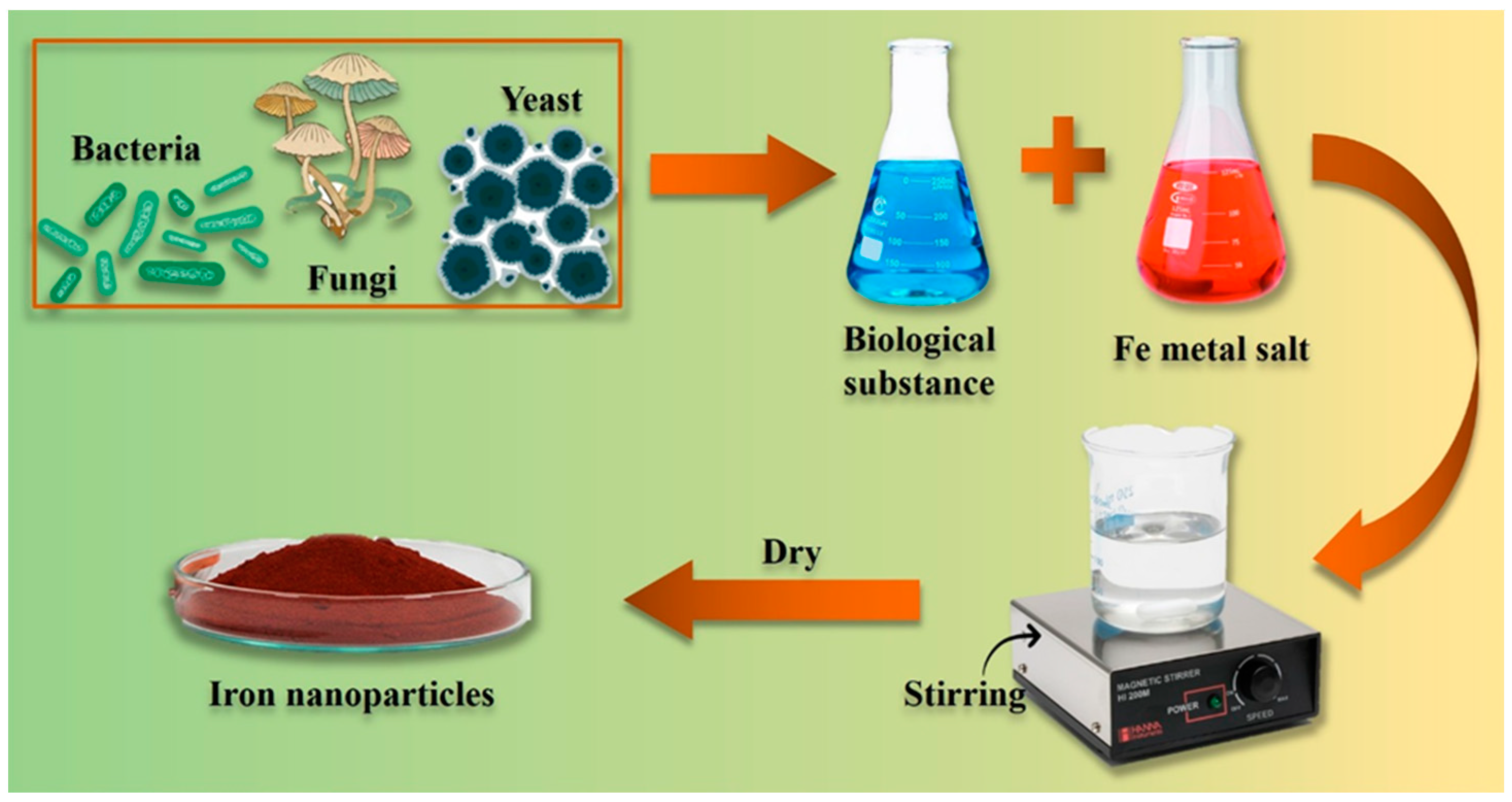

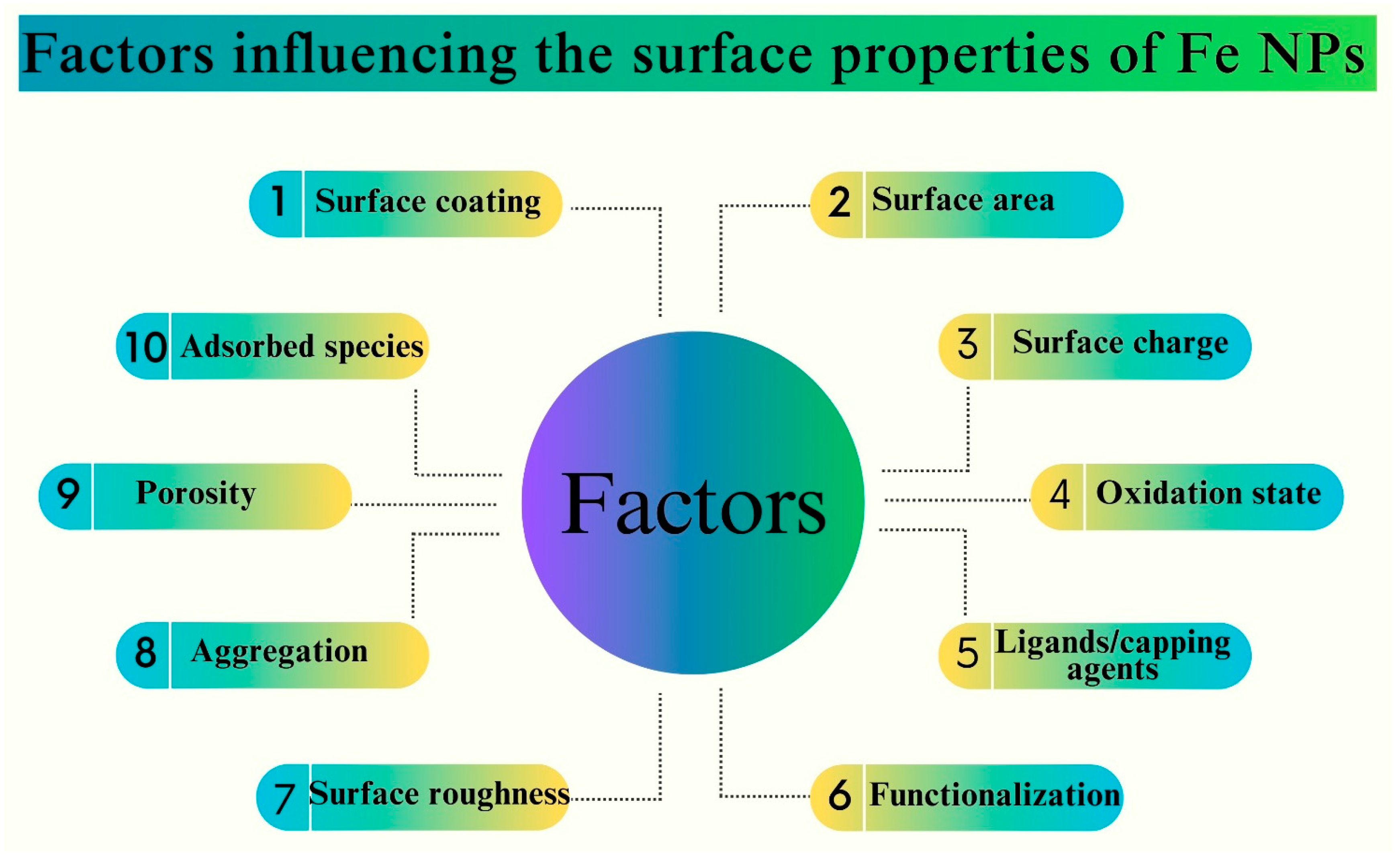
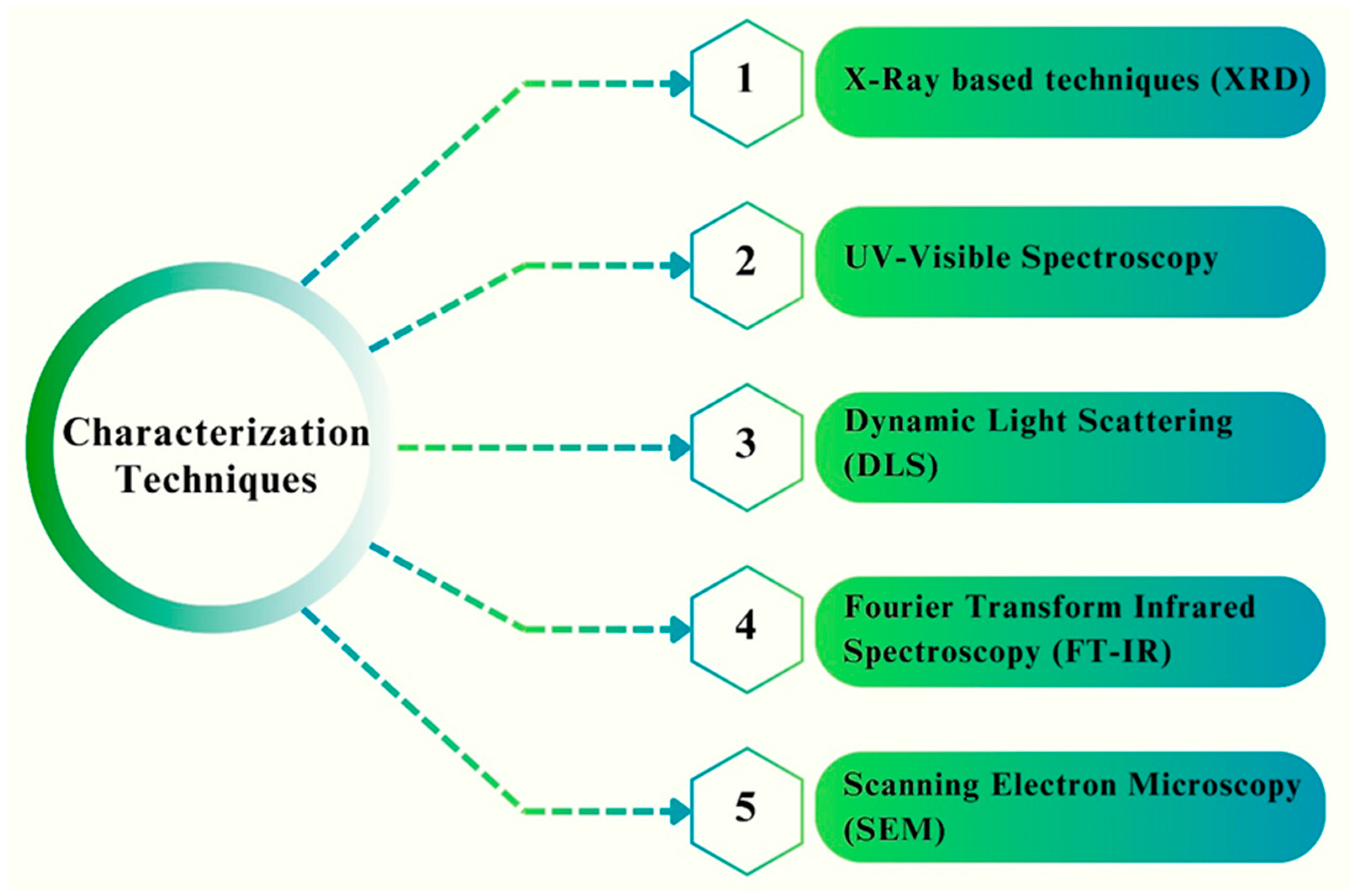
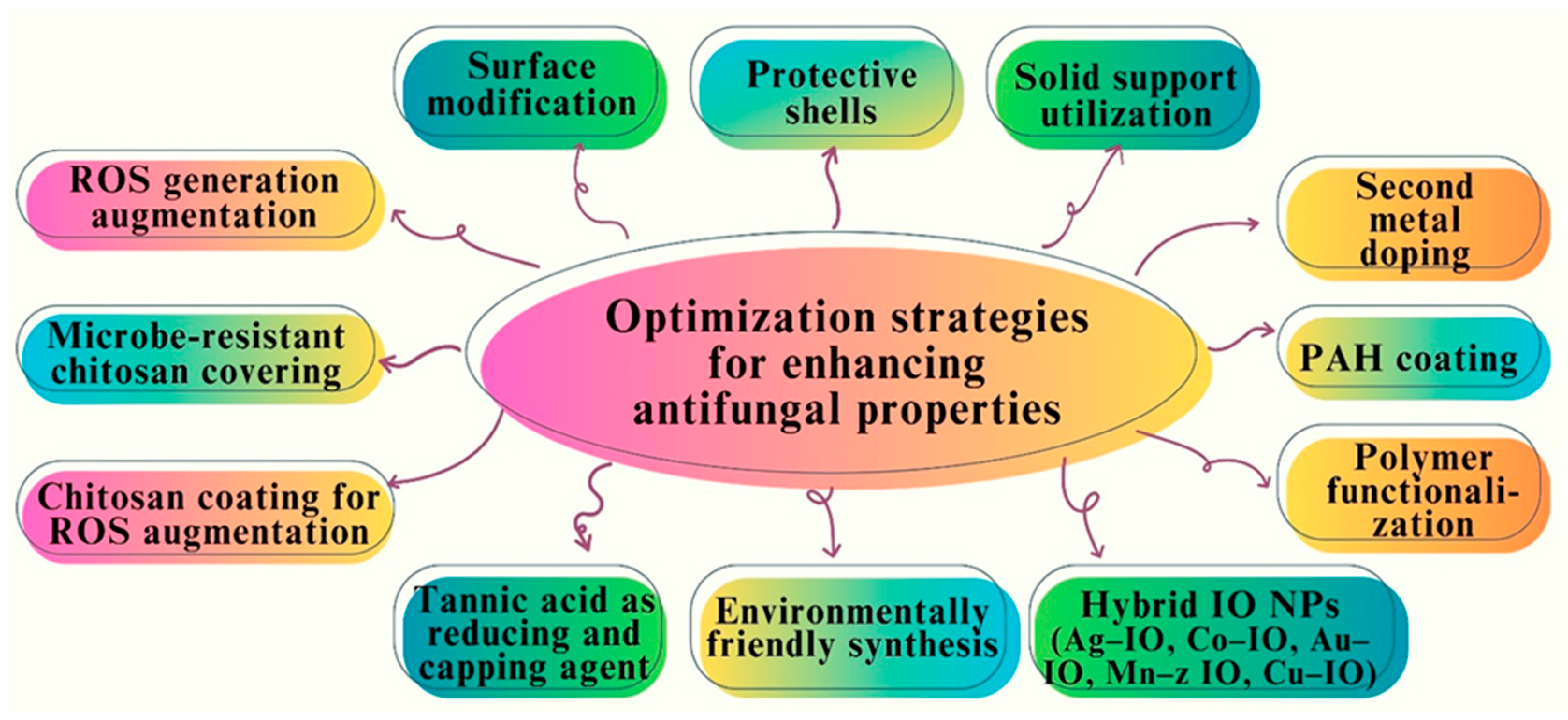



| Nanoparticle | Synthesis Method | NP Size (nm) | Surface Functionalization | Antifungal Activity/Fungal Strains | Biocompatibility Assessment | Ref |
|---|---|---|---|---|---|---|
| Fe2O3 | Chemical co-precipitation | 100–130 | Oleic acid functionalization, tagged with Itraconazole and encapsulation with PHB. | Against C. albicans at the highest concentration of 20 µg/mL. | Encapsulation with Polyhydroxybutyrate (PHB). | [74] |
| Fe2O3 | Green Method | 10–30 | Iron oxide NPs (1%) were fabricated using tannic acid in an alkaline medium. | Trichothecium roseum, Cladosporium herbarum, Penicillium chrysogenum | The highest inhibition was caused by P. chrysogenum (28.67 mm) followed by A. niger (26.33 mm). | [75] |
| Chitosan-coated Fe2O3 | Co-precipitation | 10.4 ± 4.9 | Antimicrobial activity was tested by agar well diffusion and analyzed by measuring the diameter of the inhibition zone. | Against Aspergillus niger (A. niger) and Fusarium solani (F. solani. | Inhibition zone of chitosan-coated Fe2O3 NPs = 14.5 to 18.5 mm. | [76] |
| Fe2O3 | Biosynthesis | 30.98 | - | A. brasiliensis, A. alternata, F. oxysporum, C. albicans | Diameters of inhibition zone, A. brasiliensis = 36.08 ± 1.37, A. alternata = 27.59 ± 1.32, F. oxysporum = 26.11 ± 1.11, C. albicans = 53.67 ± 3.18 | [77] |
| Fe2O3 | Green Synthesis | 20–86 | 10 g of Wedelia urticifolia leaves was utilized for surface functionalization. | Against Candida albicans. | - | [78] |
| Ag, Cu, Fe, Zn | Biosynthesis | 18.33 | Ginger and garlic extract. | C. albicans | Inhibition one diameter = 7 mm | [79] |
| Fe | Green Synthesis | 50 | Aqueous leaves extract of Plumeria obtuse. | A. niger and S. commune. | Doxorubicin and Cisplatin were used as standards while 2.1 ± 0.01% hemolysis is shown by Fe NPs. | [80] |
| Fe2O3 | Green synthesis | 76 | Microalgal proteins, carbohydrates, and polyphenols are responsible for bio fabrication. | Fusarium oxysporum, Fusarium tricinctum, Fusarium maniliforme, Rhizoctonia solani, and Phythium sp. | Inhibition one diameter = 10–25 mm | [81] |
| Fe2O3 | Green synthesis | 38 | Agar well diffusion method with week-old fungal cultures grown on potato dextrose medium. | Aspergillus niger and Mucor piriformis | Activity against Aspergillus niger = 16 mm and Mucor piriformis = 26 mm | [82] |
| Fe2O3/Ag@Fe2O3 | Green synthesis | 4 ± 1 | Agar well diffusion and macro dilution broth method. | Candida albicans | Inhibition zone Fe2O3 = 10 mm | [83] |
| SrFeO3-δ | Sol–gel method | 91.28 | Nystatin was used as a standard. | Candida albicans and Aspergillus brasiliensis | Inhibition zone diameter Candida albicans = 7.81 ± 0.91. Aspergillus brasiliensis = 20.2 ± 0.05 | [84] |
| Ag–Fe bimetallic | Green synthesis | 3–30 | Micro dilution. | C. albican | MIC value = 62.5 ppm | [85] |
| Fe NPs | Green Synthesis | 40.4 | - | C. albican | Inhibition zone = 21 mm | [86] |
| Fe/Cu/Ag | Green synthesis | - | Aflatoxins (B1, B2, G1 and G2) standards used. | Aspergillus flavus and A. parasiticus | - | [87] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sandhu, Z.A.; Raza, M.A.; Alqurashi, A.; Sajid, S.; Ashraf, S.; Imtiaz, K.; Aman, F.; Alessa, A.H.; Shamsi, M.B.; Latif, M. Advances in the Optimization of Fe Nanoparticles: Unlocking Antifungal Properties for Biomedical Applications. Pharmaceutics 2024, 16, 645. https://doi.org/10.3390/pharmaceutics16050645
Sandhu ZA, Raza MA, Alqurashi A, Sajid S, Ashraf S, Imtiaz K, Aman F, Alessa AH, Shamsi MB, Latif M. Advances in the Optimization of Fe Nanoparticles: Unlocking Antifungal Properties for Biomedical Applications. Pharmaceutics. 2024; 16(5):645. https://doi.org/10.3390/pharmaceutics16050645
Chicago/Turabian StyleSandhu, Zeshan Ali, Muhammad Asam Raza, Abdulmajeed Alqurashi, Samavia Sajid, Sufyan Ashraf, Kainat Imtiaz, Farhana Aman, Abdulrahman H. Alessa, Monis Bilal Shamsi, and Muhammad Latif. 2024. "Advances in the Optimization of Fe Nanoparticles: Unlocking Antifungal Properties for Biomedical Applications" Pharmaceutics 16, no. 5: 645. https://doi.org/10.3390/pharmaceutics16050645
APA StyleSandhu, Z. A., Raza, M. A., Alqurashi, A., Sajid, S., Ashraf, S., Imtiaz, K., Aman, F., Alessa, A. H., Shamsi, M. B., & Latif, M. (2024). Advances in the Optimization of Fe Nanoparticles: Unlocking Antifungal Properties for Biomedical Applications. Pharmaceutics, 16(5), 645. https://doi.org/10.3390/pharmaceutics16050645









